PURPOSE:
Although electronic patient-reported outcomes (ePROs) are efficacious in symptom management, much is unknown about the utility of vital signs surveillance. We examined the feasibility of a remote patient monitoring platform that integrates ePROs and biometrics into the ambulatory management of symptom burden.
METHODS:
Using a decentralized workflow, patients with gastrointestinal or thoracic cancer were approached for a 1-month study. Patients reported symptom burden via ePROs and biometrics (blood pressure, oxygen saturation, pulse, weight, and temperature) using bluetooth-enabled devices daily. Alerts on the basis of prespecified thresholds were managed via nurse-led triage. Adherence was defined as the completion of > 70% of daily symptom and biometric reporting requirements. Pilot acceptability, appropriateness, and feasibility were measured using validated instruments. Net promoter score, system usability scale, and emergency department (ED) admission rates were collected.
RESULTS:
Over 8 months, 36 patients were enrolled and 25 (60% gastrointestinal) completed the study. Participants had a mean age of 58.0 years, mean Eastern Cooperative Oncology Group score of 0.88, were 52% female, and predominantly had stage IV or recurrent disease (72%). Program adherence was 73% and associated with high acceptability (4.63), feasibility (4.56), and appropriateness (4.46). System usability scale and net promoter score scores were 88 and 55, respectively. Seventy percent of alerts were generated by biometrics, 28% for symptoms, and 2% were patient-initiated communication. Finally, the ED visitation rate over the pilot period was 8%.
CONCLUSION:
Our remote patient monitoring pilot program was highly acceptable, feasible, and appropriate. It had high rates of patient adherence and satisfaction and was associated with low ED visitation rates.
Leveraging a decentralized study workflow, our remote patient monitoring pilot program successfully integrates biometric capture and electronic PROs for the ambulatory management of chemotherapy-related symptoms
INTRODUCTION
Despite advances in the management of disease trajectory, many patients with cancer still suffer from many debilitating symptoms either as a result of tumor progression or treatment side effects.1,2 The persistence and/or escalation of these symptoms is associated with poor health-related quality of life (HRQoL), physical functioning, increased health resource utilization, treatment nonadherence, and diminished overall survival.3-5 Unfortunately, many symptoms are poorly characterized by clinicians and underreported by patients, highlighting the need for more robust approaches for surveillance.6,7 Remote symptom monitoring, via electronic patient-reported outcomes (PROs), has been associated with significant improvements in HRQoL, health resource utilization, and clinical outcomes.8
The COVID-19 pandemic has accelerated the dissemination of digital technologies in oncology because of the exigent need to maintain care continuity in the context of immunosuppression and social distancing requirements.9,10 Remote patient monitoring (RPM) and decentralized clinical trials are two prominent examples that have been implemented in many cancer institutions over the course of the pandemic.11,12 Decentralized trials leverage remote technologies and a supply chain to conduct study procedures in the homes of participants.11,13 RPM, defined as the deployment of point-of-need technologies that allow for the triage and management of patients with acute or chronic conditions, has already been embedded in the management paradigm for other chronic diseases.14,15
The utility of integrating biometric data (ie, vital signs) into the ambulatory management of treatment- or disease-related symptom burden remains an open question.12,16 Therefore, we investigated the feasibility and implementation outcomes regarding the use of an RPM platform for monitoring symptom burden and biometrics in high-risk patients receiving chemotherapy. We also evaluated the effectiveness of a virtual or decentralized workflow for patient recruitment, education, symptom monitoring, and study completion.11
METHODS
Study Setting, Participants, and Recruitment
This study was approved by the MD Anderson Cancer Center (MDACC) Institutional Review Board, and a detailed study protocol is publicly available.12 We conducted a single-arm pilot study (ClinicalTrials.gov identifier: NCT05038254) of adult patients (>18 years) with gastrointestinal (GI) or thoracic cancers receiving care at MDACC between July 1, 2021, and March 31, 2022. These malignancies were chosen on account of high annual patient volumes and a track record of strong clinician engagement with virtual care initiatives within our institution. Additional eligibility criteria were chemotherapy initiation within the preceding 2 months, Texas resident status (permanent or temporary), having a high risk for acute care utilization, English proficiency, and willingness to report data (PROs and biometric) for at least 30 days and to engage in televisits as needed with a triage nurse.12,17 Patients on concurrent biologic, hormonal, or immunotherapy were also eligible. We excluded patients who received investigational new drugs, were enrolled in a phase I trial, required inpatient infusions, were living in institutional environments (ie, prisons), were pregnant, had a history of dementia, physical disability, or neurological deficits that impaired the ability to use study devices.
Consistent with published work and the ORBIT framework of behavioral treatment development guidelines, we attempted to recruit a sample (n = 25) that included at least three racial minorities, three patients older than 70 years, and an even gender distribution.18,19 A digital health navigator (DHN) screened the clinic schedules of participating oncologists to identify eligible patients.20 Email communication was initiated with the treating oncologist to confirm appropriateness on the basis of published risk factors for acute care utilization.12,17
Our screening workflow was eventually automated during the last month of the pilot using a commercially available natural language processing platform (Deep6 AI, Pasadena, CA). Deep6 applies artificial intelligence techniques to clinical data to accelerate trial recruitment by identifying and matching eligible patients. Implementing the Deep6 platform significantly expedited parts of our screening process, specifically the reliable identification of adult patients with GI or thoracic malignancies and a primary residence in Texas, who were about to initiate chemotherapy. This allowed the DHN (1.0 FTE) to prioritize attention to patient onboarding and ensuring adherence with protocolized reporting of symptoms and vital signs. After clinician assent, the DHN contacted eligible patients by phone, in-person, or through the electronic medical record (EMR) patient portal. Our EMR also enabled an electronic informed consent process. The DHN provided an overview of the RPM platform, study procedures, and participant expectations.
Study Intervention
The RPM platform is a HIPAA-compliant, FDA-approved data system (Vivify health, Plano, TX) that includes a tablet preloaded with an interface for symptom reporting and bluetooth-enabled consumer-friendly devices (ie, pulse oximeter, weight scale, blood pressure monitor, and thermometer) as detailed in Appendix Table A1 (online only). Information related to symptom burden and biometrics were transmitted to a secure online dashboard that was accessible to the DHN and a nurse-led triage center (Appendix Figure A1, online only). Because of COVID-related restrictions and the added complexity of supply chain logistics related to storing the kits on MDACC premises (eg, sterile processing following kit return upon pilot completion and proper inventory management), we elected to ship the kits directly to the verified personal address for each patient. Shipment of the RPM platform to and from the patient's home was at no cost and via tracked courier services. Upon signature confirmation receipt, the DHN contacted the patient to reinforce prior teaching on device use and study protocol. Technical support for the RPM platform was provided by the vendor (Vivify). RPM retrieval occurred at the time of study completion, discontinuation of chemotherapy, patient death, or transition to hospice via scheduled pickup or prepaid postage. Free mobile hotspots were offered to all participants; however, none was requested. The DHN's workflow (screening, enrollment, kit deployment, data collection, and protocol adherence) was fully decentralized as outlined in Figure 1.
FIG 1.
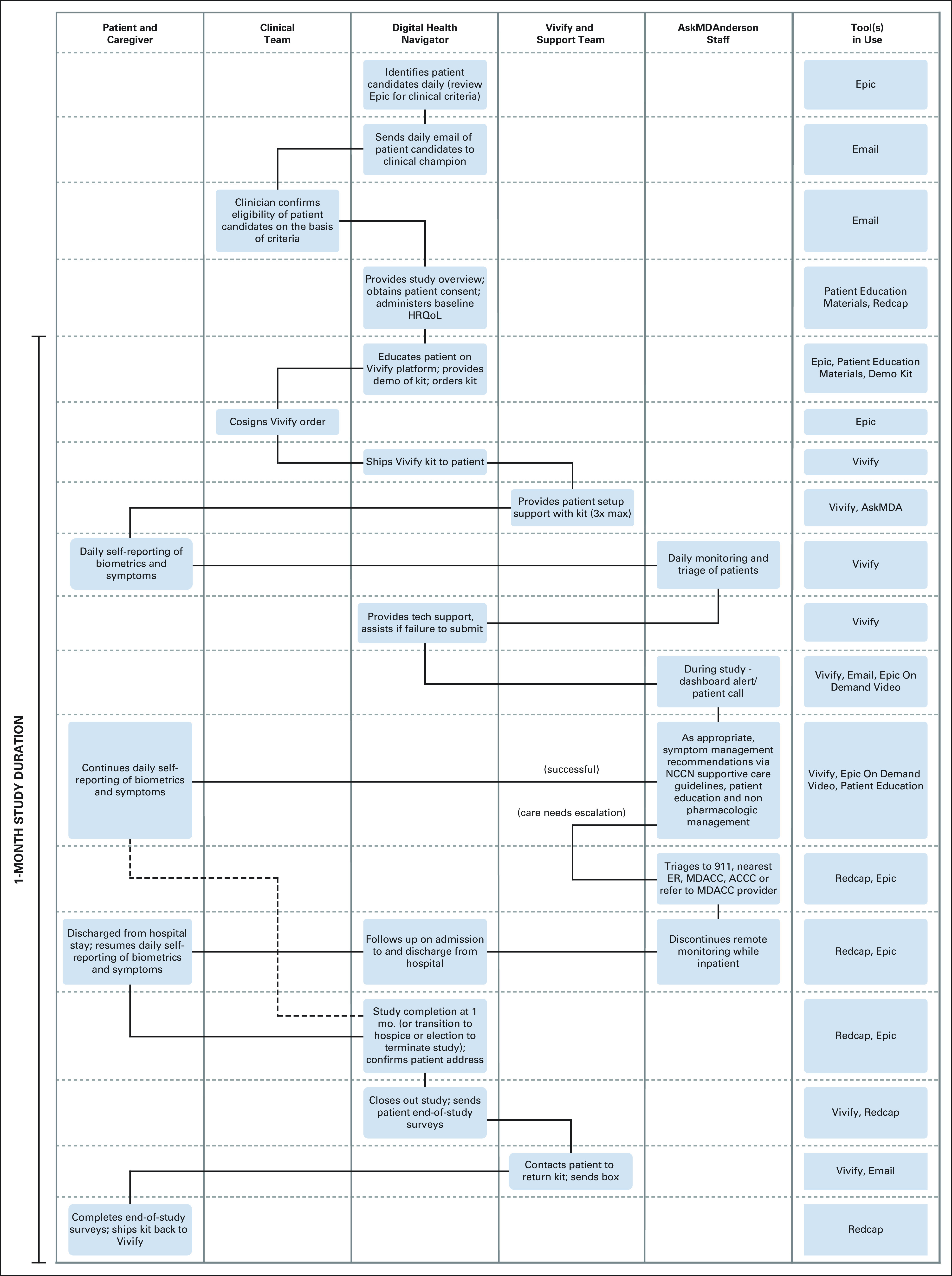
Decentralized workflow for remote patient monitoring service delivery. ACCC, Acute Cancer Care Center; ER, emergency room; HRQoL, health-related quality of life; MDACC, MD Anderson Cancer Center; NCCN, National Comprehensive Cancer Network.
The intervention entailed daily self-reporting of symptom burden and biometric status (ie, vital signs) over a 1-month period. The patient-reported outcome common terminology criteria for adverse events (PRO-CTCAE), contextualized to GI and thoracic cancers, was used for reporting symptom burden (Data Supplement).8,21 Assessed symptoms included diarrhea, dry mouth, constipation, fatigue, decreased appetite, difficulty swallowing, vomiting, nausea, and pain. Symptoms were rated on a 5-point scale from 0 (nonpresent) to 4 (very severe). Weights were reported weekly. Decisions regarding the frequency of reporting symptoms and biometric data were based on published data, guidance from clinical collaborators, and feedback from members of the MDACC patient and caregiver stakeholder community.22 The DHN monitored reported symptoms and device usage via the online dashboard and contacted patients with 3 or more days of missing data to help resolve any technical problems.
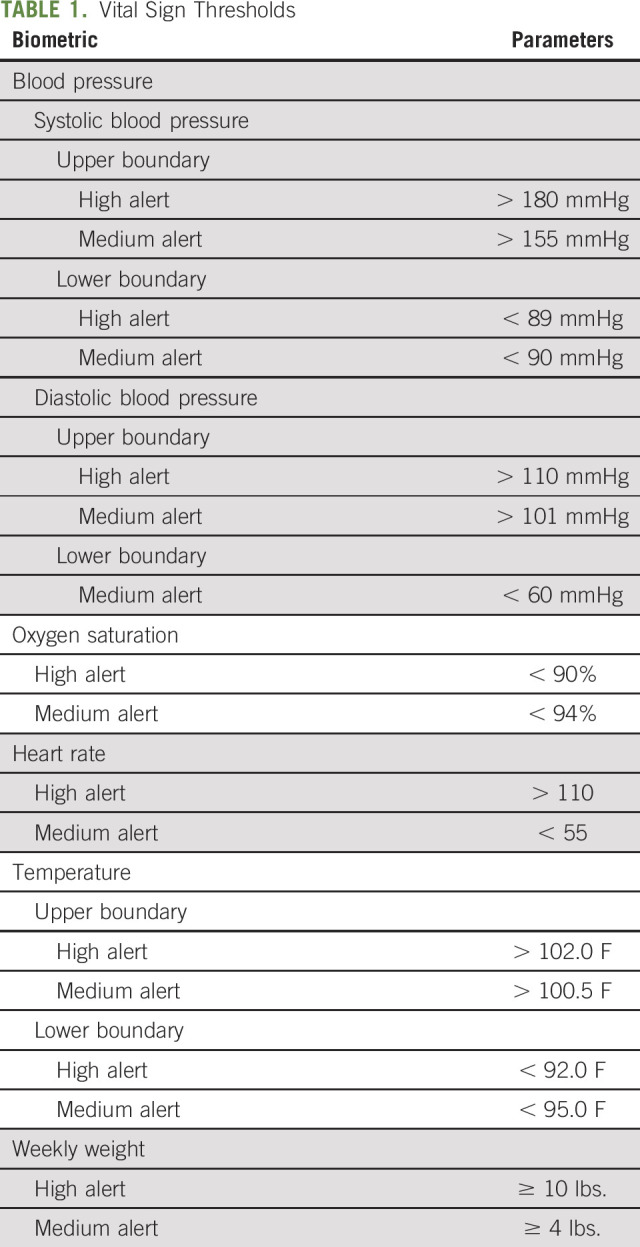
The RPM platform can generate email alerts when patient-reported data met or exceeded prespecified thresholds (Table 1). Alerts graded as high (red) necessitated a nurse triage response within 2 hours, and those graded as medium (yellow) required response within 3 hours. On receipt of an email alert, triage nurses reviewed data on the online dashboard; contacted patients via phone, text message, or patient portal; confirmed the accuracy of the alert data; and engaged with patients as clinically appropriate (eg, recommend an emergency department (ED) visit, refer a patient to his/her primary care physician, arrange for a semiurgent clinic visit). Triage nurses leveraged the National Comprehensive Cancer Network symptom management guidelines to inform nonpharmacological recommendations depending on the alert grade.23 Our nurse-led triage team also included nurse practitioners with prescriptive authority for a limited set of key medications (eg, Zofran, Senna, Miralax, Reglan, Loperamide) at prespecified doses. Physician oversight for nurse practitioners' prescriptions was also maintained in compliance with Texas state law. Because of resource limitations, triage nurses were available from 8 am to 8 pm Monday through Sunday. Participants were instructed to complete their daily symptom reporting and device usage during those hours and to contact their oncologist's office on-call coverage or 911 for after-hour emergencies or if experiencing concerning symptoms. Study participation did not alter the schedule of routine follow-up or consultation with their treating clinicians.
TABLE 1.
Vital Sign Thresholds
Outcome Measures
The primary outcome of the present feasibility study was patient adherence to the RPM protocol, defined as completion of ≥ 70% of daily symptom and biometric reporting requirements at least 4 days per week.18 This definition is consistent with previously published telemonitoring studies in oncology patients.18,24 We also leveraged the following secondary outcome measures to assess the feasibility of our largely decentralized screening, recruitment and onboarding process as defined by (1) an approach-to-consent rate of > 60% among screened eligible patients and (2) an enrollment rate of two patients monthly.18
To evaluate our overall implementation strategy, we administered the following validated instruments, via Research Electronic Data Capture: Feasibility of Intervention Measure, Intervention Appropriateness Measure, and Acceptability of Intervention Measure.25 All measures were scored on a 5-point Likert scale (completely disagree to completely agree), and a mean score ≥ 3 was consistent with a successful pilot. With respect to the usability of our RPM platform (Vivify), participants completed the 10-item System Usability Scale (SUS) via Research Electronic Data Capture.24,26 SUS items were rated on a 5-point Likert scale (strongly disagree to strongly agree) with aggregate scores > 68 shown to be associated with a usable technology system.24 Upon study completion, participants were also asked questions related to the net promoter score (NPS) and the RPM platform's ease of use. The NPS, a metric commonly used for customer satisfaction and loyalty programs, was assessed via the prompt: How likely is that you will recommend our RPM program to a friend, family member, or colleague receiving chemotherapy.27 The NPS calculation methodology has been previously described, and a net positive score was considered acceptable.27 Ease of use-related questions were scored on a 5-point Likert scale questionnaire (strongly disagree to strongly agree).
Data Collection and Follow-Up
The following participant data were abstracted from the EMR: age, race, self-reported sex, marital status (single, divorced, married, widow, or widower), body mass index, cancer type, cancer stage, baseline Eastern Cooperative Oncology Group status, and ED visits during the pilot study period. Finally, the RPM tablet was used to abstract patient responses related to satisfaction with the program, technology ease of use, and NPS.
Sample Size and Analytical Plan
Our study sample size of 25 patients was based on study time frame and previously published pilot data involving remote monitoring of patients with cancer undergoing chemotherapy.16,18 Participant characteristics and demographic information were characterized using descriptive statistics (eg, mean, counts, percentages, and standard deviations). Univariate analysis was performed via the χ2 and Fisher's exact test for categorical variables and unpaired t-test for continuous variables. All analyses were performed using SAS, version 9.4 (SAS Institute Inc, Cary, NC), and two-tailed P-values < 0.05 were considered statistically significant.
RESULTS
Decentralized Recruitment Metrics
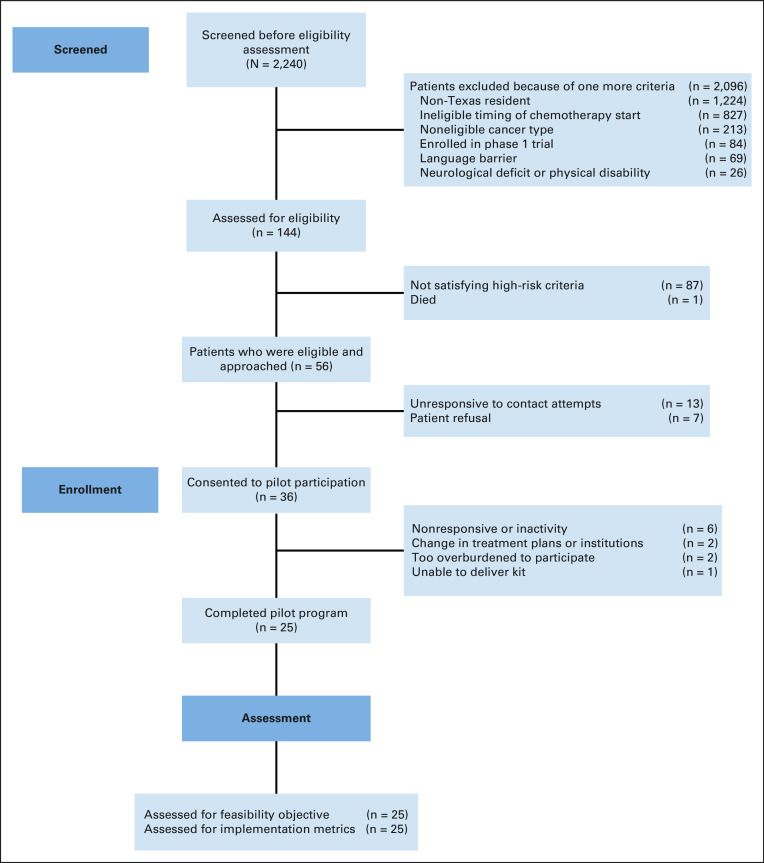
Over an 8-month period, 56 eligible patients were approached after screening and 36 patients consented for an approach-to-consent rate of 64%. Of these patients, 11 withdrew from the study for the following reasons: dropped because of inactivity and nonresponsive (n = 6), change in treatment plans or institution (n = 2), too overburdened to participate (n = 2), and unable to deliver kit to patient (n = 1). The average monthly enrollment was 4.5 patients while logistical milestones associated with our decentralized workflow were as follows: time to receipt of RPM kit after enrollment (mean, 4.5 days), initiation of data reporting after receipt of RPM kit (mean, 3.1 days), and time from screen to active status (median, 7 days; mean, 8.1 days). None of the 11 participants who withdrew self-reported any PRO or biometric information. A flow diagram, adapted to account for our nonrandomized pilot design, is presented as a flow diagram (Figure 2).
FIG 2.
Modified flow diagram outlining patient screening, enrollment, and analysis in the present single-arm pilot study.
Patient Demographics and Acute Care Utilization
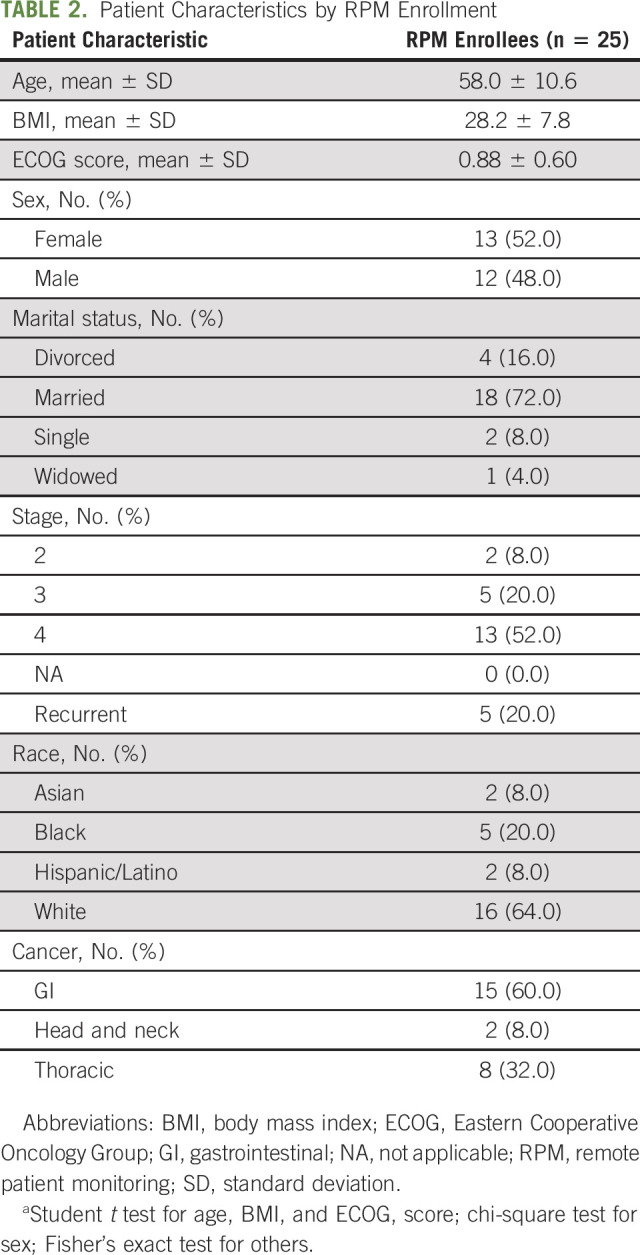
Baseline demographics and disease characteristics of the 25 participants are displayed in Table 2. The participant pool had a slight female gender predisposition (52%) with a mean age of 58 years ± 10.6, mean body mass index of 28.2 ± 7.8, and mean Eastern Cooperative Oncology Group score of 0.88 ± 0.60. There was a preponderance of GI cancers (60%) and stage IV or recurrent disease (72%). Most patients identified as being married (72%) and Caucasian (64%). The rate of all-cause ED visits and hospitalizations over the pilot period was 8% among study participants.
TABLE 2.
Patient Characteristics by RPM Enrollment
Engagement with RPM Program
The program adherence rate was 73%, and a total of 165 alert-based interactions (40% high and 60% medium alerts), across our 25 participants, were generated with a distribution as follows: 26% PROs, 72% biometrics, and 2% patient-initiated communication. The most reported vital signs were blood pressure (31%), weight (28%), and pulse (7%). The symptoms most associated with yellow alerts were nausea (37%), pain (11.54%), vomiting (11.54%), and decreased appetite (11.54%). Similarly, for red alerts the modal symptoms were nausea (28.6%), pain (20%), vomiting (14.3%), and decreased appetite (14.3%).
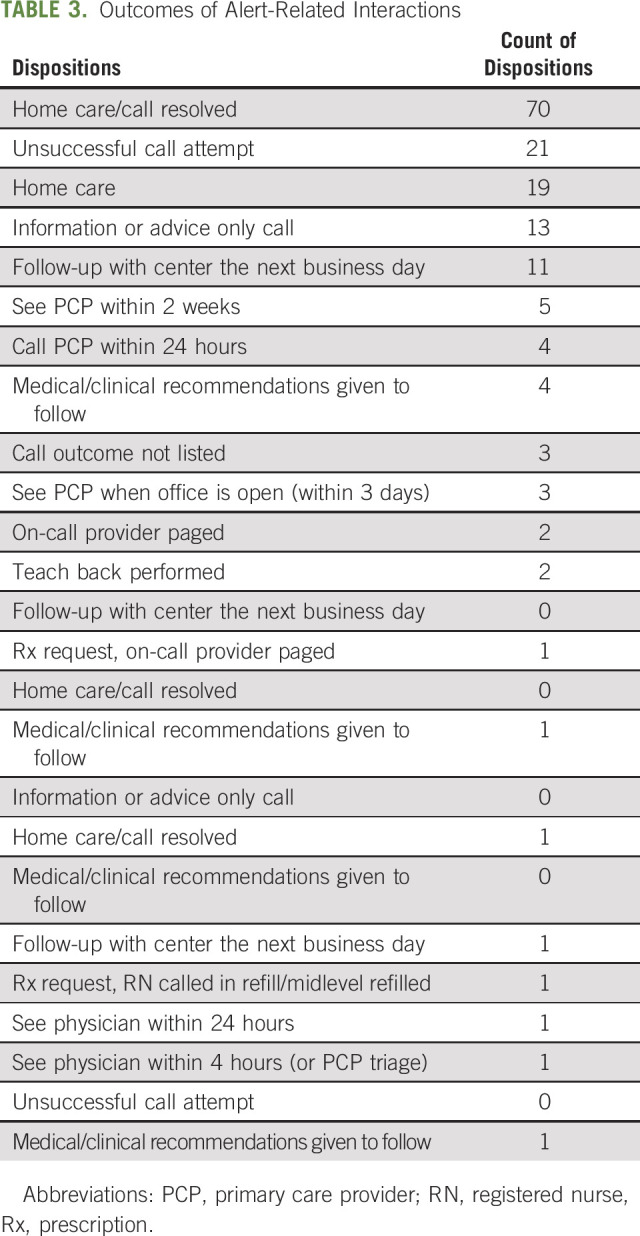
Across all alert-initiated interactions, 143 (86.6%) involved a clinical recommendation, and the overwhelming majority (n = 140, 84.5%) was performed by the nurse triage staff alone. Only three interactions involved a page request to the on-call provider, and 22 interactions (13.3%) involved unsuccessful attempts to reach the patient within the prespecified time windows. The complete outcomes of alert-related interactions are outlined in Table 3.
TABLE 3.
Outcomes of Alert-Related Interactions
Implementation Metrics
The mean Acceptability of Intervention Measure, Feasibility of Intervention Measure, Intervention Appropriateness Measure scores were 4.63, 4.56, and 4.46, respectively. The mean SUS score for our RPM platform was 88 while the NPS for our overall program was 55. Scores for patient satisfaction score and technology ease of use were 94% and 100%, respectively.
DISCUSSION
In this decentralized pilot study of an RPM paradigm that encompassed nurse-led triage of self-reported PROs and biometric measurements, we identified strong engagement (>70% adherence rates with daily assessments) and positive approval (>60% consent rate, NPS of 55, and satisfaction score of 94%) among a diverse pool of participants. Our workflow was also found to be feasible, acceptable, and appropriate. These results provide support for a future RCT exploring the effectiveness of integrating biometric and PRO data into supporting clinical workflows for ambulatory symptom management.
There is mounting interest in identifying, integrating, and scaling appropriate digital health interventions in oncology. Symptom management has emerged as the most persuasive use case because the majority (88%) of patients undergoing active treatment report at least one side effect, with fatigue (80%), pain (48%), and vomiting (48%) as the most prevalent.1 Adult patients with cancer frequently visit the ED on account of symptom burden, and approximately 60% of these encounters convert to hospitalizations, at great cost to patient, families, and society.28 Prevailing symptom management paradigms often involve two components: (1) the use of tablets, mobile applications, or web-based platforms to document PROs related to symptom burden and (2) follow-up that is often directed by nurse navigators.6,29 We posit that additional digital touchpoints (ie, biometric data) can provide supplementary information that enhances patient-provider communication and clinical decision making regarding symptom burden.12,30 For instance, the sensitivity of PRO-CTCAE entries that are concerning for dehydration (ie, difficulty swallowing, oral sores, and decreased appetite) is enhanced in the context of low systolic blood pressure or tachycardia.
We noted analogous adherence rates between PRO completion and use of sensor devices, signifying that biometric monitoring is an acceptable and useful adjunct. Ad hoc feedback from pilot participants to the call center staff revealed a perception of our RPM intervention as a crucial element of their cancer management and not research, bolstering our strategy to leverage the lived experience of PFAC members during program conceptualization.12 This approach also catalyzed the iteration of several components of our RPM program (eg, patient education materials and alert parameters) to be more patient-centered and meaningful.12
Decentralized trial is a COVID-related adaptation to the research enterprise that has the potential to increase patient accrual, reduce costs, and improve the speed of study completion.11,13 To the best of our knowledge, the present pilot is one of the first descriptions of decentralized study conduct in oncology and was borne of the need to recruit patients during the pandemic while preserving personal protective equipment for health care workers.10 By adopting remote consent, RPM capabilities, video-based clinical assessments, and shipment of kits directly to patients' homes, we were able to successfully transfer the majority of study activities to patients' homes, negating the need for a research-related visit to our facility. As experience with this research paradigm grows, regulatory guidance and processes for maintaining data quality and security must evolve to meet the needs of stakeholders, for example, patients, researchers, and trial sponsors. Finally, given the significant disparities in the United States with respect to technology literacy, access to broadband, and comfort with digital technologies, attention must be paid to the digital divide and promoting inclusivity among study participants.10
Despite high-level evidence of the benefits of electronic PRO monitoring in symptom control for patients with cancer, widespread adoption in routine practice remains limited outside of a research context.6 This points to the critical need for more data to guide the implementation of RPM paradigms for patients with cancer. This is because operationalizing RPM is a complex undertaking with workforce, cultural, technology, patient experience, and financial implications.6,12 Beyond evaluating the incremental effectiveness of biometric capture, relative to PROs alone, which will be evaluated in a future RCT, we will also attempt to delineate the contextual drivers, facilitators, and barriers to deploying RPM. Future treatment paradigms and clinical settings that might benefit from this paradigm (ie, RPM of PROs and biometrics), if shown to be effective, include rural cancer populations, palliative care, surgical prehabilitation programs, and hematologic malignancies. Finally, a sustainable financing and reimbursement strategy will be needed to recoup or offset the significant capital investments necessary.
There are several methodological limitations that warrant attention. First, this was a single-arm, single-institution implementation pilot and thus was inherently limited with respect to generalizability. Specifically, the present pilot was focused on implementation and not designed or powered to evaluate effectiveness in reducing acute care utilization. Second, our observation period of 1 month was considerably shorter than that represented in published trials; as a result, ensuing conclusions about patient adherence and impact on acute care utilization may not be reflective.6,29 Third, although we intentionally enrolled a diverse participant pool (age, sex, and race/ethnicity), our patient mix may not be representative of most oncology practices because of our traditionally high representation of secondary referrals. Finally, we did not evaluate digital health literacy or caregiver burden, factors that could influence the effects of our intervention.
In conclusion, our RPM program is feasible and acceptable and has the potential to improve symptom management. These results highlight the need for a future RCT comparing HRQoL, acute care utilization, and patient experience associated with symptom management via our RPM platform versus PROs alone. It will establish the effectiveness of integrating in-situ physiologic measurements into clinical decision making as part of the ambulatory care of patients with cancer.
APPENDIX
TABLE A1.
Description of Sensor Devices Included in the Patient Kit
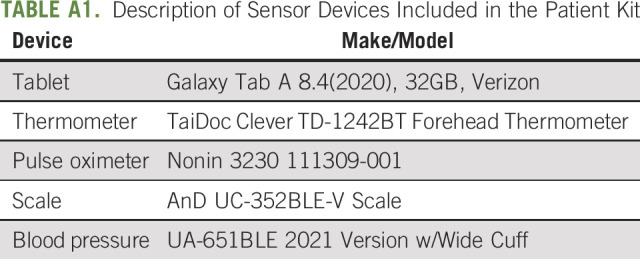
FIG A1.
Sample of the user interface for the remote patient monitoring online platform.
Anaeze C. Offodile
Other Relationship: Blue Cross Blue Shield Association, National Academy of Medicine, Patient Advocate Foundation, Indiana University
Yu-Li Lin
Research Funding: Blue Cross Blue Shield
Travel, Accommodations, Expenses: Blue Cross and Blue Shield of Texas
Sanjay Shete
Stock and Other Ownership Interests: Vertex, 23andMe
Michael J. Overman
Consulting or Advisory Role: Bristol Myers Squibb, Roche/Genentech, Gritstone Bio, MedImmune, Novartis, Promega, Spectrum Pharmaceuticals, Array BioPharma, Janssen, Pfizer, 3D Medicines, Merck, Eisai
Research Funding: Bristol Myers Squibb, Merck, Roche, MedImmune
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented as a poster at the American Society of Clinical Oncology Quality Care Symposium, Chicago, IL, October 1, 2022.
SUPPORT
Support provided, in part, by the Assessment, Intervention and Measurement (AIM) Shared Resource through a Cancer Center Support Grant (CA16672, PI: P. Pisters, MD Anderson Cancer Center), from the National Cancer Institute, National Institutes of Health, and through the Duncan Family Institute for Cancer Prevention and Risk Assessment.
CLINICAL TRIAL REGISTRATION
AUTHOR CONTRIBUTIONS
Conception and design: Anaeze C. Offodile, Domenica Delgado, Sanchita Jain, Janice P. Finder, Susan K. Peterson
Financial support: Anaeze C. Offodile, Janice P. Finder
Administrative support: Anaeze C. Offodile, Janice P. Finder, Christopher J. Miller, Susan K. Peterson
Provision of study materials or patients: Anaeze C. Offodile, Janice P. Finder, Frank V. Fossella, Michael J. Overman
Collection and assembly of data: Anaeze C. Offodile, Domenica Delgado, Danielle Geyen, Christopher J. Miller, Janice P. Finder, Susan K. Peterson
Data analysis and interpretation: Anaeze C. Offodile, Yu-Li Lin, Danielle Geyen, Janice P. Finder, Sanjay Shete, Michael J. Overman, Susan K. Peterson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Integration of Remote Symptom and Biometric Monitoring Into the Care of Adult Patients With Cancer Receiving Chemotherapy—A Decentralized Feasibility Pilot Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Anaeze C. Offodile
Other Relationship: Blue Cross Blue Shield Association, National Academy of Medicine, Patient Advocate Foundation, Indiana University
Yu-Li Lin
Research Funding: Blue Cross Blue Shield
Travel, Accommodations, Expenses: Blue Cross and Blue Shield of Texas
Sanjay Shete
Stock and Other Ownership Interests: Vertex, 23andMe
Michael J. Overman
Consulting or Advisory Role: Bristol Myers Squibb, Roche/Genentech, Gritstone Bio, MedImmune, Novartis, Promega, Spectrum Pharmaceuticals, Array BioPharma, Janssen, Pfizer, 3D Medicines, Merck, Eisai
Research Funding: Bristol Myers Squibb, Merck, Roche, MedImmune
No other potential conflicts of interest were reported.
REFERENCES
- 1.Henry DH, Viswanathan HN, Elkin EP, et al. : Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Support Care Cancer 16:791-801, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Cleeland CS: Mechanisms of treatment-related symptoms in cancer patients. EJC Suppl 11:301-302, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson TM, Ryan SJ, Bennett AV, et al. : The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): A systematic review. Support Care Cancer 24:3669-3676, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Numico G, Cristofano A, Mozzicafreddo A, et al. : Hospital admission of cancer patients: Avoidable practice or necessary care? PLoS One 10:e0120827, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newcomb RA, Nipp RD, Waldman LP, et al. : Symptom burden in patients with cancer who are experiencing unplanned hospitalization. Cancer 126:2924-2933, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Basch E, Schrag D, Henson S, et al. : Effect of electronic symptom monitoring on patient-reported outcomes among patients with metastatic cancer. JAMA 327:2413-2422, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nipp RD, Horick NK, Qian CL, et al. : Effect of a symptom monitoring intervention for patients hospitalized with advanced cancer: A randomized clinical trial. JAMA Oncol 8:571-578, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basch E, Deal AM, Kris MG, et al. : Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 34:557-565, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrag D, Hershman DL, Basch E: Oncology practice during the COVID-19 pandemic. JAMA 323:2005-2006, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Offodile AC, Aloia T: Oncology clinical transformation in response to the COVID-19 pandemic. JAMA Health Forum 1:e201126, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Kadakia KT, Asaad M, Adlakha E, et al. : Virtual clinical trials in oncology—Overview, challenges, policy considerations, and future directions. JCO Clin Cancer Inform 5:421-425, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Offodile AC, DiBrito SR, Finder JP, et al. : Active surveillance of chemotherapy-related symptom burden in ambulatory cancer patients via the implementation of electronic patient-reported outcomes and sensor-enabled vital signs capture: Protocol for a decentralised feasibility pilot study. BMJ Open 12:e057693, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadakia KT, Halperin DM, Offodile AC: Operationalizing virtual trials in oncology—From aspiration to action. JCO Clin Cancer Inform 5:953-957, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Su D, Michaud TL, Estabrooks P, et al. : Diabetes management through remote patient monitoring: The importance of patient activation and engagement with the technology. Telemed e-Health 25:952-959, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Peyroteo M, Ferreira IA, Elvas LB, et al. : Remote monitoring systems for patients with chronic diseases in primary health care: Systematic review. JMIR Mhealth Uhealth 9:e28285, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nipp RD, Shulman E, Smith M, et al. : Supportive oncology care at home interventions: Protocols for clinical trials to shift the paradigm of care for patients with cancer. BMC Cancer 22:383, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daly B, Kuperman G, Zervoudakis A, et al. : InSight care pilot program: Redefining seeing a patient. JCO Oncol Pract 16:e1050-e1059, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright AA, Raman N, Staples P, et al. : The HOPE pilot study: Harnessing patient-reported outcomes and biometric data to enhance cancer care. JCO Clin Cancer Inform 2:1-12, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czajkowski SM, Powell LH, Adler N, et al. : From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol 34:971-982, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Offodile AC, Seitz AJ, Peterson SK: Digital health navigation: An enabling infrastructure for optimizing and integrating virtual care into oncology practice. JCO Clin Cancer Inform 5:1151-1154, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basch E, Reeve BB, Mitchell SA, et al. : Development of the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 106:dju244, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daly B, Nicholas K, Flynn J, et al. : Analysis of a remote monitoring program for symptoms among adults with cancer receiving antineoplastic therapy. JAMA Netw Open 5:e221078, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storz E, Gschwend JE, Retz M: Chemotherapy-induced nausea and vomiting: Current recommendations for prophylaxis. Der Urologe Ausg A 57:532-542, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Jonker LT, Plas M, de Bock GH, et al. : Remote home monitoring of older surgical cancer patients: Perspective on study implementation and feasibility. Ann Surg Oncol 28:67-78, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiner BJ, Lewis CC, Stanick C, et al. : Psychometric assessment of three newly developed implementation outcome measures. Implement Sci 12:108, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bangor A, Kortum PT, Miller JT: An empirical evaluation of the system usability scale. Int J Hum-Comput Int 24:574-594, 2008 [Google Scholar]
- 27.Reichheld FF: The one number you need to grow. Harv Bus Rev 81:46-54, 124, 2003 [PubMed] [Google Scholar]
- 28.Rivera DR, Gallicchio L, Brown J, et al. : Trends in adult cancer–related emergency department utilization: An analysis of data from the nationwide emergency department sample. JAMA Oncol 3:e172450, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basch E, Deal AM, Kris MG, et al. : Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 34:557-565, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barber EL, Garg R, Strohl A, et al. : Feasibility and prediction of adverse events in a postoperative monitoring program of patient-reported outcomes and a wearable device among gynecologic oncology patients. JCO Clin Cancer Inform 6:e2100167, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]