Abstract
We herein report a case of hepatitis-associated aplastic anemia (HAAA) that occurred after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination. In this patient, progressive pancytopenia observed two months after acute hepatitis following the second dose of the SARS-CoV-2 vaccine indicated the development of HAAA. Although some reports have suggested that SARS-CoV-2 vaccination may be involved in the development of autoimmune diseases, no cases of HAAA developing after SARS-CoV-2 vaccination have been reported. SARS-CoV-2 vaccination in children has only started relatively recently, so the range of side effects in children has not yet been thoroughly described. Therefore, we need to strengthen surveillance for symptoms of children who are vaccinated.
Keywords: HAAA, COVID-19, SARS-CoV-2, vaccination, AIH, aplastic anemia
Introduction
The ongoing global pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a major impact across the world, leading to the rapid development of anti-COVID-19 mRNA vaccines. Although a detailed causality relationship cannot be proven, some recent reports have suggested that SARS-CoV-2 vaccination triggers the development of autoimmune diseases (1-7).
Hepatitis-associated aplastic anemia (HAAA) is an acquired type of aplastic anemia in which pancytopenia appears after a few months following an acute attack of hepatitis. This pancytopenia is lethal if left untreated.
We herein report a case of severe HAAA that developed after the administration of the SARS-CoV-2 vaccine and discuss the relationship between the development of HAAA and SARS-CoV-2 vaccination.
Case Report
We report the case of a 15-year-old girl with no medication history who presented with abdominal bloating and constipation a few days after receiving the first dose of the Pfizer-BioNTech mRNA vaccine. A week after receiving the second dose of the same vaccine (received three weeks after her first dose), the patient presented with jaundice.
Laboratory investigations showed serum total bilirubin 6.8 mg/dL, direct bilirubin 5.3 mg/dL, aspartate aminotransferase (AST) 844 U/L, and alanine aminotransferase (ALT) 1,021 U/L; the serum IgG level was elevated at 10.12 g/L. Serological tests for hepatitis B, C virus, cytomegalovirus and human immunodeficiency virus were all negative. The test for serum immunoglobulin against Epstein-Barr virus was positive but not indicative of virus reactivation. She had previously received the diphtheria, tetanus, and pertussis (DPT) combination vaccine, oral poliovirus vaccine (OPV), Bacille Calmette-Guerin (BCG) vaccine, measles and rubella (MR) vaccine, and Japanese encephalitis vaccine without any adverse events. She had a history of varicella infection and was not vaccinated against varicella or mumps.
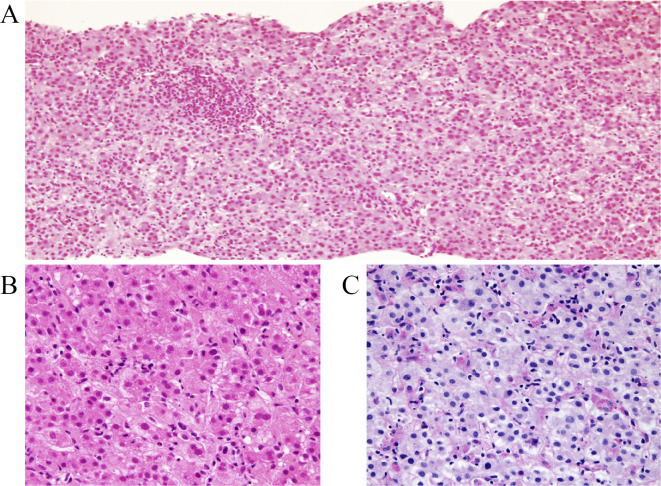
Abdominal ultrasound showed a slightly enlarged liver and gallbladder wall thickening with no biliary dilation (and thrombosis). A liver biopsy showed acute intrahepatic cholestasis: feathery degeneration and sporadic lymphocytic inflammation with frequent celloid macrophages, without eosinophilia and plasmacytosis (Fig. 1). This pattern of liver histology was not but conventional intrahepatic cholestasis, showing the damage higher gravity from view of so much frequent celloid macrophages. Here are not existed another specific finding of lobulocentral cytolytic autoimmune hepatitis (AIH), nor granulomatous primary biliary cholangitis lesions, nor primary sclerosing cholangitis lesion nor stigma of unicellular target or portal inflammation of hepatitis virus infection.
Figure 1.
Histological findings of the liver biopsy, periodic acid Schiff (PAS)-stained after amylase digestion. (A) Acute cholestatic hepatitis (arrow) of diffuse feathery hepatic corditis with celloid macrophages. (B) Diffuse secondary portal inflammation consists primary of lymphocytes without eosinophilia and plasmacytosis.
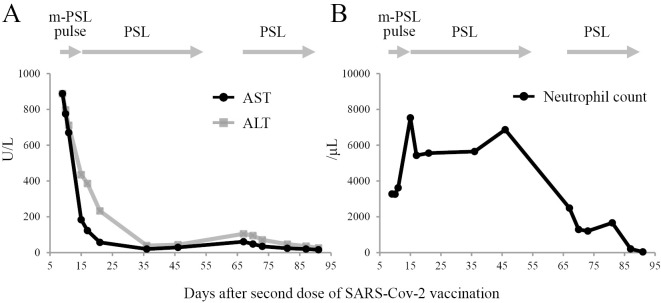
Treatment was started with intravenous methylprednisolone pulse therapy at 1,000 mg/day for 3 days followed by oral prednisolone at 40 mg/day. After one month of treatment, the liver enzyme levels had normalized, and symptoms had significantly improved (Fig. 2A). Prednisolone was tapered over a total of four weeks and then stopped.
Figure 2.
The trends of laboratory test results. (A) Diagram showing trend of AST, ALT, (B) neutrophils count days after second dose of SARS-CoV-2 vaccination. AST: aspartate aminotransferase, ALT: alanine aminotransferase, m-PSL: methylprednisolone, PSL: prednisolone
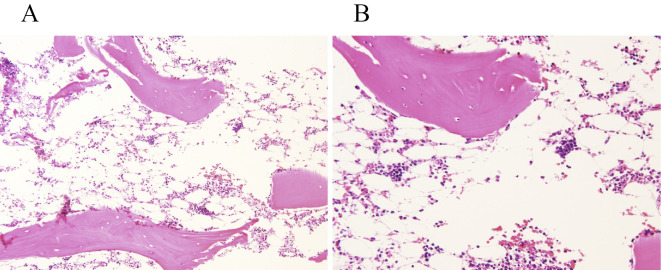
However, 2 months after contracting acute hepatitis, the patient developed progressive pancytopenia (WBC 1.9×109/L, hemoglobin 8.5 g/dL, platelets 19×109/L) (Fig. 2B). A bone marrow biopsy revealed hypocellular bone marrow (Fig. 3A, B). The patient was diagnosed with HAAA (1). While transfusing platelets frequently, further worsening of parameters (WBC 0.7×109/L, hemoglobin 7.1 g/dL, platelets 12×109/L) was observed. Allergic transfusion reactions to platelets included rash and respiratory symptoms, which were treated with antihistamines and hydrocortisone sodium succinate; we have therefore begun using washed-platelet transfusions for safety.
Figure 3.
Histological findings of bone marrow biopsy. (A) Hematoxylin and Eosin (H&E) staining (×10) of bone marrow biopsy two months after contracting acute hepatitis showed lamellar bones with fatty, and moderately hypocellular bone marrows with some cartilaginous tissue. (B) H&E staining (×20) revealed small amounts of granulocytes and histiocytic cells in the fatty bone marrow.
Discussion
Various side reactions, including the development of autoimmune disease, have been reported following SARS-CoV-2 vaccination (2,3). Some cases of AIH developing after SARS-CoV-2 vaccines have been reported (4-9), as have cases of aplastic anemia (AA) diagnosed after SARS-CoV-2 vaccines (10). However, no case of HAAA developing after SARS-CoV-2 vaccination has been reported.
In previously reported cases of AA developing after SARS-CoV-2 vaccines, the interval between vaccination and symptoms was approximately three to four weeks (10). However, in the present case, the patient developed progressive pancytopenia two months after acute hepatitis, an interval between vaccination and the onset that strongly supports the reliable diagnosis of HAAA. Furthermore, telomere shortening was not detected, nor were minor populations of glycosylphosphatidylinositol (GPI)-anchored protein-deficient granulocytes detected in the peripheral blood of this patient. Moreover, no genetic mutations that might be responsible for dyskeratosis congenita (DKC), a severe inherited bone marrow failure syndrome, were detected (11).
HAAA is a distinct variant of acquired aplastic anemia in which pancytopenia appears after a few months following acute hepatitis (12). Pancytopenia is lethal if left untreated. Although several viruses, such as hepatitis A-C, cytomegalovirus, Epstein-Barr virus, parvovirus B19, and echovirus, have been associated with the onset of HAAA, the etiology is unclear in most cases (13). To our knowledge, no report of vaccination being considered a trigger of HAAA development has been published.
The pathogenic mechanisms by which mRNA vaccination triggers the development of autoimmune disease remains unclear. mRNA vaccination triggers potential cross-reactivity between antibodies against the spike protein and self-antigens and may also activate immune responses, leading to the production of interferon I and other pro-inflammatory cytokines and chemokines (14).
Regarding the pathogenesis of HAAA, it has been postulated that cytotoxic immunity plays a prominent role in bone marrow failure (15). Common cytotoxic T cells (CTLs) are considered to recognize similar antigens between the liver and bone marrow cells in the early period of hepatitis. There are some reports that CTLs mediate the destruction of hematopoietic stem and progenitor cells (HSPCs) in AA (16). Furthermore, massive clonal expansion of CTLs has been reported to occur following vaccination (17). Therefore, one hypothesis holds that auto-reactive CTLs against similar antigens between the liver and HSPCs are induced by SARS-CoV-2 vaccination. However, whether or not SARS-CoV-2 vaccination actually triggers the auto-reactive immune responses leading to the destruction of HSPCs remains unclear. Confirming the detailed mechanisms may require analyzing the repertoire of T cell receptors after SARS-CoV-2 vaccination.
To our knowledge, this is the first report of HAAA developing after SARS-CoV-2 vaccination as a severe side effect in a child. We plan to perform allogeneic hematopoietic stem cell transplantation in this case. As it has not been long since SARS-CoV-2 vaccination for children began in many countries, the range of possible side effects after vaccination in children is not yet known. We report this case to encourage vigilance for children who are vaccinated and to also be alert for medium- to long-term side reactions from SARS-CoV-2 vaccination.
Regarding the association between SARS-CoV-2 vaccination and the development of diseases caused by abnormal autoimmune responses, more detailed investigations will be required.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol 172: 187-207, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 21: 195-197, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watad A, De Marco G, Mahajna H. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines 9: 435, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Londoño M-C, Gratacos-Gines J, Saez-Penataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination - still casualty? J Hepatol 75: 1248-1249, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol 75: 222-224, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rocco A, Sgamato C, Compare D, Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casualty. J Hepatol 75: 728-729, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan CK, Wong YJ, Wang LM, Ang TL, Kumar R. Autoimmune hepatitis following COVID-19 vaccination: true causality or mere association? J Hepatol 75: 1250-1252, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghielmetti M, Schaufelberger HD, Mieli-Vergani G, et al. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity? J Autoimmun 123: 102706, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McShane C, Kiat C, Rigby J, Crosbie O. The mRNA COVID-19 vaccine - a rare trigger of autoimmune hepatitis? J Hepatol 75: 1252-1254, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tabata S, Hosoi H, Murata S, Takeda S, Mushino T, Sonoki T. Severe aplastic anemia after COVID-19 mRNA vaccination: causality or coincidence? J Autoimmun 126: 102782, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dokal I, Vulliamy T. Dyskeratosis congenita: its link to telomerase and aplastic anaemia. Blood Rev 17: 217-225, 2003. [DOI] [PubMed] [Google Scholar]
- 12. Brown KE, Tisdale J, Barrett AJ, Dunbar CE, Young NS. Hepatitis-associated aplastic anemia. N Engl J Med 336: 1059-1064, 1997. [DOI] [PubMed] [Google Scholar]
- 13. Fuhrer M, Rampf U, Baumann I, et al. ; the German/Austrian Aplastic Anemia Working Group. Immunosuppressive therapy for aplastic anemia in children: a more severe disease predicts better survival. Blood 106: 2102-2104, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin Immunol 224: 108665, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ikawa Y, Nishimura R, Kuroda R, et al. Expansion of a liver-infiltrating cytotoxic T-lymphocyte clone in concert with the development of hepatitis-associated aplastic anaemia. Br J Haematol 161: 599-602, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Schoettler ML, Nathan DG. The pathophysiology of acquired aplastic anemia: current concepts revisited. Hematol Oncol Clin North Am 32: 581-594, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ritz C, Meng W, Stanley NL, Baroja ML, Xu C, Yan P. Postvaccination graft dysfunction/aplastic anemia relapse with massive clonal expansion of autologous CD8+ lymphocytes. Blood Adv 4: 1378-1382, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]