Abstract
Splenic diffuse red pulp small B-cell lymphoma (SDRPL) is an extremely rare B-cell lymphoma. The disease is typically indolent and treatment with splenectomy usually results in durable remissions. Here, we present a case of an extremely aggressive course of SDRPL with transformation to diffuse large B-cell lymphoma and multiple relapses immediately following cessation of immunochemotherapy. We provide results from whole-exome sequencing from debut of SDRPL and from following transformed stages and identified a novel somatic mutation in RB1 as the possible driver of this aggressive disease, which has not been reported earlier in SDRPL.
Keywords: Splenic diffuse red pulp small B-cell lymphoma, Aggressive course of splenic diffuse red pulp small B-cell lymphoma, RB1, CN loss of CDKN2A and CDKN2B
Introduction
Splenic diffuse red pulp small B-cell lymphoma (SDRPL) is a rare and indolent subgroup of splenic B-cell lymphomas [1, 2] with only a few cases previously published [3, 4]. The genomic landscape of SDRPL is not well known. Here we present an unusual aggressive course of transformed SDRPL which we investigate with sequential whole-exome sequencing (WES) during chronic and transformed stages. We identified a novel somatic mutation in the tumor suppressor gene RB1 as the potential driver of this aggressive disease, which has not been reported in SDRPL earlier [3, 5-7].
Case Report
Investigations
A 57-year-old female presented with fatigue, abdominal pain, nausea, fever, and headache through 1 week. Clinical examination revealed distended abdomen, pain in the lower left quadrant and splenomegaly.
Diagnosis
Laboratory work revealed white blood cell count (WBC) of 117,000/µL with lymphocytes of 113.76 and neutrophils of 2.32, hemoglobin (Hb) of 11.9 g/dL, platelet (PLT) count of 108,000/µL and lactate dehydrogenase (LDH) at 630 U/L. A whole-body 18F-FDG positron emission tomography-computed tomography (PET-CT) scan showed inhomogeneous FDG uptake in a markedly enlarged spleen and bone marrow (BM). Bone marrow biopsy (BMB) showed infiltration from a mature B-cell non-Hodgkin lymphoma (NHL) (Fig. 1d, e), in contrast peripheral blood (PB) smear showed signs of transformation with many large lymphocytes.
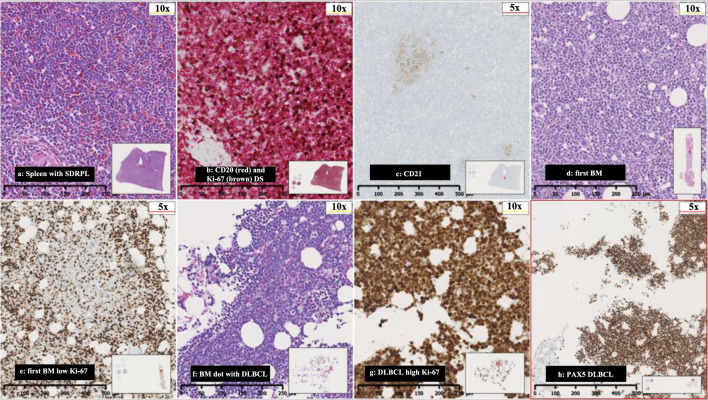
Figure 1.
(a) Spleen with SDRPL, (b) CD20 (red) and Ki-67 (brown) double stain (DS), (c) CD21-spleen showing minimal follicular dendritic networks, (d) first BM with nodular and interstitial infiltration with small and medium sized lymphocytes, (e) first BM with low proliferations rate in Ki-67 in nodular infiltration, (f) BM with DLBCL, (g) high proliferations rate in DLBCL, and (h) PAX5 with severe BM infiltration from DLBCL. SDRPL: splenic diffuse red pulp small B-cell lymphoma; BM: bone marrow; DLBCL: diffuse large B-cell lymphoma.
Due to the discrepancy in the first BM findings and PB morphology with marked lymphocytosis and many medium to large lymphocytes in addition to the enlarged spleen, a diagnostic splenectomy was performed to exclude a high-grade lymphoma.
The pathological examination of the splenic tissue revealed an expanded red pulp showing massive infiltration of small to medium sized B-lymphocytes with a variable proliferation rate ranging from 10-40% by Ki-67 index. Immunohistochemistry and flowcytometry revealed positivity for PAX5, CD20, CD79a, BCL2, and IgM-lambda clonality while CD5, CD10, BCL6, MUM1, CYD1, SOX11, CD23, CD11c, CD103, CD25, CD30, EBER and BRAF were negative and no c-MYC overexpression. There were only focal rests of follicular dendritic reticulum in areas representing the white pulp. The case was classified as an SDRPL (Fig. 1a-c).
Treatment
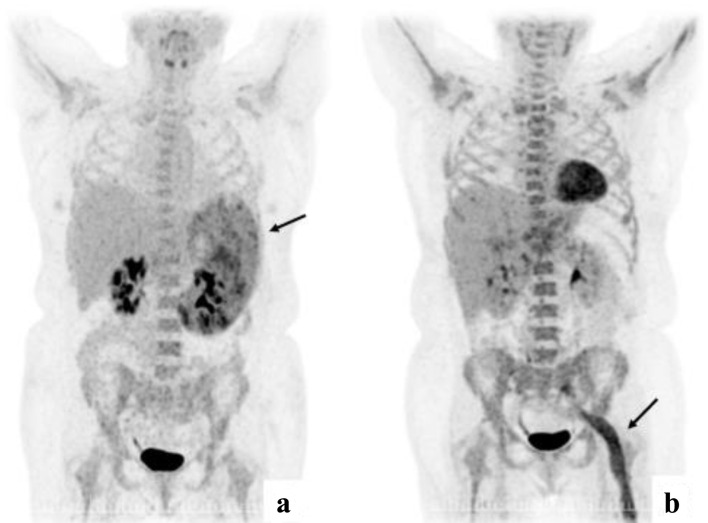
Due to the indolent nature of the lymphoma, the low proliferation rate by Ki-67 of 10-40% in BM and spleen and LDH of 238 U/L after the splenectomy was considered as sufficient treatment. After 1 month, the patient was readmitted with fever, fatigue, and night sweats. Laboratory test revealed WBC of 237,000/µL, Hb of 4.2 g/dL, PLT of 328,000/µL and LDH of 2,263 U/L. Subsequent BMB and PB examinations confirmed a diffuse large B-cell lymphoma (DLBCL) (Fig. 1d-f). A whole-body CT showed no enlarged lymph nodes. Lumbar puncture (LP) did not reveal lymphoma cells in the cerebrospinal fluid (CSF). The patient was treated with six courses of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone, and achieved complete remission (CR). Two and a half months after, the patient was readmitted with fever, decreased tonus in the left arm and pain in the left leg persisting throughout 4 weeks. A PET-CT revealed extensive FDG uptake corresponding to the course of the left sciatic nerve (Fig. 2). A BMB confirmed relapse of DLBCL. A magnetic resonance imaging (MRI) of the brain showed pararhyme meningeal thickening, whereas LP did not reveal lymphoma cells in the CSF. Second-line treatment with rituximab, ifosfamide, carboplatin and etoposide was initiated. The patient’s symptoms were reduced, and a new BMB and PET-CT showed CR. Three weeks later, the patient was readmitted with frontal headache radiating to the neck, nausea, vomiting and fatigue through 1 week. An MRI of brain showed leptomeningeal thickening and affection of the vestibular nerve, and signs of increased intracranial pressure with flattened papilla. High dose methylprednisolone was initiated. In 2 days, the patient developed a peripheral facial paresis, left oculomotor nerve palsy and neurogenic pain radiating to the lower limbs. An MRI of brain did not reveal additional pathological findings; however, an LP confirmed CNS-relapse with infiltration of DLBCL in the CSF (Fig. 1c). A new BMB displayed minimal infiltration of the DLBCL. Treatment with four series of methotrexate, cytarabine, thiotepa and rituximab was initiated, and the symptoms disappeared. A new CR was achieved (by PET-CT, MRI of brain and LP) and brain electrical activity mapping (BEAM) conditioning was initiated followed by autologous stem cell transplantation (autoSCT).
Figure 2.
(a) PET-CT at diagnosis with enlarged spleen with inhomogeneous FDG uptake. (b) PET-CT from first relapse with extensive FDG uptake corresponding to the course of the left sciatic nerve. PET-CT: positron emission tomography-computed tomography.
Follow-up and outcomes
Three weeks after autoSCT, laboratory work revealed WBC of 46,000/µL and LDH of 1,506 U/L. In 2 days, LDH increased to 5,270 U/L. The patient developed headache. A new BMB revealed relapse of the DLBCL. Prednisolone and cytarabine were initiated. Over 2 days, the patient developed speech difficulties and loss of vision, and passed away shortly after, 16 months after the diagnosis was given.
Retrospectively, WES, and RNA-sequencing were performed on diagnostic BM and spleen tissue. In addition, the same studies were done on mononuclear cells from PB from first relapse, using our personalized medicine pipeline [8]. In all samples, a somatic loss-of-function mutation in RB1 R251* (c.751C>T) with variant allele frequency (VAF) ranging 58-88% was found. Furthermore, in all samples copy number (CN) loss of CDKN2A and CDKN2B (CN = 0) was found. The diagnostic BM sample revealed a somatic mis-splicing variant in PPP2R5A (c.808-1G>T) in VAF 10%; however, this variant was not seen in samples from the spleen or PB from first relapse. Overall, no signs of microsatellite instability or gene fusions were seen.
Discussion
In previous cases, primary treatment consists of splenectomy or immunochemotherapy followed by splenectomy [3, 5, 6]. Previous cases indicate that primary treatment with splenectomy might be a sufficient treatment [5].
Although SDRPL often is indolent, more aggressive courses of SDRPL have been reported. In a case series with 17 patients with SDRPL, two patients died due to progression and four patients had clinical progression after splenectomy with liver, skin, testicular and systemic involvement [3]. In one of these patients, DLBCL transformation was found in a lymph node biopsy; however, follow-up was missing [3]. Previously, gain-of-function mutations in the NOTCH1 and NOTCH2 genes in B-cell lymphomas have been linked to a more aggressive clinical phenotype [9-11]. Mutations in NOTCH1 have also been associated with Richter’s transformation in chronic lymphoid leukaemia [12]. Furthermore, in follicular lymphoma NOTCH1 mutation has been associated with splenic involvement and transformation to DLBCL [13]. The genomic landscape of SDRPL is poorly understood and only a few studies have investigated the prognostic impact of specific somatic mutations. A study of 19 patients with SDRPL showed that among five patients with a more aggressive phenotype of SDRPL, two patients had NOTCH1 mutations, one patient had MAP2K mutations, and one patient had mutations in TP53 [5]. The study revealed a heterogenous cytogenetic spectrum including complex karyotype (two patients), del(17p) (three patients), IGH-rearrangements (three cases) and 13 patients had various copy number alterations [5]. A previous study performed WES on 10 cases of SDRPL, afterwards a panel of 106 genes were evaluated in 42 cases of SDRPL and compared with 46 cases of splenic marginal zone lymphoma and eight cases of hairy cell leukaemia. They found mutations or losses in BCOR in 10/42 cases of SDRPL and CCND3 mutations in 9/42 SDRPL cases. Mutations in NOTCH1 and NOTCH2 were found in 5/42 cases of SDRPL. Moreover, they found that SDRPL has a distinct mutations pattern. However, information on the course of the diseases, treatment and survival was not reported [7].
In our case, no mutations were found in NOTCH1, TP53 or MAP2K; however, mutations in RB1 and CN loss of CDKN2A and CDKN2B were found in all samples suggesting loss of cell cycle control by loss of key tumor suppressor genes and dysregulated Cdk4/cyclin/Rb pathway as also observed in other cancers [14, 15]. Genetic variations in this pathway are a well-known driver of malignant transformation in glioblastoma [16] and in lung cancer CN loss of CDKN2A and CDKN2B have been reported to drive progression [17, 18]. Single nucleotide variants and loss-of-function mutations in RB1 have been reported in up to 3.7% of DLBCL enriched in the germinal-center B-cell-like (GCB) cell of origin subtype [19]. In contrast, homozygous loss of CDKN2A and CDKN2B has been shown to cluster in the activated B-cell-like subtype (ABC) and has been reported in up to 50% and 30% cases of DLBCL, respectively [19, 20].
However, the impact of these genetic variants in SDRPL and DLBCL is not fully understood, but it is assumable that these mutations drive this aggressive course of SDRPL.
Conclusion
In conclusion, we present a case, with an aggressive phenotype of transformed SDRPL with mutations in RB1 and CN loss of CDKN2A and CDKN2B found in all samples indicating loss of cell cycle control by loss of key tumor suppressor genes and dysregulated Cdk4/cyclin/Rb pathway.
Learning points
This case report describes an aggressive course of a transformation of an SDRPL in a 57-year-old female with a novel and previous unreported somatic loss-of-function mutation in RB1 R251* (c.751C>T) as the possible driver of this aggressive disease.
Acknowledgments
We thank Mads Sonderkaer bioinformatician, the Department of Molecular Diagnostic, Aalborg University Hospital for support with analyzing the WES, and the Department of Radiology, Aalborg University Hospital for the whole-body 18F-FDG PET-CT scan image.
Financial Disclosure
No financial disclosure or funding.
Conflict of Interest
All authors declare no conflict of interest.
Informed Consent
Written consent for publication has been obtained from the patient.
Author Contributions
Conception and design: LMC, MTS, KD, and DTK; provision of patient material: TH, PJ, and PK; collection and assembly of data: LMC, MTS, PK, KD, and DTK; data analysis and interpretation: LMC, MTS, AO, PK, and DTK; manuscript writing: all authors; final approval of manuscript: all authors; accountable for all aspects of the work: all authors.
Data Availability
Data and materials are available from the corresponding author on reasonable request.
References
- 1.Campo E, Jaffe ES, Cook JR, Quintanilla-Martinez L, Swerdlow SH, Anderson KC, Brousset P. et al. The international consensus classification of mature lymphoid neoplasms: a report from the Clinical Advisory Committee. Blood. 2022;140(11):1229–1253. doi: 10.1182/blood.2022015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G. et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720–1748. doi: 10.1038/s41375-022-01620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanellis G, Mollejo M, Montes-Moreno S, Rodriguez-Pinilla SM, Cigudosa JC, Algara P, Montalban C. et al. Splenic diffuse red pulp small B-cell lymphoma: revision of a series of cases reveals characteristic clinico-pathological features. Haematologica. 2010;95(7):1122–1129. doi: 10.3324/haematol.2009.013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traverse-Glehen A, Baseggio L, Salles G, Coiffier B, Felman P, Berger F. Splenic diffuse red pulp small-B cell lymphoma: toward the emergence of a new lymphoma entity. Discov Med. 2012;13(71):253–265. [PubMed] [Google Scholar]
- 5.Martinez D, Navarro A, Martinez-Trillos A, Molina-Urra R, Gonzalez-Farre B, Salaverria I, Nadeu F. et al. NOTCH1, TP53, and MAP2K1 mutations in splenic diffuse red pulp small B-cell lymphoma are associated with progressive disease. Am J Surg Pathol. 2016;40(2):192–201. doi: 10.1097/PAS.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 6.Traverse-Glehen A, Baseggio L, Bauchu EC, Morel D, Gazzo S, Ffrench M, Verney A. et al. Splenic red pulp lymphoma with numerous basophilic villous lymphocytes: a distinct clinicopathologic and molecular entity? Blood. 2008;111(4):2253–2260. doi: 10.1182/blood-2007-07-098848. [DOI] [PubMed] [Google Scholar]
- 7.Jallades L, Baseggio L, Sujobert P, Huet S, Chabane K, Callet-Bauchu E, Verney A. et al. Exome sequencing identifies recurrent BCOR alterations and the absence of KLF2, TNFAIP3 and MYD88 mutations in splenic diffuse red pulp small B-cell lymphoma. Haematologica. 2017;102(10):1758–1766. doi: 10.3324/haematol.2016.160192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodker JS, Sonderkaer M, Vesteghem C, Schmitz A, Brondum RF, Sommer M, Rytter AS. et al. Development of a precision medicine workflow in hematological cancers, Aalborg University Hospital, Denmark. Cancers (Basel) 2020;12(2):312. doi: 10.3390/cancers12020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puente XS, Pinyol M, Quesada V, Conde L, Ordonez GR, Villamor N, Escaramis G. et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475(7354):101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kridel R, Meissner B, Rogic S, Boyle M, Telenius A, Woolcock B, Gunawardana J. et al. Whole transcriptome sequencing reveals recurrent NOTCH1 mutations in mantle cell lymphoma. Blood. 2012;119(9):1963–1971. doi: 10.1182/blood-2011-11-391474. [DOI] [PubMed] [Google Scholar]
- 11.Bea S, Valdes-Mas R, Navarro A, Salaverria I, Martin-Garcia D, Jares P, Gine E. et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci U S A. 2013;110(45):18250–18255. doi: 10.1073/pnas.1314608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villamor N, Conde L, Martinez-Trillos A, Cazorla M, Navarro A, Bea S, Lopez C. et al. NOTCH1 mutations identify a genetic subgroup of chronic lymphocytic leukemia patients with high risk of transformation and poor outcome. Leukemia. 2013;27(5):1100–1106. doi: 10.1038/leu.2012.357. [DOI] [PubMed] [Google Scholar]
- 13.Karube K, Martinez D, Royo C, Navarro A, Pinyol M, Cazorla M, Castillo P. et al. Recurrent mutations of NOTCH genes in follicular lymphoma identify a distinctive subset of tumours. J Pathol. 2014;234(3):423–430. doi: 10.1002/path.4428. [DOI] [PubMed] [Google Scholar]
- 14.Knudsen ES, Nambiar R, Rosario SR, Smiraglia DJ, Goodrich DW, Witkiewicz AK. Pan-cancer molecular analysis of the RB tumor suppressor pathway. Commun Biol. 2020;3(1):158. doi: 10.1038/s42003-020-0873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyson NJ. RB1: a prototype tumor suppressor and an enigma. Genes Dev. 2016;30(13):1492–1502. doi: 10.1101/gad.282145.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimaras H, Corson TW, Cobrinik D, White A, Zhao J, Munier FL, Abramson DH. et al. Retinoblastoma. Nat Rev Dis Primers. 2015;1:15021. doi: 10.1038/nrdp.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W, Zhuang C, Huang T, Yang S, Zhang M, Lin B, Jiang Y. Loss of CDKN2A at chromosome 9 has a poor clinical prognosis and promotes lung cancer progression. Mol Genet Genomic Med. 2020;8(12):e1521. doi: 10.1002/mgg3.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinnam M, Goodrich DW. RB1, development, and cancer. Curr Top Dev Biol. 2011;94:129–169. doi: 10.1016/B978-0-12-380916-2.00005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolen CR, Klanova M, Trneny M, Sehn LH, He J, Tong J, Paulson JN. et al. Prognostic impact of somatic mutations in diffuse large B-cell lymphoma and relationship to cell-of-origin: data from the phase III GOYA study. Haematologica. 2020;105(9):2298–2307. doi: 10.3324/haematol.2019.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, Roulland S. et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378(15):1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials are available from the corresponding author on reasonable request.