Abstract
Background
Premature ventricular complexes (PVCs) are a potentially reversible cause of heart failure. However, the characteristics of patients most likely to develop impaired left ventricular function are unclear. Hence, the objective of this study is to systematically assess risk factors for the development of PVC-induced cardiomyopathy.
Methods
We performed a structured database search of the scientific literature for studies investigating risk factors for the development of PVC-induced cardiomyopathy (PVC-CM). We investigated the reporting of PVC-CM risk factors (RF) and assessed the comparative association of the different RF using random-effect meta-analysis.
Results
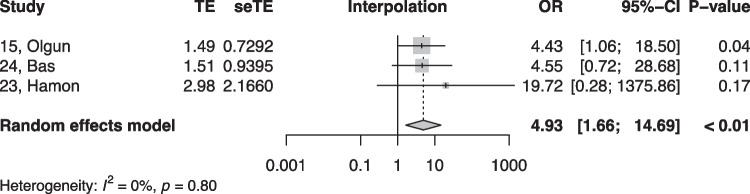
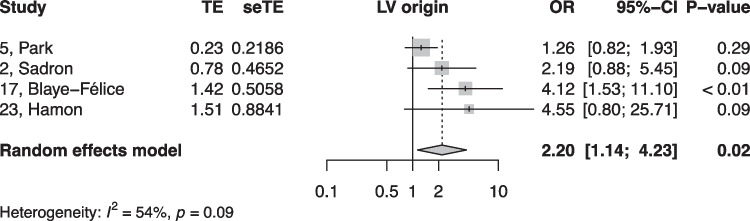
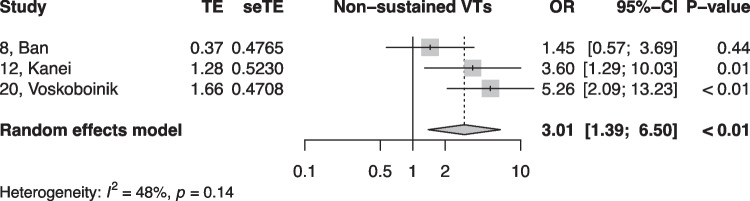
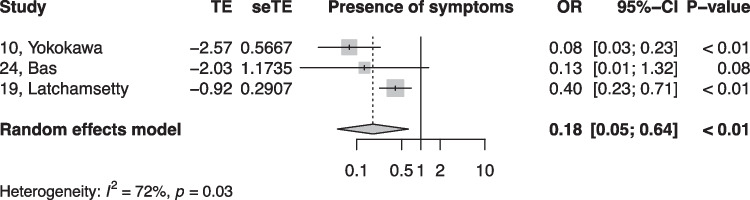
A total of 26 studies (9 prospective and 17 retrospective studies) involving 16,764,641 patients were analyzed (mean age 55 years, 58% women, mean PVC burden 17%). Eleven RF were suitable for quantitative analysis (≥ 3 occurrences in multivariable model assessing a binary change in left ventricular (LV) function). Among these, age (OR 1.02 per increase in the year of age, 95% CI [1.01, 1.02]), the presence of symptoms (OR 0.18, 95% CI [0.05, 0.64]), non-sustained ventricular tachycardias (VT) (OR 3.01, 95% CI [1.39, 6.50]), LV origin (OR 2.20, 95% CI [1.14, 4.23]), epicardial origin (OR 4.72, 95% CI [1.81, 12.34]), the presence of interpolation (OR 4.93, 95% CI [1.66, 14.69]), PVC duration (OR 1.05 per ms increase in QRS-PVC duration [1.004; 1.096]), and PVC burden (OR 1.06, 95% CI [1.04, 1.08]) were all significantly associated with PVC-CM.
Conclusions
In this meta-analysis, the most consistent risk factors for PVC-CM were age, non-sustained VT, LV, epicardial origin, interpolation, and PVC burden, whereas the presence of symptoms significantly reduced the risk. These findings help tailor stringent follow-up of patients presenting with frequent PVCs and normal LV function.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10840-022-01421-8.
Keywords: Premature ventricular contractions, Ventricular arrhythmias, PVC-induced cardiomyopathy, Hear failure
Introduction
Premature ventricular complex-induced cardiomyopathy (PVC-CM) is defined as the development of left ventricular dysfunction (left ventricular ejection fraction (LVEF) of < 50%) caused solely by frequent PVCs [1]. Superimposed PVC-CM can be defined as worsening of LVEF by at least 10% due to frequent PVCs in a previously known CM [1]. Currently, diagnosis of PVC-induced CM can only be made during follow-up, by showing documentation of complete LVEF recovery in absence of PVCs after successful treatment [2].
Clinical studies have found that a high PVC burden is associated with an increased risk of systolic heart failure (HF) (hazard ratio [HR]: 1.48 to 1.8) [3, 4]. Two main studies have shown that PVC burden > 16% and 24% best identifies patients with a diagnosis of PVC-CM [5, 6]. Nevertheless, some patients do not develop CM even with a high PVC burden, whereas other patients develop CM with a burden as low as 6% [7]. Thus, it is likely that other patients’ characteristics and/or PVC features besides PVC burden play a role in the pathophysiology of PVC-CM. Multiple predictors of PVC-CM were described including male sex, lack of symptoms or duration of palpitations [8], variability of PVC coupling interval (dispersion) [9], interpolation of PVCs [10], QRS duration of PVC > 150 ms [11], or epicardial origin [12].
Prior studies investigating risk factors for PVC-CM were retrospective and were not designed with the main objective of assessing these RFs [3–8, 11, 12]. In addition, the assessed study populations were very heterogeneous and often the main endpoint was not defined with enough precision. Thus, most predictors have been variably reported and further validation is required.
We therefore conducted a systematic review and meta-analysis of studies addressing clinical, ECG, Holter, or echocardiographic risk factors able to differentiate patients having a PVC-induced CM from other forms of CM.
Methods
This systematic review and meta-analysis received approval from the ethics committee and was registered on PROSPERO (CRD42021243622). The reporting of our results was done according to the PRISMA statement about systematic reviews and meta-analyses [13] (Supplemental Table 1) and followed the latest guidelines about reporting systematic reviews and meta-analyses of prognostic factors studies [14].
Data sources and search
A comprehensive systematic search was conducted in PubMed, MEDLINE, and Embase by combining keywords synonyms of PVC, heart failure, and risk factors as detailed in the Supplemental appendix. The study registry Clinicaltrial.gov was manually searched using the same terms. The search was conducted once on February 27, 2021, accounting for all articles published between January 1, 2000, and February 27, 2021.
Study selection
Studies that met the following pre-specified criteria were included: (1) RCTs, prospective, or retrospective observational studies and registers; (2) with at least 50 patients total (with and without PVC-CM); (3) assessing adult patients with at least part of the cohort diagnosed with PVC-CM and at least part of the cohort presenting with PVCs; (4) investigating risk factors for the development of PVC-CM (which were not defined beforehand); (5) reporting summary statistics such as regression coefficients, odds ratios (OR) or HR; (6) assessing the incidence, prevalence, or recovery of heart failure thought to be related to PVCs or the change in ejection fraction (EF) due to the presence, increase, or reduction of PVCs; (7) providing either time-to-event data or cross-sectional data; and (8) providing at least one adjusted (multivariable) risk-factor model.
Endpoints
The primary endpoint of this meta-analysis was the quantitative meta-analysis of risk factors for the development of PVC-CM. We pre-defined that risk factors should be reported in at least 3 different studies with a compatible definition in order to allow for a meaningful quantitative summary.
Secondary endpoints were either the qualitative analysis of risk factors reported in ≥ 3 different studies or important study characteristics, such as (1) the prevalence of comprehensive work-up to ensure patients diagnosed with PVC-CM did not present with another cause for heart failure; (2) the differences in the reported definitions of PVC-CM; and (3) the assessment of study quality using the validated QUIPS (Quality in Prognosis Studies) tool [15].
Primary outcome
The primary outcome of this meta-analysis was the presence of PVC-CM, which we pre-defined either as the development, presence, or recovery from heart failure with reduced ejection fraction (HFrEF) in patients with CMP in whom no other cause of heart failure was evident. Further details are available in the supplemental.
Analysis of risk factors
A meta-analysis was conducted on risk factors presenting ≥ 3 times throughout the studies. When continuous risk factors were presented using cutoffs, the exposure per group (above and below the respective cutoff) was derived as recommended in previous dose-exposure meta-analyses and corresponding guidelines [16–19].
Further details regarding the analysis of risk factors are given in the supplemental.
Assessment of study quality
Study quality was assessed according to the QUIPS tool [15] and summarized graphically.
Statistical analysis
The analysis was performed according to the recommendations of the Cochrane Collaboration [20] and the reporting was in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [13] and according to recent guidelines on the conduction of review and meta-analyses of prognostic factor studies [14].
We recorded quantitative measures of baseline characteristics as mean with standard deviation (SD) or median with interquartile range (IQR). To allow for quantitative summaries, we transformed the median with IQR into mean with SDs using a mathematical transformation as proposed in previous research [21].
For the main analysis, in order to increase the number of studies available for the quantitative summary of each risk factor, we summarized odds ratios and hazard ratios as a common measure of risk ratio, as it has been conducted in previous meta-analyses [16, 22].
To allow for the expected heterogeneity in effect measures across studies, summary relative risk estimates and their 95% CIs were estimated from a random effect model [23] that used the inverse variance method as proposed by the metagen package [24], which considers both within- and between-study variation. To estimate the between-study variance, the Tau estimator was calculated according to the DerSimonian-Laird estimator [23, 25]. Statistical heterogeneity among studies was evaluated using the I2 statistic [26].
Details of the dose–response analysis are available in the supplemental.
Significant heterogeneity was defined as an I2 statistic of > 50%.
Evidence for publication bias was assessed for PVC burden graphically using contour-enhanced funnel plots [27] and the Egger test.
The risk of bias within each study was assessed using the QUIPS tool.
All statistical analyses were performed using the Statistical Software “R” (R Foundation for Statistical Computing, Vienna, Austria). P values < 0.05 were considered as significant.
Results
Selected studies
A total of 1567 studies were identified and 1540 were excluded. There were 65 full-text publications reviewed, of which 39 were excluded: 31 studies were based on the same cohorts (mostly representing abstracts of otherwise available complete studies) and 8 studies did not provide risk factors of interest or appropriate statistics. This resulted in 26 studies included in the present systematic review and meta-analysis [5, 7–12, 28–46] (Fig. 1).
Fig. 1.
Study selection chart flow
Baseline study characteristics are presented in Table 1. The included studies reported data on patients treated between 1989 and 2019. They consisted of 9 prospective and 17 retrospective studies. One of the retrospective studies was a re-analysis of a register (the California Health Care Cost and Utilization Project (CHCCUP)) evaluating 16,757,903 patients that was qualitatively analyzed but was eventually excluded from the meta-analysis because of the bias caused by its extreme weight. The 25 other studies provided a total of 6738 patients.
Table 1.
Study baseline characteristics
| Study number | Main author | Abstract or full study | Study begin | Study end | Study duration (years) | Type | Country | Centers | Number of patients recruited | Number of patient analyzed | Is this a re-analysis of a previous trial? | Previous trial |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Altıntaş | Full study | 2019–01-01 | 2019–05-01 | 0.3 | Prospective study: cohort study | Turkey | Multicentric | 341 | 341 | No | |
| 2 | Sadron | Full study | 2003–01-01 | 2012–01-01 | 9.0 | Retrospective study: case–control study | International | Multicentric | 168 | 168 | No | |
| 3 | Lee | Full study | 2011–01-01 | 2017–01-01 | 6.0 | Retrospective study: cohort study | Australia | Unknown | 152 | 152 | No | |
| 4 | Penela | Full study | Prospective study: cohort study | International | Multicentric | 70 | 70 | No | ||||
| 5 | Park | Full study | 2000–01-01 | 2015–07-01 | 15.5 | Retrospective study: cohort study | Japan | Multicentric | 801 | 180 | No | |
| 6 | Agarwal | Full study | 2005–01-01 | 2009–12-31 | 5.0 | Retrospective study: cohort study | USA | Multicentric | 16,800,000 | 16,757,903 | Yes | California Healthcare Cost and Utilization Project |
| 7 | Dukes | Full study | 1989–01-01 | 2000–01-01 | 11.0 | Prospective study: cohort study | USA | Multicentric | 1429 | 1139 | Yes | Cardiovascular Health Study |
| 8 | Ban | Full study | Prospective study: cohort study | Korea | Monocentric | 127 | 127 | No | ||||
| 9 | Yokokawa | Full study | Prospective study: cohort study | USA | Monocentric | 315 | 294 | No | ||||
| 10 | Yokokawa | Full study | 1999–04-01 | 2010–12-31 | 11.8 | Retrospective study: cohort study | USA | Monocentric | 241 | 241 | No | |
| 11 | Baman | Full study | Retrospective study: cohort study | USA | Monocentric | 174 | 174 | No | ||||
| 12 | Kanei | Full study | 2001–01-01 | 2006–08-30 | 5.7 | Retrospective study: cohort study | Japan | Monocentric | 429 | 108 | No | |
| 13 | Kawamura | Full study | 2007–01-01 | 2013–08-30 | 6.7 | Retrospective study: cohort study | USA | Monocentric | 214 | 214 | No | |
| 14 | Mountantonakis | Full study | Retrospective study: cohort study | USA | Monocentric | 69 | 69 | No | ||||
| 15 | Olgun | Full study | Retrospective study: cohort study | USA | Monocentric | 51 | 51 | No | ||||
| 16 | Yokokawa | Abstract | Prospective study: cohort study | USA | Monocentric | 197 | 197 | No | ||||
| 17 | Blaye-Félice | Abstract | Prospective study: case–control study | International | Multicentric | 168 | 168 | No | ||||
| 18 | Yang | Full study | 2005–06-28 | 2013–06-18 | 8.0 | Prospective study: case–control study | USA | Multicentric | 5289 | 264 | No | |
| 19 | Latchamsetty | Full study | 2004–01-01 | 2013–01-01 | 9.0 | Retrospective study: cohort study | International | Multicentric | 1185 | 1185 | No | |
| 20 | Voskoboinik | Full study | 2012–01-01 | 2019–10-01 | 7.7 | Retrospective study: cohort study | International | Multicentric | 206 | 206 | No | |
| 21 | Azizi | Abstract | 2011–01-01 | 2017–01-01 | 6.0 | Prospective study: case–control study | Monocentric | 204 | 130 | No | ||
| 22 | Yamada | Full study | 2010–01-01 | 2015–01-01 | 5.0 | Retrospective study: case–control study | International | Multicentric | 130 | 130 | No | |
| 23 | Hamon | Full study | 2011–05-01 | 2013–06-01 | 2.1 | Retrospective study: cohort study | International | Multicentric | 107 | 107 | No | |
| 24 | Bas | Full study | 2005–11-01 | 2011–09-01 | 5.8 | Retrospective study: cohort study | USA | Monocentric | 107 | 107 | No | |
| 25 | Gunda | Full study | 2014–11-01 | 2016–10-01 | 1.9 | Retrospective study: cohort study | USA | Monocentric | 846 | 846 | No | |
| 26 | Del | Full study | 2005–11-01 | 2008–07-01 | 2.7 | Retrospective study: cohort study | USA | Monocentric | 70 | 70 | No |
Further details regarding inclusion and exclusion criteria for each study and definitions of both PVC-CM and PVCs are presented in Supplemental Table 3. Fifteen of 26 (57.7%) studies provided a definition of PVC-CM: the CMP was mostly defined as an LVEF < 50% and 9/26 (34.6%) studies took a time component into account (e.g., normalization or increase in the EF over time). The requirement for LVEF improvement in the PVC-CM definition varied from 10 to 15% in these studies.
Baseline patient characteristics
Often, several groups were analyzed in each study, which did not always report data for the overall cohort. The analyzed groups are presented in Table 2. In summary, the overall patient population was rather young (weighted mean age of 50.2 years old, 55.0 years old when excluding data from the predominant CHCCUP study) and with a weighted mean PVC burden of 16.5% (not reported in the CHCCUP study). The weighted mean percentage of women in the overall analyzed dataset was 57.6%, which decreased to 44.2% when excluding data from the CHCCUP. In a significant proportion of the studies and reported groups, there was no described attempt to assess for the presence of underlying structural heart disease or this detail was not reported (8/26 studies, Supplemental Tables 4 and 5).
Table 2.
Baseline characteristics of the patient groups in the 26 selected studies
| Study number | Group number | Name | Patient number | Diagnosis of arrhythmia | Diagnosis of HF | PVC-CMP | SHD | Age | LVEF | PVC burden | % women | % men |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Overall cohort | 341 | All | Part | Some | None | 50 ± 6 | 60 ± 2 | 10 ± 3 | 40.8 | 50.4 |
| 2 | 1 | Overall cohort | 168 | All | Part | Some | Some | 55 ± 15 | 48 ± 15 | 22 ± 13 | 38.1 | 61.9 |
| 2 | 2 | PVC-CMP group | 96 | All | All | Some | Some | 53 ± 16 | 38 ± 10 | 26 ± 12 | 26.0 | 74.0 |
| 2 | 3 | Control group without PVC-CMP | 72 | All | None | None | None | 56 ± 15 | 62 ± 7 | 17 ± 22 | 54.2 | 45.8 |
| 3 | 1 | Cardiomyopathy group (LVEF < 50%) | 54 | All | All | Unknown | Some | 59 ± 15 | 39 ± 3 | 30 ± 6 | 25.9 | 74.1 |
| 3 | 2 | Control group with LVEF > 50% | 98 | All | None | None | None | 50 ± 16 | 59 ± 2 | 19 ± 5 | 60.2 | 39.8 |
| 4 | 1 | Overall cohort | 70 | All | All | Some | Some | 58 ± 11 | 34 ± 9 | 24 ± 4 | 17.1 | 82.9 |
| 4 | 2 | Myocardial scar | 29 | All | All | Some | Some | 61 ± 8 | 35 ± 9 | 24 ± 4 | 3.4 | 96.6 |
| 4 | 3 | No myocardial scar | 41 | All | All | Some | Some | 56 ± 12 | 33 ± 9 | 26 ± 3 | 26.8 | 73.2 |
| 5 | 1 | Symptomatic with PVC cardiomyopathy | 28 | All | All | All | None | 52 ± 13 | 35 ± 8 | 29 ± 16 | 25.0 | 75.0 |
| 5 | 2 | Symptomatic without cardiomyopathy | 116 | All | None | None | None | 49 ± 15 | 59 ± 6 | 21 ± 15 | 56.0 | 44.0 |
| 5 | 3 | Asymptomatic with PVC cardiomyopathy | 24 | All | All | All | None | 58 ± 15 | 34 ± 9 | 31 ± 10 | 16.7 | 83.3 |
| 5 | 4 | Asymptomatic without cardiomyopathy | 12 | All | None | None | None | 55 ± 16 | 56 ± 8 | 28 ± 12 | 41.7 | 58.3 |
| 6 | 1 | PVC diagnosis | 35,817 | All | None | None | None | 66 ± 17 | 48.9 | 51.1 | ||
| 6 | 2 | No PVC diagnosis | 16,722,086 | None | None | None | None | 50 ± 19 | 57.7 | 42.3 | ||
| 7 | 1 | Below or equal to the median of percent PVC | 587 | Part | None | None | None | 70 ± 2 | 63.7 | 36.3 | ||
| 7 | 2 | Above the median of percent PVCs | 552 | All | None | Unknown | None | 71 ± 2 | 51.3 | 48.7 | ||
| 8 | 1 | LV dysfunction (LVEF < 50%) | 28 | All | All | Some | Unknown | 48 ± 14 | 44 ± 5 | 31 ± 11 | 39.3 | 60.7 |
| 8 | 2 | No LV dysfunction (LVEF > 50%) | 99 | All | None | Unknown | None | 43 ± 13 | 57 ± 3 | 22 ± 10 | 66.7 | 33.3 |
| 9 | 1 | Overall cohort | 294 | All | Part | Some | None | 48 ± NA | 52 ± 12 | 19 ± 14 | 53.4 | 46.6 |
| 9 | 2 | Reversible PVC-induced cardiomyopathy | 113 | All | Part | All | None | 49 ± 15 | 40 ± 10 | 27 ± 12 | 36.3 | 63.7 |
| 9 | 3 | No PVC-induced cardiomyopathy | 181 | All | Part | None | None | 48 ± 13 | 60 ± 4 | 14 ± 12 | 63.5 | 36.5 |
| 10 | 1 | Cardiomyopathy | 76 | All | All | All | None | 48 ± 16 | 36 ± 9 | 28 ± 12 | 32.9 | 67.1 |
| 10 | 2 | No cardiomyopathy | 165 | All | Part | None | None | 48 ± 13 | 59 ± 5 | 15 ± 13 | 61.2 | 38.8 |
| 11 | 1 | No cardiomyopathy | 117 | All | Part | None | Unknown | 48 ± 12 | 59 ± 4 | 14 ± 12 | 55.6 | 44.4 |
| 11 | 2 | Cardiomyopathy | 57 | All | All | Some | Unknown | 49 ± 12 | 35 ± 9 | 33 ± 14 | 38.6 | 61.4 |
| 12 | 1 | < 1000 PVC/24 h | 24 | All | Part | Unknown | Unknown | 47 ± 16 | 29.2 | 20.8 | ||
| 12 | 2 | 1000–10,000 PVC/24 h | 55 | All | Part | Unknown | Unknown | 52 ± 17 | 63.6 | 36.4 | ||
| 12 | 3 | > 10,000 PVC/24 h | 29 | All | Part | Unknown | Unknown | 48 ± 15 | 69.0 | 31.0 | ||
| 13 | 1 | LV dysfunction | 51 | All | All | Some | Unknown | 50 ± 13 | 42 ± 5 | 19 ± 6 | 49.0 | 51.0 |
| 13 | 2 | No LV dysfunction | 163 | All | None | Unknown | None | 46 ± 14 | 62 ± 9 | 15 ± 11 | 60.7 | 39.3 |
| 14 | 1 | Overall cohort | 69 | All | All | Unknown | None | 51 ± 16 | 35 ± 9 | 37.7 | 62.3 | |
| 14 | 2 | Patients without pre-existing cardiomyopathy | 49 | All | All | Unknown | None | 50 ± 15 | 37 ± 8 | 34.7 | 65.3 | |
| 14 | 3 | Patients with pre-existing cardiomyopathy | 20 | All | All | Unknown | None | 55 ± 16 | 28 ± 7 | 45.0 | 55.0 | |
| 15 | 1 | Patients with pre-existing cardiomyopathy | 21 | All | All | All | None | 50 ± 15 | 37 ± 10 | 30 ± 11 | 33.3 | 66.7 |
| 15 | 2 | Patients without pre-existing cardiomyopathy | 30 | All | None | None | None | 47 ± 16 | 59 ± 7 | 14 ± 15 | 40.0 | 60.0 |
| 15 | 3 | Patients with interpolation | 20 | All | Part | Some | None | 28 ± 12 | ||||
| 15 | 4 | Patients without interpolation | 31 | All | Part | Some | None | 15 ± 15 | ||||
| 16 | 1 | Overall cohort | 197 | All | Part | Some | Unknown | 48 ± 14 | 54.3 | 45.7 | ||
| 16 | 2 | Reduced LVEF | 56 | All | Part | All | Unknown | 15 ± 13 | ||||
| 16 | 3 | Normal LVEF | 141 | All | Part | None | Unknown | 29 ± 12 | ||||
| 17 | 1 | PVC-CMP group | 93 | All | None | All | None | 58 ± 14 | 25.8 | 74.2 | ||
| 17 | 2 | Non PVC-CMP control group | All | None | Unknown | None | ||||||
| 17 | 3 | Overall cohort | 168 | Part | None | Some | None | 27 ± 12 | ||||
| 18 | 1 | High burden PVC group | 66 | All | Part | Unknown | Some | 64 ± 16 | 53 ± 12 | 42.4 | 57.6 | |
| 18 | 2 | Control group | 198 | Part | Part | Unknown | Some | 58 ± 20 | 63 ± 10 | 56.1 | 43.9 | |
| 19 | 1 | Overall cohort | 1185 | Part | None | Some | None | 52 ± 15 | 55 ± 10 | 20 ± 13 | 54.9 | 45.1 |
| 20 | 1 | Derivation cohort with PVC patients | 206 | All | Part | Some | Unknown | 65 ± 16 | 57 ± 12 | 12 ± 6 | 38.3 | 61.7 |
| 20 | 2 | First validation cohort with PVC patients | All | None | None | None | ||||||
| 20 | 3 | Second validation cohort with PVC patients | 516 | All | None | None | None | 56 ± 17 | 63 ± 4 | 20 ± 10 | 54.5 | 45.5 |
| 21 | 1 | EF under 50% | All | All | Some | None | ||||||
| 21 | 2 | PVC-CMP | 15 | All | All | All | None | 60 ± 19 | 32 ± 17 | 13.3 | 86.7 | |
| 21 | 3 | Control group | 103 | All | None | None | None | 15 ± 13 | ||||
| 22 | 1 | PVC-induced cardiomyopathy | 25 | All | All | All | None | 47 ± 13 | 42 ± 5 | 24 ± 15 | 56.0 | 44.0 |
| 22 | 2 | Normal LVEF | 105 | All | None | None | None | 43 ± 12 | 60 ± 7 | 15 ± 11 | 63.8 | 36.2 |
| 23 | 1 | Overall cohort | 107 | All | Part | Unknown | Some | 56 ± 16 | 48 ± 14 | 23 ± 12 | 35.5 | 64.5 |
| 23 | 2 | Epicardial origin | 25 | All | Part | Unknown | Some | 54 ± 14 | 42 ± 10 | 25 ± 10 | 20.0 | 80.0 |
| 23 | 3 | Endocardial origin | 82 | All | Part | Unknown | Some | 56 ± 16 | 50 ± 15 | 23 ± 12 | 41.5 | 58.5 |
| 23 | 4 | With PVC-CMP | 58 | All | Part | All | Some | 56 ± 15 | 38 ± 9 | 28 ± 10 | 22.4 | 77.6 |
| 23 | 5 | Without PVC-CMP | 44 | All | None | None | None | 56 ± 16 | 62 ± 7 | 16 ± 10 | 56.8 | 43.2 |
| 24 | 1 | With CM | 43 | All | All | All | None | 48 ± 16 | 38 ± 5 | 28 ± 12 | 25.6 | 74.4 |
| 24 | 2 | Without CM | 64 | All | None | None | None | 47 ± 13 | 58 ± 4 | 20 ± 10 | 59.4 | 40.6 |
| 25 | 1 | PVC burden < 1% | 599 | All | Part | Unknown | Unknown | 53 ± 9 | 1 ± 0 | 13.9 | 86.1 | |
| 25 | 2 | PVC burden 1–2.1% | 82 | All | Part | Unknown | Unknown | 50 ± 10 | 1 ± 0 | 8.5 | 91.5 | |
| 25 | 3 | PVC burden 2.2–4.9% | 81 | All | Part | Unknown | Unknown | 47 ± 14 | 4 ± 0 | 7.4 | 92.6 | |
| 25 | 4 | PVC burden 5–24% | 83 | All | Part | Unknown | Unknown | 45 ± 14 | 14 ± 3 | 4.8 | 95.2 | |
| 25 | 5 | LVEF > 50% | 331 | All | None | None | None | 2 ± 4 | 11.8 | 88.2 | ||
| 25 | 6 | LVEF 42.5–49.6% | 38 | All | All | Unknown | Unknown | 2 ± 4 | 2.6 | 97.4 | ||
| 25 | 7 | LVEF 30–40% | 32 | All | All | Unknown | Unknown | 3 ± 6 | 12.5 | 87.5 | ||
| 25 | 8 | LVEF 12.5–27.50% | 34 | All | All | Unknown | Unknown | 6 ± 10 | 0.0 | 100.0 | ||
| 26 | 1 | EF < 50% | 17 | All | All | All | Unknown | 42 ± 17 | 38 ± 9 | 29 ± 15 | 41.2 | 58.8 |
| 26 | 2 | EF ≥ 50% | 53 | All | None | None | None | 39 ± 18 | 59 ± 6 | 17 ± 14 | 62.3 | 37.7 |
Assessment of outcomes
Most of the studies assessed the presence of PVC-CM (17/26), the recovery of LVEF after PVC-CM 4/27 (defined as a binary variable), or the worsening of LVEF suspected to be due to PVC-CM 2/27 (also defined as a binary variable). We conducted a pooled analysis for these three outcomes, as these are solely different ways to define a PVC-CM. Studies reporting continuous LVEF change over time (3/26) were rare (Table 3).
Table 3.
Derived models in the different studies and recorded outcomes and risk factors
| Study ID | First author | Uni- vs multivariable | Outcome | Summarized outcome | Type of model | Risk factors assessed |
|---|---|---|---|---|---|---|
| 1 | Altıntaş | Multivar | LVEF (continuous) | LVEF (continuous) | Linear regression | PVC burden, interpolation, age, sex, PVC type: outflow origin, PVC type: duration, coupling interval, PVC type: morphology, QRS duration |
| 2 | Sadron | Univar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Coupling interval, QRS duration, PVC burden, sex, PVC type: morphology, age, PVC type: origin, palpitations |
| 2 | Sadron | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Coupling interval, QRS duration, PVC burden, sex, PVC type: morphology, age, PVC type: origin, palpitations |
| 3 | Lee | Univar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Age, sex, coupling interval, PVC type: outflow origin, PVC type: duration, PVC burden, symptoms |
| 3 | Lee | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Age, sex, coupling interval, PVC type: outflow origin, PVC type: duration, PVC burden, symptoms |
| 4 | Penela | Univar | Recovery of LVEF (categorical) | LVEF change | Logistic regression | Sex, age, EF, PVC burden, PVC type: origin, PVC type: duration, LVED, SHD, PVC type: morphology |
| 5 | Park | Univar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Sex, PVC type: duration, QRS duration, PVC burden, PVC type: origin |
| 5 | Park | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Sex, PVC type: duration, QRS duration, PVC burden, PVC type: origin |
| 6 | Agarwal | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Cox hazard proportional model | Sex, race, age, PVC burden, HTN, DM, CAD |
| 7 | Dukes | Univar | Worsening of LVEF (categorical) | LVEF change | Logistic regression | PVC burden |
| 7 | Dukes | Multivar | Worsening of LVEF (categorical) | LVEF change | Logistic regression | PVC burden |
| 8 | Ban | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | PVC burden, non-sustained VT |
| 9 | Yokokawa | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | PVC burden, QRS duration, PVC type: origin, sex |
| 10 | Yokokawa | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Symptoms, PVC burden |
| 11 | Baman | Univar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Sex, PVC burden, PVC type: outflow origin, PVC type: morphology, non-sustained VT, PVC type: origin |
| 11 | Baman | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Sex, PVC burden, PVC type: outflow origin, PVC type: morphology, non-sustained VT, PVC type: origin |
| 12 | Kanei | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Non-sustained VT |
| 13 | Kawamura | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Age, coupling interval, PVC burden, QRS duration, other |
| 14 | Mountantonakis | Univar | Recovery of LVEF (categorical) | LVEF change | Cox hazard proportional model | Age, SHD, EF, PVC type: morphology |
| 14 | Mountantonakis | Multivar | Recovery of LVEF (categorical) | LVEF change | Cox hazard proportional model | Age, SHD, EF, PVC type: morphology |
| 15 | Olgun | Univar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | PVC burden, interpolation, BB |
| 15 | Olgun | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | PVC burden, interpolation, BB |
| 16 | Yokokawa | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Cox hazard proportional model | PVC burden |
| 17 | Blaye-Félice | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | PVC type: morphology, PVC type: outflow origin, PVC type: origin, PVC burden |
| 18 | Yang | Multivar | LVEF (continuous) | LVEF (continuous) | Logistic regression | PVC burden, QRS duration |
| 19 | Latchamsetty | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Sex, PVC burden, symptoms, age, PVC type: origin, PVC type: outflow origin, CAD, HTN, PVC type: morphology |
| 20 | Voskoboinik | Univar | Worsening of LVEF (categorical) | LVEF change | Logistic regression | Non-sustained VT, sex, coupling interval, PVC type: origin, PVC burden, PVC type: duration, CAD, age, HTN, PVC type: morphology |
| 20 | Voskoboinik | Multivar | Worsening of LVEF (categorical) | LVEF change | Logistic regression | Non-sustained VT, sex, coupling interval, PVC type: origin, PVC burden, PVC type: duration, CAD, age, HTN, PVC type: morphology |
| 21 | Azizi | Multivar | Recovery of LVEF (categorical) | LVEF change | Logistic regression | PVC burden |
| 22 | Yamada | Univar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | PVC burden, QRS duration, non-sustained VT, interpolation, coupling interval, Q wave amplitude in aVL |
| 22 | Yamada | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | PVC burden, QRS duration, non-sustained VT, interpolation, coupling interval, Q wave amplitude in aVL |
| 23 | Hamon | Multivar | Recovery of LVEF (categorical) | LVEF change | Logistic regression | Sex, SHD, interpolation, coupling interval, PVC type: origin, QRS duration, PVC burden, PVC type: duration, palpitations |
| 24 | Bas | Univar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Sex, PVC burden, PVC type: morphology, interpolation, symptoms, PVC type: duration |
| 24 | Bas | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | Sex, PVC burden, PVC type: morphology, interpolation, symptoms, PVC type: duration |
| 25 | Gunda | Univar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Linear regression | PVC burden |
| 25 | Gunda | Multivar | Presence of a PVC-induced cardiomyopathy (categorical) | LVEF change | Logistic regression | PVC burden |
| 26 | Del | Multivar | LVEF (continuous) | LVEF (continuous) | Linear regression | PVC burden, non-sustained VT, PVC type: duration, PVC type: morphology, palpitations |
Assessed risk factors
Table 4 presents the occurrence of all risk factors throughout the selected studies and the occurrence of reporting which were suitable for quantitative analysis (≥ 3 occurrences in multivariable model assessing a binary change in LV function).
Table 4.
Candidate risk factors proposed in the 26 studies and their relative occurrence (either overall or in multivariable models assessing a binary change in LVEF—either an improvement, worsening in EF, or the development of a PVC-CMP—suitable for quantitative summary analysis)
| Candidate risk factor | Occurrence in selected studies | Occurrence as multivariable model assessing binary change in LV function |
|---|---|---|
| PVC burden | 24 | 20 |
| Sex | 13 | 7 |
| PVC type: origin | 11 | 6 |
| Age | 10 | 3 |
| PVC type: morphology | 10 | 3 |
| PVC type: duration | 8 | 4 |
| QRS duration | 8 | 5 |
| Coupling interval | 7 | 3 |
| Non-sustained VT | 6 | 3 |
| Interpolation | 5 | 3 |
| CAD | 4 | 2 |
| HTN | 4 | 2 |
| Symptoms | 4 | 3 |
| EF | 3 | 1 |
| Palpitations | 3 | 2 |
| SHD | 3 | 2 |
| Symptom duration | 3 | – |
| Acute successful ablation | 2 | |
| PVC burden reduction | 2 | |
| Antiarrhythmic drug use | 1 | |
| Atrial fibrillation | 1 | |
| Beta-blocker therapy | 1 | |
| BNP (pg mL−1) | 1 | |
| Body mass index > 30 | 1 | |
| Chronic ablation outcome | 1 | |
| Coefficient of variation | 1 | |
| Coronary artery bypass graft | 1 | |
| Coupling interval dispersion | 1 | |
| Diabetes mellitus | 1 | |
| Duration of palpitations | 1 | |
| Fascicular PVC | 1 | |
| First-degree family history of sudden death | 1 | |
| History of dizziness | 1 | |
| History of myocardial infarction | 1 | |
| Inferior axis | 1 | |
| Left bundle branch block | 1 | |
| LVED | 1 | |
| Mean creatinine | 1 | |
| Myocardial scar (g) in MRI | 1 | |
| Peak deflection index | 1 | |
| PVC-CMP index | 1 | |
| Q wave amplitude in aVL | 1 | |
| Q wave ratio in leads aVL/aVR | 1 | |
| Race | 1 | |
| Residual PVC burden after ablation | 1 | |
| Retrograde P wave | 1 | |
| Superiorly directed PVC axis | 1 |
Supplemental table 6 presents the risk factors analyzed by each study. The exact definitions of each risk factor, as provided by the individual studies, are presented in the supplemental.
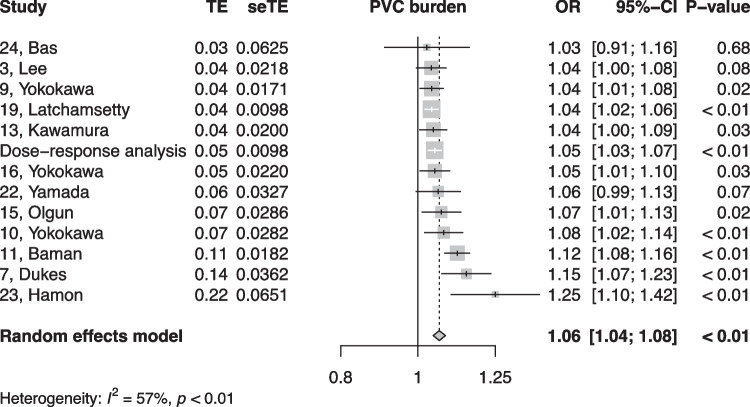
PVC burden was the most commonly analyzed risk factor (24/26 studies, 20/26 studies for quantitative summary), followed by sex (13/26), PVC origin (11/26), PVC and morphology (10/26), and PVC and QRS duration (each in 8/26 studies). Only few other risk factors (age, coupling interval, non-sustained VTs, interpolation, and the presence of symptoms) were investigated in ≥ 3 studies and suitable for quantitative summary. Further investigated risk factors were baseline LVEF, coupling interval, polymorphic PVCs, and outflow tract origins. These risk factors did not appear often enough (< 3 appearances) or were differently defined, hence not suitable for quantitative summary.
Quantitative associations of risk factors with PVC-CM
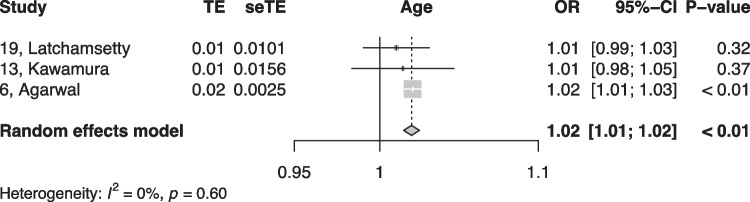
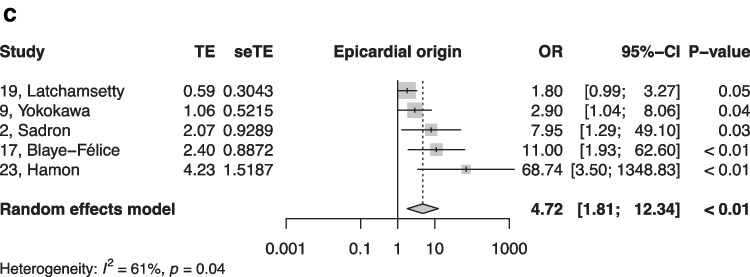
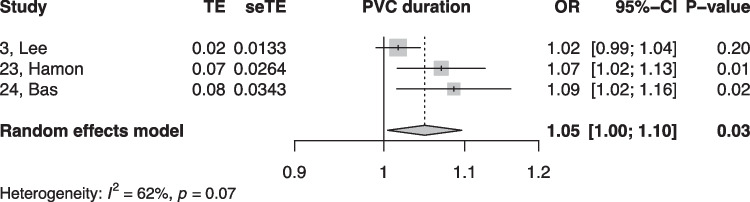
When summarized quantitatively, age (OR 1.02 per increase in year of age, 95% CI [1.01, 1.02]), the presence of symptoms (OR 0.18, 95% CI [0.05, 0.64]), non-sustained VTs (OR 3.01, 95% CI [1.39, 6.50]), LV origin (OR 2.20, 95% CI [1.14, 4.23]), epicardial origin (OR 4.72, 95% CI [1.81, 12.34]), the presence of interpolation (OR 4.93, 95% CI [1.66, 14.69]), PVC burden (OR 1.06 per percent increase in burden, 95% CI [1.04, 1.08]), and PVC duration (OR 1.05 per ms increase in QRS-PVC duration [1.004; 1.096]) were all significantly associated with PVC-CM (Figs. 2, 3, 4, 5, 6, 7, 8, and 9). Coupling interval, polymorphic PVCs, outflow tract origin, sex, and QRS duration did not display a significant association (Supplemental Fig. 1).
Fig. 2.
Random effects model showing the overall effect of age on the risk of developing PVC-CM. TE, estimate of treatment effect; seTE, standard error of treatment estimate; OR, odds ratio; CI, confidence interval
Fig. 3.
Random effects model showing the overall effect of overall PVC burden on the risk of developing PVC-CM. TE, estimate of treatment effect; seTE, standard error of treatment estimate; OR, odds ratio; CI, confidence interval
Fig. 4.
Random effects model showing the overall effect of epicardial origin of the PVC on the risk of the developing PVC-CM. TE, estimate of treatment effect; seTE, standard error of treatment estimate; OR, odds ratio; CI, confidence interval
Fig. 5.
Random effects model showing the overall effect of interpolated PVCs on the risk for PVC-CM. TE, estimate of treatment effect; seTE, standard error of treatment estimate; OR, odds ratio; CI, confidence interval
Fig. 6.
Random effects model showing the overall effect of left ventricular origin of the PVC on the risk of the developing PVC-CM. TE, estimate of treatment effect; seTE, standard error of treatment estimate; OR, odds ratio; CI, confidence interval
Fig. 7.
Random effects model showing the overall effect of non-sustained ventricular tachycardia on the risk for PVC-CM. TE, estimate of treatment effect; seTE, standard error of treatment estimate; OR, odds ratio; CI, confidence interval
Fig. 8.
Random effects model showing the overall effect of symptoms on the risk for PVC-CM. TE, estimate of treatment effect; seTE, standard error of treatment estimate; OR, odds ratio; CI, confidence interval
Fig. 9.
Random effects model showing the effect of PVC duration (per ms increase in QRS PVC duration) on the risk for PVC-CM. TE, estimate of treatment effect; seTE, standard error of treatment estimate; OR, odds ratio; CI, confidence interval
Dose–response analysis of PVC burden
In the dose–response analysis encompassing 7 studies reporting PVC burden at different cutoffs, there was a highly significant association between increase in PVC burden and an exponential increase in risk for PVC-CM (at 10% PVC burden, beta-coefficient 1.54 [1.3, 1.8], at 20% PVC burden beta-coefficient 1.5 [1.7, 3.6], at 30% PVC burden beta-coefficient 4 [2.3, 7], Fig. 10). A univariate Cochran Q test for residual heterogeneity was highly significant, with an I2 statistic of 89.7%.
Fig. 10.
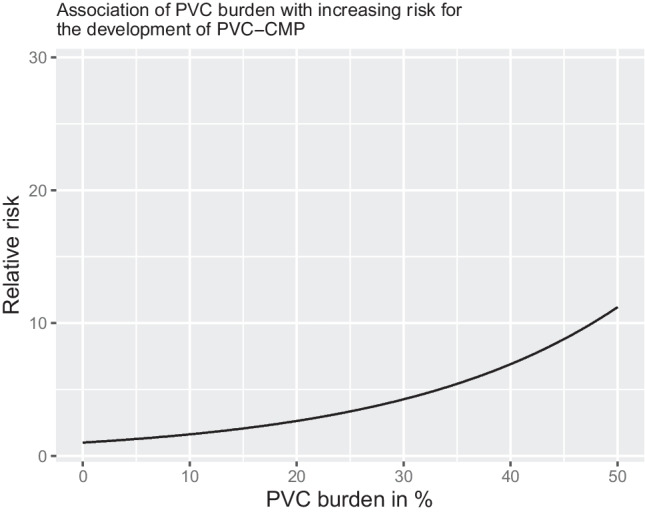
Dose–response plot of PVC burden and association with PVC-CMP. Based on 7 studies reporting PVC burden with a cutoff, a dose–response analysis was conducted. The black line represents the predicted increase in PVC-CMP risk associated with an increase in PVC burden in %. The gray ribbon represents the confidence interval of the prediction
Modification of the risk associated with PVC burden through meta-regression
When assessing the risk modification associated with the publication year or with study quality, older studies and studies with higher quality were associated with a non-significant trend in increased risk for the development of PVC-CM with a growing PVC burden.
The PVC-CM risk associated with PVC burden decreased of 0.28% (− 0.28%, 95% CI [− 1.02%, 0.46%], P = 0.462, Supplemental Fig. 2) with each increase in publication year, meaning that studies published in 2020 displayed a non-significant 2.8% lower risk association of PVC-CM with PVC burden as compared with the studies published in 2010.
Inversely, the PVC-CM risk associated with PVC burden increased of 0.09% (95% CI [− 0.13%, 0.31%], P = 0.413, Supplemental Fig. 3) with each increase in quality point of the summed QUIPS tool, meaning that studies with a low risk of bias (in mean 45 points in the summed QUIPS tool) presented a 2.7% higher risk association of PVC-CM with PVC burden as compared with the studies with high risk of bias (in mean 15 points in the summed QUIPS tool).
Publication bias
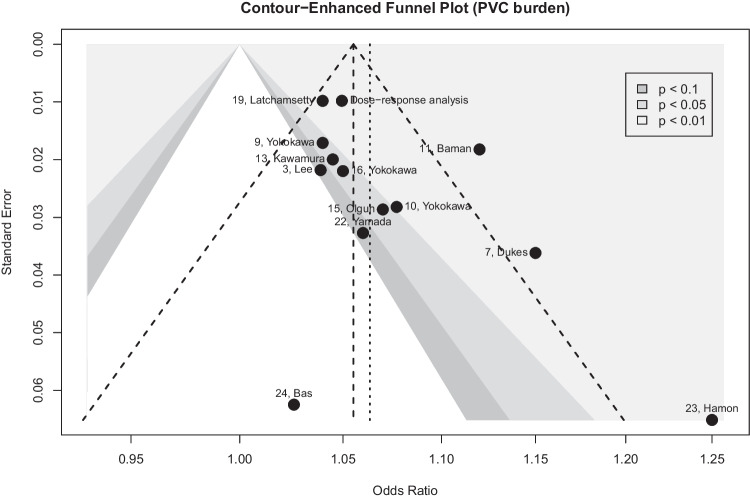
On funnel plot analysis of PVC burden, study distribution was mildly asymmetric (Fig. 11) but the Egger test did not suggest any publication bias (P = 0.07).
Fig. 11.
Assessment of publication bias using a contour-enhanced funnel plot. The contour-enhanced funnel plot represents the different studies reporting estimated for the association between PVC burden (continuous increase in %) and assess the risk for publication bias. The 7 studies reporting a cutoff of PVC burden were summarized beforehand as the “dose–response analysis.” The dotted line represents the overall estimate using all available studies and the dashed line represents a classical funnel plot with the expected distribution of the studies if no publication bias is present. The contour-enhanced funnel plot is centered at 0 (i.e., the value under the null hypothesis of no relationship) and various levels of statistical significance are indicated by the shaded region. The white region corresponds to non-significant P values. Highly significant P values appear in the light gray region
Quality assessment
As presented in Fig. 12, all of the studies presented with at least a moderate risk of bias. The uncontrolled risk of confounding appeared as the most problematic throughout all recorded studies.
Fig. 12.
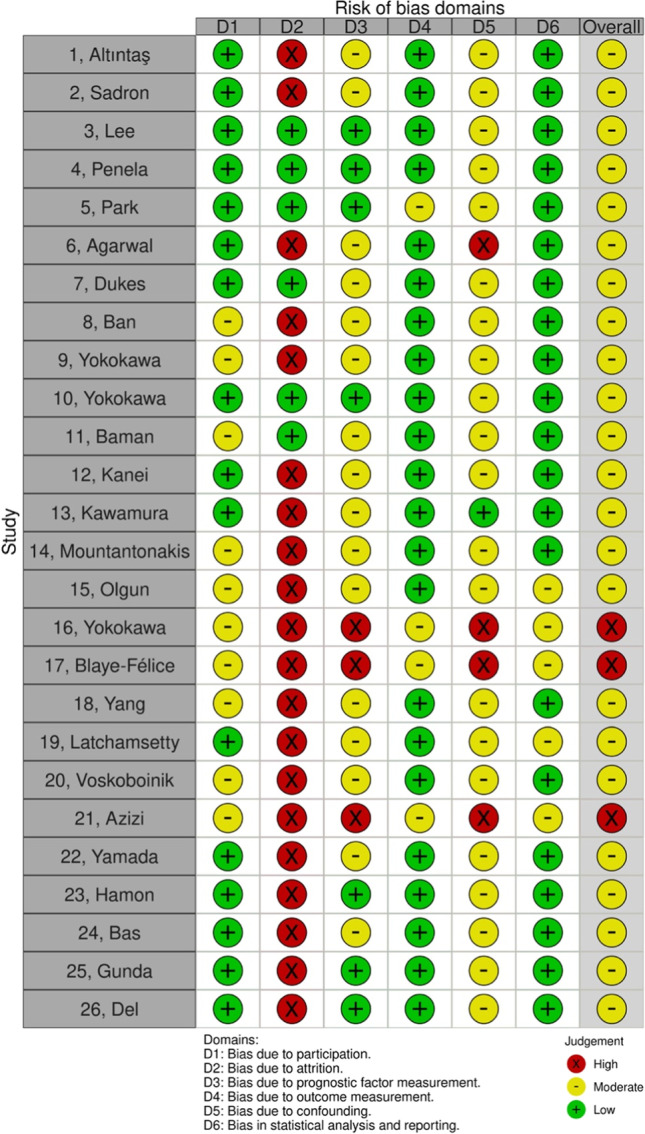
Assessment of study quality. Evaluation of study quality according to the QUIPS tool. Five domains of bias (participation, attrition, prognostic factor measurement, outcome measurement, confounding and statistical analysis and reporting) are represented with the associated risk of bias (high in red, moderate in yellow, and low in green). The overall column represents the mean risk of bias from the 6 domains
Discussion
This systematic review and meta-analysis investigated 26 studies to investigate risk factors associated with the development of PVC-CM. We report four major findings. First, despite screening abstracts published over 30 years of scientific research, only few studies presented a multivariable assessment of risk factors potentially associated with PVC-CM and the quality of the research currently does not allow for definitive conclusion. Second, although many candidate risk factors were proposed by the analyzed studies, only 13 risk factors (age, PVC burden, PVC origin from epicardial, outflow tract or LV, interpolation, non-sustained VTs, presence of symptoms, coupling interval, PVC morphology and duration, QRS duration, and sex) were reported often enough with appropriate statistics to allow for a quantitative summary. Many other predictors remain possible candidates for the risk stratification of PVC-CM development. Third, age, non-sustained VTs, LV and epicardial origin, interpolation, PVC duration, and PVC burden were all associated with an increased risk for PVC-CM, whereas the presence of symptoms significantly reduced the risk. Fourth, there was a clear association between increasing PVC burden and increasing PVC-CM risk. In the dose–response analysis encompassing 7 studies reporting PVC burden at different cutoffs, there was a highly significant association between increase in PVC burden and an increasing risk for PVC-CM. Specifically, per % increase in PVC burden, there was an exponential increase in the absolute risk of PVC-CM. This association was not significantly impacted by the study publication year, suggesting that despite improvements in heart failure treatments and prevention over years, the burden remains an important predictor of PVC-CM development.
To the best of our knowledge, this is the first systematic review and meta-analysis comprehensively assessing the risk factors for the development of PVC-induced cardiomyopathy. The optimal approach to frequent PVCs (> 10% burden) without LV dysfunction, symptoms, or idiopathic ventricular fibrillation is unclear, but patients should probably be monitored every 6–12 months with echocardiography and PVC burden assessment [47]. Therefore, until PVC-induced cardiomyopathy can be predicted, these results help to focus on patients at the highest risk of developing PVC-CM. The role of early rhythm control with catheter ablation or AAD of frequent PVCs without LV dysfunction and symptoms, but risk factors, needs to be defined.
Several studies have confirmed a correlation between a higher PVC burden and development of cardiomyopathy, although no precise burden of PVCs consistently predicts the development of a cardiomyopathy. In this meta-analysis, we found a highly significant association between an increase in PVC burden and increasing risk for PVC-CM.
Limitations
This systematic review and meta-analysis has several limitations. First, the quantitative summary of risk factors we are presenting summarizes different measures of risks (odds and hazard ratios) together. While this has been conducted in previous research and is acknowledged by recent guidelines as a possible necessary simplification [14], this might have biased absolute risk estimated. Second, most of the articles had different definitions for the risk factors. As such, only 15 of the 26 analyzed studies (57%) provided a definition for PVC-CM and only 9 of the 26 (34.6%) assessed the evolution of EF into the model. The latest literature on PVC-CM [2, 48, 49] recommends assessing the temporal course of worsening or recovery of EF over time. Thus, about three fourth of the studies we investigated did not define their main endpoint with enough precision. At the same time, none of the three included studies provided a standardized definition for non-sustained tachycardia, limiting the credibility of the result.
Third, as several studies did not thoroughly assess other underlying heart failure etiologies in their patients collectively, our estimates may have been occasionally confounded by other causes of heart failure. Fourth, as most of the studies providing a PVC burden cutoff only provided two categories, we had to assume a linear trend between PVC exposure and the associated increase in risk (thereby leading to an exponentially growing risk after back-transformation of the log-odds). With more detailed data, quadratic estimations could lead to more accurate dose–response relationship modelling.
Conclusion
In this meta-analysis, the most consistent risk factors for PVC-CM were age, non-sustained VTs, LV and epicardial origin, interpolation, PVC duration, and PVC burden, while the presence of symptoms significantly reduced the risk. These findings help tailor stringent follow-up to patients presenting with frequent PVCs and normal LV function.
Supplementary information
Below is the link to the electronic supplementary material.
Funding
Open access funding provided by University of Basel
Declarations
Ethics approval
The study received approval of the ethics’ committee.
Consent to participate
Not applicable.
Conflict of interest
Sven Knecht has received funding of the “Stiftung für Herzschrittmacher und Elektrophysiologie.”
Michael Kühne reports personal fees from Bayer, personal fees from Böhringer Ingelheim, personal fees from Pfizer BMS, personal fees from Daiichi Sankyo, personal fees from Medtronic, personal fees from Biotronik, personal fees from Boston Scientific, personal fees from Johnson & Johnson, personal fees from Roche, grants from Bayer, grants from Pfizer, grants from Boston Scientific, grants from BMS, grants from Biotronik, and grants from Daiichi Sankyo, all outside the submitted work.
Christian Sticherling is a Member of Medtronic Advisory Board Europe and Boston Scientific Advisory Board Europe, received educational grants from Biosense Webster and Biotronik and a research grant from the European Union’s FP7 program and Biosense Webster, and lecture and consulting fees from Abbott, Medtronic, Biosense-Webster, Boston Scientific, Microport, and Biotronik, all outside the submitted work.
Patrick Badertscher has received research funding from the “University of Basel,” the “Stiftung für Herzschrittmacher und Elektrophysiologie,” the “Freiwillige Akademische Gesellschaft Basel,” and Johnson & Johnson, all outside the submitted work and reports personal fees from Abbott.
Jeanne du Fay de Lavallaz has received research funding from the “University of Basel” and from the “Swiss Heart Foundation.”
Henry Huang has received research funding from Medtronic, educational grants from Medtronic, Biotronik, Abbott, and Boston Scientific, and consulting fees from Biosense-Webster and Cardiofocus.
Michael Gold is a consultant to Boston Scientific and Medtronic, as well as on steering committees with Boston Scientific, Abbott, and Medtronic.
Others have nothing to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bozkurt, B. et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016; 134: e579–e646 Preprint at 10.1161/CIR.0000000000000455 [DOI] [PubMed]
- 2.Huizar JF, Ellenbogen KA, Tan AY, Kaszala K. Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2328–2344. doi: 10.1016/j.jacc.2019.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogun F, et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm. 2007;4:863–867. doi: 10.1016/j.hrthm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Takemoto M, et al. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol. 2005;45:1259–1265. doi: 10.1016/j.jacc.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 5.Baman TS, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 6.Hasdemir, C. et al. Tachycardia-induced cardiomyopathy in patients with idiopathic ventricular arrhythmias: the incidence, clinical and electrophysiologic characteristics, and the predictors. 10.1111/j.1540-8167.2010.01986.x. [DOI] [PubMed]
- 7.Blaye-felice MS, et al. Premature ventricular contraction-induced cardiomyopathy: related clinical and electrophysiologic parameters. Heart Rhythm. 2016;13:103–110. doi: 10.1016/j.hrthm.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Yokokawa M, et al. Relation of symptoms and symptom duration to premature ventricular complex-induced cardiomyopathy. Heart Rhythm. 2012;9:92–95. doi: 10.1016/j.hrthm.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Kawamura M, et al. Coupling interval dispersion and body mass index are independent predictors of idiopathic premature ventricular complex-induced cardiomyopathy. J Cardiovasc Electrophysiol. 2014;25:756–762. doi: 10.1111/jce.12391. [DOI] [PubMed] [Google Scholar]
- 10.Olgun H, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm. 2011;8:1046–1049. doi: 10.1016/j.hrthm.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Yokokawa M, et al. Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm. 2012;9:1460–1464. doi: 10.1016/j.hrthm.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 12.Latchamsetty R, et al. Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC Clin Electrophysiol. 2015;1:116–123. doi: 10.1016/j.jacep.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. The BMJ. 2021; 372. [DOI] [PMC free article] [PubMed]
- 14.Riley, R. D. et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ (Online). 2019;364. [DOI] [PubMed]
- 15.Grooten WJA, et al. Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using QUIPS—aspects of interrater agreement. Diagn Progn Res. 2019;3:1–11. doi: 10.1186/s41512-019-0050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartemink N, Boshuizen HC, Nagelkerke NJD, Jacobs MAM, Van Houwelingen HC. Combining risk estimates from observational studies with different exposure cutpoints: a meta-analysis on body mass index and diabetes type 2. Am J Epidemiol. 2006;163:1042–1052. doi: 10.1093/aje/kwj141. [DOI] [PubMed] [Google Scholar]
- 17.Pandey A, et al. Continuous dose-response association between sedentary time and risk for cardiovascular disease a meta-analysis. JAMA Cardiol. 2016;1:575–583. doi: 10.1001/jamacardio.2016.1567. [DOI] [PubMed] [Google Scholar]
- 18.Sattelmair J, et al. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124:789–795. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding M, Bhupathiraju SN, Satija A, Van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129:643–659. doi: 10.1161/CIRCULATIONAHA.113.005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Online) 2011;343:1–9. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:1–13. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemingway, H. et al. Evaluating the quality of research into a single prognostic biomarker: a systematic review and metaanalysis of 83 studies of C-reactive protein in stable coronary artery disease. PLoS Med. 2010;7. [DOI] [PMC free article] [PubMed]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Schwarzer G, Carpenter JR, Rücker G. Meta-analysis with R. (Springer International Publishing, 2015). 10.1007/978-3-319-21416-0.
- 25.Peters JL. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61:991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Altıntaş B. et al. The effect of idiopathic premature ventricular complexes on left ventricular ejection fraction. Annals of Noninvasive Electrocardiology. 2020;25. [DOI] [PMC free article] [PubMed]
- 29.Lee A, Denman R, Haqqani HM. Ventricular ectopy in the context of left ventricular systolic dysfunction: risk factors and outcomes following catheter ablation. Heart Lung Circ. 2019;28:379–388. doi: 10.1016/j.hlc.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Penela D, et al. Influence of myocardial scar on the response to frequent premature ventricular complex ablation. Heart. 2019;105:378–383. doi: 10.1136/heartjnl-2018-313452. [DOI] [PubMed] [Google Scholar]
- 31.Park K-M, Im SI, Park S-J, Kim JS, On YK. Risk factor algorithm used to predict frequent premature ventricular contraction-induced cardiomyopathy. Int J Cardiol. 2017;233:37–42. doi: 10.1016/j.ijcard.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal V, et al. Relation between ventricular premature complexes and incident heart failure. Am J Cardiol. 2017;119:1238–1242. doi: 10.1016/j.amjcard.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 33.Dukes JW, et al. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol. 2015;66:101–109. doi: 10.1016/j.jacc.2015.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ban J-E, et al. Electrocardiographic and electrophysiological characteristics of premature ventricular complexes associated with left ventricular dysfunction in patients without structural heart disease. Europace. 2013;15:735–741. doi: 10.1093/europace/eus371. [DOI] [PubMed] [Google Scholar]
- 35.Kanei Y, et al. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol. 2008;13:81–85. doi: 10.1111/j.1542-474X.2007.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mountantonakis SE, et al. Reversal of outflow tract ventricular premature depolarization-induced cardiomyopathy with ablation: effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm. 2011;8:1608–1614. doi: 10.1016/j.hrthm.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Yokokawa M, et al. Predictors of left ventricular dysfunction in patients with frequent premature ventricular complexes. Heart Rhythm. 2011;8:S261–S284. [Google Scholar]
- 38.Sadron M, et al. Premature ventricular beat-induced cardiomyopathy. Characteristics and prognosis factor for recovery after radio-frequency ablation. Arch Cardiovasc Dis Suppl. 2015;7:58–75. [Google Scholar]
- 39.Yang J, Dudum R, Mandyam MC, Marcus GM. Characteristics of unselected high-burden premature ventricular contraction patients. Pacing Clin Electrophysiol. 2014;37:1671–1680. doi: 10.1111/pace.12476. [DOI] [PubMed] [Google Scholar]
- 40.Azizi Z, et al. Clinical predictors of ventricular tachycardia induced cardiomyopathy. Circulation. 2019;140:A15539. [Google Scholar]
- 41.Yamada S, et al. Electrocardiographic characteristics for predicting idiopathic right ventricular outflow tract premature ventricular complex-induced cardiomyopathy. J Interv Card Electrophysiol. 2018;53:175–185. doi: 10.1007/s10840-018-0384-5. [DOI] [PubMed] [Google Scholar]
- 42.Hamon D, et al. A new combined parameter to predict premature ventricular complexes induced cardiomyopathy: impact and recognition of epicardial origin. J Cardiovasc Electrophysiol. 2016;27:709–717. doi: 10.1111/jce.12967. [DOI] [PubMed] [Google Scholar]
- 43.Bas HD, et al. Effect of circadian variability in frequency of premature ventricular complexes on left ventricular function. Heart Rhythm. 2016;13:98–102. doi: 10.1016/j.hrthm.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 44.Gunda S, et al. Consequences of chronic frequent premature atrial contractions: association with cardiac arrhythmias and cardiac structural changes. J Cardiovasc Electrophysiol. 2019;30:1952–1959. doi: 10.1111/jce.14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carpio DEL, Munoz F, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol. 2011;22:791–798. doi: 10.1111/j.1540-8167.2011.02021.x. [DOI] [PubMed] [Google Scholar]
- 46.Voskoboinik A, et al. Predictors of adverse outcome in patients with frequent premature ventricular complexes: the ABC-VT risk score. Heart Rhythm. 2020;17:1066–1074. doi: 10.1016/j.hrthm.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 47.Cronin EM, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm. 2020;17:e2–e154. doi: 10.1016/j.hrthm.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcus GM. Evaluation and management of premature ventricular complexes. Circulation. 2020;1404–1418. [DOI] [PubMed]
- 49.Latchamsetty R, Bogun F. Premature ventricular complex–induced cardiomyopathy. JACC Clin Electrophysiol. 2019;5:537–550. doi: 10.1016/j.jacep.2019.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.