Abstract
This study evaluates the effect of nursing staff's renewed consistent pressure ulcer (PU) prevention practice on PU prevalence and the PU prevention implemented for residents. A quasi‐experimental intervention study was conducted. The data were collected from 232 residents (n = 115 in intervention and 117 in comparison group) in two public long‐term older people care (LOPC) facilities in Finland using the Pressure Ulcer Patient instrument (PUP‐Instrument). The facilities were chosen with convenience sampling, after which they were randomly allocated as either intervention or comparison facility. Based on international guidelines for PU prevention, the renewed, consistent PU prevention practice with six areas was developed and implemented using the operational model for evidence‐based practices (OMEBP). After the intervention, a significant difference between the intervention and the comparison facility was seen in the prevalence of PUs and in the residents’ highest stage of PUs in the sacrum, buttock and hip areas, and heels. Between the facilities, a significant difference was seen in the use of PU and nutrition risk assessment instruments and nutritional supplements, time used for repositioning in the daytime and at night‐time, lifting belt use, and avoiding shearing or stretching residents’ skin. The successful intervention improved skin integrity in LOPC facilities.
Keywords: evidence‐based practice, intervention, long‐term care, pressure ulcer, prevention
1. INTRODUCTION
Consistent practice based on evidence has been seen as a guarantee for quality of care. 1 , 2 Consistent practice means that no variation occurs in patient care regardless of the person or organization providing it. 3 In pressure ulcer (PU) prevention, consistent practice can be improved by using clinical practice guidelines. 4 , 5 The European Pressure Ulcer Advisory Panel (EPUAP), National Pressure Injury Advisory Panel (NPIAP), and Pan Pacific Pressure Injury Alliance (PPPIA) have drawn up international PU prevention guidelines. 6 These guidelines include several recommendations concerning various PU prevention areas such as risk assessment, skin assessment and care, nutrition, repositioning, and support surfaces.
However, PU prevalence in long‐term older people care (LOPC) is persistently high, varying across Europe. 6 In addition, despite various reported PU prevention interventions, the effectiveness of the preventive interventions in LOPC has varied. 7 This indicates a continued need for further allocation of resources into promoting PU prevention. In the context of LOPC, effective PU prevention interventions and implementation models for PU prevention practice are needed. A consistent practice intervention for PU prevention that is commonly agreed based on evidence and when developed and implemented with the OMEBP model produces new knowledge to ensure PU prevention of sufficient quality in LOPC.
Older people are at higher risk for PUs because of a wide range of characteristics, such as age, multiple comorbidities, and living in an aged care facility 6 , 8 , 9 For example, in Japan, in 2017, the age‐specific number of people with PUs including PU stage I was 9.2 per 1,000 population in those aged ≥65 years, and 44.6 in those aged ≥80 years. 9 In New Zealand, in 2016, PU prevalence in nursing home facilities was 8% in those over 65 years, but increased to 12% in those older than 85. 10 Older people with comorbidities such as cancer, 11 , 12 cardiovascular diseases, 13 dementia, 12 or diabetes 14 are at higher risk of PU. With age, physical activity and moving decrease as well, 15 and limited mobility, long‐lasting sitting or lying may lead to the development of PUs. 8 , 12 , 16 Immobility may also weaken the general health status 17 and lead to poorer condition of the skin and the appearance of PUs. 15 In addition, PU prevalence in older people has been reported to be associated with memory disorders or proximity to death. 18 , 19 , 20
In a review of long‐term conditions, the PU prevalence reported in care homes or nursing homes varied from 3.4% to 32.4%. 21 In the last 5 years, the reported PU prevalence numbers in LOPC facilities varied depending on the country or setting, but also on whether PU stage I was included or not for prevalence accounting. PU stage I means intact skin with non‐blanchable redness of a localised area, usually over a bony prominence. PU stage II and higher include stages from partial skin loss to full tissue loss with exposed fat, bone, tendon, or muscle. 6 PU prevalence including stage I and higher was reported in Finland, Switzerland, New Zealand, Portugal, and Germany. In these studies, in 10 to 75 LOPC facilities, in the years 2014 to 2018, PU prevalence rates of 4.3% to 12% were identified. 10 , 22 , 23 , 24 , 25 PU prevalence including PUs of stage II or higher was reported in the United States and Germany. In 2012 to 2018, in these studies, including 7662 to 2,936,146 LOPC residents, the PU prevalence was 4.0% to 10.1%. 26 , 27 In older residents with severe care dependency as well as in older people with a life expectancy of at most 6 months eligible for palliative home care service, most PUs were located in the sacrum followed by the heels. 18 , 25
Previously, prevention of PUs in LOPC consisted of various interventions such as repositioning, 28 , 29 nutrition, 30 pressure relieving devices, 31 , 32 or PU prevention bundles or programmes. 33 , 34 , 35 Effective interventions aim at preventing PUs in LOPC included repositioning with change of back position and 30 degrees tilting every 3 hours combined with the heels offloaded from the bed, 29 nutritional intervention where extra protein and calories were served, 30 use of advanced mattresses, overlays or cushions in beds or wheelchairs. 31 , 32 , 36 PU prevention bundles or programmes consisting of components of best practices have also been effective. 34 , 35
In addition, risk assessment, skin assessment, and nursing staff education have usually been an essential part of previous PU prevention interventions. In international PU prevention guidelines, risk assessment has been recommended to identify individuals at potential risk of PU. Risk assessment should first be made at every admission to health care, identifying those who are at risk of PU followed by full screening with a PU risk assessment tool. 6 The advantage of risk assessment scales, such as Braden scale, 37 is that they provide a structural approach to risk assessment in practice. 6 Skin assessment is a recommended practice as a component of any PU risk assessment and should be conducted as soon as possible after admission and, during repositioning, briefly for pressure points. 6 Education of nursing staff has usually been part of PU prevention interventions in LOPC. Education sessions of PU prevention topics or protocol compliance have been part of or used as a supportive structure for interventions. 28 , 29 , 32 , 33 , 34 , 35 , 38 Education of nursing staff was the most often reported supporting structure to promote the implementation of PU prevention interventions in LOPC. 7
The aim of this study was to evaluate the effect of nursing staff's renewed consistent PU prevention practice on PU prevalence and the impact of PU prevention implemented for residents. The research questions were as follows: (1) What is the prevalence of PU and the residents’ highest stages of PUs at baseline and after the renewed consistent pressure ulcer prevention practice? (2) What PU prevention practices have been implemented for the residents at baseline and after the renewed consistent pressure ulcer prevention practice? The hypothesis was that after the renewed consistent PU prevention practice intervention, the prevalence of PU and the residents’ highest stages of PUs would decrease and PU prevention implemented for residents improve in the intervention facility compared with the comparison facility. The ultimate goal is to decrease suffering and to achieve cost savings in PU treatment by improving the quality of PU prevention care in long‐term older people care.
2. METHODS
2.1. Study design, participants, and setting
A quasi‐experimental intervention study was conducted between January 2016 and January 2017 in two public LOPC facilities in Finland. The two LOPC facilities, including a total of 13 care units, were chosen with convenience sampling, after which the facilities were randomly allocated as intervention or comparison group. For convenience sampling, facilities with more than a hundred beds and observed PUs were chosen after a cross‐sectional study of older people with PUs, conducted prior to this study, in private and public LOPC facilities in the area. 22 In total, the intervention facility included five and the comparison facility eight care units. All residents (n = 122 intervention/n = 133 comparison) in the facilities were invited to participate in the study. The invitation included permission to assess their skin and to use their patient records. Of these, 232 (91.0%), (n = 115 in intervention facility/117 in comparison facility) residents participated. In addition, all RNs and PNs (n = 161, 76 intervention/85 comparison) were invited to participate in the study. Of these, 141 (88%, n = 69/72) RNs and PNs participated. The TREND guidelines were followed. 39
In the Finnish health care system, municipalities are responsible for organizing health care and forming the basis of the health care system. 40 In 2018, municipalities provided over 50% of the sheltered housing with 24‐h assistance. 41 In Finland, nursing staff in LOPC facilities are led by head nurses and comprise registered nurses (8.4%) and practical nurses (74%). 41 Practical nurses have vocational education in social and health care, lasting 2 to 3 years, with varied competence areas, such as care and rehabilitation for older people. 42
2.2. Intervention
The intervention was aimed at changing the PU prevention practice of nursing staff. The intervention, which included development and implementation of a renewed consistent practice for PU prevention, 43 was based on international guidelines. The content of the renewed consistent practice was a bundle of six PU prevention areas: risk assessment, skin assessment and skin care, nutrition, repositioning, pressure relieving devices, and documentation. 43 , 44 The operational model for developing evidence‐based practices (OMEBP) (Figure 1) was used for developing and implementing the intervention. In the comparison care units, usual standard PU practice prevention was continued.
FIGURE 1.
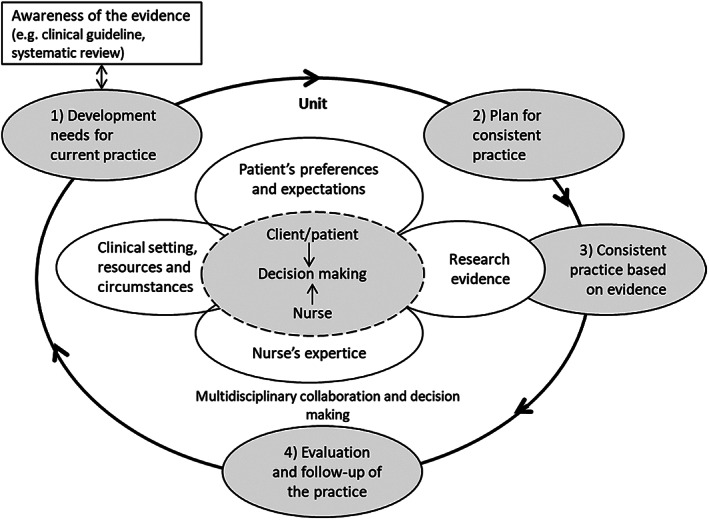
The operational model for evidence‐based practices, OMEBP 45
2.2.1. Development and implementation of a renewed consistent PU prevention practice using OMEBP
The operational model for developing evidence‐based practices 45 , 46 (OMEBP, Figure 1) supports the implementation of the best available evidence. In the OMEBPs, the development and implementation of practice proceeded in four phases. 43 The phases I and II took place partially at the same time.
In the first phase (two months; January‐February), “Development needs for current practice”, the researchers arranged two meetings for head nurses, RNs and PNs. During the meetings, the participants were informed about the research and given presentations on evidence‐based practices, OMEBPs, 45 international guidelines regarding PU prevention and early identification, 44 and the international PU classification system. 44 The results of the assessed current PU prevention practice in the facility 43 were also reported, and by comparing the current practice with international PU prevention guidelines, development needs in the current practice were identified.
The second phase (one month; February), “Plan for consisting practice”, included two meetings attended by head nurses and two wound contact persons from each unit. In these meetings, consistent practice in six content areas of the international PU prevention guidelines—risk assessment, skin assessment and skin care, nutrition, repositioning, pressure relieving devices, and documentation—was planned, and the “Procedure for PU Prevention in LOPC Facility” 43 was written.
In the third phase (10 months; March‐December), “Consistent practice”, the renewed consistent PU prevention practice was first described to nursing staff and then implemented. At unit meetings, the researcher went through the renewed practice together with the nursing staff. A copy of the “Procedure for PU Prevention in LOPC Facility” 43 was also sent to the nursing staff members’ personal e‐mail addresses. In addition, the procedure was presented on the facility's internal web pages. Following this, the renewed consistent practice for PU prevention started. Immediately after unit meetings, the whole nursing staff worked on PU prevention as agreed in “Procedure for PU Prevention 43 in LOPC Facility”.
As a supporting structure, nursing staff were educated in six 90‐minute sessions. PU prevention topics related to risk assessment, skin assessment and skin care, nutrition, pressure relieving devices, and, as secondary prevention of PUs, wound care were taught. The participants were also given written information, such as laminated pocket‐size Braden scale instructions and the international PU classification system. The nursing staff members were also advised to use existing web material on PU prevention. In addition, the nursing staff were encouraged to consult the researcher and authorised wound care nurse.
In the fourth phase (one month; January), “Evaluation and follow‐up of the practice”, the second data set of the nursing staff's PU prevention practice was collected. 43 This happened after 10 months use of renewed consistent PU prevention practice (phase three). Also, the researcher made regular weekly visits to the intervention units at random times to promote the fidelity of the implemented renewed consistent practice.
2.3. Outcomes, data collection, and instrument
Outcomes of the intervention were as follows: (1) prevalence of PU, (2) the residents’ highest PU stage, and (3) PU prevention practices implemented for residents. The data were collected at baseline in January 2016 and after the intervention in January 2017 using the Pressure Ulcer Patient instrument (PUP‐Ins). 47 , 48 , 49 PUP‐Ins, a structured questionnaire, was completed for every resident as the resident's skin was assessed. The same measurers, ie, a registered nurse specialised in wound care and the researcher, assessed the skins of all the residents and also collected the data on characteristics of the residents and the PU prevention practices implemented for residents from patient records or by interviewing the nursing staff both in the intervention and the comparison facility. EPUAP Category stage I‐IV PUs were included in the study. 44
The Pressure Ulcer Patient instrument (PUP‐Ins), developed and used in previous studies, 22 , 47 , 48 , 49 was updated in 2015, based on international PU prevention guidelines. 44 PUP‐Ins includes13 items of background questions (such as gender, age, weight, height, diagnosed diseases, and length of stay in a facility) and 48 items of dichotomous, multiple‐choice, and open‐ended questions: 16 items on PUs: localization, stage, and description of PUs, 2 items of mental state, 3 items on urinary continence or bowel retention, and 27 items on PU prevention practices implemented for the resident. These 27 items include movement and repositioning (15 items), pressure relieving devices (2 items), skin assessment (4 items), and nutrition (6 items). The items of resident's PU prevention practices movement and repositioning, and pressure relieving devices differ and are used in part, depending on whether the resident is a person who is bedridden, seated, or walking.
2.4. Analysis
Data were analysed with IBM SPSS Statistics for Windows 26 (IBM Corp., Armonk, NY). The prevalence of PU stages II‐IV after the intervention was estimated to be 10% in the control group and 1% in the intervention group. The required sample size to detect this difference was 100 residents in both groups with 80% power and alpha of 0.05.
Independent samples t‐test for normally distributed continuous variables, Mann‐Whitney U‐test for non‐normally distributed continuous variables, and Pearson chi square test or Fisher's exact test for categorical variables were used in comparison of participants’ characteristics between the groups. Differences in PU prevalence, the residents’ highest PU stage, and PU prevention practices implemented for residents within the groups were tested by using Wilcoxon signed rank test for non‐normally distributed continuous variables and for ordinal variables, and McNemar's test for dichotomous variables. The differences between groups in PU prevalence, the residents’ highest PU stage, and PU prevention practices implemented for residents were compared with Mann‐Whitney U‐test for non‐normally distributed continuous variables and for ordinal variables, and with Pearson chi‐square test or Fisher's exact test for dichotomous variables. The level of significance was set at P < .05.
2.5. Ethical considerations
The research followed good scientific practices as determined by the Finnish Advisory Board on Research Integrity 50 and conforms with the Declaration of Helsinki. 51 Ethical approval (43/2015) was obtained from the Ethics Committee of the University. Permissions to conduct the study were given by the participating organizations. The nursing staff were informed, they were asked for informed consent to participate, and they had an opportunity to ask questions before and during the research. Residents were asked for a written informed consent and they were informed about voluntariness to consent to check their skin and the use of their patient records and possibility to discontinue participation at any stage of skin assessment. If the resident was unable to understand the consent question presented to him or her because of illness, permission was sought from the resident's close relative or legal representative.
3. RESULTS
At baseline, data on skin assessment and data from patient records of 232 participated residents were collected (n = 115 in the intervention group and n = 117 in the comparison group). After the intervention, one year after the baseline, data of 176 residents were collected (n = 96 in the intervention group and n = 80 in the comparison group). In the data collection after the intervention, 53 of 232 participants were missing because 48 (n = 19 in intervention/29 in comparison group) residents had died, one was in hospital, and four had been moved to another facility.
3.1. Characteristics of participants
At baseline, no statistical differences were found between the intervention group and the comparison group with respect to most characteristics of residents (Table 1) or nursing staff. 43 However, at baseline, some statistical differences were found between the residents in some diseases (P = .026 to .048), in health status and movement, and between the nursing staff members in work experience in the current work unit and in the amount of reading guidelines about PU prevention. After the intervention, no statistical differences were found between the intervention group and the comparison group with respect to residents’ characteristics (Supplement 1).
TABLE 1.
Characteristics of residents, baseline
| Characteristics of residents | Intervention group (n = 115) | Comparison group (n = 117) | P‐value a |
|---|---|---|---|
| Gender, n (%), All | 114 (100) | 117 (100) | .123 |
| Male | 24 (21.1) | 35 (29.9) | |
| Female | 90 (78.9) | 82 (71.1) | |
| Age, Mean (SD) | 83.05 (7.33) b | 84.74 (6.07) b | .058 c |
| Length of stay in facility in years, Median [IQR] | 1.08 [1.92] d | 1.75 [4.00] d | .602 e |
| Diseases, n (%), All | 112 (100) | 117 (100) | |
| Cardiovascular/vascular disease | 89 (79.5) | 79 (67.5) | .041 |
| Neurological disease | 95 (84.8) | 109 (93.2) | .043 |
| Memory disease/disorder | 79 (70.5) | 91 (77.8) | .210 |
| Endocrine disease | 37 (33.0) | 37 (31.6) | .819 |
| DM II or decreased sugar tolerance | 24 (21.4) | 22 (18.8) | .620 |
| Musculoskeletal disease | 42 (37.5) | 28 (23.9) | .026 |
| Psychiatric illness | 21 (18.8) | 12 (10.3) | .067 |
| Height, Mean (SD) | 163.43 (11.535) f | 134.13, (9.799) f | .814 c |
| Weight, Mean (SD) | 68.17 (14.478) g | 66.99 (14.742) g | .557 c |
| Fever, Yes, n (%) | 2 (1.8) | 4 (3.5) | .683 h |
| Smoking, Yes, n (%) | 1 (0.9) | 1 (0.9) | 1.000 h |
| Health condition, n (%) | 109 (100) | 117 (100) | .002 |
| Good | 31 (28.4) | 16 (13.7) | |
| Satisfied | 50 (45.9) | 47 (40.2) | |
| Week | 25 (22.9) | 40 (34.2) | |
| Very week | 3 (2.8) | 14 (12.0) | |
| Mobility, n (%) | 113 (100) | 117 (100) | .013 |
| Bedbound | 20 (17.7) | 39 (33.3) | |
| Seated | 35 (31.0) | 36 (30.8) | |
| Walking | 58 (51.3) | 42 (35.9) | |
| Diet, n (%) | 112 (100) | 116 (100) | .134 |
| Basic diet | 68 (60.7) | 59 (50.9) | |
| Structure‐modified diet | 58 (100) | 56 (100) | .027 h |
| Soft | 5 (8.6) | 9 (16.1) | |
| Puree | 44 (75.9) | 45 (80.4) | |
| Liquid | 2 (3.4) | 2 (3.6) | |
| Special diet | 7 (12.1) | 0 (0.0) | |
| Urinary incontinence, n (%) | 102 (89.5) | 106 (90.6) | .775 |
Pearson chi square‐test.
n = 113 in intervention group and 117 in comparison group.
Independent samples t‐test.
n = 109 in intervention group and 115 in comparison group.
Mann‐Whitney U‐test.
n = 31 in intervention group and 23 in comparison group.
n = 111 in intervention group and 101 in comparison group.
Fisher's exact test.
3.2. Prevalence of PU and the residents’ highest stage of PUs
Five skin areas were assessed for PUs. Three areas had the most PUs (Tables 2 and 3).
TABLE 2.
Prevalence of PU baseline and after the intervention
| Intervention group (Baseline n = 113 residents, After the intervention n = 95 residents) | Comparison group (Baseline n = 116 residents, After n = 80 residents) | Difference between groups | ||||||
|---|---|---|---|---|---|---|---|---|
| Assessed skin area for PUs | Baseline PU stage I‐IV n (%) | After PU stage I‐IV n (%) | Difference within groups P‐value | Baseline PU stage I‐IV n (%) | After PU stage I‐IV n (%) | Difference within groups P‐value | Baseline, P‐value | After, P‐value |
| Sacrum, buttock and hip areas | 4 (3.5) | 2 (2.1) | 1.000 a | 9 (7.8) | 10 (12.5) | .109 a | .168 b | .007 b |
| 1 (1.1) | 2 (2.1) | 4 (5.1) | 10 (12.7) | |||||
| Other areas of the feet | 6 (5.3) | 7 (7.3) | .754 a | 2 (1.7) | 3 (3.8) | .625 a | .167 c | .515 c |
| 5 (5.3) | 7 (7.4) | 1 (1.3) | 3 (3.8) | |||||
| Heels | 3 (2.7) | 0 (0.0) | .754 a | 3 (2.6) | 8 (10.1) | .0625 a | 1.000 c | .001 c |
| 1 (1.1) | 0 (0.0) | 2 (2.5) | 8 (10.1) | |||||
| All areas, PU stages I‐IV | 12 (10.6) | 9 (9.4) | .774 a | 10 (8.6) | 17 (21.3) | .008 a | .608 b | .027 b |
| 7 (7.4) | 9 (9.5) | 5 (6.3) | 17 (21.5) | |||||
| All areas, PU stages II‐IV | 5 (4.4) | 0 (0.0) | NA | 3 (2.6) | 6 (7.5) | .063 a | .495 b | .008 b |
| 2 (2.1) | 0 (0.0) | 1 (1.3) | 6 (7.6) | |||||
Note: The level of significance for bold values was set at <0.05.
McNemar's test, included residents assessed both at baseline and after the intervention.
Pearson chi square test.
Fisher's exact test.
TABLE 3.
The residents' highest stages of PUs at baseline and after the intervention
| Intervention group (Baseline n = 113 residents, After the intervention, n = 95 residents) | Comparison group (Baseline n = 116 residents, After n = 80 residents) | Difference between groups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Assessed skin area for PUs | PU stage | Baseline PUs n (%) | After PUs n (%) | Difference within groups P‐value | Baseline PUs n (%) | After PUs n (%) | Difference within groups P‐value | Baseline, P‐value | After, P‐value |
| Sacrum, buttock and hip areas | I | 1 (0.9) | 2 (2.1) | 1.000 a | 6 (5.2) | 7 (8.8) | .088 a | .176 b | .006 b |
| 0 (0.0) | 2 (2.1) | 3 (3.8) | 7 (8.9) | ||||||
| II | 3 (2.7) | 0 (0.0) | 1 (0.9) | 2 (2.5) | |||||
| 1 (1.1) | 0 (0.0) | 0 (0.0) | 2 (2.5) | ||||||
| III | 0 (0.0) | 0 (0.0) | 1 (0.9) | 1 (1.3) | |||||
| 0 (0.0) | 0 (0.0) | 0 (0.0) | 1(1.3) | ||||||
| IV | 0 (0.0) | 0 (0.0) | 1 (0.9) | 0 (0.0) | |||||
| 0 (0.0) | 0 (0.0) | 1 (1.3) | 0 (0.0) | ||||||
| Other areas of the feet | I | 4 (3.5) | 7 (7.3) | .782 a | 2 (1.7) | 2 (2.5) | .257 a | .137 b | .336 b |
| 4 (4.2) | 7 (7.4) | 1 (1.3) | 2 (2.5) | ||||||
| II | 2 (1.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||||
| III | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.3) | |||||
| 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.3) | ||||||
| IV | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||||
| Heels | I | 3 (2.7) | 0 (0.0) | .317 a | 1 (0.9) | 5 (6.4) | .033 a | .646 b | .003 b |
| 1 (1.1) | 0 (0.0) | 1 (1.3) | 5 (6.5) | ||||||
| II | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.6) | |||||
| 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.6) | ||||||
| III | 0 (0.0) | 0 (0.0) | 1 (0.9) | 0 (0.0) | |||||
| 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||||
| IV | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||||
| All | I | 7 (6.2) | 9 (9.4) | .967 a | 7 (6.0) | 11(13.8) | .006 a | .604 b | 0.020 b |
| 5 (5.3) | 9 (9.5) | 4 (5.1) | 11 (13.9) | ||||||
| II | 5 (4.4) | 0 (0.0) | 1 (0.9) | 4 (5.0) | |||||
| 2 (2.1) | 0 (0.0) | 0 (0.0) | 4 (5.1) | ||||||
| III | 0 (0.0) | 0 (0.0) | 1 (0.9) | 2 (2.5) | |||||
| 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.5) | ||||||
| IV | 0 (0.0) | 0 (0.0) | 1 (0.9) | 0 (0.0) | |||||
| 0 (0.0) | 0 (0.0) | 1 (1.3) | 0 (0.0) | ||||||
Note: The level of significance for bold values was set at <0.05.
Wilcoxon signed rank test; included residents assessed both at baseline and after the intervention.
Mann‐Whitney U‐test.
At baseline, no statistical differences were found between the intervention group and the comparison group with respect to the prevalence of PUs (P = .167‐1.00) (Table 2). Before the intervention, most PUs in both groups were located in the sacrum, buttock and hip areas, other areas of the feet, and heels. A statistically significant difference between the intervention and the comparison group was seen after the intervention in the prevalence of PU in the sacrum, buttock and hip areas (P = .007), n = 2 (2.1%) intervention, n = 10 (12.5%) comparison, and heels (P = .001), n = 0 (0.0%) intervention, n = 8 (10.1%) comparison. A statistically significant difference between the intervention and the comparison group was also seen after the intervention in all areas in the prevalence of PU stages I‐IV (P = .027) and stages II‐IV (P = .008) (Table 2).
At baseline, no statistical differences were found between the intervention group and the comparison group with respect to the residents’ highest stage of PUs (P = .137 to .646) (Table 3). Stage I was the most common PU stage. A statistically significant difference between the intervention and the comparison group was seen after the intervention in the residents’ highest stage of PUs in the sacrum, buttock and hip areas (P = .006), heels (P = .003), and in all areas (P = .020). In addition, in the comparison group, residents’ PU stages showed an increased negative trend in the heels and all areas.
3.3. PU prevention practices implemented for resident
At baseline (Table 4), no statistical differences were found between the intervention group and the comparison group with respect to most variables of resident's PU prevention. However, at baseline, there was a significant difference between the intervention group and the comparison group in the following variables: PU risk assessment instrument used, skin assessment daily used time for, skin assessment duration, weight monitoring, nutrition risk assessment instrument in use, duration of repositioning at night, use of sliding sheet, use of lifting belt, and mattresses, (P < .001 to .023).
TABLE 4.
PU prevention practices implemented for resident baseline and after the intervention
| Intervention group, n (%) Baseline, 113 (100), After the intervention, 95 (100) | Comparison group, n (%) Baseline, 117 (100), After, 80 (100) | Difference between groups | ||||||
|---|---|---|---|---|---|---|---|---|
| PU prevention | Baseline | After | Difference within groups, P‐value | Baseline | After | Difference within groups, P‐value | Baseline, P‐value | After, P‐value |
| PU Risk assessment instrument used, All n | 112 | 94 | 116 | 80 | ||||
| n (%) | 10 (8.9) | 73 (77.7) | 0 (0.0) | 0 (0.0) | .001 a | <.001 a | ||
| All n | 91 | 91 | 79 | 79 | ||||
| n (%) | 7 (7.7) | 72 (79.1) | <.001 b | 0 (0.0) | 0 (0.0) | NA | ||
| Skin assessment, time (min) used daily, median[IQR] | 0.3[0.25] | 0.2 [0.12] | 0.0 [0.05] | 0.2 [0.17] | <.001 c | .018 c | ||
| 10.0[18.0] | 2.0[6.0] | .903 d | 10.0[7.0] | 10.0[10.0] | .096 d | |||
| Skin assessment duration | ||||||||
| All n | 113 | 93 | 117 | 80 | <.001 c | .209 c | ||
| Daily, every care session or repositioning, n(%) | 52(46.0) | 63 (67.7) | 92 (78.6) | 62 (77.5) | ||||
| Daily, every work‐shift, n (%) | 39 (34.5) | 21 (22.6) | 21 (17.9) | 10 (12.5) | ||||
| Weekly during care session or repositioning, n(%) | 22 (19.5) | 9 (9.7) | 4 (3.4) | 8 (10.0) | ||||
| All n | 91 | 91 | .002 d | 80 | 80 | .553 d | ||
| Daily, every care session or repositioning, n(%) | 43 (47.3) | 62 (68.1) | 61 (76.3) | 62 (77.5) | ||||
| Daily, every work‐shift, n (%) | 29 (31.9) | 20 (22.0) | 15 (18.8) | 10 (12.5) | ||||
| Weekly during care session or repositioning, n(%) | 19 (20.9) | 9 (9.9) | 4 (5.0) | 8 (10.0) | ||||
| Weight monitoring, All n | 108 | 95 | 116 | 78 | <.001 c | .023 c | ||
| No | 26 (24.1) | 13 (13.7) | 52 (44.8) | 13 (16.7) | ||||
| 1‐6 times a year | 31 (28.7) | 67 (70.5) | 48 (41.4) | 64 (82.1) | ||||
| Monthly | 51 (47.2) | 15 (15.8) | 16 (13.8) | 1 (1.3) | ||||
| All n | 89 | 89 | .024 d | 77 | 77 | .139 d | ||
| No | 23 (25.8) | 11 (12.4) | 32 (41.6) | 13 (16.9) | ||||
| 1‐6 times a year | 23 (25.8) | 65 (73.0) | 34 (44.2) | 63 (81.8) | ||||
| Monthly | 43 (48.3) | 12 (14.6) | 11 (14.3) | 1 (1.3) | ||||
| Nutrition risk assessment instrument in use, All n | 113 | 92 | 117 | 80 | ||||
| n (%) | 18 (15.9) | 46 (50) | 0 (0.0) | 1 (1.3) | <.001 a | <.001 a | ||
| All n | 90 | 90 | 80 | 80 | ||||
| n (%) | 14(15.6) | 46 (51.1) | .001 b | 0 (0.0) | 1 (1.3) | NA | ||
| Nutritional supplements, All n | 113 | 94 | 117 | 80 | ||||
| Yes, n (%) | 9 (8.0) | 16 (17.0) | 5 (4.3) | 5 (6.3) | .242 a | .030 a | ||
| All n | 92 | 92 | 80 | 80 | ||||
| Yes, n (%) | 9(9.8) | 16 (17.4) | .118 b | 2 (2.5) | 5 (6.3) | .375 b | ||
| Bedbound resident, Repositioning | ||||||||
| Duration of repositioning daytime, hours, median[IQR] | 2.5 [1.0] | 2.5 [1.0] | 2.8 [0.5] | 3.0 [2.0] | .108 c | .255 c | ||
| 2.0[8.0] | 2.5[6.0] | .705 d | 2.0[1.0] | 2.5[2.0] | .343 d | |||
| Daytime, time used for repositioning in minutes, median[IQR] | 0.1 [4.2] | 0.5(0.25] | 0.0 [0.47] | 0.3 [0.13] | .083 c | <.001 c | ||
| 10.0[26.0] | 2.0[28.0] | .116 d | 27.5[15.0] | 15.0[8.0] | .266 d | |||
| Duration of repositioning night‐time, hours, median(IQR) | 3.0 [1] | 3.0 [1] | 4.0 [0] | 4.0 [0] | <.001 c | <.001 c | ||
| 3.0[1.0] | 4.0[0.0] | .276 d | 3.0[1.0] | 4.0[0.0] | .066 d | |||
| Night‐time, time used for repositioning in minutes, median[IQR] | 0.2[0.18] | 0.3 [0.13] | 0.0 [0.22] | 0.1 [0.13] | .086 c | <.001 c | ||
| 10.0[12.0] | 2.0[13.0] | .065 d | 17.5[9.0] | 8.0[4.0] | 0.536 d | |||
| Bedbound resident, While transferring resident, All n | 24 e | 25 | 44 e | 36 | ||||
| Avoid shearing or stretching skin n (%) | 17 (70.8) | 12 (48) | 28 (63.6) | 18 (50) | .549 a | .878 a | ||
| Transfer plate is used n (%) | 1 (4.2) | 1 (4.0) | 1 (2.3) | 2 (5.6) | .659 a | .782 a | ||
| Sliding sheet is used n (%) | 15 (62.5) | 24 (96.0) | 38 (86.4) | 34 (94.4) | .023 a | .782 a | ||
| Lifting belt is used n (%) | 3 (12.5) | 1 (4.0) | 0 (0.0) | 1 (2.8) | .016 a | .792 a | ||
| Lifter is used n (%) | 2 (8.7) | 3 (12.0) | 4 (9.1) | 6 (16.7) | .957 a | .613 a | ||
| All n | 10 | 10 | 24 | 24 | ||||
| Avoid shearing or stretching skin n (%) | 6 (60.0) | 5 (50.0) | 1.000 | 16 (6.67) | 14 (58.3) | 0.727 | ||
| Transfer plate is used n (%) | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.00) | NA | ||
| Sliding sheet is used n (%) | 10 (100) | 10 (100) | NA | 20 (83.3) | 22 (91.7) | 0.687 | ||
| Lifting belt is used n (%) | 0 (0.0) | 1 (10.0) | NA | 0 (0.0) | 0 (0.0) | NA | ||
| Lifter is used n (%) | 0 (0.0) | 0 (0.0) | NA | 4 (16.7) | 3 (12.5) | 1.000 | ||
| Bedbound resident, In the lateral position, All n | 21 e | 26 | 45 e | 36 | ||||
| Direct pressure at bony prominences is avoided n (%) | 11 (52.4) | 23 (88.5) | 28 (62.2) | 29 (80.6) | .449 a | .404 a | ||
| A pressure relieving back cushion is used n (%) | 11 (52.4) | 7 (26.9) | 24 (53.3) | 12 (33.3) | .942 a | .399 a | ||
| Pressure relieving posture cushions are used n (%) | 16 (76.2) | 12 (46.2) | 36 (80.0) | 18 (50.0) | .724 a | .765 a | ||
| All n | 11 | 11 | 24 | 24 | ||||
| Direct pressure at bony prominences is avoided n (%) | 5 (45.5) | 9 (81.8) | .289 b | 16 (66.7) | 20 (83.3) | .219 b | ||
| A pressure relieving back cushion is used n (%) | 7 (63.6) | 2 (18.2) | .180 b | 13 (54.2) | 9 (37.5) | .289 b | ||
| Pressure relieving posture cushions are used n (%) | 9 (81.8) | 5 (45.5) | .289 b | 20 (83.3) | 13 (54.2) | .065 b | ||
| Seated resident, Repositioning | ||||||||
| Sits in a chair daily continuously for a maximum of hours, median(IQR) | 3.0[2.3] | 3.5 [1.0] | 3.8[2.9] | 3.8 [1.5] | .497 c | .962 c | ||
| 4.0[3.3] | 3.8[2.6] | .543 d | 4.0[2.0] | 4.0[2.9] | .243 d | |||
| Assisted in changing position approximately, duration, hours, median[IQR] | 2.0 [1] | 3.5 [3] | 2.0 [2] | 2.0 [0] | .285 c | .135 c | ||
| 2.0[−] | 2.8[−] | .285 d | 2.0[−] | 1.8[−] | .180 d | |||
| Time used daily for repositioning, minutes, median[IQR] | 0.2[0.11] | 0.1 [0.26] | 0.1[0.40] | 0.1 [0.21] | .628 c | .636 c | ||
| 15.0[0.0] | 2.0[0.0] | .157 d | 2.0[0.0] | 7.5[−] | .180 d | |||
| Seated resident, the resident is advised to change position, All n | 41 f | 31 | 31 | 26 | ||||
| Yes, n (%) | 7 (17.1) | 5 (16.1) | 5 (16.1) | 1 (3.8) | .915 a | .132 a | ||
| All n | 18 | 18 | 10 | 10 | ||||
| Yes, n (%) | 3 (16.7) | 3 (16.7) | 1.000 b | 3 (30.0) | 0 (0.0) | NA | ||
| Seated resident, All n In chair with | 52 | 29 | 37 | 28 | ||||
| Support belt n (%) | 5 (9.6) | 3 (10.3) | 8 (21.6) | 10 (35.7) | .114 a | .022 a | ||
| Crotch wedge n (%) | 4 (7.7) | 0 (0.0) | 1 (2.7) | 0 (0.0) | .314 a | NA | ||
| Anti‐ slip cloth n (%) | 1 (2.0) | 1 (3.4) | 3 (8.1) | 0 (0.0) | .172 a | .322 a | ||
| All n | 20 | 20 | 12 | 12 | ||||
| Support belt n (%) | 1 (5.0) | 2 (10.0) | 1.000 b | 3 (25.0) | 6 (50.0) | .250 b | ||
| Crotch wedge n (%) | 2 (10.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA | ||
| Anti‐ slip cloth n (%) | 0 (0.0) | 1 (5.0) | NA | 2 (16.7) | 0 (0.0) | NA | ||
| Seated resident, All n While transferring | 45 | 29 | 35 | 28 | ||||
| Shearing or stretching resident's skin is avoided n (%) | 42 (93.3) | 29 (100) | 29 (100.0) | 23 (82.1) | .074 a | .017 a | ||
| Transfer plate is used n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | NA | ||
| Sliding sheet is used n (%) | 3 (6.7) | 6 (20.7) | 0 (0.0) | 3 (10.7) | .119 a | .302 a | ||
| Lifting belt is used n (%) | 1 (2.2) | 4 (13.8) | 2 (5.7) | 0 (0.0) | .415 a | .042 a | ||
| Lifter is used n (%) | 9 (20.5) | 10 (34.5) | 10 (28.6) | 6 (21.4) | .402 a | .273 a | ||
| All n | 20 | 20 | 11 | 11 | ||||
| Shearing or stretching resident's skin is avoided n (%) | 19 (95.0) | 20 (100) | NA | 9 (81.8) | 10 (90.9) | 1.000 b | ||
| Transfer plate is used n (%) | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA | ||
| Sliding sheet is used n (%) | 1 (5.0) | 6 (30) | .125 b | 0 (0.0) | 1 (9.1) | NA | ||
| Lifting belt is used n (%) | 1 (5.0) | 4 (20.0) | .375 b | 1 (9.1) | 0 (0.0) | NA | ||
| All n | 19 | 19 | .250 b | 11 | 11 | |||
| Lifter is used n (%) | 5 (26.3) | 8 (42.1) | 3 (27.3) | 3 (27.3) | 1.000 b | |||
| At night ensured that resident does not sleep in the same position for more than 4 hours, All n | 57 | 29 | 52 | 28 | ||||
| n (%) | 31 (54.4) | 17 (58.6) | 36 (69.2) | 13 (46.4) | .112 a | .357 a | ||
| All n | 19 | 19 | 15 | 15 | ||||
| n (%) | 10 (52.6) | 12 (63.2) | .687 b | 10 (66.7) | 6 (40.0) | .219 b | ||
| Walking resident, is activated to independent moving, All n | 69 | 40 | 47 | 30 | ||||
| n (%) | 41 (59.4) | 21 (52.5) | 20 (42.6) | 6 (20.0) | .074 a | .006 a | ||
| All n | 34 | 34 | 1.000 b | 25 | 25 | .065 b | ||
| n (%) | 20 (58.8) | 20 (58.8) | 13 (52.0) | 6 (24.0) | ||||
| Mattresses, All n | 113 | 95 | 117 | 80 | <.001 a | .032 a | ||
| Foam mattress n (%) | 38 (33.0) | 30 (31.6) | 73 (62.4) | 39 (48.8) | <.001 a | .021 a | ||
| A pressure‐distributing mattress, Foam gel mattress n (%) | 67 (58.8) | 57 (60.0) | 44 (37.6) | 39 (48.8) | .003 a | .136 a | ||
| A pressure‐distributing mattress, Static air overlay n (%) | 9 (7.9) | 8 (8.4) | 1 (0.9) | 2 (2.5) | .027 a | .093 a | ||
| All n | 94 | 94 | .247 d | 80 | 80 | .006 d | ||
| Foam mattress n (%) | 33 (35.1) | 30 (31.9) | .508 b | 50 (62.5) | 39 (48.8) | .003 b | ||
| A pressure‐distributing mattress, Foam gel mattress n (%) | 56 (59.6) | 56 (59.6) | 1.000 b | 29 (36.3) | 39 (48.8) | .013 b | ||
| A pressure‐distributing mattress, Static air overlay n (%) | 5 (5.3) | 8 (8.5) | .375 b | 1 (1.3) | 2 (2.5) | 1.000 b | ||
| Cushion in wheelchair/chair | ||||||||
| Pressure‐distributing cushion in wheelchair, All n | 113 | 95 | 117 | 80 | ||||
| n (%) | 8 (7.0) | 12 (12.6) | 6 (5.1) | 5 (6.3) | .547 a | .156 a | ||
| All n | 94 | 94 | 80 | 80 | ||||
| n (%) | 3 (3.7) | 12 (12.8) | .092 b | 4 (5.3) | 5 (6.3) | 1.000 b | ||
| Diaper changes per day, median[IQR] | 3.0[1.0] | 3.0[1.0] | 3.0[1.5] | 2.0 [0.5] | .557 c | <.001 c | ||
| .253 d | .100 d | |||||||
Note: The level of significance for bold values was set at <0.05.
Chi‐square test.
McNemar's test; included residents assessed both at baseline and after the intervention.
Mann‐Whitney U‐test.
Wilcoxon signed rank test; included residents assessed both at baseline and after the intervention.
Part of seated residents also included here.
Part of bedbound residents also included here.
After the intervention, there was significant improvement in the intervention group in the use of PU risk assessment instrument (7.7% vs 79.1%, P < .001), skin assessment duration (P = .002), weight monitoring (P = .024), and in the use of nutritional supplements (15.6% vs 51.1%, P < .001).
After the intervention, there was a significant difference between the intervention group and comparison group in variables PU risk assessment instrument used, (77.7% vs 0.0%, P < .001), skin assessment time (P = .018), weight monitoring (P = .023), nutrition risk assessment instrument used (50.0% vs 1.3%, P < .001), nutritional supplements used (17.0% vs 6.3%, P = .030), time (minutes) used for repositioning in daytime (P < .001), time (minutes) used for repositioning at night‐time (P < .001), seated resident, when transferring, shearing or stretching resident's skin is avoided (100% vs 82.1%, P = .017), and lifting belt is used (13.8% vs 0.0%, P = .042), walking resident is activated to independent moving (52.5% vs 20.0%, P = 006), mattresses (P = .032), foam mattress (31.6% vs 48.8%, P = .021), and diaper changes per day (P < .001).
4. DISCUSSION
The purpose of this study was to evaluate the effect of nursing staff's renewed consistent PU prevention practice on PU prevalence, the residents’ highest stages of PUs, and the impact on PU prevention implemented for residents. The intervention, which included development and implementation of the renewed consistent PU prevention practice, was based on international PU prevention guidelines. The content of the developed renewed consistent practice comprised a bundle of six PU prevention areas: risk assessment, skin assessment and skin care, nutrition, repositioning, pressure relieving devices, and documentation. The intervention was shown to be effective in reducing the prevalence of PUs and the residents’ highest stages of PUs in the sacrum, buttock and hip areas, and heels.
As hypothesised, PU prevalence decreased more in the intervention facility compared with the comparison facility. At baseline, PU prevalence in the facilities (10.6% intervention/8.6% comparison) including PUs stage I and higher was consistent with the PU prevalence reported previous five years. 10 , 22 PU prevalence including PUs of stage II and higher was 4.4% in the intervention facility, while 2.6% in the comparison facility, which was lower compared with the PU prevalence reported in earlier studies. 26 , 27 After the intervention, PU prevalence in the facilities (9.4% in intervention /21.3% in comparison facility) including PUs of stage I and higher was consistent with the PU numbers reported in the previous 5 years, 10 , 22 but PU prevalence in the intervention facility was significantly lower compared with the comparison facility. After the intervention, the prevalence of PU including PUs of stage II and higher changed to the opposite: PU prevalence was 0.0% in the intervention facility, which was lower than earlier studies reporting PU prevalence, 26 , 27 but rose to 7.5% in the comparison facility. The prevalence of PUs including stage II and higher was significantly lower in the intervention facility compared with the comparison facility. This shows the effectiveness of the conducted intervention.
As hypothesised, the PU stages decreased more in the intervention facility compared with the comparison facility. At baseline, PUs in both facilities were mostly stage I and II. After the intervention, in the intervention facility, all the PUs were stage I, and there were no PUs of stage II or higher. This means that PUs were detected at an early stage and none of them developed into second stage or broke the skin. In contrast, in the comparison facility, the stages of PUs worsened significantly during the study, and after the intervention, there was a significant difference between the intervention and comparison facility in terms of PU stages. The results are in line with a previous study of 28 LOPD residents 33 where after the PU prevention bundle in four areas—heel protection/skin assessment, repositioning, incontinence, and nutrition—only stage I PUs existed. The results of this study are opposite to those reported in earlier studies conducted in LOPC facilities of PU prevention interventions consisting of repositioning, 29 mattresses, overlays or cushions, 31 , 32 and PU prevention programmes, 34 where stage II PUs still existed. This shows the effectiveness of the conducted intervention and also recommends the use of bundles in PU prevention.
As shown in earlier studies conducted in LOPC, 18 , 25 , 29 also in this study most PUs were located in the sacrum, buttock and hip areas, and at the heels. However, unlike in previous studies, in this study, PUs were also found in other areas of the feet, which can be explained by the higher number of walking residents who were in better condition. In contrast to previous studies, 18 , 25 , 29 after the intervention, there were no PUs in the heels and only two stage I PUs in the sacrum, buttock, and hip areas. This indicates the effectiveness of the conducted intervention in decreasing PUs in the most common PU areas. In the comparison facility, most PUs were still located in the sacrum, buttock, and hip areas.
The intervention was shown to be effective in PU prevention practices implemented for residents—by improving the use of the PU risk assessment instrument and, in the prevention area of nutrition, in weight monitoring and the use of nutritional supplements. In repositioning there was a significant difference after the intervention between the intervention group and the comparison group time used for repositioning in daytime and at night‐time. In pressure relieving devices, when transferring a seated resident, shearing or stretching of the resident's skin was avoided and lifting belt was used. However, after the intervention, the use of more advanced mattresses had not changed. This may be because many residents already had advanced mattresses in use.
Although the residents’ PU risk‐increasing characteristics were multiple, the intervention worked well under these conditions, and after the intervention, none of the intervention facility residents had broken skin caused by PUs. In several earlier studies, residents’ characteristics increasing the PU risk have been reported: their mean age was high, over 83/85 years, and they lived in older people care facilities. 9 , 10 They had multiple PU risk‐increasing co‐morbidities 12 , 13 , 14 , 15 , 18 ; three‐quarters of the residents had cardiovascular or vascular disease, memory disease or disorders, and a quarter or more of the residents had type 2 diabetes or musculoskeletal disease. In addition, only up to a third of the residents had good health status while the rest of the residents had satisfactory, poor, or very poor health status; additionally, approximately half of the residents were seated or bedridden and thus had limited mobility, all of which increases the risk for PU15.
This study had some limitations: all the answers to the PUP‐instrument were not reported in patient records and they were asked by the nursing staff who cared for the residents, which may have had an impact on the results. However, the same two persons, a registered nurse specialised in wound care and the researcher, collected all the data on participants' characteristics and the PU prevention practices implemented for residents from patient records and interviewed the nursing staff both in the intervention and the comparison facility, which may have improved the consistency of the data. Furthermore, the content of the PUP‐instrument was based on good level of evidence from international PU prevention guidelines, and had been used and evaluated previously. 22 , 47 , 48 , 49 However, further testing of the instrument used is required. In addition, the PUP‐instrument may also work as a slightly shorter and concise version. These could be topics of future research. The consistency of the results was improved by the fact that the same two persons also assessed the skin of all the residents in the intervention and the comparison facility and by the use of the systematic instrument, the EPUAP scale of PUs stages 1– 444 in the assessment. The difference in the degree of mobility at baseline between the intervention and comparison group was also a limitation. In the comparison group, there were more bedbound residents than in the intervention group, which may have had an impact on the results. However, 49% of the residents in the intervention group were totally bedbound or seated, and the effect of the intervention could be seen in their unbroken skin. After the intervention, walking residents had PUs located in other areas of the feet caused by shoes, and prevention of PUs in walking residents requires more investment.
The international PU prevention guidelines 6 consist of more than a hundred pages of recommendations, and their direct use in practical work is impossible. For daily practical use, shorter, concise, renewed instructions tailored for LOPC facility level are needed. After the intervention, the intervention facility had an effective PU prevention practice, which was commonly agreed, evidence‐based, and tailored for LOPC facility level. In addition, for implementation, a systematic implementation model and expertise of the nursing staff as experts in the local context were required. The use of the OMEBP model enabled this.
In conclusion, the successful intervention improved older people's skin integrity in the LOPC facilities. This study supports the implementation of a commonly agreed, renewed consistent PU prevention practice based on international guidelines, tailored for facility level to improve the skin integrity of older people in LOPC facilities. This study recommends the use of bundles in PU prevention. The results of this study also support the use of the OMEBP model for systematic development and implementation of evidence‐based practice as well as use of nursing staff as experts on local conditions when developing and implementing evidence‐based practice. This study produced new knowledge for the purpose of PU prevention of sufficient quality in LOPC aimed at decreasing suffering and achieving cost savings in PU treatment.
AUTHOR CONTRIBUTIONS
Sirpa Mäki‐Turja‐Rostedt, Helena Leino‐Kilpi, and Elina Haavisto conceptualised and designed the study; Sirpa Mäki‐Turja‐Rostedt and Elina Haavisto developed consistent practice; Sirpa Mäki‐Turja‐Rostedt collected the data; Sirpa Mäki‐Turja‐Rostedt, Tero Vahlberg, and Elina Haavisto analysed the data; Sirpa Mäki‐Turja‐Rostedt, Helena Leino‐Kilpi, Marita Koivunen, Tero Vahlberg, and Elina Haavisto wrote or revised the manuscript. All authors approved the final manuscript.
CONFLICT OF INTEREST
No conflict of interest has been declared by the authors.
Supporting information
Data S1: Supporting Information
ACKNOWLEDGEMENTS
Sincere thanks to the nursing staff and residents of the facilities for taking part in this study. Anna Vuolteenaho is acknowledged for editing the English language.
Mäki‐Turja‐Rostedt S, Leino‐Kilpi H, Koivunen M, Vahlberg T, Haavisto E. Consistent pressure ulcer prevention practice: The effect on PU prevalence and PU stages, and impact on PU prevention—A quasi‐experimental intervention study. Int Wound J. 2023;20(6):2037‐2052. doi: 10.1111/iwj.14067
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Barradas Cavalcante T, Carvalho Moura EC, Barros Araújo Luz MH, Luz Nunes Queiroz AAF, Barbosa Furtado L, da Silva Monte BK. Updating of the assistance protocol for pressure prevention: evidence based practice. J Nurs UFPE. 2016;3(Suppl. 3):1498‐1506. [Google Scholar]
- 2. Jylhä V, Oikarainen A, Perälä M‐L, Holopainen A. Facilitating evidence‐based practice in nursing and midwifery in the WHO European Region. Euro.who.int. 2017. http://www.euro.who.int/__data/assets/pdf_file/0017/348020/WH06_EBP_report_coua=1 [Google Scholar]
- 3. Kennedy P, Leathley CM, Hughes CF. Clinical practice variation. Med J Aust. 2010;193(8):S97. [DOI] [PubMed] [Google Scholar]
- 4. Beal ME, Smith K. Pressure ulcer prevalence in an acute care hospital using evidence‐based practice. Worldviews Evid Based Nurs. 2016;13(2):112‐117. [DOI] [PubMed] [Google Scholar]
- 5. Martin D, Albensi L, Haute S, et al. Healthy skin wins: A glowing pressure ulcer prevention program that can guide evidence‐based practice. Worldviews Evid Based Nurs. 2017;14(6):473‐483. [DOI] [PubMed] [Google Scholar]
- 6. European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline. Emily Haesler (Ed.). EPUAP/NPIAP/PPPIA. 2019.
- 7. Mäki‐Turja‐Rostedt S, Stolt M, Leino‐Kilpi H, Haavisto E. Preventive interventions for pressure ulcers in long‐term older people care facilities: A systematic review. J Clin Nurs. 2019;28(13–14):2420‐2442. doi: 10.1111/jocn.14767 [DOI] [PubMed] [Google Scholar]
- 8. Latimer S, Chaboyer W, Thalib L, McInnes E, Bucknall T, Gillespie BM. Pressure injury prevalence and predictors among older adults in the first 36 hours of hospitalisation. J Clin Nurs. 2019;28(21–22):4119‐4127. doi: 10.1111/jocn.14967 [DOI] [PubMed] [Google Scholar]
- 9. Nakashima S, Yamanashi H, Komiya S, Tanaka K, Maeda T. Prevalence of pressure injuries in Japanese older people: a population‐based cross‐sectional study. PLoS One. 2018;13(6):e0198073. doi: 10.1371/journal.pone.0198073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carryer J, Weststrate J, Yeung P, Rodgers V, Towers A, Jones M. Prevalence of key care indicators of pressure injuries, incontinence, malnutrition, and falls among older adults living in nursing homes in New Zealand. Res Nurs Health. 2017;40(6):555‐563. doi: 10.1002/nur.21835 [DOI] [PubMed] [Google Scholar]
- 11. Aljezawi M, Tubaishat A. Pressure injuries among hospitalized patients with cancer: prevalence and use of preventive interventions. J Wound Ostomy Continence Nurs. 2018;45(3):227‐232. doi: 10.1097/WON.0000000000000429 [DOI] [PubMed] [Google Scholar]
- 12. Cai J‐Y, Zha M‐L, Yuan B‐F, Xie Q, Chen H‐L. Prevalence of pressure injury among Chinese community‐dwelling older people and its risk factors: anational survey based on Chinese Longitudinal Healthy Longevity Survey. J Adv Nurs. 2019;75(11):2516‐2525. doi: 10.1111/jan.14008 [DOI] [PubMed] [Google Scholar]
- 13. Gillespie BM, Chaboyer WP, McInnes E, Kent B, Whitty JA, Thalib L. Repositioning for pressure ulcer prevention in adults. Cochrane Syst Rev—Intervention. 2014;3(4):CD009958. doi: 10.1002/14651858.CD009958.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Børsting TE, Tvedt CR, Skogestad IJ, Granheim TI, Gay CL, Lerdal A. Prevalence of pressure ulcer and associated risk factors in middle‐ and older‐aged medical inpatients in Norway. J Clin Nurs. 2018;27(3‐4):e535‐e543. doi: 10.1111/jocn.14088 [DOI] [PubMed] [Google Scholar]
- 15. Coleman S, Gorecki C, Nelson EA, et al. Patient risk factors for pressure ulcer development: Systematic review. Int J Nurs Stud. 2012;50(7):974‐1003. doi: 10.1016/j.ijnurstu.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 16. Moore Z, Cowman S. Pressure ulcer prevalence and prevention practices in care of the older person in the Republic of Ireland. J Clin Nurs. 2012;21(3‐4):367‐371. doi: 10.1111/j.1365-2702.2011.03749.x [DOI] [PubMed] [Google Scholar]
- 17. Skogestad I, Martinsen L, Borsting T, et al. Supplementing the Braden scale for pressure ulcer risk among medical inpatients: The contribution of self‐reported symptoms and standard laboratory tests. J Clin Nurs. 2016;26(1‐2):202‐214. doi: 10.1111/jocn.13438 [DOI] [PubMed] [Google Scholar]
- 18. Artico M, Dante A, D'Angel D, et al. Prevalence, incidence and associated factors of pressure ulcers in home palliative care patients: a retrospective chart review. Palliat Med. 2018;32(1):299‐307. doi: 10.1177/0269216317737671 [DOI] [PubMed] [Google Scholar]
- 19. Estabrooks CA, Hoben M, Poss JW, et al. Dying in a nursing home: treatable symptom burden and its link to modifiable features of work context. J Am Med Dir Assoc. 2015;16(6):515‐520. doi: 10.1016/j.jamda.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 20. Martinsson L, Lundström S, Sundelöf J. Quality of end‐of‐life care in patients with dementia compared to patients with cancer: a population‐based register study. PLoS One. 2018;13(7):e0201051. doi: 10.1371/journal.pone.0201051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anthony D, Alosoumi D, Safari R. Prevalence of pressure ulcers in long‐term care: a global review. Wound Care. 2019;28(11):702‐709. doi: 10.12968/jowc.2019.28.11.702 [DOI] [PubMed] [Google Scholar]
- 22. Stolt M, Hjerppe A, Hietanen H, Puukka P, Haavisto E. Local treatment of pressure ulcers in long‐term care: a correlational cross‐sectional study. J Wound Care. 2019;28(6):409‐415. doi: 10.12968/jowc.2019.28.6.409 [DOI] [PubMed] [Google Scholar]
- 23. Courvoisier DS, Righi L, Béné N, Rae A‐C, Chopard P. Variation in pressure ulcer prevalence and prevention in nursing homes: A multicenter study. Appl Nurs Res. 2018;42:45‐50. doi: 10.1016/j.apnr.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 24. Lopes TS, Marques dos Santos Videira LM, Ricardo Fonseca Saraiva DM, Agostinho ES, Bandarra AJF. Multicentre study of pressure ulcer point prevalence in a Portuguese region. J. Tissue Viability. 2020;29:12‐18. doi: 10.1016/j.jtv.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 25. Hahnel E, Blume‐Peytavi U, Trojahn C, Kottner J. Associations between skin barrier characteristics, skin conditions and health of aged nursing home residents: a multi‐center prevalence and correlational study. BMC Geriatr. 2017;17(1):263. doi: 10.1186/s12877-017-0655-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahn H, Cowan L, Garvan C, Lyon D, Stechmiller J. Risk factors for pressure ulcers including suspected deep tissue injury in nursing home facility residents: analysis of national minimum data set 3.0. Adv Skin Wound Care. 2016;29(4):178‐190; quiz E1. doi: 10.1097/01.ASW.0000481115.78879.63 [DOI] [PubMed] [Google Scholar]
- 27. Raeder K, Jachan DE, Müller‐Werdan U, Lahmann NA. Prevalence and risk factors of chronic wounds in nursing homes in Germany: a cross‐sectional study. Int Wound J. 2020;17(5):1128‐1134. doi: 10.1111/iwj.13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bergstrom N, Smout R, Horn S, Spector W, Hartz A, Limcangco MR. Stage 2 pressure ulcer healing in nursing homes. J Am Geriatr Soc. 2008;56(7):1252‐1258. doi: 10.1111/j.1532-5415.2008.01765.x [DOI] [PubMed] [Google Scholar]
- 29. Moore Z, Cowman S, Conroy RM. A randomised controlled clinical trial of repositioning, using the 30 degrees tilt, for the prevention of pressure ulcers. J Clin Nurs. 2011;20(17–18):2633‐2644. [DOI] [PubMed] [Google Scholar]
- 30. Pouyssegur V, Brocker P, Schneider SM, et al. An innovative solid oral nutritional supplement to fight weight loss and anorexia: open, randomised controlled trial of efficacy in institutionalised, malnourished older adults. Age Ageing. 2015;44(2):245‐251. doi: 10.1093/ageing/afu150 [DOI] [PubMed] [Google Scholar]
- 31. Brienza D, Kelsey S, Karg P, et al. A randomized clinical trial on preventing pressure ulcers with wheelchair seat cushions. J Am Geriatr Soc. 2010;58(12):2308‐2314. Version of Record online: 10 NOV 2010. doi: 10.1111/j.1532-5415.2010.03168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Leen M, Schols J, Hovius S, Halfens RJG. The effect of a simple 3‐step pressure relieving strategy for preventing pressure ulcers: an explorative longitudinal study from 2002–11. Wounds. 2014;26(10):285‐292. [PubMed] [Google Scholar]
- 33. Keen DC, Gaudario M. Implementing pressure ulcer prevention in a welsh nursing home. J. Community Nurs. 2014;28(4):38‐48. [Google Scholar]
- 34. Kwong EW‐Y, Lau AT‐Y, Lee RL‐P, Kwan RY‐C. A pressure ulcer prevention program specially designed for nursing homes: does it work? J Clin Nurs. 2011;20:2777‐2786. doi: 10.1111/j.1365-2702.2011.03827 [DOI] [PubMed] [Google Scholar]
- 35. Tippett AW. Reducing the incidence of pressure ulcers in nursing home residents: a prospective 6‐year evaluation. Ostomy Wound Manage. 2009;55(11):52‐58. [PubMed] [Google Scholar]
- 36. HamptonS CF. Reducing pressure ulcer incidence in a long‐term setting. Br J Nurs. 2005;14(15):S6‐S12. [PubMed] [Google Scholar]
- 37. Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden score for predicting pressure sore risk. Nurs Res. 1987;36(4):205‐210. [PubMed] [Google Scholar]
- 38. Rosen J, Mittal V, Degenholtz H, et al. Pressure ulcer prevention in black and white nursing home residents: a QI initiative of enhanced ability, incentives, and management feedback. Adv Skin Wound Care. 2006;19(5):262‐268. doi: 10.1097/00129334-200606000-00011 [DOI] [PubMed] [Google Scholar]
- 39. Des Jarlais DC, Lyles C, Crepaz N. Trend Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94:36‐366. doi: 10.2105/ajph.94.3.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Finnish Institute for Health and Welfare (THL), Finland . Ageing. 2018, https://thl.fi/en/web/ageing
- 41. Ministry of Social Affair and Health (STM), Finland . Social and health services. https://stm.fi/sotepalvelut/jarjestelma‐vastuut?p_p_id=56_INSTANCE_7SjjYVdYeJHp&p_p_lifecycle=0&p_p_state=normal&p_p_mode=view&p_p_col_id=column‐2&p_p_col_count=2&_56_INSTANCE_7SjjYVdYeJHp_languageId=en_US
- 42. Ministry of Education and Culture (OKM), Finland . Vocational training. https://minedu.fi/en/vocational-education-and-training
- 43. Mäki‐Turja‐Rostedt S, Leino‐Kilpi H, Korhonen T, Vahlberg T, Haavisto E. Consistent practice for pressure ulcer prevention in long‐term older people care: a quasi‐experimental intervention study. Scand J Caring Sci. 2021;35(3):962‐978. doi: 10.1111/scs.12917 [DOI] [PubMed] [Google Scholar]
- 44. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Haesler E ed. 2014, Cambridge Media, Osborne Park, Western Australia.
- 45. Nursing Research Foundation (NRF), Finland . An operational model for evidence‐based practice. https://www.hotus.fi/supporting-structures-in-ebp/?lang=en
- 46. Korhonen T, Holopainen A, Kanerva A‐M, Petman S, Haavisto E. The role of ward managers when developing consistent evidence‐based practices in long‐term care facility: a qualitative study. Malays J Nurs. 2020;11(4):54‐62. [Google Scholar]
- 47. Eriksson E, Hietanen H, Asko‐Seljavaara S. Prevalence and characteristics of pressure ulcers. Clin Nurse Spec. 2000;14(3):119‐125. [DOI] [PubMed] [Google Scholar]
- 48. Lepistö M. Pressure ulcer risk assessment in long‐term care. Developing an instrument. Annales Universitatis Turkuensis D588. Doctoral dissertation. Turku. Painosalama Oy, 2004.
- 49. Lepistö M, Eriksson E, Hietanen H, Asko‐Seljavaara S. Patients with pressure ulcers in Finnish hospitals. Int J Nurs Pract. 2001;7(4):280‐287. [DOI] [PubMed] [Google Scholar]
- 50. Finnish National Board on Research Integrity (TENK) . Responsible conduct of research (RCR). 2012, https://www.tenk.fi/en/responsible-conduct-of-researcch
- 51. World Medical Association (WMA) . Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. 2013, https://www.wma.net/policies‐post/wma‐declaration‐of‐helsinki‐ethical‐principles‐for‐medical‐research‐involving‐human‐subjects/ [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


