PURPOSE:
We surveyed oncologists who treat classic Hodgkin lymphoma (cHL) as part of the CONNECT study to understand the treatment decision‐making process, including the impact of positron emission tomography/computed tomography (PET/CT) imaging.
METHODS:
US physicians self-identifying as oncologists, hematologists, or hematologists/oncologists with ≥2 years of practice experience who treated ≥1 adult with stage III/IV cHL in the frontline setting in the last year were surveyed (October 19-November 16, 2020). Physician demographics, guideline adherence, and PET/CT utilization, interpretation, and access barriers were assessed.
RESULTS:
In total, 301 physicians participated in the survey. Eighty-eight percent of physicians gave somewhat-to-significant consideration to NCCN guidelines. Most physicians (94%; n = 284) reported obtaining a PET/CT scan at diagnosis; of these physicians, 97% reported obtaining an interim PET/CT scan for stage III/IV cHL, with 65% typically obtaining an interim PET/CT scan after cycle 2. The Deauville 5-point scale (5PS) was the primary scoring system used to review PET/CT results by 62% of physicians, with a positive score defined as ≥3 by 44%, ≥4 by 37%, and ≥2 by 12% of physicians. Fifty-five percent of physicians reported difficulty in obtaining PET/CT scans.
CONCLUSION:
Although most physicians considered NCCN guidelines when treating patients with stage III/IV cHL, interim PET/CT scans after cycle 2 were not universally obtained. When PET/CT scans were obtained, Deauville 5PS scores were not always provided, and variability existed on what defined a positive score. These findings suggest that opportunities exist for education and improved PET-adapted treatment approaches.
INTRODUCTION
Hodgkin lymphoma (HL), a rare cancer,1 is one of the most common cancers among adolescents and young adults (age 15-39 years), who compose the largest affected group.2 Among patients with HL, 95% have classic HL (cHL).1
Guidelines recommend one of the following regimens frontline for stage III/IV cHL in adult patients3: ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine); A + AVD (brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine); and escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone).
As real-world data suggest that young adults with HL are treated with ABVD or escalated BEACOPP, an unmet need remains to reduce long-term treatment-related toxicities among adolescents and young adults treated with either regimen.4 To minimize bleomycin exposure, positron emission tomography (PET)–adapted treatments, including integrated PET and computed tomography (CT) scans, such as those used in the Risk-Adapted Treatment of HL (RATHL) and SWOG S0816 trials, have emerged as potential alternatives to six cycles of ABVD.5-9 In these approaches, treatment intensity is escalated or de-escalated on the basis of response measured using an interim PET/CT scan. As adverse events are reduced when bleomycin is removed after two cycles, guidelines recommend a PET-adapted approach to assess ABVD response after two treatment cycles.3
Recommendations are to perform PET/CT scans under the supervision of and interpreted by a physician with specific qualifications.3,10 Recommendations after a negative PET/CT scan (Deauville score of 1-3 on the corresponding 5-point scale [5PS]) after two cycles of ABVD are to de-escalate therapy to AVD (doxorubicin, vinblastine, and dacarbazine), and recommendations after a positive PET/CT scan (Deauville 5PS score of 4 or 5) after two cycles of ABVD are to change therapy to escalated BEACOPP.3 PET-adapted therapies can be challenging to implement when timely access, interpretation, and reimbursement of PET/CT scans are limited.11
We surveyed oncologists who treat patients with cHL as part of CONNECT (Classic Hodgkin Lymphoma: Real-World Observations from Physicians, Patients, and Caregivers on the Disease and Its Treatment), which is, to our knowledge, the first real-world survey of physicians, patients, and caregivers about cHL, to understand their treatment decision-making process and how PET/CT scan utilization and interpretation, and barriers to PET/CT scan access, reimbursement, and comprehension, influence treatment choices.
METHODS
Study Design
The anonymous, online CONNECT physician survey was administered between October 19, 2020, and November 16, 2020. This study received exemption status from the New England Institutional Review Board. Physicians were blinded to the study sponsor, and participants were blinded to the sponsor and researchers.
Study Participants
Physicians from the United States were recruited via e-mail using existing large online panels of health care providers. The panels seek to represent the US physician population and leverage multiple sources for physician recruitment to increase reach and capacity, improve consistency, and minimize bias. Physicians were screened at survey initiation to ensure that they met study inclusion criteria.
Eligible US physicians self-identified as medical oncologists, hematologists/oncologists, or hematologists having two or more years in medical practice and having treated at least one patient at least age 18 years with cHL with active disease (≥1 patient with stage III/IV cHL and ≥1 patient treated in the frontline setting) in the past 12 months. Physicians practicing in Maine or Vermont were excluded, as Sunshine Act laws in these states would unblind study participants to the study sponsor.
Study Survey
Data were obtained on physician demographics, clinical trial and guideline adherence, overall PET/CT and interim PET/CT scan utilization and interpretation, and barriers to PET/CT scan access.
Study Analysis
Descriptive analyses were conducted using WinCross software. Univariate statistics were used to describe physician demographics. Continuous data were reported as mean and standard deviation or median and range on the basis of distribution; categorical data were reported as numbers and percentage of total. Nonmutually exclusive data were reported as numbers and percentages of total sample size.
Consent and Compliance
This study was conducted in full compliance with the Guidelines for Good Pharmacoepidemiology Practice (GPP), published by the International Society of Pharmacoepidemiology.12 Physician participation and responses were anonymous, and no identifying information was included in the analyses.
RESULTS
Participant Sample and Practice Characteristics
A total of 301 US-based physicians completed the survey. Eighty percent of respondents self-identified as hematologists/oncologists, with the remainder identifying as medical oncologists. On average, participating physicians had been practicing for 16 years. Physicians were from all four regions of the United States (South, 34%; Northeast, 26%; West, 21%; Midwest, 20%) and on average reported spending approximately 90% of their professional time in direct patient care.
In the 12 months preceding survey participation, 16% of physicians saw more than 100 patients (high volume), 37% of physicians saw 31 to 100 patients (medium volume), and 47% of physicians saw 30 or fewer patients (low volume) with active disease or cHL survivors. Physicians saw a median of 16 adult patients with cHL with active disease (including newly diagnosed and relapsed or refractory disease) and 15 adult cHL survivors; 41% of these patients were age 18-39 years, 34% were age 40-59 years, and 25% were age 60 years or older. Fifty-nine percent of these patients had stage III/IV disease. Sixty-two percent of physicians practiced in a community setting (42% office/clinic-based private practice, 12% cancer center affiliated with a community hospital, and 8% nonteaching/community hospital–based practice). The remainder of physicians worked in an academic setting (22% teaching/university hospital–based practice and 16% cancer center affiliated with an academic hospital).
Physicians who practiced in academic settings, versus community settings, saw a greater number of patients with active disease (median [IQR], 20 [10-46] v 15 [6-34] patients; P = .036) and who were younger in age (20 [8-30] v 11 [5-25] age 18-39 years; P = .012).
Clinical Trial and Guideline Adherence
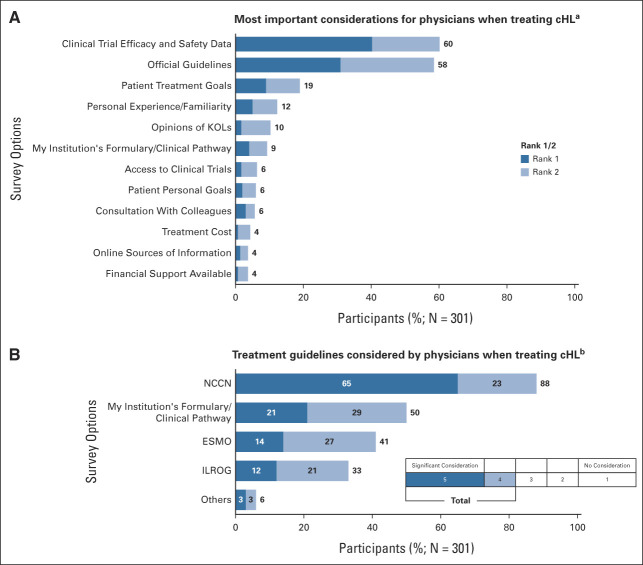
Physicians reported that efficacy and safety data from clinical trials (60%) or official guidelines (58%) were their most important considerations when treating patients with cHL (Fig 1A); patient treatment goals were considered important by 19% of physician respondents. Fewer than 5% of physicians responded that costs, online sources of information, or available financial support were important considerations.
FIG 1.
Physician considerations when treating cHL. (A) Question: What are the most important considerations for you when treating cHL? Please rank up to five. (B) Question: When treating cHL, how much do you consider the following guidelines? Response on the basis of the percentage of physicians selecting either four or five on the 5-point scale shown. cHL, classic Hodgkin lymphoma; ESMO, European Society for Medical Oncology; ILROG, International Lymphoma Radiation Oncology Group; KOLs, key opinion leaders; NCCN, National Comprehensive Cancer Network.
The importance that physicians placed on guidelines was inversely associated with the volume of patients they saw in the 12 months preceding the survey. Thirty-nine percent of physicians who saw more than 100 patients ranked guidelines as the first or second most important consideration versus 54% of physicians who saw 31 to 100 patients and 69% of physicians who saw fewer than 30 patients. When participating physicians were asked how much they consider specific guidelines when treating patients with cHL, 65% of physicians reported that they gave significant consideration to NCCN guidelines; an additional 23% reported somewhat considering NCCN guidelines (Fig 1B).
Overall PET/CT and Interim PET/CT Scan Utilization and Interpretation
When participating physicians were asked specifically about using a combined PET/CT scan, 94% (n = 284) reported using a combined scan to diagnose/stage patients with cHL at the time of initial diagnosis. Of those, the majority (97%) also reported obtaining an interim PET/CT scan for patients with stage III/IV cHL.
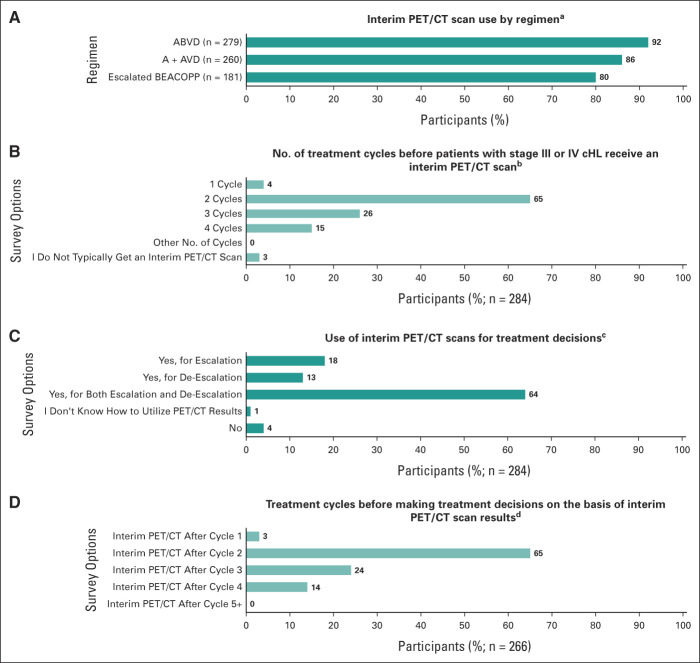
Of physicians using a PET/CT scan to diagnose/stage patients with stage III/IV cHL, 98% of physicians (n = 279) reported using ABVD, 92% (n = 260) reported using A + AVD, and 64% (n = 181) reported using escalated BEACOPP. Interim PET/CT scans for patients with stage III/IV cHL were obtained by most physicians during these treatments (ABVD, 92%; A + AVD, 86%; escalated BEACOPP, 80%; Fig 2A). When physicians who reported using PET/CT scans were asked after what cycle patients with stage III/IV cHL received an interim PET/CT scan, 65% reported typically obtaining an interim PET/CT scan after cycle 2 and 41% after cycle 3 or 4 (26% after cycle 3 and 15% after cycle 4; Fig 2B). Most physicians (64%) reported making treatment decisions on the basis of the interim PET/CT scan results for both escalation and de-escalation of treatment; 18% reported making treatment decisions on the basis of results only for escalation, and 13% reported making treatment decisions on the basis of results only for de-escalation (Fig 2C). Of physicians using an interim PET/CT scan for treatment decisions (n = 266), 65% reported doing so after cycle 2, 24% after cycle 3, and 14% after cycle 4 (Fig 2D).
FIG 2.
Interim PET/CT scan utilization. (A) Question: Do you use an interim PET/CT scan for your advanced-stage cHL patients on ABVD, A + AVD, escalated BEACOPP? Select one response for each regimen: Yes, No, and I don't use this regimen. The number of physicians who reported using each regimen is given in parentheses. (B) Question: After how many cycles of treatment do your advanced-stage cHL patients typically get an interim PET/CT scan? Select all that apply: One cycle, two cycles, three cycles, four cycles, and other number of cycles (specify between five and 15), I typically do not get an interim PET/CT scan. (C) Question: Are you making treatment decisions based on the interim PET/CT results? Select one response: Yes, for escalation; Yes, for de-escalation; Yes, for both escalation and de-escalation; I don't know how to utilize PET/CT results; No. (D) Question: After which cycle of treatment do you make treatment decisions based on interim PET/CT results? Select all that apply: Cycle 1, cycle 2, cycle 3, cycle 4, and cycle (specify between 5 and 15). A + AVD, brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine; ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; cHL, classic Hodgkin lymphoma; escalated BEACOPP, escalated doses of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; PET/CT, positron emission tomography/computed tomography.
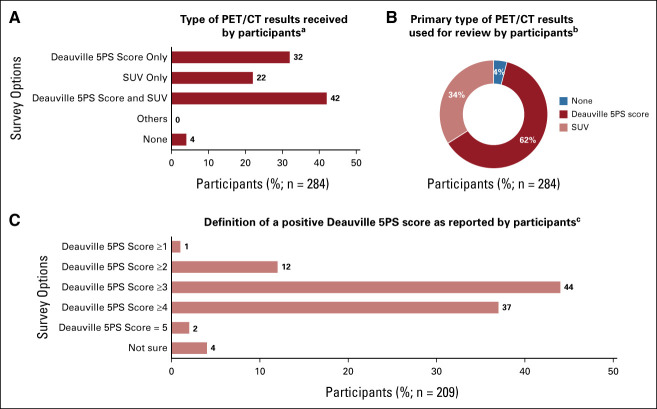
Of physicians who reported using a PET/CT scan (n = 284), 42% of physicians reported receiving both a Deauville 5PS score and a standardized uptake value (SUV) from the radiologist, 32% reported receiving only a Deauville 5PS score, 22% reported receiving only an SUV, and 4% reported not receiving either a Deauville 5PS or SUV (Fig 3A). Most physicians (62%) reported primarily using the Deauville 5PS scoring system when reviewing PET/CT scan results; 34% reported primarily using an SUV (Fig 3B).
FIG 3.
PET/CT scan results received and interpretation. (A) Question: Which diagnostic scoring system(s), if any, do you typically receive from the radiologist for PET/CT scan results? Select all that apply (all responses reported in the figure). (B) Question: Which diagnostic scoring system do you primarily use when reviewing PET/CT scan results? Select one (response options shown in the figure). (C) Question: How do you define a positive PET/CT scan using the Deauville scoring system? Select one (response options shown in the figure). 5PS, 5-point scale; PET/CT, positron emission tomography/computed tomography; SUV, standardized uptake value.
There was no consensus on the definition of a positive Deauville 5PS score among physicians who receive a Deauville 5PS score from the radiologist for PET/CT scan results (n = 209); a positive Deauville 5PS score was defined as 3 or higher by 44%, 4 or higher by 37%, and 2 or higher by 12% of these physicians (Fig 3C). Of physicians who received SUV scores from the radiologist for PET/CT scan results (n = 181), 84% defined a positive PET/CT scan using the SUV scoring system as a new area of metabolic activity relative to a prior scan, 75% defined it as a worse SUV score than the previous scan, and 59% defined it as persistent elevation compared with the liver.
Among the 209 physicians who used a Deauville 5PS score (academic based, n = 87; community based, n = 122), 54% (n = 47) of academic-based physicians and 36% (n = 44) of community-based physicians defined a positive score as 3 or higher, 33% (n = 29) and 39% (n = 48) as 4 or higher, and 1% (n = 1) and 3% (n = 4) as 5. Difficulty in interpreting PET/CT scan results was reported by 19% (53 of 284) of physicians who use a PET/CT scan to diagnose/stage their patients with cHL.
Barriers to Obtaining PET/CT Imaging
Of all participating physicians, 55% reported experiencing difficulty in obtaining a PET/CT scan for patients with advanced-stage cHL for any reason; 21% said that the greatest barrier to treating cHL was difficulty in obtaining a PET/CT scan, 17% said that the greatest barrier was interpreting PET/CT scan results, and 36% agreed that it was more difficult to get patients with cHL access to PET/CT scans because of the COVID-19 pandemic. Physicians who reported experiencing difficulty in obtaining a PET/CT scan (n = 166) reported that they were unable to get a PET/CT scan a mean (SD) of 20% (20) of the time.
Of physicians using a PET/CT scan to diagnose/stage patients with cHL (n = 284), 16% reported challenges in accessing PET/CT scans; 86% reported challenges in typically receiving PET/CT scan results within 2 days, whereas 14% reported waiting three to five business days for results; and 21% reported that delays in obtaining PET/CT scan results affected their ability to use a PET-adaptive approach. In the absence of a PET/CT scan, 63% of physicians used a CT scan alone, 36% obtained a biopsy, and 22% did not use alternative diagnostics.
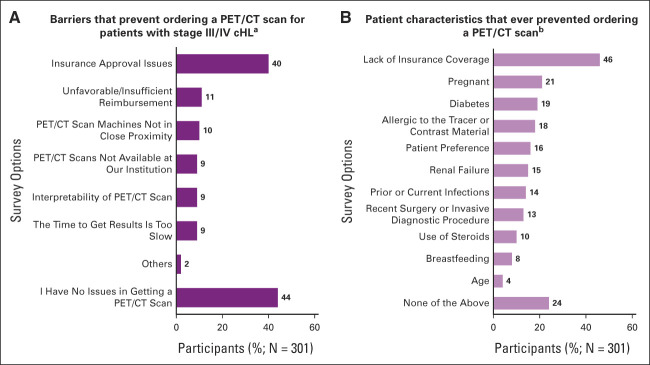
Barriers that prevented ordering a PET/CT scan for patients with stage III/IV cHL identified by participating physicians (N = 301) included insurance approval issues (40%), unfavorable/insufficient reimbursement (11%), PET/CT machines not in close proximity (10%), PET/CT scan not available at institution (9%), interpretability (9%), and time to get results is too slow (9%; Appendix Fig A1A [online only]). The most common patient characteristics that these physicians reported had ever prevented them from ordering a PET/CT scan included lack of insurance coverage (46%), pregnancy (21%), diabetes (19%), and allergies to tracer materials (18%; Appendix Fig A1B).
DISCUSSION
In total, 301 medical oncologists and hematologists/oncologists with experience in treating cHL in the United States participated in CONNECT, which is, to our knowledge, the first real-world survey of physicians, patients, and caregivers about cHL treatment decision making. Although 65% of physicians reported giving significant consideration to NCCN guidelines when treating stage III/IV cHL, interim PET/CT scans were not universally obtained after two cycles of chemotherapy.
Guidelines for stage III/IV cHL recommend an interim PET/CT scan after cycle 2 of ABVD or escalated BEACOPP, with results used to change treatment.3,13 For patients initially treated with ABVD for two cycles with a negative interim PET/CT scan (Deauville 5PS score of 1, 2, or 3), the NCCN guidelines recommend four additional cycles of AVD per the RATHL study.3 For patients initially treated with ABVD for two cycles with a positive interim PET/CT scan (Deauville 5PS score of 4 or 5), the NCCN guidelines recommend three cycles of escalated BEACOPP followed by a restaging PET/CT scan, with subsequent treatment on the basis of scan results.3
Among physicians prescribing ABVD or escalated BEACOPP, 92% reported obtaining an interim PET/CT scan during ABVD, and 80% during treatment with escalated BEACOPP. However, 36% of physicians who obtained an interim PET/CT scan reported not using escalated BEACOPP as a regimen for stage III/IV cHL.
Although most physicians obtained an interim PET/CT scan with ABVD therapy, approximately 40% obtained an interim PET/CT scan later than guideline-recommended. In addition, although most physicians requesting an interim PET/CT scan used results for both escalation and de-escalation of therapy, 18% reported using an interim PET/CT scan for therapy escalation only, and 13% for therapy de-escalation only. As the CONNECT study surveyed participating physicians on their approach to treating patients with cHL, individual patient-level information on treatment de-escalation/escalation with ABVD was not obtained. However, results from two real-world analyses in the United States found that interim PET/CT scans and Deauville 5PS scores to inform treatment modification were not universally obtained for patients with stage III/IV cHL treated with frontline ABVD.14,15 In these two studies, de-escalation to AVD was observed and escalation to BEACOPP was rare regardless of Deauville 5PS score. Taken together, CONNECT physician survey results and real-world data highlight inconsistent use of guideline-directed PET-adapted therapy.
Among physicians who prescribe A + AVD for stage III/IV cHL, 86% reported obtaining an interim PET/CT scan during A + AVD therapy, reflecting guideline-based therapy during the CONNECT survey.13 Guidelines published after survey completion no longer recommend an interim PET/CT scan with A + AVD as supported by 5-year results from the ECHELON-1 trial, which did not require treatment adaption on the basis of interim PET/CT scan results. In ECHELON-1 (median follow-up: 60.9 months), patients treated with A + AVD versus ABVD had a significant improvement in 5-year progression-free survival (PFS) (82.2% v 75.3%; HR, 0.68; 95% CI, 0.53 to 0.87; P = .0017).16 This improvement was evident in high-risk subgroups on the basis of disease stage, age, and International Prognostic Score. Six-year ECHELON-1 results (median follow-up: 73.0 months) continued to show PFS improvement and a significant 41% reduction in the risk of death (HR, 0.59; 95% CI, 0.40 to 0.88; P = .009) with A + AVD versus ABVD, regardless of interim PET/CT scan results.17
We found that Deauville 5PS scores were not universally received by physicians for PET/CT scans. Guidelines recommend that final PET/CT scan reports that define response should include a Deauville 5PS score3 and support the American College of Radiology and Society of Nuclear Medicine and Molecular Imaging recommendations for PET/CT interpretation by a physician with specific qualifications. Qualifications include board certification and education requirements and that the interpreting radiology or nuclear medicine physician has adequate training and continuing education/experience in interpreting PET/CT scans for patients with lymphoma, including the Deauville 5PS.3,10
One-third of physicians in the CONNECT study reported primarily using the SUV, a diagnostic scoring system previously used for assessing patients with cHL, but no longer included in guidelines.3 CONNECT study results align with those from a US Oncology Network real-world, observational study that also showed discordance between study results and the NCCN guidelines as Deauville 5PS scores were absent for two-thirds of interim PET/CT scans in patients receiving frontline ABVD for stage III/IV cHL.18
We also found in the CONNECT study that physician definitions of a positive Deauville 5PS score varied, with 44% of physicians using a threshold of 3 or higher and 37% of physicians using a threshold of 4 or higher, with variation between community and academic settings. Low rates of therapy escalation on the basis of Deauville 5PS scores of 4 or 5 have been observed. Results from one study showed that only 24% of patients with a Deauville 5PS score of 4 or 5 after an interim PET/CT scan received escalated therapy.19 These findings highlight an opportunity to educate radiologists and oncologists on using interim PET/CT Deauville 5PS scores to optimize patient outcomes.
Approximately 20% of physicians reported that barriers to accessing PET/CT scans affected their ability to implement a PET-adaptive strategy. Physicians noted that insurance issues were both a barrier and a patient characteristic that hindered obtaining a PET/CT scan. Although physicians were not asked to differentiate between baseline and interim PET/CT scans when reporting barriers, barriers that may result in the absence of a baseline PET/CT scan can hinder radiologists in formulating an accurate response assessment on the basis of an interim PET/CT scan. Physicians reported increased difficulty in accessing a PET/CT scan since the start of the COVID-19 pandemic. To our knowledge, this is the first study to assess barriers that prevent physicians from using PET/CT scans during cHL management.
Outcomes measured represent the practices of physicians who participated in this study and may vary from nonparticipating physicians. Recall bias may affect outcomes, as physicians were asked to recall practice patterns over the previous 12 months. Although the intention was to collect practice patterns related to newly diagnosed patients, it is likely, on the basis of the number of patients reported by respondents, that respondents might have included the number of patients with active disease inclusive of those newly diagnosed and those with relapsed or refractory disease.
In conclusion, in CONNECT, which is, to our knowledge, the first real-world survey of physicians, patients, and caregivers about cHL, physicians' most reported important considerations when treating cHL were clinical trial safety and efficacy data and treatment guidelines. Despite this, physicians reported inconsistent adherence to PET-adapted strategies described by NCCN guidelines, with limited consensus on the interpretation of interim PET/CT scan results.
When PET/CT scans were obtained, Deauville 5PS scores were not universally provided and variability existed among physicians regarding the definition of a positive Deauville 5PS score. Challenges in obtaining PET/CT scans, with increased difficulty because of the COVID‐19 pandemic, were reported. In addition, other barriers, such as lack of insurance coverage and PET/CT scan costs, may prohibit optimal adherence to guidelines on PET/CT scan utilization.
These results suggest that there is an opportunity to educate oncologists and radiologists on the importance of consistently reporting PET/CT Deauville 5PS scores and the use of response-adapted imaging to optimize treatment modifications.
ACKNOWLEDGMENT
Medical writing support was provided by Sarah Criddle, PharmD, and Beth A. Lesher, PharmD, from OPEN Health, and was funded by Seagen Inc.
APPENDIX
FIG A1.
Barriers that prevent ordering a PET/CT scan for patients with stage III/IV cHL. (A) Question: Which of the following inhibit you from getting a PET/CT scan for your advanced-stage cHL patients? Response options shown in the figure. Responses are not mutually exclusive. (B) Question: What specific patient characteristics have ever prevented you from ordering a PET/CT scan? Response options shown in the figure. Responses are not mutually exclusive. cHL, classic Hodgkin lymphoma; PET/CT, positron emission tomography/computed tomography.
Susan K. Parsons
Consulting or Advisory Role: Seagen Inc.
Kristina S. Yu
Employment: Seagen Inc.
Stock and Other Ownership Interests: Seagen Inc.
Research Funding: Seagen Inc.
Travel, Accommodations, Expenses: Seagen Inc.
Nicholas Liu
Employment: Seagen Inc.
Stock and Other Ownership Interests: Seagen Inc.
Research Funding: Seagen Inc.
Travel, Accommodations, Expenses: Seagen Inc.
Michelle A. Fanale
Employment: Seagen Inc.
Stock and Other Ownership Interests: Seagen Inc.
Carlos Flores
Employment: Genesis Research
Darcy R. Flora
Employment: GRYT Health
Leadership: GRYT Health
Stock and Other Ownership Interests: GRYT Health
Research Funding: Amgen, Pfizer, Seagen Inc., Syneos Health
Andrew M. Evens
Honoraria: Seagen Inc., Pharmacyclics, Research to Practice, Miltenyi Biotec, Epizyme, Novartis, MorphoSys, Cota Healthcare, Curio Science, Targeted Oncology, WebMD, AbbVie/Pharmacyclics, HMP, Takeda, Patient Power, PER, OncLive Clinical Congress Consultants, HUTCHMED, Incyte, MorphoSys, Daiichi Sankyo/Astra Zeneca
Consulting or Advisory Role: Seagen Inc., Novartis, Pharmacyclics, Miltenyi Biotec, Epizyme, MorphoSys, Cota Healthcare, AbbVie, Incyte, MorphoSys
Speakers' Bureau: Research to Practice, Curio Science
Travel, Accommodations, Expenses: Seagen Inc., Novartis, Curio Science
No other potential conflicts of interest were reported.
Footnotes
Some of these data were presented at the American Society of Hematology Annual Meeting 2021 (Atlanta, GA, December 10-14, 2021) and the Pan Pacific Lymphoma Conference (Koloa, HI, July 18-22, 2022).
AUTHOR CONTRIBUTIONS
Conception and design: Susan K. Parsons, Kristina S. Yu, Nicholas Liu, Supriya Kumar, Katie Holmes, Andy Surinach, Darcy R. Flora, Andrew M. Evens
Financial support: Kristina S. Yu, Nicholas Liu
Administrative support: Nicholas Liu, Supriya Kumar, Katie Holmes
Provision of study materials or patients: Kristina S. Yu, Nicholas Liu, Supriya Kumar, Katie Holmes, Darcy R. Flora
Collection and assembly of data: Supriya Kumar, Katie Holmes, Darcy R. Flora
Data analysis and interpretation: Susan K. Parsons, Kristina S. Yu, Nicholas Liu, Supriya Kumar, Michelle A. Fanale, Katie Holmes, Carlos Flores, Andy Surinach, Darcy R. Flora, Andrew M. Evens
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Observations of Oncologists on Treatment Selection With Interim Positron Emission Tomography–Adapted Approaches in Classic Hodgkin Lymphoma: The Real-World CONNECT Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Susan K. Parsons
Consulting or Advisory Role: Seagen Inc.
Kristina S. Yu
Employment: Seagen Inc.
Stock and Other Ownership Interests: Seagen Inc.
Research Funding: Seagen Inc.
Travel, Accommodations, Expenses: Seagen Inc.
Nicholas Liu
Employment: Seagen Inc.
Stock and Other Ownership Interests: Seagen Inc.
Research Funding: Seagen Inc.
Travel, Accommodations, Expenses: Seagen Inc.
Michelle A. Fanale
Employment: Seagen Inc.
Stock and Other Ownership Interests: Seagen Inc.
Carlos Flores
Employment: Genesis Research
Darcy R. Flora
Employment: GRYT Health
Leadership: GRYT Health
Stock and Other Ownership Interests: GRYT Health
Research Funding: Amgen, Pfizer, Seagen Inc., Syneos Health
Andrew M. Evens
Honoraria: Seagen Inc., Pharmacyclics, Research to Practice, Miltenyi Biotec, Epizyme, Novartis, MorphoSys, Cota Healthcare, Curio Science, Targeted Oncology, WebMD, AbbVie/Pharmacyclics, HMP, Takeda, Patient Power, PER, OncLive Clinical Congress Consultants, HUTCHMED, Incyte, MorphoSys, Daiichi Sankyo/Astra Zeneca
Consulting or Advisory Role: Seagen Inc., Novartis, Pharmacyclics, Miltenyi Biotec, Epizyme, MorphoSys, Cota Healthcare, AbbVie, Incyte, MorphoSys
Speakers' Bureau: Research to Practice, Curio Science
Travel, Accommodations, Expenses: Seagen Inc., Novartis, Curio Science
No other potential conflicts of interest were reported.
REFERENCES
- 1.Piccaluga PP, Agostinelli C, Gazzola A, et al. : Pathobiology of Hodgkin lymphoma. Adv Hematol 2011:920898, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Cancer Institute : Section 32: Adolescent and Young Adult Cancer by Site Incidence, Survival and Mortality. National Cancer Institute; https://seer.cancer.gov/archive/csr/1975_2015/results_merged/sect_32_aya.pdf [Google Scholar]
- 3.Hoppe RT, Advani RH, Ai WZ, et al. : NCCN Guidelines® insights: Hodgkin lymphoma, version 2.2022: Featured updates to the NCCN Guidelines. J Natl Compr Cancer Netw 20:322-334, 2022 [Google Scholar]
- 4.Bigenwald C, Galimard JE, Quero L, et al. : Hodgkin lymphoma in adolescent and young adults: Insights from an adult tertiary single-center cohort of 349 patients. Oncotarget 8:80073-80082, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen PB, Gordon LI: Frontline therapy for classical Hodgkin lymphoma by stage and prognostic factors. Clin Med Insights Oncol 11:1179554917731072, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansell SM: Hodgkin lymphoma: 2018 update on diagnosis, risk-stratification, and management. Am J Hematol 93:704-715, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Gallamini A, Barrington SF, Biggi A, et al. : The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica 99:1107-1113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson P, Federico M, Kirkwood A, et al. : Adapted treatment guided by interim PET-CT scan in advanced Hodgkin's lymphoma. N Engl J Med 374:2419-2429, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Press OW, Li H, Schoder H, et al. : US intergroup trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: Southwest Oncology Group S0816. J Clin Oncol 34:2020-2027, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boellaard R, Delgado-Bolton R, Oyen WJG, et al. : FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur J Nucl Med Mol Imaging 42:328-354, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Relecom A, Federico M, Connors JM, et al. : Resources-stratified guidelines for classical Hodgkin lymphoma. Int J Environ Res Public Health 17:1783, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Public Policy Committee, International Society of Pharmacoepidemiology : Guidelines for good pharmacoepidemiology practice (GPP). Pharmacoepidemiol Drug Saf 25:2-10, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Hoppe RT, Advani RH, Ai WZ, et al. : Hodgkin lymphoma, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 18:755-781, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Winter A, Liu N, Surinach A, et al. : Real-world patient characteristics, treatment patterns, and outcomes in patients with stage III/IV classic Hodgkin lymphoma treated with frontline ABVD: A retrospective analysis using a real-world database. Blood 140:8054-8055, 2022. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 15.Yasenchak C, Liu N, Beeks A, et al. : Real-world Adherence to National Comprehensive Cancer Network (NCCN) Guidelines Regarding the Usage of PET/CT and Reported Deauville Scores in Advanced Stage Classical Hodgkin Lymphoma: A Community Oncology Practice Perspective. Presented at: Pan Pacific Lymphoma Conference, Big Island, Hawaii (2022)
- 16.Straus DJ, Długosz-Danecka M, Connors JM, et al. : Brentuximab vedotin with chemotherapy for stage III or IV classical Hodgkin lymphoma (ECHELON-1): 5-year update of an international, open-label, randomised, phase 3 trial. Lancet Haematol 8:e410-e421, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Ansell SM, Radford J, Connors JM, et al. : Overall survival with brentuximab vedotin in stage III or IV Hodgkin’s lymphoma. N Engl J Med 387:310-320, 2022 [DOI] [PubMed] [Google Scholar]
- 18.Yasenchak CA, Clark J, Liu N, et al. : Real-world adherence to National Comprehensive Cancer Network (NCCN) guidelines regarding the usage of PET/CT and reported Deauville scores in advanced stage classical Hodgkin lymphoma: A community oncology practice perspective. Blood 136:32-33, 2020. (suppl 1) [Google Scholar]
- 19.Hamid MS, Rutherford SC, Jang H, et al. : Outcomes among classical Hodgkin lymphoma patients after an interim PET scan: A real-world experience. Clin Lymphoma Myeloma Leuk 22:e435-e442, 2022 [DOI] [PubMed] [Google Scholar]