Abstract
For over 300 million years, insects have relied on symbiotic microbes for nutrition and defence. However, it is unclear whether specific ecological conditions have repeatedly favoured the evolution of symbioses, and how this has influenced insect diversification. Here, using data on 1,850 microbe–insect symbioses across 402 insect families, we found that symbionts have allowed insects to specialize on a range of nutrient-imbalanced diets, including phloem, blood and wood. Across diets, the only limiting nutrient consistently associated with the evolution of obligate symbiosis was B vitamins. The shift to new diets, facilitated by symbionts, had mixed consequences for insect diversification. In some cases, such as herbivory, it resulted in spectacular species proliferation. In other niches, such as strict blood feeding, diversification has been severely constrained. Symbioses therefore appear to solve widespread nutrient deficiencies for insects, but the consequences for insect diversification depend on the feeding niche that is invaded.
Subject terms: Social evolution, Coevolution, Evolutionary ecology
Insects rely on symbiotic microbes for nutrition and defence. Analysing a large dataset of microbe–insect symbioses, the authors show that symbiosis evolved in response to nutrient deficiencies but its impacts on insect diversification depend on their feeding niche.
Main
Across the tree of life, microbial symbionts have enabled organisms to harness new forms of energy, access unobtainable nutrients and outsource critical functions such as defence1–4. So valuable are symbiotic partnerships that they have repeatedly led to organisms becoming obligately dependent on each other for survival5. Such interdependence between hosts and symbionts has led to the evolution of new levels of organismal complexity that have ultimately shaped the diversity of life on Earth3,6.
The essential metabolic services provided by symbionts have enabled hosts to expand into previously uninhabitable environments1,4,7. For example, sulfur-oxidizing bacteria enable giant marine tubeworms to live in deep-sea vents, root-associated fungi helped plants colonize land and nutrient-supplementing symbionts have allowed insects to live solely on the imbalanced diets of plant sap and vertebrate blood2,8,9. However, it is unclear whether there are unifying factors that guide how and why symbiotic relationships evolve.
Insects are an excellent system to study the evolution of obligate symbiosis. Multiple insect families have acquired microbes to perform a range of functions, including defence and nutrition10. Defensive symbionts protect their hosts from attack by natural enemies11, whereas nutritional symbioses allow insects to feed on specialized resources that lack essential nutrients, such as plant sap, blood (haematophagy) and wood (xylophagy)2. It is therefore widely accepted that symbiotic partnerships have opened new ecological niches and helped the incredible diversification of insects7. However, previous work has primarily focused on the functional role and impact of obligate symbiosis within single groups of insects. Consequently, whether we can generalize about the ecological causes and consequences of obligate symbiosis across different groups of insects is unknown. Are there consistent nutrient limitations that have repeatedly selected for the evolution of symbioses across different feeding niches? Do symbioses influence diversification in a consistent or niche-dependent way?
In this article, we address these questions by examining the macro-evolutionary patterns of obligate symbiosis across 1,850 microbe-insect combinations from 402 insect families. Data were collated across bacteria, fungi and protist symbionts with nutritional and defensive functions (Supplementary Tables 1–4). First, we estimated how often insect lineages within different feeding niches have evolved obligate symbiosis, where the host cannot survive without symbionts. We are interested in cases where hosts are obligately or highly dependent (effectively obligately) on their symbionts. Obligate dependence is ideally proven experimentally, but only a limited number of such studies exist5. To allow comparison across a wider range of species, we used two criteria to establish putative obligate dependence, hereafter referred to as obligate dependence, both of which had to be fulfilled: (1) the symbiont is universally present in reproductive females; and (2) the insect possesses morphological structures that are predominantly associated with symbionts being required for survival (for example, bacteriocytes10), or where information on symbiont housing organs was lacking, data on the impact of symbiont removal and patterns of host–symbiont co-speciation were used to determine obligate dependence (‘Insect and symbiont data’ in Methods). Known parasitic symbionts, such as reproductive manipulators (for example, Spiroplasma, Cardinium and Wolbachia), that have not evolved beneficial functions were excluded from our dataset.
Second, we examined the composition of insect diets to determine whether specific nutrient deficiencies have consistently led to the evolution of obligate symbiosis across different feeding niches. The nutritional composition of diets was determined by collating literature on the food sources used by adults and juveniles (nfood sources = 362) and extracting information on carbohydrates, fats, proteins, essential amino acids, non-essential amino acids and vitamins A, B, C and E from as many example foods as possible (range 1–24) from nutritional databases (‘Nutrient data’ in Methods, Supplementary Table 4 and Extended Data Fig. 1). Data on other vitamins were collected but had >30% missing data and so were excluded from analyses (‘Nutrient data’ in Methods). We differentiate between insect families that specialize on single plant-based resources (phloem, xylem or wood) from families that exploit various plant parts (phytophagy, referred to here as herbivores), as there were large differences in the nutrients of these diets (Supplementary Tables 1 and 4). Third, we tested if the acquisition of obligate symbionts has increased or decreased host species richness after radiating into different feeding niches. We circumvent the problem of poorly resolved species level phylogenies by reconstructing the evolutionary history of obligate symbioses at the family level.
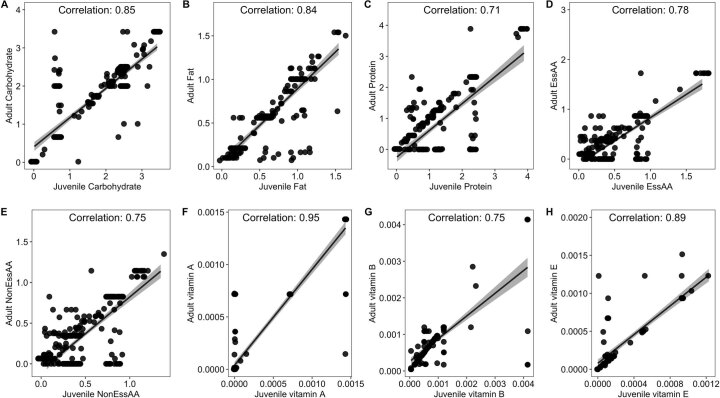
Extended Data Fig. 1. The correspondence between the composition of adult and juvenile diets across insect families.
The relationship between adult and juvenile dietary concentrations of (A) Carbohydrate, (B) Fat, (C) Protein, (D) Essential Amino Acids (EssAA), (E) Non-Essential Amino Acids (NonEssAA), (F) Vitamin A, (G) Vitamin B and (H) vitamin E are presented. Each point is one insect family and the lines are linear regressions with 95% confidence intervals (shaded bands). Pearson correlation coefficients (‘Correlation’) are presented for each nutrient.
Evolutionary origins of obligate symbiosis
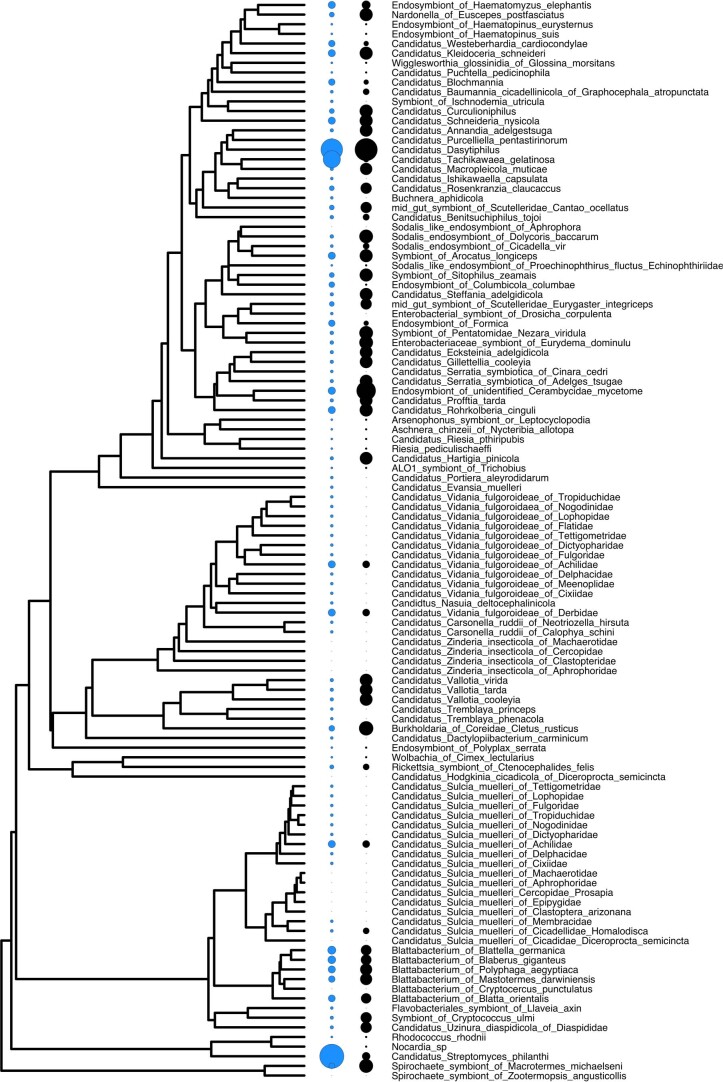
We found at least 16 independent origins of obligate symbiosis spread across 89 insect families (Bayesian phylogenetic mixed model (BPMM): Fig. 1 and Supplementary Table 5). These origins were estimated on the time-calibrated phylogeny12 to date back as far as 336 million years. Within insect families, there were also several more recent transitions to obligate symbiosis. For example, 15 families were found to contain species with and without obligate symbionts (Fig. 1 and Supplementary Tables 1 and 2) but without species phylogenies the exact number of origins cannot be resolved. Our analyses therefore focus on the deeper, family-level origins of symbiosis, while accounting for variation within families by modelling the percentage of species within families with obligate symbionts (‘Specific analyses’ in Methods).
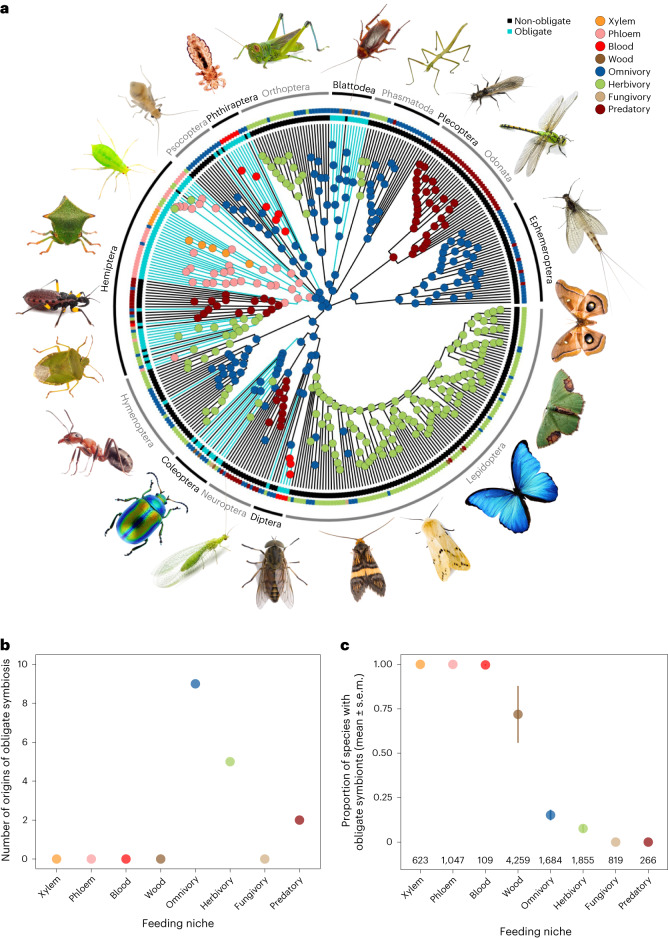
Fig. 1. The evolutionary origins of obligate symbionts and their association with different feeding niches.
a, The phylogenetic distribution of obligate symbionts across insect families investigated for symbiosis and their feeding niches. Turquiose tips and branches represent obligate symbiosis and different coloured dots represent different feeding niches. Ancestral feeding niches were estimated using SCM, and obligate symbiosis states were estimated using a BPMM (Supplementary Table 5; for tree with tip labels, see Extended Data Fig. 4). b, The number of times obligate symbiosis evolved in different ancestral feeding niches of insects estimated using a BPMM. c, Proportion of species within families with obligate symbionts (mean ± standard error of the mean (s.e.m.)) in relation to the feeding niches of insects. The average number of species within families is given along the x axis.
Reconstructing the ancestral feeding niches of insect families showed that obligate symbioses evolved from omnivorous, herbivorous and predatory ancestors (respective percentage of origins estimated using stochastic character mapping (SCM): 75%, 8% and 17%; Fig. 1 and Supplementary Table 5). Following the acquisition of obligate symbionts, 60% of lineages switched to a single food source (phloem 42%, blood 12%, xylem 6%; Fig. 1 and Supplementary Table 5). This pattern of food utilization explains the current distribution of obligate symbiosis remarkably well, where over 90% of insect species feeding on blood, phloem, xylem and wood have obligate symbionts (Fig. 1 and Supplementary Tables 1, 2 and 6). Conversely, there are no known cases of obligate symbioses in insect families that are predominantly predators or fungivores (Fig. 1 and Supplementary Tables 1, 2 and 6).
Only five insect families are known to have endosymbionts with defensive functions. This is probably influenced by sampling effort, as defensive symbionts residing within insect hosts have only been discovered relatively recently11,13, in taxa such as aphids, drosophilids, psyllids, crabronids (beewolves) and beetles (reviewed in ref. 14). There are well-known examples of defensive mutualisms that reside outside of the host (ectosymbionts), such as the antimicrobial producing actinobacteria of fungus-farming ants15, but these were excluded as our analyses focus on endosymbiosis. Out of the 13 endosymbiont species shown to provide insects with protective services, nearly all maintain facultative relationships with their hosts. There is only one exception in our database, the Asian citrus psyllid, Diaphorina citri, which has evolved obligate dependence on a defensive symbiont, which is housed in bacteriocytes alongside a putative nutrient provisioning symbiont16.
Several defensive symbioses show evidence of strong vertical inheritance and are associated with hosts at high frequencies, such as those found in lagriid beetles17, beewolves18 and fungus-growing ants19, suggesting they may be near obligate in nature. However, the absence of the symbiont in some individuals18,20, existence of multiple symbiont strains within the same host individual20 and evidence of frequent acquisitions from environmental sources19,21 demonstrates that most defensive symbioses have not reached the high degree of mutual dependence observed in obligate nutritional associations. While more work is clearly needed, these data support the hypothesis that selection for protection against natural enemies is too inconsistent across generations to favour the evolution of obligate dependence5,11.
Nutrient deficiencies and obligate symbiosis
Our results show that the evolution of obligate symbioses in insects is associated with transitions to specialized feeding niches (Fig. 1). Studies have shown that symbionts have enabled these transitions by synthesizing a range of essential nutrients missing in their hosts’ diet including vitamins, carotenoids and amino acids, as well as digestive enzymes that aid in nutrient recycling10,22–24. However, it is not clear if certain key nutrients are consistently deficient in the diets of insects with obligate symbionts across different feeding niches.
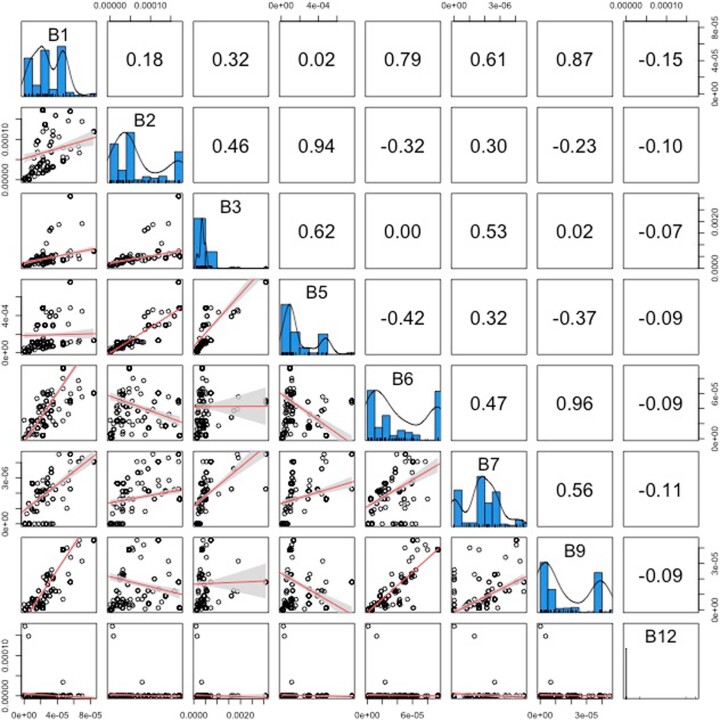
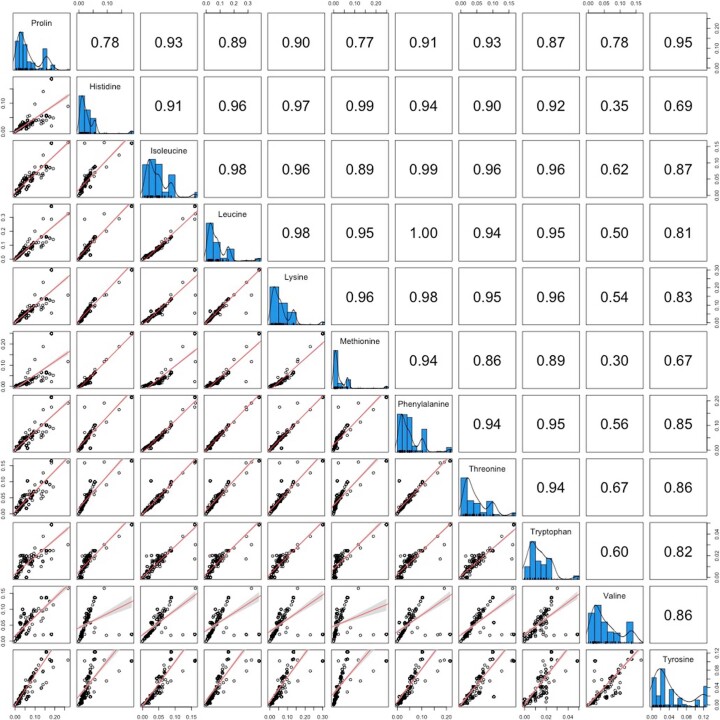
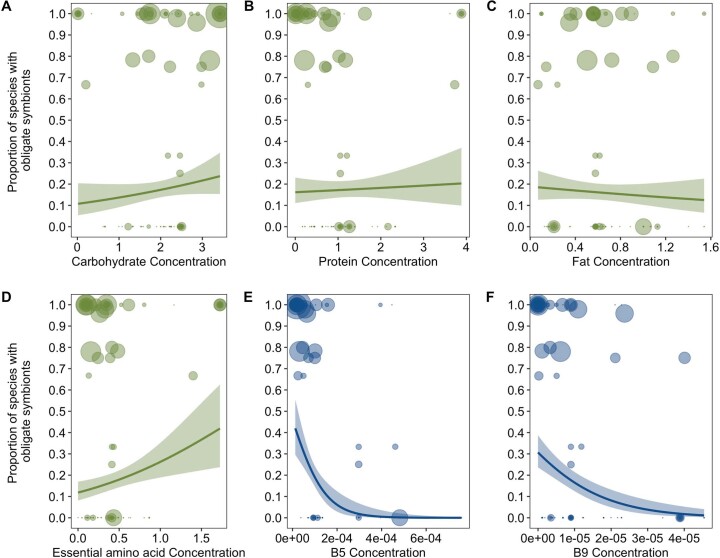
We found that only one dietary component was significantly correlated with the presence of obligate symbionts across all feeding niches: low levels of B vitamins (Fig. 2; BPMM: phylogenetic correlation −0.32, credible interval (CI) −0.54 to −0.09, proportion of iterations above or below a test value correcting for the finite sample size of posterior samples (pMCMC) = 0.006; Supplementary Table 7). This pattern held across hosts with diets that are highly variable in carbohydrates, proteins, fats, vitamins and amino acids (Fig. 2 and Supplementary Table 7). Examining types of B vitamins further showed that specifically B5 and B9 are phylogenetically negatively correlated with the evolution of obligate symbiosis (Fig. 2 and Supplementary Table 8). Different types of B vitamins were, however, highly correlated, indicating that sets of B vitamins are often concurrently absent from the diets of some insects (Extended Data Fig. 2). For example, vitamins B1 and B6 were correlated with B9 (Pearson’s correlation coefficients r = 0.89 and 0.96) and vitamins B2 and B3 were correlated with B5 (r = 0.92 and 0.64; data on vitamins B7 and B12 had >30% missing data and so were not analysed).
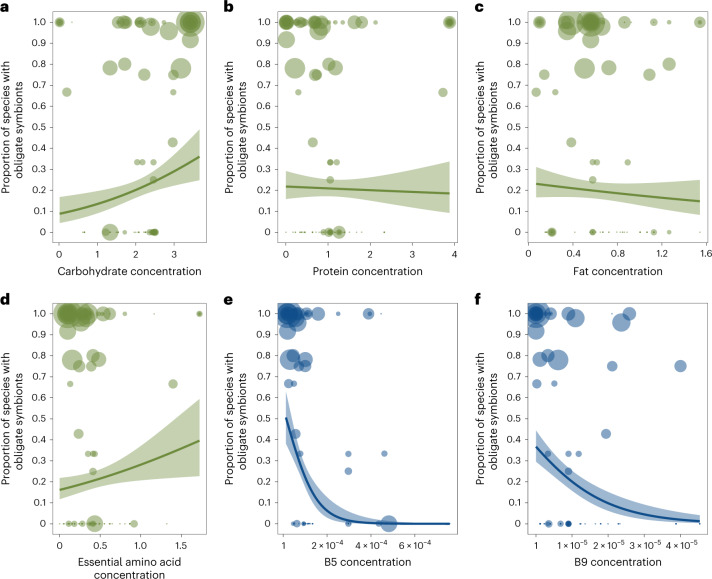
Fig. 2. Nutrient deficiencies and the evolution of obligate symbioses.
a–d, Across all diets, macronutrients (a–c) and essential amino acids (d) were not consistently associated with the proportion of species within families that had obligate symbionts. e,f, Insect families with diets deficient in B5 (e) and B9 (f) vitamins had a significantly higher proportions of species with obligate symbionts than families feeding on diets with high levels of B vitamins (B5 phylogenetic correlation (CI) −0.45 (−0.61 to −0.22), pMCMC = 0.001; B9 phylogenetic correlation (CI) −0.25 (−0.48 to −0.05), pMCMC = 0.03). Nutrient values on x axes are standardized amounts per gram (‘Nutrient data’ in Methods). The size of points represents the number of host species (log-transformed) examined for obligate symbionts per family. Lines represent logistic regressions with 95% confidence intervals (shaded bands) plotted for illustrative purposes. Analyses of individual amino acids confirmed that obligate symbiosis was not consistently associated with any amino acid deficiencies (‘Obligate symbiosis and types of amino acids’ in Methods and Supplementary Table 29).
Extended Data Fig. 2. The relationship between different B vitamins across diets.
The frequency distributions of the different B vitamins are plotted in the diagonal panels. Scatter plots of the correlation between different B vitamins are plotted in the panels below the diagonal, where each point represents one insect family and lines represent linear regressions with 95% confidence intervals (shaded bands). Pearson correlation coefficients are given in the panels above the diagonal.
No other macro- or micronutrients were significantly correlated with obligate symbiosis across insect families (Fig. 2 and Supplementary Table 7). This is not to say that obligate symbionts are not important for provisioning other nutrients, but rather these nutrients are restricted to specific niches. For example, essential amino acids are deficient in certain feeding niches associated with obligate symbiosis, such as phloem (BPMM: phloem versus background levels β = −1.11, CI = −1.53 to −0.67, pMCMC = 0.001), but are enriched in other niches with symbionts, such as blood (BPMM: blood versus background levels β = 3.25, CI = 2.77 to 3.86, pMCMC = 0.001; Supplementary Table 9). Note that all amino acids concentrations were highly correlated across insect diets (Extended Data Fig. 3).
Extended Data Fig. 3. The relationship between different amino acids across diets.
The frequency distributions of the different amino acids are plotted in the diagonal panels. Scatter plots of the correlation between different amino acids are plotted in the panels below the diagonal, where each point represents one insect family and lines represent linear regressions with 95% confidence intervals (shaded bands). Pearson correlation coefficients are plotted above the diagonal.
Our results are consistent with detailed studies that have demonstrated the fitness consequences of providing B vitamins to specific insect species. For example, the fitness of tsetse flies depends on B9 and B6 vitamins provided by Wigglesworthia bacteria25,26, and Buchnera supplements aphids with B5 and B2 vitamins, with B5 having a particularly strong effect on host survival27. Similarly, the Baumania symbiont of xylem-feeding sharpshooters has retained the capacity to synthesize six B vitamins (all but B3 and B12), and the coordinated host–symbiont biosynthesis of B7 and B5 in whiteflies has been shown to be critical for host fitness28,29. Dietary studies have also confirmed that mutualistic Wolbachia provide essential B vitamins for Cimex bed bugs30; and metabolic homeostasis is restored in symbiont-free Dysdercus cotton stainers when B vitamins are supplemented, or hosts are re-infected with their actinobacterial symbionts31. In addition, genome studies have shown that the metabolic pathways to biosynthesize B vitamins have been retained in the genomes of symbionts from insects that occupy diverse feeding niches (for example, blood32, plant sap33,34 and seeds35), suggesting their widespread importance in maintaining symbioses. A useful future step would be to assess whether B-vitamin pathways are more highly conserved in symbiont genomes, compared with pathways that encode other host-beneficial factors.
Evolutionary transitions to nutrient-deficient diets
Our results suggest that B-vitamin deficiencies are widespread and important for the evolution of obligate symbiosis in insects. There are, however, two evolutionary scenarios for why such transitions occur. One possibility is that insects feeding on diets low in vitamin B recruited symbionts to supply B vitamins. The alternative is that insects first acquired obligate symbionts that could synthesize B vitamins, possibly for some other benefit, which then enabled them to invade feeding niches where B vitamins were scarce. The question is therefore whether the evolution of obligate symbioses were triggered by low B vitamins in diets or whether obligate symbioses facilitated specialization on these diets.
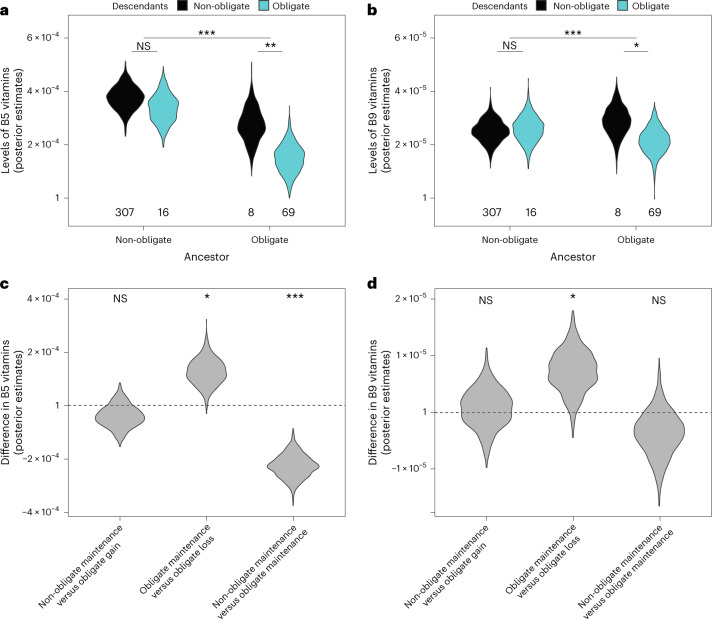
We tested these competing hypotheses by estimating the amount of B5 and B9 vitamins in ancestral diets before, and following, transitions to obligate symbiosis. A high phylogenetic signal of B vitamins across insects allowed us to relatively accurately estimate ancestral values (Supplementary Table 8 and Extended Data Fig. 4). We found little evidence that levels of B5 and B9 vitamins were reduced in the diets of insects before they acquired obligate symbionts (Fig. 3 and Supplementary Tables 10 and 11). Instead, we found that hosts that recruited obligate symbionts subsequently evolved to specialize on diets with low levels of B5 and B9 vitamins (Fig. 3 and Supplementary Tables 10 and 11). Once obligate symbioses evolved, shifts to diets deficient in B vitamins were much more frequent, particularly for B5, where transition rates to low levels of B5 were 30 times higher than for lineages without obligate symbionts (Supplementary Table 11).
Extended Data Fig. 4. Reconstruction of ancestral levels of B vitamins, obligate symbiosis and feeding niches across 402 insect families.
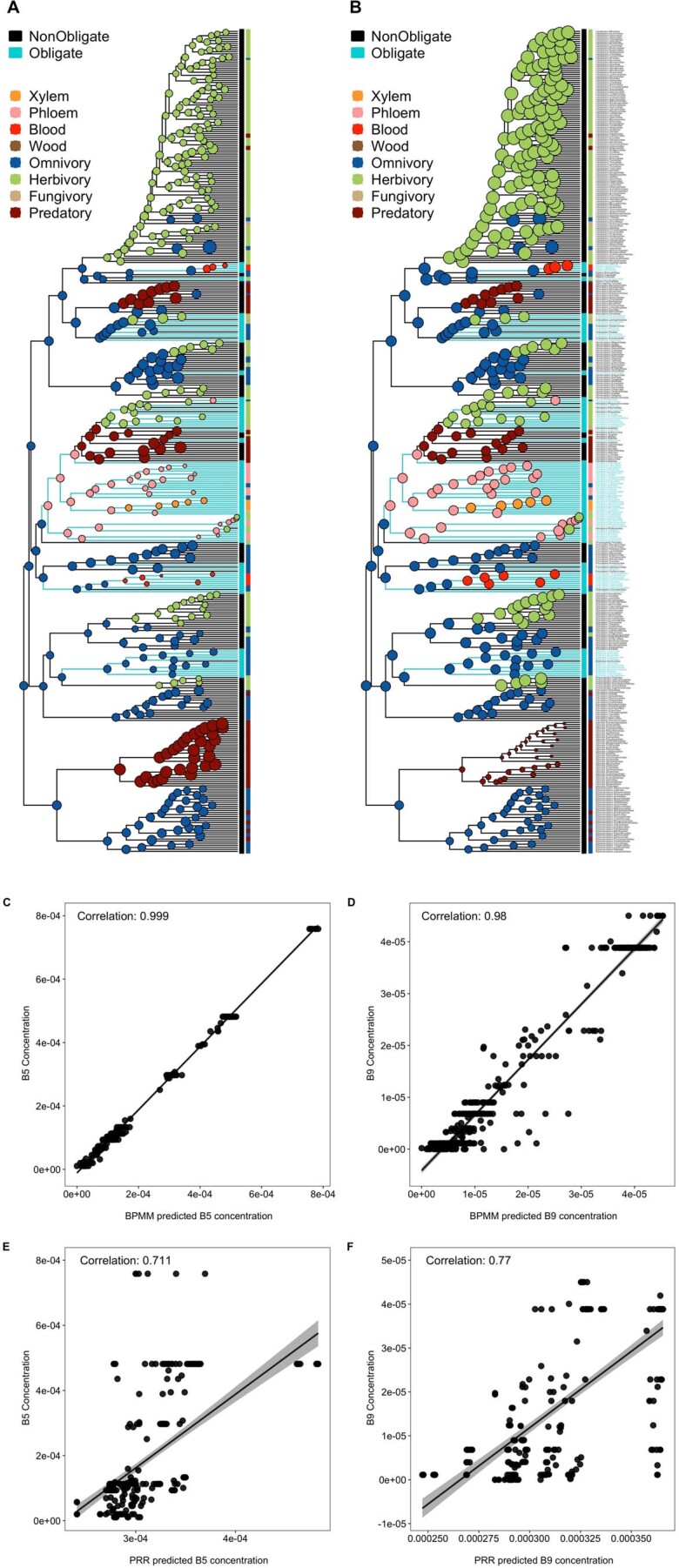
Ancestral concentrations of (A) B5 and (B) B9 vitamins were estimated using BPMMs and are shown by the size of circles at each node. Turquoise tips and branches indicate obligate symbionts and different coloured dots represent different feeding niches. Ancestral feeding niches were estimated using SCM and states of obligate symbiosis were estimated using a BPMM (Supplementary Table 5). There was greater correspondence between predictions from BPMMs and raw concentrations of (C) B5 and (D) B9 vitamins than there was for predictions from phylogenetic ridge regressions (PRR, E-F), which allowed for rate shifts in B vitamins across the phylogeny (E-F). In C-F lines represent linear regressions with 95% confidence intervals (shaded bands).
Fig. 3. Obligate symbiosis enables evolutionary shifts to diets deficient in B vitamins.
a,b, The ancestors of lineages that evolved obligate symbioses had similar levels of B5 (a) and B9 vitamins (b) in their diets compared with lineages that did not evolve obligate symbioses. c,d, After acquiring obligate symbionts, lineages evolved diets with significantly lower levels of B5 (c) and B9 vitamins (d) (‘non-obligate maintenance versus obligate maintenance’ comparison). The evolutionary loss of obligate symbiosis was also associated with increases in dietary levels of vitamin B5 and B9 compared with where obligate symbiosis was maintained (c,d: ‘obligate maintenance versus obligate loss’ comparison). Vitamin B concentrations are standardized amounts per gram for each ancestral node, estimated from the diets of extant insect families (‘Nutrient data’ in Methods; for reconstructed levels of B vitamins plotted on the tree and robustness of estimates to rate shifts in B vitamins, see Extended Data Fig. 4). Numbers along the x axis in a and b indicate the numbers of transitions. Violin density curves represent the posterior distribution of estimated ancestral levels of B5 and B9 vitamins (1000 samples) estimated using a BPMM. The violin width corresponds approximately to the most likely estimate of B vitamins. A BPMM was used to test for significant differences between transitions (*pMCMC <0.05, **pMCMC <0.01, ***pMCMC <0.0001; exact pMCMC values are given in Supplementary Table 10): In a and b we tested if the posterior distribution of B-vitamin estimates for a given transition was above or below the comparison transition, and in c and d we tested if the posterior distribution of the difference in B-vitamin estimates for transition comparisons was above or below 0. NS, not significant.
The importance of obligate symbionts in supplying B vitamins was further supported by the loss of obligate symbioses when insects switched to diets with elevated B-vitamin levels (Fig. 3 and Supplementary Tables 10 and 11). Insect lineages with above-average levels of B5 and B9 vitamins in their diets were more likely to lose their obligate symbionts (Fig. 3a,b and Supplementary Table 11). Our results match with observations from specific taxa, where obligate symbiont losses have been associated with dietary changes in their insect hosts. In the mealybug genus, Hippeococcus, symbiont losses are thought to be associated with nutrient provisioning by Dolichoderus ants, and Typhlocybides plant hoppers lost their ancestrally acquired obligate symbionts when switching from plant sap to more nutrient-rich parenchyma36.
Symbiont specialization in nutrient provisioning
Given the importance of B-vitamin provisioning by symbionts in insects, we examined whether specific lineages of symbiotic bacteria specialize in providing B vitamins to hosts. Have hosts relied on a restricted set of symbiotic partners, or have a variety of symbionts converged to provide B vitamins? To address this question, we constructed a phylogeny for symbionts to quantify the amount of variation in dietary B vitamins explained by symbiont ancestry and their co-evolutionary relationships with hosts.
We found that hosts have evolved dependence on a broad range of microbes (Supplementary Tables 12 and 13). Less than 1% of variation in B5 and B9 vitamins in host diets was explained by symbiont phylogenetic history and the co-evolutionary relationships between symbionts and hosts (BPMM: symbiont phylogeny (% variance) B5 0.08, CI = 0.02 to 0.15; B9 0.1, CI = 0.03 to 0.2; co-evolutionary interaction (% variance) B5 0.06 CI = 0.02 to 0.12; B9 0.09 CI = 0.03 to 0.16; Supplementary Tables 12 and 13). Instead, divergent symbiotic lineages appear to have become convergently associated with insects feeding on low-vitamin-B diets (Extended Data Fig. 5). Following the establishment of obligate symbioses, hosts and symbionts tend to co-evolve, as related insect families were significantly more likely to be partnered with phylogenetically similar symbionts (BPMM: co-evolutionary interaction (% variance) 22.65, CI = 10.47 to 37.35; Parafit: P = 0.05; Supplementary Tables 14 and 15). These results match with research showing that diverse symbiotic bacteria have retained the genes for synthesizing B vitamins37, and those insects whose bacteria lose the capacity to provide B vitamins recruit new symbiont lineages to compensate for the loss38.
Extended Data Fig. 5. Levels of B vitamins in host diets in relation to symbiont phylogenetic history.
B5 vitamins are presented in blue and B9 vitamins are in black with the size of the circles indicating the amount of B5 and B9 vitamins in the diets of insect hosts.
Obligate symbiosis and insect diversification
Finally, we tested if obligate symbioses have influenced patterns of diversification across insects. The current paradigm, based on observations from specific lineages, such as sap-feeding Hemipterans, is that the acquisition of symbionts opens up new niches and increases host species proliferation39,40. Host–symbiont co-evolution can also generate incompatibilities between populations that may increase speciation rates4. Dependence on symbionts may, however, ‘trap’ hosts in specific niches, leading to the opposite prediction that symbionts reduce diversification4. For example, hosts can be restricted to feeding on specific resources because of symbiont-assisted specialization41, or limited by the sensitivity of their obligate symbionts to environmental conditions, such as temperature42,43. Mutation accumulation can also degrade symbiont functioning, resulting in hosts being stranded with maladapted symbionts that may increase extinction risk44.
Although symbioses are thought to have facilitated adaptive radiations in specific insect lineages7,45, these competing hypotheses have not been systematically tested across insects, generating debate over the general role of symbiosis in insect diversification. We therefore analysed the relationship between obligate symbiosis and species richness in three ways: across all families, across families with and without obligate symbionts that have the same feeding niche, and between sister lineages.
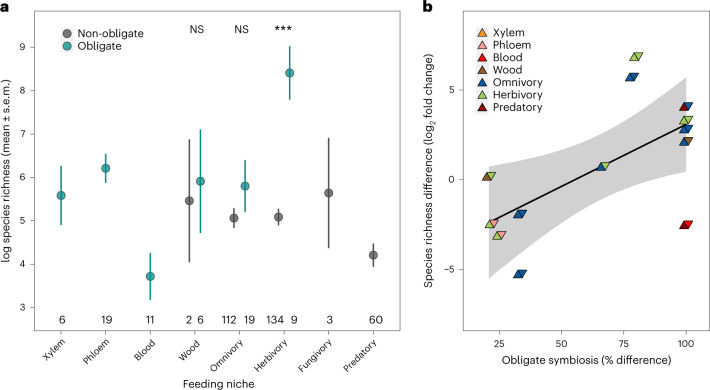
Across all insects, we found that obligate symbiosis was associated with extreme highs and lows of species richness compared with background rates (Fig. 4a and Supplementary Tables 16 and 17). At the extreme high, herbivorous insect families with obligate symbionts had 12 times as many species compared with the average across families (Fig. 4a; BPMM: herbivores with obligate symbionts versus background species richness 2.75, CI = 0.97 to 4.01, pMCMC = 0.001). At the other extreme, extraordinarily low species richness was associated with insect families feeding on blood, which had eight times fewer species than the average (Fig. 4a; BPMM: blood feeders with obligate symbionts versus background species richness −1.95, CI = −3.64 to −0.26, pMCMC = 0.01). This resulted in a 51-fold difference in the number of species in herbivorous insect families with obligate symbionts versus those in blood-feeding niches. These estimates of species richness were after accounting for differences between holo- and hemi-metabolism, the age of insect families and insect phylogenetic history, all of which can affect the number of species in families12,46.
Fig. 4. Obligate symbioses and the evolutionary potential for diversification.
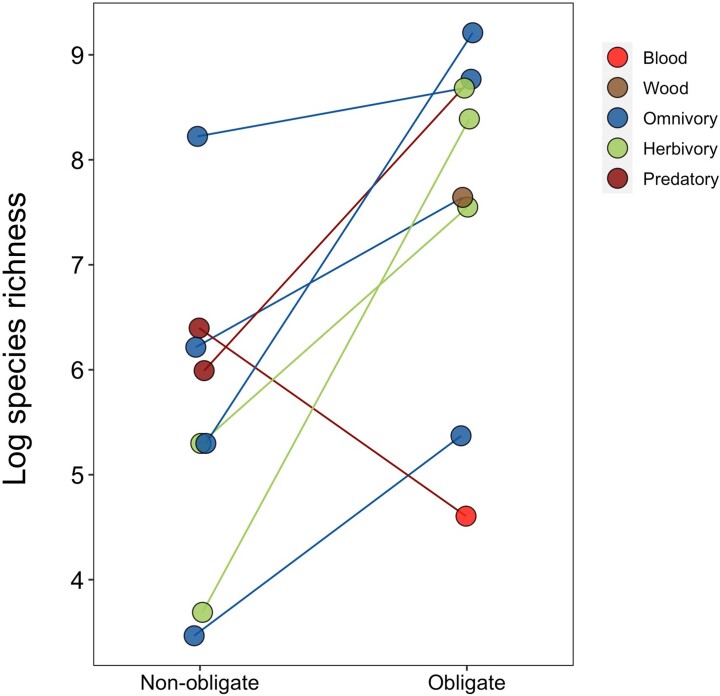
a, Diversification was measured as the number of species within families presented on a natural logarithmic scale (mean ± s.e.m.). BPMMs were used to test if the species richness of each feeding niche was significantly higher or lower than the average of all other niches (*pMCMC <0.05, **pMCMC <0.01, ***pMCMC <0.0001. Exact pMCMC values are given in Supplementary Table 17), and if families with (>50%) and without (<50%) obligate symbionts feeding within the same niche had higher or lower species richness (Supplementary Table 17). BPMMs controlled for differences in family age, holo- and hemi-metabolism and insect phylogenetic history. The number of insect families are given along the x axis. b, Sister lineage comparisons showing families with a higher percentage of species with obligate symbionts are associated with greater species richness (y axis: log2 fold change: 0, no difference; 1, double the number of species; −1, half the number of species; Extended Data Fig. 6 shows differences in absolute numbers of species). Details of the taxa involved in sister comparisons are presented in Supplementary Table 29. Symbols are coloured by the feeding niches of the sister families, with colours on the left indicating the feeding niche of the family with the lowest rates of obligate symbiosis and the highest on the right. Regression line with 95% confidence intervals (shaded band) plotted for illustrative purposes. NS, not significant.
Obligate symbiosis promotes diversification within niches
Patterns of species richness across insects appear to be niche specific (Supplementary Table 17). However, within feeding niches, symbionts may directly promote diversification if they allow species to exploit different resources. For example, in insect families feeding on more varied diets, such as generalist herbivores and omnivores, symbionts may enable resource partitioning between species, fuelling the speciation process. There were three feeding niches where families with and without obligate symbionts could be compared: herbivores, omnivores and wood feeders. If true, then families within these niches with obligate symbionts should have higher species richness than families without them.
We found that herbivorous insect families with obligate symbionts had 15 times as many species as families without symbionts (Fig. 4a; BPMM: families with versus without obligate symbionts 3.47, CI = 1.57 to 4.79, pMCMC = 0.001; Supplementary Table 17). Omnivorous and wood feeding families of insects with obligate symbionts also had two to three times as many species as families that lacked symbionts, but these differences were not statistically significant (Fig. 4a; BPMM: omnivorous families with versus without obligate symbionts 0.17, CI = −1.08 to 1.34, pMCMC = 0.78; wood families with versus without obligate symbionts 0.13, CI = −2.78 to 3.71, pMCMC = 0.71; Supplementary Table 17).
In support of obligate symbiosis playing a role in promoting diversification at finer evolutionary scales, we found that among more closely related sister taxa, lineages with higher percentages of obligate symbionts (n = 13) were more specious (Fig. 4b; BPMM: percentage of species with obligate symbionts 7.43, CI = −0.03 to 12.79, pMCMC = 0.05; Supplementary Tables 18 and 19 and Extended Data Fig. 6). Our results are consistent with findings from specific taxonomic groups. For example, symbionts allowed Curculionidae weevils, now one of the most diverse families of insects, to feed and radiate exclusively on plants47,48. Similarly, the success of certain highly specious ant lineages has been facilitated by nutrient-provisioning symbionts that have allowed them to thrive on primarily plant-derived diets49.
Extended Data Fig. 6. Sister comparisons of families with and without obligate symbionts.
Obligate symbiosis was associated with increased species richness in all comparisons apart from one, where there has been a switch to blood feeding from predation. Species richness was measured as the number of species within families and is presented on the natural logarithmic scale. There were 13 sister families with different percentages of species with obligate symbionts. Of these, 5 comparisons had small differences in rates of obligate symbiosis: <35% difference in percentage of species with obligate symbionts (not plotted, see Fig. 4 for all 13 comparisons).
Sensitivity analyses
We examined the robustness of our analyses to different methodological approaches. First, we examined the robustness of our estimates of obligate symbiosis and ancestral feeding niches to different analytical approaches (Supplementary Table 20 and ‘The number of origins of obligate symbiosis’ and ‘Estimating ancestral feeding niches’ in Methods). Second, we tested how inserting families (n = 23) that were not included in the published phylogeny12 influenced our results (Supplementary Table 21 and Extended Data Fig. 7). Third, we examined the robustness of our results to excluding non-bacterial symbionts and families that had multiple co-occurring obligate symbionts (Supplementary Tables 22–24 and Extended Data Fig. 8). Fourth, we repeated our analyses using a second dataset restricted to species where dependence on symbionts had been directly studied, rather than inferred from microscopy studies on the presence of bacteriocytes within certain insect orders and superfamilies (Supplementary Tables 25–27 and Extended Data Fig. 9). Fifth, 15 out of the 402 insect families had species with and without obligate symbionts. This was accounted for by modelling the percentage of species with obligate symbionts within families. However, in the transition rate analyses, families had to be classified as having obligate symbionts (>50% of species with obligate symbionts) or not (<50% of species with obligate symbionts). We tested the sensitivity of these analyses to this binary classification by re-running models including only families where 100% of species had, or did not have, obligate symbionts (Supplementary Table 28). All of these different analyses provided qualitatively and quantitatively similar conclusions (‘Sensitivity analyses’ in Methods).
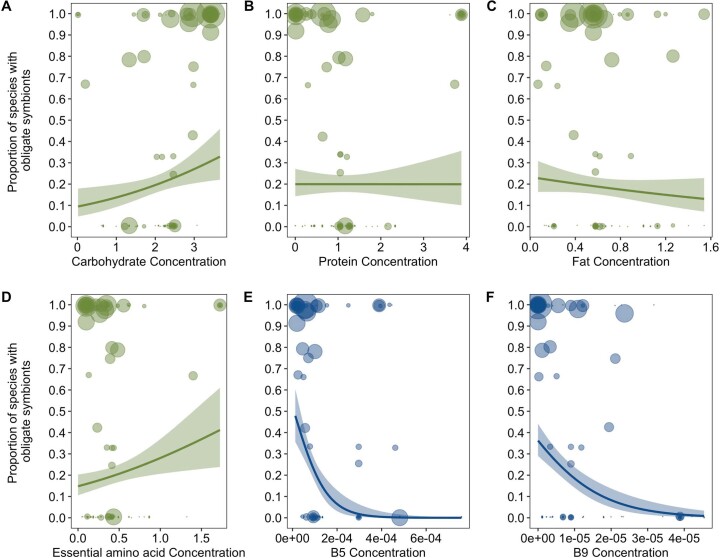
Extended Data Fig. 7. The evolution of obligate symbioses in relation to nutrient deficiencies after removing families that were added to the Rainford tree.
The proportion of species with obligate symbionts in families is plotted in relation to dietary concentrations of (A) Carbohydrate, (B) Protein, (C) Fat (D) Essential Amino Acids, (E) Vitamin B5 and (F) vitamin B9. Obligate symbioses was negatively phylogenetically correlated to concentrations of B vitamins (B phylo r (CI) = −0.36 (−0.53, −0.11), pMCMC = 0.008. Supplementary Table 21). Values of macro- and micro-nutrients are standardized amounts per gram (‘Nutrient data’ in Methods). The size of points represents the mean number of host species (log transformed) examined for obligate symbionts per family. Lines represent logistic regressions with 95% confidence intervals (shaded bands) plotted for illustrative purposes.
Extended Data Fig. 8. The evolution of obligate symbioses in relation to nutrient deficiencies including only bacterial symbionts.
The proportion of species with obligate symbionts in families is plotted in relation to dietary concentrations of (A) Carbohydrate, (B) Protein, (C) Fat (D) Essential Amino Acids, (E) Vitamin B5 and (F) vitamin B9. Obligate symbioses was negatively phylogenetically correlated to concentrations of B vitamins (B phylo r (CI) = −0.35 (−0.49, −0.06), pMCMC = 0.008. Supplementary Table 22). Values of macro- and micro-nutrients are standardized amounts per gram (‘Nutrient data’ in Methods). The size of points represents the mean number of host species (log transformed) examined for obligate symbionts per family. Lines represent logistic regressions with 95% confidence intervals (shaded bands) plotted for illustrative purposes.
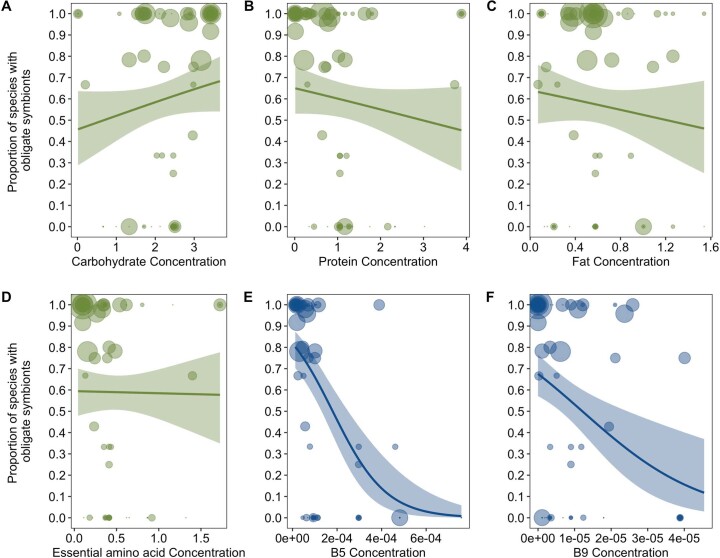
Extended Data Fig. 9. The evolution of obligate symbioses in relation to nutrient deficiencies after restricting data to families where obligate symbioses had been directly studied.
The proportion of species with obligate symbionts in families is plotted in relation to dietary concentrations of (A) Carbohydrate, (B) Protein, (C) Fat (D) Essential Amino Acids, (E) Vitamin B5 and (F) vitamin B9. Obligate symbioses was negatively phylogenetically correlated to concentrations of B vitamins (B phylo r (CI) = −0.58 (−0.79, −0.04), pMCMC = 0.05. Supplementary Table 25). Values of macro- and micro-nutrients are standardized amounts per gram (‘Nutrient data’ in Methods). The size of points represents the mean number of host species (log transformed) examined for obligate symbionts per family. Lines represent logistic regressions with 95% confidence intervals (shaded bands) plotted for illustrative purposes.
Discussion
Genomic and experimental studies have provided important insights into the metabolic function of obligate symbioses for specific insects. This previous work has focused on well-studied groups—mostly plant-feeding hemipterans containing symbionts with highly reduced genomes10,24,50,51, but also herbivorous beetles23,47,52, omnivorous ants53, cockroaches54 and blood-feeding insects (flies55,56, bed bugs30 and lice57). Our approach complements this work by allowing tests of the importance of obligate symbioses in overcoming nutrient limitations at a much broader taxonomic scale, including lineages where symbioses have not been studied in-depth using detailed molecular methods. This enables contrasts to be made between insect lineages with and without symbionts, circumventing potential biases that arise from studying only positive associations.
Our analyses highlight that B-vitamin deficiencies are the primary nutrient limitation associated with obligate symbioses. While B vitamins are consistently important, this does not mean that other nutritional deficiencies are not important for specific clades. For example, certain ant and beetle lineages have evolved tyrosine-supplementing symbioses that are thought to help thicken their heavily sclerotized cuticles47,53,58. Specialized herbivores have evolved symbioses to solve a variety of problems, from including the production of digestive enzymes to breakdown of plant cell walls23 and the synthesis of specific amino acids missing from plant-based diets35. Similarly, transitions to feeding on plant phloem and xylem are well known to be associated with essential amino acid provisioning by bacteria in insects2,24,52. These feeding niches are, however, also deficient in B vitamins, a common feature of symbiont-associated diets, including those rich in amino acids, such as blood feeding. The widespread need for B-vitamin supplementation is also reflected in the genomes of symbionts that retain metabolic pathways to synthesize B vitamins across a diversity of host feeding niches32–35. Further insights into the consistent need for B-vitamin supplementation may be gained by extending such genomic analyses to compare the synthesis pathways of B vitamins with those of other nutrients, such as amino acids, in both symbionts and their insect hosts.
To conclude, it appears possible to make relatively broad inferences about the causes and consequences of obligate symbioses in insects. Vitamin B supplementation by microbial partners is widespread in insects and has helped insect hosts to exploit novel food resources. In some cases, such as herbivorous insects, the shift to feeding on new resources appears to have facilitated adaptive radiations, analogous to textbook examples such as Darwin’s finches59. In other cases, such as strict blood feeding, new niches seem to have severely constrained diversification. The intricate relationships between hosts and their nutritional symbionts therefore appear key to shaping patterns of global insect diversity.
Methods
Data collection
Insect and symbiont data
Literature searches
We compiled a database on insect–microbe symbioses by: (1) searching published literature using the following key words [order name] OR [family name] AND ‘symbio’* using the search engines Web of Science and Google scholar during 2015–2017 and again in 2020, (2) searching prominent reviews (for example, Ries60, Schneider61, Müller62, Buchner36, Douglas63, Abe et al.64, Bourtzis and Miller65–67, Baumann68 and Baumann et al.69) and (3) forward and backward searches from the resulting papers. A full list of the papers screened can be found in Supplementary Table 2.
The insect families included in the literature search were those listed in Bouchard et al.70, Davis et al.71 and Rainford et al.12, and those included in published phylogenies investigating insect biodiversity: Hedges et al.72, Misof et al.73 and Rainford et al.12. For symbiont detection, we considered only studies using methods capable of capturing phylogenetically diverse bacteria species (for example, deep-coverage sequencing, or cloning, using ‘universal’ 16S ribosomal RNA (rRNA) primers), or microscopy studies investigating whole insects for the presence of symbionts.
Specific clades of insects are known to carry the same obligate symbionts due to strict vertical transmission (Supplementary Table 2 ‘reference obligate criteria’). We therefore searched Genbank to recover all insect species that have been associated with specific vertically transmitted symbionts (identified taxonomically by symbiont genus name in most cases) to increase our coverage of host–symbiont associations (Supplementary Table 3). Search results were checked manually to ensure host species belonged to the insect clade known to harbour the symbiont (Supplementary Table 3). In families that have species both with and without obligate symbionts, we considered only species directly studied for obligate symbiosis. Vertically transmitted symbionts were included only in analyses of host evolution (‘Specific analyses’ in Methods). In analyses of host–symbiont co-evolution we were interested in testing how host and symbiont phylogenetic history influence symbiont recruitment (‘Nutrient deficiencies and host–symbiont co-specialisation’ in Methods). Vertically transmitted symbionts were excluded as they inflate the signature of co-evolution, not because of symbiont recruitment, but because of inheritance from a common ancestor.
Data inclusion and exclusion
The aim of our paper was to investigate the evolution of beneficial obligate symbioses. We therefore excluded studies: (1) on parasitic symbionts, such as those that manipulate host reproduction (for example, Spiroplasma, Cardinium and Wolbachia) that have not evolved beneficial functions; (2) that failed to screen the entire insect (for example, performed only insect gut analyses); and (3) on symbionts with presumed beneficial functions, but that lacked data needed to assess our obligate criteria (see below). Fungal and protist symbionts were included where data on host dependency were available. Analyses of host–symbiont co-evolution were restricted to symbionts for which a phylogeny could be constructed (bacteria with 16S rRNA genetic data: for details, see ‘Insect and symbiont phylogenies’ in Methods).
For each insect–microbe association we collected data on: the insect species; juvenile and adult insect diets; whether insects were holo- versus hemi-metabolous; the identity of symbionts; symbiont domain; whether symbionts were intra- or extracellular; whether symbionts were housed within specialized structures (for example, bacteriocytes); whether symbionts are thought to have a defensive or nutritional function in nature; and whether insects were obligately dependent on symbionts (for assessment criteria, see below).
Criteria for assessing obligate symbiosis
We are interested in cases where hosts are obligately or highly dependent upon their symbionts (effectively obligate). Obligate dependence is usually proven experimentally, but only a limited number of such studies have been carried out5. Consequently, to allow comparison across a wider range of species, we used the following criteria as indicators of putative obligate dependence, both of which must be fulfilled: (1) Symbiont is universally present in reproductive females; and (2) symbionts are housed in a bacteriome (or mycetome) within bacteriocytes (specialized symbiont-housing cells), or insect-symbiont phylogenies are concordant, or symbiont removal results in significant reduction in host fitness.
In well-studied systems, insects with bacteriocytes typically cannot survive without their symbionts under natural conditions, as observed in aphids74, coccids75, carpenter ants76, cockroaches77, anobiid beetles78, cerambycid beetles79, tsetse flies80 and lice81 (for examples under other conditions, see refs. 75,82,83). In these cases, stable mutual dependence is evident from extreme partner fidelity, genetic uniformity of symbionts within a host species, and concordance of host and symbiont phylogeny as the microbe is faithfully maternally transmitted from a common ancestor (reviewed in refs. 10,84). Consequently, species where a single symbiont is not universally present in all reproductive females, or where specialized symbiont housing organs are lacking were classified as not having obligate symbionts. If symbionts were universally present, but cophylogenetic and/or host fitness data were unavailable the relationship was classified as unresolved and were not included in analyses.
Data on individual species were used to estimate the percentage of species in each family that have evolved dependency on symbionts, which is summarized in Supplementary Table 1. Data on the insect species examined, their associated symbionts and the criteria to assess dependency are provided in Supplementary Table 2.
Feeding niche classification
The feeding niches of species were classified using information on their diets. Omnivores were defined as species that feed on both plant and animal matter, or those that scavenged on detritus. Due to large differences in the nutrient contents of different plant tissues, insect species that specialize on phloem, xylem and wood (xylophagy) were considered separately from species that exploit non-vascular/non-woody plant tissues (for example, leaves, flowers, fruits, seeds and/or root tips), which we refer to as generalist herbivores (also known as phytophagous).
The feeding niches of families were classified using the information on species feeding niches (Supplementary Table 1). Families were described as having omnivorous diets if they contained species that were omnivores/detritivores, or if single species within families utilized multiple food sources that combined plant, animal or fungal material. Families containing species that fed on multiple plant tissues were classified as generalist herbivores. Families were assigned to the feeding categories of haematophagy, phloem feeding, xylem feeding and predatory where most species in the family, if not all, feed exclusively on those resources. Families assigned as xylophagous were those where most species fed on wood as their primary food source. In cases where species-specific diets were not available, we based diets on family-level feeding habits published in books and reviews listed in Supplementary Table 2.
Nutrient data
To estimate the nutrient composition of each insect family, we performed the following steps:
The food types (for example, fruit and roots) utilized by adults and juveniles of all species in our obligate symbiosis dataset were collected from published literature (Supplementary Table 2). Literature was searched using the terms [species name] and [adult diet] or [juvenile diet] in Web of Science and Google Scholar. Where possible, we cross-validated diet assignments using multiple published studies (Supplementary Table 2).
The nutrient composition of all food types that species were found to feed on (Nfood types = 362) was estimated by searching dietary databases for as many examples of those food types as possible (range per food type 1–24, total number of nutrient estimates 5,446; Supplementary Table 4). From dietary databases, we extracted information on the concentration of carbohydrate, fat, protein, essential amino acids (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine), non-essential amino acids (arginine, cystine, glycine, glutamic acid, proline and tyrosine) and vitamins (A, B, C, D, E, K, choline and betaine). Nutrient contents were from a range of foods and are therefore an approximation of insect diets. Where possible, micronutrients were broken down into their subcomponents, for example, individual B vitamins. There were substantial missing data for vitamins C, D, K, choline and betaine (>30% of insect families missing data), which were excluded from analyses.
For each food type, the median concentration of each nutrient across example foods was calculated. Median nutrient values were combined with information on insect diets to calculate a nutrient profile for adults and juveniles of each species (Extended Data Fig. 1). For omnivorous species, nutrients were calculated by averaging across all foods.
Estimates of the nutrients in the diets of each species were calculated by taking the median across adults and juveniles.
Estimates of the dietary nutrients for each family were calculated by taking the median across all species within families. In cases where species-specific diets were not available, we based diets on family-level feeding habits published in books and reviews listed in Supplementary Table 2.
Standardization of nutrient data
Reported data on nutrients (carbohydrates, fats, proteins, amino acids and vitamins) were converted to amount per gram. For some foods, however, this was wet weight and for others it was dry weight. We therefore standardized nutrient values to make them comparable across food types. Values were standardized by dividing each nutrient by the mean weight of each food type (for details, see R script ‘DataConstruction.R’). The mean was used rather than the sum to avoid the non-independence of percentages when analysing all nutrients together.
Diversification
Diversification is typically modelled using three different approaches when species level phylogenies are not available: (1) diversification rates are calculated using clade age and species richness information (also known as ‘age richness rate’); (2) raw estimates of species richness are used; and (3) species richness of sister taxa are compared. It has recently been shown that calculating diversification rates should be avoided when examining patterns of biodiversity85. We therefore examined patterns of diversification using species richness, including family age as an explanatory variable (‘Species richness and obligate symbiosis’ in Methods), and by testing differences in species richness between sister lineages. The ages of families were extracted from the time-calibrated Rainford phylogeny12. Data on the number of extant species in insect families (species richness) were taken from ref. 12. It is likely that species richness for all insect families is underestimated as new species are continually being described. Our analyses do not, however, rely on exact absolute numbers of species, but rather that estimates are representative of relative species richness across families, which has been argued as reasonable46. Variation in estimates of species richness, for example, due to differences in research effort, are also unlikely to correlate to the proportion of species with obligate symbionts, likely adding noise to our results rather than systematic bias.
Insect and symbiont phylogenies
Insects
Our analyses were conducted using the dated insect phylogeny published in ref. 12. The tree was constructed using maximum likelihood (RAxML) and dated with a relaxed molecular clock in MrBayes calibrated with 86 fossils12. A single consensus tree was published, and therefore our analyses do not account for uncertainty in tree topology or branch lengths. A useful next step will be to incorporate tree uncertainty into analyses, which can be done, for example, by integrating results over a sample of candidate trees. More in-depth details of topological uncertainties and dating methods are available in ref. 12.
Families that lacked data on obligate symbioses were pruned from the tree. There were 23 families for which there were data on obligate symbioses that were not included in ref. 12. We therefore added these families to the phylogeny at branches corresponding to published sister taxa (Supplementary Table 1) using the bind.tip function in the R package ‘phytools’86 (for details, see R script ‘Rainford_adding_tips.R’). Added families were not included for diversification analyses due to uncertainty of the age of these families.
Symbionts
We estimated the phylogenetic relationships for bacterial symbionts for which genetic data were available. A ~1,500 bp region of the bacterial 16S rRNA gene downloaded from the SILVA RNA database was aligned with MUSCLE and edited in the alignment software Geneious 8.1.8 (https://www.geneious.com). We constructed a maximum likelihood phylogeny for the bacterial lineages using the on-line PhyML server87, and the best-fitting models of evolution were estimated using the Aikake information criterion. The symbiont phylogeny was bootstrapped 100 times and rooted to Thermus thermophilus, which is basal to all the bacterial lineages presented in this study.
General statistical methods
Data were analysed using Bayesian phylogenetic mixed models with single (BPMM) and multiple response variables (MR-BPMM), SCM and transition rate models with Markov chain Monte Carlo (MCMC) estimation. In this section we provide general details of modelling approaches, and in ‘Specific analyses’ in Methods, we outline the details of the analyses conducted. All analyses were performed in R 4.1.3 (ref. 88), apart from transition rate models that were conducted in BayesTraits V4 (ref. 89). Continuous response and explanatory variables were Z-transformed before analyses (mean 0, standard deviation 1).
BPMMs
Model construction, parameter estimates and assessing significance
To estimate phylogenetic signature, co-evolutionary relationships and ancestral trait values we used BPMMs and MR-BPMMs with MCMC estimation in the R package MCMCglmm90. Obligate symbiosis was analysed as the number of species with and without obligate symbionts (proportion) within each family using a binomial error distribution with a logit link function. Analysing obligate symbiosis in this way enables variation in the number of species examined for obligate symbionts across insect families to be accounted for. Nutrient concentrations were Z-transformed before analysis and modelled as Gaussian response variables and species richness was modelled using a Poisson error distribution with log link function.
The global intercept was removed from MR-BPMMs to allow trait specific intercepts to be estimated. Parameter estimates from models are presented as posterior modes with 95% CIs, together with approximate P values (pMCMC)90.
The non-independence of data resulting from phylogenetic relatedness between insect hosts and phylogenetic relatedness between symbiont lineages was modelled using random effects. For phylogenetic effects we fitted a variance–covariance matrix constructed from the insect and bacteria phylogenies. Phylogenetic and residual correlations between traits were calculated using the variance and covariance estimates from unstructured phylogenetic and residual variance–covariance matrices. We estimated the amount of variation in response variables explained by random effects (RE), including phylogenetic effects, as the intraclass correlation coefficient on the latent scale estimated as
where Vi is the focal random effect, VRE is the sum of all random effects and Ve is the residual variance on the latent scale. For binomial error distributions Ve was calculated as the observed residual variance plus the variance associated with the link function (logit = pi2/3; for discussion, see refs. 91,92).
Prior settings
For random effects we began prior selection by assessing model convergence using inverse-gamma priors (V = diag(n), nu = n − 1 + 0.002, where n was equivalent to the number of response traits). If the mixing properties of the MCMC chain were poor, which was often the case for binomial response variables, we examined parameter expanded priors (V = diag(n), nu = n − 1, alpha.mu = rep(0, n), alpha.V = diag(n) × 252) (ref. 92). For fixed effects the default priors in MCMCglmm (independent normal priors with zero mean and large variance (1010)) were used apart from in models with binomial response variables where a prior of mu = 0, V = σ2 units + π2/3 was specified. This is approximately flat on the probability scale when a logit link function is defined90,93, and in all cases improved the mixing of chains. The final prior settings used for each analysis are specified in the supplementary R code (R script ‘Analyses.R’).
Model convergence
Models were run for 2 million iterations with a burn-in of 1 million iterations, and chains were sampled every 1,000 iterations. We examined the convergence of models by repeating each analysis three times and examining the correspondence between chains using the R package ‘coda’94 in the following ways: (1) visually inspecting the traces of the MCMC posterior estimates and their overlap; (2) calculating the autocorrelation and effective sample size of the posterior distribution of each chain; and (3) using Gelman and Rubin’s convergence diagnostic test that compares within- and between-chain variance using a potential scale reduction factor. Potential scale reduction factor values substantially higher than 1.1 indicate chains with poor convergence properties. For convergence checking, see R script ‘ModelCheckingCombining.R’.
Transition rate models
SCM
SCM was used to estimate ancestral states of obligate symbiosis and feeding niches across the insect phylogeny in the R package ‘phytools’86. In brief, this approach calculates the conditional likelihood that each ancestral node is in a given state depending on the estimated transition rate matrix (Q) between states and the length of the branch associated with that node. On the basis of these conditional likelihoods, ancestral states at each node are stochastically simulated and used in combination with observations at the tips to reconstruct a character history along each branch. Each character history is simulated using a continuous-time Markov chain where changes between states and the time spent in each state are modelled as a Poisson process (for more details, see ref. 95).
BayesTraits
BayesTraits V4 was used to reconstruct ancestral values of feeding niches and examine co-evolutionary relationships between B vitamins and obligate symbiosis (for details, see ‘Specific analyses’ in Methods). We used hyper priors where values are drawn from a uniform distribution with a range 0 to 10 to seed the mean and variance of an exponential prior to reduce uncertainty over prior selection89. We ran each model three times for a total of 11,000,000 iterations with a burn-in of 1,000,000 iterations and sampled every 1000 iterations. We examined the convergence of models in the same way as ‘Transition rate models’ in Methods.
Bayes factors (2(log marginal likelihood of complex model − log marginal likelihood of simple model)) were used to test if models where traits were allowed to co-evolve provided a better fit to the data than models that assumed independent evolution. To calculate the log marginal likelihood, we used the stepping-stones procedure as described in the BayesTraits V4 manual where 100 stones were run for 1,000 iterations each. Bayes factors over 2 are considered to offer positive evidence, over 5 strong evidence and over 10 very strong evidence89. To test whether transition rates were significantly different from each other, we calculated the posterior mode, 95% CIs and pMCMC value of the posterior distribution of differences between transition rates.
Specific analyses
Evolutionary history of obligate symbioses
The number of origins of obligate symbiosis
The probability of each node in the insect phylogeny having an obligate symbiont was estimated using a BPMM with the proportion of species in families with obligate symbionts as a response variable. The posterior probability of each node having obligate symbionts was estimated using the ‘predict’ function in MCMCglmm and nodes with a posterior probability greater than 0.5 were classified as ‘obligate’. We found support for 16 origins and 8 losses of obligate symbiosis.
We examined the robustness of the estimated number of origins and losses of obligate symbiosis using SCM. As SCM requires categorical states (obligate versus non-obligate), insect families (n = 402) were classified as having an obligate symbiosis where over 50% species in families had symbionts. Data on obligate symbiosis were used to build 1,000 stochastic character maps across the insect phylogeny using an all-rates different Q matrix estimated with MCMC. Nodes were classified as having obligate symbionts if the proportion of the 1,000 stochastic character maps was above 50%. We found high correspondence between the SCM and BPMM analyses: 98% of ancestral states had the same predicted state of obligate symbiosis across analyses, indicating that our results are robust to different statistical approaches (Supplementary Table 20).
Estimating ancestral feeding niches
Ancestral feeding niches were estimated using SCM. Data on the feeding niches of insect families (n = 402) were used to build 1,000 stochastic character maps across the insect phylogeny using an equal-rates Q matrix estimated with MCMC. Ancestral estimates of nodes were assigned according to the feeding niche with the highest proportion of the 1,000 stochastic character maps. Transitions between feeding niches were identified where ancestral and descendant nodes were in different states (Supplementary Table 5).
We examined the robustness of ancestral feeding niches estimated using SCM by performing a second set of ancestral reconstructions. We used the MULTISTATE module in BayesTraits V4 with reversible-jump MCMC estimation and compared them with the estimates gained by SCM. There was very good correspondence between SCM and MULTISTATE, with 94% of ancestral nodes predicted to have the same feeding niche (Supplementary Table 20).
Rates of obligate symbiosis across different feeding niches
The probability that insects with different feeding niches had obligate symbionts was modelled using a BPMM with the proportion of species in families with obligate symbionts as a response variable. The feeding niche of each family was fitted as an eight-level fixed effect (Supplementary Table 6). To determine if rates of obligate symbiosis were significantly different across niches, we calculated the pairwise differences between niches and examined if the 95% CIs spanned 0 (Supplementary Table 6).
Nutritional deficiencies and the evolution of obligate symbiosis
Phylogenetic correlations between obligate symbiosis and nutrients
The phylogenetic correlations between obligate symbiosis and nutrients within diets was estimated using a MR-BPMM with the proportion of species in families with obligate symbionts and concentrations of carbohydrate, fat, protein, essential amino acids (sum of histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine), non-essential amino acids (sum of arginine, cystine, glycine, glutamic acid, proline and tyrosine), vitamin A, vitamin B (sum of individual B vitamins) and vitamin E as response variables (Supplementary Table 7). For analyses of individual amino acids, see ‘Obligate symbiosis and types of amino acids’ in Methods and Supplementary Table 29. There were missing values for some nutrients for some families in our dataset (Supplementary Table 4). In BPMMs, missing data are permitted in response variables and are predicted with an accuracy relative to the phylogenetic signature in traits and magnitude of trait correlations. This can enable missing values to be predicted with high accuracy93,96. All nutrients had high phylogenetic signature (phylogenetic heritability (phylo H2) 0.71–0.96; Supplementary Table 7), and therefore nutrients with up to 30% missing values were included in analyses (vitamin A = 21%, vitamin E = 29%; all other nutrients had less than 5% missing data).
Differences in the nutrient contents of feeding niches
Differences in the nutritional composition of feeding niches were estimated using a MR-BPMM with carbohydrate, fat, protein, essential amino acids, non-essential amino acids, vitamin A, vitamin B and vitamin E as response variables. To test if nutrient levels of each feeding niche differed from background rates, we sequentially fitted two-level factors of focal feeding niche versus all other niches as a fixed effect (Supplementary Table 9).
Phylogenetic correlations between obligate symbiosis and B vitamins
The phylogenetic correlations between obligate symbiosis and individual B vitamins were analysed using a MR-BPMM with the proportion of species in families with obligate symbionts and concentrations of vitamins B5 and B9 as response variables (Supplementary Table 8). Data on vitamins B7 and B12 were not analysed as there were missing values for over 30% of insect families. Data on B1, B2 and B3 were highly correlated with vitamin B5 levels (r > 0.9), but there were more data on vitamin B5. As a result, only vitamin B5 was analysed, but it is worth noting that B1, B2 and B3 may contribute to any association with obligate symbiosis.
Nutrients and the gains and losses of obligate symbiosis
Ancestral B vitamins in relation to the gains and losses of symbionts
We examined how levels of B5 and B9 vitamins differed between the ancestors of families with and without obligate symbionts using a two-step approach: first, we used the output of the model in ‘The number of origins of obligate symbiosis’ in Methods to classify nodes as: (1) non-obligate node with non-obligate descendants (Non to Non); (2) non-obligate node with at least one obligate descendant (Non to Ob); (3) obligate node with obligate descendants (Ob to Ob); and (4) obligate node with at least one non-obligate descendant (Ob to Non). Second, node classifications were entered as a four-level fixed factor in an MR-BPMM with B5 and B9 vitamin concentrations as response variables (Supplementary Table 10). B5 and B9 vitamin levels were compared across nodes that preceded the origin (1 versus 2), maintenance (1 versus 3) and loss of obligate symbiosis (3 versus 4). Unstructured phylogenetic and residual variance–covariance matrices were fitted as random effects with the phylogenetic covariance matrix being linked to node labels.
To account for uncertainty in our node classifications, we repeated the analysis 100 times, each time reclassifying nodes as ‘obligate’ or ‘non-obligate’ by resampling from the posterior distribution of model 4.1.1. Posterior samples from across the 100 models were combined. Each model was run for 1,100,000 iterations with a burn-in of 1,000,000 iterations and a thinning interval of 10,000 samples, which across the re-samplings resulted in 1,000 posterior samples (100 re-samplings × 10 samples per resampling). To verify that our estimates of B vitamins were robust to rate shifts in B vitamins across the tree, we compared our BPMM estimates with phylogenetic ridge regression models that allowed for rate shifts97. We found high correspondence between estimates from BPMM and phylogenetic ridge regression models and between BPMM estimates and actual B vitamin values for insect families (Extended Data Fig. 4).
Transition rates between obligate symbiosis and B vitamins
We tested if models that allowed for co-evolution between obligate symbiosis and B5 and B9 vitamins better explained our data than models that assumed independent evolution of each trait using transition rate models in BayesTraits. Co-evolution was modelled using an all-rates different Q matrix with separate models run for B5 and B9 vitamins. For transition rate models, only binary classifications can be modelled. Insect families were therefore classified as obligate (>50% of species within families have obligate symbionts) and non-obligate (<50% of species within families have obligate symbionts), and having high and low B5 and B9 vitamins. For B-vitamin classifications, two different cut-offs were analysed to establish the sensitivity of our results to different thresholds: above and below the 25% and 50% quantile for high and low B vitamins, respectively (Supplementary Table 11). It was not necessary to examine the sensitivity of our results to the classification of obligate symbiosis as 96% of 402 insect families had 100% of species with or without obligate symbionts. Reversible-jump MCMC models were also run to further test if transitions occurred (percentage of models where transition rate was above 0) and if transition were different from each other (percentage of models where transition rates were assigned to different rate categories).
Nutrient deficiencies and host–symbiont co-specialization
Host–symbiont interactions and the evolution of obligate symbiosis
To examine how obligate symbiosis has been influenced by the co-evolutionary history between insects and bacteria, we constructed a dataset of pairwise combinations between all insect families and all symbionts (excluding vertically transmitted symbionts). For each combination, the number of insect species within a family with a particular obligate symbiont versus the number of species sampled without that symbiont was calculated and analysed using a BPMM with a binomial error distribution. This enabled differences in the sampling effort across different insect–bacteria associations to be accounted for. Whether symbionts were intra- and extracellular was included as a two-level fixed effect. Three different variance–covariance matrices were fitted as random effects to quantify the amount of variation in obligate symbiosis explained by: (1) insect hosts independent of their phylogenetic history (‘h’), for example, certain hosts are more likely to form obligate relationships than others; (2) insect hosts phylogenetic history (‘[h]’), for example, certain host lineages are more likely to form obligate relationships than others; and (3) phylogenetic interactions between hosts and symbionts (‘[hs]’), for example, particular host phylogenetic lineages are more likely to form obligate symbioses with particular bacterial phylogenetic lineages (Supplementary Table 14). For details of model fitting, see ref. 98 (the variance–covariance matrices outlined in ref. 98 that relate to the number of hosts symbionts associate with were not fitted in models as each symbiont lineage was found in only one insect family).
To further examine whether phylogenetically related lineages of bacteria are more likely to form obligate symbioses with phylogenetically related lineages of insects, we used parafit in the R package ‘ape’ (Supplementary Table 15). This tests the correlation between host- and symbiont-shared branch lengths against a randomized distribution generated from 1,000 permutations of the data99.
Host–symbiont interactions and levels of B vitamins in insect diets
To test if specific lineages of symbiotic bacteria specialize in providing B5 and B9 vitamins to hosts, we used the same BPMM approach described in ‘Host–symbiont interactions and the evolution of obligate symbiosis’ in Methods. We estimated variation in levels of B vitamins (Gaussian responses) explained by h, [h], [s] and [hs]. Separate models were run for B5 and B9 vitamins, and data were restricted to combinations of hosts and bacteria that formed obligate symbioses (Supplementary Tables 12 and 13).
Obligate symbiosis and diversification
Species richness and obligate symbiosis
The relationship between obligate symbioses and species richness was estimated using an MR-BPMM with the proportion of species in families with obligate symbionts and species richness as response variables. Family age and whether insect families were holo- or hemimetabolous (two-level factor previously shown to influence diversification rates12) were fitted as fixed effects (Supplementary Table 16).
Species richness across symbiont-associated feeding niches
To test if species richness differed between insect families with and without obligate symbionts occupying different feeding niches, a BPMM with species richness as a response variable was used. Each insect family was classified according to whether it had obligate symbionts (>50% of species with symbionts) and its feeding niche (11-level factor), which was fitted as a fixed effect along with family age and holo–hemi metabolism (Supplementary Table 17). To test if species richness of each obligate symbiosis-feeding niche combination differed from background levels, we re-ran models fitting two-level fixed factor of the focal obligate symbiosis-feeding niche combination versus all other data (Supplementary Table 17).
Species richness and obligate symbiosis among sister taxa
Sister comparisons were extracted from ref. 12 phylogeny using the ‘extract_sisters’ function in the R package ‘diverge’100 (Supplementary Table 30). We analysed data on all sister comparisons (n = 123) using a BPMM to test if feeding niche and obligate symbiosis was related to species richness at a finer taxonomic scale than in ‘Species richness and obligate symbiosis’ in Methods. Species richness was the response variable and the percentage of species within families with obligate symbionts (% logit transformed), age of the sister comparison (million years) and feeding niche were included as fixed effects. The non-independence of data from sister taxa was modelled by including sister pair identity as a random effect and the non-independence of sister pairs across the phylogeny was modelled by including node identities of each sister comparison linked to the phylogeny as a random effect (Supplementary Table 18).
Species richness changes and the evolution of obligate symbiosis
To further examine if the evolution of obligate symbiosis is associated with increased species richness, we restricted our sister comparison dataset to cases where sister families differed in the percentage of species with obligate symbionts. Differences in species richness was calculated as the log2 of the ratio of species richness between sister taxa and analysed as a Gaussian response variable using a BPMM. The difference in the percentage of species with obligate symbionts between sister taxa and pair age were fitted as fixed effects. Node identity of sister comparisons, linked to the phylogeny, was included as a random effect (Supplementary Table 19).
Sensitivity analyses
We tested the robustness of our conclusions to several underlying data assumptions. These sensitivity analyses provided quantitatively similar results to our main analysis (Supplementary Tables 21–27).
Removing families added to the Rainford tree
There were 23 families within our obligate symbiont dataset that were not represented in the Rainford phylogeny that were added to the phylogeny (‘Insect and symbiont phylogenies’ in Methods). To examine the robustness of our results to including these families, we re-ran the analyses detailed in ‘Phylogenetic correlations between obligate symbiosis and nutrients’ in Methods (Supplementary Table 21) with the 23 families excluded.
Including only bacterial symbionts
Bacteria made up the vast majority of obligate symbionts (79 out of 84 insect families had bacterial symbionts, 94%). To verify that our results were not explained by a few outlying eukaryotic symbionts, we re-ran the analyses detailed in ‘Phylogenetic correlations between obligate symbiosis and nutrients’ in Methods including only insect families with bacterial symbionts (nfamilies = 395; Supplementary Table 22).
Removing co-occurring obligate symbionts
There were 112 unique host–bacterial symbiont combinations. Of these 49% (n = 55) had multiple co-occuring symbionts. It is possible that any signature of bacteria specializing in B5 and B9 vitamin production is obscured by the presence of co-residing obligate symbionts that may change nutrient provisioning roles. We therefore repeated the analyses in ‘Host symbiont interactions and levels of B vitamins in insect diets’ in Methods section after removing hosts that had multiple co-occurring symbionts (Supplementary Table 23).
Excluding data from microscope studies
Out of the 402 insect families included in our analyses, 260 were inferred to lack obligate symbionts from microscopy by Buchner and colleagues that showed an absence of specialized symbiont organs. Insects in the orders Ephemeroptera, Plecoptera, Odontata, Neuroptera, Orthoptera and Lepidoptera, superfamily Tenthredinoidea and subclade Aculeata (excluding Formicidae) all lacked bacteriocytes and in general do not depend on endosymbionts for survival23. We tested the sensitivity of our results to including this inferred data by re-running analyses outlined in 4.2.1, 4.2.3 and 4.5.2 on data where obligate symbiosis had only been directly studied (for more details, see ‘Criteria for assessing obligate symbiosis’ in Methods). This also tested the robustness of our results to removing Lepidoptera that contribute many tips to the phylogenetic tree, lack obligate symbionts and are predominately herbivorous.
Obligate symbiosis and types of amino acids
In 4.2.1 we analysed the sum of essential and non-essential amino acids. All amino acids were highly correlated (r > 0.8. Extended Data Fig. 3), but to further check if minor differences in the concentration of each amino acid influenced the relationship with obligate symbiosis we ran a second set of analyses. The phylogenetic correlation between obligate symbiosis and each amino acid was examined using a series of MR-BPMMs. The proportion of species with obligate symbionts in families and the concentration of each amino acid were response variables in each analysis (nine different bivariate models for essential amino acids and six different bivariate models of non-essential amino acids; Supplementary Table 29).
Removing families with and without obligate symbionts
Out of the 402 insect families, 15 families contained species with and without obligate symbionts (3.7%). For transition rate analyses, these mixed families had to be classified as having obligate symbionts or not. We tested the sensitivity of our results to this classification by removing these families and re-running the analyses described in ‘Transition rates between obligate symbiosis and B vitamins’ in Methods.
The conclusions from the sensitivity analyses were quantitatively similar to our main analyses (Supplementary Tables 21–29).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Supplementary Tables 1–30 and R session information (versions and packages loaded).
Acknowledgements
We are very grateful to funding from the Knut and Alice Wallenberg Foundation (Wallenberg Academy fellowship 2018.0138 to C.K.C.), the Swedish Research Council (grant number 2017-03880 to C.K.C.), the European Research Council (grant numbers 335542 to E.T.K. and 834164 to S.A.W.), the Ammodo Foundation (funding to E.T.K.) and Natural Environmental Research Council (grant number NE/M018016/1 to L.H.).
Extended data
Source data
Source data for Fig. 1.
Source data for Fig. 2.
Source data for Fig. 3.
Source data for Fig. 4.
Source data for Extended Data Fig. 1.
Source data for Extended Data Fig. 2.
Source data for Extended Data Fig. 3.
Source data for Extended Data Fig. 4.
Source data for Extended Data Fig. 5.
Source data for Extended Data Fig. 6.
Source data for Extended Data Fig. 7.
Source data for Extended Data Fig. 8.
Source data for Extended Data Fig. 9.
Author contributions
Conceptualization: C.K.C., A.V.P., J.E., E.T.K., S.A.W and L.M.H. Methodology: C.K.C., A.V.P., J.E., M.K., R.J., E.T.K., S.A.W. and L.M.H. Investigation: C.K.C., A.V.P., J.E., M.K., R.J., E.T.K., S.A.W. and L.M.H. Visualization: C.K.C., A.V.P., E.T.K., S.A.W. and L.M.H. Funding acquisition: C.K.C., J.E., E.T.K., S.A.W. and L.M.H. Project administration: J.E., E.T.K. and L.M.H. Supervision: J.E., E.T.K. and L.M.H. Writing—original draft: C.K.C., E.T.K., S.A.W. and L.M.H. Writing—review and editing: C.K.C., A.V.P., J.E., M.K., R.J., E.T.K., S.A.W. and L.M.H.
Peer review
Peer review information
Nature Ecology & Evolution thanks Hassan Salem, Mariana Braga and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Funding
Open access funding provided by Lund University.
Data availability
All data are provided in Supplementary Tables 1–4. Full citations of references in supplementary tables are given in the method references7,12,16,101–404. All supplementary tables are available at the Open Science Framework (osf.io project number TYK7C; 10.17605/OSF.IO/TYK7C)405. Source data are provided with this paper.
Code availability
R code, BayesTraits code and analysis results are available at the Open Science Framework (osf.io project number TYK7C; 10.17605/OSF.IO/TYK7C)405.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/3/2023
A Correction to this paper has been published: 10.1038/s41559-023-02137-2
Contributor Information
Charlie K. Cornwallis, Email: charlie.cornwallis@biol.lu.se
Lee M. Henry, Email: l.henry@qmul.ac.uk
Extended data
is available for this paper at 10.1038/s41559-023-02058-0.
Supplementary information
The online version contains supplementary material available at 10.1038/s41559-023-02058-0.
References
- 1.Bronstein, J. L. Mutualism (Oxford Univ. Press, 2015).
- 2.Douglas AE. The microbial dimension in insect nutritional ecology. Funct. Ecol. 2009;23:38–47. doi: 10.1111/j.1365-2435.2008.01442.x. [DOI] [Google Scholar]
- 3.Estrela S, Kerr B, Morris JJ. Transitions in individuality through symbiosis. Curr. Opin. Microbiol. 2016;31:191–198. doi: 10.1016/j.mib.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Bennett GM, Moran NA. Heritable symbiosis: the advantages and perils of an evolutionary rabbit hole. Proc. Natl Acad. Sci. USA. 2015;112:10169–10176. doi: 10.1073/pnas.1421388112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher RM, Henry LM, Cornwallis CK, Kiers ET, West SA. The evolution of host–symbiont dependence. Nat. Commun. 2017;8:1. doi: 10.1038/ncomms15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maynard-Smith, J. & Szathmary, E. The Major Transitions in Evolution (Oxford Univ. Press, 1997).
- 7.Moran NA, Tran P, Gerarado NM. Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial phylum bacteroidetes. Appl. Environ. Microbiol. 2005;71:8802–8810. doi: 10.1128/AEM.71.12.8802-8810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleiner M, et al. Metaproteomics of a gutless marine worm and its symbiotic microbial community reveal unusual pathways for carbon and energy use. Proc. Natl Acad. Sci. USA. 2012;109:E1173–E1182. doi: 10.1073/pnas.1121198109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris JL, et al. The timescale of early land plant evolution. Proc. Natl Acad. Sci. USA. 2018;115:E2274–E2283. doi: 10.1073/pnas.1719588115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 2008;42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 11.Oliver KM, Smith AH, Russell JA. Defensive symbiosis in the real world advancing ecological studies of heritable, protective bacteria in aphids and beyond. Funct. Ecol. 2014;28:341–355. doi: 10.1111/1365-2435.12133. [DOI] [Google Scholar]
- 12.Rainford JL, Hofreiter M, Nicholson DB, Mayhew PJ. Phylogenetic distribution of extant richness suggests metamorphosis is a key innovation driving diversification in insects. PLoS ONE. 2014;9:e109085. doi: 10.1371/journal.pone.0109085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliver KM, Russell JA, Moran NA, Hunter MS. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl Acad. Sci. USA. 2003;100:1803–1807. doi: 10.1073/pnas.0335320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flórez LV, Biedermann PHW, Engl T, Kaltenpoth M. Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Nat. Prod. Rep. 2015;32:904–936. doi: 10.1039/C5NP00010F. [DOI] [PubMed] [Google Scholar]
- 15.Currie CR, Scott JA, Summerbell RC, Malloch D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature. 1999;398:701–704. doi: 10.1038/19519. [DOI] [Google Scholar]
- 16.Nakabachi A, et al. Defensive bacteriome symbiont with a drastically reduced genome. Curr. Biol. 2013;23:1478–1484. doi: 10.1016/j.cub.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Flórez LV, et al. Antibiotic-producing symbionts dynamically transition between plant pathogenicity and insect-defensive mutualism. Nat. Commun. 2017;8:15172. doi: 10.1038/ncomms15172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaltenpoth M, et al. Partner choice and fidelity stabilize coevolution in a Cretaceous-age defensive symbiosis. Proc. Natl Acad. Sci. USA. 2014;111:6359–6364. doi: 10.1073/pnas.1400457111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cafaro MJ, et al. Specificity in the symbiotic association between fungus-growing ants and protective Pseudonocardia bacteria. Proc. R. Soc. B. 2011;278:1814–1822. doi: 10.1098/rspb.2010.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flórez LV, Kaltenpoth M. Symbiont dynamics and strain diversity in the defensive mutualism between Lagria beetles and Burkholderia. Environ. Microbiol. 2017;19:3674–3688. doi: 10.1111/1462-2920.13868. [DOI] [PubMed] [Google Scholar]
- 21.Mueller UG, Dash D, Rabeling C, Rodrigues A. Coevolution between attine ants and actinomycete bacteria: a reevaluation. Evolution. 2008;62:2894–2912. doi: 10.1111/j.1558-5646.2008.00501.x. [DOI] [PubMed] [Google Scholar]
- 22.Sloan DB, Moran NA. Endosymbiotic bacteria as a source of carotenoids in whiteflies. Biol. Lett. 2012 doi: 10.1098/rsbl.2012.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salem H, et al. Drastic genome reduction in an herbivore’s pectinolytic symbiont. Cell. 2017;171:1520–1531.e13. doi: 10.1016/j.cell.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Douglas AE. Phloem-sap feeding by animals: problems and solutions. J. Exp. Bot. 2006;57:747–754. doi: 10.1093/jxb/erj067. [DOI] [PubMed] [Google Scholar]
- 25.Snyder AK, Rio RVM. ‘Wigglesworthia morsitans’ folate (vitamin B9) biosynthesis contributes to tsetse host fitness. Appl. Environ. Microbiol. 2015;81:5375–5386. doi: 10.1128/AEM.00553-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalkova V, Benoit JB, Weiss BL, Attardo GM, Aksoy S. Vitamin B6 generated by obligate symbionts is critical for maintaining proline homeostasis and fecundity in tsetse flies. Appl. Environ. Microbiol. 2014;80:5844–5853. doi: 10.1128/AEM.01150-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blow F, et al. B-vitamin nutrition in the pea aphid-Buchnera symbiosis. J. Insect Physiol. 2020;126:104092. doi: 10.1016/j.jinsphys.2020.104092. [DOI] [PubMed] [Google Scholar]
- 28.Ren F-R, et al. Biotin provisioning by horizontally transferred genes from bacteria confers animal fitness benefits. ISME J. 2020;14:2542–2553. doi: 10.1038/s41396-020-0704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren F-R, et al. Pantothenate mediates the coordination of whitefly and symbiont fitness. ISME J. 2021;15:1655–1667. doi: 10.1038/s41396-020-00877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikoh N, et al. Evolutionary origin of insect nutritional mutualism. Proc. Natl Acad. Sci. USA. 2014;111:10257–10262. doi: 10.1073/pnas.1409284111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salem H, et al. Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proc. R. Soc. B. 2014;281:20141838. doi: 10.1098/rspb.2014.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manzano-Marín A, Oceguera-Figueroa A, Latorre A, Jiménez-García LF, Moya A. Solving a bloody mess: B-vitamin independent metabolic convergence among gammaproteobacterial obligate endosymbionts from blood-feeding arthropods and the leech haementeria officinalis. Genome Biol. Evol. 2015;7:2871–2884. doi: 10.1093/gbe/evv188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCutcheon JP, McDonald BR, Moran NA. Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc. Natl Acad. Sci. USA. 2009;106:15394–15399. doi: 10.1073/pnas.0906424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Husnik F, et al. Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell. 2013;153:1567–1578. doi: 10.1016/j.cell.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 35.Stever H, Eiben J, Bennett GM. Hawaiian nysius insects rely on an obligate symbiont with a reduced genome that retains a discrete nutritional profile to match their plant seed diet. Genome Biol. Evol. 2021;13:evab189. doi: 10.1093/gbe/evab189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchner, P. Endosymbiosis of Animals with Plant Microorganisms (Interscience Publishers, 1965).
- 37.Douglas AE. The B vitamin nutrition of insects: the contributions of diet, microbiome and horizontally acquired genes. Curr. Opin. Insect Sci. 2017;23:65–69. doi: 10.1016/j.cois.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Monnin D, et al. Parallel evolution in the integration of a co-obligate aphid symbiosis. Curr. Biol. 2020;30:1949–1957.e6. doi: 10.1016/j.cub.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Sudakaran S, Kost C, Kaltenpoth M. Symbiont acquisition and replacement as a source of ecological innovation. Trends Microbiol. 2017;25:375–390. doi: 10.1016/j.tim.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Bell-Roberts L, Douglas AE, Werner GDA. Match and mismatch between dietary switches and microbial partners in plant sap-feeding insects. Proc. R. 2019;286:20190065. doi: 10.1098/rspb.2019.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manzano-Marín A, et al. Serial horizontal transfer of vitamin-biosynthetic genes enables the establishment of new nutritional symbionts in aphids’ di-symbiotic systems. ISME J. 2020;14:259–273. doi: 10.1038/s41396-019-0533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan Y, Wernegreen JJ. Can’t take the heat: high temperature depletes bacterial endosymbionts of ants. Microb. Ecol. 2013;66:727–733. doi: 10.1007/s00248-013-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunbar HE, Wilson ACC, Ferguson NR, Moran NA. Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol. 2007;5:e96. doi: 10.1371/journal.pbio.0050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCutcheon JP, Boyd BM, Dale C. The life of an insect endosymbiont from the cradle to the grave. Curr. Biol. 2019;29:R485–R495. doi: 10.1016/j.cub.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 45.Sudakaran S, Retz F, Kikuchi Y, Kost C, Kaltenpoth M. Evolutionary transition in symbiotic syndromes enabled diversification of phytophagous insects on an imbalanced diet. ISME J. 2015;9:2587–2604. doi: 10.1038/ismej.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiens JJ, Lapoint RT, Whiteman NK. Herbivory increases diversification across insect clades. Nat. Commun. 2015;6:8370. doi: 10.1038/ncomms9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anbutsu H, et al. Small genome symbiont underlies cuticle hardness in beetles. Proc. Natl Acad. Sci. USA. 2017;114:E8382–E8391. doi: 10.1073/pnas.1712857114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toju H, Tanabe AS, Notsu Y, Sota T, Fukatsu T. Diversification of endosymbiosis: replacements, co-speciation and promiscuity of bacteriocyte symbionts in weevils. ISME J. 2013;7:1378–1390. doi: 10.1038/ismej.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feldhaar H, et al. Nutritional upgrading for omnivorous carpenter ants by the endosymbiont Blochmannia. BMC Biol. 2007;5:48. doi: 10.1186/1741-7007-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moran NA, Bennett GM. The tiniest tiny genomes. Annu. Rev. Microbiol. 2014;68:195–215. doi: 10.1146/annurev-micro-091213-112901. [DOI] [PubMed] [Google Scholar]
- 51.McCutcheon JP. The genomics and cell biology of host-beneficial intracellular infections. Annu. Rev. Cell Dev. Biol. 2021;37:115–142. doi: 10.1146/annurev-cellbio-120219-024122. [DOI] [PubMed] [Google Scholar]
- 52.Reis F, et al. Bacterial symbionts support larval sap feeding and adult folivory in (semi-)aquatic reed beetles. Nat. Commun. 2020;11:2964. doi: 10.1038/s41467-020-16687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson R, et al. Convergent evolution of a labile nutritional symbiosis in ants. ISME J. 2022;16:2114–2122. doi: 10.1038/s41396-022-01256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patiño-Navarrete R, Moya A, Latorre A, Peretó J. Comparative genomics of Blattabacterium cuenoti: the frozen legacy of an ancient endosymbiont genome. Genome Biol. Evol. 2013;5:351–361. doi: 10.1093/gbe/evt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akman L, et al. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat. Genet. 2002;32:402–407. doi: 10.1038/ng986. [DOI] [PubMed] [Google Scholar]
- 56.Hosokawa T, et al. Reductive genome evolution, host–symbiont co-speciation and uterine transmission of endosymbiotic bacteria in bat flies. ISME J. 2012;6:577–587. doi: 10.1038/ismej.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirkness EF, et al. Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. Proc. Natl Acad. Sci. USA. 2010;107:12168–12173. doi: 10.1073/pnas.1003379107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiefer, J. S. T. et al. Inhibition of a nutritional endosymbiont by glyphosate abolishes mutualistic benefit on cuticle synthesis in Oryzaephilus surinamensis. Commun. Biol.4, 554 (2021). [DOI] [PMC free article] [PubMed]
- 59.Weiner, D. J. The Beak of the Finch: A Story of Evolution in Our Time (Vintage, 1995).
- 60.Ries E. Die symbiose der Läuse und Federlinge. Z. Morphol. Ökol. Tiere. 1931;20:233–367. doi: 10.1007/BF00444101. [DOI] [Google Scholar]
- 61.Schneider, G. Beitrage zur Kenntis der Symbiontischen Einrichtuingen der Heteroperen (Springer, 1939).
- 62.Müller HJ. Neuere Vorstellungen über Verbreitung und Phylogenie der Endosymbiosen der Zikaden. Z. Morphol. Ökol. Tiere. 1962;51:190–210. doi: 10.1007/BF00409635. [DOI] [Google Scholar]
- 63.Douglas AE. Mycetocyte symbiosis in insects. Biol. Rev. 1989;64:409–434. doi: 10.1111/j.1469-185X.1989.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 64.Abe, T., Bignell, D. E. & Higashi, M. Termites: Evolution, Sociality, Symbioses, Ecology (Springer, 2000).
- 65.Bourtzis, K. & Miller, T. A. Insect Symbiosis Vol. 1 (CRC Press, 2003).
- 66.Bourtzis, K. & Miller, T. A. Insect Symbiosis Vol. 2 (CRC Press, 2006).
- 67.Bourtzis, K. & Miller, T. A. Insect Symbiosis Vol. 3 (CRC Press, 2009).
- 68.Baumann P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 2005;59:155–189. doi: 10.1146/annurev.micro.59.030804.121041. [DOI] [PubMed] [Google Scholar]
- 69.Baumann, P., Moran, N. A. & Baumann, L. C. in The Prokaryotes: Prokaryotic Biology and Symbiotic Associations (eds Rosenberg, E. et al.) 465–496 (Springer, 2013).
- 70.Bouchard, P. et al. Family-group names in Coleoptera (Insecta). ZooKeys10.3897/zookeys.88.807 (2011). [DOI] [PMC free article] [PubMed]
- 71.Davis RB, Baldauf SL, Mayhew PJ. The origins of species richness in the Hymenoptera: insights from a family-level supertree. BMC Evol. Biol. 2010;10:109. doi: 10.1186/1471-2148-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hedges SB, Marin J, Suleski M, Paymer M, Kumar S. Tree of life reveals clock-like speciation and diversification. Mol. Biol. Evol. 2015;32:835–845. doi: 10.1093/molbev/msv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Misof B, et al. Phylogenomics resolves the timing and pattern of insect evolution. Science. 2014;346:763–767. doi: 10.1126/science.1257570. [DOI] [PubMed] [Google Scholar]
- 74.Wilkinson TL. The elimination of intracellular microorganisms from insects: an analysis of antibiotic-treatment in the pea aphid (Acyrthosiphon pisum) Comp. Biochem. Physiol. A. 1998;119:871–881. doi: 10.1016/S1095-6433(98)00013-0. [DOI] [Google Scholar]
- 75.Köhler M, Schwartz W. Untersuchungen über die symbiose von Schildläusen mit pflanzlichen Mikroorganismen. Z. Allg. Mikrobiol. 1961;1:173–174. doi: 10.1002/jobm.3630010209. [DOI] [Google Scholar]
- 76.Zientz E, Beyaert I, Gross R, Feldhaar H. Relevance of the endosymbiosis of Blochmannia floridanus and carpenter ants at different stages of the life cycle of the host. Appl. Environ. Microbiol. 2006;72:6027–6033. doi: 10.1128/AEM.00933-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brooks MA, Richards AG. Intracellular symbiosis in cockroaches. I. Production of aposymbiotic cockroaches. Biol. Bull. 1955;109:22–39. doi: 10.2307/1538656. [DOI] [Google Scholar]
- 78.Koch A. Über das verhalten symbiontenfreier Sitodrepalarven. Biol. Zentr. 1933;53:199–203. [Google Scholar]
- 79.Schomann H. Die Symbiose der Bockkäfer. Z. Morphol. Ökol. Tiere. 1937;32:542–611. doi: 10.1007/BF00406840. [DOI] [Google Scholar]
- 80.Nogge G. Sterility in tsetse flies (Glossina morsitans Westwood) caused by loss of symbionts. Experientia. 1976;32:995–996. doi: 10.1007/BF01933932. [DOI] [PubMed] [Google Scholar]
- 81.Aschner M. Studies on the symbiosis of the body louse: I. Elimination of the symbionts by centrifugalisation of the eggs. Parasitology. 1934;26:309–314. doi: 10.1017/S0031182000023611. [DOI] [Google Scholar]
- 82.Hirota B, et al. A novel, extremely elongated, and endocellular bacterial symbiont supports cuticle formation of a grain pest beetle. mBio. 2017;8:e01482–17. doi: 10.1128/mBio.01482-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huger A. Experimentelle Untersuchungen über die künstliche Symbiontenelimination bei Vorratsschädlingen: Rhizopertha dominica F. (Bostrychidae) und Oryzaephilus surinamensis L. (Cucujidae) Z. Morphol. Ökol. Tiere. 1956;44:626–701. doi: 10.1007/BF00390698. [DOI] [Google Scholar]
- 84.Zientz E, Dandekar T, Gross R. Metabolic interdependence of obligate intracellular bacteria and their insect hosts. Microbiol. Mol. Biol. Rev. 2004;68:745–770. doi: 10.1128/MMBR.68.4.745-770.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rabosky DL, Benson RBJ. Ecological and biogeographic drivers of biodiversity cannot be resolved using clade age-richness data. Nat. Commun. 2021;12:2945. doi: 10.1038/s41467-021-23307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Revell LJ. Phytools: an R package for phylogenetic comparative biology (and other things) Methods Ecol. Evol. 2012;3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- 87.Guindon S, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 88.R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2020).
- 89.Pagel M, Meade A. Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain Monte Carlo. Am. Nat. 2006;167:808–825. doi: 10.1086/503444. [DOI] [PubMed] [Google Scholar]
- 90.Hadfield, J. D. MCMC methods for multi-response generalized linear mixed models: the mcmcglmm R package. J. Stat. Software33, 1–22 (2010).
- 91.Nakagawa, S. & Schielzeth, H. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev.10.1111/j.1469-185X.2010.00141.x (2010). [DOI] [PubMed]
- 92.Villemereuil P, de Schielzeth H, Nakagawa S, Morrissey M. General methods for evolutionary quantitative genetic inference from generalized mixed models. Genetics. 2016;204:1281–1294. doi: 10.1534/genetics.115.186536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hadfield JD, Nakagawa S. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 2010;23:494–508. doi: 10.1111/j.1420-9101.2009.01915.x. [DOI] [PubMed] [Google Scholar]
- 94.Plummer M, Best N, Cowles K, Vines K. CODA: convergence diagnosis and output analysis for MCMC. R. News. 2006;6:7–11. [Google Scholar]
- 95.Bollback JP. SIMMAP: stochastic character mapping of discrete traits on phylogenies. BMC Bioinformatics. 2006;7:88. doi: 10.1186/1471-2105-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakagawa, S. in Ecological Statistics: Contemporary Theory and Application (eds Fox, G. A. et al.) 81–105 (Oxford Univ. Press, 2015).
- 97.Castiglione S, et al. A new method for testing evolutionary rate variation and shifts in phenotypic evolution. Methods Ecol. Evol. 2018;9:974–983. doi: 10.1111/2041-210X.12954. [DOI] [Google Scholar]
- 98.Hadfield JD, Krasnov BR, Poulin R, Nakagawa S. A tale of two phylogenies: comparative analyses of ecological interactions. Am. Nat. 2014;183:174–187. doi: 10.1086/674445. [DOI] [PubMed] [Google Scholar]
- 99.Legendre, P., Desdevises, Y. & Bazin, E. A statistical test for host–parasite coevolution. Syst. Biol.51, 217–234 (2002). [DOI] [PubMed]
- 100.Anderson, S. A. S. & Weir, J. T. diverge: evolutionary trait divergence between sister species and other paired lineages. Rpackage version 2.0.4. (2022); https://CRAN.R-project.org/package=diverge
- 101.SELFNutritionDatahttps://nutritiondata.self.com/ (2003).
- 102.Slismhttps://slism.com/calorie/ (2012).
- 103.Fat Secrethttps://platform.fatsecret.com/ (2007).
- 104.Aksoy S, Chen X, Hypsa V. Phylogeny and potential transmission routes of midgut-associated endosymbionts of tsetse (Diptera: Glossinidae) Insect Mol. Biol. 1997;6:183–190. doi: 10.1111/j.1365-2583.1997.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 105.Allen JM, Reed DL, Perotti MA, Braig HR. Evolutionary relationships of ‘Candidatus riesia spp.,’ endosymbiotic Enterobacteriaceae living within hematophagous primate lice. Appl. Environ. Microbiol. 2007;73:1659–1664. doi: 10.1128/AEM.01877-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Allen JM, Burleigh JG, Light JE, Reed DL. Effects of 16S rDNA sampling on estimates of the number of endosymbiont lineages in sucking lice. PeerJ. 2016;4:e2187. doi: 10.7717/peerj.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Andersen PC, Brodbeck VB, Mizell RF. Metabolism of amino acids, organic acids and sugars extracted from the xylem fluid of four host plants by adult Homalodisca coagulata. Entomol. Exp. Appl. 1989;50:149–159. doi: 10.1111/j.1570-7458.1989.tb02384.x. [DOI] [Google Scholar]
- 108.Andersen PC, Brodbeck BV, Mizell RF. Feeding by the leafhopper, Homalodisca coagulate, in relation to xylem fluid chemistry and tension. J. Insect Physiol. 1992;38:611–622. doi: 10.1016/0022-1910(92)90113-R. [DOI] [Google Scholar]
- 109.Andersen PC. Diurnal vitiations in tension, osmolarity, and the composition of nitrogen and carbon assimilates in xylem fluid of Prunus persica, Vitis hybrid, and Pyrus communis. J. Am. Soc. Horticult. Sci. 1995;120:600–606. doi: 10.21273/JASHS.120.4.600. [DOI] [Google Scholar]
- 110.Andersen JC, et al. A phylogenetic analysis of armored scale insects (Hemiptera: Diaspididae), based upon nuclear, mitochondrial, and endosymbiont gene sequences. Mol. Phylogenet. Evol. 2010;57:992–1003. doi: 10.1016/j.ympev.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 111.Antoń S, Komoń-Janczara E, Denisow B. Floral nectary, nectar production dynamics and chemical composition in five nocturnal Oenothera species (Onagraceae) in relation to floral visitors. Planta. 2017;246:1051–1067. doi: 10.1007/s00425-017-2748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aohara T, et al. Presence of a basic secretory protein in xylem sap and shoots of poplar in winter and its physicochemical activities against winter environmental conditions. J. Plant Res. 2019;132:655–665. doi: 10.1007/s10265-019-01123-9. [DOI] [PubMed] [Google Scholar]
- 113.Arab DA, Bourguignon T, Wang ZQ, Ho SYW, Lo N. Evolutionary rates are correlated between cockroach symbionts and mitochondrial genomes. Biol. Lett. 2020;16:20190702. doi: 10.1098/rsbl.2019.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arnett, R. H., Thomas, M. C., Skelley, P. E. & Franks, J. H. American Beetles, Volume II Polyphaga: Scarabaeoidea through Curculionoidea (CRC Press, 2002).
- 115.Ayayee P, et al. Gut microbes contribute to nitrogen provisioning in a wood-feeding cerambycid. Environ. Entomol. 2014;43:903–912. doi: 10.1603/EN14045. [DOI] [PubMed] [Google Scholar]
- 116.Back EA. Biology of the saw-toothed grain beetle, Oryzaephilus surinamensis Linné. J. Agric. Res. 1926;33:435–452. [Google Scholar]
- 117.Baena M. Unusual feeding habits in four Iberian heteroptera (Hemiptera) Bol. Soc. Entomol. Aragonesa. 2011;48:399–401. [Google Scholar]
- 118.Ball DW. The chemical composition of honey. J. Chem. Educ. 2007;84:1643–1646. doi: 10.1021/ed084p1643. [DOI] [Google Scholar]
- 119.Barker SC, Whiting M, Johnson KP, Murrell A. Phylogeny of the lice (Insecta, Phthiraptera) inferred from small subunit rRNA. Zool. Scr. 2003;32:407–414. doi: 10.1046/j.1463-6409.2003.00120.x. [DOI] [Google Scholar]
- 120.Bechly, G. & Szwedo, J. in The Crato Fossil Beds of Brazil: Window into an Ancient World (eds Martill, D. M. et al.) 313–318 (Cambridge Univ. Press, 2007).
- 121.Begon M. in The Genetics and Biology of Drosophila (eds Ashburner, M. et al.) 345–384 (Academic Press, 1982).
- 122.Bell, W. J. et al. Cockroaches: Ecology, Behavior, and Natural History (Johns Hopkins Univ. Press, 2007).
- 123.Bennett GM, Moran NA. Small, smaller, smallest: the origins and evolution of ancient dual symbioses in a phloem-feeding insect. Genome Biol. Evol. 2013;5:1675–1688. doi: 10.1093/gbe/evt118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berlanga M, Paster BJ, Guerrero R. Coevolution of symbiotic spirochete diversity in lower termites. Int. Microbiol. 2007;10:133–139. [PubMed] [Google Scholar]
- 125.Bertsch A, Coello N. A biotechnological process for treatment and recycling poultry feathers as a feed ingredient. Bioresour. Technol. 2005;96:1703–1708. doi: 10.1016/j.biortech.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 126.Bieńkowski, A. O. & Orlova-Bienkowskaja, M. J. in New Developments in the Biology of Chrysomelidae (eds Jolivet, P. et al.) 481–502 (SPB Academic Publishing, 2004).
- 127.Bienkowski AO. Feeding behavior of leaf-beetles (Coleoptera, Chrysomelidae) Entomol. Rev. 2010;90:1–10. doi: 10.1134/S001387381001001X. [DOI] [Google Scholar]
- 128.Bistolas KSI, Sakamoto RI, Fernandes JAM, Goffredi SK. Symbiont polyphyly, co-evolution, and necessity in pentatomid stinkbugs from Costa Rica. Front. Microbiol. 2014;5:349. doi: 10.3389/fmicb.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health: a review. J. Am. Coll. Nutr. 2008;27:677–689. doi: 10.1080/07315724.2008.10719745. [DOI] [PubMed] [Google Scholar]
- 130.Boyd BM, et al. Two bacterial genera, Sodalis and Rickettsia, associated with the seal louse Proechinophthirus fluctus (Phthiraptera: Anoplura) Appl. Environ. Microbiol. 2016;82:3185–3197. doi: 10.1128/AEM.00282-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Braendle C, et al. Developmental origin and evolution of bacteriocytes in the aphid–Buchnera symbiosis. PLoS Biol. 2003;1:70–76. doi: 10.1371/journal.pbio.0000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bressan A, Arneodo J, Simonato M, Haines WP, Boudon-Padieu E. Characterization and evolution of two bacteriome-inhabiting symbionts in cixiid planthoppers (Hemiptera: Fulgoromorpha: Pentastirini) Environ. Microbiol. 2009;11:3265–3279. doi: 10.1111/j.1462-2920.2009.02055.x. [DOI] [PubMed] [Google Scholar]
- 133.Buchner P. Studien an intracellularen sSymbionten V. Die symbiontischen Einrichtungen der Zikaden. Z. Morphol. Ökol. Tiere. 1925;4:88–245. doi: 10.1007/BF00407627. [DOI] [Google Scholar]
- 134.Buchner, P. Endosymbiosis of Animals with Plant Microorganisms (Interscience Publishers, 1965).
- 135.Buhtz A, Kolasa A, Arlt K, Walz C, Kehr J. Xylem sap protein composition is conserved among different plant species. Planta. 2004;219:610–618. doi: 10.1007/s00425-004-1259-9. [DOI] [PubMed] [Google Scholar]
- 136.Bukkens SGF. The nutritional value of edible insects. Ecol. Food Nutr. 1997;36:287–319. doi: 10.1080/03670244.1997.9991521. [DOI] [Google Scholar]
- 137.Burke GR, Normark BB, Favret C, Moran NA. Evolution and diversity of facultative symbionts from the aphid subfamily lachninae. Appl. Environ. Microbiol. 2009;75:5328–5335. doi: 10.1128/AEM.00717-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buxton PA. The biology of a blood-sucking bug, Rhodmius prolixus. Trans. R. Entomol. Soc. Lond. 1930;78:227–256. doi: 10.1111/j.1365-2311.1930.tb00385.x. [DOI] [Google Scholar]
- 139.Calderon O, Berkov A. Midgut and fat body bacteriocytes in neotropical cerambycid beetles (Coleoptera: Cerambycidae) Environ. Entomol. 2012;41:108–117. doi: 10.1603/EN11258. [DOI] [PubMed] [Google Scholar]
- 140.Campbell AM, Lawrence AJ, Hudspath CB, Gruwell ME. Molecular identification of Diaspididae and elucidation of non-native species using the genes 28s and 16s. Insects. 2014;5:528–538. doi: 10.3390/insects5030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Campos MGR, et al. Pollen composition and standardisation of analytical methods. J. Apic. Res. 2008;47:154–161. doi: 10.1080/00218839.2008.11101443. [DOI] [Google Scholar]
- 142.Capinera, J. L. et al. Encyclopedia of Entomology Vol. 1 (Springer, 2008).
- 143.Chanbusarakum LJ, Ullman DE. Distribution and ecology of Frankliniella occidentalis (Thysanoptera: Thripidae) bacterial symbionts. Environ. Entomol. 2009;38:1069–1077. doi: 10.1603/022.038.0414. [DOI] [PubMed] [Google Scholar]
- 144.Chen X, Li S, Aksoy S. Concordant evolution of a symbiont with its host insect species: molecular phylogeny of genus Glossina and its bacteriome-associated endosymbiont, Wigglesworthia glossinidia. J. Mol. Evol. 1999;48:49–58. doi: 10.1007/PL00006444. [DOI] [PubMed] [Google Scholar]
- 145.Chen, S. et al. Value-added chemicals from animal manure. OSTI.GOVhttp://www.osti.gov/servlets/purl/15009485/ (2003).
- 146.Chen X, Stansly PA. Biology of Tamarixia radiata (Hymenoptera: Eulophidae), parasitoid of the citrus greening disease vector Diaphorina citri (Hemiptera: Psylloidea): a mini review. Fla. Entomol. 2014;97:1404–1413. doi: 10.1653/024.097.0415. [DOI] [Google Scholar]
- 147.Chinery, M. Insects of Britain and Western Europe (Domino Books, 2012).
- 148.Cho G, Malenovsky I, Lee S. Higher-level molecular phylogeny of jumping plant lice (Hemiptera: Sternorrhyncha: Psylloidea) Syst. Entomol. 2019;44:638–651. doi: 10.1111/syen.12345. [DOI] [Google Scholar]
- 149.Claridge MF, Wilson MR. Host plant associations, diversity and species–area relationships of mesophyll-feeding leafhopper or trees and shrubs in Britain. Ecol. Entomol. 1981;6:217–238. doi: 10.1111/j.1365-2311.1981.tb00610.x. [DOI] [Google Scholar]
- 150.Clark JW, Kambhampati S. Phylogenetic analysis of Blattabacterium, endosymbiotic bacteria from the wood roach, Cryptocercus (Blattodea: Cryptocercidae), including a description of three new species. Mol. Phylogenet. Evol. 2003;26:82–88. doi: 10.1016/S1055-7903(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 151.Clements JC, Doucet DA, McCorquodale DB. Establishment of a European cockroach. J. Acadian Entomol. Soc. 2013;7:4–7. [Google Scholar]
- 152.Cleveland LR. The physiological and symbiotic relationships between intenstinal protozoa of termites and their host, with special reference to Reticulitermes flavipes Kollar. Biol. Bull. 1924;46:178–201. doi: 10.2307/1536507. [DOI] [Google Scholar]
- 153.Condamine FL, Clapham ME, Kergoat GJ. Global patterns of insect diversification: towards a reconciliation of fossil and molecular evidence? Sci. Rep. 2016;6:19208. doi: 10.1038/srep19208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Coscarón, M. C. & Contreras, E. F. in True Bugs (Heteroptera) of the Neotropics (eds Panizzi, A. R. & Grazia, J.) 423–458 (Springer, 2015).
- 155.Crowson, R. A. The Biology of the Coleoptera (Academic Press, 1981).
- 156.Cryan JR, Svenson GJ. Family-level relationships of the spittlebugs and froghoppers (Hemiptera: Cicadomorpha: Cercopoidea) Syst. Entomol. 2010;35:393–415. doi: 10.1111/j.1365-3113.2009.00520.x. [DOI] [Google Scholar]
- 157.de Arruda VAS, Pereira AAS, de Freitas AS, Barth OM, de Almeida-Muradian LB. Dried bee pollen: B complex vitamins, physicochemical and botanical composition. J. Food Compos. Anal. 2013;29:100–105. doi: 10.1016/j.jfca.2012.11.004. [DOI] [Google Scholar]
- 158.Degnan PH, Lazarus AB, Brock CD, Wernegreen JJ. Host–symbiont stability and fast evolutionary rates in an ant–bacterium association: cospeciation of Camponotus species and their endosymbionts, Candidatus blochmannia. Syst. Biol. 2004;53:95–110. doi: 10.1080/10635150490264842. [DOI] [PubMed] [Google Scholar]
- 159.Deibert P, Konig D, Kloock B, Groenefeld M, Berg A. Glycaemic and insulinaemic properties of some German honey varieties. Eur. J. Clin. Nutr. 2010;64:762–764. doi: 10.1038/ejcn.2009.103. [DOI] [PubMed] [Google Scholar]
- 160.Dem FF, Stewart AJA, Gibson A, Weiblen GD, Novotny V. Low host specificity in species-rich assemblages of xylem- and phloem-feeding herbivores (Auchenorrhyncha) in a New Guinea lowland rain forest. J. Trop. Ecol. 2013;29:467–476. doi: 10.1017/S0266467413000540. [DOI] [Google Scholar]
- 161.Denno, R. F. & Perfect, J. R. Planthoppers: Their Ecology and Management (Springer, 1994).
- 162.de Vries EJ, Jacobs G, Breeuwer JAJ. Growth and transmission of gut bacteria in the Western flower thrips, Frankliniella occidentalis. J. Invertebr. Pathol. 2001;77:129–137. doi: 10.1006/jipa.2001.5010. [DOI] [PubMed] [Google Scholar]
- 163.Dhami MK, Buckley TR, Beggs JR, Taylor MW. Primary symbiont of the ancient scale insect family Coelostomidiidae exhibits strict cophylogenetic patterns. Symbiosis. 2013;61:77–91. doi: 10.1007/s13199-013-0257-8. [DOI] [Google Scholar]
- 164.Dinant S, Bonnemain JL, Girousse C, Kehr J. Phloem sap intricacy and interplay with aphid feeding. C. R. Biol. 2010;333:504–515. doi: 10.1016/j.crvi.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 165.Downie DA, Gullan PJ. Phylogenetic congruence of mealybugs and their primary endosymbionts. J. Evol. Biol. 2005;18:315–324. doi: 10.1111/j.1420-9101.2004.00834.x. [DOI] [PubMed] [Google Scholar]
- 166.Duarte RT, Carvalho Simões MC, Sgarbieri VC. Bovine blood components: fractionation, composition, and nutritive value. J. Agric. Food Chem. 1999;47:231–236. doi: 10.1021/jf9806255. [DOI] [PubMed] [Google Scholar]
- 167.Duff A. Identification—longhorn beetles: part 1. Br. Wildl. 2006;18:406–414. [Google Scholar]
- 168.Duron O, et al. Origin, acquisition and diversification of heritable bacterial endosymbionts in louse flies and bat flies. Mol. Ecol. 2014;23:2105–2117. doi: 10.1111/mec.12704. [DOI] [PubMed] [Google Scholar]
- 169.D’Urso V, Uliana M. Acanalonia conica (Hemiptera, Fulgoromorpha, Acanaloniidae), a Nearctic species recently introduced in Europe. Dtsch. Entomol. Z. 2006;53:103–107. doi: 10.1002/mmnd.200600010. [DOI] [Google Scholar]
- 170.Ebino KY. Studies on coprophagy in experimental animals. Exp. Anim. 1993;42:1–9. doi: 10.1538/expanim1978.42.1_1. [DOI] [PubMed] [Google Scholar]
- 171.Eichler S, Schaub GA. Development of symbionts in triatomine bugs and the effects of infections with trypanosomatids. Exp. Parasitol. 2002;100:17–27. doi: 10.1006/expr.2001.4653. [DOI] [PubMed] [Google Scholar]
- 172.Emmet AD, Grindley HS. The chemstry of animal feces—first paper. A comparison of the analysis of fresh and air-dried feces. J. Am. Chem. Soc. 1909;31:569–579. doi: 10.1021/ja01935a011. [DOI] [Google Scholar]
- 173.Estes AM, et al. Brood ball-mediated transmission of microbiome members in the dung beetle, Onthophagus taurus (Coleoptera: Scarabaeidae) PLoS ONE. 2013;8:e79061. doi: 10.1371/journal.pone.0079061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eutick ML, Veivers P, Obrien RW, Slaytor M. Dependence on higher termites, Nasutitermes exitosus and lower termite, Coptotermes lactues on the gut flora. J. Insect Physiol. 1978;24:363–368. doi: 10.1016/0022-1910(78)90076-8. [DOI] [Google Scholar]
- 175.Evans HE. Notes on the prey and nesting behavior of some solitary wasps of Mexico and southwestern United States. J. Kans. Entomol. Soc. 1966;37:35–40. [Google Scholar]
- 176.Evans HE. The natural history and behavior of north american beewolves. J. N. Y. Entomol. Soc. 1988;96:487–489. [Google Scholar]
- 177.Every D, Farrell JAK, Stufkens MW. Wheat-bug damage in New Zealand wheats: the feeding mechanism of nysius huttoni and its effect on the morphological and physiological development of wheat. J. Sci. Food Agric. 1990;50:297–309. doi: 10.1002/jsfa.2740500303. [DOI] [Google Scholar]
- 178.Foottit, R. G. Insect Biodiversity: Science and Society, 2nd Edition Vol. 1 (Wiley Blackwell, 2017).
- 179.Franzini PZN, et al. The gut microbiomes of two Pachysoma Macleay desert dung beetle species (Coleoptera: Scarabaeidae: Scarabaeinae) feeding on different diets. PLoS ONE. 2016;11:e0161118. doi: 10.1371/journal.pone.0161118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fukatsu T. Secondary intracellular symbiotic bacteria in aphids of the genus Yamatocallis (Homoptera: Aphididae: Drepanosiphinae) Appl. Environ. Microbiol. 2001;67:5315–5320. doi: 10.1128/AEM.67.11.5315-5320.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fukatsu T, et al. Bacterial endosymbiont of the slender pigeon louse, Columbicola columbae, allied to endosymbionts of grain weevils and tsetse flies. Appl. Environ. Microbiol. 2007;73:6660–6668. doi: 10.1128/AEM.01131-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fukatsu T, et al. Intestinal endocellular symbiotic bacterium of the macaque louse Pedicinus obtusus: distinct endosymbiont origins in anthropoid primate lice and the Old World Monkey louse. Appl. Environ. Microbiol. 2009;75:3796–3799. doi: 10.1128/AEM.00226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gardener MC, Gillman MP. Analyzing variability in nectar amino acids: composition is less variable than concentration. J. Chem. Ecol. 2001;27:2545–2558. doi: 10.1023/A:1013687701120. [DOI] [PubMed] [Google Scholar]
- 184.Gesse F, Ribes J, Goula M. Belonochilus numenius, the sycamore seed bug, new record for the Iberian fauna. Bull. Insectol. 2009;62:121–123. [Google Scholar]
- 185.Girousse C BJLDS, Bournoville R. Sugar and amino acid composition of phloem sap of Medicago sativa: a comparative study of two collecting methods. Plant Physiol. Biochem. 1991;21:41–48. [Google Scholar]
- 186.Glad C, Regnard JL, Queroe Y, Brun O, Motorgaudry JF. Flux and chemical-composition of xylem exudates from chardonnay grapevines—temporal evolution and effect of recut. Am. J. Enol. Vitic. 1992;43:275–282. doi: 10.5344/ajev.1992.43.3.275. [DOI] [Google Scholar]
- 187.Gonella E, Orru B, Alma A. Egg masses treatment with micronutrient fertilizers has a suppressive effect on newly-emerged nymphs of the brown marmorated stink bug Halyomorpha halys. Entomol. Generalis. 2019;39:231–238. doi: 10.1127/entomologia/2019/0819. [DOI] [Google Scholar]
- 188.Goodchild AJP. Some observations on growth and egg production of the blood-sucking reduviids, Rhodnius prolixus and Triatoma infestans. Proc. R. Entomol. Soc. Lond. 1955;30:137–144. [Google Scholar]
- 189.Gordon ERL, McFrederick Q, Weirauch C. Phylogenetic evidence for ancient and persistent environmental symbiont reacquisition in Largidae (Hemiptera: Heteroptera) Appl. Environ. Microbiol. 2016;82:7123–7133. doi: 10.1128/AEM.02114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gottsberger G, Schrauwen J, Linskens HF. Amino acids and sugars in nectar, and their putative evolutionary significance. Plant Syst. Evol. 1984;145:55–77. doi: 10.1007/BF00984031. [DOI] [Google Scholar]
- 191.Gregory BR, Wilder OHM, Ostby PC. Studies on the amino acid and vitamin composition of feather meal. Poult. Sci. 1956;35:234–235. doi: 10.3382/ps.0350234. [DOI] [Google Scholar]
- 192.Grimaldi, D. & Engel, M. S. Evolution of the Insects (Cambridge Univ. Press, 2005).
- 193.Gruwell ME, Morse GVE, Normark BB. Phylogenetic congruence of armored scale insects (Hemiptera: Diaspididae) and their primary endosymbionts from the phylum Bacteroidetes. Mol. Phylogenet. Evol. 2007;44:267–280. doi: 10.1016/j.ympev.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 194.Gueguen G, et al. Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol. Ecol. 2010;19:4365–4378. doi: 10.1111/j.1365-294X.2010.04775.x. [DOI] [PubMed] [Google Scholar]
- 195.Gullan PJ, Kosztarab M. Adaptations in scale insects. Annu. Rev. Entomol. 1997;42:23–50. doi: 10.1146/annurev.ento.42.1.23. [DOI] [PubMed] [Google Scholar]
- 196.Gullan PJ, Cook LG. Phylogeny and higher classification of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea) Zootaxa. 2007;425:413–425. doi: 10.11646/zootaxa.1668.1.22. [DOI] [Google Scholar]
- 197.Gutiérrez A, Del Río JC, González-Vila FJ, Martín F. Chemical composition of lipophilic extractives from eucalyptus globulus Labill. wood. Holzforschung. 1999;53:481–486. doi: 10.1515/HF.1999.079. [DOI] [Google Scholar]
- 198.Hagedorn HH. Effect of the age of pollen used in pollen supplements on their nutritive value for the honeybee. II. Effect of vitamin content of pollens. J. Apic. Res. 1968;7:97–101. doi: 10.1080/00218839.1968.11100196. [DOI] [Google Scholar]
- 199.Hall SM, Baker DA. The chemical composition of Ricinus phloem exudate. Planta. 1972;106:131–140. doi: 10.1007/BF00383992. [DOI] [PubMed] [Google Scholar]
- 200.Hamilton KGA. A new family of froghoppers from the American tropics (Hemiptera: Cercopoidea: Epipygidae) Biodiversity. 2001;2:15–21. doi: 10.1080/14888386.2001.9712551. [DOI] [Google Scholar]
- 201.Hamilton PT, Perlman SJ. Host defense via symbiosis in Drosophila. PLoS Pathog. 2013;9:e1003808. doi: 10.1371/journal.ppat.1003808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hanelová J, Vilímová J. Behaviour of the central European Acanthosomatidae (Hemiptera: Heteroptera: Pentatomoidea) during oviposition and parental care. Acta Musei Morav. Sci. Biol. 2013;98:433–457. [Google Scholar]
- 203.Hayashi H, Chino M. Collection of pure phloem sap from wheat and its chemical composition. Plant Cell Physiol. 1986;27:1387–1393. doi: 10.1093/oxfordjournals.pcp.a077237. [DOI] [Google Scholar]
- 204.Hayashi H, Chino M. Chemical composition of phloem sap from the uppermost internode of the rice plant. Plant Cell Physiol. 1990;31:247–251. [Google Scholar]
- 205.Hendrick RL, Pregitzer KS. The dynamics of fine root length, biomass, and nitrogen content in two northern hardwood ecosystems. Can. J. Res. 1993;23:2507–2520. doi: 10.1139/x93-312. [DOI] [Google Scholar]
- 206.Henry TJ. Phylogenetic analysis of family groups within the infraorder Pentatomomorpha (Hemiptera: Heteroptera), with emphasis on the Lygaeoidea. Ann. Entomol. Soc. Am. 1997;90:275–301. doi: 10.1093/aesa/90.3.275. [DOI] [Google Scholar]
- 207.Henry, T. J. in Insect Biodiversity: Science and Society (eds Foottit, R. G. & Adler, P. H.) Ch. 10 (Wiley, 2009).
- 208.Henry LM, et al. Horizontally transmitted symbionts and host colonization of ecological niches. Curr. Biol. 2013;23:1713–1717. doi: 10.1016/j.cub.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hijaz F, Killiny N. Collection and chemical composition of phloem sap from Citrus sinensis L. Osbeck (sweet orange) PLoS ONE. 2014;9:e101830. doi: 10.1371/journal.pone.0101830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hirota, B., Meng, X. Y. & Fukatsu, T. Bacteriome-associated endosymbiotic bacteria of Nosodendron tree sap beetles (Coleoptera: Nosodendridae). Front. Microbiol.11, 588841 (2020). [DOI] [PMC free article] [PubMed]
- 211.Hlavjenková I, Šefrová H. Chrysomphalus aonidum (Linnaeus, 1758), a new alien pest of ornamental plants in the Českáeská Republikach republic (Hemiptera: Coccoidea: Diaspididae) Acta Univ. Agric. Silvic. Mendelianae Brun. 2012;60:69–78. doi: 10.11118/actaun201260050069. [DOI] [Google Scholar]
- 212.Hodkinson ID. Biology of Psylloidea (Homoptera): a review. Bull. Entomol. Res. 1974;64:325–338. doi: 10.1017/S0007485300031217. [DOI] [Google Scholar]
- 213.Horsfield D. Evidence for xylem feeding by Philaenus spumarius (L.) (Homoptera: Cercopidae) Entomol. Exp. Appl. 1978;24:95–99. doi: 10.1111/j.1570-7458.1978.tb02759.x. [DOI] [Google Scholar]
- 214.Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T. Strict host–symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 2006;4:1841–1851. doi: 10.1371/journal.pbio.0040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hosokawa T, Koga R, Kikuchi Y, Meng XY, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl Acad. Sci. USA. 2010;107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hosokawa T, Kikuchi Y, Nikoh N, Fukatsu T. Polyphyly of gut symbionts in stinkbugs of the family cydnidae. Appl. Environ. Microbiol. 2012;78:4758–4761. doi: 10.1128/AEM.00867-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hosokawa T, Imanishi M, Koga R, Fukatsu T. Diversity and evolution of bacterial symbionts in the gut symbiotic organ of jewel stinkbugs (Hemiptera: Scutelleridae) Appl. Entomol. Zool. 2019;54:359–367. doi: 10.1007/s13355-019-00630-4. [DOI] [Google Scholar]
- 218.Howe, M. A. A Provisional Checklist of the Invertebrates Recorded in Wales 4. True Bugs. (Countryside Council for Wales, 2004).
- 219.Huang CY, Sabree ZL, Moran NA. Genome sequence of Blattabacterium sp. strain BGIGA, endosymbiont of the Blaberus giganteus cockroach. J. Bacteriol. 2012;194:4450–4451. doi: 10.1128/JB.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hunt T, et al. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science. 2007;318:1913–1916. doi: 10.1126/science.1146954. [DOI] [PubMed] [Google Scholar]
- 221.Husnik F, McCutcheon JP. Repeated replacement of an intrabacterial symbiont in the tripartite nested mealybug symbiosis. Proc. Natl Acad. Sci. USA. 2016;113:E5416–E5424. doi: 10.1073/pnas.1603910113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ischayek JI, Kern M. US honeys varying in glucose and fructose content elicit similar glycemic indexes. J. Am. Diet. Assoc. 2006;106:1260–1262. doi: 10.1016/j.jada.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 223.Ishii Y, Matsuura Y, Kakizawa S, Nikoh N, Fukatsu T. Diversity of bacterial endosymbionts associated with Macrosteles leafhoppers vectoring phytopathogenic phytoplasmas. Appl. Environ. Microbiol. 2013;79:5013–5022. doi: 10.1128/AEM.01527-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Itoh H, Matsuura Y, Hosokawa T, Fukatsu T, Kikuchi Y. Obligate gut symbiotic association in the sloe bug Dolycoris baccarum (Hemiptera: Pentatomidae) Appl. Entomol. Zool. 2017;52:51–59. doi: 10.1007/s13355-016-0453-0. [DOI] [Google Scholar]
- 225.Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science. 2010;329:212–215. doi: 10.1126/science.1188235. [DOI] [PubMed] [Google Scholar]
- 226.Jagota SK, Dani HM. A new calorimetric technique for the estimation of vitamin Cusing folin phenol reagent. Anal. Biochem. 1982;182:178–182. doi: 10.1016/0003-2697(82)90162-2. [DOI] [PubMed] [Google Scholar]
- 227.Jousselin E, Desdevises Y, Coeur D’Acier A. Fine-scale cospeciation between Brachycaudus and Buchnera aphidicola: bacterial genome helps define species and evolutionary relationships in aphids. Proc. R. Soc. B. 2009;276:187–196. doi: 10.1098/rspb.2008.0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jung S, Lee S. Molecular phylogeny of the plant bugs (Heteroptera: Miridae) and the evolution of feeding habits. Cladistics. 2012;28:50–79. doi: 10.1111/j.1096-0031.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- 229.Jungen H. Endosymbionten bei Ameisen. Insect. Soc. 1968;15:227–232. doi: 10.1007/BF02225845. [DOI] [Google Scholar]
- 230.Kafil M, Bandani AR, Kaltenpoth M, Goldansaz SH, Alavi SM. Role of symbiotic bacteria in the growth and development of the Sunn pest, Eurygaster integriceps. J. Insect Sci. 2013;13:99. doi: 10.1673/031.013.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kaiwa N, et al. Symbiont-supplemented maternal investment underpinning host’s ecological adaptation. Curr. Biol. 2014;24:2465–2470. doi: 10.1016/j.cub.2014.08.065. [DOI] [PubMed] [Google Scholar]
- 232.Kaltenpoth M, Göttler W, Herzner G, Strohm E. Symbiotic bacteria protect wasp larvae from fungal infestation. Curr. Biol. 2005;15:475–479. doi: 10.1016/j.cub.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 233.Karamipour N, Mehrabadi M, Fathipour Y. Gammaproteobacteria as essential primary symbionts in the striped shield bug, Graphosoma lineatum (Hemiptera: Pentatomidae) Sci. Rep. 2016;6:21–25. doi: 10.1038/srep33168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kashima T, Nakamura T, Tojo S. Uric acid recycling in the shield bug, Parastrachia japonensis (Hemiptera: Parastrachiidae), during diapause. J. Insect Physiol. 2006;52:816–825. doi: 10.1016/j.jinsphys.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 235.Kashkouli M, Fathipour Y, Mehrabadi M. Heritable gammaproteobacterial symbiont improves the fitness of Brachynema germari Kolenati (Hemiptera: Pentatomidae) Environ. Entomol. 2019;48:1079–1087. doi: 10.1093/ee/nvz089. [DOI] [PubMed] [Google Scholar]
- 236.Kashkouli M, Fathipour Y, Mehrabadi M. Habitat visualization, acquisition features and necessity of the gammaproteobacterial symbiont of pistachio stink bug, Acrosternum heegeri (Hem.: Pentatomidae) Bull. Entomol. Res. 2020;110:22–33. doi: 10.1017/S0007485319000245. [DOI] [PubMed] [Google Scholar]
- 237.Keilin D. On the life-history of Dasyhelea obscura, Winertz (Diptera, Nematocera, Ceratopogonidae), with some remarks on the parasites and hereditary bacterian symbiont of this midge. Ann. Mag. Nat. Hist. 1921;8:576–589. doi: 10.1080/00222932108632621. [DOI] [Google Scholar]
- 238.Kellner RLL. Molecular identification of an endosymbiotic bacterium associated with pederin biosynthesis in Paederus sabaeus (Coleoptera: Staphylinidae) Insect Biochem. Mol. Biol. 2002;32:389–395. doi: 10.1016/S0965-1748(01)00115-1. [DOI] [PubMed] [Google Scholar]
- 239.Khan ZR, Saxena RC. Effect of steam distillate extracts of resistant and susceptible rice cultivars on behavior of Sogatella furcifera (Homoptera: Delphacidae) J. Econ. Entomol. 1986;79:928–935. doi: 10.1093/jee/79.4.928. [DOI] [Google Scholar]
- 240.Kikuchi Y, et al. Host–symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 2009;7:2. doi: 10.1186/1741-7007-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kikuchi Y, Hosokawa T, Fukatsu T. An ancient but promiscuous host–symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J. 2011;5:446–460. doi: 10.1038/ismej.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kikuchi Y, Hosokawa T, Nikoh N, Fukatsu T. Gut symbiotic bacteria in the cabbage bugs Eurydema rugosa and Eurydema dominulus (Heteroptera: Pentatomidae) Appl. Entomol. Zool. 2012;47:1–8. doi: 10.1007/s13355-011-0081-7. [DOI] [Google Scholar]
- 243.Kim KC, Ludwig HW. The family classification of the Anoplura. Syst. Entomol. 1978;3:249–284. doi: 10.1111/j.1365-3113.1978.tb00120.x. [DOI] [Google Scholar]
- 244.Kimura M, Fujita T, Itokawa Y. Liquid-chromatographic determinationof the total thiamin content of blood. Clin. Chem. 1982;28:29–31. doi: 10.1093/clinchem/28.1.29. [DOI] [PubMed] [Google Scholar]
- 245.Kleespies RG, Nansen C, Adouhoun T, Huger AM. Ultrastructure of bacteriomes and their sensitivity to ambient temperatures in Prostephanus truncatus (Horn) Biocontrol Sci. Technol. 2001;11:217–232. doi: 10.1080/09583150120035648. [DOI] [Google Scholar]
- 246.Klein A, et al. A novel intracellular mutualistic bacterium in the invasive ant Cardiocondyla obscurior. ISME J. 2016;10:376–388. doi: 10.1038/ismej.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Koga R, Bennett GM, Cryan JR, Moran NA. Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environ. Microbiol. 2013;15:2073–2081. doi: 10.1111/1462-2920.12121. [DOI] [PubMed] [Google Scholar]
- 248.Koga R, Nikoh N, Matsuura Y, Meng XY, Fukatsu T. Mealybugs with distinct endosymbiotic systems living on the same host plant. FEMS Microbiol. Ecol. 2013;83:93–100. doi: 10.1111/j.1574-6941.2012.01450.x. [DOI] [PubMed] [Google Scholar]
- 249.Kölsch G, Matz-Grund C, Pedersen VB. Ultrastructural and molecular characterization of endosymbionts of the reed beetle genus Macroplea (Chrysomelidae, Donaciinae), and proposal of ‘Candidatus Macropleicola appendiculatae’ and ‘Candidatus Macropleicola muticae’. Can. J. Microbiol. 2009;55:1250–1260. doi: 10.1139/W09-085. [DOI] [PubMed] [Google Scholar]
- 250.Kölsch GK, Kubiak M. The aquatic leaf beetle species Macroplea mutica and M. appendiculata (Coleoptera, Chrysomelidae, Donaciinae) differ in their use of Myriophyllum spicatum as a host plant. Aquat. Insects. 2011;33:13–26. doi: 10.1080/01650424.2011.572558. [DOI] [Google Scholar]
- 251.Krenn HW, Plant JD, Szucsich NU. Mouthparts of flower-visiting insects. Arthropod Struct. Dev. 2005;34:1–40. doi: 10.1016/j.asd.2004.10.002. [DOI] [Google Scholar]
- 252.Krenn HW. Feeding mechanisms of adult lepidoptera: structure, function, and evolution of the mouthparts. Annu. Rev. Entomol. 2010;55:307–327. doi: 10.1146/annurev-ento-112408-085338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Küchler SM, Dettner K, Kehl S. Molecular characterization and localization of the obligate endosymbiotic bacterium in the birch catkin bug Kleidocerys resedae (Heteroptera: Lygaeidae, Ischnorhynchinae) FEMS Microbiol. Ecol. 2010;73:408–418. doi: 10.1111/j.1574-6941.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 254.Kuechler SM, Dettner K, Kehl S. Characterization of an obligate intracellular bacterium in the midgut epithelium of the bulrush bug Chilacis typhae (Heteroptera, Lygaeidae, Artheneinae) Appl. Environ. Microbiol. 2011;77:2869–2876. doi: 10.1128/AEM.02983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kuechler SM, Renz P, Dettner K, Kehl S. Diversity of symbiotic organs and bacterial endosymbionts of: Lygaeoid bugs of the families Blissidae and Lygaeidae (Hemiptera: Heteroptera: Lygaeoidea) Appl. Environ. Microbiol. 2012;78:2648–2659. doi: 10.1128/AEM.07191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kuechler SM, Gibbs G, Burckhardt DH, Dettner K, Hartung V. Diversity of bacterial endosymbionts and bacteria-host co-evolution in Gondwanan relict moss bugs (Hemiptera: Coleorrhyncha: Peloridiidae) Environ. Microbiol. 2013;15:2031–2042. doi: 10.1111/1462-2920.12101. [DOI] [PubMed] [Google Scholar]
- 257.Kuechler SM, Matsuura Y, Dettner K, Kikuchi Y. Phylogenetically diverse Burkholderia associated with midgut crypts of spurge bugs, Dicranocephalus spp. (Heteroptera: Stenocephalidae) Microbes Environ. 2016;31:145–153. doi: 10.1264/jsme2.ME16042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kumar H, Sharma S. Determination of chlorophyll and carotenoid loss in Dalbergia sissoo caused by Aonidiella orientalis (Newstead) [Homoptera: Coccoidea: Diaspididae] J. Entomol. Zool. Stud. 2014;2:104–106. [Google Scholar]
- 259.Lamelas A, et al. Serratia symbiotica from the aphid Cinara cedri: a missing link from facultative to obligate insect endosymbiont. PLoS Genet. 2011;7:e1002357. doi: 10.1371/journal.pgen.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lefèvre C, et al. Endosymbiont phylogenesis in the Dryophthoridae weevils: evidence for bacterial replacement. Mol. Biol. Evol. 2004;21:965–973. doi: 10.1093/molbev/msh063. [DOI] [PubMed] [Google Scholar]
- 261.Leschen, R. A. B., Beutel, R. G. & Lawrence, J. F. Volume 2 Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia partim) (De Gruyter, 2010).
- 262.Liu L-Y, Schonitzer K, Yang J-T. A review of the literature on the life history of Bostrichidae. Mitt. Münch. Entomol. Ges. 2008;98:91–97. [Google Scholar]
- 263.Lo N, Beninati T, Stone F, Walker J, Sacchi L. Cockroaches that lack Blattabacterium endosymbionts: the phylogenetically divergent genus Nocticola. Biol. Lett. 2007;3:327–330. doi: 10.1098/rsbl.2006.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Loper GM, Standifer LN, Thompson MJ, Gilliam M. Biochemistry and microbiology of bee collected almond (Prunus dulcis) pollen and bee bread. I-fatty acids, sterols, vitamins and minerals. Apidologie. 1980;11:63–73. doi: 10.1051/apido:19800108. [DOI] [Google Scholar]
- 265.Lukasik P, van Asch M, Guo HF, Ferrari J, Godfray HCJ. Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol. Lett. 2013;16:214–218. doi: 10.1111/ele.12031. [DOI] [PubMed] [Google Scholar]
- 266.Machtelinckx T, et al. Microbial community of predatory bugs of the genus Macrolophus (Hemiptera: Miridae) BMC Microbiol. 2012;12:S9-2180–12-S1-S9. doi: 10.1186/1471-2180-12-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Magallon S, Sanderson MJ. Absolute diversification rates in angiosperm clades. Evolution. 2001;55:1762–1780. doi: 10.1111/j.0014-3820.2001.tb00826.x. [DOI] [PubMed] [Google Scholar]
- 268.Maier CT. The behavior of Hydrometra championana (Hemiptera: Hydrometridae) and resource partitioning with Tenagogonus quadrilineatus (Hemiptera: Gerridae) J. Kans. Entomol. Soc. 1977;50:263–271. [Google Scholar]
- 269.Majka C. The flat bark beetles (Coleoptera, Silvanidae, Cucujidae, Laemophloeidae) of Atlantic Canada. ZooKeys. 2008;2:221–238. doi: 10.3897/zookeys.2.14. [DOI] [Google Scholar]
- 270.Majka CG, Lesage L. Introduced leaf beetles of the maritime provinces, 7. Zootaxa. 2008;56:37–56. doi: 10.11646/zootaxa.1811.1.3. [DOI] [Google Scholar]
- 271.Mann J. Cactus-feeding insects and mites. Bull. US Natl Mus. 1969;256:1–158. [Google Scholar]
- 272.Mao M, Yang XS, Poff K, Bennett G. Comparative genomics of the dual-obligate symbionts from the treehopper, Entylia carinata (Hemiptera: Membracidae), provide insight into the origins and evolution of an ancient symbiosis. Genome Biol. Evol. 2017;9:1803–1815. doi: 10.1093/gbe/evx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Marche T, et al. Chemical changes during composting of a paper mill sludgehardwood sawdust mixture. Geoderma. 2003;116:345–356. doi: 10.1016/S0016-7061(03)00108-3. [DOI] [Google Scholar]
- 274.Marshall, S. A. Flies: The Natural History and Diversity of Diptera (Firefly Books, 2012).
- 275.Martin M. Life history and habits of the pigeon louse (Columbicola columbae [Linnaeus]) Can. Entomol. 1934;66:6–16. doi: 10.4039/Ent666-1. [DOI] [Google Scholar]
- 276.Martínez M, Canneva B, Ronderos MM. Diptera, Ceratopogonidae, Dasyhelea necrophila Spinelli and Rodriguez, 1999: detection of eggs in ovitraps, in Uruguay. Check List. 2010;6:239–241. doi: 10.15560/6.2.239. [DOI] [Google Scholar]
- 277.Martinez AJ, et al. Angiosperm to gymnosperm host–plant switch entails shifts in microbiota of the Welwitschia bug, Probergrothius angolensis (Distant, 1902) Mol. Ecol. 2019;28:5172–5187. doi: 10.1111/mec.15281. [DOI] [PubMed] [Google Scholar]
- 278.Martins DB, de Oliveira EZ, Valandro MA, Franco M, de Souza J. Trichodectes canis in puppy and adult dogs. Comp. Clin. Pathol. 2014;23:1485–1489. doi: 10.1007/s00580-013-1810-9. [DOI] [Google Scholar]
- 279.Matsuura Y, et al. Huge symbiotic organs in giant scale insects of the genus drosicha (coccoidea: monophlebidae) harbor flavobacterial and enterobacterial endosymbionts. Zool. Sci. 2009;26:448–456. doi: 10.2108/zsj.26.448. [DOI] [PubMed] [Google Scholar]
- 280.Matsuura Y, et al. Evolution of symbiotic organs and endosymbionts in lygaeid stinkbugs. ISME J. 2012;6:397–409. doi: 10.1038/ismej.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Matsuura Y, Kikuchi Y, Meng XY, Koga R, Fukatsu T. Novel clade of alphaproteobacterial endosymbionts associated with stinkbugs and other arthropods. Appl. Environ. Microbiol. 2012;78:4149–4156. doi: 10.1128/AEM.00673-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.McClure, M. S. in Armoured Scale Insects: Their Biology, Natural Enemies and Control (ed. Rosen, D.) 289–291 (Elsevier, 1990).
- 283.McCutcheon JP, McDonald BR, Moran NA. Origin of an alternative genetic code in the extremely small and GC-rich genome of a bacterial symbiont. PLoS Genet. 2009;5:e1000565. doi: 10.1371/journal.pgen.1000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.McCutcheon JP, von Dohlen CD. An interdependent metabolic patchwork in the nested symbiosis of mealybugs. Curr. Biol. 2011;21:1366–1372. doi: 10.1016/j.cub.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Mead, F. W. & Fasulo, T. R. Scentless plant bugs, Jadera sp. Insecta: Hemiptera. Dtsch. Entomol. Z. 1–3 (2000).
- 286.Merritt SZ. Within-plant variation in concentrations of amino acids, sugar, and sinigrin in phloem sap of black mustard, Brassica nigra (L.) Koch (Cruciferae) J. Chem. Ecol. 1996;22:1133–1145. doi: 10.1007/BF02027950. [DOI] [PubMed] [Google Scholar]
- 287.Merville A, et al. Endosymbiont diversity among sibling weevil species competing for the same resource. BMC Evol. Biol. 2013;13:28. doi: 10.1186/1471-2148-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Meyer JM, Hoy MA. Molecular survey of endosymbionts in Florida populations of Diaphorina citri (Hemiptera: Psyllidae) and its parasitoids Tamarixia radiata (Hymenoptera: Eulophidae) and Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae) Fla. Entomologist. 2008;91:294–304. doi: 10.1653/0015-4040(2008)91[294:MSOEIF]2.0.CO;2. [DOI] [Google Scholar]
- 289.Michalik A, Jankowska W, Szklarzewicz T. Ultrastructure and transovarial transmission of endosymbiotic microorganisms in Conomelus anceps and Metcalfa pruinosa (Insecta, Hemiptera, Fulgoromorpha) Folia Biologica (Krak.ów) 2009;57:131–137. doi: 10.3409/fb57_3-4.131-137. [DOI] [PubMed] [Google Scholar]
- 290.Michalik A, Jankowska W, Kot M, Gołas A, Szklarzewicz T. Symbiosis in the green leafhopper, Cicadella viridis (Hemiptera, Cicadellidae): association in statu nascendi? Arthropod Struct. Dev. 2014;43:579–587. doi: 10.1016/j.asd.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 291.Michalik K, Szklarzewicz T, Kalandyk-Kolodziejczyk M, Jankowska W, Michalik A. Bacteria belonging to the genus Burkholderia are obligatory symbionts of the eriococcids Acanthococcus aceris Signoret, 1875 and Gossyparia spuria (Modeer, 1778) (Insecta, Hemiptera, Coccoidea) Arthropod Struct. Dev. 2016;45:265–272. doi: 10.1016/j.asd.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 292.Miles PW. The salivary secretions of a plant-sucking bug, Oncopeltus fascwitus (Dall.) Heteroptera: Lygaeidae)—I the types of secretions and their roles during feeding. J. Insect Physiol. 1958;3:243–255. doi: 10.1016/0022-1910(59)90004-6. [DOI] [Google Scholar]
- 293.Millett MA, Baker AJ, Feist WC, Mellenberger RW, Satter LD. Modifying wood to increase its in vitro digestibility. J. Anim. Sci. 1970;31:781–788. doi: 10.2527/jas1970.314781x. [DOI] [Google Scholar]
- 294.Møller HB, Sommer SG, Ahring BK. Methane productivity of manure, straw and solid fractions of manure. Biomass Bioenergy. 2004;26:485–495. doi: 10.1016/j.biombioe.2003.08.008. [DOI] [Google Scholar]
- 295.Moran NA, Telang A. Bacteriocyte-associated symbionts of insects—a variety of insect groups harbor ancient prokaryotic endosymbionts. Bioscience. 1998;48:295–304. doi: 10.2307/1313356. [DOI] [Google Scholar]
- 296.Moran NA, Dale C, Dunbar HE, Smith WA, Ochman H. Intracellular symbionts of sharpshooters (Insecta: Hemiptera: Cicadellinae) form a distinct clade with a small genome. Environ. Microbiol. 2003;5:116–126. doi: 10.1046/j.1462-2920.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- 297.Morlon H. Phylogenetic approaches for studying diversification. Ecol. Lett. 2014;17:508–525. doi: 10.1111/ele.12251. [DOI] [PubMed] [Google Scholar]
- 298.Morse SF, Bush SE, Patterson BD, Dick CW, Gruwell ME. Evolution, multiple acquisition, and localization of endosymbionts in bat flies (Diptera: Hippoboscoidea: Streblidae and Nycteribiidae) Appl. Environ. Microbiol. 2013;79:2952–2961. doi: 10.1128/AEM.03814-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Mukesh DJ. Production of feather protein concentrate from feathers by in vitro enzymatic treatment, its biochemical characterization and antioxidant. Nat. Middle-East J. Sci. Res. 2012;11:881–886. [Google Scholar]
- 300.Munson MA, et al. Evidence for the establishment of aphid-eubacterium endosymbiosis in an ancestor of four aphid families. J. Bacteriol. 1991;173:6321–6324. doi: 10.1128/jb.173.20.6321-6324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Nasu S, Kusumi T, Suwa Y, Kita H. Symbiotes of planthoppers: II. Isolation of intracellular symbiotic microorganisms from the brown planthopper, Nilaparata lugens STAL, and immunological comparison of the symbiotes associated with rice planthoppers (Hemiptera: Delphacidae) Appl. Entomol. Zool. 1981;16:88–93. doi: 10.1303/aez.16.88. [DOI] [Google Scholar]
- 302.Nepi M, et al. Amino acids and protein profile in floral nectar: much more than a simple reward. Flora Morphol. Distrib. Funct. Ecol. Plants. 2012;207:475–481. [Google Scholar]
- 303.New TR. Biology of the Psocoptera. Orient. Insects. 1987;21:1–109. doi: 10.1080/00305316.1987.11835472. [DOI] [Google Scholar]
- 304.Nishino T, Tanahashi M, Lin CP, Koga R, Fukatsu T. Fungal and bacterial endosymbionts of eared leafhoppers of the subfamily Ledrinae (Hemiptera: Cicadellidae) Appl. Entomol. Zool. 2016;51:465–477. doi: 10.1007/s13355-016-0422-7. [DOI] [Google Scholar]
- 305.Noda H. Histological and histochemical observation of intracellular yeast-like symbiotes in the fat body of the smaller brown planthopper, Laodelphax striatellus (Homoptera: Delphacidae) Appl. Entomol. Zool. 1977;12:134–141. doi: 10.1303/aez.12.134. [DOI] [Google Scholar]
- 306.Noda H, et al. Bacteriome-associated endosymbionts of the green rice leafhopper Nephotettix cincticeps (Hemiptera: Cicadellidae) Appl. Entomol. Zool. 2012;47:217–225. doi: 10.1007/s13355-012-0110-1. [DOI] [Google Scholar]
- 307.Novakova E, Husnik F, Sochova E, Hypsa V. Arsenophonus and Sodalis symbionts in louse flies: an analogy to the Wigglesworthia and Sodalis system in tsetse flies. Appl. Environ. Microbiol. 2015;81:6189–6199. doi: 10.1128/AEM.01487-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Nováková E, Hypša V, Nguyen P, Husník F, Darby AC. Genome sequence of Candidatus Arsenophonus lipopteni, the exclusive symbiont of a blood sucking fly Lipoptena cervi (Diptera: Hippoboscidae) Stand. Genom. Sci. 2016;11:72. doi: 10.1186/s40793-016-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Novotny V, Wilson MR. Why are there no small species among xylem-sucking insects? Evol. Ecol. 1997;11:419–437. doi: 10.1023/A:1018432807165. [DOI] [Google Scholar]
- 310.O’Brien, L. B. in The Leafhoppers and Planthoppers (ed. Nault, L. R.) 61–102 (Wiley, 1985).
- 311.Ohbayashi T, Itoh H, Lachat J, Kikuchi Y, Mergaert P. Burkholderia gut symbionts associated with European and Japanese populations of the dock bug Coreus marginatus (Coreoidea: Coreidae) Microbes Environ. 2019;34:219–222. doi: 10.1264/jsme2.ME19011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ohshima T, Hayashi H, Chino M. Collection and chemical composition of pure phloem sap from Zea mays L. Plant Cell Physiol. 1990;31:735–737. [Google Scholar]
- 313.Okanovic D, Ristic M, Kormanjos S, Filipovic S, Zivkovic B. Chemical characteristics of poultry slaughterhouse byproducts. Biotechnol. Anim. Husb. 2009;25:143–152. doi: 10.2298/BAH0902143O. [DOI] [Google Scholar]
- 314.Okude G, et al. Novel bacteriocyte-associated pleomorphic symbiont of the grain pest beetle Rhyzopertha dominica (Coleoptera: Bostrichidae) Zool. Lett. 2017;3:13. doi: 10.1186/s40851-017-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Overholt WA, Diaz R, Rosskopf E, Green SJ, Overholt WA. Deep characterization of the microbiomes of Calophya spp. (Hemiptera: Calophyidae) gall-inducing psyllids reveals the absence of plant pathogenic bacteria and three dominant endosymbionts. PLoS ONE. 2015;10:e0132248. doi: 10.1371/journal.pone.0132248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Pant NC, Fraenkel G. The function of the symbiotic yeasts of two insect species, Lasioderma serricorne F. and Stegobium (Sitodrepa) paniceum L. Science. 1950;112:498–500. doi: 10.1126/science.112.2913.498. [DOI] [PubMed] [Google Scholar]
- 317.Patil VS, Magar NG. Fatty acids of human blood. Biochem. J. 1959;74:427–429. doi: 10.1042/bj0740427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Peeters PJ, Read J, Sanson GD. Variation in the guild composition of herbivorous insect assemblages among co-occurring plant species. Austral. Ecol. 2001;26:385–399. doi: 10.1046/j.1442-9993.2001.01123.x. [DOI] [Google Scholar]
- 319.Pennell MW, et al. Geiger v2.0: an expanded suite of methods for fitting macroevolutionary models to phylogenetic trees. Bioinformatics. 2014;30:2216–2218. doi: 10.1093/bioinformatics/btu181. [DOI] [PubMed] [Google Scholar]
- 320.Pérez-Ruiz M, Martínez-Rodríguez P, Herranz J, Bella JL. A survey of Wolbachia, Spiroplasma and other bacteria in parthenogenetic and non-parthenogenetic phasmid (Phasmatodea) species. Eur. J. Entomol. 2015;112:409–418. doi: 10.14411/eje.2015.061. [DOI] [Google Scholar]
- 321.Perotti MA, Clarke HK, Turner BD, Braig HR. Rickettsia as obligate and mycetomic bacteria. FASEB J. 2006;20:2372–2374. doi: 10.1096/fj.06-5870fje. [DOI] [PubMed] [Google Scholar]
- 322.Peuke AD. Correlations in concentrations, xylem and phloem flows, and partitioning of elements and ions in intact plants. A summary and statistical re-evaluation of modelling experiments in Ricinus communis. J. Exp. Bot. 2010;61:635–655. doi: 10.1093/jxb/erp352. [DOI] [PubMed] [Google Scholar]
- 323.Powell, J. A, Mitter, C & Farrell, B. in Teilband/Part 35 Volume 1: Evolution, Systematics, and Biogeography (ed. Kükenthal, W.) 403–422 (De Gruyter, 1998).
- 324.Putman WL. The feeding habits of certain leafhoppers. Can. Entomol. 1941;73:39–53. doi: 10.4039/Ent7339-3. [DOI] [Google Scholar]
- 325.Rabosky DL. Ecological limits and diversification rate: alternative paradigms to explain the variation in species richness among clades and regions. Ecol. Lett. 2009;12:735–743. doi: 10.1111/j.1461-0248.2009.01333.x. [DOI] [PubMed] [Google Scholar]
- 326.Rahman NA, et al. A molecular survey of Australian and North American termite genera indicates that vertical inheritance is the primary force shaping termite gut microbiomes. Microbiome. 2015;3:5. doi: 10.1186/s40168-015-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ramírez-Puebla ST, et al. Molecular phylogeny of the genus Dactylopius (Hemiptera: Dactylopiidae) and identification of the symbiotic bacteria. Environ. Entomol. 2010;39:1178–1183. doi: 10.1603/EN10037. [DOI] [PubMed] [Google Scholar]
- 328.Raven JA. Phytophages of xylem and phloem: a comparison of animal and plant sap-feeders. Adv. Ecol. Res. 1983;13:135–234. doi: 10.1016/S0065-2504(08)60109-9. [DOI] [Google Scholar]
- 329.Rebijith KB, et al. Reconstructing the macroevolutionary patterns of aphids (Hemiptera: Aphididae) using nuclear and mitochondrial DNA sequences. Biol. J. Linn. Soc. 2017;121:796–814. doi: 10.1093/biolinnean/blx020. [DOI] [Google Scholar]
- 330.Resh, V. Encyclopedia of Insects 2nd edn (Academic Press, 2009).
- 331.Rivault C, Cloarec A. Exploitation of food resources by the cockroach Blattella germaica in an urban habitat. Entomol. Exp. Appl. 1991;61:149–158. doi: 10.1111/j.1570-7458.1991.tb02407.x. [DOI] [Google Scholar]
- 332.Rivera-Torres V, Noblet J, van Milgen J. Changes in chemical composition in male turkeys during growth. Poult. Sci. 2011;90:68–74. doi: 10.3382/ps.2010-00633. [DOI] [PubMed] [Google Scholar]
- 333.Robbins, C. R. Chemical and Physical Behavior of Human Hair 105–176 (Springer, 2012).
- 334.Rop O, Mlcek J, Jurikova T, Neugebauerova J, Vabkova J. Edible flowers of mineral elements in human nutrition. Molecules. 2012;17:6672–6683. doi: 10.3390/molecules17066672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Rosas-Pérez T, Rosenblueth M, Rincón-Rosales R, Mora J, Martínez-Romero E. Genome sequence of ‘Candidatus Walczuchella monophlebidarum’ the flavobacterial endosymbiont of Llaveia axin axin (Hemiptera: Coccoidea: Monophlebidae) Genome Biol. Evol. 2014;6:714–726. doi: 10.1093/gbe/evu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Rosenblueth M, Sayavedra L, Sámano-Sánchez H, Roth A, Martínez-Romero E. Evolutionary relationships of flavobacterial and enterobacterial endosymbionts with their scale insect hosts (Hemiptera: Coccoidea) J. Evolut. Biol. 2012;25:2357–2368. doi: 10.1111/j.1420-9101.2012.02611.x. [DOI] [PubMed] [Google Scholar]
- 337.Rosengaus RB, Zecher CN, Schultheis KF, Brucker RM, Bordenstein SR. Disruption of the termite gut microbiota and its prolonged consequences for fitness. Appl. Environ. Microbiol. 2011;77:4303–4312. doi: 10.1128/AEM.01886-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Rumpold BA, Schlüter OK. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013;57:802–823. doi: 10.1002/mnfr.201200735. [DOI] [PubMed] [Google Scholar]
- 339.Sabree ZL, et al. Genome shrinkage and loss of nutrient-providing potential in the obligate symbiont of the primitive termite Mastotermes darwiniensis. Appl. Environ. Microbiol. 2012;78:204–210. doi: 10.1128/AEM.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Sadof, C. S. & Neal, J. J. Use of host plant resources by the Euonymus scale, Unaspis euonymi (Homoptera: Diaspididae). Ann. Entomol. Soc. Am.86, 614–620 (1993).
- 341.Salem H, Kreutzer E, Sudakaran S, Kaltenpoth M. Actinobacteria as essential symbionts in firebugs and cotton stainers (Hemiptera, Pyrrhocoridae) Environ. Microbiol. 2013;15:1956–1968. doi: 10.1111/1462-2920.12001. [DOI] [PubMed] [Google Scholar]
- 342.Santos-Garcia D. The all-rounder Sodalis: a new bacteriome-associated endosymbiont of the lygaeoid bug Henestaris halophilus (Heteroptera: Henestarinae) and a critical examination of its evolution. Genome Biol. Evol. 2017;9:2893–2910. doi: 10.1093/gbe/evx202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Sanz ML, Gonzalez M, de Lorenzo C, Sanz J, Martinez-Castro I. Carbohydrate composition and physico chemical properties of artisanal honeys from Madrid (Spain): occurrence of Echium sp honey. J. Sci. Food Agric. 2004;84:1577–1584. doi: 10.1002/jsfa.1823. [DOI] [Google Scholar]
- 344.Sariyildiz T, Anderson JM. Variation in the chemical composition of green leaves and leaf litters from three deciduous tree species growing on different soil types. For. Ecol. Manag. 2005;210:303–319. doi: 10.1016/j.foreco.2005.02.043. [DOI] [Google Scholar]
- 345.Sasaki-Fukatsu K, et al. Symbiotic bacteria associated with stomach discs of human lice. Appl. Environ. Microbiol. 2006;72:7349–7352. doi: 10.1128/AEM.01429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Sauer C, Stackebrandt E, Gadau J, Holldobler B, Gross R. Systematic relationships and cospeciation of bacterial endosymbionts and their carpenter ant host species: proposal of the new taxon Candidatus blochmannia gen. nov. Int. J. Syst. Evol. Microbiol. 2000;50:1877–1886. doi: 10.1099/00207713-50-5-1877. [DOI] [PubMed] [Google Scholar]
- 347.Saxena AK. Further observations on the freely circulating hemocytes of Liperus lawrensis tropicalis Peters (Phthiraptera, Ischnocera) Curr. Sci. 1981;50:551–551. [Google Scholar]
- 348.Saxena AK, Agarwal GP. Mycetocytes in Aegypoecus perspicuus (Phthiraptera) Curr. Sci. 1985;54:763–764. [Google Scholar]
- 349.Schaefer CW, Chopra NP. Cladistic analysis of the rhopalidae, with a list of food plants. Ann. Entomol. Soc. Am. 1982;75:224–233. doi: 10.1093/aesa/75.3.224. [DOI] [Google Scholar]
- 350.Scurfield G, Nicholls PW. Amino-acid composition of wood proteins. J. Exp. Bot. 1970;21:857–868. doi: 10.1093/jxb/21.4.857. [DOI] [Google Scholar]
- 351.Şen A, Miranda I, Santos S, Graça J, Pereira H. The chemical composition of cork and phloem in the rhytidome of Quercus cerris bark. Ind. Crops Prod. 2010;31:417–422. doi: 10.1016/j.indcrop.2010.01.002. [DOI] [Google Scholar]
- 352.Seo PJ, et al. Natural variation in floral nectar proteins of two Nicotiana attenuata accessions. BMC Plant Biol. 2013;13:101. doi: 10.1186/1471-2229-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Shelomi M, Lo WS, Kimsey LS, Kuo CH. Analysis of the gut microbiota of walking sticks (Phasmatodea) BMC Res. Notes. 2013;6:368. doi: 10.1186/1756-0500-6-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Shibata K, et al. Values of water-soluble vitamins in blood and urine of Japanese young men and women consuming a semi-purified diet based on the Japanese Dietary Reference Intakes. J. Nutr. Sci. Vitaminol. 2005;51:319–328. doi: 10.3177/jnsv.51.319. [DOI] [PubMed] [Google Scholar]
- 355.Shils ME, Baker H, Frank O. Blood vitamin levels of long-term adult home total parenteral nutrition patients: the efficacy of the AMA–FDA parenteral multivitamin formulation. J. Parenter. Enter. Nutr. 1985;9:179–188. doi: 10.1177/0148607185009002179. [DOI] [PubMed] [Google Scholar]
- 356.Singh SK, Arya S, Singh SK, Khan V. Feeding and reproductive behaviour of pigeon slender louse, Columbicola. J. Appl. Nat. Sci. 2010;1:126–133. doi: 10.31018/jans.v2i1.111. [DOI] [Google Scholar]
- 357.Sirimanne SR, Patterson DG, Ma L, Justice JB. Application of cloud-point extraction-reversed-phase high-performance liquid chromatography: a preliminary study of the extraction and quantification of vitamins A and E in human serum and whole blood. J. Chromatogr. B. 1998;716:129–137. doi: 10.1016/S0378-4347(98)00287-4. [DOI] [PubMed] [Google Scholar]
- 358.Skaljac M, Zanic K, Ban SG, Kontsedalov S, Ghanim M. Co-infection and localization of secondary symbionts in two whitefly species. BMC Microbiol. 2010;10:142. doi: 10.1186/1471-2180-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Slater JA. A contribution to the biology of the subfamily Cyminae (Heteroptera: Lygaeidae) Ann. Entomol. Soc. Am. 1952;45:315–326. doi: 10.1093/aesa/45.2.315. [DOI] [Google Scholar]
- 360.Slater JA. Monocots and chinch bugs: a study of host plant relationships in the lygaeid subfamily Blissinae (Hemiptera: Lygaeidae) Biotropica. 1976;8:143–165. doi: 10.2307/2989681. [DOI] [Google Scholar]
- 361.Somerville, D. C., Rural Industries Research and Development Corporation (Australia) & NSW Agriculture. Nutritional Value of Bee Collected Pollens: A Report for the Rural Industries Research and Development Corporation (RIRDC, 2001).
- 362.de Souza, N. R. Host Plant associations of Two Cochineal Insect Species, Dactylopius ceylonicus and D. opuntiae (Dactylopiidae: Hemiptera), on the Invasive Cactus Species Opuntia monacantha, O. ficus-indica and a Possible Hybrid Cactus, in South Africa. Thesis, Univ. Cape Town (2014).
- 363.Stammer H-J. Studien an Symbiosen zwischen Käfern und Mikroorganismen I. Die Symbiose der Donaciien (Coleopt. chrysomel.) Z. Morphol. Ökol. Tiere. 1935;29:585–608. doi: 10.1007/BF00407434. [DOI] [Google Scholar]
- 364.Stammer, H. J. Studien an Symbiosen zwischen Käfern und Mikroorganismen. II. Die Symbiose der Bromius obscuius L. und der Cassidaarten (Coleopt. Chrysomel.). Zool. Inst. Univ. Breslau, 31 (1936).
- 365.Stock AMW, Lattin JD. Biology of intertidal Saldula palustris (Douglas) on the Oregon Coast (Heteroptera: Saldidae) J. Kans. Entomol. Soc. 1976;49:313–326. [Google Scholar]
- 366.Sudan V. A rare documentation of Haematomyzus elephantis lice from elephants of Mathura. J. Parasit. Dis. 2015;39:793–794. doi: 10.1007/s12639-014-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sutton MF. On the food, feeding mechanism and alimentary canal of Corixidae (Hemiptera, Heteroptera) Proc. Zool. Soc. Lond. 1951;121:465–499. doi: 10.1111/j.1096-3642.1951.tb00749.x. [DOI] [Google Scholar]
- 368.Sweet MH. The seed bugs: a contribution to the feeding habits of the Lygaeidae (Hemiptera: Heteroptera) Ann. Entomol. Soc. Am. 1960;53:317–321. doi: 10.1093/aesa/53.3.317. [DOI] [Google Scholar]
- 369.Szabo G, et al. Convergent patterns in the evolution of mealybug symbioses involving different intrabacterial symbionts. ISME J. 2017;11:715–726. doi: 10.1038/ismej.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Szklarzewicz T, Jankowska W, Wieczorek K, Wgierek P. Structure of the ovaries of the primitive aphids Phylloxera coccinea and Phylloxera glabra (Hemiptera, Aphidinea: Phylloxeridae) Acta Zool. 2009;90:123–131. doi: 10.1111/j.1463-6395.2008.00335.x. [DOI] [Google Scholar]
- 371.Tada A, et al. Obligate association with gut bacterial symbiont in Japanese populations of the southern green stinkbug Nezara viridula (Heteroptera: Pentatomidae) Appl. Entomol. Zool. 2011;46:483–488. doi: 10.1007/s13355-011-0066-6. [DOI] [Google Scholar]
- 372.Taha EKA. Chemical composition and amounts of mineral elements in honeybee-collected pollen in relation to botanical origin. J. Apicult. Sci. 2015;59:75–81. doi: 10.1515/jas-2015-0008. [DOI] [Google Scholar]
- 373.Teixeira L, Ferreira A, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008;6:2753–2763. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Thao MLL, et al. Cospeciation of psyllids and their primary prokaryotic endosymbionts. Appl. Environ. Microbiol. 2000;66:2898–2905. doi: 10.1128/AEM.66.7.2898-2905.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Thao MLL, Gullan PJ, Baumann P. Secondary (γ-Proteobacteria) endosymbionts infect the primary (β-Proteobacteria) endosymbionts of mealybugs multiple times and coevolve with their hosts. Appl. Environ. Microbiol. 2002;68:3190–3197. doi: 10.1128/AEM.68.7.3190-3197.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Thao MLLL, Baumann P. Evolutionary relationships of primary prokaryotic endosymbionts of whiteflies and their hosts. Appl. Environ. Microbiol. 2004;70:3401–3406. doi: 10.1128/AEM.70.6.3401-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Theis RC. The protein content of the whole blood and plasma in cancer. J. Cancer Res. 1921;6:127–130. [Google Scholar]
- 378.Thompson V. Associative nitrogen fixation, C4 photosynthesis, and the evolution of spittlebugs (Hemiptera: Cercopidae) as major pests of neotropical sugarcane and forage grasses. Bull. Entomol. Res. 2004;94:189–200. doi: 10.1079/BER2004293. [DOI] [PubMed] [Google Scholar]
- 379.Tian G, Kang BT, Brussaard L. Biological effects of plant residues with contrasting chemical compositions under humid tropical conditions—decomposition and nutrient release. Soil Biol. Biochem. 1992;24:1051–1060. doi: 10.1016/0038-0717(92)90035-V. [DOI] [Google Scholar]
- 380.Tillman PG, Mullinix BG. Effect of prey species on plant feeding behavior by the big-eyed bug, Geocoris punctipes (Say) (Heteroptera: Geocoridae), on cotton. Environ. Entomol. 2003;32:1399–1403. doi: 10.1603/0046-225X-32.6.1399. [DOI] [Google Scholar]
- 381.Toenshoff ER, Gruber D, Horn M. Co-evolution and symbiont replacement shaped the symbiosis between adelgids (Hemiptera: Adelgidae) and their bacterial symbionts. Environ. Microbiol. 2012;14:1284–1295. doi: 10.1111/j.1462-2920.2012.02712.x. [DOI] [PubMed] [Google Scholar]
- 382.Toenshoff ER, Szabo G, Gruber D, Horn M. The pine bark adelgid, Pineus strobi, contains two novel bacteriocyte-associated gammaproteobacterial symbionts. Appl. Environ. Microbiol. 2014;80:878–885. doi: 10.1128/AEM.03310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Toenshoff ER, et al. Bacteriocyte-associated gammaproteobacterial symbionts of the Adelges nordmannianae/piceae complex (Hemiptera: Adelgidae) ISME J. 2012;6:384–396. doi: 10.1038/ismej.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Toju H, et al. ‘Candidatus Curculioniphilus buchneri,’ a novel clade of bacterial endocellular symbionts from weevils of the genus Curculio. Appl. Environ. Microbiol. 2010;76:275–282. doi: 10.1128/AEM.02154-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Trivedi MC, Sharma S, Rawat BS, Saxena AK. Haematophagous nature of an amblyceran phthirapteran, Menacanthus cornutus Schommer, infesting poultry bird Gallus domesticus L. In India. J. Appl. Entomol. 1990;110:107–111. doi: 10.1111/j.1439-0418.1990.tb00102.x. [DOI] [Google Scholar]
- 386.Tsuchida T, Koga R, Shibao H, Matsumoto T, Fukatsu T. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 2002;11:2123–2135. doi: 10.1046/j.1365-294X.2002.01606.x. [DOI] [PubMed] [Google Scholar]
- 387.Tsuchida T, Koga R, Fujiwara A, Fukatsu T. Phenotypic effect of ‘Candidatus Rickettsiella viridis,’ a facultative symbiont of the pea aphid (Acyrthosiphon pisum), and its interaction with a coexisting symbiont. Appl. Environ. Microbiol. 2014;80:525–533. doi: 10.1128/AEM.03049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Urban JM, Cryan JR. Two ancient bacterial endosymbionts have coevolved with the planthoppers (Insecta: Hemiptera:Fulgoroidea) BMC Evol. Biol. 2012;12:87. doi: 10.1186/1471-2148-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.van Helden M, Tjallingh WF, van Beek TA. Phloem sap collection from lettuce (Lactuca sativa L.): chemical comparison among collection methods. J. Chem. Ecol. 1994;20:3191–3206. doi: 10.1007/BF02033720. [DOI] [PubMed] [Google Scholar]
- 390.Van Huis, A. et al. Nutritional Values of Insects for Human Consumption (2013).
- 391.Vasconcelos EJR, et al. Assessing cat flea microbiomes in Northern and Southern California by 16S rRNA next-generation sequencing. Vector-Borne Zoon. Dis. 2018;18:491–499. doi: 10.1089/vbz.2018.2282. [DOI] [PubMed] [Google Scholar]
- 392.Vea IM, Grimaldi DA. Putting scales into evolutionary time: the divergence of major scale insect lineages (Hemiptera) predates the radiation of modern angiosperm hosts. Sci. Rep. 2016;6:23487. doi: 10.1038/srep23487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.von Dohlen CD, et al. Diversity of proteobacterial endosymbionts in hemlock woolly adelgid (Adelges tsugae) (Hemiptera: Adelgidae) from its native and introduced range. Environ. Microbiol. 2013;15:2043–2062. doi: 10.1111/1462-2920.12102. [DOI] [PubMed] [Google Scholar]
- 394.Waldkircher G, Webb MD, Maschwitz U. Description of a new shieldbug (Heteroptera: Plataspidae) and its close association with a species of ant (Hymenoptera: Formicidae) in Southeast Asia. Tijdschr. Entomol. 2004;147:21–28. doi: 10.1163/22119434-900000133. [DOI] [Google Scholar]
- 395.Weiss B, Kaltenpoth M. Bacteriome-localized intracellular symbionts in pollen-feeding beetles of the genus Dasytes (Coleoptera, Dasytidae) Front. Microbiol. 2016;7:1486. doi: 10.3389/fmicb.2016.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Wiens JJ. The causes of species richness patterns across space, time, and clades and the role of ‘ecological limits’. Q. Rev. Biol. 2011;86:75–96. doi: 10.1086/659883. [DOI] [PubMed] [Google Scholar]
- 397.Williams, D. Aquatic Insects. vols Aquatic Conservation: Marine and Freshwater Ecosystems (C. A. B. Int., 1992).
- 398.Wilson ACC, Duncan RP. Signatures of host/symbiont genome coevolution in insect nutritional endosymbioses. Proc. Natl Acad. Sci. USA. 2015;112:10255–10261. doi: 10.1073/pnas.1423305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Winnick CG, Holwell GI, Herberstein ME. Internal reproductive anatomy of the praying mantid Ciulfina klassi (Mantodea: Liturgusidae) Arthropod Struct. Dev. 2009;38:60–69. doi: 10.1016/j.asd.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 400.Woodard HQ, White DR. The composition of body-tissues. Br. J. Radiol. 1986;59:1209–1219. doi: 10.1259/0007-1285-59-708-1209. [DOI] [PubMed] [Google Scholar]
- 401.Zhou WW, et al. Identification and expression profiles of nine glutathione S-transferase genes from the important rice phloem sap-sucker and virus vector Laodelphax striatellus (Fallén) (Hemiptera: Delphacidae) Pest Manag. Sci. 2012;68:1296–1305. doi: 10.1002/ps.3297. [DOI] [PubMed] [Google Scholar]
- 402.Ziegler H, Ziegler I. Die wasserlöslichen Vitamine in den Siebröhrensäften einiger Bäume. Flora Allg. Bot. Ztg. 1962;152:257–278. [Google Scholar]
- 403.Zimmerman, M. H. The Formation of Wood in Forest Trees (Academic Press, 1964).
- 404.Voedingswaarde tabel.nl.
- 405.Cornwallis, C. K. Symbioses shape feeding niches and diversification across insects. Open Science Frameworkhttps://doi.org/10.17605/OSF.IO/TYK7C (2023). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1–30 and R session information (versions and packages loaded).
Data Availability Statement
All data are provided in Supplementary Tables 1–4. Full citations of references in supplementary tables are given in the method references7,12,16,101–404. All supplementary tables are available at the Open Science Framework (osf.io project number TYK7C; 10.17605/OSF.IO/TYK7C)405. Source data are provided with this paper.
R code, BayesTraits code and analysis results are available at the Open Science Framework (osf.io project number TYK7C; 10.17605/OSF.IO/TYK7C)405.