Abstract
Negative pressure wound therapy (NPWT) is a wound‐dressing system that applies sub‐atmospheric pressure on the surface of a wound to promote healing. An evolution of this technology, NPWT with solution instillation and dwell time (NPWTi‐d), is increasingly being used to maximise wound closure and reduce failure rates. However, there is still a lack of evidence concerning its use in orthoplastic surgery. Therefore, the aim of this study is to compare NPWTi‐d with NPWT and standard of care for wound management in orthoplastic surgery. A comprehensive literature search using PubMed, Web of Science, and Cochrane databases up to 15 March 2022 was performed, including studies describing the outcomes of NPWTi‐d for traumatic/orthopaedic injuries. A meta‐analysis on the number of surgical debridements, as well as the rate of complete wound closure and complications was carried out, although for other outcomes, a descriptive statistic was applied. Risk of bias and quality of evidence were assessed using the Downs& Black's Checklist for Measuring Quality. Thirteen studies with a total number of 871 patients were included, in which NPWTi‐d demonstrated significantly higher primary wound closure and lower complication rates (P < .05). No difference in the number of surgical procedures required for final wound healing was observed. Moreover, five out of six studies showed better results for NPWTi‐d when the change of the bioburden and bacterial count of the wound were analysed. A singular study investigating the length of the hospital stay of patients treated with NPWTi‐d showed a reduction in the latter. The present meta‐analysis proves that NPWTi‐d is superior to NPTW or conventional dressings in orthoplastic wound care management, in terms of complete wound closure rate and the reduced number of complications. Still, the limited quality of the studies analysed shows that future randomised studies are needed to confirm the benefits and to identify the most appropriate recommendations for using NPWTi‐d in orthoplastic surgery, as well as to investigate the cost‐effectiveness of this wound‐dressing system.
Keywords: negative pressure wound therapy, NPWT, NPWTi‐d, acute wound, chronic wound, infection, orthoplastic surgery
1. INTRODUCTION
Negative pressure wound therapy (NPWT), also called vacuum‐assisted wound therapy, is a wound‐dressing concept used worldwide that continuously or intermittently applies sub‐atmospheric pressure to the surface of a wound in order to promote healing. 1 An up‐and‐coming extension of this technology, the so‐called NPWT with instillation and dwell time (NPWTi‐d), has been developed in recent years with the aim of maximising outcomes and reducing failure rates (i.e. the impossibility of achieving complete healing).
NPWTi‐d consists of the local instillation of a solution and its dwelling for a planned interval of time. 2 In addition to the known effects of standard NPWT, which boost healing through increased local blood flow, reduced tissue edema, reduction of local bacterial load, and the possible development of tissue granulation, NPWTi‐d may provide complementary benefits when it comes to wound cleansing. 1 , 2 , 3 In fact, NPWTi‐d has been associated with an increased development of granulation tissue and improved healing rate of wounds that have not responded adequately to traditional NPWT. 4 , 5 , 6 For all the above‐mentioned reasons, NPWTi‐d continues to gain popularity and the list of indications for its use has been steadily growing since its first conception. Nowadays, it can be applied to the treatment of a wide range of acute and chronic, closed and open, infected and non‐infected wounds, as well as any combination thereof. 7
Best practices for the use of the different forms of NPWT have shifted in recent years, based on a growing body of evidence and extensive worldwide experience with this technology. 8 However, although there are several published studies comparing NPWTi‐d outcomes with NPWT alone, there is a lack of broad‐based evidence regarding its benefits within the setting of orthoplastic surgery, in particular with regard to a variety of complex wound types in which wet‐to‐moist dressing changes or advanced wound dressings are applied. Therefore, there is a need to synthesise existing data across multiple studies, hence providing a more precise estimate of the effects of NPWTi‐d in this field.
The aim of this systematic literature review and meta‐analysis is to compare the various outcomes of NPWTi‐d versus NPWT and standard of care from the perspective of wound care in the field of orthoplastic surgery.
2. MATERIALS AND METHODS
2.1. Literature search
A review protocol was developed based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement (www.prisma-statement.org) (Table 1). A comprehensive literature search was performed by two independent authors (L.DP. and M.DM.) in the bibliographic databases PubMed, Web of Science, and Cochrane Library on 15 March 2022. The following research terms were used: ‘NPWTid’ or ‘NPWTi‐d’ or ‘NPWT with instillation’ or ‘Negative pressure wound therapy with instillation’. Randomised controlled trials, case–control studies, prospective and retrospective cohort studies, and case series with at least five patients were included. Regarding the follow‐up period, there were no limitations. Studies not including injuries of traumatic or orthopaedic origin were excluded. Pre‐clinical studies, ex‐vivo studies, literature studies, and studies in languages other than English were also excluded.
TABLE 1.
Included studies
| Study | Country | Design | N° of pts. (case–control) | F‐up (months) | Control therapy | Cause of injury | Outcomes evaluated |
|---|---|---|---|---|---|---|---|
| Kim et al., 2020 10 | USA | Retrospective comparative | 116 (42–74) | 1 | NPWT |
NPWTi‐d: 17 Ischemic, 16 Neuropathic, 16 Decubitus, 17 Surgical, 3 VI, 4 Trauma, 3 Other NPWT: 6 Ischemic, 14 Neuropathic, 4 Decubitus, 13 Surgical, 2 VI, 1 Trauma, 1 Other |
N° of operations, length of hospital stay, time to final procedure, wound closure/coverage, wounds closed at one month. |
| Chowdhry et al., 2019 11 | USA | Retrospective comparative | 30 (15–15) | 3 | Wet to moist dressing | NPWTi‐d/ NPWT: 15 sternal wounds after sternotomy | Therapy / dressing days, debridement/dressing changes, time to 1° closure |
|
Cole et al., 2020 12 |
USA |
Retrospective Comparative |
10 (5–5) |
NA | Advanced wound dressing |
NPWTid: 1 Trauma, 1 VLU, 2 PU, 1 DFU NPWT: 3 DFU, 1 VLU, 1 Trauma |
Number of debridements and wound complications, final outcome |
| Gabriel et al., 2014 13 | USA | Retrospective comparative | 82 (48–34) | 6 | NPWT |
NPWTid: 13 UE, 14 LE, 21 Trunk NPWT: 11 UE, 4 LE, 19 Trunk |
Length of hospital stay, time to wound closure, N° of surgical debridement in the OR, LOT |
| Schreiner et al., 2020 14 | Germany | Retrospective comparative | 27 (11–16) | NA | NPWT | NPWTi‐d/NPWT: 27 SJI | Length of hospital stay, N° NPWT, N° sponge dressing changes, time to wound closure, chest wall resection, muscle flap transposition, complications, bacterial status |
| Kim et al., 2020 15 | USA | RCT | 181 (93–88) | NA | NPWT |
NPWTi‐d: Ulcers: 2 Arterial, 39 Diabetic, 2 Radiation, 19 Pressure, 2 Venous. 1 Fasciitis, 1 Other, 3 Surgical dehisced, 10 Surgical non‐dehisced, 3 Trauma, 1 Burn NPWT: Ulcers: 3 Arterial, 39 Diabetic, 12 Pressure, 1 Radiation, 3 Venous. 1 Other, 10 Surgical dehisced, 14 Surgical non‐dehisced, 5 Trauma |
N° of inpatient OR debridements, bacterial status, high versus low bacteria count at first dressing change in pts. with initial high bacteria, closed wounds and time to closure/coverage in pts. with high versus low bacteria count, type of wound closure |
| Omar et al., 2016 16 | Germany | Prospective comparative | 20 (10–10) | 4 | NPWT | NPWTi‐d/NPWT: 10 acute wounds LE (traumatic/infected) | Surgeries, time to wound closure, length of hospital stay, wound size |
| Goss et al., 2014 17 | USA, Italy | Prospective Comparative | 13 (7–7) * | 7 days | NPWT |
NPWTi‐d: 1 AI, 1 Trauma, 1 NF, 1 Ischemic, 8 CVU, 4 DFU NPWT: NA |
Bioburden reduction at post‐op. day 7, maintenance of bioburden reduction after debridement to closure. |
| Kim et al., 2014 18 | USA | RCT | 142 (34–74) | 1 | NPWT |
NPWTi‐d 6 min: 7 Ischemic, 6 Neuropathic, 6 Decubitus, 9 Surgical, 2 VI, 2 Trauma, 2 Other NPWT: 17 Ischemic, 16 Neuropathic, 16 Decubitus, 17 Surgical, 3 Venous, 4 Traumatic, 3 Other |
N°. of OR visits, length of hospital stay, time to final surgical procedure, closing rate, remained closed at 1 mo, culture improvement, culture improvement without Gram‐, corynebacterium, and yeast. |
| Kim et al., 2014 18 | USA | RCT | 142 (34–74) | 1 | NPWT | NPWT‐id 20 min: 8 Ischemic, 7 Neuropathic, 4 Decubitus, 10 Surgical, 1 VI, 1 Trauma, 3 Other | ‐“‐ |
| Gabriel et al., 2008 19 | USA | Prospective comparative | 30 (15–15) | 6 | NPWT |
NPWTi‐d: 3 NF, 2 PU, 3 Expos. Hardware, 2 SW, 5 Trauma NPWT: 5 DU, 4 NF, 6 Expos. Hardware |
Days treated, infection cleared, day wound cleared of infection, wound closed, wound closure method (%), 1°/secondary intention, skin graft, local flap, time to wound closure, time to discharge. |
| Timmers et al., 2008 20 | Netherlands | Retrospective comparative | 124 (30–94) | 43–89 | NPWT | NPWTi‐d: 33 Osteomyelitis, 13 Soft tissue, 12 Trauma, 3 NF, 1 Pilonidal sinus | N° of hospital admissions, N° operations, N° of operations per admission, duration of hospital stay, recurrence of osteomyelitis |
| Burusapat et al., 2021 21 | Thailand | Prospective Comparative | 48 (24–24) | NA | NPWT |
NPWTi‐d: 12 PU, 4 DU, 2 Tumour surgery, 4 NF, 2 Trauma NPWT: 12 PU, 3 DU, 2 Tumour surgery, 4 NF, 3 Trauma |
Wound depth and % of reduction, wound volume and % of reduction, pathogens |
| Giri et al., 2020 22 | India | RCT | 48 (25–23) | 10 days | NPWT | NPWTi‐d/NPWT: DFU, Trauma, Insect/snake bite | Change in histological parameters between day 1/10, comparison of CFU and % of reduction in wound size at day 1/ 10. |
2.2. Data extraction
Two independent reviewers (L.DP. and M.DM.) screened all the titles and abstracts. After this initial screening, the articles that met the inclusion criteria were analysed for full‐text eligibility and excluded if they met any one of the exclusion criteria (Figure 1). In case of disagreement between the two reviewers, a third reviewer (P.F.) was consulted in order to reach consensus.
FIGURE 1.
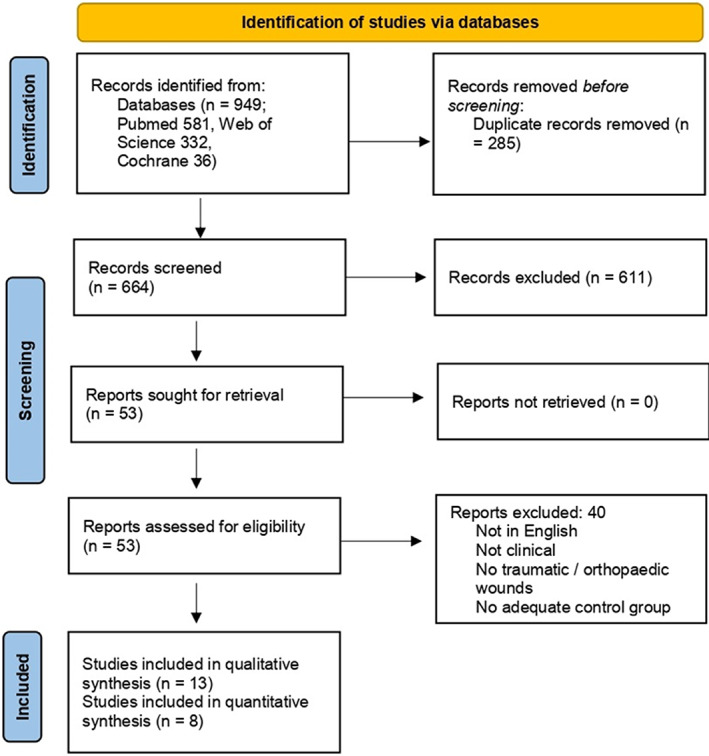
PRISMA of included studies
An electronic table for data extraction was created prior to the study using Excel (Microsoft). The following data were extracted from each included study: country; number of surgical procedures; number, gender and age of the participants; duration of follow‐up; wound characteristics; therapy settings; infection rate; time until final wound closure in days; complication rate; total duration of treatment in days; and length of hospital stay in days. Following this independent data collection, the reviewers compared the extracted data.
2.3. Assessment of risk of bias and quality of evidence of the included studies
The Downs and Black's “Checklist for Measuring Quality” (PMID: 9764259) was used to evaluate the risk of bias. The checklist contains 27 ‘yes’ or ‘no’ questions across five sections. This checklist is easy to use and provides a numeric score out of a scale of 32 points. The five sections include questions about the overall quality of the study (10 items), the ability to generalise the findings of the study (three items), the study bias (seven items), the confounding and selection bias (six items), and the power of the study (one item). Assessment of risk of bias and quality of evidence were completed independently for all outcomes by two authors (L.DP. and P.F.) and a third author (M.DM.) resolved any discrepancies in reaching consensus.
2.4. Statistical analysis
The statistical analysis was carried out according to Neyeloff et al. 9 using Excel (Microsoft) by an independent professional statistician. The Mantel–Haenszel method was used to provide pooled rates across the studies. A statistical test for heterogeneity was first conducted with the Cochran Q statistic and I2 metric and the presence of significant heterogeneity was considered with I2 values ≥25%. When no heterogeneity was found with I2 < 25%, a fixed effect model was used to estimate the pooled rates and 95% CIs. Otherwise, a random‐effect model was applied and an I2 metric was evaluated for the random effect to check the correction of heterogeneity. The studies confidence intervals were carried out using the continuity‐corrected Wilson interval.
3. RESULTS
3.1. Literature search results
Initially, a total of 949 publications were identified according to our inclusion criteria. After the removal of duplicates and screening of the titles and abstracts, 53 articles were chosen for full‐text review. Forty of these did not meet the inclusion criteria, and, therefore, 13 studies (eight from the USA, two from Germany, one from India, one from Thailand, and one from the Netherlands) were included in this systematic review (Figure 1 PRISMA). One study (Kim et al., 2020) was considered twice, because it included two NPWTi‐d groups with two different dwell times. Details of the included studies are summarised in Table 1.
3.2. Details of the included studies
A total of 871 patients were included in this review (511 males, 317 females, and 43 gender unknown); of them, 393 patients were treated with NPWTi‐d (237 males, 134 females, and 22 gender unknown), 478 patients (274 males, 183 females, and 21 gender unknown) were in the control group, being treated either with standard NPWT (used as a control in 12 studies) or wet‐to‐moist dressings and advanced wound dressings (each used once as a control). ‘Advanced wound dressing’ was defined as an alginate or collagen dressing, applied to a wound and changed one to three times per week in accordance with the manufacturers' instructions. The follow‐up time reported for each study varied significantly, from seven days to 89 months (a mean of 2.3 months).
3.3. Therapeutic settings
The mean length of therapy in the NPWTi‐d groups was 10.3 days, resulting in a mean hospital stay of 21.3 days, whereas NPWT was used for a mean of 20.9 days (30.5 days in the hospital). Different instillation solutions were used in the NPWTi‐d groups, the most common one being saline (five out of 13). Other forms of antibacterial solutions were as follows: 1/8 and 1/4 Dakins solution (dilute solution of sodium hypochlorite [0.4% to 0.5%] and other stabilising ingredients), polyhexanide antiseptic solution, Prontosan wound cleansing solution, silver nitrate, and tetrachloride deca oxygen‐anion complex.
3.4. Meta‐analysis outcomes
Out of the 13 studies reviewed, six studies were suitable for the meta‐analysis regarding the rate of complete wound closure: overall, there was a significantly higher rate in NPWTi‐d patients compared with the control group (P = .023, O.R. 2.006, 95% C.I. 1.315–3.058) (Figure 2). Patients who received NPWTi‐d had a significantly lower rate of complication compared with the control group (P = .025, O.R. 0.421, 95% C.I. 0.260–0.683) (Figure 3), whereas there was no significant difference statistically within the number of surgical debridements performed in NPWTi‐d patients compared with the control group (P = .2146, 95% C.I. 0.4–1.3) (Figure 4.).
FIGURE 2.
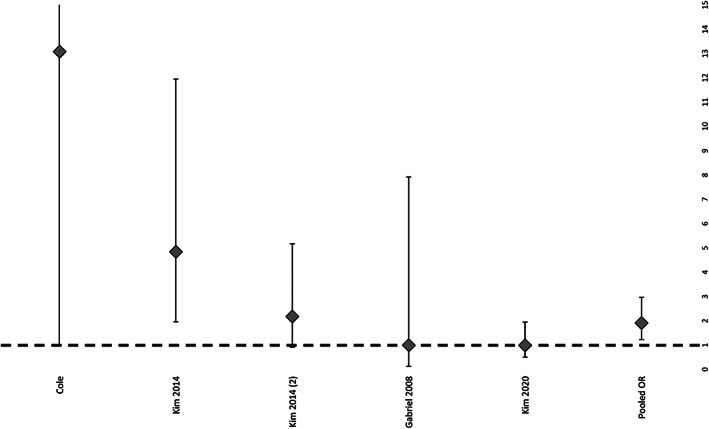
Rate of primary closure of patients treated with NPWTi‐d
FIGURE 3.
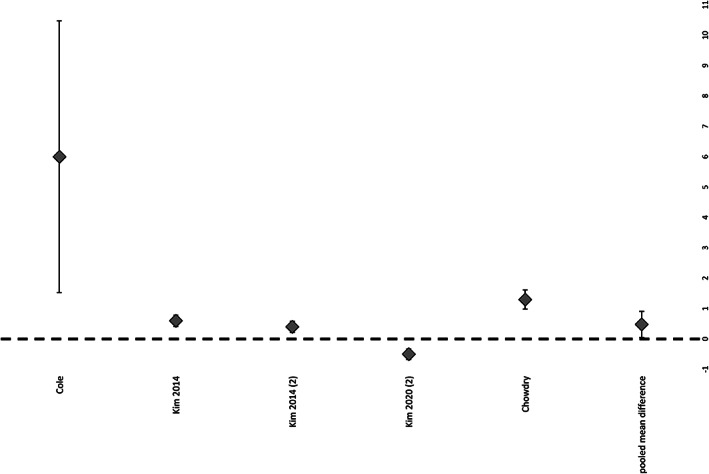
Rate of surgical debridements of patients treated with NPWTi‐d
FIGURE 4.
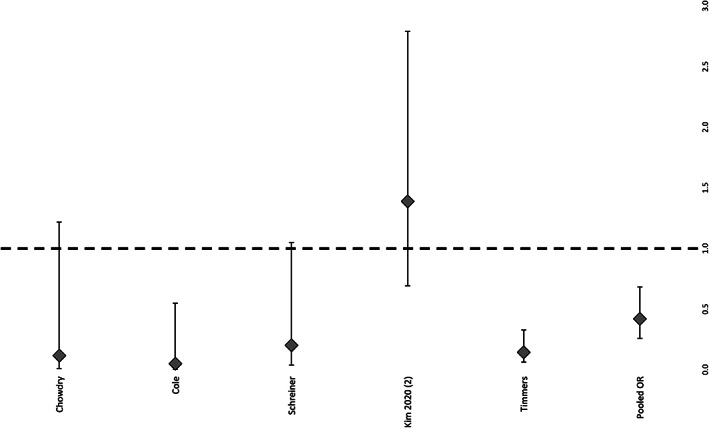
Rate of complications of patients treated with NPWTi‐d
No meta‐analysis could be performed on the time to readiness for surgical closure, length of therapy, costs of the therapy modalities, and length of hospital stay due to the heterogeneity of the data retrieved from the included studies.
3.5. Length of hospital stay and costs of NPWTi‐d
The length of recovery is the time until the final surgical procedure, which within the study published by Kim et al., 2014, is shown to be significantly shorter for the 6‐ and 20‐minute dwell time groups (7.8 ± 5.2 and 7.5 ± 3.1 days, respectively) compared with the standard NPWT group (9.23 ± 5.2 days) (P ≤ .05). 2
With regard to the cost‐effectiveness of NPWTi‐d and NPWT, only one study compared the two therapeutic approaches. Gabriel et al. used a theoretical economic model, which took the costs of the therapy unit, the canisters used, and the dressings for both treatment modalities into consideration. 13 The authors showed that the daily cost of NPWTi‐d amounted to $194.80 per patient, whereas the daily cost of NPWT without instillation amounted to $106.08. However, the reduced rate of surgical debridements (a mean of two debridements in the NPWTi‐d group versus 4.4 debridements in the NPWT group), as well as the possible reduced need to use the operating theatre associated with a decreased total length of treatment and potentially decreased reduction in the length of hospital stay (NPWTi‐d 8.1 day versus NWPT: 27.4 days) resulted in an overall reduced treatment cost of $799 for NPWTi‐d treatment compared with $2217 for NPWT. 1 No cost‐effectiveness comparison between NPWTi‐d and other methods was feasible.
3.6. Change in bioburden and clinical infection
Six studies reported on clinical signs of infection and change in the bioburden of the wound, where ‘bioburden’ is defined as the number of bacteria living on a surface that has not been sterilised. Gabriel et al. showed a significant reduction in the time required for wounds to be clear of clinical infection (25.9 ± 6.6 d NPWTi‐d versus 6.0 ± 1.5 d standard moist wound care therapy P < .01). 2 Although the culture number for Gram+ bacteria was significantly higher for the six‐minute dwell time group compared with the non‐instillation group (90% versus 63%, P ≤ .05), 2 the results of Schreiner et al. showed only a trend to a lower bacterial presence in the NPWTi‐d group (nine out of 11 patients) compared with the NPWT group (eight out of 16 patients), but without this reaching statistical significance (P = .093). 6 Kim et al. achieved similar results, showing a significant mean decrease in the total bacterial count between the time of initial surgical debridement of the wound and the first dressing change in NPWTi‐d subjects (n = 69) compared with NPWT subjects (n = 63) (−0.18 Log10 CFU/g versus 0.6 Log10 CFU/g, respectively; P = .02). 1 This was also shown with the results of Goss et al., which demonstrated a significant difference between the mean absolute reduction in bioburden for the NPWTi‐d group (10.6 × 10°6 bacteria/g of tissue) and the mean absolute increase for the NPWT group (28.7 × 10°6 bacteria/g of tissue) (P = .016). 3 On the other hand, one study by Fluieraru et al. in 2013 described no statistically‐significant difference in the reduction of the microbial load of the wound between the two groups. 5 It was not possible to meta‐analyse the outcomes of these five studies as they reported different outcomes with regard to both the clinical signs of infection and changes in the bioburden of the wound.
4. DISCUSSION
The main finding of this systematic review and meta‐analysis is that NPWTi‐d has been shown to produce significantly higher primary wound closure and lower complication rates (P < .05) compared with routine care, in the setting of orthoplastic wound care. Moreover, five out of six studies showed better results for NPWTi‐d when analysing the change in the bioburden and bacterial count of wounds. A singular study investigating the length of the hospital stay of patients treated with NPWTi‐d showed a reduction in the latter.
Many factors are taken into account by surgeons when it comes to choosing the appropriate therapy for a patient: the first is most likely the complete wound closure rate of the wound, which is crucial, as it means the possibility of closing the wound without further revision. Our current study found a significant advantage in terms of the complete wound closure rate in favour of NPWTi‐d, further elaborating previous literature suggesting that NPWTi‐d used as a supplement was superior in the treatment of complex orthoplastic cases compared with the wound dressings currently used, as demonstrated in the management of osteomyelitis of the proximal femur and spinal wounds. 23 Secondly, the rate of complications is of importance, because it is directly correlated with the risks for the patient and treatment cost, both in the short‐term and the long‐term. The analysis demonstrated a decreased rate of complications when using NPWTi‐d, although the literature does not differentiate between major and minor complications. Moreover, the current analysis confirmed the previous findings of Gabriel et al., Goss et al., and Kim et al, 6 , 15 , 17 namely that NPWTi‐d was superior in reducing the bacterial load of wounds compared with NPWT.
The present study found no difference in the number of surgical debridements, another important aspect, between NPTWi‐d and conventional methods. This finding is interesting given the fact that previous meta‐analyses reported the number of surgical debridements to be lower in NPTWi‐d settings. 8 , 24 However, the present study was conducted in the specific setting of orthoplastic wounds. Therefore, it would be interesting to understand why there is no difference between NPTWi‐d and NPTW in the number of debridements in orthopaedic injuries. In fact, the lack of difference could either be due to the heterogeneity and poor quality of the studies—as demonstrated by the risk of bias evaluation and as suggested by the significant benefits documented in terms of primary closures—or also the possibility of having heterogeneous outcomes in different patients. Future studies should better explore the specific orthoplastic settings in which it is best to use NPTWi‐d.
Future studies also ought to target the lack of evidence regarding the appropriate duration of dwell time, with the current ranges proposed by literature varying from one second to 30 minutes. 19 , 25 , 26 Initially, based on the positive outcomes obtained in the first applications of these therapeutic devices, a shorter span of dwell time, lasting around six minutes, was implemented. 19 , 26 This recommendation was sustained by the concern of longer dwell times increasing the risk of fluid leakage and maceration of the surrounding tissues in contact with the irrigation fluid. These problems have not been clearly documented, and 10‐ to 30‐minute dwell intervals have also been proposed, 2 , 20 , 26 , 27 although unfortunately, current literature makes it difficult to draw definitive conclusions in this regard. In detail specifically, Lehner et al., when studying the treatment of periprosthetic implant infections, reported 5–30 min of dwell time infiltrating polyhexanide 0.04%, resulting in a salvage rate of 80% for acute and 86.4% for chronic infections. 26 Another study reporting a longer dwell time is the one conducted by Timmers et al. using 10–15 min of polyhexanide 0.04% for traumatic bone infections. 20 In this study, a recurrence rate of infection of 10% in the NPWTi‐d group is clearly in contrast with a rate of 58.5% in the controls treated with NPWT or ‘classic’ wound dressings. Furthermore, this study also demonstrated a significantly shorter hospital stay (36 versus 73 days) and a lower number of surgical procedures (2 versus 5) required to achieve complete wound closure. A similar result was shown in the study of Kim et al. in which the time until final surgical procedure was proven to be significantly shorter for groups treated with either a dwell time of six or 20 minutes (7.8 ± 5.2 and 7.5 ± 3.1 d, respectively), compared with the non‐instillation group (9.23 ± 5.2 days) (P ≤ .05). 2 Based on the wide range of dwell times leading to positive outcomes that have been reported, it is to be assumed that a specific dwell time (out of the time range analysed in literature) may not be a crucial factor in the overall effectiveness of NPWTi‐d.
The choice of instillation solution is another debated aspect in NPWTi‐d treatment. The ideal solution for topical instillation should, on one hand, be effective at reducing wound bioburden and, on the other hand, should induce limited cytotoxicity locally. 19 , 28 Achieving this balance is quite difficult, as bactericidal agents commonly inhibit the cellular growth of normal, healthy tissue. 29 , 30 , 31 An agent used on a regular basis is Prontosan, composed of 0.1% polyhexanide, thus implying antimicrobial qualities, and 0.1% betaine as a surfactant. Prontosan has a high tolerability profile with in vivo and in vitro benefits at low concentrations and at the same time is highly effective against a wide variety of pathogens. 32 However, a variety of other solutions and combinations of these have been reported, including Dakin's solution, silver nitrate, and mixed antibiotic solution. 33 , 34 , 35 For example, in the study of Goss et al., a dilute solution of sodium hypochlorite (0.4% to 0.5%) and other stabilising ingredients (Dakin's solution) has proven successful in the bacterial clearance of large wounds of the lower extremity, with only minimal local tissue toxicity. 17 Other studies have suggested the use of normal saline as an instillation solution, showing no significant difference in the endpoints compared with Dakin's solution. 20 , 26 Another study suggesting favourable results for the use of normal saline as instillation liquid is the randomised controlled trial of Kim et al., who compared the use of normal saline versus 0.1% polyhexadine plus 0.1% betadine solution in a total of100 patients, where the saline group was found to require less time to complete wound closure (5.6 d versus 7.5 d, P = .04) as well as no significant differences within length of hospital stay (d), proportion of wounds with complete closure, and number of subsequent debridements required. 36 This may indicate that the combination of negative pressure on the wound tissues and repeated cycles of instillation of the wound may enhance wound closure, rather than the specific instillation solution.
Finally, cost‐effectiveness analysis is another important matter of debate, because it allows for an understanding of the sustainability of a medical or surgical procedure. Up to now, literature has not provided any evidence of the cost‐effectiveness of NPTWi‐d compared with other approaches of wound management in different fields. This is also in line with the current meta‐analysis, in which the data regarding the cost‐effectiveness of NPWTi‐d is based on a single study only. Future studies should be conducted specifically on this important aspect, where the costs resulting from its consumables may be outweighed by the higher effectiveness in achieving final wound closure.
The current study shows interesting findings, nevertheless, several limitations must be highlighted. Firstly, the studies using NPWTi‐d applied the latter to different anatomical regions of the body, treating wounds of variable size and comparing different wound closure techniques. Secondly, many different wound care products were used throughout the studies with regard to the control group, which may also have had an influence on the results. Several studies with a shorter follow‐up time that did not report on the duration of treatment may have biased the results by under‐reporting the complication rate in the long run. Finally, the overall heterogeneity of the available studies and their limited quality made it difficult to properly investigate all outcomes related to these kind of treatments. In view of this, a meta‐analysis could only be performed on several of the outcomes. Future studies are needed to confirm the study findings, as well as to better document and quantify the potential benefits of NPWTi‐d for wound care in the orthoplastic field.
5. CONCLUSIONS
The present meta‐analysis proves that NPWTi‐d is superior to NPTW or conventional dressings in orthoplastic wound care management, in terms of complete wound closure rate and the reduced number of complications. Still, the limited quality of the studies analysed shows that future randomised studies are needed to confirm the benefits and to identify the most appropriate recommendations for using NPWTi‐d in orthoplastic surgery, as well as to further investigate the cost‐effectiveness of this wound‐dressing system.
ACKNOWLEDGEMENTS
None.
De Pellegrin L, Feltri P, Filardo G, et al. Effects of negative pressure wound therapy with instillation and dwell time (NPWTi‐d) versus NPWT or standard of care in orthoplastic surgery: A systematic review and meta‐analysis . Int Wound J. 2023;20(6):2402‐2413. doi: 10.1111/iwj.14072
DATA AVAILABILITY STATEMENT
The present data is a comprehensive literature search in the bibliographic databases PubMed, Web of Science, and Cochrane Library.
REFERENCES
- 1. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563‐576. discussion 577. [PubMed] [Google Scholar]
- 2. Kim PJ, Attinger CE, Constantine T, et al. Negative pressure wound therapy with instillation: international consensus guidelines update. Int Wound J. 2020;17:174‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553‐562. [DOI] [PubMed] [Google Scholar]
- 4. Brinkert D, Ali M, Naud M, Maire N, Trial C, Téot L. Negative pressure wound therapy with saline instillation: 131 patient case series. Int Wound J. 2013;10(Suppl 1):56‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fluieraru S, Bekara F, Naud M, et al. Sterile‐water negative pressure instillation therapy for complex wounds and NPWT failures. J Wound Care. 2013;22:293‐294. 296, 298‐299. [DOI] [PubMed] [Google Scholar]
- 6. Gabriel A. Integrated negative pressure wound therapy system with volumetric automated fluid instillation in wounds at risk for compromised healing. Int Wound J. 2012;9(Suppl 1):25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Apelqvist J, Willy C, Fagerdahl AM, et al. EWMA document: negative pressure wound therapy. J Wound Care. 2017;26:S1‐s154. [DOI] [PubMed] [Google Scholar]
- 8. Gabriel A, Camardo M, O'Rorke E, Gold R, Kim PJ. Effects of negative‐pressure wound therapy with instillation versus standard of Care in Multiple Wound Types: systematic literature review and meta‐analysis. Plast Reconstr Surg. 2021;147:68s‐76s. [DOI] [PubMed] [Google Scholar]
- 9. Neyeloff JL, Fuchs SC, Moreira LB. Meta‐analyses and Forest plots using a microsoft excel spreadsheet: step‐by‐step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim PJ, Silverman R, Attinger CE, Griffin L. Comparison of negative pressure wound therapy with and without instillation of saline in the Management of Infected Wounds. Cureus. 2020;12:e9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chowdhry SA, Wilhelmi BJ. Comparing negative pressure wound therapy with instillation and conventional dressings for sternal wound reconstructions. Plast Reconstr Surg Glob Open. 2019;7:e2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cole W. Early‐stage Management of Complex Lower Extremity Wounds Using Negative Pressure Wound Therapy with Instillation and a reticulated open cell foam with through holes. Wounds. 2020;32:159‐163. [PubMed] [Google Scholar]
- 13. Gabriel A, Kahn K, Karmy‐Jones R. Use of negative pressure wound therapy with automated, volumetric instillation for the treatment of extremity and trunk wounds: clinical outcomes and potential cost‐effectiveness. Eplasty. 2014;14:e41. [PMC free article] [PubMed] [Google Scholar]
- 14. Schreiner W, Ludolph I, Dudek W, Horch RE, Sirbu H. Negative pressure wound therapy combined with instillation for sternoclavicular joint infection. Ann Thorac Surg. 2020;110:1722‐1725. [DOI] [PubMed] [Google Scholar]
- 15. Kim PJ, Lavery LA, Galiano RD, et al. The impact of negative‐pressure wound therapy with instillation on wounds requiring operative debridement: pilot randomised, controlled trial. Int Wound J. 2020;17:1194‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Omar M, Gathen M, Liodakis E, et al. A comparative study of negative pressure wound therapy with and without instillation of saline on wound healing. J Wound Care. 2016;25:475‐478. [DOI] [PubMed] [Google Scholar]
- 17. Goss SG, Schwartz JA, Facchin F, Avdagic E, Gendics C, Lantis JC 2nd. Negative pressure wound therapy with instillation (NPWTi) better reduces post‐debridement bioburden in chronically infected lower extremity wounds than NPWT alone. J Am Coll Clin Wound Spec. 2012;4:74‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim PJ, Attinger CE, Steinberg JS, et al. The impact of negative‐pressure wound therapy with instillation compared with standard negative‐pressure wound therapy: a retrospective, historical, cohort, controlled study. Plast Reconstr Surg. 2014;133:709‐716. [DOI] [PubMed] [Google Scholar]
- 19. Gabriel A, Shores J, Heinrich C, et al. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J. 2008;5:399‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Timmers MS, Graafland N, Bernards AT, Nelissen RG, van Dissel JT, Jukema GN. Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen. 2009;17:278‐286. [DOI] [PubMed] [Google Scholar]
- 21. Burusapat C, Sringkarawat S. Efficacy of negative‐pressure wound therapy with Tetrachlorodecaoxygen‐anion complex instillation compared with standard negative‐pressure wound therapy for accelerated wound healing: a prospective, randomized, controlled Trial. Plast Reconstr Surg. 2021;148:339‐352. [DOI] [PubMed] [Google Scholar]
- 22. Giri P, Krishnaraj B, Sistla S, et al. Does negative pressure wound therapy with saline instillation improve wound healing compared to conventional negative pressure wound therapy?—a randomized controlled trial in patients with extremity ulcers. Ann Med Surg. 2021;61:73‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jain N, Horn CB, Andrade EG, Punch L. Combination of Girdlestone Pseudoarthroplasty and negative pressure wound therapy with instillation and dwell in the treatment of invasive osteomyelitis of the proximal femur. Cureus. 2018;10:e3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kanapathy M, Mantelakis A, Khan N, Younis I, Mosahebi A. Clinical application and efficacy of negative pressure wound therapy with instillation and dwell time (NPWTi‐d): a systematic review and meta‐analysis. Int Wound J. 2020;17:1948‐1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fleischmann W, Russ M, Westhauser A, Stampehl M. Vacuum sealing as carrier system for controlled local drug administration in wound infection. Unfallchirurg. 1998;101:649‐654. [DOI] [PubMed] [Google Scholar]
- 26. Lehner B, Fleischmann W, Becker R, Jukema GN. First experiences with negative pressure wound therapy and instillation in the treatment of infected orthopaedic implants: a clinical observational study. Int Orthop. 2011;35:1415‐1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fleischmann W, Strecker W, Bombelli M, Kinzl L. Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurg. 1993;96:488‐492. [PubMed] [Google Scholar]
- 28. Wilson JR, Mills JG, Prather ID, Dimitrijevich SD. A toxicity index of skin and wound cleansers used on in vitro fibroblasts and keratinocytes. Adv Skin Wound Care. 2005;18:373‐378. [DOI] [PubMed] [Google Scholar]
- 29. McCauley RL, Li YY, Poole B, et al. Differential inhibition of human basal keratinocyte growth to silver sulfadiazine and mafenide acetate. J Surg Res. 1992;52:276‐285. [DOI] [PubMed] [Google Scholar]
- 30. McCauley RL, Linares HA, Pelligrini V, Herndon DN, Robson MC, Heggers JP. In vitro toxicity of topical antimicrobial agents to human fibroblasts. J Surg Res. 1989;46:267‐274. [DOI] [PubMed] [Google Scholar]
- 31. Robson MC, Payne WG, Ko F, et al. Hypochlorous acid as a potential wound care agent: part II. Stabilized hypochlorous acid: its role in decreasing tissue bacterial bioburden and overcoming the inhibition of infection on wound healing. J Burns Wounds. 2007;6:e6. [PMC free article] [PubMed] [Google Scholar]
- 32. Heggers JP, Sazy JA, Stenberg BD, et al. Bactericidal and wound‐healing properties of sodium hypochlorite solutions: the 1991 Lindberg award. J Burn Care Rehabil. 1991;12:420‐424. [DOI] [PubMed] [Google Scholar]
- 33. Ford C, Reinhard E, Yeh D, et al. Interim analysis of a prospective, randomized Trial of vacuum‐assisted closure versus the Healthpoint system in the Management of Pressure Ulcers. Ann Plast Surg. 2002;49:55‐61. discussion 61. [DOI] [PubMed] [Google Scholar]
- 34. Mouës CM, van den Bemd GJ, Heule F, Hovius SE. Comparing conventional gauze therapy to vacuum‐assisted closure wound therapy: a prospective randomised trial. J Plast Reconstr Aesthet Surg. 2007;60:672‐681. [DOI] [PubMed] [Google Scholar]
- 35. Mouës CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum‐assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11‐17. [DOI] [PubMed] [Google Scholar]
- 36. Kim PJ, Attinger CE, Oliver N, et al. Comparison of outcomes for Normal saline and an antiseptic solution for negativepressure wound therapy with instillation. Plast Reconstruct Surg. 2015;136(5):657e‐664e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The present data is a comprehensive literature search in the bibliographic databases PubMed, Web of Science, and Cochrane Library.


