Abstract
Ferroptosis is a newly discovered cell death type which is different from apoptosis, autophagy, pyroptosis as well as necrosis in the following aspects: morphology, biochemistry, gene and regulatory mechanisms. Ferroptosis is regulated by multiples of mechanisms such as system Xc− mechanism, glutathione peroxidase 4 (GPX4) mechanism, iron metabolism and lipid metabolism. Currently, ferroptosis has been revealed to be significant in wound healing such as diabetic wound, irradiated wound and ultraviolet (UV)‐driven wound. Hence, how to intervene in the pathogenesis as well as the development of wounds and promote the wound healing by the regulation of ferroptosis have become a research hotspot. This review systematically summarises the latest scientific advances of ferroptosis and wound healing fields, with hoping to propose a new insight and advance in the wound treatment.
Keywords: biomaterials, diabetic wound, ferroptosis, iron metabolism, lipid peroxidation, wound healing
1. INTRODUCTION
It has been widely accepted that wound is one of the most common and enduring clinical diseases or complications, which has burdened patients with huge economic costs and social interaction problems, especially chronic wounds. It has been estimated that the cost of chronic wounds care in the medical system is more than US $25 billion per year. 1 More importantly, with the growing number of patients who suffer with diabetes mellitus, the incidence of diabetic wound such as diabetic foot has experienced a great growth globally (>6.5 million yearly) and the cost of diabetic wound has been no less than US $50 000 every year. 2 Although numerous methods have been applied to overcome this clinical issue, the outcomes of wound healing are still poor and the exact mechanisms underlying wound healing are still unclarified. 3
In 2012, Dixon first coined the term ferroptosis, a unique iron‐dependent form of regulated cell death, which was different from apoptosis, autophagy, pyroptosis as well as necrosis in the following aspects: morphology, biochemistry, gene and regulatory mechanisms 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 (Table 1). Morphologically, unlike other four types of cell death, ferroptosis mainly shows as an apparent shrinkage and dwindled‐sized of mitochondria with increased membrane density as well as the reduction or loss of mitochondrial crista and outer membrane. Biochemically, ferroptosis mainly involves iron accumulation, lipid peroxidation, Feton reaction and cystine deprivation, which is different from other types of cell death.
TABLE 1.
Characteristics of ferroptosis, apoptosis, autophagy, pyroptosis and necroptosis
| Ferroptosis | Apoptosis | Autophagy | Pyroptosis | Necroptosis | |
|---|---|---|---|---|---|
| Discovery time | 2012 | 1972 | 1992 | 2009 | 2005 |
| Discoverer | Scott J. Dixon, USA | John F R Kerr, Andrew H Wyllie and Alastair R Currie, UK | Yoshinori Ohsumi, Japan | Tessa Bergsbaken, USA | Alexei Degterev, USA |
| Morphology |
|
|
|
|
|
| Biochemistry |
|
|
|
|
|
| Key genes | GPX4, ACSL4, TFRC, SLC7A11, SLC3A2, FSP1, ATM, MTF1, FPN1, NRF2, NCOA4, HSPB1, LPCAT3, ALOX12/15, JNK, p38, p53, NOXs, ATGs | Caspase‐3, Bcl‐2, Bak, Bax, Fas, ASK1, JNK, p38, p53 | ATGs, LC3, Beclin‐1, p62/SQSTM1, mTOR, AMPK, PI3K, VPS34, DRAM3, TFEB | Inflammatory caspase family, NLR family, Gasdermin family, NLRP1/3, p10, IL‐1β, IL‐18 | RIPK1, RIPK3, MLKL, TNFR1, Fas, TRADD, NOXs, RFK, HMOX‐1, TLR4, JNK |
| Regulatory mechanisms |
|
|
|
|
|
| Inducers | Erastin, Piperazine erastin, Imidazole ketone erastin, RSL3, Sorafenib, Sulfasalazine, Iron, Cyst(e)inase, DPI family, FIN56, FINO2, Artesunate, Cisplatin, Doxorubicin | Various drugs or compounds and diseases | Rapamycin, MG‐132, SB 203580, Cisplatin, Sorafenib, Olaparib | Inflammatory caspase family, Gasdermin family, Cisplatin, Metformin, Anthocyanin, DHA, Paclitaxel | Sorafenib, Artesunate, Shikonin, 5‐FU, Iron, Resibufogenin, Doxorubicin |
| Inhibitors | Ferrostatin‐1, Deferoxamine, Deferiprone, Ciclopirox, β‐mercaptoethanol, Cycloheximide, Liproxststatin‐1, Vitamin E, SRS11‐9, SRS16‐86, U0126 | Caspase family inhibitors | 3‐Methyladenine, Chloroquine, Cycloheximide, Bafilomycin A1, Resatorvid | Inflammatory caspase family inhibitors, Gasdermin family inhibitors | Necrostatin‐1 |
Abbreviations: ACSL4, acyl‐CoA synthetase long‐chain family member 4; ALOX12/15, arachidonate lipoxygenase 15; ATGs, autophagy‐related genes; COQ10, coenzyme Q10; ERK, extracellular signal‐regulated kinase; FSP1, ferroptosis suppressor protein 1; GPX4, glutathione peroxidase 4; HSPB1, heat shock protein family B (small) member 1; IRP1 and IRP2, iron‐regulatory proteins 1 and 2; Keap1, kelch‐like ECH‐associated protein 1; LPCAT3, lysophosphatidylcholine acyltransferase 3; MAPK, mitogen‐activated protein kinase; mTOR, mammalian target of rapamycin; NCOA4, nuclear receptor coactivator; NRF2, nuclear factor erythroid 2‐related factor4; PUFA‐PLs, polyunsaturated‐fatty‐acid–containing phospholipids; RSL3, RAS synthetic lethal 3.
Current studies have confirmed that the inhibition of ferroptosis can have an efficient therapeutical effectiveness on various diseases such as ischemia–reperfusion injury, cancer, stroke, neurodegenerative disorders, traumatic brain injury, lung fibrosis, liver fibrosis and acute kidney injury, etc. 22 In addition, many studies have showed that ferroptosis plays a significant role in the occurrence, development, treatment and prognosis of many types of wounds and has become the research hotspots in the field of burn and plastic surgery. Considering this, we reviewed and summarised the latest research progress on the relations between ferroptosis and wound to propose some new insights and advances for the improvement and promotion of wound healing.
2. THE PROCESS OF WOUND HEALING
Wound healing is a complex process that contains the repair of tissue injury, the restoration of skin barrier and the maintenance of skin integrity, involving the dynamic interactions of multiples of cells, extracellular matrix and cytokine. 23 Currently, it has been confirmed that the process of wound healing can be divided into four stages: haemostasis, inflammation, proliferation, remodelling and maturation. 24
On the first stage of wound healing, platelets adhere to exposed fibrinogen underneath the broken blood vessel endothelium, and begin to be activated to release adenosine diphosphate (ADP), thromboxane A2, prostaglandin 2‐α, 5‐hydroxytryptamine (5‐HT) and platelet factor IV. 25 Subsequently, the coagulation system is activated and fibrinogen is transformed to an insoluble fibrin network, producing blood clots. 26 Thus, damaged blood vessels are sealed and bleeding is stopped.
On the second stage of wound healing, attracted by such chemoattractive agents as tumour necrosis factor (TNF), interleukin‐1 (IL‐1), C3a, C5a and LTB4, inflammatory cells such as neutrophils, macrophages and lymphocytes begin to gather and accumulate in the wound sites, 27 which triggers a strong inflammatory reaction. Various inflammatory mediators such as vasoactive amines, arachidonic acid and its byproducts, platelet‐activating factor, cytokines, active oxygen, NO, neuropeptide and bradykinin, etc, are key materials that participate and mediate inflammatory reactions. The important roles of this stage of wound healing include combating infection, removing harmful and necrotic substances and laying proper foundations for proliferation process.
On the third stage of wound healing, bleeding is stopped as well as immune and inflammatory responses are in balance, continual injury is transformed to repair phase. Epithelialization occurs initially in repair phase, which depends on the integrity of the basement membrane. 28 Subsequently, regulated by vascular endothelial growth factor (VEGF), angiopoietin and fibroblast growth factor (FGF), endothelial cells start to proliferate and migrate to the capillary formation, inducing angiogenesis, which is significant for the proper wound healing. 29 At the same time, neogenetic myofibroblasts on the edge of the wound begin to migrate to the centre of the wound and secrete a huge amount of collagen as well as extracellular matrix (ECM), 30 with the purpose to diminish the size of the wounds. Hence, signalled by platelet‐derived growth factor (PDGF), epidermal growth factor (EGF), tissue growth factor (TGF)‐β, FGF, IL‐1 and TNF, capillary as well as fibroblasts proliferate quickly and more and more fibroblasts start to transform into myofibroblasts. Thus, granulation tissue are formed, which fill in the defects of injured tissue, indicating the completion of the repair process.
On the final stage of wound healing, granulation tissue start to transform to scar, which involves the processes of synthesis and degradation of collagen and ECM, 31 of which the key enzymes are matrix metalloproteinase (MMP) family. 32 MMP family can be divided into four types: interstitial collagenase, gelatinase or type IV collagenase, stromelysin and membrane type I matrix metalloproteinase (MT1‐MMP). Induced by PDGF, FGF, IL‐1, TNF‐α and phagocytosis, MMP can be secreted by fibroblasts, macrophages, neutrophils and epithelial cells to degrade collagen and ECM. However, this biological process is regulated strictly. All types of MMP are secreted in a non‐active form of zymogen and only can be activated by such chemical stimuli as HOCL and fibrinolysin. 33 Otherwise, activated MMP can be suppressed by tissue inhibitor of metalloproteinase (TIMP), which can by secreted by the majority of mesenchymal cells. 4 Clinically, the imbalance of this phase can cause improper wound healing. For example, patients with matrix deposition problems will suffer with the poor strength of the wound. And patients with excessive collagen synthesis are common with the problems of hypertrophic scar or keloid. 25
3. OVERVIEW OF FERROPTOSIS
It has been widely that system Xc− mechanism, GPX4 mechanism, iron mechanism and lipid mechanism are four main mechanisms of ferroptosis. In addition, there are other mechanisms such as transsulfuration mechanism, p62/Kelch‐like ECH‐associated protein 1 (Keap1)/nuclear factor erythroid 2‐related factor (NRF2) pathway and ATG5/ATG7/nuclear receptor coactivator 4 (NCOA4) pathway also playing important roles in ferroptosis (Figure 1).
FIGURE 1.
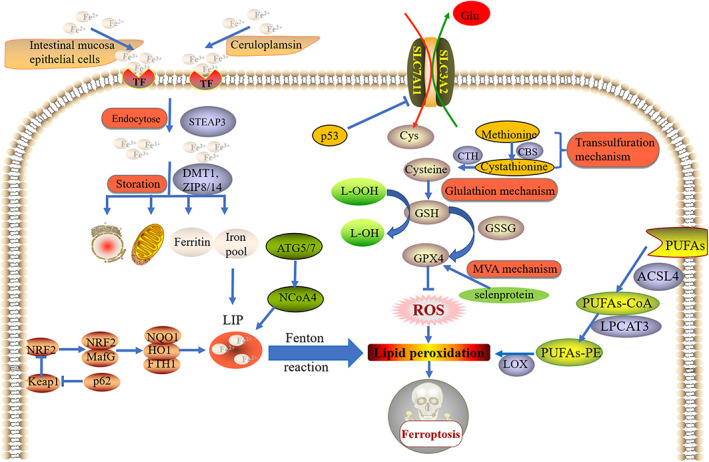
Mechanisms of ferroptosis. System Xc− mechanism, GPX4 mechanism, iron mechanism and lipid mechanism are four main mechanisms of ferroptosis. In addition, there are other mechanisms such as transsulfuration mechanism, p62/Keap1/NRF2 pathway and ATG5/ATG7/NCOA4 pathway also playing important roles in ferroptosis
3.1. System Xc−
System Xc− is an amino acid antitransporter, also called cystine/glutamate exchange system, which is mostly distributed in membrane phospholipids. System Xc− is a necessary antioxidant system and is consisted of two subunits (SLC7A11 and SLC3A2). 34 Normally, system Xc− could exchange extracellular cystine and intracellular glutamate at a ratio of 1:1, which is essential for the synthesis of GSH. 35 Driven by glutathione peroxidases (GPXs), GSH is important for the reductions of ROS and reactive nitrogen, maintaining the intracellular redox balance. As ferroptosis inducers, erastin, sorafenib and sulfasalazine could induce ferroptosis via the suppression of system Xc−, which causes the impediment of the absorption of cystine, leading to the decrease of GPXs activity, the lethal accumulation of lipid ROS and ultimately the oxidative damage. 22 Moreover, p53, one of the key genes regulating ferroptosis, can inhibit the functions of system Xc− through the downregulation of the expression of SLC7A11, which affects the normal structure of system Xc−, leading to the cystine deprivation, cellular redox balance disruption and the accumulation of lipid ROS, thereby inducing ferroptosis. 36
3.2. GPX4
As one of the members of GPX family, GPX4 is the key gene of the occurrence and development of ferroptosis, which mainly acts its guardian role by converting GSH into oxidised glutathione (GSSG), thus reducing the cytotoxic lipid peroxides (L‐OOH) to the non‐cytotoxic alcohols (L‐OH). 37 Therefore, the inhibition of GPX4 activity or the suppression of GPX4 expression can cause ROS accumulation and lipid peroxidation, thus inducing ferroptosis. Such ferroptosis inducers as RSL3, altretamine, DPIs, FIN56, JKE‐1674, ML162 and ML210 have been confirmed to induce ferroptosis by inhibiting GPX4 activity. In addition, various studies have revealed that cells (BJ‐fibroblast‐derived cells, HT‐1080 cells) with down‐regulated GPX4 expression or GPX4 knockdown are more sensitive to ferroptosis compared with control cells. 38 , 39 Moreover, it has been accepted that selenoprotein plays a pivotal role in the synthesis of GPX4 and protecting GPX4 from irreversible inactivation. 40 To ensure GPX4 can exert its proper functions, the metabolisms of selenoprotein are strictly regulated by mevalonate (MVA) pathway. Down‐regulation of MVA pathway is responsible for the impediment of the synthesis of selenoprotein, thus inhibiting GPX4 activity and inducing ferroptosis. 22 , 40
3.3. Iron metabolism
There are two forms of iron in the body include Fe2+ and Fe3+. In the normal status, the content and distribution of Fe2+ and Fe3+ are in homeostasis and some pathological conditions will be induced if this homeostasis is interrupted. 41 Intestinal absorption and erythrocyte degradation are the two main sources of Fe2+. On one side, Fe2+ absorpted from food can be oxidised by intestinal mucosa epithelial cells to Fe3+ and binds to transferrin (TF) to form TF–Fe3+. On the other side, Fe2+ from erythrocyte degradation can be oxidised by ceruloplasmin to Fe3+ and binds to TF to form TF–Fe3+. After the endocytose of TF–Fe3+, Fe3+ is reduced to Fe2+ by six‐transmembrane epithelial antigen of the prostate 3 (STEAP3), and Fe2+ can be stored in mitochondria, endoplasmic reticulum, ferritin and iron pool, of which the processes are strictly regulated by divalent metal transporter 1 (DMT1) and Zinc‐Iron regulatory protein family 8/14 (ZIP8/14). 42 Once the iron metabolism is disordered, Fe2+ stored in mitochondria, endoplasmic reticulum and ferritin will be released and iron pool will convert to unstable iron pool (LIP), which leads to the excessive content of Fe2+ in cytoplasm. 43 Then, iron accumulation induces Fenton peroxidation and lipid ROS accumulation, which triggers ferroptosis.
3.4. Lipid metabolism
Lipid peroxidation is essential for ferroptosis and lipid peroxidation is closely related to lipid metabolism. Lipid metabolism participate in the process of lipid peroxidation and ferroptosis mainly by transforming free polyunsaturated fatty acids (PUFAs) to PUFAs‐phosphatidylethanolamine (PUFAs‐PE). 44 Free PUFAs is widely distributed phospholipid bilayers and functioned as an esterified form, which has nothing to do with ferroptosis. However, PUFAs‐PE involves arachidonic acid (AA) and its derivative adrenaline, which are important substrates of lipid peroxidation. 45 Studies have revealed that acyl‐CoA synthetase long‐chain family member 4 (ACSL4) as well as lysophosphatidylcholine acyltransferase 3 (LPCAT3) are the key enzymes of lipid metabolism. First, ACSL4 combines PUFAs with CoA, thus producing PUFAs‐CoA. Subsequently, LPCAT3 combines PUFAs‐CoA with PE, thus producing PUFAs‐PE. Finally, catalysed by lipoxygenase (LOX), PUFAs‐PE can be oxidised, contributing to lipid peroxidation and thereby inducing ferroptosis. 46 Therefore, the expressions of ACSL4 in those ferroptosis‐sensitive cells such as HepG2 and HL60 are higher than that in such ferroptosis‐resistant cells as LNCaP as well as K562. 47 In addition, compared with control MLE cells, LPCAT3 knockdown MLE cells are resistant to RSL3‐induced ferroptosis, showing lower rate of cell death. 48
3.5. Other mechanisms
There are other mechanisms regulating the occurrence and development of ferroptosis. Apart from system Xc− mechanism, transsulfuration mechanism is another important source of cysteine for GSH. 49 Cystathionine‐β‐synthase (CBS) and cystathionine‐γ‐lyase (CTH) are key enzymes of transsulfuration mechanism. Catalysed by CBS, methionine can be converted to cystathionine. Subsequently, CTH promotes the transformation from cystathionine to cysteine, thus providing the essential substrate for GSH generation. 49 , 50 In addition, activating p62/Keap1/NRF2 pathway is demonstrated to fight against ferroptosis via the functions of various enzymes such as quinone oxidoreductase 1 (NQO1), heme oxygenase‐1 (HO1) and ferritin heavy chain 1 (FTH1). 51 Otherwise, investigations have found that autophagy can contribute to ferroptosis by degrading ferritin, increasing intercellular iron levels, and promoting Fenton reaction through ATG5/ATG7/NCOA4 pathway. 52 , 53 , 54
4. RELATIONSHIP BETWEEN FERROPTOSIS AND WOUND
4.1. Diabetic wound
Diabetic wound always has some problems of iron metabolism such as reduced iron levels and dysregulated expression of iron genes, which may delay ECM deposition. Exogenous iron administration can promote ECM deposition of collagen type I and collagen type III. However, the knockdown of STEAP3 leads to impaired ECM deposition. 55 In addition, iron stored in FPN of skin macrophages is of significance for wound healing and FPN deletion in skin macrophages can impair wound healing by the compromises of angiogenesis and stromal cells proliferation. 56 Apart from abnormal iron metabolism, excessive oxidative and lipid peroxidation are also commonly observed in diabetic wound. For example, Li, et al. 57 has found that compared with control groups, the levels of ROS, malondialdehyde (MDA) and lipid peroxidation of fibroblasts as well as vascular endothelial cells that exposed to high glucose conditions are elevated obviously, and the cells display reduced survival rate and impaired migration. In addition, they also have revealed that ferroptosis is strongly induced in diabetic wound both in vitro and vivo aspects, which are confirmed by low level of GPX4, SLC7A11 expressions and high level of TFRC.
Moreover, various studies have demonstrated that ferroptosis inhibitors can promote diabetic wound by inhibiting ferroptosis. For example, the local application of ferrostatin‐1 (ferroptosis inhibitor) can accelerate the rat diabetic wound healing by inhibiting ferroptosis through the upregulation of PI3K/Akt signalling pathway. 57 In addition, many investigations have showed that the administration of DFO, another type of ferroptosis inhibitors, can promote the diabetic wound healing. Gao, et al. 58 have found that the co‐application of DFO and hydroxysafflor yellow A in hydrogel can accelerate rat diabetic wound healing via enhanced angiogenesis. Hopfner U, et al. 59 have found that DFO can promote the regenerative capacity of adipose‐derived stem cells (ADSCs) in rat diabetic wound via the enhancement of hypoxia‐inducible factor‐α (HIF‐α) and VEGF expressions. Furthermore, the combined applications of DFO and biomaterials also have been showed to have an efficient therapeutic effectiveness on diabetic wound. Kong, et al. 60 have showed that the application of injectable hydrogel combining with DFO and bioglass containing Si ions can promote the repair of diabetic wound by enhancing revascularization. In addition, Chen, et al. 61 have found that the sustained release of DFO from hydrogel nanofibrous scaffolds can enhance angiogenesis in diabetic wound by upregulating the expressions of HIF‐1α, VEGF and SDF‐1α. Dominik Duscher, et al. 62 have revealed that the transdermal delivery of DFO can also significantly accelerate diabetic wound healing by promoting angiogenesis via the upregulation of HIF‐1α as well as VEGF expressions. Furthermore, this novel DFO delivery method can overcome such disadvantages of DFO as potential toxicity and short plasma half‐life.
4.2. Irradiated wound
Irradiated wound is one of the common skin lesions in clinical, which mainly caused by irradiation therapies of tumours and unexpected irradiation accidents. Excessive irradiation can impair local vessels and cause angiosclerosis by damaging the structures and activities of proteins and DNA, thus delaying the wound healing. 63 Gan, et al. 64 have demonstrated that the local injection of plasma‐derived exosomes (RP‐Exos) can promote the healing process of rat irradiated wounds by boosting the proliferation, migration, cycle progression as well as survival rate of fibroblasts. They also have found that RP‐Exos can disrupt ferroptosis pathway, thus inhibiting ferroptosis in irradiated fibroblasts. In addition, various investigations have confirmed that irradiation can trigger cell death by inducing ferroptosis. For example, Stockwell, et al. 65 have found that applying Cs‐137 gamma radiation to intervene in HT‐1080 cells can trigger cell death by inducing ferroptosis, which can be reversed by ferroptosis inhibitors such as sorafenib and ferrostatin‐1. Yuki Shibata, et al. 66 also have found that compared with normal cells, human cervical adenocarcinoma cells and lung adenocarcinoma cells that are irradiated by x‐ray are more sensitive to ferroptosis, which can be reversed by erastin. Moreover, Khorsandi, et al. 67 have revealed that the laser irradiation can cause ROS accumulation in breast cancer cells and melanoma cells.
4.3. UV‐driven wound
Apart from irradiation treatments for tumour, excessive ultraviolet (UV) exposure, especially UVA and UVB, can also cause UV‐driven cutaneous wound. Kavita Vats, et al. 68 have showed that excessive UVB exposure can initiate the inflammation and cell death of human keratinocytes by inducing ferroptosis, which can be inhibited by ferrostatin‐1. Moreover, excessive UV exposure can cause GSH deprivation, thereby disrupting redox homeostasis. Feng, et al. 69 have showed that in UV‐driven wounds, the content of GSH is lower than normal so that GPX4 mechanism is comprised, thus ferroptosis is induced. However, this pathological process can be alleviated by GSH recruitment using nicotinamide mononucleotide. 69 Furthermore, excessive UV exposure can also lead to ROS accumulation in skin, thus inducing ferroptosis by lipid peroxidation mechanism. 70 Taken together, excessive UV exposure can induce ferroptosis by GSH deprivation mechanism, GPX4 mechanism and lipid peroxidation mechanism. Hence, in UV‐driven wound, caused by frequent ferroptosis, weak capacity to keep redox homeostasis and high rate of cell death led to the impairment of DNA repair and dysregulation of wound healing‐related genes (e.g. VEGF, FGF, PDGF and MMPs, etc), thus delaying the healing processes of UV‐driven wound.
5. CONCLUSIONS AND PROSPECTS
In summary, wound healing, especially chronic and refractory wounds healing, is an extremely complex process with the involvement of multitudes of mechanisms. As an emerging cell death mechanism, ferroptosis has been found to have strong relationships with wound healing. Taken together, abnormal contexts of iron, ROS and oxidative stress are the common features of both wound healing and ferroptosis, and ferroptosis inducing can be observed in different degrees in different types of wounds. Furthermore, inhibiting ferroptosis by ferroptosis inhibitors such as ferrostatin‐1, DFO and sorafenib or dysregulating ferroptosis‐related mechanisms has been confirmed to have an efficient acceleration and boost on wound healing. With the developments of engineering and biotechnology, biomaterials are regarded as a promising therapy for wound healing. Ferroptosis inhibitors‐loaded biomaterials will potentially serve as a novel strategy for wound care and treatment.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Bi M, Li D, Zhang J. Research progress and insights on the role of ferroptosis in wound healing. Int Wound J. 2023;20(6):2473‐2481. doi: 10.1111/iwj.14102
DATA AVAILABILITY STATEMENT
Data is available.
REFERENCES
- 1. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brem H, Stojadinovic O, Diegelmann RF, et al. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007;13(1–2):30‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beyene RT, Derryberry SL Jr, Barbul A. The effect of comorbidities on wound healing. Surg Clin North Am. 2020;100(4):695‐705. [DOI] [PubMed] [Google Scholar]
- 4. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron‐dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen PH, Wu J, Ding CC, et al. Kinome screen of ferroptosis reveals a novel role of ATM in regulating iron metabolism. Cell Death Differ. 2020;27(3):1008‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35(6):600‐604. [DOI] [PubMed] [Google Scholar]
- 7. Shen J, Cheng J, Zhu S, et al. Regulating effect of baicalin on IKK/IKB/NF‐kB signaling pathway and apoptosis‐related proteins in rats with ulcerative colitis. Int Immunopharmacol. 2019;73:193‐200. [DOI] [PubMed] [Google Scholar]
- 8. Lee S, Hirohama M, Noguchi M, Nagata K, Kawaguchi A. Influenza a virus infection triggers Pyroptosis and apoptosis of respiratory epithelial cells through the type I interferon signaling pathway in a mutually exclusive manner. J Virol. 2018;92(14):e00396‐e00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang YY, Yu W, Zhou B. Hippo signaling pathway in cardiovascular development and diseases. Yi Chuan. 2017;39(7):576‐587. [DOI] [PubMed] [Google Scholar]
- 10. Vegliante R, Ciriolo MR. Autophagy and Autophagic cell death: uncovering new mechanisms whereby Dehydroepiandrosterone promotes beneficial effects on human health. Vitam Horm. 2018;108:273‐307. [DOI] [PubMed] [Google Scholar]
- 11. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lage SL, Amarante‐Mendes GP, Bortoluci KR. Evaluation of pyroptosis in macrophages using cytosolic delivery of purified flagellin. Methods. 2013;61(2):110‐116. [DOI] [PubMed] [Google Scholar]
- 13. Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821‐832. [DOI] [PubMed] [Google Scholar]
- 14. Jia C, Zhang J, Chen H, et al. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis. 2019;10(10):778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu L, Chen M, Chen X, et al. Chemotherapy‐induced pyroptosis is mediated by BAK/BAX‐caspase‐3‐GSDME pathway and inhibited by 2‐bromopalmitate. Cell Death Dis. 2020;11(4):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li D, Ren W, Jiang Z, Zhu L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol Med Rep. 2018;18(5):4399‐4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury [published correction appears in Nat Chem Biol. 2005;1(4):234]. Nat Chem Biol. 2005;1(2):112‐119. [DOI] [PubMed] [Google Scholar]
- 18. Karki R, Sharma BR, Tuladhar S, et al. Synergism of TNF‐α and IFN‐γ triggers inflammatory cell death, tissue damage, and mortality in SARS‐CoV‐2 infection and cytokine shock syndromes. Cell. 2021;184(1):149‐168.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fu G, Wang B, He B, Feng M, Yu Y. LPS induces cardiomyocyte necroptosis through the Ripk3/Pgam5 signaling pathway. J Recept Signal Transduct Res. 2021;41(1):32‐37. [DOI] [PubMed] [Google Scholar]
- 20. Seong D, Jeong M, Seo J, et al. Identification of MYC as an antinecroptotic protein that stifles RIPK1‐RIPK3 complex formation. Proc Natl Acad Sci U S A. 2020;117(33):19982‐19993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lou X, Zhu H, Ning L, et al. EZH2 regulates intestinal inflammation and necroptosis through the JNK signaling pathway in intestinal epithelial cells. Dig Dis Sci. 2019;64(12):3518‐3527. [DOI] [PubMed] [Google Scholar]
- 22. Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang M, Xu S, Du C, et al. Novel PLCL nanofibrous/keratin hydrogel bilayer wound dressing for skin wound repair [published online ahead of print, 2022 Dec 28]. Colloids Surf B Biointerfaces. 2022;222:113119. [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Yang X, Liu Y, et al. NRF2 signalling pathway: new insights and progress in the field of wound healing [published online ahead of print, 2021 Jun 18]. J Cell Mol Med. 2021;25(13):5857‐5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Liu W, Wang Q. Positive effects of low‐dose photodynamic therapy with aminolevulinic acid or its methyl ester in skin rejuvenation and wound healing: an update [published online ahead of print, 2023 Jan 5]. J Biophotonics. 2023:e202200293. [DOI] [PubMed] [Google Scholar]
- 26. Seidel D, Bunse J. Der postoperative Wundinfekt: diagnose, Klassifikation und Behandlung [postoperative wound infections: diagnosis, classification and treatment]. Chirurg. 2017;88(5):385‐394. [DOI] [PubMed] [Google Scholar]
- 27. Gantwerker EA, Hom DB. Skin: histology and physiology of wound healing. Facial Plast Surg Clin North Am. 2011;19(3):441‐453. [DOI] [PubMed] [Google Scholar]
- 28. Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ. Wound healing. J Chin Med Assoc. 2018;81(2):94‐101. [DOI] [PubMed] [Google Scholar]
- 29. Kogan S, Sood A, Garnick MS. Zinc and wound healing: a review of zinc physiology and clinical applications. Wounds. 2017;29(4):102‐106. [PubMed] [Google Scholar]
- 30. Kim SY, Nair MG. Macrophages in wound healing: activation and plasticity. Immunol Cell Biol. 2019;97(3):258‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pasaribu KM, Ilyas S, Tamrin T, et al. Bioactive bacterial cellulose wound dressings for burns with collagen in‐situ and chitosan ex‐situ impregnation [published online ahead of print, 2023 Jan 1]. Int J Biol Macromol. 2023;230:123118. [DOI] [PubMed] [Google Scholar]
- 32. Tejada S, Manayi A, Daglia M, et al. Wound healing effects of curcumin: a short review. Curr Pharm Biotechnol. 2016;17(11):1002‐1007. [DOI] [PubMed] [Google Scholar]
- 33. Kasuya A, Tokura Y. Attempts to accelerate wound healing. J Dermatol Sci. 2014;76(3):169‐172. [DOI] [PubMed] [Google Scholar]
- 34. Xia X, Fan X, Zhao M, Zhu P. The Relationship between Ferroptosis and tumors: a novel landscape for therapeutic approach. Curr Gene Ther. 2019;19(2):117‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Conrad M, Sato H. The oxidative stress‐inducible cystine/glutamate antiporter, system x c −: cystine supplier and beyond. Amino Acids. 2012;42(1):231‐246. [DOI] [PubMed] [Google Scholar]
- 36. Jiang L, Kon N, Li T, et al. Ferroptosis as a p53‐mediated activity during tumour suppression. Nature. 2015;520(7545):57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hirschhorn T, Stockwell BR. The development of the concept of ferroptosis. Free Radic Biol Med. 2019;133:130‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron‐dependent, nonapoptotic cell death in oncogenic‐RAS‐harboring cancer cells. Chem Biol. 2008;15(3):234‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Friedmann Angeli JP, Conrad M. Selenium and GPX4, a vital symbiosis. Free Radic Biol Med. 2018;127:153‐159. [DOI] [PubMed] [Google Scholar]
- 41. Dev S, Babitt JL. Overview of iron metabolism in health and disease. Hemodial Int. 2017;21(Suppl 1):S6‐S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hassannia B, Vandenabeele P, Vanden BT. Targeting Ferroptosis to iron out cancer. Cancer Cell. 2019;35(6):830‐849. [DOI] [PubMed] [Google Scholar]
- 43. Latunde‐Dada GO. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 2017;1861(8):1893‐1900. [DOI] [PubMed] [Google Scholar]
- 44. Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26(3):165‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Su LJ, Zhang JH, Gomez H, et al. Reactive oxygen species‐induced lipid peroxidation in apoptosis, autophagy, and Ferroptosis. Oxidative Med Cell Longev. 2019;2019:5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tan X, Huang X, Niu B, Guo X, Lei X, Qu B. Targeting GSTP1‐dependent ferroptosis in lung cancer radiotherapy: existing evidence and future directions. Front Mol Biosci. 2022;9:1102158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478(3):1338‐1343. [DOI] [PubMed] [Google Scholar]
- 48. Kagan VE, Mao G, Qu F, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McBean GJ. The transsulfuration pathway: a source of cysteine for glutathione in astrocytes. Amino Acids. 2012;42(1):199‐205. [DOI] [PubMed] [Google Scholar]
- 50. The Latest View on the Mechanism of Ferroptosis and Its Research Progress in Spinal Cord Injury. [DOI] [PMC free article] [PubMed]
- 51. Sun X, Ou Z, Chen R, et al. Activation of the p62‐Keap1‐NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hou W, Xie Y, Song X, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509(7498):105‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nakamura T, Naguro I, Ichijo H. Iron homeostasis and iron‐regulated ROS in cell death, senescence and human diseases. Biochim Biophys Acta Gen Subj. 2019;1863(9):1398‐1409. [DOI] [PubMed] [Google Scholar]
- 55. Wilkinson HN, Upson SE, Banyard KL, Knight R, Mace KA, Hardman MJ. Reduced iron in diabetic wounds: an oxidative stress‐dependent role for STEAP3 in extracellular matrix deposition and remodeling. J Invest Dermatol. 2019;139(11):2368‐2377.e7. [DOI] [PubMed] [Google Scholar]
- 56. Recalcati S, Gammella E, Buratti P, et al. Macrophage ferroportin is essential for stromal cell proliferation in wound healing. Haematologica. 2019;104(1):47‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li S, Li Y, Wu Z, Wu Z, Fang H. Diabetic ferroptosis plays an important role in triggering on inflammation in diabetic wound. Am J Physiol Endocrinol Metab. 2021;321(4):E509‐E520. [DOI] [PubMed] [Google Scholar]
- 58. Gao SQ, Chang C, Li JJ, et al. Co‐delivery of deferoxamine and hydroxysafflor yellow a to accelerate diabetic wound healing via enhanced angiogenesis. Drug Deliv. 2018;25(1):1779‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hopfner U, Maan ZN, Hu MS, et al. Deferoxamine enhances the regenerative potential of diabetic adipose derived stem cells. J Plast Reconstr Aesthet Surg. 2020;73(9):1738‐1746. [DOI] [PubMed] [Google Scholar]
- 60. Kong L, Wu Z, Zhao H, et al. Bioactive injectable hydrogels containing Desferrioxamine and bioglass for diabetic wound healing. ACS Appl Mater Interfaces. 2018;10(36):30103‐30114. [DOI] [PubMed] [Google Scholar]
- 61. Chen H, Jia P, Kang H, et al. Upregulating Hif‐1α by hydrogel Nanofibrous scaffolds for rapidly recruiting angiogenesis relative cells in diabetic wound. Adv Healthc Mater. 2016;5(8):907‐918. [DOI] [PubMed] [Google Scholar]
- 62. Duscher D, Neofytou E, Wong VW, et al. Transdermal deferoxamine prevents pressure‐induced diabetic ulcers. Proc Natl Acad Sci U S A. 2015;112(1):94‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ye LF, Chaudhary KR, Zandkarimi F, et al. Radiation‐induced lipid peroxidation triggers Ferroptosis and synergizes with Ferroptosis inducers. ACS Chem Biol. 2020;15(2):469‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gan F, Wang R, Lyu P, et al. Plasma‐derived exosomes boost the healing of irradiated wound by regulating cell proliferation and Ferroptosis. J Biomed Nanotechnol. 2021;17(1):100‐114. [DOI] [PubMed] [Google Scholar]
- 65. Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shibata Y, Yasui H, Higashikawa K, Miyamoto N, Kuge Y. Erastin, a ferroptosis‐inducing agent, sensitized cancer cells to X‐ray irradiation via glutathione starvation in vitro and in vivo. PLoS One. 2019;14(12):e0225931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Khorsandi K, Kianmehr Z, Hosseinmardi Z, Hosseinzadeh R. Anti‐cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int. 2020;20:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vats K, Kruglov O, Mizes A, et al. Keratinocyte death by ferroptosis initiates skin inflammation after UVB exposure [published online ahead of print, 2021 Sep 25]. Redox Biol. 2021;47:102143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Feng Z, Qin Y, Huo F, et al. NMN recruits GSH to enhance GPX4‐mediated ferroptosis defense in UV irradiation induced skin injury [published online ahead of print, 2021 Oct 6]. Biochim Biophys Acta Mol basis Dis. 2021;1868(1):166287. [DOI] [PubMed] [Google Scholar]
- 70. Gao Y, Hou Q, Guo R, Ying J, Xiong J, Jiang H. Effect of Sun exposure‐induced ferroptosis mechanisms on pathology and potential biological processes of primary melanoma by microarray data analysis. Front Genet. 2022;13:998792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available.


