Abstract
A new recombinant proteolytic enzyme, isolated from maggot saliva, with fibrinolytic action has been investigated through a series of non‐clinical toxicology and in‐vitro/in‐vivo pharmacology studies to explore its potential safety and efficacy as an enzymatic debridement agent for use in chronic wounds. Studies indicate that the enzyme has a good safety profile. When locally administered, it is not detrimental to wound healing, is non‐sensitising and is rapidly inactivated in the systemic circulation. Adverse effects are limited, at very high concentrations, to transient erythema at the site of application. In‐vitro testing indicates that the enzyme, whilst selective for fibrin, has additional proteolytic action against collagen and elastin, with enzymatic action for all three substrates being dose dependent. In‐vivo, we used an established MRSA biofilm model, in which microbiological counts were used as a surrogate for debridement efficacy. Here, we showed that higher concentrations of the enzyme in a formulated proprietary gel, significantly reduced MRSA counts over a period of 2 to 14 days, and significantly improved the vascularity of the wound at 14 days. Together, these data support the potential for this maggot‐derived proteolytic enzyme as a clinically effective debriding agent.
Keywords: chronic, debridement, pharmacology, toxicology, wounds
1. INTRODUCTION
Maggots, notably of the green bottle fly Lucilia sericata, have been clinically recognised for their ability to debride wounds for centuries 1 and result in an improved rate of wound healing. 2 The logistics associated with delivering Maggot Debridement Therapy (MDT), combined with patient antithesis towards using maggots in open wounds has however limited the viability of MDT as a routine means of debridement for chronic wounds. Clinical practice is therefore largely limited to slow autolytic debridement, fast but painful sharp or mechanical debridement (also requiring expert clinical staff) or existing proteolytic enzymes (eg, collagenase, bromelain, papain, urea, papain‐urea etc.). The latter are only marginally effective, have none‐specific effects (ie, resulting in digestion of both eschar and granulation tissue) and/or have resulted in sensitization and/or significant localised adverse effects (eg, pain).
The debridement efficacy of MDT is considered to be the consequence of two primary mechanisms of action; mechanical (achieved by mouth hooks and/or rough bodies that scratch wound surfaces) and specific proteolytic enzymes 2 expressed within maggot saliva and which have evolved over millennia to allow effective digestion of wound debris, slough and eschar by maggots. In turn, these allow the maggot to feed off the liquefied material (also known as alimentary secretion and excretions, ASE). 3 In‐vitro and in‐vivo evidence with BioBag (Biomonde), 4 , 5 , 6 , 7 , 8 which employs sterile maggots in a bag (thereby negating the mechanical effects of maggots) supports the hypothesis that maggot‐expressed proteolytic enzymes can facilitate debridement directly without the need of direct contact with the larvae. 8
Using a biomimicry approach, we have identified a key proteolytic enzyme (25 kDa) expressed in maggot saliva and responsible for eschar digestion, we have sequenced the gene responsible for its production, and cloned this into a Pichia pastoris yeast. The resulting recombinant enzyme, “aurase,” has been used in an aqueous solution (for in‐vitro experiments and in‐vivo intravenous studies) and within a proprietary gel (for topical delivery within in‐vivo studies) to assess its potential safety and efficacy for use as a topically applied clinical wound debridement agent.
2. MATERIALS AND METHODS
2.1. Animals
Animal toxicology studies were performed using freshly prepared aqueous solutions of aurase (for intravenous studies) or aurase within a proprietary hydrogel for topical studies to wounded skin.
All in‐vivo animal studies were approved by the institutional animal care committees. Toxicology (safety) studies, utilising Sprague Dawley rats, and Gottingen mini‐pigs were performed in the UK by Covance Ltd or Aurigon Toxicological Research Centre Ltd, Hungary whilst sensitisation studies were performed in Germany by Bioserv Analytik und Medizinprodukte GmbH, all in accordance with the principles of OECD Good Laboratory Practice (GLP) and with the requirements of national regulations.
The in‐vivo porcine experiments performed for efficacy were performed at the University of Miami, Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery, according to the department's Standard Operating Procedures. The study was also approved by the Animal Use Committee.
2.2. Evaluation of the mechanism of deactivation
To explore potential mechanisms of deactivation of aurase arising from absorption into the systemic circulation, we evaluated the action of three key endogenous serine protease inhibitors (Serpins); alpha‐2 macroglobulin (A2M), alpha‐2 antiplasmin (A2AP) and alpha‐1 antitrypsin (A1AT) all obtained from Sigma‐Aldrich, UK. In these experiments, aurase was incubated at 1, 2, 5, 15, 60, 240 minutes or 16 hours in 2 M excess of the respective serpin and then subjected to analysis by SDS PAGE (with and without dithiothreitol [DTT]—Applichem) and employing a heat denaturation step (8 minutes at 100°C) in the presence of SDS (sodium dodecyl sulphate). Incubated samples were additionally subjected to a chromogenic bioassay used to determine the protease activity of aurase. In this assay, the catalytic activity was determined by cleaving the substrate Z‐Gly‐Gly‐Arg‐βNA directly after the arginine residue. Therefore, the increase of the released β‐naphthylamine (βNA) was measured spectrophotometrically over 2 minutes by kinetic measurement of the optical density at 335 nm in 5‐second intervals. The proteolytic activity was defined as the amount of substrate (Z‐Gly‐Gly‐Arg‐βNA) in μmol that is converted per minute per enzyme amount or volume.
2.3. Evaluation of in‐vitro efficacy
A disk of fluorescent artificial wound eschar (fAWE) was prepared composed of collagen‐FITC, fibrin‐coumarin, elastin‐rhodamine and fibrinogen and then clotted with thrombin. In summary, fluorescent fibrin‐coumarin was prepared by labelling fibrinogen from bovine plasma (Sigma‐Aldrich) with 7‐Hydroxycoumarin‐3‐carboxylic acid N‐succinimidyl ester (Sigma‐Aldrich), followed by conversion with thrombin from bovine plasma (Sigma‐Aldrich). The final solid was washed, dried, ground and weighed. Artificial wound eschar (AWE) was then prepared by mixing 650 mg collagen‐FITC, 100 mg fibrin‐coumarin and 100 mg elastin rhodamine thoroughly in 10 mL Tris buffer for 10 minutes. The fibrinogen solution was prepared by dissolving 150 mg fibrinogen in 10 mL Tris buffer. The two prepared solutions were mixed thoroughly and 0.5 mL thrombin at 50 U/mL was added to the solution and quickly mixed. The mixture was poured into a petri dish containing a 90 mm Nylon membrane filter. After 30 minutes clotting, the AWE substrate was rinsed with deionised water for 15 minutes to remove any unreacted thrombin. Excess water was removed by tissue paper.
All incubations were performed in 6 well cell culture plates at 37°C and 30 rpm. Samples were collected by taking 200 μL solution from the 6 well plates then adding 200 μL Tris‐buffer to the 6 well plates. The collected samples were added into a black flat‐bottomed 96 well plate (n = 3), reading at 485‐20/520‐20 nm (collagen‐FITC), 360‐40/460‐40 nm (fibrin‐coumarin) and 530‐20/590‐20 nm (elastin‐rhodamine).
2.4. In‐vivo wound efficacy
An in‐vivo efficacy study using pigs was selected because of the established morphological similarities between swine skin and human skin 9 and the prior development of methodology to create sloughy wounds in pigs containing an MRSA biofilm. 10
Specifically, two young specific pathogen free pigs (one male and one female) weighing 35 to 45 kg were used in the study and kept in‐house for at least 5 days prior to initiating the experiment. The backs of the animals were clipped with standard animal clippers on the day of wounding. The skin on both sides of each animal was prepared by washing with a non‐antibiotic soap and sterile water. Each animal was anesthetised and given analgesics until the end of the study. Thirty‐three deep reticular wounds measuring 22 mm × 22 mm × 3 mm deep were made in the paravertebral and thoracic area of the animals with a specialised electrokeratome fitted with a 22 mm blade. Each wound was separated from another by 5 to 7 cm of unwounded skin. Within 20 minutes of wounding, an inoculum of 25 μL of 1010 Methicillin Resistant Staphylococcus aureus (MRSA USA 300) diluted into 35 mL of Tryptic Soy Broth (TSB) was introduced into the centre of each wound site and covered with an occlusive dressing (Tegaderm, 3 M) for 3 days to form a sloughy wound, containing a MRSA biofilm.
After 3 days, treatment was commenced with a low dose aurase (0.05 g/L) wound gel, a medium dose aurase (0.125 g/L) wound gel, a high‐dose aurase (0.25 g/L) wound gel, as well as a placebo control (hydrogel + diluent solution) and an untreated Tegaderm control. Each wound received 1.2 mL per wound (equivalent to ~0.19 mL/cm2) to mimic the application rate to humans when calculating for equivalent body surface areas. On Days 2, 4, 7, 10 and 14 before treatment application or recovery, wounds were gently wiped with moisturised sterile PBS gauze. Wounds were re‐treated and re‐dressed with the occlusive Tegaderm dressing.
Because of the small size of each wound, microbiological counts were used as a surrogate for debridement efficacy in line with previous findings in which the level of debridement correlates with the decrease in pathogen counts. 11 Therefore, on days 2 and 14 (after treatment application), three wounds were biopsied for microbiology enumeration from each treatment group and immediately placed in 1 mL of “All‐Purpose Neutralising Solution.” The sample was combined with an additional 4 mL of Neutralising Solution and homogenised in a sterile homogenisation tube. Serial dilutions were made, and scrub solutions were quantified using the Spiral Plater System, which deposits a small defined amount (50 μL) of suspension over the surface of a rotating agar plate. ORSAB selective media was used for count of MRSA USA300 present in the Neutralising Solution. After plating, all samples were incubated aerobically for 48 hours at 37°C. After the incubation period, colonies on the plates were counted and the CFU/mL calculated.
Histological samples collected from biopsies were fixed in formalin, processed and stained by haematoxylin and eosin for histological analyses.
2.5. Statistical analysis
Data were combined and statistical analysis for significance using a one‐way ANOVA (IBM SPSS Statistics 22) was used to determine difference among treatment groups.
3. RESULTS
3.1. Safety
To establish initial systemic and dermal safety, prior to clinical testing, several GLP compliant toxicology studies were performed with aurase using either bolus intravenous administration (rats), topical application to intact skin (rats and guinea‐pigs) and topical application to full‐thickness and partial‐thickness wounds (mini‐pigs) all utilising concentrations of aurase at least 10 times greater than the maximum clinical exposure anticipated, assuming the maximum chronic wound size to be treated clinically was 50 to 100 cm2 and assuming a maximum clinical dose equivalent to 9.8 μg of aurase per cm2 of wound area (ie, approximately 9 times the natural level of aurase expressed in maggot saliva). Details of each study and their principal findings are presented in Table 1.
TABLE 1.
Summary of non‐clinical GLP toxicity studies performed with aurase containing formulations
| Species | Route of administration | Dose | Duration of Exposure | Highlighted findings |
|---|---|---|---|---|
| Rat (Sprague Dawley) | Topical (intact skin) | 0, 250 μg/kg/day administered as gel formulation | 7 days |
No localised or systemic clinical signs that were treatment related. No effects on body weight or organ weights. No macroscopic findings |
| Rat (Sprague Dawley) | Topical (intact skin) | 0, 250 μg/kg/day administered as gel formulation | 14 days |
No systemic clinical signs that were treatment related Observations of erythema observed for up to 4 consecutive days in 4/15 females receiving 250 μg/kg/day. Slight erythema observed for up to 5 consecutive days in 10/30 animals receiving 250 μg/kg/day compared with 5/30 control animals. No effects on body weight, food consumption or changes in haematology. No macroscopic or histopathology findings relating to treatment. |
| Mini‐pig (Gottingen) | Topical (to eight 2.5 cm × 2.5 cm full thickness wounds) | 0, 175 and 250 μg/g/day administered as gel formulation | 14 days (14 days recovery) |
No effects on body weight, food consumption, ophthalmic examination, neurological observation, ECG. Haematology at week 2 revealed an increase in RBC and reticulocytes with minor fluctuations in WBC. All parameters showed full recovery during 2 weeks off dose. Localised observations included thrombus and blood (day 2), slough (day 3), granulation tissue (day 4) and re‐epithelization and closure (day 9). |
| Mini‐pig (Gottingen) | Topical (to two 10 cm × 7.5 cm partial thickness wounds) | 0, 514 μg/day and 6.81 mg/day administered as gel formulation | 28 days |
No systemic clinical signs that were treatment related. No effects on body weight, food consumption or changes in haematology. Mild, transient erythema (Grade 2) observed in some animals at the highest concentration, and observed macroscopically at necropsy. |
| Rat (Sprague Dawley) | Intravenous | 0, 200 μg/kg/day administered as a pH 5 citrate buffered solution | 7 days |
There were no unscheduled deaths There was no clinical observations or differences in body weight, food consumption, ophthalmic observations, haematology and blood chemistry parameters or organ weights. There were no macroscopic or histopathology findings. |
| Guinea‐pig | Topical to intact skin (Buhler) | Test patches loaded with 514 μg/day | 7 × 6 hours exposures on Day 0, 6‐8 and 13‐15 |
Systemically well tolerated and did not cause skin irritation. No evidence of sensitization |
| Guinea‐pig | Topical to intact skin | Test patches loaded with 11.95 mg | 2 × 6 hours exposures | Systemically well tolerated and did not cause skin irritation. |
3.2. In‐vivo kinetics
During the GLP 7‐day rat bolus IV study (administered dose of 200 μg/kg/day), plasma concentrations of aurase were measured serially on day 1 and day 7 using a validated LC‐MS/MS method (LLOQ 50 ng/mL) immediately pre‐dose, and then at 2, 3, 5, 15 and 30 mins post‐administration. Results indicated that the systemic Tmax was 2.0 minutes in males on day 1 (no data was collected for females) and in both males and females on day 7. Importantly, in all animals at day 1 and 7 the reported plasma levels were below the limit of quantitation (LOQ) after 5 minutes. Comparable kinetic testing, following topical application to mini‐pigs in the GLP 14‐day repeat dose toxicity study in full‐thickness wounds (equivalent to 2 mg/day) and in the GLP 28‐day repeat dose toxicity study in partial thickness wounds (equivalent to 6.81 mg/day) resulted in levels below the limit of quantification at all time points. Hence, no toxicokinetic profile could be established for topical administration.
The rapid loss of aurase observed in‐vivo is readily explained through the rapid action of inhibitory endogenous protease inhibitors (serpins), notably A1AT. In the proteolytic bioassay, all three serpins tested (in the absence of aurase) showed no or negligible enzymatic activity. At 2 M excess, and following incubation with aurase, the proteolytic activity of aurase alone was reduced by 0%, 42.2% and 99.5% in combination with A2AP, A2M A1AT respectively (Figure 1). Supporting analytical methods including SDS Gel Zymographs, SDS‐PAGE, HPLC analysis and SEC (data not shown) equally showed a reduced intensity of 25 kDa aurase peaks/bands following incubation with A1AT along with the emergence of a corresponding band/peak at 76.6 kDa consistent with the molecular weight of a complex of aurase and A1AT. Binding studies with A1AT in the presence of benzamidine (a small molecular inhibitor of aurase that binds at the active catalytic site) also led to less complex formation indicating that aurase likely binds to A1AT at the active site or at least requires an empty active site to change into an energetic favourable level.
FIGURE 1.

Residual proteolytic activity of aurase with and without incubation with 2 M excess of alpha‐2‐macroglobulin (A2M), alpha‐2‐antiplasmin (A2AP) and alpha‐1‐antitrypsin (A1AT). Error bars are mean ± SD
Importantly, the timed incubations of aurase and A1AT, indicate that complex formation is fully complete within 1 minute (with the corresponding loss of aurase peaks). No increase in the intensity of complex staining is observed with prolonged incubation, consistent with timings observed in the in‐vivo data (Figure 2).
FIGURE 2.

Reducing SDS PAGE showing aurase incubated with A1AT after 1, 2, 5, 15, 60, 240 minutes in SDS buffer
3.3. In‐vitro efficacy
Incubation of negative control (tris buffer) resulted in intact fAWE disks that remained floating within the wells at 24 hours and took until 48 hours to fully disperse. By contrast, a number of fluorescent particles were observed to have peeled off the edge of the fAWE disc within 2 hours in the 5 μg/mL aurase and within 1 hour in the 25 μg/mL aurase solutions. The fAWE disks were fully dispersed at 4 hours in the 25 μg/mL aurase group and 7 hours in the 5 μg/mL aurase group. Intact fAWE discs remained in the collagenase group up to 7 hours and had fully dispersed by 24 hours (Figure 3).
FIGURE 3.

Illustrative representations of the dispersal of collagen/fibrin/elastin fAWE disks after a 7 hours incubation period, at 37°C with (LEFT) 5 mL tris‐buffer (CENTRE LEFT) 5 mL aurase solution (5 μg/mL), (CENTRE RIGHT) 5 mL aurase solution (25 μg/mL) and (RIGHT) collagenase solution (200 μg/mL)
Aurase, as would be expected of a fibrin selective proteolytic enzyme, resulted in statistically significant increases in fibrin degradation compared with control at all time points with the rate of degradation observed being dose dependent; the 25 μg/mL concentration of aurase achieving significantly more degradation compared with the 5 μg/mL group and compared with 200 μg collagenase after 4, 7, 24 and 32 hours. After 48 hours, there was no difference between the three active enzymes (Figure 4A). More surprisingly, aurase solutions at both 5 and 25 μg/mL was found to be equally effective as 200 μg/mL of collagenase in degrading collagen (Figure 4B) and elastin (Figure 4C) over the period of 4 to 72 hours.
FIGURE 4.
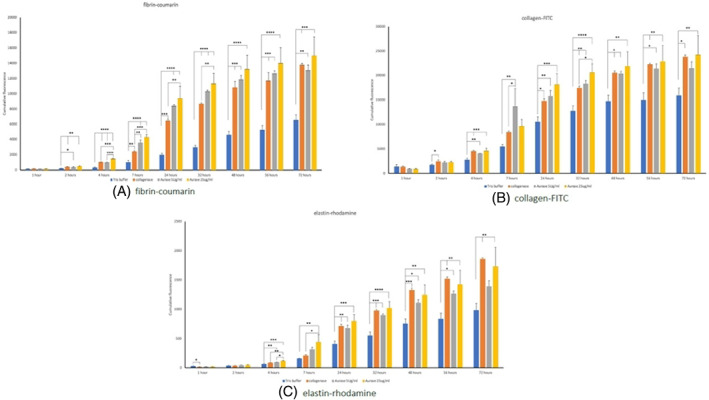
Cumulative degradation of fluorescent artificial wound eschar (fAWE) proteins by tris‐buffer (control), Aurase 5 μg/mL, Aurase 25 μg/mL and collagenase 200 μg/mL against A, fibrin‐coumarin; B, collagen‐FITC and C, Elastin‐rhodamine. *P < .05; **P < .01; ***P < .0001 ****P < .0001.
3.4. In‐vivo efficacy
After 72 hours inoculation (ie, baseline prior to initiation of treatment) MRSA bacterial count in pig wounds was 7.33 ± 0.51 Log CFU/g. Wounds treated with placebo and untreated Tegaderm control wounds showed the highest MRSA counts 2 days after the first treatment application. Wounds treated with high dose aurase showed the lowest MRSA counts among all treatments and was significant (P < .05) compared with baseline counts and all other treatments. Low‐dose, mid‐dose and high dose aurase wound gels allowed for the reduction of MRSA counts by 90.82% 97.95% and 99.4% by day 2 respectively, compared with baseline controls. Relative to placebo, low medium and high dose aurase wound gels showed 73.04%, 93.97% and 99.82% reductions in MRSA counts respectively and 75.95%, 94.62% and 99.84% relative to untreated controls. Further reductions in MRSA counts were observed out to day 14, with significance being maintained in the high dose group compared with baseline and all other treatments (Figure 5).
FIGURE 5.

MRSA USA300 Counts following treatment with aurase, placebo and untreated controls compared with baseline. □ P < .05 compared with baseline and all treatments; * P < .05 compared with baseline, placebo and untreated controls; • P < .05 compared with baseline only.
The evaluation of histological samples collected after 2 days revealed similar extent of inflammation, granulation tissue and crust formation regardless of treatment group. At 14 days, treatment with high dose aurase noted a significant induction of neovascularisation (P < .05) compared with control untreated Tegaderm covered wounds and wounds treated with low‐dose aurase. The extent of angiogenesis for the wounds treated with mid‐dose aurase wound gel and placebo were similar with no significant differences compared with other treatment groups. All wounds had fully re‐epithelialized at Day 14, thus limiting observations of differences in wound closure (Figure 6).
FIGURE 6.

Treatment with high dose aurase (0.25 g/L) was noted to increase wound neovascularization. Morphological scores of vascularization in H&E‐stained histological sections of wounds were used for quantification. 0 = normal vascularization, 1 = discrete, 2 = moderate and 3 = high vascularity
4. DISCUSSION
Before clinical testing of a new recombinant protein can commence, it is important to establish safety in relevant animal species. Collectively, the toxicology studies performed have indicated that aurase results in no observable systemic effects, even when at high concentrations administered by bolus intravenous injection. The lack of systemic adverse events correlates well with the observation that aurase is rapidly bound to A1AT, an endogenous protease inhibitor, to form a high affinity (possibly covalent) complex which in turn leads to a loss of biological activity. Given the significant excess of A1AT in the plasma (reference range 100 to 273 mg/dL 12 ), compared with administered doses of aurase, there is little chance of the system becoming saturated.
When administered topically, no sensitisation, irritation or maceration was observed when applied to intact skin or to wounded skin, with the only adverse events noted being transient erythema at very high concentrations in some animals. Importantly, no delays in wound healing were observed in either full thickness or partial thickness wounds as a result of topical application of aurase concentrations. This likely aligns well with the established enzymatic activity of aurase which is noted to have optimal enzymatic activity at around pH 7‐9, drops to approximately 50% of enzymatic activity by pH 6 and has <10% of biological activity at pH 5. Within the context of topical application to wounds, this means that aurase will likely work best within chronic wounds, where the pH has previously been measured within the range of 7.15 to 8.9. 13 , 14 Importantly, as the wound progresses towards healing, the pH typically reduces to neutral and then acidifies, 14 , 15 providing a natural mechanism to inactivate the proteolytic action within a readily healing wound.
Wound Slough is essentially the by‐product of the inflammatory phase of wound healing comprising fibrin, leucocytes, dead and living cells, microorganisms and proteinaceous material. 16 Wound eschar has additionally been reported to contain a heterogenous mix of collagen, elastin, fibrin as well as other minor components. 17 The ability of an enzymatic agent to effectively debride a sloughy wound in‐vivo likely requires a wider range of substrates in the wound than solely fibrin, although fibrin is the prime substrate anchoring material to the wound bed. In this regard aurase, although fibrinolytic, has shown the ability to act against other substrates in wound eschar, including collagen and elastin to an extent comparable with collagenase.
In‐vitro performance of the enzyme has been further validated by reference to an established in‐vivo animal (pig) MRSA biofilm model, which has previously been used to demonstrate the effectiveness of debridement methods by correlating debridement efficacy with the decrease in pathogen counts. In this model, occlusion alone provides for autolytic debridement and indeed a small but significant reduction in MRSA counts was observed over a 14‐day period in response to Tegaderm dressing or when the vehicle gel was applied under occlusive dressing, consistent with an autolytic effect. The addition of aurase gels under occlusion resulted in further dose‐dependent significant reductions in MRSA bacteria over a shorter period of time (2 days) and these reductions in pathogen counts was maintained over the entire 14‐day period of the study. Given the prevalence of biofilms in chronic wounds, the ability of aurase to reduce pathogen counts whilst simultaneously clearing slough from the wound, may have significant clinical implications.
Histopathology of wound sites treated with high‐dose aurase also showed a significant induction of neovascularisation of the wound compared with untreated Tegaderm covered wounds (P < .05) as well as a dose response increase in angiogenesis suggesting that enzymatic activity of aurase may additionally promote tissue remodelling, resulting in stimulation of new blood vessel formation. Whilst further work with aurase is required to elaborate on this potential mechanism, it is noted that other authors have reported similar effects arising from matrix‐derived bioactive peptides following enzymatic debridement. 18 , 19 , 20
In conclusion, aurase may represent an additional enzyme for the safe and effective debridement of chronic wounds. We eagerly await the results of clinical testing.
FUNDING INFORMATION
Funding for all reported studies was provided by SolasCure Ltd, Wellington House, East Road, Cambridge, CB1 1BH, UK.
CONFLICT OF INTEREST
All authors are associated with SolasCure Ltd either as employees, shareholders and/or company advisers.
ACKNOWLEDGEMENTS
The authors acknowledge the involvement of Covance Ltd, UK; Bioserv Ltd, Germany; Aurigon, Hungary; 5D Health Protection Group Ltd, UK; Alderley Analytical Ltd, UK; Peak Proteins Ltd, UK and staff at the University of Miami, Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery, notably Joel Gil, Michael Solis and Alex Higa for their contributions towards executing the reported studies.
Fairlamb DM, Kelety B, Bachert A, et al. Preliminary evidence supporting a new enzymatic debridement product for use in chronic wounds. Int Wound J. 2023;20(6):2095‐2104. doi: 10.1111/iwj.14079
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gottrup F, Jorgensen B. Maggot debridement: an alternative method for debridement. Eplasty. 2011;11:e33. [PMC free article] [PubMed] [Google Scholar]
- 2. Naik G, Harding K. Maggot debridement therapy: the current perspectives. Chronic Wound Care Manag Res. 2017;4:121‐128. [Google Scholar]
- 3. Naafs MA. The Maggots—Professional Wound Cleaners: Global Biofilm Advisory Council; 2017; https://infectioncontrol.tips/2017/10/31/maggots-professional-wound-cleaners.
- 4. Grassberger M, Fleischmann W. The biobag ‐ a new device for the application of medicinal maggots. Dermatology. 2002;204(4):306. [DOI] [PubMed] [Google Scholar]
- 5. Preuss SF, Stenzel MJ, Esriti A. The successful use of maggots in necrotizing fasciitis of the neck: a case report. Head Neck. 2004;26(8):747‐750. [DOI] [PubMed] [Google Scholar]
- 6. Jukema GN, Steenvoorde P, Wong CY, Bernards AT, van Dissel JT. Maggot therapy for treatment of severe infections in trauma surgery: "back to the future!". Zentralbl Chir. 2006;131(Suppl 1):S75‐S78. [DOI] [PubMed] [Google Scholar]
- 7. Blake FA, Abromeit N, Bubenheim M, Li L, Schmelzle R. The biosurgical wound debridement: experimental investigation of efficiency and practicability. Wound Repair Regen. 2007;15(5):756‐761. [DOI] [PubMed] [Google Scholar]
- 8. Gazi U, Taylan‐Ozkan A, Mumcuoglu KY. The effect of Lucilia sericata larval excretion/secretion (ES) products on cellular responses in wound healing. Med Vet Entomol. 2021;35(3):257‐266. [DOI] [PubMed] [Google Scholar]
- 9. Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Rep Regen. 2001;9(2):66‐76. [DOI] [PubMed] [Google Scholar]
- 10. Davis SC, Ricotti C, Cazzaniga A, Welch E, Mertz P. Microscopic and physiological evidence for biofilm‐associated wound colonization in‐vivo. Wound Rep Regen. 2008;16:23‐29. [DOI] [PubMed] [Google Scholar]
- 11. Nusbaum AG, Gil J, Rippy MK, et al. Effective method to remove wound bacteria: comparison of various debridement modalities in an in vivo porcine model. J Surg Res. 2012;176(2):701‐707. [DOI] [PubMed] [Google Scholar]
- 12. Donato LJ, Jenkins SM, Smith C, Katzmann JA, Snyder MR. Reference and interpretive ranges for alpha(1)‐antitrypsin quantitation by phenotype in adult and pediatric populations. Am J Clin Pathol. 2012;138(3):398‐405. [DOI] [PubMed] [Google Scholar]
- 13. Wilson M, Henry M, Quill R, Byrne P. The pH of varicose ulcer surfaces and its relationship to healing. VASA. 1979;8:339‐342. [PubMed] [Google Scholar]
- 14. Tsukada K, Togunaga K, Iwama T, Mishima Y. The pH changes of pressure ulcers related to the healing process of wounds. Wounds. 1992;4(1):16‐20. [Google Scholar]
- 15. Kaufman T, Eichenlaub EH, Angel MF, Levin M, Futrell JW. Topical acidification promotes healing of experimental deep partial thickness skin burns: a randomized double‐blind preliminary study. Burns Incl Therm Inj. 1985;12(2):84‐90. [DOI] [PubMed] [Google Scholar]
- 16. Percival SL, Suleman L. Slough and biofilm: removal of barriers to wound healing by desloughing. J Wound Care. 2015;24(11): 498, 500‐3, 506‐10. [DOI] [PubMed] [Google Scholar]
- 17. Thomas AM, Harding KG, Moore K. The structure and composition of chronic wound eschar. J Wound Care. 1999;8(6):285‐287. [DOI] [PubMed] [Google Scholar]
- 18. Riley KN, Herman IM. Collagenase promotes the cellular responses to injury and wound healing in vivo. J Burns Wounds. 2005;4:e8. [PMC free article] [PubMed] [Google Scholar]
- 19. Sheets AR, Demidova‐Rice TN, Shi L, Ronfard V, Grover KV, Herman IM. Identification and characterization of novel matrix‐derived bioactive peptides: a role for collagenase from Santyl(R) ointment in post‐debridement wound healing? PLoS One. 2016;11(7):e0159598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi L, Ermis R, Garcia A, Telgenhoff D, Aust D. Degradation of human collagen isoforms by Clostridium collagenase and the effects of degradation products on cell migration. Int Wound J. 2010;7(2):87‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


