Abstract
We treated a case of gastroesophageal varices due to decompensated liver cirrhosis associated with Wilson's disease. The varicose veins penetrated the paraesophageal vein. We performed endoscopic variceal ligation (EVL) on the perforating vein and endoscopic injection sclerotherapy distally. However, 5 days after treatment, the patient vomited blood. Esophagogastroduodenoscopy showed bleeding from the ulcer after EVL at the perforating vein. We performed EVL and stopped the bleeding. However, the next day, she vomited blood again and developed hemorrhagic shock. We were able to achieve hemostasis and save the patient's life with combination therapy consisting of percutaneous transhepatic obliteration and Sengstaken-Blakemore tube placement.
Keywords: gastroesophageal varices, endoscopic injection sclerotherapy, endoscopic variceal ligation, perforating vein, percutaneous transhepatic obliteration, Sengstaken-Blakemore tube
Introduction
Hemorrhaging from gastroesophageal varices is a serious complication of portal hypertension (1,2). The mortality rate depends on whether the gastroesophageal varices present as an isolated complication of cirrhosis (5-year mortality rate, 20%) or in association with other complications (5-year mortality rate, over 80%) (3). Bleeding can easily induce hepatic failure. Therefore, prevention of bleeding with endoscopic therapy is important. When there are perforating veins between the esophageal varices and the paraesophageal vein, 5% ethanolamine oleate (EO) does not stagnate adequately during endoscopic injection sclerotherapy (EIS), making treatment challenging (4). In such situations, performing endoscopic variceal ligation (EVL) on the perforating vein and EIS distal to the EVL site may be successful. However, re-bleeding sometimes occurs from the ulcer after EVL, which is a matter of concern in clinical practice.
We herein report a patient with bleeding from the post-EVL ulcer on esophageal varices with a perforating vein. The patient developed shock. Life-saving hemostasis was achieved with percutaneous transhepatic obliteration (PTO) under Sengstaken-Blakemore (S-B) tube pressure.
Case Report
A 44-year-old woman was diagnosed with Wilson disease in X-27. She visited a local doctor. In November X-1, she underwent esophagogastroduodenoscopy (EGD) as part of a diagnostic evaluation for anemia. Gastroesophageal varices were identified. She was admitted to our hospital in January X for endoscopic treatment of gastroesophageal varices accompanied by decompensated liver cirrhosis due to Wilson disease. Her mental status was normal, blood pressure was 90/61 mmHg, and pulse was 77 bpm. Her palpebral conjunctivas were not pale, and there was no jaundice. An abdominal examination was normal. She had neurological findings associated with Wilson disease consisting of dysarthria, masked face, and upper limb dyskinesia. There were no flapping tremors.
Table shows the patient's laboratory data at the time of hospitalization. Blood tests showed mild anemia with hemoglobin (Hb) of 11.8 g/dL, but blood urea nitrogen/creatinine was not elevated. Her prothrombin time (PT) was 95%, albumin (Alb) was 3.9 g/dL, and total bilirubin was 0.9 mg/dL. Diuretics (azosemide 20 mg and spironolactone 25 mg) were used to treat edema of the lower extremities and ascites, with good control achieved. There was no history of hepatic encephalopathy.
Table.
Laboratory Data at the Time of Hospitalization.
| Blood cell count | Blood chemistry | ||
| WBC, /µL | 3,470 | AST, U/L | 39 |
| RBC, ×104/µL | 371 | ALT, U/L | 34 |
| Hb, g/dL | 11.8 | LDH, U/L | 215 |
| Plt, ×104/µL | 37.1 | γ-GTP, U/L | 59 |
| ALP, U/L | 247 | ||
| Blood coagulation | T-Bil, mg/dL | 0.9 | |
| PT% | 95 | Alb, g/dL | 3.9 |
| PT-INR | 1.18 | BUN, mg/dL | 13.2 |
| Cr, mg/dL | 0.58 | ||
| Hepatitis virus markers | Na, mEq/L | 137 | |
| HBsAg | (-) | K, mEq/L | 3.4 |
| HCVAb | (-) | Cl, mEq/L | 101 |
| NH3, µg/dL | 50 | ||
| Child-Pugh score | 6 | Cu (urine), µg/L | 15 |
| Child-Pugh grade | B | Cu (serum), µg/dL | 20 |
| ceruloplasmin, mg/dL | 2.5 | ||
| M2BPGi | 3.54 |
WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, Plt: platelet count, PT: prothrombin time, PT-INR: prothrombin time-international normalized ratio, HBsAg: hepatitis B surface antigen, HCVAb: HCV antibody, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, γ-GTP: γ-glutamyltransferase, ALP: alkaline phosphatase, T-Bil: total bilirubin, Alb: albumin, BUN: blood urea nitrogen, Cr: creatinine, Na: sodium, K: potassium, Cl: chlorine, NH3: ammonia, Cu: copper, M2BPGi: Mac-2 binding protein glycosylation isomer
The Child-Pugh classification score was 6 points, corresponding to grade A disease. The platelet count was low at 83,000 /μL. Progression of hepatic fibrosis was considered with a high Mac-2-binding protein glycosylation isomer value of 3.54. Aspartate aminotransferase was 39 U/L, alanine transaminase was 34 U/L, and γ-glutamyl transpeptidase was 59 U/L; hepatobiliary enzyme levels were mildly high. Serum copper was low at 20 μg/dL because she was taking a zinc formulation. Ceruloplasmin was low at 2.5 mg/dL, and urinary copper was high at 15 μg/L.
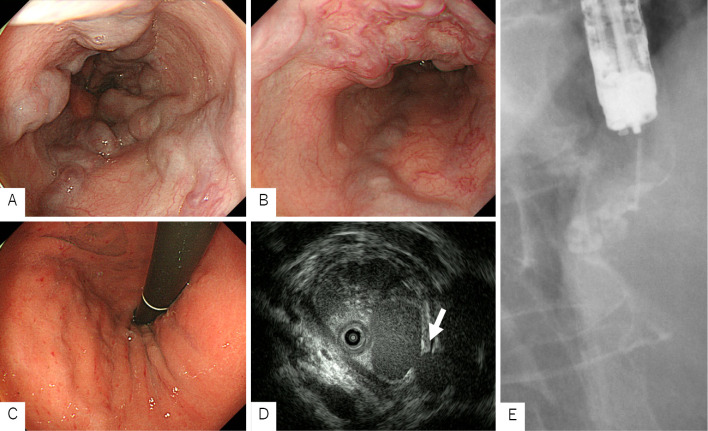
During EGD, form F2 esophageal varices were found in the upper esophagus, which were positive for the red-color sign. Gastric varices were found in the cardia [Ls, F2, Cb, RC1 (CRS, RWM), Lg-c, F1, Cw, RC0] (Fig. 1A-C). Endoscopic ultrasonography (EUS) showed that the esophageal varix at the 3 o'clock position was continuous with Lg-c, and a perforating vein with a diameter of 3.2 mm was interposed between the esophageal varix and the paraesophageal vein (Fig. 1D).
Figure 1.
Esophagogastroduodenoscopy and endoscopic injection sclerotherapy. A, B: EGD: Form F2 esophageal varices starting in the upper esophagus with a positive red-color sign. C: EGD: Form F1 gastric varices in the cardia. D: EUS: A perforating vein between the esophageal varices and the paraesophageal vein (arrow). E: EIS: We performed EVL on the perforating vein and injected EO distally. As a result, stagnation of EO was observed in Lg-c. EGD: esophagogastroduodenoscopy, EIS: endoscopic injection sclerotherapy, EO: ethanolamine oleate, EUS: endoscopic ultrasonography, EVL: endoscopic variceal ligation
We performed EIS using EO on the varix at the 3 o'clock position, but EO leaked into the perforating vein, making it difficult to visualize the Lg-c. Therefore, we injected 1 mL of absolute ethanol (ET) during the second EIS and injected EO 1 minute later. The Lg-c was visualized, but stagnation of EO was not achieved. At the time of the third EIS, the perforating vein was obscured by EUS, but stagnation of EO into the Lg-c was not observed. During the fourth EIS, the perforating vein was again clearly depicted by EUS, but sufficient EO could not be injected into the Lg-c. Therefore, during the fifth EIS, we performed EVL on the perforating vein and injected EO distally. As a result, stagnation of EO was observed in the Lg-c (Fig. 1E). We performed EVL in 12 locations on the circumference and finished the treatment session.
After EVL-EO combination therapy, oral intake was resumed on the following day with rice gruel twice which was diluted more than usual. After three days on this diet, she was given usual rice gruel, but since she reported pain while swallowing, she was therefore again given rice gruel which was diluted more than usual. Sodium alginate and the proton pump inhibitor rabeprazole sodium were given before treatment and continued even after treatment. Subsequently, the patient had a good clinical course, and she was discharged from the hospital four days after the treatment.
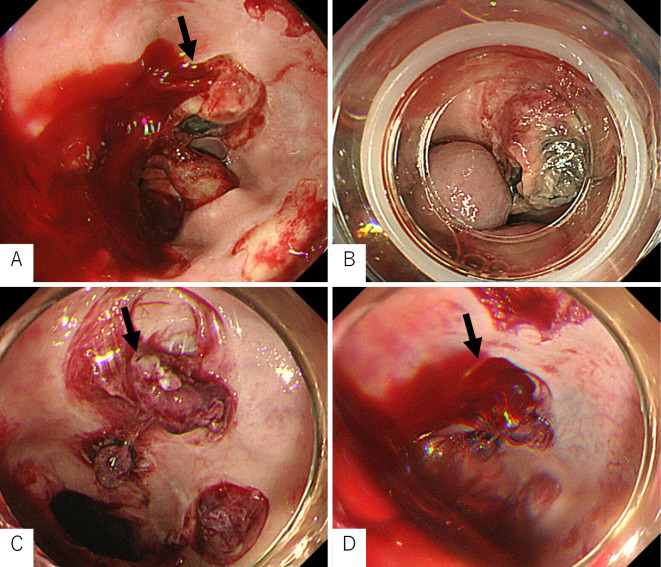
On the day after discharge, she vomited blood again at home and was emergently re-hospitalized at our hospital. Her mental state was normal, but she was in shock with a blood pressure of 78/45 mmHg and a pulse of 113 bpm. Blood tests showed a remarkably low Hb level of 7.1 g/dL. Her liver reserve was markedly decreased with PT of 54% and Alb of 2.8 g/dL. Her condition was serious and had a risk of leading to liver failure. We performed emergency EGD, which revealed eruptive bleeding from the post-EVL ulcer on the perforating vein (Fig. 2A). We performed EVL distal to the site of bleeding, which stopped the bleeding (Fig. 2B). It was useful to perform EVL farther away from the site of bleeding because the area around the re-bleeding site was vulnerable due to EVL. In addition, blood in the esophageal varices flowed from the stomach, which was distal to the esophagus.
Figure 2.
Esophagogastroduodenoscopy after endoscopic variceal ligation. A: Eruptive hemorrhaging was observed from the post-EVL ulcer on the perforating vein (arrow). B: We performed EVL distal to the source of bleeding, which stopped the bleeding. C, D: On the day after EVL, hemorrhaging was observed again from the post-EVL ulcer on the perforating vein (arrow). EVL: endoscopic variceal ligation
However, the next day, she vomited a large amount of blood again. She suddenly fell unconscious and went into hemorrhagic shock. Her transcutaneous arterial oxygen saturation decreased. We performed emergency EGD while administering blood transfusion, hypertensive drugs, and oxygen. EGD showed recurrent eruptive bleeding from the post-EVL ulcer at the perforating vein (Fig. 2C, D). We inserted an S-B tube and also performed intubation because we could not measure her transcutaneous arterial oxygen saturation. Her blood pressure remained low, and she remained in a critical condition. There was no fresh bleeding seen in the mouth on ballooning with the S-B tube. However, slight relaxation of the S-B tube resulted in hematemesis of fresh blood. Recompression with the S-B tube stopped the bleeding. Compression with the balloon was considered appropriate. We suspected that the site of re-bleeding after EVL was greatly ulcerated, even though this was not possible to confirm. We therefore determined that the S-B tube could no longer be released. Thus, we determined that further endoscopic hemostasis would be difficult. Since her vital signs were unstable, we concluded that surgical treatment including Hassab surgery would be difficult. We made the decision to conduct emergency hemostasis with PTO.
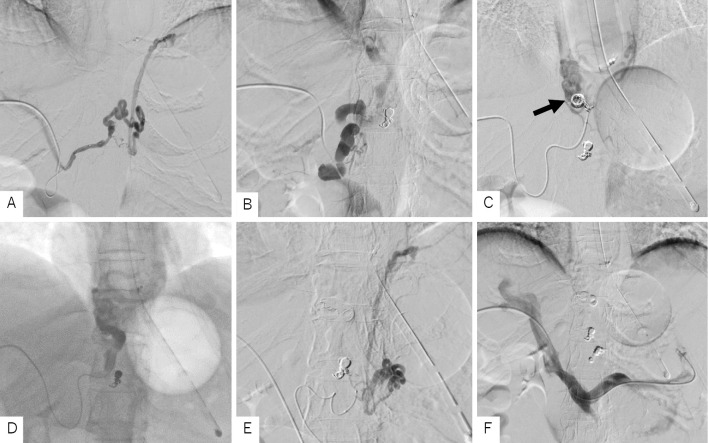
Percutaneous transhepatic puncture of portal vein P5 was performed under ultrasound guidance. Portal angiography first revealed a branch of the right gastric vein, considered one of the vessels supplying the site of the hemorrhaging (Fig. 3A). We performed coil embolization, which eliminated blood flow. After imaging, a developed right gastric vein, considered to be the main vessel for blood supply, was visualized (Fig. 3B). We injected EO after the microcatheter was advanced to the periphery and performed embolization using a microcoil (Fig. 3C). EO stagnated (Fig. 3D) with the effect of blocking the drainage vein with the S-B tube. Immediately afterwards, the vital signs stabilized. In addition, a donor vein from the left gastric vein was also observed, so we performed coil embolization (Fig. 3E). Finally, we performed portal angiography and confirmed the disappearance of blood flow in the three embolized donor veins (Fig. 3F). Subsequently, the patient did not have any more bleeding and was discharged from the hospital on postoperative day 23.
Figure 3.
Percutaneous transhepatic obliteration. A: Percutaneous transhepatic portal angiography revealed a branch of the right gastric vein. Coil embolization was performed from the microcatheter. B: A developed right gastric vein was visualized. It was considered to be a trunk of the varices continuing to the site of bleeding. C: Coil embolization was performed from the microcatheter advanced to the periphery of the right gastric vein to decrease the blood flow (arrow). D: EO (5%) was injected with the S-B tube blocking the drainage channel, and sufficient stagnation was obtained. E: A donor vein branching from the left gastric vein was also observed. Coil embolization was performed. F: Portal angiography after PTO on three donor channels confirmed their disappearance. EO: ethanolamine oleate, PTO: percutaneous transhepatic obliteration, S-B tube: Sengstaken-Blakemore tube
Discussion
Treatment for esophageal and gastric varicose veins, such as EIS (5) and EVL (6), have become standardized and widely used. Since many patients have background cirrhosis, treatment with EIS or EVL is selected in consideration of the patient's hepatic reserve. When endoscopic treatment is difficult, transjugular intrahepatic portosystemic shunt (7,8), partial splenic embolization (9), PTO (10), or other procedures are used after recent developments in interventional radiology (IVR) technology. However, IVR treatment is considered optional; it is positioned as a treatment to be attempted when EIS and EVL are difficult.
Initially, the present patient received systemic management according to the basic treatment strategy for ruptured varices. We first performed hemostasis via compression using balloon tamponade (11,12). Primary hemostasis was initially successful due to pressure on the bleeding sites by the S-B tube. However, the patient was in critical condition with severe recurrent bleeding when the S-B tube's balloon collapsed. It was suggested that the bleeding from the ulcer after EVL was not bleeding from esophageal varices. Endoscopic treatment was already contraindicated because the S-B tube's balloon could not be released. Hassab surgery is considered as a treatment for esophageal or gastric varices, but emergency surgery under general anesthesia seemed difficult due to the decrease in liver reserve.
Surgery for hemorrhaging is not specified in any guidelines from Japan or the American Association for the Study of Liver Diseases (2). Therefore, we approached the portal vein in the percutaneous hepatic system and performed hemostasis via PTO. While bleeding was stopped with the S-B tube's balloon during PTO, hemostasis was followed by injection of 5% EO (ASKA Pharmaceutical, Tokyo, Japan) after stagnating the blood flow with a coil embolism proximal to the bleeding esophageal varices. To date, there have been no reports of PTO performed under S-B tube pressure.
In Japan, while systemic management for hemorrhagic shock is performed for bleeding due to ruptured esophageal varices, hemostasis via compression with a S-B tube is performed first with EVL performed as soon as possible as primary hemostasis. In patients with primary hemostasis, additional EIS or EVL is considered in order to prevent recurrent bleeding based on the liver reserve.
The perforating vein of the esophageal wall is a blood vessel that carries the varix inside the esophageal wall. The portion of the blood vessel outside the esophageal wall is mostly an inflow passage, feeding blood from outside of the esophagus to the varix. Although the frequency of occurrence has not been clarified, the perforating vein becomes an outflow passage for the curing agent during EIS. When a perforating vein remains after treatment, it supplies blood to a recurrent varix. Therefore, it is extremely important to confirm the perforating veins before performing endoscopic treatment for gastroesophageal varices (4).
Irisawa et al. (13) performed endoscopic donor pathway imaging for esophageal varices with perforating veins and evaluated the effect of endoscopic embolization with contrast. They showed that EO alone could block the perforating vein in patients with a donor tract that was clearly shown with the contrast agent. However, for the majority of patients in whom the donor track was not visualized, substantial blood flow to the perforating vein made it difficult to block the perforating vein with EO alone. By using ET in combination, it became possible to block the perforating vein in many patients. EVL was performed when the perforating vein could not be occluded, even with ET. When EVL was performed, recurrence of the varix was observed, but ulcer bleeding immediately after endoscopic treatment, as in our case, was not observed.
In addition, Zheng et al. investigated the prediction of esophageal variceal recurrence after endoscopic variceal eradication therapies in patients with cirrhosis using an endoscopic ultrasound probe (14). They found that the peri-esophageal collateral veins and perforating vein had a better ability to predict esophageal variceal recurrence than the Child-Pugh score. When the cut-off value for the peri-esophageal collateral vein diameter was 3.5 mm, the sensitivity and specificity of the predicted variceal recurrence at 1 year were 45% and 86%, respectively. The Japan Society for Portal Hypertension also recommended in the Clinical Manual of Portal Hypertension injecting EO into esophageal varices distally after EVL at the site of the perforating vein (combination of selective EVL and EO injection), which has indeed been shown to be effective (4).
Our patient had a perforating vein diameter of 3.2 mm and poor donor path contrast due to the influence of shunt blood flow. Thus, it was difficult to block the perforating vein even when EO and ET were used together. Therefore, we used the selective EVL-EO combination method. However, five days after EVL, bleeding from the post-EVL ulcer at the perforating vein caused shock. The reason for the delayed nature of this bleeding incident rather than occurring immediately after EVL might be due to O-ring detachment from the post-EVL ulcer. The O-ring had restricted the varices sufficiently retracted into the hood of the gastroendoscope; there did not seem to be any technical problems. The thick perforating vein and high blood flow might have led to the formation of ulcers after EVL and caused bleeding. Due to the thick perforating vein into the post-EVL ulcer, blood flow in the drainage vein was quick, and stagnation of EO was difficult. Through pressure from the S-B tube, it became possible to shut off the drainage vein, and PTO could be performed. In our patient, we feel that sufficient stagnation of EO was obtained by blocking the drainage channel with the S-B tube and performing coil embolization. In Japan, PTO under pressure from an S-B tube has not been previously reported, except for one case of elective therapy that was presented at a conference. Thus, this was an extremely rare case.
In cases presenting with no bleeding of the esophagus and gastric varices, treatment that includes vascular agonists is often performed to prevent hemorrhaging. In the Cirrhosis Guidelines 2020 (revised third edition) prepared by the Japanese Gastroenterological Society and the Japanese Hepatology Society (15), nonselective β blockers, isosorbide mononitrates, or a combination of both drug types are proposed as treatment for esophageal and gastric varicose veins in patients with cirrhosis. Nonselective β blockers might result in decreased cardiac output and β2 receptor inhibition by β1 receptor inhibitors, contraction of abdominal visceral vessels due to α1 sympathetic nerve action, and decreased portal pulse pressure due decreased portal vein blood flow. In recent years, carvedilol, which has β-blocking activity, has been said to reduce the hepatic venous gradient pressure (16).
Isosorbide mononitrate is expected to increase nitric oxide levels in the liver and reduce the risk of recurrent bleeding by lowering vascular resistance in the liver. At a dose of 40 mg, hepatic venous pressure range, bivenous blood flow, and intravenous pressure are lowered to suppress an increase in portal pulse pressure after meals; the effect on BP is small (17). Angiotensin II receptor antagonists are also reported to lower portal pulse pressure and inhibit hepatic fibrosis (18).
Drugs used during esophageal varicose bleeding include vasopressin, which has a powerful vasoconstrictor action via V1 receptors, and terlipressin, a V1 receptor agonist. In Europe, these agents are used for emergency hemostasis. They work by constricting the abdominal visceral artery system and reducing the portal vein blood flow. In addition, somatostatin and its synthetic analog octreotide are considered to have a portal pulse pressure-lowering effect (19), and their usefulness has been reported. Many patients with acute esophageal varix rupture are not treated with internal use of these agents in order to prevent rupture. In the present patient, combination therapy with medication was not performed in order to prevent hemorrhaging before or after treatment of the esophageal varices, although that could have been considered.
EVL-EO combination therapy for thick esophageal varices in perforating vein is considered a useful treatment based on the hemodynamics of varices. In the case of esophageal varices when the timing of rupture is unknown, EVL-EO combination therapy is considered to be a rupture-preventing treatment. However, before treatment, EUS should be performed, the shape of the perforating veins should be fully understood, and a treatment plan should be carefully made. It is also necessary to fully inform patients that complications such as those seen in this case might occur and to obtain consent for EVL-EO combination therapy. Furthermore, even after successful treatment, it is essential to thoroughly consider the introduction of drug therapy that takes into account liver stiffness.
In conclusion, PTO should also be considered as endoscopic treatment for esophageal gastric varices with perforating vein when vascular occlusion with EO and ET is difficult.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Tripathi D, Stanley AJ, Hayes PC, et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 64: 1680-1704, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 65: 310-335, 2017. [DOI] [PubMed] [Google Scholar]
- 3. D'Amico G, Pasta L, Morabito A, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther 39: 1180-1193, 2014. [DOI] [PubMed] [Google Scholar]
- 4.The Japan Society for Portal Hypertension. Clinical Manual of Portal Hypertension: diagnosis treatment of esophageal and gastric varices. 15-16, 65-66, 2015. (in Japanese). [Google Scholar]
- 5. Sivak MV Jr, Stout DJ, Skipper G. Endoscopic injection sclerosis (EIS) of esophageal varices. Gastrointest Endosc 27: 52-57, 1981. [DOI] [PubMed] [Google Scholar]
- 6. Stiegmann GV, Goff JS, Michaletz-Onody PA, et al. Endoscopic sclerotherapy as compared with endoscopic ligation for bleeding esophageal varices. N Engl J Med 326: 1527-1532, 1992. [DOI] [PubMed] [Google Scholar]
- 7. Boyer TD, Haskal ZJ; American Association for the Study of Liver Disease. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 41: 386-400, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Tripathi D, Therapondos G, Jackson E, Redhead DN, Hayes PC. The role of the transjugular intrahepatic portosystemic stent shunt (TIPSS) in the management of bleeding gastric varices: clinical and haemodynamic correlations. Gut 51: 270-274, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshida H, Mamada Y, Taniai N, Tajiri T. Partial splenic embolization. Hepatol Res 38: 225-233, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Lunderquist A, Vang J. Transhepatic catheterization and obliteration of the coronary vein in patients with portal hypertension and esophageal varices. N Engl J Med 291: 646-649, 1974. [DOI] [PubMed] [Google Scholar]
- 11. Teres J, Planas R, Panes J, et al. Vasopressin/nitroglycerin infusion vs. esophageal tamponade in the treatment of acute variceal bleeding: a randomized controlled trial. Hepatology 11: 964-968, 1990. [DOI] [PubMed] [Google Scholar]
- 12. Paquet KJ, Feussner H. Endoscopic sclerosis and esophageal balloon tamponade in acute hemorrhaging from esophagogastric varices: a prospective controlled randomized trial. Hepatology 5: 580-583, 1985. [DOI] [PubMed] [Google Scholar]
- 13. Irisawa A, Obara K, Sakamoto H, Takiguchi F, Tojo J, Saito A, Kasukawa R. The selection and evaluation of the manipulation for endoscopic injection sclerotherapy against esophageal varices with extra esophageal shunt. Jpn J Portal Hypertens Esophageal Varices 3: 147-154, 1997. [Google Scholar]
- 14. Zheng J, Zhang Y, Li P, et al. The endoscopic ultrasound probe findings in prediction of esophageal variceal recurrence after endoscopic variceal eradication therapies in cirrhotic patients: a cohort prospective study. BMC Gastroenterol 19: 32, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoshiji H, Nagoshi S, Akahane T, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2020. J Gastroenterol 56: 593-619, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta V, Rawat R, Shalimar, Saraya A. Carvedilol versus propranolol effect on hepatic venous pressure gradient at 1 month in patients with index variceal bleed: RCT. Hepatol Int 11: 181-187, 2017. [DOI] [PubMed] [Google Scholar]
- 17. Escorsell A, Feu F, Bordas JM, et al. Effects of isosorbide-5-mononitrate on variceal pressure and systemic and splanchnic haemodynamics in patients with cirrhosis. J Hepatol 24: 423-429, 1996. [DOI] [PubMed] [Google Scholar]
- 18. Vorobioff JD, Gamen M, Kravetz D, et al. Effects of long-term propranolol and octreotide on postprandial hemodynamics in cirrhosis: a randomized, controlled trial. Gastroenterology 122: 916-922, 2002. [DOI] [PubMed] [Google Scholar]
- 19. Bunchorntavakul C, Reddy KR. Pharmacologic management of portal hypertension. Clin Liver Dis 23: 713-736, 2019. [DOI] [PubMed] [Google Scholar]