Abstract
Rationale:
Escalation of drug intake and craving are two DSM-5 hallmark symptoms of opioid use disorder (OUD).
Objectives:
This study determined if escalation of intake as modeled by long-access (LgA) self-administration (SA) and craving measured by reinstatement are related.
Methods:
Adult male and female Sprague-Dawley rats were trained to self-administer fentanyl across 7 daily 1-h short access (ShA) sessions, followed by 21 SA sessions of either 1- or 6-h duration (ShA or LgA). Following 14 1-h extinction sessions, Experiment 1 assessed reinstatement induced by either fentanyl (10 or 30 μg/kg) or yohimbine (1 or 2 mg/kg) and Experiment 2 assessed reinstatement induced by a drug-associated cue light.
Results:
Females acquired fentanyl SA faster than males. When shifted to LgA sessions, LgA rats escalated fentanyl intake, but ShA rats did not; no reliable sex difference in the rate of escalation was observed. In extinction, compared to ShA rats, LgA rats initially responded less and showed less decay of responding across sessions. A fentanyl prime induced reinstatement, with LgA rats reinstating more than ShA rats at the 30 μg/kg dose. Yohimbine (1 mg/kg) also induced reinstatement, but there was no effect of access group or sex. With cue-induced reinstatement, LgA females reinstated less than LgA males and ShA females.
Conclusion:
Among the different reinstatement tests assessed, escalation of fentanyl SA increased only drug-primed reinstatement, suggesting a limited relationship between escalation of drug intake and craving (reinstatement) for OUD.
Keywords: fentanyl, escalation, sex, reinstatement, cue, yohimbine, male, female
1. Introduction
The DSM-5 characterizes opioid use disorder (OUD) as a chronic relapsing disorder where individuals typically consume opioids in larger amounts and over longer periods of time than intended, combined with intense opioid craving during abstinence, thus producing a high relapse rate (American Psychiatric Association 2013). A study of chronic non-cancer pain therapy and “street users” of opioids found that most pain therapy patient users of morphine do not escalate their intake and only 9.5% express withdrawal symptoms after controlled use. In contrast, street users initially use heroin intermittently and then escalate their intake, often accompanied by switching from smoking to intravenous injection (Cowan et al. 2001). In addition, those on long-term opioid therapy for pain who escalate their dose within their first year of treatment are more likely to be diagnosed with an OUD (Henry et al. 2015).
While escalation and relapse are often considered to be separate characteristics of OUD that may involve dissociable mechanisms, evidence suggests that intake amount may influence the propensity to relapse. For example, one study found that high levels of heroin use prior to treatment was associated with high relapse rates (Smyth et al. 2010). Similarly, another study found that patients most likely to relapse were those showing signs of dependence and with a recent history of compulsive use (Grau-Lopez et al. 2012), suggesting that escalation of intake is an antecedent condition for vulnerability to relapse. There is also at least one preclinical study indicating that conditions that produce escalated SA also increase reinstatement of cocaine seeking in rats (Mantsch et al. 2004). The positive relationship between escalation and reinstatement suggests that the mechanisms underlying escalation and reinstatement may overlap, at least in part.
The purpose of the current study was to examine more directly the relationship between escalation and relapse in a controlled laboratory setting using a preclinical model of fentanyl self-administration and reinstatement. While both escalation of opioid use and reinstatement of opioid seeking have been modeled in rats and mice, there have been no systematic studies to determine if there is a relation between these DSM-like characteristics of OUD. In one study, rats given access to vaporized sufentanil in long access (LgA; 12-h) sessions showed escalated intake and expressed heightened naloxone-precipitated signs of withdrawal compared to rats maintained on short access (ShA; 1-h) daily sessions (Vendruscolo et al. 2018); however, propensity for relapse was not assessed. To model escalation in the current study, we used the general methods for LgA fentanyl self-administration as described previously (Wade et al. 2015; Towers et al. 2019). To model relapse, we used the extinction-reinstatement model with forced abstinence as described previously (De Vries et al. 1998; Shalev 2002; Stairs et al. 2006). Reinstatement was assessed by either a fentanyl prime or a pharmacological stressor (yohimbine), as well as by a fentanyl-associated cue.
The second purpose of the current study was to determine if sex differences exist in the relation between escalation and relapse with fentanyl self-administration. In both humans and rats, escalation of drug intake and reinstatement of drug seeking are generally higher in females than in males (Becker 2016), although much of this work has been conducted with stimulants and may depend on the schedule of reinforcement. In the case of opioids, females show greater self-administration than males on a FR5 schedule of reinforcement for 0.32 and 1 ug/kg/injection of fentanyl and show greater performance on a progressive ratio (PR) schedule for 3.2 and 10 ug/kg/injection of fentanyl. However, this sex effect reverses when males and females are given concurrent access to 3.2 ug/kg/injection of fentanyl and 18% diluted Ensure® using a choice procedure (Townsend et al. 2019). Using an escalation model, another study found that female C57BL/6J mice escalate heroin intake with LgA (6-h) sessions more than male mice, at least when a low unit dose (30 μg/kg) is used (Towers et al. 2019). Following the oral self-administration of oxycodone, females show greater footshock-induced reinstatement at 0.4 and 1.0 mA, but males show greater footshock-induced reinstatement at 0.8 mA (Fulenwider et al. 2020). Lynch and Carroll found no sex differences in ShA (2-h) cocaine SA, but an increase in female sensitivity to cocaine-induced reinstatement compared to males (2000). Females also show heightened susceptibility to yohimbine- and cue-induced reinstatement of cocaine seeking (Becker & Koob 2016) and alcohol seeking (Bertholomey et al. 2016). A similar effect is found following drug- and cue-induced reinstatement of methamphetamine seeking (Becker & Koob 2016).
Further complicating these sex differences, responding during reinstatement in females may depend on the estrus cycle, as females in estrus reinstate more to a cocaine prime than females in diestrus or proestrus, as well as compared to males (Kippin et al., 2005). With opioids, estrus cycles do not affect oral oxycodone self-administration in Long Evans rats but may be dysregulated by self-administration (Fulenwider et al. 2020). Considering this, further research should investigate the impact of estrus cycles during reinstatement. Based on these overall findings, we hypothesized that female rats would show greater escalation of fentanyl intake and greater reinstatement of fentanyl seeking compared to males.
2. Materials and Methods
2.1. Animals
Male (n = 34) and female (n = 37) Sprague-Dawley rats from Envigo Laboratories (Indianapolis, IN) were delivered at 8–9 weeks of age and individually housed in a humidity- and temperature-controlled colony room maintained on a 12-h light/dark cycle (lights on at 0700-h). All animals had access to food and water ad libitum throughout the experiment. All rats were acquired and tested in cohorts of 12 or 24. All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (University of Kentucky). Details regarding rat weights across all phases of Experiment 1 and 2 are provided in Online Resource 1.
2.2. Drugs
Fentanyl HCl was acquired from the National Institute on Drug Abuse (NIDA Drug Supply Program, Rockville, MD) and dissolved in 0.9% bacteriostatic saline to make the doses used for self-administration (2.5 μg/kg per 0.1 ml infusion volume) or fentanyl-induced reinstatement (15 or 30 μg/kg delivered via subcutaneous [s.c.] injection). The α2 adrenergic antagonist yohimbine HCl (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile water to prepare the two yohimbine-induced reinstatement doses (1 or 2 mg/kg delivered via intraperitoneal [i.p.] injection). Yohimbine solutions were mixed the day prior to reinstatement and stored at 4°C under aluminum foil until needed. All doses were based on salt weight.
2.3. Surgical Procedures
All rats were implanted with chronic indwelling jugular catheters with the port secured to the head using dental acrylic, as described previously (Weiss et al. 2018). For 5 days after surgery, catheters were flushed daily using antibiotic gentamycin solution (0.2 ml), and a post-flush solution (0.2 ml; consisting of gentamycin, heparin, and saline). Rats received 2–4 additional recovery days, where their catheters were flushed with 0.2 ml post-flush solution daily.
2.4. Operant Conditioning Chambers
Operant conditioning chambers (MED-Associates, St. Albans, VT) were used and controlled using computers equipped with MED-PC software. Chambers were enclosed in sound-attenuating cabinets with exhaust fans, and were equipped with two levers, two cue lights, a food receptacle, and house light. Levers were located on both sides of one wall and cue lights were mounted above each lever. The food receptacle was positioned between both levers. A house light was located in the upper middle portion of the opposite wall. Syringe pumps outside the sound-attenuating cabinet connected to a leash inside the operant chamber that moved freely via a swivel connection and attached to rat catheters. During autoshaping and acquisition, fentanyl was delivered in 10 ml syringes at an infusion rate of 0.1 ml per 5.9 sec. During the escalation phase, 20 ml syringes were used and fentanyl was delivered at an infusion rate of 0.1 ml per 3.4 sec. Syringes were changed as needed during the escalation sessions to avoid syringe emptying.
2.5. Experiment 1 and 2: Autoshaping and Acquisition
For both Experiments 1 (n= 16 males and n=19 females) and 2 (n=18 males and n=19 females), rats were trained to self-administer fentanyl using an autoshaping procedure for 7 consecutive days. Each day consisted of a 1-h autoshaping session, a 2-h rest in the home cage, and a 1-h operant conditioning session. During the autoshaping session, rats were given 5 non-contingent infusions on a 6-min random time schedule across a 35-min period. For each infusion, the active lever would extend for 15 sec and then would retract concomitant with an infusion and illumination of both cue lights for 20 sec; if the rat pressed the lever during the 15-sec lever extension, the lever was retracted and followed immediately by an infusion and cue light illumination. The active lever was counterbalanced across rats. Following delivery of the 5 infusions, the remaining 25 min of the autoshaping session was spent with both levers retracted. The house light was turned on throughout the autoshaping session. Immediately following each autoshaping session, all rats were placed in their home cage (with ad libitum food and water) for 2 h before returning to the operant chambers for a 1-h operant acquisition session. On this operant session, both the active and inactive levers were continually available, and rats self-administered on a FR1 schedule; the active lever was the same one used during the autoshaping session. Each infusion was paired with a 20-sec illumination of both cue lights and a time-out period, during which responses were not reinforced with an infusion. The house light was turned off throughout the operant acquisition session.
2.6. Experiment 1 and 2: Escalation
After 7 days of acquisition, rats were divided randomly into ShA (Experiment 1: n= 8 males and n= 8 females, Experiment 2: n= 8 males and n= 9 females) or LgA (Experiment 1: n= 8 males and n= 9 females, Experiment 2: n= 8 males and n= 9 females) self-administration groups. For the next 21 daily self-administration sessions, the ShA group was maintained on 1-h sessions, while the LgA group had the session length increased to 6 h. Both access groups continued to self-administer on a FR1 schedule. All procedures were the same as described for initial acquisition, except that the autoshaping phase was omitted and session length was changed for LgA rats.
During escalation, some rats began to engage in self-injurious behaviors (digit biting and aggressive grooming). These behaviors were redirected with aspen chew blocks (large, 2 × 1.5 × 1.5 inch: Lomir Biomedical Inc., Malone, NY) placed in the home cage and operant chambers at the start of escalation. Wounds were treated using hibiclens antimicrobial skin liquid soap, 0.9% bacteriostatic saline, and triple antibiotic ointment after sessions. Areas with persistent wounding were swabbed with a metronidazole and New Skin liquid bandage mixture before, during, and/or after the session as needed.
2.7. Estrus Cycle Testing
Immediately prior to each reinstatement session, females received vaginal swabs for estrus cycle testing and males were prodded with swabs to simulate estrus cycle testing. Each female was swabbed twice, once to clean the area of previous dead cells, and the second to collect vaginal wall cells for estrus cycle examination. Following vaginal cell collection, swabs were immediately rolled onto slides and examined under light microscope on the same day. There was no significant difference in females based on metestrus/diestrus vs proestrus/estrus phases during Experiment 1 fentanyl- and yohimbine-induced reinstatement, or Experiment 2 cue-induced reinstatement (results shown in Online Resource 2).
2.8. Experiment 1: Fentanyl- and Yohimbine-induced Reinstatement
Three cohorts of rats (total n= 8 ShA males, n= 8 ShA females, n= 8 LgA males, n= 8 LgA females) were used to examine both fentanyl- and yohimbine-induced reinstatement. For both ShA and LgA groups, rats underwent 14 1-h extinction sessions, which were identical to the acquisition sessions, except that fentanyl infusions were omitted. On these sessions, rats were not connected to the metal leash. Reinstatement sessions followed the same format as extinction training, except that rats received one of 5 treatment combinations before the session: (1) 1 mg/kg yohimbine + vehicle; (2) 2 mg/kg yohimbine + vehicle; (3) vehicle + 10 μg/kg fentanyl; (4) vehicle + 20 μg/kg fentanyl; or (5) vehicle + vehicle. On each of these reinstatement tests, rats received two injections, with the first injection being yohimbine or water vehicle (i.p.) and the second injection being fentanyl or saline vehicle (s.c.). Injections were separated by 30 min and rats were placed into the operant conditioning chambers immediately after the second injection. The order of these test treatments was counterbalanced such that all rats received each treatment in a counterbalanced order across the 5 reinstatement sessions. Each test session was separated by 5 maintenance extinction sessions (no injections).
2.9. Experiment 2: Cue-induced Reinstatement
Two cohorts (total n= 8 ShA males, n= 9 ShA females, n= 7 LgA males, n= 9 LgA females) were used to examine cue-induced reinstatement. For both ShA and LgA groups, rats underwent 14 1-h extinction sessions, which were identical to the acquisition sessions, except that fentanyl infusions, cue light illuminations and leash attachments were omitted. Cue-induced reinstatement was assessed on the day after the final extinction session. During this 1-h reinstatement session, active lever presses resulted in illumination of both cue lights and activation of the syringe pump, but fentanyl was not delivered.
2.10. Statistical Analyses
Analyses were performed with SAS version 9.4. Multilevel modeling (MLM) procedures were used to assess acquisition, escalation, extinction, and reinstatement. Initial analyses determined that behavior did not differ across cohorts. The general form of the models of acquisition, escalation, and extinction was for lever presses to be modeled as a function of time (session number). Models for extinction data differed in that they took the form of a power decay model (log of lever pressing predicted by log of session). Two sets of analyses were conducted for each Experiment 1 and 2 escalation. The first set included data from the full session for each subject (1-h for ShA group and 6-h for LgA group). The second set accounted for the different lengths of time between the two access groups by only analyzing the first hour of data from the LgA group (1 h of data from each access group per session). The general form of the models for reinstatement was for active lever presses to be modeled as a function of drug condition (vehicle, fentanyl dose, or yohimbine dose) or cue condition, with vehicle or last session of extinction as the reference group, respectively. In all analyses for acquisition, escalation, extinction and reinstatement, the number of active lever presses did not include the number of presses on the active lever during the time-out period.
A standard model taxonomy was followed (Singer and Willett 2003), starting with an unconditional means model (UMM), followed by an unconditional growth model (UGM) where necessary, and subsequent models including variables such as reinstatement condition, access group (ShA or LgA), lever type (active or inactive), sex, or interactions between these variables as predictors of initial status and change in lever pressing across session. To determine the preferred model, we examined the improvement in model deviance using the likelihood ratio test (Δ deviance). If a variable was unrelated to the dependent variable and its removal did not significantly worsen model fit, it was removed in the final model to maximize parsimony.
Model assumptions were evaluated for preferred models; there were mild to moderate violations of normal residuals and/or homoscedasticity found in each analysis. However, no remedial action successfully alleviated these problems. Therefore, remedial actions were not employed, as MLM is robust to mild to moderate violations of assumptions. Data were examined for multivariate outliers, and these observations were replaced as missing in the data analyzed (n = 1 observations across the entire study). An additional outlier was found in Experiment 1 fentanyl-induced reinstatement, but it did not affect model conclusions and there was no justification for its replacement as missing, thus it was kept in analyses. All missing data were handled using full information maximum likelihood estimations.
Hypotheses were examined as follows. Acquisition of fentanyl self-administration was demonstrated by a difference between active and inactive lever pressing across session, where active lever pressing increases and inactive lever pressing decreases. Escalation was shown by an increase in active lever pressing across sessions, but extinction was shown by a decrease in lever pressing across sessions. Reinstatement was demonstrated by a significant increase in active lever pressing from the control condition. Differences on these measures between males and females, and LgA and ShA rats were examined. Coefficients for the final preferred models are listed in Tables 1, 2, and 3. Complete details on all Experiment 1 and 2 model taxonomies are provided in Online Resource 3.
Table 1.
Preferred model results for acquisition, escalation, and extinction for Experiment 1.
| Model | ||||
|---|---|---|---|---|
| Fixed Effects | Acquisition | Escalation | 1st h Escalation | Extinction |
| Initial Status | ||||
| Intercept | 3.28**** | 14.17* | 14.18**** | 1.55**** |
| LgA | 59.70**** | 0.73 | −0.10* | |
| Female | 3.00** | 0.04 | ||
| Session | ||||
| Intercept | −0.31 | 0.28 | 0.28 | |
| LgA | 5.04**** | 0.78* | ||
| Female | −0.70** | |||
| Session × Session | ||||
| Intercept | −0.004 | −0.004 | ||
| LgA | −0.04 | −0.005 | ||
| Log(Session) | ||||
| Intercept | −0.40**** | |||
| LgA | 0.18*** | |||
| Female | 0.059 | |||
| Active | ||||
| Intercept | 1.94* | |||
| Female | −2.85* | |||
| Active × Session | ||||
| Intercept | 1.41**** | |||
| Female | 1.22*** | |||
| Random Effects | ||||
| L1 Residual | 14.88**** | 202.84**** | 14.47**** | 0.0092**** |
| Cov (I,S) | −9.19 | 0.30 | −0.0098* | |
| Var (I) | 1.17* | 545.88*** | 15.72** | 0.018*** |
| Var (S) | 8.42** | 0.96** | 0.012** | |
| Cov (I, Q) | 0.24 | 0.01 | ||
| Cov (S, Q) | −0.32* | −0.04** | ||
| Var (Q) | 0.02** | 0.002** | ||
| −2 LL | 2695.0 | 5716.7 | 3909.7 | −706.5 |
The coefficients for the final preferred models for Experiment 1 acquisition, escalation, 1st h escalation, and extinction are shown above. Cov = covariance, Var = variance, −2 LL = −2 Log Likelihood, I = intercept, S = slope, Q = quadratic.
(p < .05),
(p < .01),
(p < .001), and
(p < .0001).
Table 2.
Preferred model results for acquisition, escalation, and extinction in Experiment 2.
| Model | ||||
|---|---|---|---|---|
| Fixed Effects | Acquisition | Escalation | 1st h Escalation | Extinction |
| Initial Status | ||||
| Intercept | 3.66**** | 3.54 | 11.29**** | 1.82**** |
| LgA | 37.23**** | −2.17 | −0.21** | |
| Female | 0.96 | 17.30** | 2.54* | −0.077 |
| Session | ||||
| Intercept | −0.50** | 0.06 | 0.22 | |
| LgA | 6.08** | 0.79* | ||
| Female | −0.16 | 0.78 | 0.49 | |
| Session × Session | ||||
| Intercept | 0.002 | −0.004 | ||
| LgA | −0.13 | −0.01 | ||
| Female | −0.03 | −0.02 | ||
| Log(session) | ||||
| Intercept | −0.68**** | |||
| LgA | 0.29**** | |||
| Female | −0.04 | |||
| Active | ||||
| Intercept | −0.004 | |||
| Female | 0.39 | |||
| Active × Session | ||||
| Intercept | 1.37**** | |||
| Female | 0.72* | |||
| Random Effects | ||||
| L1 Residual | 11.08**** | 174.43**** | 12.44**** | 0.025**** |
| Cov (I,S) | −0.39 | 6.14 | −0.7 | −0.02* |
| Var (I) | 3.34** | 230.20*** | 9.49** | 0.026** |
| Var (S) | 0.08 | 28.65*** | 0.71** | 0.025** |
| Cov (I, Q) | −0.57 | 0.01 | ||
| Cov (S, Q) | −1.14*** | −0.02* | ||
| Var (Q) | 0.05*** | 0.001** | ||
| −2 LL | 2663.2 | 5638.0 | 3804.8 | −295.8 |
The coefficients for the final preferred models for Experiment 2 acquisition, escalation, 1st h escalation, and extinction are shown above. Cov = covariance, Var = variance, −2 LL = −2 Log Likelihood, I = intercept, S = slope, Q = quadratic.
(p < .05),
(p < .01),
(p < .001), and
(p < .0001).
Table 3.
Preferred models for reinstatement data.
| Model | |||
|---|---|---|---|
| Fixed Effects | Fentanyl | Yohimbine | Cue |
| Initial Status | |||
| Intercept | 7.39* | 15.09**** | 10.80**** |
| LgA | 7.19 | 2.43 | |
| Female | 13.25** | −4.18 | |
| LgA × Female | −10.06 | ||
| Fent 10 ug/kg | |||
| Intercept | 13.09**** | ||
| Fent 30 ug/kg | |||
| Intercept | 9.86* | ||
| LgA | 13.06* | ||
| Female | 9.37 | ||
| LgA × Female | −13.69 | ||
| Yoh 1 mg/kg | |||
| Intercept | 8.34*** | ||
| Yoh 2 mg/kg | |||
| Intercept | 4.09 | ||
| Cue | |||
| Intercept | 29.60**** | ||
| LgA | 4.58 | ||
| Female | 2.43 | ||
| LgA × Female | −20.18** | ||
| Random Effects | |||
| L1 Residual | 81.81**** | 71.33**** | 75.99**** |
| Var (I) | 48.48** | 59.72** | 12.59 |
| −2 LL | 727.9 | 722.3 | 482.6 |
The coefficients for the final preferred models for fentanyl-, yohimbine-, and cue-induced reinstatement are shown above. Vehicle is the reference category for the Experiment 1 fentanyl and yohimbine models. The last extinction session is the reference category for the Experiment 2 cue model. Fent = fentanyl, Yoh = yohimbine, Cov = covariance, Var = variance, −2 LL = −2 Log Likelihood, I = intercept.
(p < .05),
(p < .01),
(p < .001), and
(p < .0001).
3. Results
3.1. Experiment 1
3.1.1. Acquisition (Fig 1A)
Figure 1: Acquisition, Escalation, 1st h Escalation, and Extinction inExperiment 1.
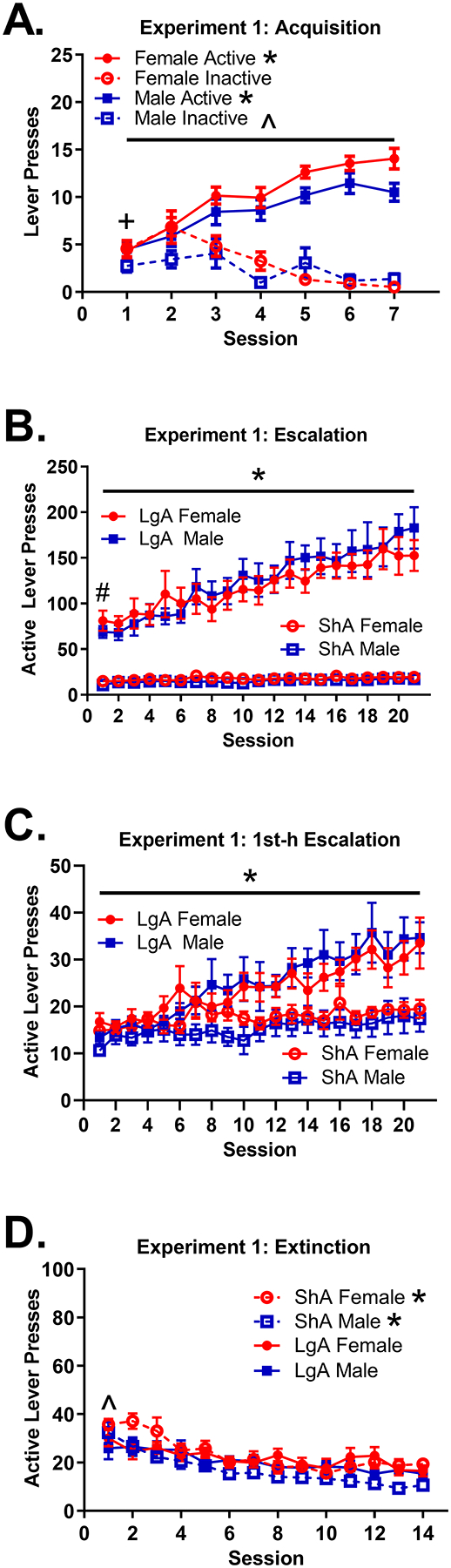
(A) Mean (±SEM) number of active and inactive lever presses for females and males across the 1-h sessions during the Experiment 1 acquisition. The difference between active and inactive lever pressing change was significant for both males and females *(both p < .0001). The difference between the active and inactive lever pressing change across sessions was greater in females than males ^(p < .001). There was a significant difference between active and inactive lever pressing on session 1 for males only +(p < .05). (B) Mean (±SEM) number of active lever presses for ShA and LgA females and males across sessions during the Experiment 1 escalation. LgA rats pressed the active lever more on session 1 than ShA rats #(p < .0001). LgA rats escalated intake across sessions *(p < .0001), but ShA rats did not (p > .05). (C) Mean (±SEM) number of active lever presses for ShA and LgA females and males across sessions during the 1st h of Experiment 1 escalation. LgA rats escalated intake across sessions *(p < .001), but ShA rats did not (p > .05). (D) Mean (±SEM) number of active lever presses for ShA and LgA females and males across sessions during the Experiment 1 extinction. LgA rats pressed the active lever less than ShA rats on session 1 ^(p ≤ .05). LgA rats showed less decay of active lever pressing than ShA rats *(p < .001). Note that in all figure panels, presses on the active lever during the time-out period were not included.
On session 1, females (M = 6.29) pressed the inactive lever more than the males (M = 3.28); difference in means = 3.00, p < .01; however, there was no difference in active lever pressing between females (M = 5.38) and males (M = 5.23), p > .05. The difference between active and inactive lever pressing on session 1 was significantly greater in males (difference in means = 1.94, p < .05), but not in females (difference in means= −0.91, p > .05); difference in mean differences = 2.85, p < .05.
Active lever pressing increased significantly more across sessions than inactive lever pressing for both females (difference in change = 2.62, p < .0001) and males (difference in change = 1.41, p < .0001), indicating that all animals acquired fentanyl self-administration. However, acquisition was more pronounced for females, as the difference between active and inactive lever pressing across sessions was greater for females than males (lever × session × sex interaction, 1.22, p < .001). There was a significant sex difference in change in inactive lever pressing (female change = −1.01, p < .001; male change = −0.31, p > .05); difference in change = 0.70, p < .01. Similarly, there was a significant sex difference in change in active lever pressing (female change = 1.61, p < .001; male change = 1.10, p < .001); difference in change = 0.51, p < .05.
3.1.2. Escalation: Results of Model Using Complete Data (Fig 1B)
For the escalation phase, the preferred model included a quadratic term, indicating that the change in active lever pressing across sessions followed a curvilinear trajectory for some subjects. LgA rats (M = 73.87) had greater active lever pressing on session 1 than ShA rats (M = 14.17); difference in means = 59.70, p < .0001. There was a significantly different trajectory of active lever pressing for ShA and LgA rats; difference in rectilinear change = 5.04, p < .0001. There was no significant change in active lever pressing for ShA rats (rectilinear change = 0.28, p > .05); however, active lever pressing significantly increased for LgA rats (rectilinear change = 5.32, p < .0001), indicating escalation for only LgA rats.
Interestingly, there was a significant session × access group × sex interaction on rat weights during escalation, where LgA males lost weight, LgA females remained consistent, and both ShA males and females gained weight across session. Details on this weight analysis are provided in Online Resource 1.
3.1.3. Escalation: Results of Model Using Only 1st Hour of Data Per Session (Fig 1C)
Results were similar to those of the prior set of analyses. The preferred model included a quadratic term, indicating that the change in active lever pressing across sessions followed a curvilinear trajectory for some subjects. There was a significantly different trajectory of active lever pressing for ShA and LgA rats; difference in rectilinear change = 0.78, p < .05. There was no significant change in active lever pressing for ShA rats (rectilinear change = 0.28 p > .05); however, active lever pressing significantly increased for LgA rats (rectilinear change = 1.06, p < .001), indicating escalation for only LgA rats.
3.1.4. Extinction (Fig 1D)
For the extinction phase, there was an effect of access group on session 1 active lever pressing and change in active lever pressing across sessions. LgA rats (M = 1.46) engaged in less log(active lever presses) than ShA rats (M = 1.57) on session 1; difference in means = −0.10, p < .05. LgA rats (change = −0.19, p < .001) also exhibited less decay in log(active lever presses) than ShA rats (change = −0.37, p < .0001); difference in change = 0.18, p < .001.
3.1.5. Fentanyl-Induced Reinstatement (Fig 2A)
Figure 2: Fentanyl- and Yohimbine-induced reinstatement inExperiment 1.
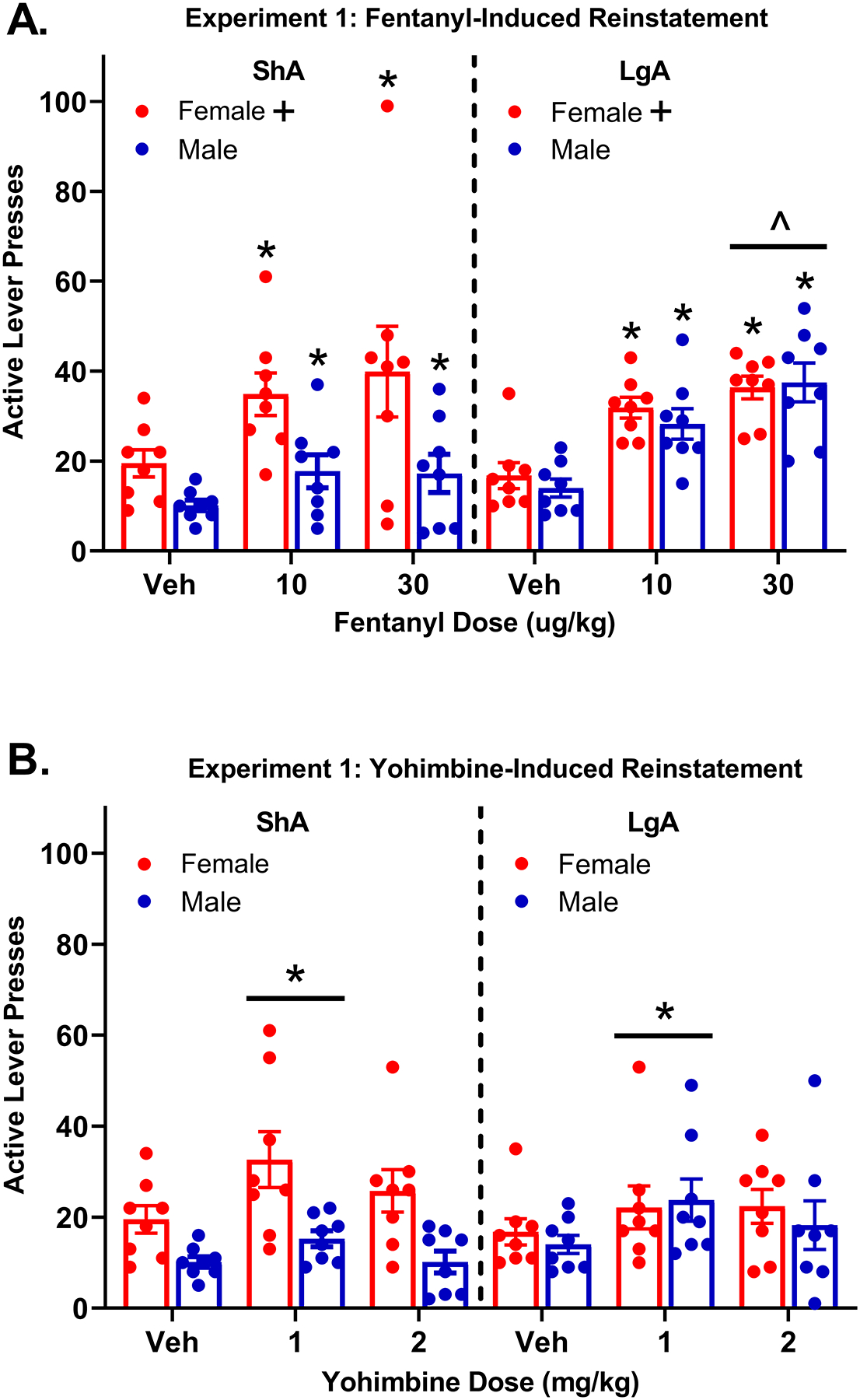
(A) Mean (±SEM) number of active lever presses for ShA and LgA females and males following vehicle, 10 μg/kg fentanyl, and 30 μg/kg fentanyl. All rats reinstated to 10 μg/kg and 30 μg/kg fentanyl in comparison to vehicle *(p < .05). The difference between vehicle and 30 ug/kg fentanyl responding was greater for the LgA group than the ShA group ^(p < .05); note the extreme outlier ShA female following 30 μg/kg fentanyl. Females had higher active lever pressing than males overall +(p < .01). (B) Mean (±SEM) number of active lever presses for ShA and LgA females and males following vehicle, 1 mg/kg yohimbine, and 2 mg/kg yohimbine. The 1 mg/kg yohimbine dose produced reinstatement in comparison to vehicle, collapsed across access group and sex *(p < .001). Note that in both figure panels, presses on the active lever during the time-out period were not included.
Results indicated that active lever pressing was greater after 10 μg/kg fentanyl (M = 28.19) compared to vehicle (M = 15.10); difference in means = 13.09, p < .001, indicating reinstatement for all rats at this dose. There was also an interaction between access group and the effect of 30 μg/kg fentanyl; difference in reinstatement = 13.06, p < .05. ShA rats exhibited greater active lever pressing after 30 μg/kg fentanyl than after vehicle (difference in means= 14.55, p < .05), indicating reinstatement. However, the difference between 30 μg/kg fentanyl and vehicle active lever responding was even larger in LgA rats (difference in means = 20.77, p < .001), indicating greater reinstatement for the LgA group than for the ShA group. Regardless of access group, females exhibited greater active lever pressing than males; difference in means = 8.22, p < .05.
3.1.6. Yohimbine-Induced Reinstatement (Fig 2B)
The preferred model included only the dummy variables for 1 and 2 mg/kg yohimbine as predictors of active lever pressing. Active lever pressing was greater after 1 mg/kg yohimbine (M = 23.43) compared to vehicle (M = 15.09); difference in means = 8.34, p < .001, indicating that all rats reinstated at this yohimbine dose. Active lever pressing was marginally greater following 2 mg/kg yohimbine group (M = 19.18) compared to vehicle (M = 15.09); difference in means = 4.09, p = .06. However, sex, access group, and interactions between sex and access group did not improve model fit and were not significant predictors of active lever pressing.
3.2. Experiment 2
3.2.1. Acquisition (Fig 3A)
Figure 3: Acquisition, Escalation, 1st h Escalation, and Extinction inExperiment 2.
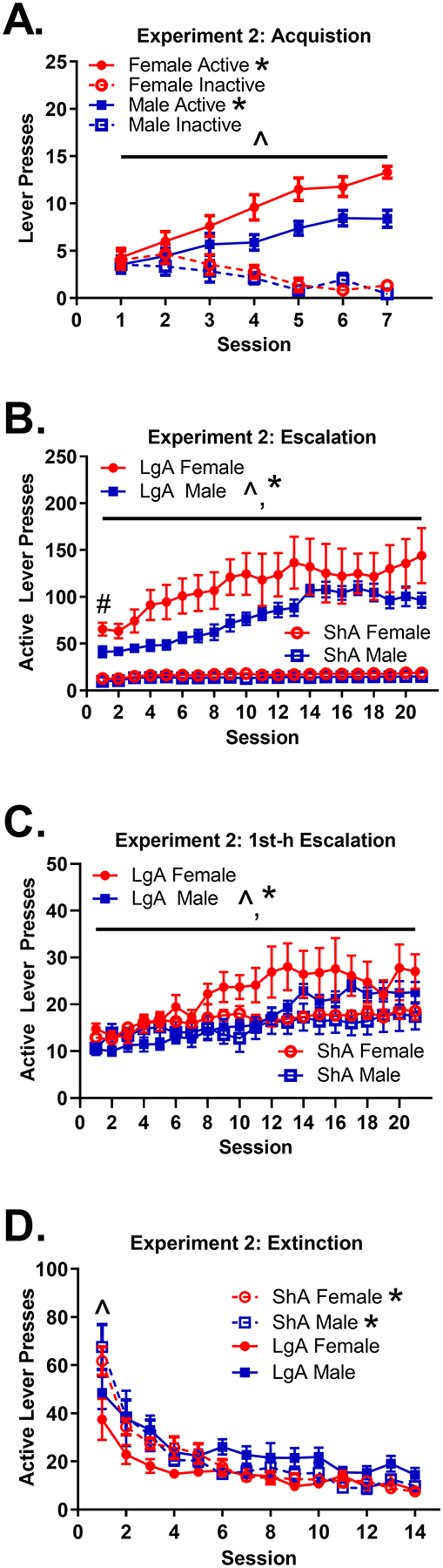
(A) Mean (±SEM) number of active and inactive lever presses for females and males across the 1-h sessions during Experiment 2 acquisition. The difference between active and inactive lever pressing change was significant for both males and females *(both p < .0001). The difference between the active and inactive lever pressing change across sessions was greater in females than males ^(p < 0.05). (B) Mean (±SEM) number of active lever presses for ShA and LgA females and males across sessions during Experiment 2 escalation. The LgA group pressed the active lever more on session 1 than the ShA group #(p < .0001). Females had more active lever presses than males overall ^(p < .05). LgA rats escalated intake across sessions *(p < .01), but ShA rats did not (p > .05). (C) Mean (±SEM) number of active lever presses for ShA and LgA females and males across sessions during the 1st h of Experiment 2 escalation. Females had more active lever presses than males overall ^(p < .05). LgA rats escalated intake across sessions *(p < .001), but ShA rats did not (p > .05). (D) Mean (±SEM) number of active lever presses for ShA and LgA females and males across sessions during Experiment 2 extinction. LgA rats pressed the active lever less than ShA rats on session 1 ^(p < .01). LgA rats showed less decay of active lever pressing than the ShA group *(p < .0001). Note that in all figure panels, presses on the active lever during the time-out period were not included.
On session 1, there was no difference between males (M = 3.66) and females (M = 4.64) in inactive lever pressing, p > .05. Similarly, there was no difference between males (M = 3.66) and females (M = 5.01) in active lever pressing on session 1, p > .05.
There was a significant 3-way interaction between sex, access group, and session (interaction = 0.72, p < .05). Active lever pressing increased significantly more across sessions than inactive lever pressing for both females (difference in change = 2.09, p < .0001) and males (difference in change = 1.37, p < .0001), indicating that all animals acquired fentanyl self-administration. There was no sex difference in change in inactive lever pressing (female change = −0.66, p < .001; male change = −0.50, p < .01), p > .05. However, there was a sex difference in change for active lever pressing, with females increasing active lever pressing across sessions more rapidly (change = 1.43, p < .0001) than males (change = 0.87, p < .0001); difference in change = 0.57 p < .05.
3.2.2. Escalation: Results of Model Using Complete Data (Fig 3B)
For the escalation phase, the preferred model included a quadratic term, indicating that the change in active lever pressing across sessions followed a curvilinear trajectory for some subjects (probing of this effect indicated that female LgA rats reached a point where their lever pressing leveled off). LgA rats (M = 49.43) had greater active lever pressing on session 1 than ShA rats (M = 12.20); difference in means = 37.23, p < .0001. There was also a significantly different trajectory of active lever pressing for ShA and LgA rats; difference in rectilinear change = 6.08, p < 01. There was no significant change in active lever pressing for ShA rats (rectilinear change = 0.48, p > .05); however, active lever pressing significantly increased for LgA rats (rectilinear change = 6.55, p < .01), indicating escalation for only LgA rats. In addition, females (M = 39.47) pressed the active lever significantly more than males (M = 22.16) overall; difference in means = 17.30, p < .01.
Interestingly, there was a significant session × access group × sex interaction on rat weights during escalation, where LgA males lost weight, LgA females remained consistent, and both ShA males and females gained weight across session. Details on this weight analysis are provided in Online Resource 1.
3.2.3. Escalation: Results of Model Using Only 1st Hour of Data Per Session (Fig 3C)
Results were similar to those of the prior set of analyses. The preferred model included a quadratic term, indicating that change in active lever pressing followed a curvilinear trajectory for some rats (probing of this effect showed that female rats reached a point where their lever pressing leveled off). There was a significant difference in trajectories of active lever pressing for ShA and LgA rats; difference in rectilinear change = 0.79, p < .05. There was no significant change in active lever pressing for ShA rats (rectilinear change = 0.48, p > .05); however, active lever pressing significantly increased for LgA rats (rectilinear change = 1.28, p < .001), indicating escalation for only LgA rats. Females (M = 12.76) pressed the active lever significantly more than males (M = 10.21) overall; difference in means = 2.54, p = .05.
3.2.4. Extinction (Fig 3D)
For the extinction phase, results were similar to Experiment 1. There was an effect of access group on session 1 active lever pressing and a change in active lever pressing across sessions. LgA rats (M = 1.57) engaged in less log(active lever presses) than ShA rats (M = 1.78, p < .0001) during session 1; difference in means = −.21, p < .01. LgA rats (change = −0.41, p < .001) also exhibited less decay in log(active lever presses) than ShA rats (change = −0.70, p < .001); difference in change = 0.29, p < .0001.
3.2.5. Cue-Induced Reinstatement (Fig 4)
Figure 4: Cue-induced reinstatement inExperiment 2.
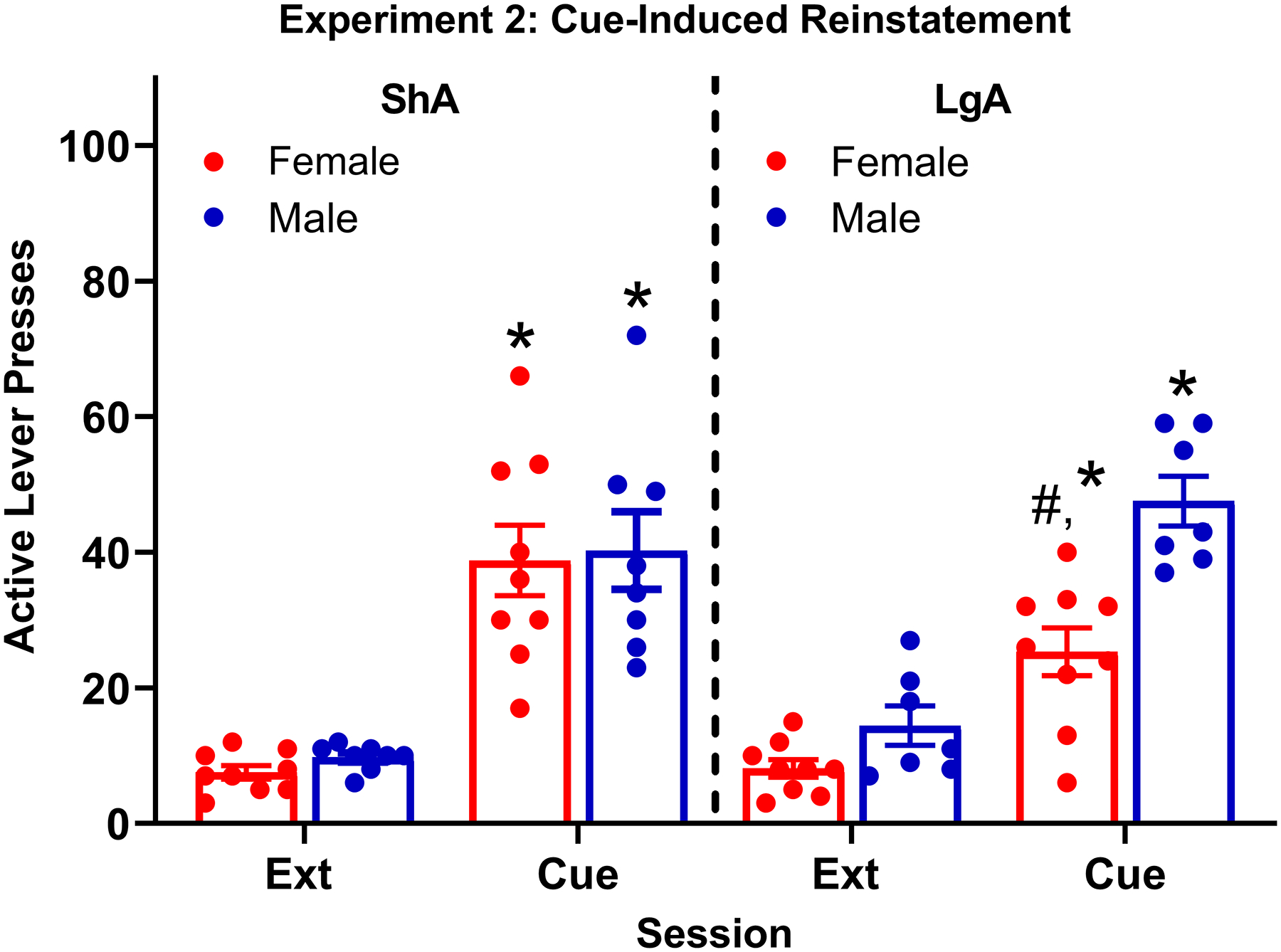
Mean (±SEM) number of active lever presses for ShA and LgA females and males following the final extinction session (Ext) or cue. All rats reinstated to the cue in comparison to their final extinction session *(p < .0001). There was also a significant cue × access group × sex interaction, indicating the in reinstatement between access groups was moderated by sex, with LgA females showing less reinstatement than LgA males and ShA females #(p < .01). Note that presses on the active lever during the time-out period were not included.
There was a 3-way interaction between cue condition, sex, and access group, −20.18, p < .01. All groups exhibited cue-induced reinstatement. Cue-induced reinstatement in LgA females (difference between cue and extinction = 16.42, p < .0001) was significantly less than in ShA females (difference between cue and extinction = 32.02, p < .0001); difference in reinstatement = 15.60, p < .01. However, cue-induced reinstatement in LgA males (difference between cue and extinction = 34.17, p < .0001) was not significantly different from ShA males (difference between cue and extinction = 29.60, p < .0001); difference in reinstatement = 4.58, p > .05. Thus, the effect of access group on cue-induced reinstatement was only observed for females.
Additional probing of the 3-way interaction examined how sex differences varied across the access groups. Cue-induced reinstatement for LgA males (difference between cue and extinction = 34.17, p < .0001) was significantly greater than LgA females (difference between cue and extinction = 16.42, p < .0001); difference in reinstatement = 17.75, p < .01. However, cue-induced reinstatement for ShA males (difference between cue and extinction = 29.60, p < .0001) was not significantly different from ShA females (difference between cue and extinction = 32.02, p < .0001); difference in reinstatement = 2.43, p > .05. Thus, a sex difference was only observed for LgA rats.
4. Discussion
With the goal of determining if escalation of fentanyl intake and reinstatement of fentanyl seeking are related in males and females, the current study revealed several key findings. First, during acquisition using an autoshaping procedure in ShA (1-h) sessions, females acquired self-administration at a faster rate than males. Second, when switched to LgA (6-h) sessions, both sexes escalated intake similarly across the 21 sessions. No escalation was obtained with either sex when rats were maintained on ShA sessions. Third, during the extinction phase, LgA rats responded less than ShA rats on the first session and they experienced a slower decay of responding. Fourth, and most importantly, ShA and LgA groups showed a differential pattern of response to the stimuli used during reinstatement testing (fentanyl, yohimbine, cue). With drug-primed reinstatement, the difference in responding between vehicle and 30 μg/kg fentanyl responding was greater in LgA rats than ShA rats. With yohimbine, only the lower dose of yohimbine (1 mg/kg) produced reinstatement and no difference between ShA and LgA groups was observed. With cue-primed reinstatement, there was a 3-way interaction (cue condition × access group × sex) effect which indicated that cue-induced reinstatement in LgA females was reduced compared to both LgA males and ShA females. Thus, while LgA sessions were related to greater drug-primed reinstatement, LgA sessions were not related to greater reinstatement induced by either yohimbine or a drug-associated cue.
Evidence indicates that females may be more susceptible than males to the reinforcing effect of drugs of abuse in both clinical and preclinical settings (Lynch et al. 2002). In rats, previous studies indicate that females have increased vulnerability to the acquisition of cocaine and heroin self-administration, and successfully acquire self-administration in fewer days than males (Lynch and Carroll 1999; Carroll et al. 2001; Carroll et al. 2002). A similar effect is found with other substances of abuse such as nicotine, methamphetamine, and alcohol (Lynch et al. 2002). Evidence suggests that accelerated acquisition of drug self-administration in females may be a result of estradiol activity (Hu et al. 2004; Jackson et al. 2006; Lynch 2006). Similar to previous results obtained with cocaine and heroin, the current results demonstrate that females acquire the self-administration of fentanyl at a faster rate than males. However, when shifted to LgA sessions, there was no reliable sex difference in the rate of escalation of fentanyl intake.
Although prior research indicates that rats given extended access (6- to 12-h) to drug-reinforced responding will escalate their intake across sessions, much of that work has been conducted with stimulant drugs. For example, cocaine self-administration in rats escalates when maintained on 6- and 12-h sessions, but not when maintained on 1- or 3-h sessions (Wee et al. 2007). LgA females also show a more robust escalation in cocaine self-administration compared to LgA males (Roth and Carroll 2004). More recent work in male rats has demonstrated that escalation of intake also occurs with opioid self-administration. Rats placed on LgA self-administration sessions escalate heroin self-administration across 18 sessions, but not when placed on ShA self-administration sessions (Ahmed et al. 2000). Furthermore, when rats are placed on LgA fentanyl self-administration sessions, they escalate their intake across sessions (Wade et al. 2015; Morgan et al. 2002). Although escalation patterns differ based upon opioid type and dose, male rats given 12-h LgA sessions show rapid escalation of intake at 2.5 ug/kg/inf of fentanyl and will also escalate their intake using intermediate doses of heroin and oxycodone, and high doses of fentanyl and oxycodone (Wade et al., 2015). The current study, while limited because only a single unit dose of fentanyl was used, extends those findings to demonstrate that both males and females escalate opioid intake similarly when given LgA but not ShA sessions.
It is well known that rats extinguish drug seeking when the emitted behavior does not lead to drug delivery (McNally 2014). A few studies have shown that extinction of self-administration behavior may vary depending on the length of the self-administration session. For example, one study found that LgA rats extinguished slower than ShA rats (Lenoir and Ahmed 2007). Consistent with that report, the current study found that when extinction was performed with or without previous drug-associated cues, LgA rats showed a less rapid decay of non-reinforced responding compared ShA rats. Paradoxically, however, ShA rats showed higher responding than LgA rats on the first extinction session. This latter result contradicts a previous report that ShA rats show less active lever pressing than LgA rats early in extinction of responding established with heroin (Ahmed et al. 2000). While several procedural differences exist between studies that prevents a firm explanation of the discrepant findings, the current results indicate that the transient “extinction burst” that occurs on the first session of reward omission is greatest in ShA rats, perhaps because they temporally learn during acquisition to emit all of their responses within one hour. Alternatively, since LgA rats were exposed to greater levels of fentanyl during escalation compared to ShA rats, they may have been in withdrawal during early extinction, thus leading to a general suppression of behavior.
Drug-primed reinstatement is a common method of inducing drug seeking following a period of extinction. Rats that previously self-administered cocaine, nicotine, alcohol, or heroin all show reinstatement after extinction when the self-administered drug is reintroduced (De Vries et al. 1998; Feltenstein et al. 2012; Lê et al. 1998; Shaham et al. 1994). While it is known that drug seeking can be achieved following a drug prime, little research has examined how extended access can alter this type of reinstatement. Mantsch et al. (2004) found that rats previously maintained on LgA (7-h) sessions were more sensitive to cocaine-induced reinstatement than rats previously maintained on ShA (1-h) sessions. Similarly, more robust heroin-induced reinstatement was found in rats trained on LgA (6-h) sessions than rats trained on ShA (1-h) sessions (Lenoir and Ahmed 2007). Consistent with those previous reports, which were in only males, the current results demonstrate that LgA rats show greater fentanyl-induced reinstatement after fentanyl (30 μg/kg) compared to ShA rats. This effect appeared to be driven primarily by a difference in reinstatement between LgA and ShA males, but a larger sample size may be needed to distinguish this effect.
Yohimbine has been used in previous research to trigger drug seeking following a period of extinction (Mantsch et al. 2014; See and Waters 2011). Yohimbine-induced reinstatement occurs in rats following self-administration of methamphetamine (Shepard et al. 2004), cocaine (Feltenstein and See 2006), alcohol (Lee et al. 2004), and nicotine (Feltenstein et al. 2012), although fentanyl seeking has not been examined. The current results indicate that reliable reinstatement occurred with 1 mg/kg yohimbine, but not 2 mg/kg yohimbine. However, this low-dose reinstatement effect did not differ between access group or sex, which contrasts with drug-primed reinstatement. Importantly, recent evidence indicates that yohimbine appears to produce anxiety- and stress-like symptoms that may produce response enhancement that is independent of past drug use (Mantsch et al. 2016; Chen et al. 2015), thus calling into question its validity as a marker of stress-induced reinstatement. Furthermore, the use of stress-induced reinstatement methods other than yohimbine following escalation, such as footshock, may produce different results. For example, one study found that rats maintained on LgA (11-h) sessions showed greater heroin seeking following footshock compared to rats maintained on ShA (1-h) sessions (Ahmed et al., 2000).
In another group of rats (Experiment 2), cue-induced reinstatement was also assessed. Reintroducing a cue previously associated with drug taking is another well-known method of producing drug seeking following self-administration of alcohol (Schroeder et al. 2008), cocaine (Sutton et al. 2000), nicotine (Le Foll et al. 2012), heroin (Rubio et al. 2019), and methamphetamine (Yan et al. 2007). However, little is known about cue-induced reinstatement after the escalation of drug taking. One study found no difference between ShA and LgA adult or adolescent male rats in cue-induced morphine seeking (Doherty 2009). Similarly, the current study found no difference in cue-induced reinstatement between ShA and LgA males. Interestingly, however, LgA females showed reduced cue-induced reinstatement compared to either ShA females or LgA males. The decrease in cue-induced reinstatement observed in LgA females may involve a failure in recognition memory, as morphine withdrawal is associated with impaired memory (Rabbani et al. 2009). Further work is needed to determine if escalation of fentanyl intake in females leads to lasting changes in cue-recognition memory or other reward-relevant systems underlying cue-induced fentanyl seeking. In any case, the effect of session length on cue-induced reinstatement observed in females did not vary based on phase of the estrus cycle, indicating that altered sex-dependent hormonal differences do not likely play a role.
In conclusion, the current study provides only limited support for the hypothesis that escalation of fentanyl intake and reinstatement of drug seeking are related processes since LgA sessions produced greater reinstatement than ShA session to a drug prime, but not to either yohimbine or a cue. In addition, LgA males displayed greater cue-induced reinstatement than LgA females. These results are consistent with clinical data indicating that males are more reactive to cocaine-associated cues than females (Frick 2020). In addition, while some human research indicates that females are more susceptible to craving and relapse (Becker 2016; Robbins et al. 1999; Fox et al. 2014; Hitschfeld et al. 2015), other studies support that males have worse outcomes following treatment, despite predicted advantages (Walitzer and Dearing 2006). Sex differences also occur in relapse patterns, with males oscillating more quickly between relapse and abstinence compared to females (Gallop et al. 2007). Further work is needed to uncover the potential mechanisms involved in the sex-dependent differences in reinstatement observed here following LgA sessions.
Supplementary Material
Acknowledgements:
Support provided by National Institute of Health grants P50 DA05312, T32 DA035200, and T32 DA016176
Footnotes
There are no conflicts of interest to report.
References
- Ahmed SH, Walker JR, Koob GF (2000) Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22:413 doi: 10.1016/S0893-133X(99)00133-5 [DOI] [PubMed] [Google Scholar]
- American Psychiatric A, American Psychiatric Association DSMTF (2013) Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, Fifth edition, Fifth edition. edn. Arlington, VA: : American Psychiatric Association, Arlington, VA [Google Scholar]
- Becker JB (2016) Sex differences in addiction. Dialogues in clinical neuroscience 18:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF (2016) Sex differences in animal models: Focus on addiction. Pharmacol Rev 68:242–263 doi: 10.1124/pr.115.011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey ML, Nagarajan V, Torregrossa MM (2016) Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology (Berl) 233:2277–2287 doi: 10.1007/s00213-016-4278-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M, Morgan A, Lynch W, Campbell U, Dess N (2002) Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology 161:304–313 doi: 10.1007/s00213-002-1030-5 [DOI] [PubMed] [Google Scholar]
- Carroll ME, Campbell UC, Heideman P (2001) Ketoconazole suppresses food restriction–induced increases in heroin self-administration in rats: Sex differences experimental and clinical. Psychopharmacology 9:307–316 doi: 10.1037/1064-1297.9.3.307 [DOI] [PubMed] [Google Scholar]
- Chen YW, Fiscella KA, Bacharach SZ, Tanda G, Shaham Y, Calu DJ (2015) Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history. Addiction Biology 20:690–700 doi: 10.1111/adb.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan DT, Allan LG, Libretto SE, Griffiths P (2001) Opioid drugs: A comparative survey of therapeutic and “street” use. Pain Medicine 2:193–203 doi: 10.1046/j.1526-4637.2001.01026.x [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer ANM, Binnekade R, Mulder AH, Vanderschuren LJMJ (1998) Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. European Journal of Neuroscience 10:3565–3571 doi: 10.1046/j.1460-9568.1998.00368.x [DOI] [PubMed] [Google Scholar]
- Doherty J, Ogbomnwan Y, Williams B, Frantz K (2009) Age-dependent morphine intake and cue-induced reinstatement, but not escalation in intake, by adolescent and adult male rats. Pharmacology, Biochemistry and Behavior 92:164–172 doi: 10.1016/j.pbb.2008.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE (2012) Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug and Alcohol Dependence 121:240–246 doi: 10.1016/j.drugalcdep.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE (2006) Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behavioural Brain Research 174:1–8 doi: 10.1016/j.bbr.2006.06.039 [DOI] [PubMed] [Google Scholar]
- Fox HC, Morgan PT, Sinha R (2014) Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology 39:1527–1537 doi: 10.1038/npp.2014.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM (2020) Estrogens and memory: Basic research and clinical implications. Oxford University Press, New York, New York [Google Scholar]
- Fulenwider HD et al. (2020) Sex differences in oral oxycodone self-administration and stress-primed reinstatement in rats. Addict Biol 25:e12822 doi: 10.1111/adb.12822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallop RJ, Crits-Christoph P, Ten Have TR, Barber JP, Frank A, Griffin ML, Thase ME (2007) Differential transitions between cocaine use and abstinence for men and women. Journal of Consulting and Clinical Psychology 75:95–103 doi: 10.1037/0022-006X.75.1.95 [DOI] [PubMed] [Google Scholar]
- Grau-López L et al. (2012) [Risk factors for relapse in drug-dependent patients after hospital detoxification]. Adicciones 24:115–122 [PubMed] [Google Scholar]
- Henry SG, Wilsey BL, Melnikow J, Iosif A-M (2015) Dose escalation during the first year of long-term opioid therapy for chronic pain. Pain Medicine 16:733–744 doi: 10.1111/pme.12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitschfeld MJ et al. (2015) Female smokers have the highest alcohol craving in a residential alcoholism treatment cohort. Drug and Alcohol Dependence 150:179–182 doi: 10.1016/j.drugalcdep.2015.02.016 [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB (2004) Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 29:81–85 doi: 10.1038/sj.npp.1300301 [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB (2006) Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology 31:129–138 doi: 10.1038/sj.npp.1300778 [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE (2005) Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 182:245–252 doi: 10.1007/s00213-005-0071-y [DOI] [PubMed] [Google Scholar]
- Lê AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y (1998) Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology 135:169–174 doi: 10.1007/s002130050498 [DOI] [PubMed] [Google Scholar]
- Le Foll B et al. (2012) Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacol 15:1265–1274 doi: 10.1017/s1461145711001398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD (2004) Pharmacological Blockade of α2-Adrenoceptors Induces Reinstatement of Cocaine-Seeking Behavior in Squirrel Monkeys. Neuropsychopharmacology 29:686–693 doi: 10.1038/sj.npp.1300391 [DOI] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH (2007) Heroin-Induced Reinstatement is Specific to Compulsive Heroin Use and Dissociable from Heroin Reward and Sensitization. Neuropsychopharmacology 32:616–624 doi: 10.1038/sj.npp.1301083 [DOI] [PubMed] [Google Scholar]
- Lynch W, Roth M, Carroll M (2002) Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology 164:121–137 doi: 10.1007/s00213-002-1183-2 [DOI] [PubMed] [Google Scholar]
- Lynch WJ (2006) Sex differences in vulnerability to drug self-administration experimental and clinical. Psychopharmacology 14:34–41 doi: 10.1037/1064-1297.14.1.34 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology 144:77–82 doi: 10.1007/s002130050979 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME (2000) Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology 148:196–200 doi: 10.1007/s002130050042 [DOI] [PubMed] [Google Scholar]
- Mantsch J, Yuferov V, Mathieu-Kia A-M, Ho A, Kreek M (2004) Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology 175:26–36 doi: 10.1007/s00213-004-1778-x [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y (2016) Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology 41:335–356 doi: 10.1038/npp.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Vranjkovic O, Twining RC, Gasser PJ, McReynolds JR, Blacktop JM (2014) Neurobiological mechanisms that contribute to stress-related cocaine use. Neuropharmacology 76:383–394 doi: 10.1016/j.neuropharm.2013.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP (2014) Extinction of drug seeking: Neural circuits and approaches to augmentation. Neuropharmacology 76 doi: 10.1016/j.neuropharm.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Morgan AD, Campbell UC, Fons RD, Carroll ME (2002) Effects of agmatine on the escalation of intravenous cocaine and fentanyl self-administration in rats. Pharmacology, Biochemistry and Behavior 72:873–880 doi: 10.1016/S0091-3057(02)00774-8 [DOI] [PubMed] [Google Scholar]
- Rabbani M, Hajhashemi V, & Mesripour A. (2009) Increase in brain corticosterone concentration and recognition memory impairment following morphine withdrawal in mice. Stress: The International Journal on the Biology of Stress 12(5):451–456 doi: 10.1080/10253890802659612 [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP (1999) Comparing levels of cocaine cue reactivity in male and female outpatients. Drug and Alcohol Dependence 53:223–230 doi: 10.1016/S0376-8716(98)00135-5 [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME (2004) Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacology, Biochemistry and Behavior 78:199–207 doi: 10.1016/j.pbb.2004.03.018 [DOI] [PubMed] [Google Scholar]
- Rubio FJ et al. (2019) Prelimbic cortex is a common brain area activated during cue-induced reinstatement of cocaine and heroin seeking in a polydrug self-administration rat model. The European journal of Neuroscience 49:165–178 doi: 10.1111/ejn.14203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW (2008) Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK 1/2 phosphorylation in specific limbic brain regions: Blockade by the mGluR5 antagonist MPEP. Neuropharmacology 55:546–554 doi: 10.1016/j.neuropharm.2008.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- See R, Waters R (2011) Pharmacologically-induced stress: a cross-species probe for translational research in drug addiction and relapse. American Journal of Translational Research 3:81–89 [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Rodaros D, Stewart J (1994) Reinstatement of heroin-reinforced behavior following long-term extinction: implications for the treatment of relapse to drug taking. Behavioural Pharmacology 5:360–364 doi: 10.1097/00008877-199406000-00015 [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm J, Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: A review. Pharmacological Reviews 54:1–42 doi: 10.1124/pr.54.1.1 [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y (2004) The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biological Psychiatry 55:1082–1089 doi: 10.1016/j.biopsych.2004.02.032 [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB (2003) Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press, New York, NY, US. doi: 10.1093/acprof:oso/9780195152968.001.0001 [DOI] [Google Scholar]
- Smyth BP, Barry J, Keenan E, Ducray K (2010) Lapse and relapse following inpatient treatment of opiate dependence. Irish medical journal 103:176–179 [PubMed] [Google Scholar]
- Stairs JD, Klein DE, Bardo TM (2006) Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behavioural Pharmacology 17:597–604 doi: 10.1097/01.fbp.0000236271.72300.0e [DOI] [PubMed] [Google Scholar]
- Sutton MA, Karanian DA, Self DW (2000) Factors that determine a propensity for cocaine-seeking behavior during abstinence in rats. Neuropsychopharmacology 22:626–641 doi: 10.1016/S0893-133X(99)00160-8 [DOI] [PubMed] [Google Scholar]
- Towers EB, Tunstall BJ, McCracken ML, Vendruscolo LF, Koob GF (2019) Male and female mice develop escalation of heroin intake and dependence following extended access. Neuropharmacology 151:189–194 doi: 10.1016/j.neuropharm.2019.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Caine SB, Thomsen M, & Banks ML (2019) Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology 44(12); 2022–2029 doi: 10.1038/s41386-019-0356-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo JCM et al. (2018) Compulsive-Like Sufentanil Vapor Self-Administration in Rats. Neuropsychopharmacology 43:801–809 doi: 10.1038/npp.2017.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF (2015) Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40:421–428 doi: 10.1038/npp.2014.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walitzer KS, Dearing RL (2006) Gender differences in alcohol and substance use relapse. Clinical Psychology Review 26:128–148 doi: 10.1016/j.cpr.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Wee S, Specio S, Koob G (2007) Effects of Dose and Session Duration on Cocaine Self-Administration in Rats. The Journal of pharmacology and experimental therapeutics 320:1134–1143 doi: 10.1124/jpet.106.113340 [DOI] [PubMed] [Google Scholar]
- Weiss V, Yates J, Beckmann J, Hammerslag L, Bardo M (2018) Social reinstatement: a rat model of peer-induced relapse. Psychopharmacology 235:3391–3400 doi: 10.1007/s00213-018-5048-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Yamada K, Nitta A, Nabeshima T (2007) Transient drug-primed but persistent cue-induced reinstatement of extinguished methamphetamine-seeking behavior in mice. Behavioural Brain Research 177:261–268 doi: 10.1016/j.bbr.2006.11.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


