Abstract
AIM
To explore the relationship between ocular and systemic conditions and the impact of ocular complications on the quality of life (QOL) in patients after allogeneic hematopoietic stem cell transplantation (ALLO-HSCT).
METHODS
Forty-four patients with severe hematopoietic disease were enrolled after ALLO-HSCT at our center from July 2018 to October 2020. They completed two questionnaires: the Ocular Surface Disease Index (OSDI) and the quality-of-life scale for Chinese patients with visual impairment (SQOL-DV1). Ocular conditions and systemic conditions were also assessed.
RESULTS
Eye damage was correlated with total bilirubin (P=0.005), and gamma-glutamyl transferase (GGT) (P=0.021). There was no significant correlation between the overall QOL score and OSDI (P=0.8226) or SQOL-DV1 (P=0.9526) scores. The OSDI and the overall QOL score were not correlated with ocular conditions, including best-corrected visual acuity (BCVA), intraocular pressure, Schirmer tear test II, sodium fluorescein staining, tear film breakup time, and tear meniscus height. SQOL-DV1 was correlated with BCVA (P=0.0007), sodium fluorescein staining (P=0.007), and tear film breakup time (P=0.0146).
CONCLUSION
In some patients, early ocular symptoms are not evident after ALLO-HSCT, while ocular surface complications can be observed after a comprehensive ophthalmological examination. Especially for those with elevated total bilirubin or GGT, regular ophthalmic follow-up visits are essential to diagnose and treat ocular graft versus host disease (oGVHD), especially for patients with elevated total bilirubin or GGT.
Keywords: allogeneic stem cell transplantation, ocular graft versus host disease, ocular surface disease, quality of life, quality-of-life scale for Chinese patients with visual impairment, Ocular Surface Disease Index
INTRODUCTION
Currently, allogeneic hematopoietic stem cell transplantation (ALLO-HSCT) is an effective treatment for many hematological malignancies and several benign hematopoietic diseases (such as lymphoma, leukemia, severe aplastic anemia and very severe aplastic anemia)[1]–[3]. However, ALLO-HSCT is associated with an array of complications, including severe infection, edema and hemorrhage[4]. However, graft-versus-host disease (GVHD) remains one of the most frequent and severe complications of ALLO-HSCT, and it can affect multiple organ systems, especially the skin, gastrointestinal tract, liver, and eyes. Therefore, it often places a significant burden on patients and imposes huge costs on families and society[5]–[6]. Approximately 40%-60% patients with chronic GVHD (cGVHD) after ALLO-HSCT will have ocular GVHD (oGVHD)[7]. Acute GVHD with ocular manifestations is often considered a marker of poor prognosis[8]–[9]. With early identification of oGVHD patients and proper treatment, ocular conditions became mild or moderate in severity, permanent visual loss might be prevented. However, it might have a negative impact on many aspects of their quality of life (QOL), including physical and psychological states, social relationships, and daily activities[10]–[11]. Moreover, oGVHD can affect almost all eye structures and causes no specific signs or symptoms[12].
Ocular structures such as the lacrimal glands, eyelids, conjunctiva, and cornea are the areas where structural or functional abnormalities often occur in oGVHD[7], with dry eye being the most common form of oGVHD[13]–[14]. Typical symptoms of dry eye comprise foreign body sensation, photophobia, red eye, photophobia, tearing, ocular fatigue and other ocular discomfort. Thus, oGVHD affects patient's QOL[15]–[18]. QOL is defined as the general well-being of individuals and societies[19]. Many studies have explored QOL in patients with oGVHD, thereby revealing the effect of the disease on vision-related QOL[20]–[21]. oGVHD affects QOL in terms of general vision, ocular pain and some vision-specific activities, explaining its impact on daily activities[21]. Therefore, many doctors use QOL as a measurement of treatment efficacy in oGVHD patients[22].
Hence, we used regular ophthalmological examinations and QOL questionnaires after surgery to better explore the eye conditions of patients after ALLO-HSCT and their impact on QOL. Additionally, we evaluated the relationship between the systemic and ophthalmic conditions, consideration that there might be differences in the general condition between those with ocular lesions and nonmorbid patients.
SUBJECTS AND METHODS
Ethical Approval
This study was conducted in accordance with the tenets of the Declaration of Helsinki. The protocol of this study was approved by the Sun Yat-sen University Sun Yat-sen Memorial Hospital Ethical Committee in China (approval number: SYSKY-2022-434-01). All participants or their guardians gave written informed consent.
This prospective cross-sectional descriptive study was conducted at Sun Yat-sen Memorial hospital, Sun Yat-sen University, from July 2018 to October 2020. The patients underwent ophthalmic examination after they accepted ALLO-HSCT in the Department of Hematology. The selected patients were ≥18 and <60 years old and able to read and complete the QOL questionnaires. Some of these patients were diagnosed with GVHD according to the National Institutes of Health (NIH) criteria[23] and had been receiving stable systemic and topical therapy after ALLO-HSCT. Patients with immune deficiency and immune system diseases, such as those with sarcoidosis and systemic lupus erythematosus (SLE), as well as ophthalmic infections, retinal disease and any other surgery in the past three months (including ocular surgery) were excluded.
A total of 44 patients were eventually enrolled in the study. Before ALLO-HSCT, 22 patients had acute myelocytic leukemia, 7 patients had myelodysplastic syndrome, four patients had severe aplastic anemia (SAA), 4 patients had chronic myelocytic leukemia (CML), 3 patients had T lymphoblastic lymphoma/leukemia (TLBL), 2 patients had acute lymphoblastic leukemia (ALL), 1 patient had myeloid sarcoma (MS) and 1 patient had mixed lineage acute leukemia (MAL). They all received busulfex/cyclophosphamide pretreatment and GVHD prophylaxis with cyclosporine (CsA), methotrexate (MTX), and mycophenolate mofetil (MMF) before surgery. Posttransplant GVHD prophylaxis consisted of CsA and MMF.
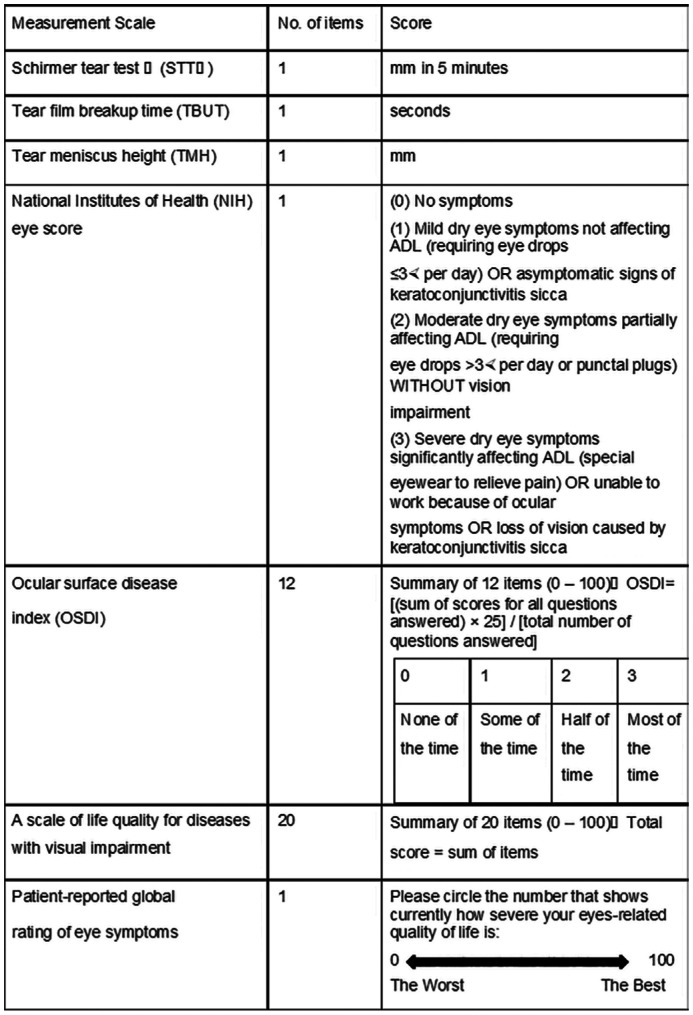
The data were collected at the patients' first ophthalmology clinic visits and including four categories: the patients' basic information, physical condition, eye condition and the impact of eye condition on QOL. The basic information and physical condition including age, sex, duration after ALLO-HSCT, systemic conditions, disease status, platelet, alkaline phosphatase, alanine aminotransferase (ALT), globulin, platelet, and basophil count at enrollment, were analyzed. The patients' eye conditions included the best corrected visual acuity (BCVA), intraocular pressure (IOP), tear film breakup time (TBUT), Schirmer tear test II (STTII), corneal fluorescein staining (FL), tear meniscus height (TMH), bulbar conjunctival hyperemia, and edema. Each ocular examination was performed by the same two ophthalmologists. oGVHD was diagnosed according to the criteria of the NIH[23]. We used the NIH scoring system to assess the severity of oGVHD. The score ranged from 0 to 3 and depended on the severity of dry eye symptoms, frequency of eye drops or intervention for dry eye, and impact on activities of daily living[24]–[25]. In addition to NIH eye scores, we have also used two QOL questionnaires. One was Ocular Surface Disease Index (OSDI), which measures three aspects (the frequency of dry eye symptoms, visual impact of dry eye, and triggers) to denote the severity of dry eye. Higher scores usually indicate greater severity[26]–[27]. The other questionnaire was the QOL scale for Chinese patients with visual impairment (SQOL-DV1), which has 20 questions assessing symptoms, visual function, physical function, social activity, and psychology. The scores of each question range from 0 to 10. The higher the score is, the greater the severity of depressive symptoms, and the total score of the scale is 200 points[28]. In the end, each patient gave their own assessment of the overall QOL score, which ranged from 0 (worst) to 100 (best). These specific evaluation scales are shown in Figure 1.
Figure 1. Evaluation items of oGVHD patients' ocular symptoms and quality of life measurement scale.
ADL: Activity of daily living; oGVHD: Ocular graft-versus-host disease.
All the data were analyzed. Taking into account multiple testing, we set the statistical significance at 0.05. The data collection was performed by using EpiData 3.1 software with two investigators. The statistical analysis of the data was perfoemed by the software Statistical Package for Social Sciences (SPSS) version 25.
RESULTS
Basic Characteristics of the Study Population
A total of 44 participants (23 men and 21 women) with a mean age of 39y participated in the study. The patients' post-ALLO-HSCT period during this study varied: 31 cases were at 1-7mo post-ALLO-HSCT, 12 cases were at 7mo -5y post-ALLO-HSCT, and one case was at >5y post-ALLO-HSCT. According to the NIH consensus criteria, 28 patients were diagnosed with oGVHD-related dry eye, which varied from mild to moderate to severe. Furthermore, we divided these 28 patients into different onset groups. They all received anti-GVHD therapy for presumed GVHD. Nine patients were self-medicated or treated by an ophthalmologist through some methods (tacrolimus eye drops, artificial tear drops, etc.). The patients' clinical characteristics were shown in Table 1.
Table 1. Characteristics of the study population.
| Variables | Mean±SD/median | Normal values |
| Age (y) | 39.12±11.48 | - |
| Albumin | 37.85±5.57 | 40-55 |
| Globulin | 28.86±8.69 | 20-40 |
| Total bilirubin | 12.57±5.84 | 3.4-22.2 |
| Alkaline phosphatase | 86.00 (50.75-109.25) | 45-125 |
| White blood cell | 3.91 (2.96-5.55) ×109 | (3.50-9.50)×109 |
| Red blood cell | (3.37±0.84)×1012 | (3.80-5.10) ×1012 |
| Hemoglobin | 105.2±22.68 | 115-150 |
| Platelets | 112.00 (87.75-180.00)×109 | (125-350)×109 |
| Eosinophil | 0.07 (0.028-0.155) ×109 | (0.000-0.060) ×109 |
| ALT | 30.5 (20.5-53.5) | 15-40 |
| AST | 30.00 (22.00-39.75) | 9-50 |
| GGT | 45.50 (25.75-134.50) | 10-60 |
| BCVA | 0.75 (0.60-1.00) | - |
| IOP (mm Hg) | 15.36±2.66 | 10-21 |
| Schirmer II test (mm/5min) | 7.00 (5.75-11.25) | ≥10 |
| Inflammatory index total score | 0.50 (0.00-2.00) | 0 |
| Sodium fluorescein stain | 0.00 (0.00-4.00) | 0 |
| Tear film rupture time (s) | 3.00 (0.00-5.00) | ≥10 |
| lachrymal height (mm) | 0.286±0.067 | - |
| Visual function QOL scores | 64.00 (46.25-85.25) | 0-200 |
| Whole body quality of life assessment | 70.90±18.40 | 0-100 |
| OSDI | 14.58 (8.33-28.72) | 0-100 |
ALT: Alanine transaminase; AST: Aspartate aminotransferase; GGT: Gamma-glutamyl transpeptidase; BCVA: Best corrected visual acuity; IOP: Intraocular pressure; OSDI: Ocular Surface Disease Index; QOL: Quality of Life.
Relationship Between General Clinical Data and Eye Damage
Clinical data are shown by group according to the NIH eye score in Table 2, which also included total bilirubin (TB; P=0.005), and gamma-glutamyl transferase (GGT; P=0.021).
Table 2. Comparison of two groups.
| Variables | ALLO-HSCT |
χ2/t/W | P | |
| Without ocular manifestation (n=16) | With ocular manifestation (n=28) | |||
| Male | 8 (50.00) | 15 (53.57) | 0 | 1.00 |
| Age, y | 43.27±12.85 | 36.81±10.18 | 1.674 | 0.107 |
| Albumin | 38.16±3.93 | 37.67±6.38 | 0.312 | 0.757 |
| Globulin | 31.43±5.49 | 27.39±9.87 | 7.742 | 0.089 |
| Total bilirubin | 9.73±3.72 | 14.19±6.26 | -2.967 | 0.005 |
| Alkaline phosphatase | 64.5 (45.5-101.5) | 87.5 (56.5-123.5) | 169 | 0.184 |
| White blood cell | 4.07 (3.50-5.11) | 3.87 (2.89-5.67) | 241.5 | 0.678 |
| Red blood cell | 3.26±0.83 | 3.44±0.85 | -0.662 | 0.513 |
| Hemoglobin | 99.00±21.83 | 108.82±22.76 | -1.414 | 0.167 |
| Platelets | 96.0 (75.5-137.0) | 125.0 (96.5-185.5) | 155 | 0.095 |
| Eosinophil | 0.10 (0.045-0.195) | 0.055 (0.015-0.090) | 290 | 0.109 |
| ALT | 25.5 (17.0-40.5) | 32.0 (23.0-66.5) | 173.5 | 0.222 |
| AST | 24.0 (21.0-33.0) | 32.0 (24.5-59.0) | 144 | 0.052 |
| GGT | 32.0 (16.0-68.0) | 91.5 (31.0-331.5) | 129 | 0.021 |
ALLO-HSCT: Allogeneic hematopoietic stem cell transplantation; ALT: Alanine transaminase; AST: Aspartate aminotransferase; GGT: Gamma-glutamyl transpeptidase.
QOL Questionnaire and Objective Index Score
Table 3 shows the correlation of ocular assessment with SQOL-DV1, OSDI and the overall QOL score. The correlation between QOL and ocular involvement was significant despite adjusting for clinical factors. We found that OSDI or the overall QOL score was not correlated with the ocular conditions including BCVA, IOP, STTII, FL, TBUT, and TMH. SQOL-DV1 was correlated with BCVA (P=0.0007), FL (P=0.007), and TBUT (P=0.0146).
Table 3. Ocular questionnaires and objective index score.
| Variables | OSDI |
SQOL-DV1 |
Overall QOL |
||||||
| r | β | P | r | β | P | r | β | P | |
| BCVA | -0.480 | -1.166 | 0.143 | -0.560 | -0.911 | 0.0007 | 0.088 | 0.156 | 0.339 |
| IOP | 0.037 | 0.026 | 0.796 | -0.216 | -0.047 | 0.183 | -0.164 | -0.035 | 0.082 |
| STT II | -0.308 | -0.051 | 0.341 | -0.220 | -0.028 | 0.143 | -0.135 | -0.007 | 0.513 |
| FL | 0.271 | 0.133 | 0.054 | 0.494 | 0.065 | 0.007 | 0.067 | 0.005 | 0.727 |
| TBUT | -0.099 | -0.069 | 0.530 | -0.356 | -0.092 | 0.0146 | 0.008 | -0.005 | 0.819 |
| TMH | -0.339 | -5.336 | 0.160 | -0.211 | -2.132 | 0.115 | -0.174 | -0.329 | 0.673 |
OSDI: Ocular Surface Disease Index; SQOL-DV1: Quality of Life scale for Chinese patients with visual impairment; QOL: Quality of Life; BCVA: Best corrected visual acuity; IOP: Intraocular pressure; FL: Cornea fluorescein stain; TBUT: Tear film breakup time; STT II: Schirmer II test; TMH: Tear meniscus height.
Relationship Between SQOL-DV1 and OSDI Scores and the Overall QOL Score
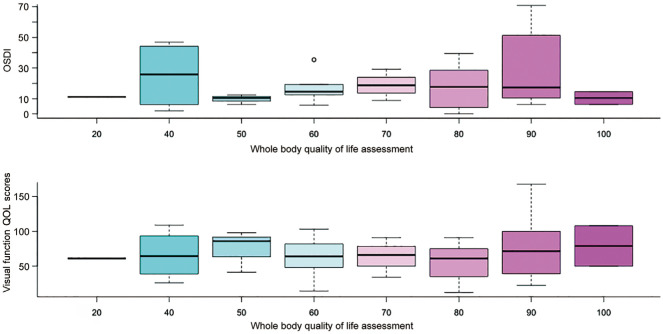
There were no significant correlation between the overall QOL score and OSDI or SQOL-DV1 (P=0.8226, P=0.9526; Figure 2).
Figure 2. Relationship between QOL, OSDI and overall QOL score.
QOL: Quality of Life; OSDI: Ocular Surface Disease Index.
DISCUSSION
Our patients received ophthalmic examinations regularly after ALLO-HSCT. Most patients were systemic disease-free or stable, but their clinical ocular symptoms differed. This suggests that systemic and ocular conditions are not parallel. It has also been confirmed by previous studies[12].
Many studies[25],[29] have examined the relationship between systemic and ocular condition. Skin, gastrointestinal, and liver GVHD could be risk factors for developing oGVHD. When skin or liver GVHD occurs, it may advance the onset of oGVHD and aggravate ocular symtons. Even if some individuals were in a generally better physical health state, hepatic function differed between the ocular-onset group and nonocular-onset group. Prior research[30] has suggested that liver function changes might be related to underlying ocular alterations. Our study also confirmed their finding. There was a significant difference in TB and GGT between the two groups. The marked increase in GGT and TB levels has been associated with cholestasis and bile duct damage[31]–[32]. We consider that oGVHD might be associated with cholestasis and bile duct damage and that GGT and TB levels might be ocular and systemic risk factors. Further studies are needed to prove this hypothesis.
We selected two questionnaires for them in this study to evaluate the relationship between subjective and objective measurements of ocular performance in patients after ALLO-HSCT. One was OSDI[33], which was mainly used to evaluate the performance of the ocular surface and the impact of ocular discomfort on life and then to investigate the patients' QOL. Regarding this questionnaire, there was no statistically significant difference. The other questionnaire we selected was SQOL-DV1[28]. It mainly evaluated patients' subjective symptoms and the impact of eye discomfort on the economy, psychology, and daily activities. Our research showed that ocular discomfort had a significant correlation with BCVA and FL but not with other ocular conditions. This is probably because our research subjects were the first time to visit the ophthalmology clinic, and some of them might have felt very mild ocular discomfort and had been accustomed to it when they were suffering from a more serious condition. Only a few patients developed unbearable ocular symptoms, and their eye lesions severely affected their QOL. However, the number of patients with moderate-to-severe ocular lesions was relatively small at the initial visit.
Patients with oGVHD perceive significant differences in QOL effects related to their ocular conditions[34]–[36]. The worse the ocular condition, the worse the QOL. Some patients even rely more on others and stay at home longer than before because of eye discomfort. For some patients with cGVHD, their subjective perception of the patient is not directly related to the ocular disease[37]. Although some patients did not feel any ocular discomfort, they had severe ocular lesions.
Kurosawa et al[38] have reported that patients without systemic GVHD symptoms have a higher QOL. Acute GVHD often means a worse QOL. For those with chronic GVHD, a good treatment response could cause a significant improvement in QOL. The literature has shown that there are no severe systemic diseases and only mild changes in the skin or eye for some patients with mild GVHD. For patients with milder systematic conditions, the general condition cannot become an important factor affecting their QOL[29]. Our study proved that the general QOL did not play an important role for mild systemic GVHD patients.
There were some limitations in our study. First, because GVHD rarely occurs in the whole population, the number of our patient samples was small, and further studies with larger sample sizes are needed. Second, there were some subjective indicators in our study, such as FL and TBUT. Although we designated two physicians to evaluate patients at the same time, there still might have been some possibility of errors. Additionally, this study was a cross-sectional study, and further prospective studies could better evaluate the ocular surface conditions and related QOL for patients after ALLO-HSCT.
In this study, we confirmed that systemic and ocular conditions were not parallel and that liver function changes were related to oGVHD. We first found that elevated GGT and TB were related to oGVHD and that Chinese patients' QOL was correlated with BCVA and FL. However, the QOL of mild oGVHD patients was not directly related to ocular damage. When oGVHD patients feel obvious ocular discomfort, they might already have severe oGVHD that is difficult to cure. Therefore, early, timely and regular assessment of the patient's eye condition after ALLO-HSCT is very important, especially when the patients' TB or GGT levels are elevated. We consider that oGVHD might be associated with cholestasis and bile duct damage, GGT and TB levels may be ocular and systemic risk factors. Moreover, further studies with large sample sizes are needed.
Acknowledgments
Authors' contributions: Fan SX, Wang WH, Li YQ, and Xiao JH drafted the manuscript and participated in information collection and editing of the manuscript. Zeng P, Huang KZ, Hu YX and Wang J were responsible for data collection. All authors read and approved the final manuscript.
Foundations: Supported by Natural Science Foundation of Guangdong Province, China (No.2019A1515011212); Beijing Bethune Charitable Foundation (No.BJ-GY2021014J).
Conflicts of Interest: Fan SX, None; Wang WH, None; Zeng P, None; Huang KZ, None; Hu YX, None; Wang J, None; Li YQ, None; Xiao JH, None.
REFERENCES
- 1.Chang YJ, Pei XY, Huang XJ. Haematopoietic stem-cell transplantation in China in the era of targeted therapies: current advances, challenges, and future directions. Lancet Haematol. 2022;9(12):e919–e929. doi: 10.1016/S2352-3026(22)00293-9. [DOI] [PubMed] [Google Scholar]
- 2.Kelkar AH, Antin JH, Shapiro RM. Long-term health outcomes of allogeneic hematopoietic stem cell transplantation. Front Oncol. 2023;13:1175794. doi: 10.3389/fonc.2023.1175794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iftikhar R, Chaudhry QUN, Anwer F, et al. Allogeneic hematopoietic stem cell transplantation in aplastic anemia: current indications and transplant strategies. Blood Rev. 2021;47:100772. doi: 10.1016/j.blre.2020.100772. [DOI] [PubMed] [Google Scholar]
- 4.Wang YQ, Huang RH, Wang Z, Xiong JK, Wang XQ, Zhang X. Facing challenges with hope: universal immune cells for hematologic malignancies. Cancer Biol Med. 2023;20(4):229–247. doi: 10.20892/j.issn.2095-3941.2022.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Giuseppe G, Thacker N, Schechter T, Pole JD. Anxiety, depression, and mental health-related quality of life in survivors of pediatric allogeneic hematopoietic stem cell transplantation: a systematic review. Bone Marrow Transplant. 2020;55(7):1240–1254. doi: 10.1038/s41409-020-0782-z. [DOI] [PubMed] [Google Scholar]
- 6.Janicsák H, Ungvari GS, Gazdag G. Psychosocial aspects of hematopoietic stem cell transplantation. World J Transplant. 2021;11(7):263–276. doi: 10.5500/wjt.v11.i7.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannaccare G, Pellegrini M, Bernabei F, et al. Ocular surface system alterations in ocular graft-versus-host disease: all the pieces of the complex puzzle. Graefes Arch Clin Exp Ophthalmol. 2019;257(7):1341–1351. doi: 10.1007/s00417-019-04301-6. [DOI] [PubMed] [Google Scholar]
- 8.Aladağ E, Kelkitli E, Göker H. Acute graft-versus-host disease: a brief review. Turk J Haematol. 2020;37(1):1–4. doi: 10.4274/tjh.galenos.2019.2019.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee D. A brief account on ocular graft versus host disease. Indian J Ophthalmol. 2023;71(4):1115–1122. doi: 10.4103/IJO.IJO_2839_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riemens A, Te Boome LC, Kalinina Ayuso V, et al. Impact of ocular graft-versus-host disease on visual quality of life in patients after allogeneic stem cell transplantation: questionnaire study. Acta Ophthalmol. 2014;92(1):82–87. doi: 10.1111/aos.12047. [DOI] [PubMed] [Google Scholar]
- 11.Tabbara KF, Al-Ghamdi A, Al-Mohareb F, Ayas M, Chaudhri N, Al-Sharif F, Al-Zahrani H, Mohammed SY, Nassar A, Aljurf M. Ocular findings after allogeneic hematopoietic stem cell transplantation. Ophthalmology. 2009;116(9):1624–1629. doi: 10.1016/j.ophtha.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 12.Baumrin E, Loren AW, Falk SJ, Mays JW, Cowen EW. Chronic graft-versus-host disease. Part I: Epidemiology, pathogenesis, and clinical manifestations. J Am Acad Dermatol. 2022:S0190-9622(22)03314. doi: 10.1016/j.jaad.2022.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kezic JM, Wiffen S, Degli-Esposti M. Keeping an ‘eye’ on ocular GVHD. Clin Exp Optom. 2022;105(2):135–142. doi: 10.1080/08164622.2021.1971047. [DOI] [PubMed] [Google Scholar]
- 14.Soleimani M, Mahdavi Sharif P, Cheraqpour K, Koganti R, Masoumi A, Baharnoori SM, Salabati M, Djalilian AR. Ocular graft-versus-host disease (oGVHD): from A to Z. Surv Ophthalmol. 2023:S0039-6257(23)00042. doi: 10.1016/j.survophthal.2023.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang SD, Singh RB, Yuksel E, Musayeva A, Sinha S, Taketani Y, Dohlman TH, Dana R. Ocular pain in ocular graft-versus-host disease patients with neurotrophic keratopathy. Ocul Surf. 2022;26:142–147. doi: 10.1016/j.jtos.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Inamoto Y, Valdés-Sanz N, Ogawa Y, et al. Ocular graft-versus-host disease after hematopoietic cell transplantation: expert review from the late effects and quality of life working committee of the center for international blood and marrow transplant research and transplant complications working party of the European society of blood and marrow transplantation. Biol Blood Marrow Transplant. 2019;25(2):e46–e54. doi: 10.1016/j.bbmt.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng XJ, Huang RH, Huang SQ, Fan W, Yuan RD, Wang XQ, Zhang X. Recent advances in ocular graft-versus-host disease. Front Immunol. 2023;14:1092108. doi: 10.3389/fimmu.2023.1092108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carreno-Galeano JT, Dohlman TH, Kim S, Yin J, Dana R. A review of ocular graft-versus-host disease: pathophysiology, clinical presentation and management. Ocul Immunol Inflamm. 2021;29(6):1190–1199. doi: 10.1080/09273948.2021.1939390. [DOI] [PubMed] [Google Scholar]
- 19.Schipper H, Clinch J, Olweny C. Quality of life studies: definitions and conceptual issues. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials, 2nd ed. Philadelphia, PA: Lippincott-Raven; 1996. pp. 11–23. [Google Scholar]
- 20.Liao YL, Zhao WX, Yang J, Wu SW, Jin L, Huang F, Liang LY. Vision-specific and cancer-specific quality of life in ocular graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Graefes Arch Clin Exp Ophthalmol. 2023;261(2):453–465. doi: 10.1007/s00417-022-05812-5. [DOI] [PubMed] [Google Scholar]
- 21.Pezzotta S, Rossi GC, Scudeller L, Antoniazzi E, Bianchi PE, Perotti C, Del Fante C. A cross-sectional study on vision-related quality of life in patients with ocular GvHD. Bone Marrow Transplant. 2015;50(9):1224–1226. doi: 10.1038/bmt.2015.24. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Zhao WX, Liao YL, Wu SW, Li J, Jin L, Liu QF, Huang F, Liang LY. Ocular surface disease index questionnaire as a sensitive test for primary screening of chronic ocular graft-versus-host disease. Ann Transl Med. 2022;10(16):855. doi: 10.21037/atm-21-6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagasia MH, Greinix HT, Arora M, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. the 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inamoto Y, Valdés-Sanz N, Ogawa Y, et al. Ocular graft-versus-host disease after hematopoietic cell transplantation: expert review from the Late Effects and Quality of Life Working Committee of the CIBMTR and Transplant Complications Working Party of the EBMT. Bone Marrow Transplant. 2019;54(5):662–673. doi: 10.1038/s41409-018-0340-0. [DOI] [PubMed] [Google Scholar]
- 25.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Trindade M, Rodrigues M, Pozzebon ME, Aranha FJP, Colella MP, Fernandes A, Fornazari DO, de Almeida Borges D, Vigorito AC, Alves M. A plethora of ocular surface manifestations in a multidisciplinary ocular graft-versus-host disease unit. Sci Rep. 2022;12(1):15926. doi: 10.1038/s41598-022-19990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa Y, Kawakami Y, Tsubota K. Cascade of inflammatory, fibrotic processes, and stress-induced senescence in chronic GVHD-related dry eye disease. Int J Mol Sci. 2021;22(11):6114. doi: 10.3390/ijms22116114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Q, Li S, Chen H. The development of a scale of life quality for diseases with visual impairment. Zhonghua Yan Ke Za Zhi. 1997;33(4):307–310. [PubMed] [Google Scholar]
- 29.Aldebasi T, Bashir R, Gangadharan S, Shaheen NA, Alhussain B, Almudhaiyan T, Alahmari B. Incidence of ocular manifestations in patients with graft versus host disease after allogeneic stem cell transplant in Riyadh, Saudi Arabia. Int J Ophthalmol. 2022;15(7):1149–1156. doi: 10.18240/ijo.2022.07.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JCC, Teichman JC, Mustafa M, O'Donnell H, Broady R, Yeung SN. Risk factors for the development of ocular graft-versus-host disease (GVHD) dry eye syndrome in patients with chronic GVHD. Br J Ophthalmol. 2015;99(11):1514–1518. doi: 10.1136/bjophthalmol-2014-306438. [DOI] [PubMed] [Google Scholar]
- 31.Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18–35. doi: 10.1038/ajg.2016.517. [DOI] [PubMed] [Google Scholar]
- 32.Brennan PN, Dillon JF, Tapper EB. Gamma-Glutamyl Transferase (γ-GT) - an old dog with new tricks? Liver Int. 2022;42(1):9–15. doi: 10.1111/liv.15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiffman RM. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 34.Pallua S, Giesinger J, Oberguggenberger A, Kemmler G, Nachbaur D, Clausen J, Kopp M, Sperner-Unterweger B, Holzner B. Impact of GvHD on quality of life in long-term survivors of haematopoietic transplantation. Bone Marrow Transplant. 2010;45(10):1534–1539. doi: 10.1038/bmt.2010.5. [DOI] [PubMed] [Google Scholar]
- 35.Sun YC, Chai XY, Inamoto Y, Pidala J, Martin PJ, Flowers MED, Shen TT, Lee SJ, Jagasia M. Impact of ocular chronic graft-versus-host disease on quality of life. Biol Blood Marrow Transplant. 2015;21(9):1687–1691. doi: 10.1016/j.bbmt.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uchino M, Ogawa Y, Uchino Y, Mori T, Okamoto S, Tsubota K. Comparison of stem cell sources in the severity of dry eye after allogeneic haematopoietic stem cell transplantation. Br J Ophthalmol. 2012;96(1):34–37. doi: 10.1136/bjophthalmol-2011-300514. [DOI] [PubMed] [Google Scholar]
- 37.Qiu Y, Hong J, Peng RM. Manifestation of clinical categories of ocular graft-versus-host disease. J Ophthalmol. 2018;2018:6430953. doi: 10.1155/2018/6430953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurosawa S, Yamaguchi T, Mori T, Kanamori H, Onishi Y, Emi N, Fujisawa S, Kohno A, Nakaseko C, Saito B, Kondo T, Hino M, Nawa Y, Kato S, Hashimoto A, Fukuda T. Patient-reported quality of life after allogeneic hematopoietic cell transplantation or chemotherapy for acute leukemia. Bone Marrow Transplant. 2015;50(9):1241–1249. doi: 10.1038/bmt.2015.137. [DOI] [PubMed] [Google Scholar]