Abstract
Purpose This study aims to assess and validate the role and cost-effectiveness of indocyanine green angiography (ICGA) in free flap surgery outcomes. A new intraoperative protocol of whole-body surface warming (WBSW) for all free flap surgeries during the strategic “microbreaks” is also described.
Methods A retrospective analysis of 877 consecutive free flaps, performed over 12 years, is presented. The results of the ICGA group ( n = 438) were compared with the historical No-ICGA group ( n = 439), and statistical significance was calculated for three crucial flap-related adverse outcomes and cost-effectiveness. ICGA was also used as a tool to show the effect of WBSW on free flaps.
Results ICGA showed a notably strong statistical significance in decreasing two outcome parameters, namely, partial flap loss and re-exploration rate. It was also cost-effective. ICGA also demonstrated the positive role of WBSW in increasing flap perfusion.
Conclusions Our study shows that the usage of ICGA for intraoperative assessment of flap perfusion can significantly reduce the partial flap loss and re-exploration rate in free flap surgeries in a cost-effective manner. A new protocol of WBSW is also described and recommended to increase flap perfusion in all free flap surgeries.
Keywords: indocyanine green angiography, SPY angiography, free flap outcomes, partial flap loss, flap warming, head and neck flaps, whole-body surface warming
Microsurgery is one of the most significant innovations in reconstructive plastic surgery. Microvascular free flap reconstructions have immensely improved functional and therapeutic outcomes, especially in complex cancer and trauma surgeries. Clinical monitoring, the mainstay of free flap perfusion, is quite subjective. Multiple technologies are now available to monitor flap tissue perfusion objectively, including indocyanine green angiography (ICGA), optical diffusion imaging spectroscopy, and hand-held and implantable Doppler. 1 2 Laser-assisted ICGA is an intraoperative imaging modality that shows the dynamic real-time vascular perfusion of the flap, which can help identify significant complications even before they occur. We have been using the SPY Elite system (Stryker Corporation, Kalamazoo, Michigan, United States; earlier Novadaq, Toronto, Canada) for ICGA to evaluate free flap perfusion intraoperatively.
Pafitanis et al concluded that ICGA helps in demonstrating the patency of vessels and preventing complications. 3 Zenn considered it a valuable tool for all microsurgeons. 4
Phillips et al have suggested that ICGA decreases reoperations. 1 Green et al compared a small number of free flaps ( n = 61) and showed lower flap failure and partial necrosis with ICGA. 5 Burnier et al extensively reviewed all published studies of ICGA uses. 6
The aim of our large retrospective study was threefold:
To assess and validate the role of ICGA in reducing major free flap–related complications by comparing with our historical control (No-ICGA).
To validate our intraoperative protocol of whole-body surface warming (WBSW) using ICGA.
To assess the cost-effectiveness of ICGA in all free flap surgeries.
Materials and Methods
We retrospectively analyzed 877 consecutive free flaps performed in 875 patients (2 patients had double free flaps) over a period of approximately 12 years (January 2010 to October 2022). Data from our hospital's electronic health records, the ICGA computer database, video recordings of microsurgical cases, and patient safety and quality audits were collated and analyzed for the study period.
All the consecutive free flaps done by us in our center over the last 12 years, irrespective of age and indications, were included. A team of four experienced microsurgeons performed all the free flaps.
A total of 439 consecutive free flaps were performed before ICGA was introduced in our department (January 2010 to October 2015). After the introduction of ICGA, 438 consecutive free flaps were performed and evaluated with ICGA. The two cohorts (ICGA and No-ICGA) were reasonably matched for age groups, indications, type of flaps, and risk factors ( Table 1 ). Patients with iodine or ICG dye allergy were excluded.
Table 1. Comparative patient data of the two study groups (No-ICGA and ICGA).
| No-ICGA ( n = 439) | ICGA ( n = 438) | |
|---|---|---|
| Average age (range), y | 50 (3–83) | 53 (7–89) |
| Gender (male:female), % | 81:19 | 79:21 |
| Defect site, % | ||
| Head and neck | 80 | 76 |
| Breast | 6 | 9 |
| Upper limb | 6 | 6 |
| Lower limb | 6 | 8 |
| Trunk | 2 | 1 |
| Indication, % | ||
| Tumor | 82 | 79 |
| Trauma | 10 | 10 |
| Others | 8 | 11 |
| Flap type, % | ||
| Radial forearm | 30 | 29 |
| Fibula | 19 | 28 |
| Anterolateral thigh | 35 | 29 |
| DIEP | 6 | 9 |
| Others | 10 | 5 |
| Comorbidities, % | ||
| Obesity (BMI ≥ 30 kg/m 2 ) | 25 | 26 |
| Diabetes | 74 | 76 |
| ASA class ≥ 3 | 39 | 41 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; DIEP, deep inferior epigastric perforator; ICGA, indocyanine green angiography.
Note: The two groups can be seen to be evenly matched in their patient demographics, flap choices, indications, and comorbidity profiles.
The results of the ICGA group ( n = 438) were compared with the historical No-ICGA group ( n = 439) by collecting data for three important flap-related adverse outcomes within the first 30 days: complete flap loss , partial flap loss , and re-exploration :
Complete flap loss was an irretrievable total flap loss.
Partial flap loss was partial flap necrosis that required a return to the operating room for reintervention. Cases of minor skin necrosis treated in the outpatient department were not included.
Re-exploration included those cases that involved patients reoperated for flap-related hemorrhage, infection, and thrombosis in the immediate postoperative period with a successful flap salvage .
Donor site complications were excluded. All results were analyzed statistically using a chi-square test conducted using IBM's SPSS software (version 29) with p < 0.05 considered as significant.
ICGA was performed intraoperatively using the SPY Elite system. ICGA is easy to perform, and the findings were interpreted by the operating team themselves. It involves no radiation.
The locally affordable ICG Aurogreen (Aurolab, Madurai, India) is used after reconstitution with 10 mL of sterile water, of which usually 3.5 to 4 mL is injected intravenously through a filter. The dye can be seen coursing through the arterial anastomosis and draining across the venous anastomosis. This flow and the flap perfusion are sequentially visualized as bright white fluorescence in grayscale on the screen. 7 Lack of flow or perfusion is easy to identify, with poor perfusion zones seen as dark areas that either do not light up or are significantly less bright compared with the surrounding normal tissues. Fig. 1 shows a typical ICGA flow of bright dye through the arterial and venous anastomosis. Fig. 2 shows excellent vascular perfusion of the deep inferior epigastric perforator (DIEP) flap skin island postanastomosis. In color imaging, a yellow–orange–red color denotes high perfusion , while a green–aqua–blue color denotes low perfusion .
Fig. 1.
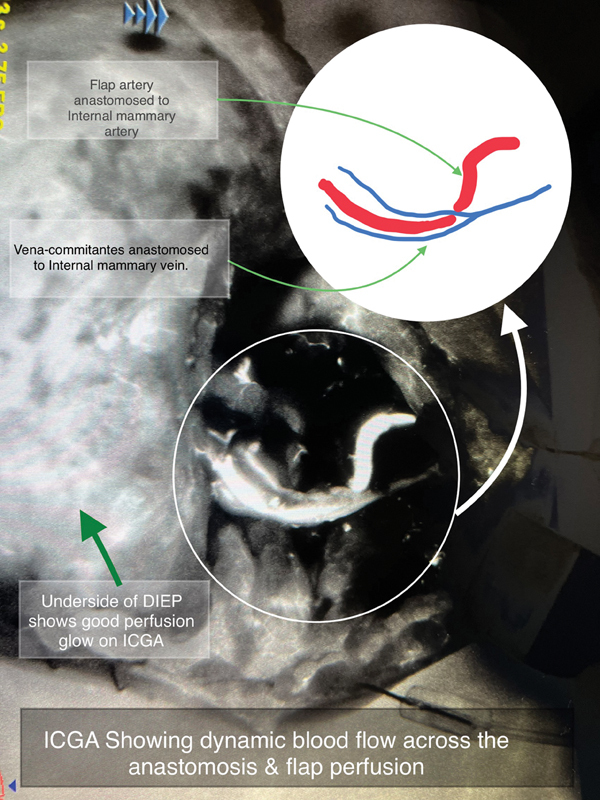
ICG dye coursing through the arterial and venous anastomosis in a DIEP free flap as bright white fluorescence.
Fig. 2.
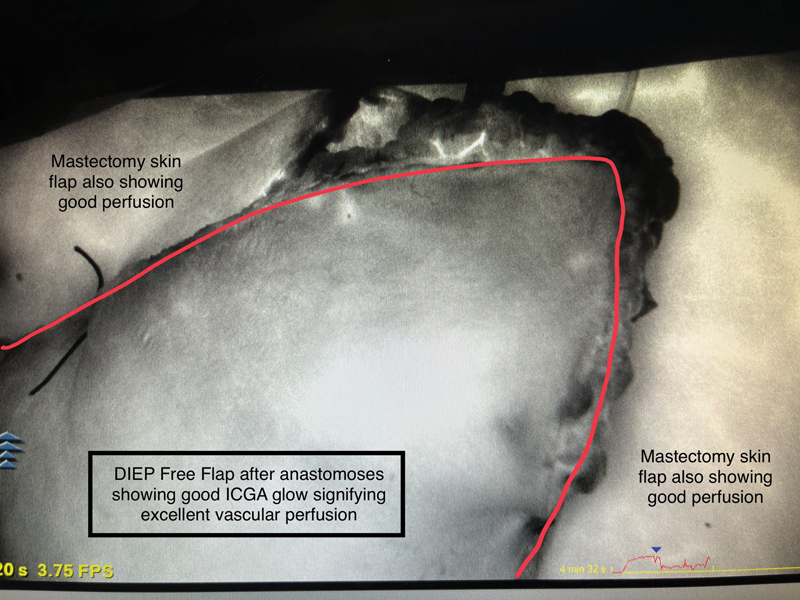
Surface of a DIEP free flap skin island immediately after microvascular anastomosis, revealing the bright glow of ICGA, which denotes excellent vascular perfusion.
The software also has a relative scoring system called SPY-Q , compared to a “surgeon-selected” reference point assigned as 100% from the surrounding normally perfused tissues. Based on our experience and previous studies, if any significant area of the flap skin or soft tissue component continues to show a poor ICGA glow and a low SPY-Q score of <40% even after our warming protocol, it is usually discarded before the inset. 8 9 Fig. 3 shows the ICGA imaging of a fully isolated fibular osteocutaneous flap just before its detachment from the donor site, which reveals poor vascular perfusion of a part of its skin island (the area has a poor SPY-Q score and appears darker in comparison to well-perfused areas). As can be seen in the figure, ICGA is capable of showing perfusion of the bony and subcutaneous components of the fibular flap and adipose tissues as well. The same holds true for the de-epithelialized free flaps. We have been following a simple WBSW protocol for all free flap surgeries. For performing WBSW, the patient, with his/her dissected surgical areas exposed, is covered entirely with a sterile, impermeable plastic drape, topped up with a sterile drape, and then covered top to toe with a thermal air blanket warmer used at 42°C for 20 minutes.
Fig. 3.
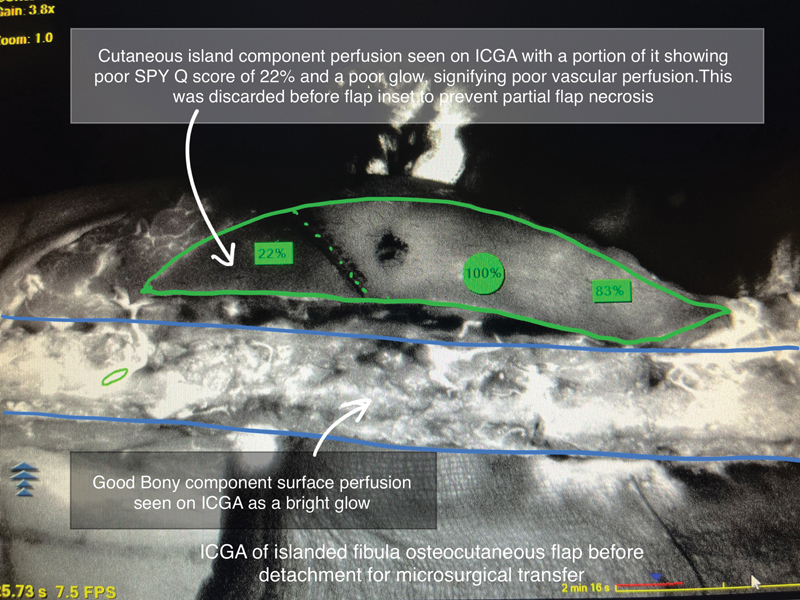
ICGA of a fibular osteocutaneous flap after whole-body surface warming (WBSW) but before its detachment for free tissue transfer. At this stage, ICGA is extremely useful in assessing the vascularity of individual chimeric flap components.
WBSW is strategically synchronized with the two 20-minute “microbreaks” that our surgical team routinely takes for lunch and relief in such long 6- to 8-hour surgeries ( Fig. 4 ).
Fig. 4.
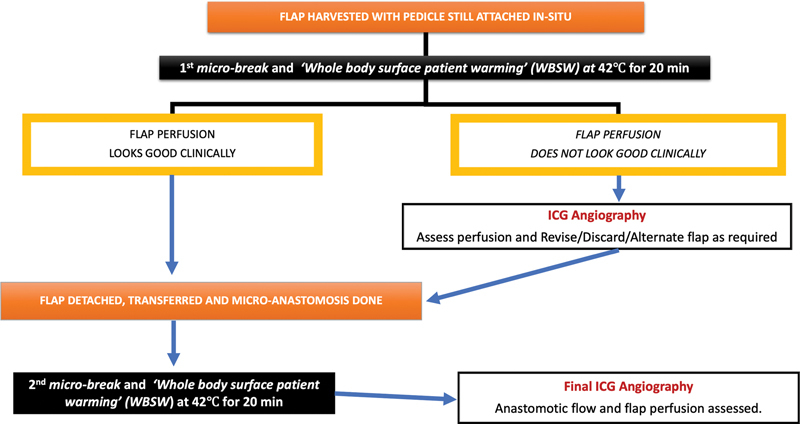
Algorithm of sequence and timing for performing whole-body surface warming (WBSW) at 42°C followed by ICGA during the strategic 20-min “microbreaks” taken by the surgical team while performing long free flap surgeries.
To ascertain whether WBSW had a positive effect in improving flap perfusion, 2 study points on each of 10 consecutive large-size free flaps, namely, 8 anterolateral thigh and 2 DIEP flaps, were specifically chosen and ICGA was performed before and after warming on these flaps.
SPY-Q scores of these 20 matched (Before-WBSW and After-WBSW) paired study points were compared and analyzed for statistical significance ( Figs. 5 6 7 8 ) using IBM's SPSS software (version 29). A p < 0.05 was considered significant.
Fig. 5.
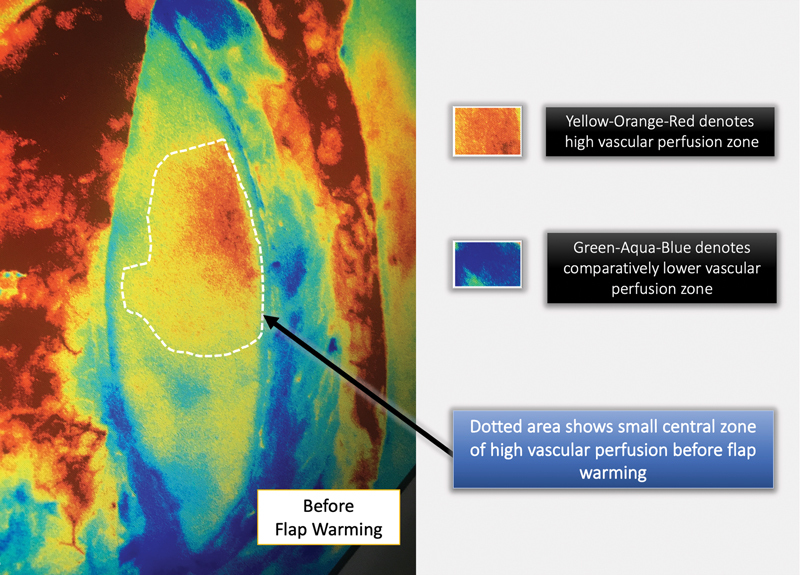
ICGA color-rendered image of an islanded anterolateral thigh flap before whole-body surface warming (WBSW). Note that the flap has been dissected entirely but is still left attached to its original pedicle.
Fig. 6.
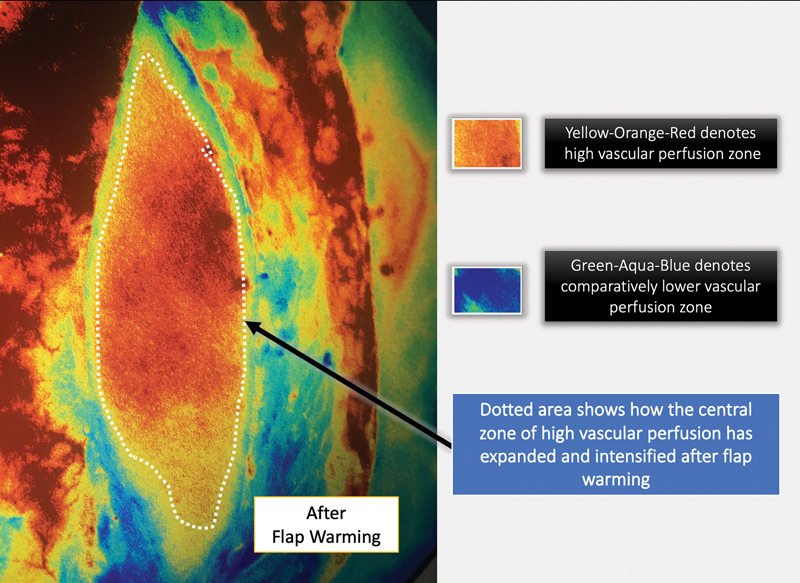
ICGA image of the same flap as in Fig. 5 after whole-body surface warming (WBSW). The improvement in the perfusion of the flap due to WBSW is visible in the expansion of the yellow–orange–red zone and its intensified glow.
Fig. 7.
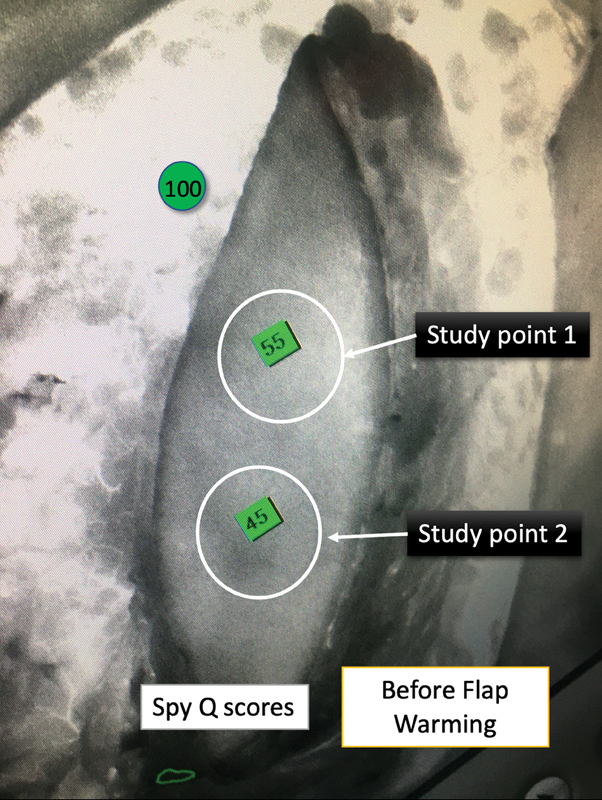
Original grayscale ICGA image, with SPY-Q scores of two study points on the same islanded anterolateral thigh flap before whole-body surface warming (WBSW) as in Fig. 5 .
Fig. 8.
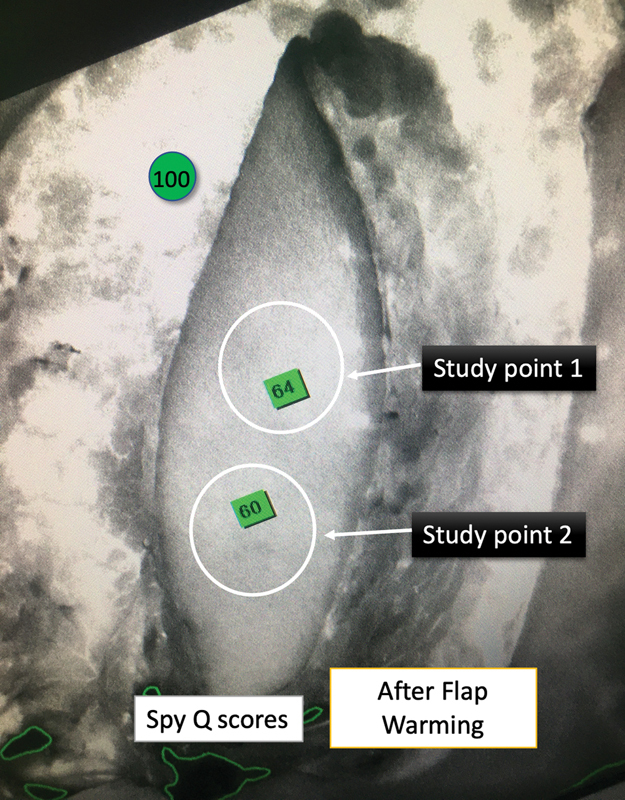
ICGA image of the same flap as in Fig. 7 after whole-body surface warming (WBSW). The flap's perfusion improvement due to WBSW can be deduced from the increase in the SPY-Q scores of the two paired study points.
It is to be noted that these flaps were studied once they had been islanded on their pedicle but had not been detached for transfer.
Detailed cost calculations and comparisons of the ICGA versus No-ICGA group were made based on hospital package estimates. The cost of uncomplicated free flap surgery, on average, was $13,545, but the same increased exponentially in the event of a major complication to as high as $24,490. The additional cost was $136 ( Table 3 ).
Table 3. This table shows detailed costing calculations and comparisons of the ICGA vs No-ICGA groups in dollar.
| Surgery type | Index hospital cost for No-ICGA patient ( C ) | Number of No-ICGA patients ( n ) | Total costing of the surgery for No-ICGA group (= C × n ) |
|---|---|---|---|
| Free flap without any complication | 13,545 | 364 | 4,930,380 |
| Complete free flap failure | 24,490 | 9 | 220,410 |
| Partial free flap failure | 23,150 | 22 | 509,300 |
| Re-exploration free flap | 22,900 | 44 | 1,007,600 |
| Total cost of all the surgeries in the No-ICGA group | 6,667,690 | ||
| Average cost of all the surgeries in the No-ICGA group | 15,188 | ||
| Index hospital cost for ICGA patient ( C ' = C + $136) a |
Number of ICGA patients
( n ') |
Total costing of the surgery for ICGA group (= C' × n ') | |
| Free flap without any complication | 13,681 | 414 | 5,663,934 |
| Complete free flap failure | 24,626 | 6 | 147,756 |
| Partial free flap failure | 23,286 | 2 | 46,572 |
| Re-exploration free flap | 23,036 | 16 | 368,576 |
| Total cost of all the surgeries in the ICGA group | 6,226,838 | ||
| Average cost of all the surgeries in the ICGA group | 14,216 | ||
| Average total saving on every free flap patient due to usage of ICGA (= 15,188–14,216) | 972 |
Abbreviation: ICGA, indocyanine green angiography.
$136 = additional cost of ICGA procedure.
The average costs of all the surgeries (with and without complications) in both the ICGA and No-ICGA groups were statistically analyzed.
Results
Among the 439 consecutive free flaps in the No-ICGA group, 9 patients had complete flap loss, 22 had partial flap loss, and 44 underwent re-exploration.
Among the 438 consecutive free flaps in the ICGA group, 6 patients had complete flap loss, 2 patients had partial flap loss, and 16 underwent re-exploration.
Flap ischemia time ranged 32 to 90 minutes (average: 62 minutes). Two of the three outcome parameters, namely, partial flap loss and re-exploration, showed a notably strong statistical significance.
Table 2 summarizes all the results of the three major adverse outcomes as a comparison between the No-ICGA and ICGA groups and enlists the associated p- value.
Table 2. This table shows all the results of the three major adverse outcomes as a comparison between the No-ICGA and ICGA groups and enlists the associated p- value .
| Free flap adverse outcomes | No-ICGA group ( n = 439) | ICGA group ( n = 438) | Calculated p -value | Significance at 5% level ( p < 0.05) |
|---|---|---|---|---|
| Partial flap loss | 5.00% ( n = 22) | 0.45% ( n = 2) | <0.001 | Yes |
| Complete flap loss | 2.10% ( n = 9) | 1.36% ( n = 6) | 0.44 | No |
| Re-exploration | 10.00% ( n = 44) | 3.65% ( n = 16) | <0.001 | Yes |
Abbreviation: ICGA, indocyanine green angiography.
Partial flap loss rate dropped from 5% (22/439) in the No-ICGA group to 0.45% (2/438) in the ICGA group with a p- value < 0.001, which is highly significant.
Complete flap loss rate decreased from 2.1% (9/439) in the No-ICGA group to 1.36% (6/438) in the ICGA group. However, this was statistically insignificant since the p- value = 0.44.
Re-exploration rate decreased from 10% (44/439) in the No-ICGA group to 3.65% (16/438) in the ICGA group with a p- value < 0.001, which is highly significant
Using a paired two-tailed t -test considering unequal variances of the Before-WBSW and After-WBSW SPY-Q scores, we found that the difference between the Before-WBSW and After-WBSW SPY-Q scores was highly significant with a p- value < 0.00001. This clearly shows that there was increased vascular perfusion due to WBSW in all the flaps that were studied.
Using a two-tailed t -test considering unequal variances of the No-ICGA and ICGA samples, we found that the difference between the average costs for the No-ICGA and ICGA groups was highly significant with a p- value ≈ 0.000002. Thus, there was a significant amount of average saving of $972 for every ICGA patient ( Table 3 ).
Serious anaphylaxis or adverse reactions from the ICG dye are very rare. 10 We encountered only one case of intraoperative anaphylactic shock immediately after the administration of the ICG dye. It must be noted that the patient did not have any history of iodine or ICG allergy in the past. The case was managed successfully per standard clinical protocol. The patient suffered no adverse consequences. The level of serum tryptase, a good biomarker of anaphylaxis, obtained 1 hour after the anaphylaxis episode was found to be highly elevated (11 ng/mL; normal serum tryptase levels are less than 1 ng/mL). 10
Discussion
Intraoperative clinical assessment and judgment of flap perfusion from its color, blanching, and capillary bleed has always been an all-time favorite of all microsurgeons. Still, there are obvious limitations to this subjective method. ICGA technology adds a hitherto invisible dimension to this by allowing us to “SPY” inside the vessel lumen to visualize directional blood flow across the anastomosis and capillary blood perfusion of the flap surface during real-time dynamic imaging. 11
Multiple studies have proven that ICGA can reliably predict skin necrosis in mastectomy flaps and flaps with doubtful skin paddles. 9 12 Lee et al have successfully utilized this technology to trim the transverse rectus abdominis myocutaneous (TRAM) flaps for safer breast reconstruction. 13 In 2020, Momeni and Sheckter published a study of 137 flaps for microsurgical breast reconstruction to predict fat necrosis. 14
Varela et al have also shown in 51 DIEP flaps that ICGA reduced the incidence of fat necrosis from 59.3 to 8.3% and reduced partial flap loss reoperations from 18.2% in the No-ICGA group to 0% in the ICGA group. 15 Similar results were shown by Malagón-López et al in 2019. 16
Studies have claimed that ICGA overpredicts necrosis and hence it should be done selectively in high-risk cases. 17 18 We do not subscribe to this view. We sincerely believe that overdebridement of the flap is due to ICGA being performed soon after harvesting the flap without any prior warming, as ICGA will exaggerate the areas of hypoperfusion in a cold flap. However, if ICGA is performed after surface warming, it can predict the perfusion of the flap more accurately, and the surgeon can thus avoid overdebridement. A recent experimental animal study by Muntean et al has also proven that local flap warming at 42°C allows a more realistic analysis of flap vascularity by ICGA. 19
McNally et al in 2017 compared 77 patients who underwent SPY for pedicled or free TRAM flaps and showed that though the free TRAM flap had 77.8% higher perfusion compared with the pedicled flap after the transfer, before flap division the perfusion of the pedicled flap was 54.7% greater than the free TRAM flap. 20 Universally, the prevention of hypothermia is part of standard intraoperative patient care and includes conventional methods of administering intravenous fluids and products using warm gel mattresses and covering unexposed areas outside the surgical field. Despite all these measures, there remains a large exposed operative surgical field with the flap and its surrounding areas, which inevitably suffers deleterious cooling and hardly benefits from the conventional warming methods. This is where WBSW, which involves warming the entire body in the two microbreak periods, turns out to be successful in mitigating these adverse effects of hypothermia on flap vascularity at all the three levels: cellular, local, and systemic. This phenomenon is nicely illustrated in Fig. 9 .
Fig. 9.
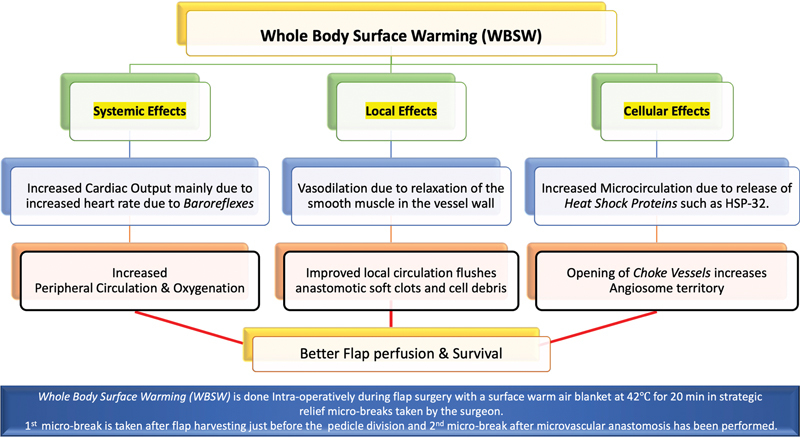
Beneficial effects of whole-body surface warming (WBSW) on flap vascularity at all the three levels: cellular, local, and systemic.
Further credence to our strategy of WBSW can also be found in prior studies that have proven that subjecting tissues and flaps to local hyperthermia of up to 42°C releases “heat shock proteins,” which precondition the flap to tolerate ischemia insult much better. 21 22 23 24 Kellogg succinctly demonstrates the effects of warming in his article. He explains that surface warming increases the heart rate via a direct impact on the sinoatrial node with increased sympathetic neural activity, thus increasing the cardiac output. 25 26
In our study, SPY-Q scores were higher by a value in the range of 8 to 15 in flaps “after WBSW” compared with those “before WBSW.” We also observed an increase in the area of the “central zone of high perfusion,” by 40 to 110%. Figs. 3 4 5 6 clearly show how vascular perfusion increases qualitatively (zone of high perfusion) and quantitatively (SPY-Q score) in a flap islanded on its pedicle but not yet detached from its origin. This demonstrates the effectiveness of warming in increasing the quality and quantity of vascular perfusion by dilating and opening choke vessels.
The SPY-Q mode helps compare the relative ICGA perfusion percentage of any doubtful flap area within the whole flap. 13 Moyer and Losken studied 118 mastectomy flaps wherein 14 patients suffered postoperative skin necrosis. The study concluded that SPY-Q values of 25 to 45% for any flap region denote poor vascularity, and such areas are unlikely to survive. 27 The score varies with skin area, race, history of smoking, and hypertension. 9 18
In our experience, if a flap region shows a low SPY-Q score of <40% even after adequate surface warming, that region of the flap is unlikely to survive and may need to be debrided. 8 9
ICGA is primarily a diagnostic modality and can never be a substitute for good surgical skills, experience, and clinical judgment. However, ICGA can provide significant help in detecting technical problems intraoperatively, allowing the surgeon to revise anastomosis, unkink the pedicle, adjust flap orientation, or discard poorly perfused regions.
In a series of 438 patients with head and neck free flap reconstructions reported by Chang et al, the average cost of free flap surgery in their center exponentially escalated by 81% in the event of a reintervention for a major complication. This study, however, did not use ICGA. 28 Taylor and Jorgensen have demonstrated the use of ICGA in assessing donor site perfusion before harvesting a free flap. They have calculated the total additional ICGA cost to be $1,300. 29 Duggal et al compared their outcome analysis in postmastectomy breast reconstruction with various methods involving the use of ICGA and found a reduction in flap necrosis from 22 to 4%, while the perfusion-related re-exploration rate fell from 14.1 to 5.9%. This led to an overall average per-patient cost saving of $614 in their study. 30 Kanuri et al studied the cost-effectiveness in assessing mastectomy flaps and found ICGA to be cost-effective. 31
On introducing ICGA in our protocol, the total cost of free flap surgery increased by $136 per patient on average, which literally translated to only a 1% total cost escalation.
A possible limitation of this study is that it is a retrospective study of a mixed cohort of patients, and sensitivity and specificity were not separately studied. However, there is already a recently published small study of 88 consecutive free flaps that showed the sensitivity of ICGA to be 100% (95% confidence interval) and specificity to be 98.8% (95% confidence interval). 32
The effect of ischemia time was not separately analyzed in this study, but as our ischemia time in the entire series ranged between 32 and 90 minutes, it is unlikely to be a significant factor in the outcomes of flap perfusion. Two recent studies by Lee et al and Chang et al studied the effect of ischemia time on 86 DIEP free flap outcomes and 116 fibular osteocutaneous free flaps and suggested maximum ischemia time limits of 99.5 minutes and 5 hours, respectively, to prevent major complications. 33 34 Although multifactorial, a hematoma can happen due to an anastomotic leak, which is possible to detect using ICGA and hence may be preventable. This thus justifies the inclusion of hematoma as a cause of re-exploration in the study. We admit that ICGA being a perfusion study surely cannot have any predictive value for infection-related adverse outcomes but we had only two cases of flap-related infection in the No-ICGA group and one case in ICGA group and did not have significant statistical effect on the results. A prospective future study would be better suited to look into some of the extra variables such as smoking and chemotherapy.
To the best of our knowledge, our study may be one of the largest single-center global comparative studies that evaluates the role and cost-effectiveness of ICGA in the outcomes of free flap surgeries.
Conclusion
Our study shows that the usage of ICGA for intraoperative assessment of flap perfusion can significantly reduce the partial flap loss and re-exploration rate in free flap surgeries in a cost-effective manner. A new protocol of WBSW at two strategic microbreaks taken by the surgeons during the surgery is also described and recommended in our study as a simple tool to increase flap perfusion in all free flap surgeries. We strongly believe that we have made a strong case for the adoption of ICGA evaluation by microsurgeons as a “standard of care” in all free flap surgeries.
Conflict of Interest None declared.
The paper has not been presented at any meetings.
References
- 1.Phillips B T, Munabi N CO, Roeder R A, Ascherman J A, Guo L, Zenn M R. The role of intraoperative perfusion assessment: what is the current state and how can I use it in my practice? Plast Reconstr Surg. 2016;137(02):731–741. doi: 10.1097/01.prs.0000475765.83901.80. [DOI] [PubMed] [Google Scholar]
- 2.Ono S, Hayashi H, Ohi H, Ogawa R. Imaging studies for preoperative planning of perforator flaps: an overview. Clin Plast Surg. 2017;44(01):21–30. doi: 10.1016/j.cps.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Pafitanis G, Raveendran M, Myers S, Ghanem A M. Flowmetry evolution in microvascular surgery: a systematic review. J Plast Reconstr Aesthet Surg. 2017;70(09):1242–1251. doi: 10.1016/j.bjps.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Zenn M R. Fluorescent angiography. Clin Plast Surg. 2011;38(02):293–300. doi: 10.1016/j.cps.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Green J M, III, Thomas S, Sabino J. Use of intraoperative fluorescent angiography to assess and optimize free tissue transfer in head and neck reconstruction. J Oral Maxillofac Surg. 2013;71(08):1439–1449. doi: 10.1016/j.joms.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Burnier P, Niddam J, Bosc R, Hersant B, Meningaud J P. Indocyanine green applications in plastic surgery: a review of the literature. J Plast Reconstr Aesthet Surg. 2017;70(06):814–827. doi: 10.1016/j.bjps.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Eguchi T, Kawaguchi K, Basugi A, Kanai I, Hamada Y. Intraoperative real-time assessment of blood flow using indocyanine green angiography after anastomoses in free-flap reconstructions. Br J Oral Maxillofac Surg. 2017;55(06):628–630. doi: 10.1016/j.bjoms.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Phillips B T, Fourman M S, Rivara A. Comparing quantitative values of two generations of laser-assisted indocyanine green dye angiography systems: can we predict necrosis? Eplasty. 2014;14:e44. [PMC free article] [PubMed] [Google Scholar]
- 9.Newman M I, Jack M C, Samson M C. SPY-Q analysis toolkit values potentially predict mastectomy flap necrosis. Ann Plast Surg. 2013;70(05):595–598. doi: 10.1097/SAP.0b013e3182650b4e. [DOI] [PubMed] [Google Scholar]
- 10.Kim M, Lee S, Park J C. Anaphylactic shock after indocyanine green video angiography during cerebrovascular surgery. World Neurosurg. 2020;133:74–79. doi: 10.1016/j.wneu.2019.09.135. [DOI] [PubMed] [Google Scholar]
- 11.Ludolph I, Horch R E, Arkudas A, Schmitz M. Enhancing safety in reconstructive microsurgery using intraoperative indocyanine green angiography. Front Surg. 2019;6:39. doi: 10.3389/fsurg.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones G E, Garcia C A, Murray J.Fluorescent intraoperative tissue angiography for the evaluation of the viability of pedicled TRAM flaps Plast Reconstr Surg 20091245319568044 [Google Scholar]
- 13.Lee S K, Lee D W, Lew D H, Song S Y. Determining the trimming layer in breast reconstruction with a free TRAM flap using intraoperative video-angiography. Plast Reconstr Surg Glob Open. 2017;5(03):e1266. doi: 10.1097/GOX.0000000000001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Momeni A, Sheckter C. Intraoperative laser-assisted indocyanine green imaging can reduce the rate of fat necrosis in microsurgical breast reconstruction. Plast Reconstr Surg. 2020;145(03):507e–513e. doi: 10.1097/PRS.0000000000006547. [DOI] [PubMed] [Google Scholar]
- 15.Varela R, Casado-Sanchez C, Zarbakhsh S, Diez J, Hernandez-Godoy J, Landin L. Outcomes of DIEP flap and fluorescent angiography: a randomized controlled clinical trial. Plast Reconstr Surg. 2020;145(01):1–10. doi: 10.1097/PRS.0000000000006393. [DOI] [PubMed] [Google Scholar]
- 16.Malagón-López P, Vilà J, Carrasco-López C. Intraoperative indocyanine green angiography for fat necrosis reduction in the deep inferior epigastric perforator (DIEP) flap. Aesthet Surg J. 2019;39(04):NP45–NP54. doi: 10.1093/asj/sjy256. [DOI] [PubMed] [Google Scholar]
- 17.Mattison G L, Lewis P G, Gupta S C, Kim H Y. SPY imaging use in postmastectomy breast reconstruction patients: preventative or overly conservative? Plast Reconstr Surg. 2016;138(01):15e–21e. doi: 10.1097/PRS.0000000000002266. [DOI] [PubMed] [Google Scholar]
- 18.Phillips B T, Lanier S T, Conkling N. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg. 2012;129(05):778e–788e. doi: 10.1097/PRS.0b013e31824a2ae8. [DOI] [PubMed] [Google Scholar]
- 19.Muntean M V, Ardelean F, Strilciuc S, Pestean C, Georgescu A V, Muntean V. Flap warming improves intraoperative indocyanine green angiography (ICGA) assessment of perfusion. An experimental study. J Plast Reconstr Aesthet Surg. 2019;72(07):1150–1156. doi: 10.1016/j.bjps.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 20.McNally R, Rimler J, Laurence V. Comparative perfusion analysis of free muscle-sparing versus pedicle transverse rectus abdominis myocutaneous (TRAM) flaps in vivo in the perioperative and late postoperative periods. World J Plast Surg. 2017;6(02):144–151. [PMC free article] [PubMed] [Google Scholar]
- 21.Krauss S, Rothenberger J, Mayer J. Tissue conditioning – strategies to improve perfusion and reduce ischemia-reperfusion injury. Plast Aesthet Res. 2018;5:39. [Google Scholar]
- 22.Harder Y, Amon M, Schramm R.Heat shock preconditioning reduces ischemic tissue necrosis by heat shock protein (HSP)-32-mediated improvement of the microcirculation rather than induction of ischemic tolerance Ann Surg 200524206869–878., discussion 878–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B H, Ye C, Stagg C A. Improved free musculocutaneous flap survival with induction of heat shock protein. Plast Reconstr Surg. 1998;101(03):776–784. doi: 10.1097/00006534-199803000-00029. [DOI] [PubMed] [Google Scholar]
- 24.Minh T C, Ichioka S, Nakatsuka T. Effect of hyperthermic preconditioning on the survival of ischemia-reperfused skin flaps: a new skin-flap model in the mouse. J Reconstr Microsurg. 2002;18(02):115–119. doi: 10.1055/s-2002-19892. [DOI] [PubMed] [Google Scholar]
- 25.Kellogg D L., Jr In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol. 2006;100(05):1709–1718. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- 26.Wilson T E, Crandall C G. Effect of thermal stress on cardiac function. Exerc Sport Sci Rev. 2011;39(01):12–17. doi: 10.1097/JES.0b013e318201eed6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyer H R, Losken A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: the gray area defined. Plast Reconstr Surg. 2012;129(05):1043–1048. doi: 10.1097/PRS.0b013e31824a2b02. [DOI] [PubMed] [Google Scholar]
- 28.Chang C S, Chu M W, Nelson J A. Complications and cost analysis of intraoperative arterial complications in head and neck free flap reconstruction. J Reconstr Microsurg. 2017;33(05):318–327. doi: 10.1055/s-0037-1598618. [DOI] [PubMed] [Google Scholar]
- 29.Taylor S R, Jorgensen J B. Use of fluorescent angiography to assess donor site perfusion prior to free tissue transfer. Laryngoscope. 2015;125(06):E192–E197. doi: 10.1002/lary.25190. [DOI] [PubMed] [Google Scholar]
- 30.Duggal C S, Madni T, Losken A. An outcome analysis of intraoperative angiography for postmastectomy breast reconstruction. Aesthet Surg J. 2014;34(01):61–65. doi: 10.1177/1090820X13514995. [DOI] [PubMed] [Google Scholar]
- 31.Kanuri A, Liu A S, Guo L. Whom should we SPY? A cost analysis of laser-assisted indocyanine green angiography in prevention of mastectomy skin flap necrosis during prosthesis-based breast reconstruction. Plast Reconstr Surg. 2014;133(04):448e–454e. doi: 10.1097/PRS.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 32.Bigdeli A K, Thomas B, Falkner F, Gazyakan E, Hirche C, Kneser U. The impact of indocyanine-green fluorescence angiography on intraoperative decision-making and postoperative outcome in free flap surgery. J Reconstr Microsurg. 2020;36(08):556–566. doi: 10.1055/s-0040-1710552. [DOI] [PubMed] [Google Scholar]
- 33.Lee K T, Lee J E, Nam S J, Mun G H. Ischaemic time and fat necrosis in breast reconstruction with a free deep inferior epigastric perforator flap. J Plast Reconstr Aesthet Surg. 2013;66(02):174–181. doi: 10.1016/j.bjps.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Chang S Y, Huang J J, Tsao C K. Does ischemia time affect the outcome of free fibula flaps for head and neck reconstruction? A review of 116 cases. Plast Reconstr Surg. 2010;126(06):1988–1995. doi: 10.1097/PRS.0b013e3181f448c8. [DOI] [PubMed] [Google Scholar]


