Abstract
A major obstacle to the development of new treatments for venous leg ulcers is the difficulty in generating evidence for their effectiveness. Randomised controlled trials using complete healing as the endpoint are seldom powered to be successful, owing to the heterogeneity of cohorts. A novel approach to the evaluation of treatments is presented, using a self‐controlled trial model and two metrics of short‐term healing rate as alternate endpoints: rate of wound margin advance, and percentage area reduction over 4 weeks. Two different treatment regimens are compared: multi‐layer compression alone, versus multi‐layer compression combined with activation of the venous leg pump by neuromuscular stimulation. With 60 patients, adding neuromuscular stimulation to multilayer compression resulted in a significant two‐fold increase in the rate of wound healing over a 4‐week period, both in terms of wound margin advance and in terms of percentage area reduction. The use of these short‐term intermediate endpoint metrics together with a self‐controlled study design offers potential for distinguishing between the relative efficacies of interventions more rapidly, with greater sensitivity, and with fewer subjects than a conventional RCT cohort model.
Keywords: alternate healing endpoint, NMES, PAR, self‐controlled trial, venous leg ulcers, WMA
1. INTRODUCTION
Given the significant burden which venous leg ulcers impose on the world's health resources, 1 , 2 , 3 it is not surprising that new, effective interventions are constantly sought. 4 A systematic overview 5 examined evidence for the effectiveness of numerous interventions currently indicated for healing venous leg ulcers, including compression bandages and stockings, topical negative pressure, oral pentoxifylline, laser treatment, skin grafting, superficial vein surgery (perforator ligation, saphenous vein stripping), therapeutic ultrasound, leg ulcer clinics, leg elevation, and activity advice. Of these, only compression and pentoxifylline were deemed to have some evidence to support their use.
One reason for the apparent lack of evidence in support of wound‐healing interventions is the typical insistence on complete healing as an outcome, and a conventional randomised controlled trial (RCT) design as the standard of evidence. 6 Chronic wounds frequently take many months to heal, 7 and the heterogeneity of ulcers and their trajectories 8 makes it difficult to match control and intervention groups. These problems mean that, even for an intervention with a substantial effect, a very lengthy trial with a prohibitively large number of stratified subjects would be required to observe that effect with any validity or statistical significance. Indeed, a Cochrane review of compression for VLU found only 48 eligible RCTs out of many 1000s of studies, of which only eight had significant stand‐alone findings of effectiveness. 9 Consequently, there has been a call for alternate endpoints such as speed of wound closure to be used for evaluations. 10 , 11
The benefit of this approach is twofold: firstly, a measurement of healing rate can be made over a period of a few weeks, whereas time to complete closure may be many months or even years. This allows for a study to be feasible. Secondly, it allows for a self‐controlled model, whereby data collected post‐intervention from each subject is paired with his/her own control data collected pre‐ intervention. This eliminates much of the confounding heterogeneity between cohorts, so vastly improving the statistical sensitivity and power of the study, as well as both internal and external validity. 12 A consensus document of opinion leaders in wound healing 13 has stated that self‐controlled studies may be preferable to traditional RCTs or typical cross over study designs for measuring treatment outcomes. A prerequisite for comparing control and intervention in terms of an alternate endpoint measuring speed of healing is that the endpoint follows a linear trajectory, to allow a change in that trajectory to be observed. A method has been established for calculating the rate of advance of the wound margin, 14 which has recently been shown to follow a linear trajectory over a 4‐week period in venous leg ulcers receiving compression therapy. 15
The geko™ device (Firstkind Ltd, Daresbury) is a small, self‐adhesive, wearable neuromuscular electro stimulator (NMES) that is applied to the surface of the skin on the lateral aspect of the leg just below the knee, over the head of the fibula. It delivers a charge‐balanced electrical pulse once per second to the common peroneal nerve which passes through this locus, eliciting a muscular twitch of the leg, so activating the venous muscle pumps of the leg and foot, and thus augmenting venous, arterial, and microvascular flow. 16 [Correction added on 21 March 2023, after first online publication: the preceding paragraph was duplicated in the Methods section and was removed.]
This study is a randomised self‐controlled trial comparing the rate of wound margin advance (WMA) for venous leg ulcers receiving 12 hours per day intermittent NMES of the common peroneal nerve in addition to compression, compared with compression alone.
2. METHODS
Sample size was determined by an interim analysis of the first 20 subjects. Sixty patients in wound clinic setting with venous leg ulcers were randomised to two groups: one to receive standard of care (SOC) consisting of commercially available multi‐layer, multicomponent compression bandaging or hosiery kits indicated for treating venous leg ulcers, and the other to receive NMES for 12 hours per day in addition to SOC. Randomisation was 1:1 using the Castor EDC platform with variable block size. Differences in group size allocation were due to patient exclusion post‐randomisation. [Correction added on 21 March 2023, after first online publication: ‘withdrawal’ was changed to ‘exclusion’ in the preceding sentence.]
The geko™ device was applied as per the manufacturer's instructions to the lateral aspect of the leg just below the knee, to stimulate the common peroneal nerve as it passes by the head of fibula. The device delivers a charge‐balanced pulse at 1 second intervals, and the settings were adjusted so that a visible twitch of the foot was elicited. Each device delivers two sessions of 12 hours of treatment, used on two successive days. New devices were applied as required up to a maximum of 28 days. Note the device is removed from the leg and stored between 12‐hour treatments. Patients randomised to NMES maintained a diary of device usage.
Inclusion Criteria:
Aged 18 years or over and able to provide written informed consent.
Chronic venous leg ulcer determined to be due to underlying venous disease following evaluation in a multidisciplinary clinic setting or by a vascular surgeon, GP or Nurse specialist
39cm2 > Ulcer size >3 cm2 at study enrolment.
Ulcer present for at least 6 weeks but no more than five years prior to study entry.
Ankle‐Brachial Pressure Index (ABPI) of 0.8–1.2 at study entry or within 8 weeks of study entry.
No active wound infection for a minimum of 48 hours prior to study entry.
No systemic antimicrobial treatment for a minimum of 7 days prior to study entry prescribed for index wound infection.
Exclusion Criteria:
Known allergy to any of the protocol‐stipulated treatments, or non‐tolerance of multilayer, multicomponent compression therapy intended for the treatment of VLU. Allergy to component of electrodes.
History of significant haematological disorders (e.g. Sickle Cell disease).
History of Deep Vein Thrombosis (DVT) within 6 months preceding study entry
History of Pyoderma Gangrenosum or other inflammatory ulceration.
Pregnancy or breast feeding.
Use of investigational drug or device within 4 weeks prior to study entry that may interfere with this study.
Use of any neuro‐modulation device.
Surgery during 3 months prior to study entry (such as abdominal, gynaecological, hip or knee replacement)
Any medication deemed by the Investigator to potentially interfere with the study treatment (e.g. systemic steroids).
Participation in any other clinical study.
Nine patients were withdrawn post‐randomisation due to randomisation criteria failures, non‐compliance or withdrawn consent, leaving 22 patients in the SOC arm, and 29 in the NMES arm. Table 1 shows the breakdown of exclusions from the final analysis.
TABLE 1.
Patient exclusions from final analysis groups.
| SOC Arm | SOC + NMES 12 h Arm | |
|---|---|---|
| Subjects enrolled (n) | 26 | 34 |
| Subjects excluded due to non‐compliance to NMES device Instructions for Use (n) | NA | 2 |
| Subject excluded due to infection (n) | 1 | 0 |
| Subjects excluded due to wound being too small at randomisation (n) | 2 | 3 |
| Subject excluded; unable to qualify wound size (n) | 1 | 0 |
| Subjects analysed (n) | 22 | 29 |
In the patients receiving NMES, adherence was 94.1%, with two patients being excluded from the trial due to non‐adherence (Table 1).
A schematic of the patient journey is shown in Figure 1. In the case of both arms, each patient spent 4 weeks on a run‐in phase receiving SOC only. This run‐in phase then served as a within‐patient control for each patient. Thereafter, the SOC randomised cohort continued to receive SOC for a further 4 weeks, whereas the NMES randomised cohort received NMES in addition to SOC for a further 4 weeks. The study was powered to compare paired healing rates between treatment phase and run‐in control phase in each cohort. The study was not powered to compare healing rates between the two randomised cohorts.
FIGURE 1.
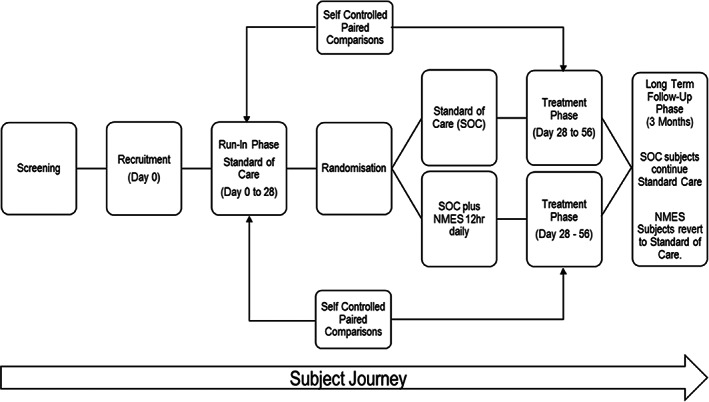
Trial design schematic.
At day 0, and at every weekly visit until day 56 (end of 8 weeks), wounds were photographed pre‐debridement using the Aranz SilhouetteStar™, a digital camera which is part of the Silhouette™ wound assessment system. It is a portable, non‐contact device for imaging and measuring ulcers. All images were sent in random order to an international independent wound expert for delineation of the wound perimeter, from which area and perimeter values were calculated. The assessor was blinded to intervention as well as to the date of each image.
According to Vidal's method, 14 a value for our primary endpoint of WMA corresponding to the rate of linear advance of the wound edge was derived by:
WMA = d/dt (A/P),
where A = area, and P = perimeter of the wound.
A value of A/P was calculated each of the 5, weekly time‐points for each wound. These values were then regressed against time to generate a gradient to represent WMA, as well as a correlation coefficient R 2.
Additionally, rate of wound healing was calculated using the metric of Percentage Area Reduction (PAR), whereby the reduction in wound area over the 4‐week intervention was presented as a percentage of the initial area of the same wound at the beginning of the 4‐week period. PAR is a commonly used metric in wound healing, and this post hoc comparison was made to examine whether the same pattern of outcomes was observed using a different metric.
Secondary endpoints collected for descriptive reporting included adherence, infection rates, percentage complete healing, quality of life scores EQ‐5D‐5L, Cardiff Wound Impact Schedule (CWIS), Venous Clinical Severity Score (VCSS), and Visual Analog Score (VAS) for pain. The study was not powered for statistical comparison of these endpoints. After the 4‐week intervention phase, patients were followed up for a 3‐month period during which SOC only was provided for both groups. Complete wound healing during the 3‐month follow‐up period was based solely on patient‐reported outcomes. No measurements or examinations were made during this period.
3. DEMOGRAPHICS
Table 2 shows the demographics of the subjects, comparing the group randomised to compression only (SOC) with the group randomised to compression plus the NMES device for 12 hours per day (SOC + NMES 12 hours). No significant differences were found between groups according to un‐paired t‐tests. The groups showed no difference in the ratio for male and female subjects. Given the immense heterogeneity of patients with venous ulcers in general, it is unsurprising to see some degree of inter‐group variation (e.g. BMI, compression duration at enrolment, age of ulcer), and this (perhaps inevitable) level of heterogeneity would be problematic in a classic inter‐cohort RCT design. However, in this self‐controlled design, each subject's intervention phase is compared with his/her own run‐in phase, so accommodating these differences.
TABLE 2.
Trial demographics.
| SOC | SOC + NMES 12 h | |||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Age (years) | 67.087 | 2.074 | 67.828 | 2.530 |
| Height (cm) | 175.539 | 2.689 | 173.577 | 2.359 |
| Weight (Kg) | 84.140 | 5.980 | 93.397 | 4.761 |
| BMI | 27.553 | 1.893 | 31.048 | 1.566 |
| ABPI | 1.112 | 0.017 | 1.100 | 0.021 |
| Wound size (cm2) | 10.390 | 1.224 | 9.961 | 1.246 |
| Age of study ulcer (days) | 477.710 | 104.198 | 522.801 | 89.684 |
| Year of First Venous Leg Ulcer | 2007 | 2.766 | 2012 | 1.962 |
| Age at First Venous Leg Ulcer (years) | 54.000 | 3.461 | 59.414 | 2.953 |
| Previous Venous Leg Ulcers (N) | 0.739 | 4.795 | 1.750 | 0.400 |
4. RESULTS
Shapiro Wilks and Kolmogorov‐Smirnof tests were performed, and showed no significant deviation from a normal distribution for the parametric outcome measures of PAR and WMA. Figure 2 shows the rate of wound margin advance in mm/week, for the run‐in phase with compression therapy alone, compared with the treatment phase with NMES in addition to compression therapy. It can be seen that the treatment phase with NMES as an adjunct to compression healed significantly faster (P = 0.016, paired t‐test) than compression alone. Meanwhile, no significant difference was found between run‐in phase (compression only) and treatment phase (also compression only) in the SOC randomised arm of the study.
FIGURE 2.

Rate of wound margin advance: run‐in phase compared with treatment phase.
In Figure 3, the same wounds are assessed using a different metric: PAR. Once again, the healing rate during treatment phase when the NMES devices were added to SOC was significantly higher than run‐in phase (P = 0.011, paired t‐test). Again, the SOC cohort showed no significant difference between run‐in and treatment phases.
FIGURE 3.

Percentage area reduction: run‐in phase versus treatment phase.
A typical series of weekly assessments is shown for a single wound in Figure 4. The wound is photographed weekly for the 4‐week run‐in phase, followed by the 4‐week treatment phase. Note that the size and perimeter of the wound remain relatively static during the run‐in phase when the patient is receiving standard of care alone, but that the area progressively reduces, and wound margin progressively advances, during the treatment phase when the NMES device is added to standard of care.
FIGURE 4.

A typical trajectory of a single wound over the 8 weeks.
Results for secondary endpoints are reported descriptively in Table 3. A substantially greater proportion of NMES patients healed completely during the course of the study and the follow‐up period. Reduction in VAS pain score was greater for patients who receive NMES when compared with patients who received SOC only. Similarly, VCSS shows an improvement for SOC plus NMES 12 hours when compared with SOC alone. Improvement in quality of life indices (EQ‐5D‐5L & Cardiff Wound Impact Schedule; not shown) was negligible in both arms and not clinically significant over the short duration of the trial.
TABLE 3.
Secondary endpoints.
| SOC | SOC + NMES 12 h | |
|---|---|---|
| Mean improvement in Vascular Clinical Severity Score(VCSS) a | 12.8% | 15.1% |
| Mean reduction in VAS pain treatment phase b | 21.1% | 30.1% |
| Percentage target wounds completely healed at 3‐month follow‐up c | 27.0% | 42.0% |
Mean improvement in score from recruitment to end of treatment period.
Mean improvement in score from randomisation to end of treatment period.
Percent healed between randomisation and end of long term follow‐up period.
[Correction added on 21 March 2023, after first online publication: In Table 3 legend, the footnote for ‘b’ and ‘c’ were switched and have been updated in this version.]
5. DISCUSSION
The recognised effectiveness of compression 17 can be explained by the pathophysiology of leg ulcers, which stem from compromised venous function. 18 Compression mitigates the detrimental effects of venous insufficiency oedema, reduced venous flow, and reflux by applying pressure to oppose hydrostatic pressures in the leg, 19 as well as increasing venous velocity by reducing vessel diameter. 20 , 21
The great majority of venous return in the lower limb is driven by the venous muscle pumps, and not by the heart. 22 , 23 Dysfunction of the calf muscle pump, either due to immobility or abnormal gait, can, therefore, exacerbate the etiological factors for VLU. 24 Mobility and exercise, activating the muscle pump, have been shown to improve VLU outcomes. 25 Patients who exercise during compression treatment see enhanced benefits of the compression in terms of venous and lymphatic return. 26 , 27 Additionally, there is evidence that compression and leg movements are mutually supportive. 28 , 29
Activation of the calf muscle pump by means of intermittent NMES of the common peroneal nerve has been shown to augment venous flow in the leg, effectively replicating the effects of exercise, 30 over a prolonged period (in this case 12 hours per day). In patients with venous leg ulcers due to chronic venous insufficiency, venous and arterial flow have been shown to be augmented by NMES, 31 as well as microcirculation and pulsatility in both the wound bed and the wound periphery. 32 Similar effects have been reported in patients with arterial leg ulcers. 33 The haemodynamic benefit of activating the muscle pump by NMES of the common peroneal nerve has been shown to exceed that of intermittent pneumatic compression (IPC). 34 , 35 Application of a rigid enclosure to the leg (in the form of a cast) further improved the benefit in terms of microvascular 36 and vascular flow. 37 Promising results have been seen when applying the NMES in this modality to lower limb wounds. 38 , 39 , 40
In this self‐controlled study, the addition of Intermittent NMES of the common peroneal nerve over a 4‐week period more than doubled the rate of wound‐margin advance (WMA) towards the centre of the wound relative to a 4‐week run‐in period with compression alone (P = 0.016). This rate of healing was also calculated using the Percentage Area Reduction method (PAR) and the same results were seen, the rate of healing more than doubled (P = 0.011). In contrast, the SOC cohort maintained the same healing rate throughout the run‐in and treatment phases of the study. This suggests that subjects' wounds were not at different stages of healing throughout duration of the trial.
The device was well tolerated by patients, with only two patients failing to adhere and participants had no issues with self‐administration of the device.
WMA has been shown to follow a linear trajectory with respect to time, 11 , 12 , 15 allowing a prediction of healing time by extrapolation after only four or five weekly measurements. It has been observed that contraction and epithelialisation occur in a linear fashion perpendicular to the wound edge, 41 and that cell fronts move at constant speed. 42 WMA is a powerful predictor of healing, 20 and it has been argued that linear healing per unit time should be preferred to measurements of percentage change in wound area to quantify wound healing in clinical trials. 43 Healing rate over 4 weeks has been used as an outcome for cost‐effectiveness in diabetic foot ulcers, 44 and has been used as the primary outcome in RCTs for comparing interventions. 45
The study was not powered for inferential statistics in the secondary endpoints. Albeit not statistically significant, it is perhaps worth noting from the descriptive statistics presented in Table 3 that patients receiving NMES showed better outcomes in terms of pain reduction and VCSS. These secondary outcomes tend to reflect the reduction in wound size during the study. Because it has been demonstrated that NMES accelerates wound healing, it seems plausible that pain will be resolved more quickly accordingly. The patients randomised to NMES were also more likely to have healed completely at the 12‐week follow‐up than patients who were randomised to standard of care alone.
This self‐controlled approach to clinical studies using intermediate endpoints for wound healing in patients with chronic wounds will add to the debate, but clearly this progressive design enables data to be collected efficiently and helps overcome many of the challenges we currently face in undertaking feasible and meaningful clinical trials.
Intermittent NMES of the common peroneal nerve significantly accelerates the healing of venous leg ulcers more than two‐fold over a 4‐week period. The effective doubling of the healing rate suggests substantial potential benefits to the patient, as well as cost savings to the health care system. NMES is well tolerated by patients and deserves serious consideration as an adjuvant to compression therapy. The use of the endpoint metrics, WMA and PAR, allow for a self‐controlled study model, which provides a means to distinguish between the relative efficacies of interventions more rapidly, with greater sensitivity, and with fewer subjects than a conventional RCT cohort model.
FUNDING INFORMATION
This study was funded by Firstkind Ltd, Daresbury, Cheshire, UK.
ETHICS STATEMENT
UK NHS REC reference: 18/LO/0219, IRAS project ID: 235092.
[Correction added on 21 March 2023, after first online publication: Reference 11 was corrected.]
ACKNOWLEDGEMENTS
Dr. Thomas E. Serena MD FACS FACHM MAPWCA., independent wound care expert responsible for the blinded assessment of anonymised wound images. Participating centres that enrolled subjects: Sarah Bradbury, Welsh Wound Innovation Centre, Rhodfa Marics, Ynysmaerdy, Pontyclun, Rhondda Cynon Taf, Wales, CF72 8UX. Isaac Nyamekye, Worcestershire Acute Hospitals NHS Trust, Worcestershire Royal Hospital, Charles Hastings Way, Worcester, WR5 1DD. Janice Tsui, Royal Free London NHS Foundation Trust, Royal Free Hospital, Pond Street, London, NW3 2QG. Karen Reay, South Tyneside and Sunderland NHS Foundation Trust, Clarendon House, Windmill Way, Hebburn, NE31 1AT. Juliet Price, Royal Devon and Exeter NHS Foundation Trust, Royal Devon and Exeter Hospital, Barrack Road, Exeter, EX2 5DW. Richard Bull, Accelerate CIC, Centenary Wing, St Joseph's Hospice, Mare St, Hackney, London, E8 4SA. Kay Baxter, Barnsley Hospital NHS Foundation Trust, Barnsley Hospital, Gawber Road, Barnsley, S75 2EP. Chandana Wijewardena, Queen Elizabeth Hospital NHS Foundation Trust, The Queen Elizabeth Hospital, Gayton Road, Kings Lynn, PE30 4ET. James Coulston, Somerset NHS Foundation Trust, Musgrove Park Hospital, Taunton, TA1 5DA. Richard Gaunt, Rowden Medical Partnership, Rowden Hill, Chippenham, SN15 2SB. Sam Davis, West Walk Surgery, Yate West Gate Centre, 21 West Walk, Yate, BS37 4AX. Amardeep Heer, Lakeside Healthcare, Lakeside Surgery, Cottingham Road, Corby, Northants, NN17 2UR. N'Jaimeh Asamoah‐Owusu, The Crouch Oak Family Practice, 45 Station Rd, Addlestone, KT15 2BH. Gordon Irvine, The Brekland Alliance, Grove Lane, Thetford, IP24 2HY. Patrick Moore, The Adam Practice, 306 Blandford Road, Poole, BH15 4JQ. Stephanie Howard, Norfolk Community Health and Care NHS Trust, Norwich Community Hospital, Bowthorpe Rd, Norwich, NR2 3TU. Katherine Morgan, Lancashire Care NHS Trust Foundation, Leyland Clinic, Yewlands Drive, Leyland, PR25 2TN. Agnes Collarte, Central London Community Healthcare NHS Trust, St Charles Centre for Health & Wellbeing, Exmoor St, London, W10 6DZ. Shruti Singh, Trafalgar Group Medical Practice, 25 Osbourne Road, Southsea, Portsmouth PO5 3ND. Heart of Bath: Tim Johnson, Heart of Bath, Oldfield Surgery, 45 Upper Oldfield Park, Bath BA2 3HT. [Correction added on 21 March 2023, after first online publication: Tim Johnson was added to acknowledgements.]
Bull RH, Clements D, Collarte AJ, Harding KG. The impact of a new intervention for venous leg ulcers: A within‐patient controlled trial. Int Wound J. 2023;20(6):2260‐2268. doi: 10.1111/iwj.14107
Trial Registration: ClinicalTrials.gov reference number: NCT03396731.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Guest JF, Ayoub N, McIlwraith T, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;5(12):e009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neumann HAM, Cornu‐Thenard A, Junger M, et al. Evidence‐based (S3) guidelines for diagnostics and treatment of venous leg ulcers. J Eur Acad Dermatol Venereol. 2016;30:1843‐1875. [DOI] [PubMed] [Google Scholar]
- 3. Harding KG. Chronic wounds: a clinical problem requiring ownership and coordination. Br J Dermatol. 2022;187:134‐135. [DOI] [PubMed] [Google Scholar]
- 4. Phillips TJ, Dover JS. Leg ulcers. J Am Acad Dermatol. 1991;25:965‐987. [DOI] [PubMed] [Google Scholar]
- 5. Nelson EA, Adderley U. Venous leg ulcers. BMJ Clin Evid. 2016;2016:1902. [PMC free article] [PubMed] [Google Scholar]
- 6. Godwin M, Ruhland L, Casson I, et al. Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity. BMC Med Res Methodol. 2003;3(28). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Falanga V. Care of venous ulcers. Ostomy Wound Manage. 1999;45(Suppl 1A):33 S‐43 S. [PubMed] [Google Scholar]
- 8. Robson MC, Hill DP, Woodske ME, Steed DL. Wound healing trajectories as predictors of effectiveness of therapeutic agents. Arch Surg. 2000;135:773‐777. [DOI] [PubMed] [Google Scholar]
- 9. O'Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012;11(11):CD000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol. 2002;119(6):1420‐1425. [DOI] [PubMed] [Google Scholar]
- 11. Driver VR, Gould LJ, Dotson P, et al. Identification and content validation of wound therapy clinical endpoints relevant to clinical practice and patient values for FDA approval. Part 1. Survey of the wound care community. Wound Rep and Reg. 2017;25(3):454‐465. [DOI] [PubMed] [Google Scholar]
- 12. Louis TA, Lavori PW, Bailar JC 3rd, Polansky M. Crossover and self‐controlled designs in clinical research. N Engl J Med. 1984;310(1):24‐31. [DOI] [PubMed] [Google Scholar]
- 13. AdvaMed Wound Healing and Tissue Regeneration Sector . Guiding Principles for Clinical Research in Chronic Wound Healing: A Consensus Document. 2010.
- 14. Vidal A, Mendieta Zerón H, Giacaman I, et al. A simple mathematical model for wound closure evaluation. J Am Coll Clin Wound Spec. 2016;7(1–3):40‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bull RH, Staines KL, Collarte AJ, Bain DS, Ivins NM, Harding KG. Measuring progress to healing: a challenge and an opportunity. Int Wound J. 2022;19(4):734‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tucker A, Maass A, Bain D, et al. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int J Angiol. 2010;19(1):e31‐e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harding K, Dowsett C, Fias L, et al. Simplifying Venous Leg Ulcer Management. Consensus Recommendations. Wounds International; 2015. [Google Scholar]
- 18. Santler B, Goerge T. Chronic venous insuffieciency ‐ a review of pathophysiology, diagnosis and treatment. J Germ soc Derm. 2017;15(5):538‐556. [DOI] [PubMed] [Google Scholar]
- 19. Wounds International . Principles of compression in venous disease: a practitioner's guide to treatment and prevention of venous leg ulcers. 2013. https://www.woundsinternational.com/resources/details/principles‐compression‐venous‐disease‐practitioners‐guidetreatment‐and‐prevention‐venous‐leg‐ulcers
- 20. Partsch H, Mortimer P. Compression for leg wounds. Br J Derm. 2015;173(2):359‐369. [DOI] [PubMed] [Google Scholar]
- 21. Brem H, Kirsner RS, Falanga V. Protocol for the successful treatment of venous ulcers. Am J Surg. 2004;188(1A Suppl):1‐8. [DOI] [PubMed] [Google Scholar]
- 22. Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2005;111(18):2398‐2409. [DOI] [PubMed] [Google Scholar]
- 23. Meissner MH. Lower extremity venous anatomy. Semin Intervent Radiol. 2005;22(3):147‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ibegbuna V, Delis KT, Nicolaides AN, Aina O. Effect of elastic compression stockings on venous hemodynamics during walking. J Vasc Surg. 2003;37(2):420‐425. [DOI] [PubMed] [Google Scholar]
- 25. Meagher H, Clarke‐Moloney G, O'Laighin G, Grace PA. An experiemental study of prescribed walking in the management of venous leg ulcers. J Wound Care. 2012;21(9):421‐430. [DOI] [PubMed] [Google Scholar]
- 26. Yang D, Vandongen YK, Stacey MC. Effect of exercise on calf muscle pump function in patients with chronic disease. Br J Surg. 1999;86(3):338‐341. [DOI] [PubMed] [Google Scholar]
- 27. Mosti G. Compression therapy in immobile or with limited mobility patients affected by leg ulcers. Poster Presented at European Wound Management Association (EWMA) Conference, Belgium, 2011.
- 28. Davies J, Bull R, Farrelly I, Wakelin M. Improving the calf muscle pump using home‐based exercises for patients with chronic venous disease. Wounds UK. 2008;4(3):48‐58. [Google Scholar]
- 29. Schuren J, Mohr K. Pascal's law and the dynamics of compression therapy: a study of healthy volunteers. Int Angiol. 2010;29(5):431‐435. [PubMed] [Google Scholar]
- 30. Griffin M, Bond D, Nicolaides A. Measurement of blood flow in the deep veins of the lower limb using the geko™ neuromuscular electro‐stimulation device. Int Angiol. 2016;35(4):406‐410. [PubMed] [Google Scholar]
- 31. Das SK, Dhoonmoon L, Chhabra S. Neuromuscular stimulation of the common peroneal nerve increases arterial and venous velocity in patients with venous leg ulcers. Int Wound J. 2021;18(2):187‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Das SK, Dhoonmoon L, Bain D, Chhabra S. Microcirculatory changes in venous leg ulcers using intermittent electrostimulation of common peroneal nerve. J Wound Care. 2021;30(2):151‐155. [DOI] [PubMed] [Google Scholar]
- 33. Bosanquet DC, Ivins N, Jones N, Harding KG. Microcirculatory flux and Pulsatility in arterial leg ulcers is increased by intermittent neuromuscular electrostimulation of the common peroneal nerve. Ann Vasc Surg. 2021;71:308‐314. [DOI] [PubMed] [Google Scholar]
- 34. Jawad H, Bain DS, Dawson H, Crawford K, Johnston A, Tucker A. The effectiveness of a novel neuromuscular electrostimulation method versus intermittent pneumatic compression in enhancing lower limb blood flow. J Vasc Surg Venous Lymphat Disord. 2014;2(2):160‐165. [DOI] [PubMed] [Google Scholar]
- 35. Williams KJ, Moore HM, Davies AH. Haemodynamic changes with the use of neuromuscular electrical stimulation compared to intermittent pneumatic compression. Phlebology. 2015;30(5):365‐372. [DOI] [PubMed] [Google Scholar]
- 36. Warwick D, Shaikh A, Worsley P, et al. Microcirculation in the foot is augmented by neuromuscular stimulation via the common peroneal nerve in different lower limb postures: a potential treatment for leg ulcers. Int Angiol. 2015;34(2):158‐165. [PubMed] [Google Scholar]
- 37. Warwick DJ, Shaikh A, Gadola S, et al. Neuromuscular electrostimulation viathe common peroneal nerve promotes lower limb blood flow in a below‐kneecast: a potential for thromboprophylaxis. Bone Joint Res. 2013;2(9):179‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones NJ, Ivins N, Ebdon V, Hagelstein S, Harding KG. Neuromuscular electrostimulation on lower limb wounds. Br J Nurs. 2018;27(20):S16‐S21. [DOI] [PubMed] [Google Scholar]
- 39. Harris C, Loney A, Brooke J, et al. Refractory venous leg ulcers: observational evaluation of innovative new technology. Int Wound J. 2017;14(6):1100‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harris C, Ramage D, Boloorchi A, Vaughan L, Kuilder G, Rakas S. Using a muscle pump activator device to stimulate healing for non‐healing lower leg wounds in long‐term care residents. Int Wound J. 2019;16(1):266‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Snowden JM. Wound closure: an analysis of the relative contributions of contraction and epithelialization. J Surg Res. 1984;37(6):453‐463. [DOI] [PubMed] [Google Scholar]
- 42. Maini P, McElwain D, Leavesley D. Traveling waves in a wound healing assay. App Math Lett. 2004;17:575‐580. [Google Scholar]
- 43. Gorin DR, Cordts PR, LaMorte WW, Menzoian JO. The influence of wound geometry on the measurement of wound healing rates in clinical trials. J Vasc Surg. 1996;23:524‐258. [DOI] [PubMed] [Google Scholar]
- 44. Waycaster CR, Gilligan AM, Motley TA. Cost‐effectiveness of Becaplermin gel on diabetic foot ulcer HealingChanges in wound surface area. J Am Podiatr Med Assoc. 2016;106(4):273‐282. [DOI] [PubMed] [Google Scholar]
- 45. Dolibog P, Franek A, Taradaj J, et al. A comparative clinical study on five types of compression therapy in patients with venous leg ulcers. Int J Med Sci. 2013;11(1):34‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


