Abstract
Excellent capability of exosome derived from human adipose‐derived stem cell (ADSC) manifested in improving the quality of wound healing with SMD (STD Mean Difference). However, it is still in the preclinical stage and its efficacy remains uncertain. Emphasised the need for a systematic review of preclinical studies to the validity of it in ameliorate wound healing quality which accelerate the clinical application translation. We performed a systematic literature review to identify all published controlled and intervention studies comparing exosome derived from human ADSC with placebo in animal models of wound closure during wound healing. PubMed, Embase and Cochrane were employed. Risk of bias assessed by the SYRCLE tool aimed at preclinical animal studies. Administration of exosome derived from human ADSC extremely improved wound closure compared with controls, which is primary outcome (SMD 1.423, 95% confidence interval (CI) 1.137–1.709 P < .001), the same effect as ADSC. The therapeutic effect is further enhanced by modified ADSC‐EV. Other outcomes: density and the number of blood vessels: (SMD 1.593 95% CI 1.007–2.179 P < .001);Fibrosis‐related protein expression was highly expressed in the early term of wound healing, decreased in shaping period, which automatically regulates wound collagen deposition. Scar size, number of fibroblast and epithelial cell migration and proliferation expressed were ranked as follows: modified adipose stem cell exosomes > adipose stem cell exosomes > controls. Exosome derived from human ADSC, especially after enrichment for specific non‐coding RNA, is a promising approach to improve healing efficiency.
Keywords: adipose‐derived stem cell, exosome, fibrosis, wound, wound healing
1. INTRODUCTION
The skin is often disturbed by various etiologies such as trauma, extensive burns lead to acute or chronic wounds which affect the lives of a large portion of the world's population, especially the elderly. Difficult‐to‐heal problems of chronic wounds, which can lead to increased pain, stress, decreased quality of life, disability, depression, and social isolation. On the other hand, chronic wounds impose a significant burden on global medical resources due to long‐term medical needs and high recurrence rates. 1 , 2 , 3 , 4 , 5 , 6 This problem of accelerating skin regeneration and restore the function of injured skin in essence demanded effective method to obtain a solution. 3 When the skin is injured, the wound healing process is initiated immediately and consists of four overlappings and spliced stages that occur at specific moments in the order of wound progress and continue with a certain intensity for a specific duration: (i) Haemostasis ‐ occurs immediately (ii) Inflammation ‐ begins immediately after injury and persists for 4–6 days, accompanied by massive production and release of pro‐angiogenic, cytokine, inflammatory and fibrotic factors. (iii) Proliferative phase ‐ including angiogenesis, fibroproliferation, and re‐epithelialization processes. Fibroblasts, epithelial cells, acquire contractile properties by migrating and proliferating close to the wound edge. (iv) ECM (Extracellular matrix) remodelling‐The ECM as well as granulation tissue are remodelled through reorganisation, degradation and resynthesis: Collagen III is replaced by Collagen I, forming collagen‐rich scar tissue. 2 , 4 , 7 , 8 Exosome of stem cell is embodied to assist in various stages of wound healing: promote new blood vessels, accelerate cell migration and proliferation, and even administer scarring. 9 Human adipose‐derived stem cells (ADSCs) are an efficient and high‐quality source of stem cells because of their convenience, wide availability, multi‐directional differentiation potential, self‐renewal potential, low immunogenicity, and high proliferation rate. 10 Particularly, paracrine effects of information transfer via exosomes which explains how they typically express flexibility in terms of time windows of wound closure exosome derived from human adipose‐derived stem cell (ADSC‐EVs) have the function of promoting tissue regeneration by activating or restraining multiple signalling pathways, involving immune regulation, 11 angiogenesis, 12 cell migration, 13 proliferation and differentiation, 14 tissue remodelling. 15
EVs are natural membrane vesicles involved in intercellular interaction. 16 , 17 , 18 They are easier to store and vehicle, protecting the bioactive substances they delivered. 19 microRNA as exosome cargo can accelerates wound closure. The prospect of using EV enriched in such microRNA to improve the scarring process is now formed and opened. 20 , 21 In previous studies, the expression profiles of microRNA in cells reflect not only donor specificity but also that exosomes containing other non‐coding RNA (e.g., lncRNA, circleRNA) are influenced by differentiated cell types 22 , 23 , 24 ;Therefore, different biological properties can be expressed using exosomes delivering different cargo components.
Approach of wound healing is a crucial affair, applications of ADSC‐EV outline the need to develop possible approaches considering complexity of clinical. 5 , 25 , 26 , 27 , 28 ADSC‐EV mechanisms have been applications being studied with classical statistical approaches. To date, most investigation has been unapproved for commercial production or clinical use, relatively few clinical studies of ADSC‐EV have been used in clinical systems. This systematic review and meta‐analyses concentrating on the efficacy of ADSC‐EV in wound closure acceleration, optimization of wound outcomes and translation to clinical research.
2. MATERIALS AND METHODS
2.1. Search strategy
This study registered on PROSPERO before starting (CRD2022327561). The research strategy was inquired after PubMed, Embase, and Cochrane until April 23rd. Any relevant literature was conducted to ensure that all relevant articles were captured. Included studies investigating the use of EV isolated from human ADSC tissue sources controlled and intervention studies comparing with placebo in animal models of wound closure during wound healing. In total, 13 studies 9 , 10 , 11 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 researches lie in the use of ADSC‐EV in wound healing was addressed and included in analysis. Our quantitative meta‐analysis included 13 researches (Table 1).
TABLE 1.
Study characteristics
| Study | Year | Species | Sex weight | Wound form | Diameter or area | Purifcation method | ADSC | Type of miRNA | Dose | Ev | Size shape | Route | Frequeny |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen Yang | 2020 | BALb/c | F (5 weeks, 170–200 g) | Cut | 1*1 | C | Flowcytometry adipogenic osteogenic | mir‐21 | / | Flow cytometry;WB | TEM\NTA100 | Subcutaneous | Immediately |
| Guo xiu Cao | 2020 | BALb/c | (20–25 g) | Cut | d = 1 | C | Flowcytometry adipogenic osteogenic | mir‐19 inhibitor | 100 μg/100 μL | WB | TEM/NTA100 | Subcutaneous | Immediately |
| Li Pi | 2021 | C57BL/6 | (6–8 weeks) | Cut | 1*1 | C | / | miR‐125a‐3p inhibitor | 100 μL | WB | TEM/NTA100 | Subcutaneous | Immediately |
| Li Qian | 2021 | BALB/c | M (4 weeks) | Cut | 1*1 | E | Flow cytometry | sh‐H19;oe‐H19 | 200ug | WB | TEM | Subcutaneous | Immediately |
| Ying jie Lu | 2020 | kunmingg | M (6–8 weeks; 18–22 g) | Cut | d = 1.2 | C | Flowcytometry three‐lineage differentiation | mir486‐5p antagomir | 200 μg/100 μL | Flow cytometry | TEM/NTA30‐100 | Subcutaneous | Immediately |
| Yan Li | 2021 | BALB/c | M (6–8 weeks) | Cut | d = 1 | C | Flowcytometry adipogenic osteogenic | / | 70/100 μL | WB | TEM/NTA:30–100 | Subcutaneous | Immediately |
| Rui hong Yuan | 2021 | kunming | M (6–8 weeks; 22 ± 2 g) | Scald | d = 2 | E\C | / | mir‐29a | 200 μg | Flow cytometry | TEM/NTA113.6 | Subcutaneous abdominal cavity | 1 time 3 d |
| Gang quan Chen | 2021 | SD | / | Cut | d = 0.9 | C | / | mir‐146a | 100 μg/80 μL 95 μg/100 μL | WB | NTA81.14 | Subcutaneous | immediately |
| Yang Zhou | 2021 | ICR | M (7 weeks 28–35 g) | Cut | 1.5*1.5 | C | Flowcytometry three‐lineage | / | 200 ug/200 μL | WB | TEM\NTA134 | Subcutaneous smear | 1 time everyday |
| Yuan zheng Zhu | 2020 | rabbit | F (2.5–3.0 kg) | Cut | d = 0.8 | C | Flowcytometry adipogenic | / | 100 μL | WB | TEM\NTA129.1 | subcutaneous | 1 time 7 d |
| Wei Zhnag | 2018 | BALb/c | M (8 weeks 20 g) | Cut | 1*1 | C | Flowcytometry adipogenic osteogenic | / | 200 μg/200 μl | WB | TEM\NTA101.7 | abdominal cavity | 1 time 3 d |
| Xin Liao | 2022 | BALb/c | M (6‐8 weeks 17‐22 g) | Cut | 2*1.5 | C | Fllowcytometry | mir‐10 binhibitor | 200 μl | WB | TEM\NTA50‐150 | intravenous | 1 time 3 d |
| Andrea da Fonseca Ferreira | 2017 | Wistar rats | M (220 g) | Cut | d = 0.5 | C | Fllowcytometry three‐lineage | / | / | WB | NTA135 | Hydroxyethyl | immediately |
Abbreviations: C, ultracentrifugation; E E‐, quick extraction kit; F, female; M, male.
Inclusion Criteria and Excluded Selection of RCTs:
The following criteria: (1) preclinical models studies involving of wound healing in vivo. (2) Any intervention‐controlled works with comparator (e.g., placebo, EV inhibition, ADSCs, etc.) was included. (3) ADSC‐EV with mimic microRNA or lncRNA and ADSC‐EV modified with microRNA or lncRNA inhibitor or antagomir transfection. (4) One or more interventions at any dose (5) Publications composed only in English.
Excluded: (1) In vitro studies only or else not wound healing studies; (2) Diabetic wounds, bleomycin‐induced, and other wounds in pathological settings were excluded. (3) Non‐single microRNA or lncRNA (4) Studies only investigating the effects of ADSC or EV from other sources were excluded. (5) Non‐comparative studies, review articles, reviews, editorials, nursing reports, and other research types were precluded. (6) Non‐English publications. (7) No relevant results are reported.
Results of topical subcutaneous ADSC‐EV administration to wounds, intraperitoneal, tail vein injection, hydrogel administration and topical application are allowed. Includes unmodified, pretreated, non‐coding RNA‐modified ADSC‐EV. The study selection process presented using the flow chart was based on PRISMA recommendations. (Figure 1).
FIGURE 1.

A flow diagram of the study selection process
2.2. Quality assessment
Each eligible study was imported into Note Express (https://rayyan.qcri.org/) used to manage records. After excising duplicates, titles and abstracts were sifted by two independent authors (Meijia Li and Xinyao Chen) to ensure that any potentially relevant studies were screened as full‐text for ultimate suitability. Once reviewers possess differences of opinion, consensus is reached through a third team member (Lijun Hao) to judge together.
Risk of bias (ROB) for included studies rated and assigned by the ROB Tool for preclinical animal research that is Consensus‐based Standards for the selection of health Measurement Instruments (COSMIN) and the Center for Systems Review of Laboratory Animal Experimentation (SYRCLE). 39 Divergence of opinion related to classification is addressed by Meijia Li and Xinyao Chen discussions.
The items were as follows: (1) Selection bias: random sequence generation, adequate allocation concealment, baseline characteristics; (2) Detection bias: intended blinding of trial caregivers and researchers, randomly housed; (3) Reporting bias: random outcome, outcome blinded; (4) Attrition bias: State completeness of outcome data; (5) Reporting bias: selective assessor outcome reporting examined; (6) Other bias from other sources.
2.3. Data extraction
Relevant data were retrieved Meijia Li and evaluated by a second person Xinyao Chen. Requested original data from authors or Enguage Digitizer11.1 Data applied to extracting from diagram in researches when the raw data were not provided. Each study was reviewed critically and the following details were collected: (1) characteristics of study: authors, year of publication (2) implementation of the animal model: species, gender, age, weight, type of wound, wound size, depth and summary of results. (3) ADSC source: Whether all ADSC meet the minimum criteria for MSC characterisation by the International Society for Cell Therapy (ISCT). 40 (4) Intervention and comparators characteristics: ADSC‐EV dose, route of administration, frequency of administration, method of ADSC‐EV isolation and characterisation sample size, type of non‐coding RNA. (5) outcome measures: wound closure, vascular density, fibrotic protein expression, thickness of the dermis, scar size, scar pathology state, adverse events, and relevant information concerned ROB was excerpted.
2.4. Statistical analysis and meta‐analysis
Results were performed using STATA 15.0 (Stata LP, Texas, USA). For continuous outcomes, a random effects model was utilised to pool the data, and the results were calculated as SMD with 95% confidence intervals (CI), involved in wound closure, blood vessel density and number, fibrosis‐related protein expression, scar size and fibroblast and epithelial cell migration and proliferation.
Since time points across studies were heterogeneous, subgroup analysis selected four time periods: 0–7 days; 8–10 days; 11–14 days and 21 days. Meta‐analyses were only performed on at least two studies reporting identical outcomes, however, any studies that did not provide sufficient data or differed in outcome measures were subjected to descriptive analyses. Explore changes in outcome by comparing ADSC‐EV with mimic microRNA or lncRNA and ADSC‐EV modified with microRNA or lncRNA inhibitor or antagomir the ability of ADSC‐EV to accelerate wound healing rates altered after modification. In the analysis of fibrotic protein expression, subgroup analyses were preconfigured in protocol and were performed to identify whether the fibrotic protein expression outcomes of ADSC‐EV varied according to the use of treatment time. Eventually, for all analyses, statistical significance at P < .05.
3. RESULTS
3.1. Article selection process
The database search yielded 246 articles. After screening titles and abstracts, 202 articles were excluded, 44 full‐text articles were retrieved and entered into the full‐text screening process according to the inclusion criteria described above. In total, 13 participants addressed the effect of with or without ADSC‐EV in wound healing quantitative meta‐analysis.19 articles were removed for the reasons can be found in Figure 1.
3.2. Study characteristics
246 unique researches were retrieved totally, of which 13 full‐text articles met the criteria (n = 254 from our animal experiment group application). Studies publication date ranged from 2015 to 2020. (Table 1) The studies used Balb/c (n = 6), C57b/c (n = 1), KM (n = 2), ICR (n = 1), Wistar rats (n = 1), SD (n = 1) and rabbit (n = 1). The methods used to establish wound were cut (n = 12) or thermal damage (n = 1). Design and implementation of wound sizing in included studies are whether wound diameters ranged from 5 to 15 mm or area size from 1 to 3 square centimetres. All (n = 13) studies allowed wounds to reach full skin thickness. All studies tested ADSCs that met ISCT 40 criteria for pluripotent differentiation potential, studies of ADSC that fully met surface markers by flow cytometry (n = 3), additionally, positive expression of one or several surface markers (n = 9).
Multidirectional differentiation potential sphere: adipogenic, osteogenic, and chondrogenic differentiation of (n = 3); adipogenic differentiation and osteogenic differentiation of ADSC (n = 4); adipogenic differentiation (n = 1). In all studies, ADSC were derived from healthy human tissue while underwent surgery. Isolation and purification of EV either make use of ultracentrifugation (n = 11) or an ExoQuick Isolation Reagent (n = 1), ultracentrifugation combined with Exo‐Quick Isolation Reagen (n = 1). The most shared characteristic of ADSC‐EV censored by electron microscopy (n = 11), followed by nanoparticle tracking analysis (n = 11). The morphological description of ADSC‐EV showed spherical, teacup‐like, and disc‐like vesicles with a depression in the middle. 41 , 42
Flow cytometry (ADSC‐EV surface markers random combinations of CD63, CD9, CD81) (n = 3), Western blot (CD9, CD63, CD81, TSG101, HSP 70 random combined) (n = 11). Eight studies reported on the measure of ADSC‐EV as a mean of 111.53 nm, Yingjie Lu and Yan Li reported between 30 and 100 nm, 10 , 31 Xin Liao reported between 50 and 150 nm. 38
ADSC‐EV compared to placebo (n = 13); a direct comparison of ADSC and their derived exosomes (n = 3); ADSC‐EV enriched specific microRNA or lncRNA that target mechanisms involved in wound closure direct compare with ADSC‐EV(n = 2); ADSC‐EV that were enriched microRNA inhibitor (n = 3) while the other one was loaded microRNA antagomir.
3.3. Quality of the individual studies
Article quality was assessed using SYRCLE's and COSMIN ROB tool. Most Articles were randomised and blinded, while some randomised or blinded. Most articles do allocation hiding is not described. Taken together, the overall ROB assessment Indicates that the included studies were of moderate quality, more details of 13 ROB summary and the quality scores are shown in Figure 2.
FIGURE 2.

Article quality assessed with SYRCLE's ROB tool
Since all preclinical animal studies were randomised and the intervention and control groups similar at baseline, however, there was no mention of sequence generation randomization methods and all studies were at an uncertain risk of sequence generation bias. Additionally, five studies 31 , 32 , 33 , 35 , 36 guaranteed the allocation of study animals. Furthermore, only one study mentioned housing randomization and the other three studies allocated animals to individual housing. 30 , 32 , 33 Only one study provided sufficient information to determine whether randomised outcome assessments were consistently applied. 34
3.4. Publication bias
Funnel plot and trim and fill analysis publication bias. Some asymmetric in funnel plot is indicative of publication bias. (Figure 3) Egger's test and trim‐and‐fill analysis were performed to confirm the result of the funnel plot. (Figure 3) Results infer that high clinical relevance in most of the studied models, whereas aspects of proliferation migration, vascularization, scar size lack uniformity in the description of results in some studies.
FIGURE 3.

Funnel plot and Trim‐and‐fill analysis publication bias
3.5. Primary outcome‐wound closure
Most of the literatures describe the wound closure rate in the form of charts, while others do not clearly state that the wound closure rate is obtained by the ratio of the wound size after treatment to the control group. ADSC exosomes significantly enhanced wound closure rates compared with placebo (11 studies, standardised mean difference SMD 1.423, 95% CI 1.137–1.709 I 2 = 18.6% P < .001 Figure 4A). Three studies directly compared ADSC‐EV with ADSC and found that ADSC‐EV could achieve the same effect compared to ADSC closure 11 , 30 , 32 (SMD‐0.140, 95% CI‐1.295–1.014; P = 0.812; Figure 4B). Since time points were not consistent across studies, we targeted Subgroup analysis performed by treatment time. And in the subgroup analysis from 0 to 7 day (SMD 1.388 95% CI 0.965–1.811; P < .001; Figure 5). Days 8–10 in subgroup analysis (SMD 1.331, 95% CI 0.657–2.005; P = 0.001; Figure 5). Days 11–14 in subgroup analysis (SMD 1.425, 95% CI 0.893–1.957; P < .001; Figure 5). Days over 21 days in subgroup analysis (SMD 1.013, 95% CI 0.430–1.596; P = 0.002; Figure 5) Two studies directly performed analyses of modified ADSC‐EV versus unmodified control ADSC‐EV. 9 , 30 In contrast, the accelerated closure observed after administration of ADSC‐EV enriched with specific non‐coding RNA receptor antagonist or inhibitor was attenuated compared to the improvement associated with unmodified ADSC‐EV. Three of studies manifested the efficacy of non‐coding inhibitor application to hinder the accelerated wound healing of ADSC‐EV. 29 , 34 , 36 (SMD 1.052, 95% CI 0.527–1.577; P < .001; Figure 6) while the other study used the non‐coding antagomir. 10
FIGURE 4.

A, The forest plot shows the effect of ADSC‐EV therapy on wound closure rates compared with placebo. B, The forest plot shows the effect of ADSC‐EV therapy on wound closure rates compared with ADSC
FIGURE 5.

Subgroup analysis performed by treatment time
FIGURE 6.

The forest plot shows the effect of therapy on wound closure rates with or without non‐coding inhibitor‐modified ADSC‐EV
3.6. Secondary outcomes
3.6.1. Vascularization
Four studies reported assessed the degree of vascularization by the expression of CD31, which is higher degree of CD31 significantly compared to controls. (SMD1.593 95% CI 1.007–2.179, P < .001 Figure 7). 9 , 30 , 32 , 36 Much of the research has examined expression of proteins such as p‐ERK‐2, SERPINH‐1, TGF‐β, α‐SMA and VEGF represent the proliferation of fibroblasts and epithelial cells as well as promote vascular density around wound. 9 , 30 , 31 , 34 One study put a value of CD31 and a‐sma protein expression reflecting the degree of vascularization. 10 ADSC‐EV enriched microRNA‐146 exceedingly increased the expression of CD31, p‐ERK‐2, SERPINH‐1 in histological examination of wounds. 9 Li Pi outlines the functions carried out by miR‐125a‐3p inhibitor reduced angiogenesis as well as CD31 expression. 29 All of the above demonstrated that reap huge fruits of using ADSC‐EV increased wound vascularization, furthermore, promoted healing compared with controls.
FIGURE 7.
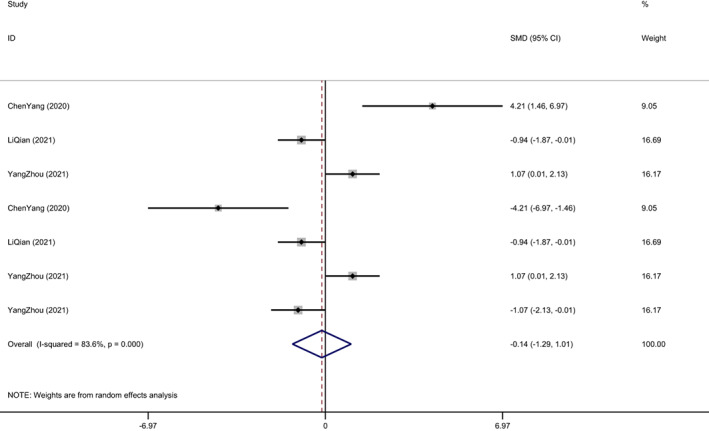
Degree of vascularization expressed by CD31
3.7. Other outcomes
3.7.1. Fibrosis
Six studies used ADSC‐EV to study the expression of fibrotic proteins during wound healing, and subgroup analysis according to monitored protein species:
Collagen I (SMD‐0.758 95% CI −2.268 to −0.752; P = 0.325; Figure 8D).
Collagen type III (SMD −0.601 95% CI −2.665 to −1.463; P = 0.568; Figure 8D).
A‐sma, (SMD −1.604 95% CI −2.244 to −0.964; P < .001; Figure 8D).
TGF‐β (SMD 1.113 95% CI −1.678 to −3.904; P = 0.434; Figure 8D).
Ratio of collagen I type to III (SMD −1.467 95% CI −2.068 to −0.866; P < .001; Figure 8).
FIGURE 8.

A, Expression of fibrotic proteins during wound healing, subgroup analysis by protein species. B, Expression of fibrotic proteins in wound healing within days 0–7. C, Expression of fibrotic proteins in Wound healing more than 14 days
Due to the large heterogeneity, the analysis was performed according to the time of tissue sampling. Analysis of results grouped by time: Wound healing early phase, especially within days 0–7, all showed accelerated collagen deposition and maturation, TGF‐β deposition, and increased collagen types I and III.
TGF‐β (SMD 2.424 95% CI 1.210‐‐3.638; P < .001; 8 E).
Wound remodelling period of more than 14 days, three studies showed suppression of expression of ECM remodelling genes 31 , 33 , 35 such as collagen I expression, collagen type III, a‐sma protein, and collagen I/III, pathological results manifested more ordered collagen arrangement. 43
Collagen I (SMD‐1.416 95% CI −2.036 to −0.796; P < .001; Figure 8F).
Collagen type III (SMD‐1.489 95% CI −2.244 to −0.734; P < .001; Figure 8F).
A‐sma, (SMD‐1.604 95% CI −2.244 to −0.964; P < .001; Figure 8F).
Ratio of collagen I to collagen type III (SMD −1.467 95% CI −2.068 to −0.866; P < .001; Figure 8F).
3.8. Thickness of the dermis
Administration of ADSC‐EV increased skin dermal thickness within days 0–7, increased re‐epithelialization rate, and favoured wound fibroblast and epithelial cell migration and proliferation. 10 , 43 , 44 Five studies described the pathological state of wound scarring: earlier wound healing, better arrangement of collagen structures, and less pathology in the ADSC‐EV‐treated group. 11 , 29 , 31 , 32 , 34 The control group had more inflammatory cell infiltration, thicker dermal tissue, disordered granulation tissue, and irregular arrangement of collagen fibres. Although ADSC‐EV administration improved collagen alignment after 14 days, the ADSC‐EV‐treated group exhibited pleasurable scar consequent compared to the control group. Three of the papers reported height of scar lift 35 ; width of scar 36 ; and reduction of dermal thickness. 33 Consequently, the dependent variables could not be unified for meta‐analysis.
3.9. Proliferation
Six papers reported that ADSC‐EV promoted skin fibroblast and epithelial cell migration and proliferation around the wound during wound healing; Chen Yang detected the expression of mir‐21, mir‐31, mir‐155 and mir‐205 at the wound edge 11 ; Li Pi detected CD31 and PI3K‐ AKT expression, indicating that ADSC‐EV promotes angiogenesis and tissue proliferation by inhibiting PTEN expression, and mir‐125a‐3p inhibitor attenuates this effect 29 ; Li Qian detected the expression of SOX9 and beta‐catenin which both can represent the ability of tissue migration and proliferation increased in periwound tissue after ADSC‐EV treatment. 30 Yingjie Lu and Guo xiu Cao quantified wound margin re‐epithelialization. 10 , 34 Gang quan Chen p‐ERK‐2, SERPINH‐1 in wounds cytokines in fibroblast proliferation and migration. 9
4. DISCUSSION
The essential problem in the design of wound healing quality is represented by how to accelerate wound closure rate and regular scaring for the measurement of variables. Therefore, Emphasis is placed on improving wound healing treatment measures by which the cell‐therapy is crucial in the medical field. Results of experimental applications of Mesenchymal stem cells stem cell therapy had become indispensable for effective wound treatment. 45 Since then, the subject that restoring the integrity of the dermal tissue with stem cell EV has been extensively explored and it is have shown rosy results. Different resource of stem cell EV is beneficial control inflammation, accelerate skin cell migration and proliferation, regular wound scarring, improve angiogenesis, and even rejuvenation of skin. 46
According to the wide availability and multi‐directional, Human adipose‐derived stem cells (hADSCs) is considered to be development prospects in the evolution source of stem cells. 47 Otherwise due to ADSC may enhance paramount immune/inflammatory processes. ADSC have been shown to benefit tumour progression in a variety of experimental tumour models. There has been a turning point in the clinical application of ADSC with the advent of ADSC‐EV. 26
ADSCs exert their biological functions through cell‐free EV without contamination of cellular components. Exosomes can replicate the functions of their parental cells in exosomes that was investigated quite intensively in recent years. ADSC‐EV may provide a superior alternative in Tissue Engineering and Regenerative Medicine in unfavourable conditions such as pH environment, high temperature, and repeated freeze–thaw cycles. 28
Exosome delivery can be used as nanocarriers to shuttle various proteins, messenger RNA (mRNA) and microRNA to regulate the activity of recipient cells, and to deliver active factors or small molecules to recipient cells to promote tissue repair. 48 , 49 Compared to the direct use of stem cells for tissue repair, exosomes have the advantage of improved safety, easier storage and transport, fast and efficient, no ethical restrictions, and a wide range of sources. ADSC‐EV have stronger biological functions than adipose stem cells, are more stable and less prone to destruction due to their plasma membrane. Advantages of ADSC‐EV are low probability of tumorigenicity or immune responses and homing abilities that migrate to target make itself serve as a new approach to achieve cell‐free therapy.
Exosomes are nanoscale extracellular vesicles containing a variety of biologically active molecules. microRNA contained in exosomes are important mediators of cell‐to‐cell interactions; exosomes can also be used as delivery mediators. After microRNA modification, the carried microRNA is transported to the surface of receptors, and the cells are exocytosed. The message is transmitted into the cell, where it is degraded and re‐expressed and the gene is regulated in the target cell. Currently, exosomes can be exploited as biomarkers for diagnosing diseases and as cargo for drug delivery. 50
MicroRNA is a non‐coding RNA that exists in various tissues of the body and can block mRNA by binding to the 3′ untranslated gene and inhibiting the target mRNA expression in the region (3′UTR), coding region or 5′UTR of the target gene interfering gene expression. Highly expressed microRNA: miR‐34a‐5p, miR124‐3p, miR‐146a‐5p, miR‐132, miR‐21 and miR‐29a which are important mediators of wound healing detected in ADSC‐EV.
Currently, substantial evidence shows that microRNA can significantly regulate various organ fibrosis processes, including skin fibroblast proliferation and ECM remodelling. MiR‐223, miR‐203, miR‐146a may get involved in anti‐inflammatory. miR‐29c, miR‐192, and miR‐215 proposed remodelling stage through the activation of SMADs, b‐catenin, E‐cadherin, and SIP1. 48 The presence of long non‐coding RNA (lncRNA) in exosomes has been reported, suggesting that lncRNA may also load into exosomes during cell–cell interactions and further regulate gene expression in host cells. ADSC‐Exo inhibits miR‐19b expression through H19, and SOX9 up‐regulation activates the Wnt/β‐catenin pathway, which accelerates HSF proliferation, migration, and invasion to accelerate skin wound healing. 30 LncRNA XIST shuttled ADSC‐EV suppresses myocardial pyroptosis in atrial fibrillation through miR‐214‐3p‐mediated Arl2. 51 lncRNA HCG11 negatively regulates cell proliferation and adipogenesis by the miR‐204‐5p/SIRT1. 23 ADSC‐EV itself carries non‐coding RNAs that improve the quality of wound healing, and non‐coding RNAs can also be engineered and packaged in exosomes to act as messengers in intercellular according to the needs of patients.
Normal angiogenesis is indispensable for normal wound closure, and its absence is one of the hallmarks of prolonged chronic wound healing in humans. It requires a tight interaction between endothelial cells and their surrounding environment. Previous studies of ADSC‐EV and skin regeneration interactions had emphasised potential advantages angiogenesis, proliferation, migration, and differentiation. Moreover, EV enhanced the proliferation, migration, and tube formation of vascular endothelial cells by stimulating the production of main cytokine involved in such as FGF, VEGF, Angiopoietin 1 (ANG‐1), E‐selectin, C‐X‐C Motif Chemokine Ligand 16 (CXCL‐16), endothelial NOS (eNOS), and C‐X‐C motif chemokine ligand 8 (IL‐8/CLCX8) in vitro. 52 Those factors are crucial angiogenesis regulators. ADSC‐EVs can transfer miR‐125a to endothelial cells and inhibit DLL4 to promote angiogenesis. 53 Sufu targeting mediated ADSCs exosomes decorated with miR‐423‐5p significantly enhanced pro‐angiogenic activity. 54 According to endothelial cell lines such as HUVEC are commonly used to assess endothelial tube formation and cell proliferation assays. Exosomes are internalised by primary human umbilical vein endothelial cells (HUVEC) and stimulate HUVEC neovascularization, endothelial cell migration and proliferation in vitro and in vivo approaches. ADSC‐Exo, as a pro‐angiogenic factor, might be a promising candidate for therapeutical tissue repair and reconstruction. 55
Statements of EV from human ADSC‐EV could accelerate the various situations involving possibility knowledge are investigated: ECM proteins, including collagen and fibronectin, and release soluble factors such as growth factors and cytokines. Bellei illustrated enhances proliferation of dermal and epidermal cells in a dose‐dependent manner. 56 The proliferation and expansion of fibroblasts were found higher treated with ADSC‐EV than in the control cells 26 EV can accelerate dermal fibroblast migration and proliferation and differentiation of major cell types involved in skin regeneration. In fact, EV from ADSC‐EV appears to optimise the characteristics of dermal fibroblasts in a dose‐dependent manner are considered. MALAT1 seems to be a regulator of the effect of ADSC‐EV, as MALAT1‐carrying ADSC‐EV was able to induce skin fibroblast migration through PI3K/Akt signalling pathway. 44
TGF‐β/Smad signalling pathway regulate wound healing and pathological scar formation is considered to be an paramount after skin trauma. Downstream genes of TGF‐β, including Smad2, α‐SMA and Collagen I, etc. ADSC‐EV can prime injured tissue and prevent end‐organ fibrosis, ultimately regulating matrix remodelling and preventing post‐wound scarring. In late wound healing phase, preventing fibroblast differentiation, enters myofibroblasts and inhibits granulation tissue reduces scarring by repressing TGF‐β1 administered ADSCs‐EV. 57 Exosome treatment inhibits myofibroblast differentiation and increases the ratio of transforming growth factor‐β3 (TGF‐β3) to TGF‐β1. ASC‐Exos increased the ratio of MMP3 to tissue inhibitor of matrix metalloproteinase‐1 (TIMP1), as well as the ratio of collagen I to III collagen, by activating the ERK/MAPK pathway to promote ECM remodelling in skin wound repair. miR‐18a‐5p is downregulated during scarring.
MiR‐18a‐5p is upregulated during scarring, and the expression levels of the proteins Smad2, Collagen I (Col I) and Col III are downregulated. MiR‐211‐5p can inhibit the proliferation, migration, invasion and ECM production of human hypertrophic scar fibroblasts by targeting the activation of TGF‐βR2/Smad3 signalling pathway. 58 LncRNA NORAD knockdown, increased miR‐26a, inhibited TGF‐β receptor apoptosis significantly increased. 59 Future research can load some of the above‐screened scar protective factors by ADSC‐EV to treat abnormal scar formation during wound healing.
A major goal of this report is to extend approaches and strategies for structuring methods of ADSC‐EV applied to wound. This paper outlines the functions carried out conclusions and future directions of research with respect that therapeutic effect of ADSC‐EV in preclinical models of wounds. We describe the preliminary results of ADSC‐EV intensely improved wound closure currently in progress and verify ADSC‐EV enriched in specific non‐coding RNA possess more rewarding functions. Meanwhile, EV loaded with non‐coding RNA inhibitors or antagomirs attenuate the function of original EV. Our observations were consistent with previous studies that contain a discussion of the implication of the efficacy of ADSC and ADSC‐EV in wound closure quality. An analysis of inference by two experiments is not statistically significant for ADSC‐EV can completely achieve the same therapeutic effect as ADSC. Specifically, several preclinical studies of ADSC‐EV whether ADSC‐EV treatment gives rise to no adverse event or complication in these works was not mentioned. None of the studies reported any loss to follow‐up or death of animals in any group.
Additionally, this research has limitation worth mentioning. In the first place, the included studies were potentially biased across many study designs. Researches in future could pay more attention to the selections of sequence generation housing, applying and reporting randomization, blinding, and assess protocols. Presence of fuzzy data is still required to figure out before final goal of application of ADSC‐EV in wound can be completed. There is one more point, I should touch on, that limited time point studies which are accurate treatment and unified efficacy evaluation for each stage of the wound were identified for specific subgroup analyses. Although a lot of effort is being spent on specific proteins, reach a unified consensus method has yet to be developed. Subgroup analysis of specific proteins requires at least two studies to evaluate the same results. Furthermore, differences were observed in some aspects of these research designs, including animal model, method of wound formation, wound diameter or area, route of administration for ADSC‐EV, dose, frequency, this may prevent a more precise estimation of wound closure.
Although the species used in animal models are inconsistent, Balb/c (n = 6), C57b/c (n = 1), KM (n = 2), ICR (n = 1), Wistar rats (n = 1), SD (n = 1) and rabbit (n = 1) (see Table 1). These results are not affected by the species studied, and it can be seen that the application of ADSC‐EV is not limited to one species and can be widely used in wound animal experiments. The doses of ADSC‐EV ranged from 70 to 200 μg (n = 7) or 100 μL (n = 2), meanwhile, most studies (n = 9) design A single local injection of ADSC‐EV was administered immediately after wound formation, other studies repeated injections were administered at intervals (Table 1). The dose of ADSC‐EV was reported Counting unclear only by cell count (n = 2). There were three routes of ADSC‐EV administration in all studies: subcutaneous injections (n = 10), abdominal cavity (n = 1), smear (n = 1) (Table 1). Yang Zhou demonstrated that wound healing efficiency was proportional to ADSC‐EV measurement and frequency. Smear application is more effective than subcutaneous injection. 32
Although standards for wound formation, wound size, and injection dose are not uniform but they have no effect on the results. Overall, in future research, more attention should be paid to the unification of the above variables. We utilise a systematic review processing that was developed to minimises researches that may be ignored. Despite heterogeneity between studies, ADSC‐EV manifested consistent positive efficacy which could be evidence to support clinical translation. The last but not the least, our research can not only facilitate the translation of ADSC‐EV in clinical applications but also guide the basic research of the pathway mechanism by which ADSC‐EV improves the quality of wound healing. Basic problems mainly in quality of wound healing are presented by the wound closure efficacy and proportion of pathological scarring. The use of ADSC‐EV seems exceedingly encouraging given the improved quality of wound healing: Wound closure profit from vascularization, fibroblast and epithelial cell migration and proliferation and dermis thickening, and re‐epithelialization at early stage, while restraining collagen deposition and reducing the refractory scarring at late stage.
In conclusion, ADSC‐EV demonstrates various outcomes and studied healing properties in severe wounds design in preclinical studies. ADSC‐EV, especially the modified ADSC‐EV is enriched in specific non‐coding and microRNA are a promising approach to improve the quality of wound healing and deserves further investigation. A considerable amount of literature will need to be designed for definitive preclinical studies protocol, which is able to reduce potential ROB, making ADSC‐EV used as an acceptable alternative in clinical research progress.
5. CONCLUSION
ADSC‐EV can effectively improve the wound closure same as ADSC by acceleratingwound healing by promoting wound vascularization, increasing fibroblast and epithelial cell migration and proliferation. ADSC‐EV manages the expression of fibrotic proteins at various stages in the wound healing process: promote fibrosis in the early stage and increase the thickness of the dermis; attenuate the fibrosis process and reduce scar formation during the ECM remodelling stage, which has achieved better therapeutic effect. ADSC‐EV modified designated non‐coding RNA performs more superior than unmodified. The function of exosomes was attenuated by loading non‐coding RNA inhibitors or antagomirs.
Yuan T, Meijia L, Xinyao C, Xinyue C, Lijun H. Exosome derived from human adipose‐derived stem cell improve wound healing quality: A systematic review and meta‐analysis of preclinical animal studies. Int Wound J. 2023;20(6):2424‐2439. doi: 10.1111/iwj.14081
DATA AVAILABILITY STATEMENT
Encourages data sharing.
REFERENCES
- 1. Manchon E, Hirt N, Bouaziz JD, Jabrane‐Ferrat N, al‐Daccak R. Stem cells‐derived extracellular vesicles: potential therapeutics for wound healing in chronic inflammatory skin diseases. Int J Mol Sci. 2021;22(6):3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosca AM, Tutuianu R, Titorencu ID. Mesenchymal stromal cells derived exosomes as tools for chronic wound healing therapy. Rom J Morphol Embryol. 2018;59(3):655‐662. [PubMed] [Google Scholar]
- 3. Hu P, Yang Q, Wang Q, et al. Mesenchymal stromal cells‐exosomes: a promising cell‐free therapeutic tool for wound healing and cutaneous regeneration. Burns Trauma. 2019;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malhotra P, Shukla M, Meena P, et al. Mesenchymal stem cells are prospective novel off‐the‐shelf wound management tools. Drug Deliv Transl Res. 2022;12(1):79‐104. [DOI] [PubMed] [Google Scholar]
- 5. Qiu H, Liu S, Wu K, Zhao R, Cao L, Wang H. Prospective application of exosomes derived from adipose‐derived stem cells in skin wound healing: a review. J Cosmet Dermatol. 2019;19:574‐581. [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez‐Menocal L, Salgado M, Ford D, van Badiavas E. Stimulation of skin and wound fibroblast migration by mesenchymal stem cells derived from normal donors and chronic wound patients. Stem Cells Transl Med. 2012;1(3):221‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shimoda A, Sawada SI, Sasaki Y, Akiyoshi K. Exosome surface glycans reflect osteogenic differentiation of mesenchymal stem cells: profiling by an evanescent field fluorescence‐assisted lectin array system. Sci Rep. 2019;9(1):11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang L, Hu L, Zhou X, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7(1):13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen MG, Wu YM, Zou LM, Zeng YM. Effect of MicroRNA‐146a modified adipose‐derived stem cell exosomes on rat Back wound healing. Int J Low Extrem Wounds. 2021;15347346211038092. [DOI] [PubMed] [Google Scholar]
- 10. Lu Y, Wen H, Huang J, et al. Extracellular vesicle‐enclosed miR‐486‐5p mediates wound healing with adipose‐derived stem cells by promoting angiogenesis. J Cell Mol Med. 2020;24(17):9590‐9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang C, Luo L, Bai X, et al. Highly‐expressed micoRNA‐21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP‐9 expression through the PI3K/AKT pathway. Arch Biochem Biophys. 2020;681:108259. [DOI] [PubMed] [Google Scholar]
- 12. Chen B, Cai J, Wei Y, et al. Exosomes are comparable to source adipose stem cells in fat graft retention with up‐regulating early inflammation and angiogenesis. Plast Reconstr Surg. 2019;144(5):816 e‐827 e. [DOI] [PubMed] [Google Scholar]
- 13. Lin R, Wang S, Zhao RC. Exosomes from human adipose‐derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383(1–2):13‐20. [DOI] [PubMed] [Google Scholar]
- 14. Skubis A, Gola J, Sikora B, et al. Impact of antibiotics on the proliferation and differentiation of human adipose‐derived mesenchymal stem cells. Int J Mol Sci. 2017;18(12):2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen T, Zheng QQ, Shen J, et al. Effects of adipose‐derived mesenchymal stem cell exosomes on corneal stromal fibroblast viability and extracellular matrix synthesis. Chin Med J. 2018;131(6):704‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kletukhina S, Neustroeva O, James V, Rizvanov A, Gomzikova M. Role of mesenchymal stem cell‐derived extracellular vesicles in epithelial‐mesenchymal transition. Int J Mol Sci. 2019;20(19):4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ching RC, Wiberg M, Kingham PJ. Schwann cell‐like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res Ther. 2018;9(1):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chinnici CM, Pietrosi G, Iannolo G, et al. Mesenchymal stromal cells isolated from human fetal liver release soluble factors with a potential role in liver tissue repair. Differentiation. 2019;105:14‐26. [DOI] [PubMed] [Google Scholar]
- 19. Fang Y, Zhang Y, Zhou J, Cao K. Adipose‐derived mesenchymal stem cell exosomes: a novel pathway for tissues repair. Cell Tissue Bank. 2019;20(2):153‐161. [DOI] [PubMed] [Google Scholar]
- 20. Yang S, Guo S, Tong S, Sun X. Promoting osteogenic differentiation of human adipose‐derived stem cells by altering the expression of Exosomal miRNA. Stem Cells Int. 2019;2019:1351860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iminitoff M, Damani T, Williams E, Brooks AES, Feisst V, Sheppard HM. microRNAs in ex vivo human adipose tissue derived mesenchymal stromal cells (ASC) undergo rapid culture‐induced changes in expression, including miR‐378 which promotes adipogenesis. Int J Mol Sci. 2020;21(4):1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang X, Chen L, Xiao B, Liu H, Su Y. Circ_0075932 in adipocyte‐derived exosomes induces inflammation and apoptosis in human dermal keratinocytes by directly binding with PUM2 and promoting PUM2‐mediated activation of AuroraA/NF‐kappaB pathway. Biochem Biophys Res Commun. 2019;511(3):551‐558. [DOI] [PubMed] [Google Scholar]
- 23. Li D, Liu Y, Gao W, et al. LncRNA HCG11 inhibits adipocyte differentiation in human adipose‐derived mesenchymal stem cells by sponging miR‐204‐5p to upregulate SIRT1. Cell Transplant. 2020;29:963689720968090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi R, Jin Y, Hu W, et al. Exosomes derived from mmu_circ_0000250‐modified adipose‐derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR‐128‐3p/SIRT1‐mediated autophagy. Am J Physiol Cell Physiol. 2020;318(5):C848‐C856. [DOI] [PubMed] [Google Scholar]
- 25. Ren S, Chen J, Duscher D, et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res Ther. 2019;10(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heo JS, Kim S, Yang CE, Choi Y, Song SY, Kim HO. Human adipose mesenchymal stem cell‐derived exosomes: a key player in wound healing. Tissue Eng Regen Med. 2021;18(4):537‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herter EK, Xu LN. Non‐coding RNAs: new players in skin wound healing. Adv Wound Care. 2017;6(3):93‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zomer HD, Jeremias TS, Ratner B, Trentin AG. Mesenchymal stromal cells from dermal and adipose tissues induce macrophage polarization to a pro‐repair phenotype and improve skin wound healing. Cytotherapy. 2020;22(5):247‐260. [DOI] [PubMed] [Google Scholar]
- 29. Pi L, Yang L, Fang BR, Meng XX, Qian L. Exosomal microRNA‐125a‐3p from human adipose‐derived mesenchymal stem cells promotes angiogenesis of wound healing through inhibiting PTEN. Mol Cell Biochem. 2022;477(1):115‐127. [DOI] [PubMed] [Google Scholar]
- 30. Qian L, Pi L, Fang BR, Meng XX. Adipose mesenchymal stem cell‐derived exosomes accelerate skin wound healing via the lncRNA H19/miR‐19b/SOX9 axis. Lab Invest. 2021;101(9):1254‐1266. [DOI] [PubMed] [Google Scholar]
- 31. Li Y, Zhang J, Shi J, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR‐192‐5p/IL‐17RA/Smad axis. Stem Cell Res Ther. 2021;12(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou Y, Zhao B, Zhang XL, et al. Combined topical and systemic administration with human adipose‐derived mesenchymal stem cells (hADSC) and hADSC‐derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther. 2021;12(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yuan R, Dai X, Li Y, Li C, Liu L. Exosomes from miR‐29a‐modified adipose‐derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF‐β2/Smad3 signaling. Mol Med Rep. 2021;24(5):758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cao G, Chen B, Zhang X, Chen H. Human adipose‐derived mesenchymal stem cells‐derived Exosomal microRNA‐19b promotes the healing of skin wounds through modulation of the CCL1/TGF‐β signaling Axis. Clin Cosmet Investig Dermatol. 2020;13:957‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu YZ, Hu X, Zhang J, Wang ZH, Wu S, Yi YY. Extracellular vesicles derived from human adipose‐derived stem cell prevent the formation of hypertrophic scar in a rabbit model. Ann Plast Surg. 2020;84(5):602‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang W, Bai X, Zhao B, et al. Cell‐free therapy based on adipose tissue stem cell‐derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res. 2018;370(2):333‐342. [DOI] [PubMed] [Google Scholar]
- 37. Ferreira A, da Silva Cunha P, Carregal VM, et al. Extracellular vesicles from adipose‐derived mesenchymal stem/stromal cells accelerate migration and activate AKT pathway in human keratinocytes and fibroblasts independently of miR‐205 activity. Stem Cells Int. 2017;2017:9841035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xin Liao FYSH, Manshu Tang JCLY. Adipose mesenchymal stem cell sheets‐derived extracellular vesicles‐microRNA‐10b promote skin wound healing by elevating expression of CDK6. J Pre‐Proof. 2022;136:212781. [DOI] [PubMed] [Google Scholar]
- 39. Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes‐Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choudhery MS, Mahmood R, Harris DT, Ahmad FJ. Minimum criteria for defining induced mesenchymal stem cells. Cell Biol Int. 2022;46:986‐989. [DOI] [PubMed] [Google Scholar]
- 41. Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;2018:8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tang YT, Huang YY, Zheng L, et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med. 2017;40(3):834‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spiekman M, Przybyt E, Plantinga JA, Gibbs S, van der Lei B, Harmsen MC. Adipose tissue‐derived stromal cells inhibit TGF‐beta1‐induced differentiation of human dermal fibroblasts and keloid scar‐derived fibroblasts in a paracrine fashion. Plast Reconstr Surg. 2014;134(4):699‐712. [DOI] [PubMed] [Google Scholar]
- 44. Hong P, Yang H, Wu Y, Li K, Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther. 2019;10(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell‐free therapy. J Extracell Vesicles. 2018;7(1):1522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Suh A, Pham A, Cress MJ, et al. Adipose‐derived cellular and cell‐derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Res Rev. 2019;54:100933. [DOI] [PubMed] [Google Scholar]
- 47. Tian M, Ticer T, Wang Q, et al. Adipose‐derived biogenic nanoparticles for suppression of inflammation. Small. 2020;16(10):e1904064. [DOI] [PubMed] [Google Scholar]
- 48. Wang X, Jiao Y, Pan Y, et al. Fetal dermal mesenchymal stem cell‐derived exosomes accelerate cutaneous wound healing by activating notch signaling. Stem Cells Int. 2019;2019:2402916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kobayashi H, Ebisawa K, Kambe M, et al. Editors' choice effects of exosomes derived from the induced pluripotent stem cells on skin wound healing. Nagoya J Med Sci. 2018;80(2):141‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. An Y, Zhao J, Nie F, et al. Exosomes from adipose‐derived stem cells (ADSCs) overexpressing miR‐21 promote vascularization of endothelial cells. Sci Rep. 2019;9(1):12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yan B, Liu T, Yao C, Liu X, du Q, Pan L. LncRNA XIST shuttled by adipose tissue‐derived mesenchymal stem cell‐derived extracellular vesicles suppresses myocardial pyroptosis in atrial fibrillation by disrupting miR‐214‐3p‐mediated Arl2 inhibition. Lab Invest. 2021;101(11):1427‐1438. [DOI] [PubMed] [Google Scholar]
- 52. Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia‐treated human adipose‐derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF‐R. Int J Biochem Cell Biol. 2019;109:59‐68. [DOI] [PubMed] [Google Scholar]
- 53. Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR‐125a. J Cell Sci. 2016;129(11):2182‐2189. [DOI] [PubMed] [Google Scholar]
- 54. Xu F, Xiang Q, Huang J, et al. Exosomal miR‐423‐5p mediates the proangiogenic activity of human adipose‐derived stem cells by targeting Sufu. Stem Cell Res Ther. 2019;10(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang J, Yi Y, Yang S, Zhu Y, Hu X. Effects of adipose‐derived stem cell released exosomes on proliferation, migration, and tube‐like differentiation of human umbilical vein endothelial cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32(10):1351‐1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bellei B, Migliano E, Tedesco M, et al. Adipose tissue‐derived extracellular fraction characterization: biological and clinical considerations in regenerative medicine. Stem Cell Res Ther. 2018;9(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li C, Wei S, Xu Q, Sun Y, Ning X, Wang Z. Application of ADSCs and their exosomes in scar prevention. Stem Cell Rev Rep. 2022;18(3):952‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tang J, Yang J, Hu H, Cen Y, Chen J. miR‐211‐5p inhibits the proliferation, migration, invasion, and induces apoptosis of human hypertrophic scar fibroblasts by regulating TGFβR2 expression. Ann Transl Med. 2021;9(10):864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Qi J, Yangyang WU Y, Zhang H, Liu Y. LncRNA NORAD regulates scar hypertrophy via miRNA‐26a mediating the regulation of TGFβR1/2. Adv Clin Exp Med. 2021;30(4):395‐403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Encourages data sharing.


