Abstract
AIM
To identify and analyze the genotype of the patients with special ocular manifestations of familial vitreous amyloidosis (FVA) in a Chinese Han family.
METHODS
Pars plana vitrectomy (PPV) surgery was performed on a 52-year-old Chinese woman presented with vitreous amyloidosis and progressive visual impairment, without evidence of cardiac, renal, gastrointestinal, central nervous system or peripheral nervous system dysfunction. During the surgery, the patient presented with a gray-white dense and thick cotton wool-like change in the vitreous body, accompanied by complete retinal detachment. Additionally, hard, free and movable yellow-white deposits were observed in the posterior pole and surrounding retina, the vitreous and subretinal deposits were examined by Congo red staining and immunohistochemical pathological examination, and whole exome sequencing was performed on blood samples from the patient and her cousin.
RESULTS
During the operation, it was discovered that there was a complete detachment of the retina and a significant amount of hard, free-floating yellow-white deposits were observed beneath the posterior pole and surrounding retina. This is an exceedingly rare ocular manifestation. Pathological examination of the vitreous and subretinal deposit specimens revealed positive Congo red staining, as well as elevated vascular endothelial growth factor (VEGF) expression in vascular endothelial cells within the sediment specimens upon immunohistochemical examination. The patient and her cousin both exhibited a heterozygous mutation in Glyl03Arg within the transthyretin (TTR) gene, resulting in a substitution of glycine (Gly) at position 103 with arginine (Arg).
CONCLUSION
FVA may present with various ocular manifestations, but panretinal detachment is a rare occurrence. In cases where retinal detachment persists for an extended period of time, amyloid deposits may form under the retina through retinal tears, leading to subretinal deposits that can impede retinal reattachment and negatively impact visual prognosis. Elevated levels of VEGF in the eyes of FVA patients may indicate an overexpression state, necessitating careful postoperative follow-up. The heterozygous mutation Gly103Arg may represent a unique pathogenic site in Chinese individuals.
Keywords: familial vitreous amyloidosis, transthyretin gene, Gly103Arg, vascular endothelial growth factor
INTRODUCTION
Hereditary thyroxine transporter amyloidosis is a rare autosomal dominant genetic disease. Familial vitreous amyloidosis (FVA) is caused by the accumulation of amyloid in the vitreous, which leads to the gradual visual impairment. The localized amyloidosis, which has different ocular clinical manifestations, is related to mutations in the transthyretin (TTR) gene.
FVA patients are mainly distributed in Portugal, Sweden, Japan, the United States and other regions[1], however, the incidence rates of FVA in China remain low. It has been reported that China is predominantly characterized by simple FVA[2], which differs from European and American countries in terms of familial amyloidpolyneuropathy (FAP) is associated with FVA.
The TTR gene is located on chromosome 18q12.1 or base pair 31 591 766 to 31 599 023, and spans 4 exons and 5 introns[3], encodes a signal peptide composed of 20 amino acids and a TTR protein composed of 127 amino acids. TTR has a molecular weight of 14 kDa in monomeric form and as a tetrameric form (55 kDa) in plasma[4]. It serves as a carrier of thyroxine and retinol[5]. However, the mutations in the TTR gene can disrupted the tetrameric structure, leading to instability and breakdown of the tetramers, the soluble tetramers dissociate into insoluble protein monomers, deposit in the tissues and induce amyloidosis[6]. Consequently, there is an increased tendency to produce the amyloid form, which favors disease development[7]–[8].
Up to now, a total of 130 mutations in the TTR gene have been documented (www.hgmd.org), with 16 of these mutations identified in Chinese patients. Most patients with FVA are typically admitted in the middle or late stages of the disease and require timely treatment through pars plana vitrectomy (PPV)[9]–[10]. Therefore, it is imperative to possess a comprehensive comprehension of the intraoperative precautions, potential postoperative complication and visual prognosis following PPV in patients with FVA. In this study, we present a rare case of vitreous amyloidosis and conducted whole exome sequencing (WES) along with a comprehensive analysis of the patient's ocular clinical characteristics.
SUBJECTS AND METHODS
Ethical Approval
The study was conducted according to the tenets of the Declaration of Helsinki and approved by the Ethics Committee of Jiaxing Traditional Chinese Medicine Hospital (Approval Number: MEC-JHTCM2020-1111). The informed consent was obtained from individual or guardian participants.
Subjects and Methods
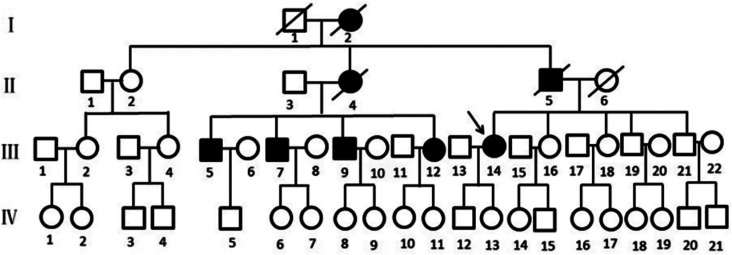
A three-generation family with TTR was identified through outpatient visits to the Jiaxing T.C.M. Hospital (Figure 1). One subject (III: 14) underwent a comprehensive consultation and rhorough ophthalmic examination, which included assessment of best corrected visual acuity (BCVA), slit lamp biomicroscopy, and dilated fundus examination. Retinal structural changes were visualized using optical coherence tomography (OCT) technology (Spectralis OCT; Heidelberg, Germany). Electroretinogram (ERG) and electrooculogram (EOG; RetiPort ERG system; Roland Consult, Wiesbaden, Germany) were performed before the vitrectomy. The ERG and EOG protocol adhered to the standards published by the International Society for Clinical Electrophysiology of Vision (ISCEV).
Figure 1. Pedigree of the family with TTR Gly103Arg mutation.
I, II, III, IV represent first, second, third and fourth generation, respectively. Normal individuals are shown as clear circles (females) and squares (males), affected individuals are shown as filled symbols. The patients passed away are shown as slash. The arrow indicates the proband. TTR: Transthyretin.
The vitreous specimens obtained during vitrectomy were first smeared and fixed with 4% paraformaldehyde, followed by staining with hematoxylin & eosin and Congo-red. The nuclei were subsequently counterstained using Mayer hematoxylin. The sections were subsequently dehydrated in absolute ethanol, cleared in xylene, and examined under a microscope after being sealed with transparent neutral gum.
Following the acquisition of informed consent, a total of 3 mL peripheral blood was collected from the female patient. Genomic DNA was extracted from peripheral blood using QIAamp DNA Blood Midi Kit (Qiagen, Hilden, Germany) according to the manufacturer's intruction. Ultrasonication was employed to disrupt the nucleic acid in the sample, followed by whole exome sequencing. The TruSeq DNA sample preparation kit was used to facilitate the ligation of both ends for the library construction. We used the medical whole exome V1 probe kit for capturing 52.9 Mb region, including 19 608 gene exons, Chinese population ancestry/copy number variation probes, and the Illumina platform were utilized for high-throughput sequencing. The data quality Q30 ratio is greater than 80%, the median sequencing depth of the target exon region is over 50×, the 95% target region sequencing depth is greater than 20×, and the exon loss rate is below 0.2%. The results were compared with a TTR reference sequence (GenBank accession number: NM_000371.3).
RESULTS
The individual affected (III:14, Figure 2) exhibited early clinical manifestations of TTR progression in a 52-year-old Chinese female. She had been experiencing blurred vision in her left eye for nearly a year, she underwent a routine vitrectomy at a hospital in Yunnan Province due to FVA affecting her right eye 6 years ago. The patient's BCVA is 0.4 in the right eye, while her left eye exhibits only perception of hand motion, however, intraocular pressure remains within normal limits.
Figure 2. Fundus examination before the operation.
A: Fundus photograph of the left eye. Degenerated vitreous body attached to the posterior capsule of the lens, showed “foot-like” vitreous opacity obscured the fundus. B: B-ultrasound showed weak echo mass detected in the vitreous cavity, which may be interspersed with non-echoic cavities, and not connected to the spherical wall. A smooth, uniform, moderately rough echo light band was detected, which is V-shaped and connected the video disc.
The anterior segment examination using slit lamp biomicroscopy revealed that cystic opacity of the posterior lens in the right eye, and a “foot-like” opacity attached to the posterior lens capsule with vitreous opacity in the left eye (Figure 2A). No apparent turbidity was observed in the vitreous of the right fundus, and the optic disc of the fundus appears clear. However, at the posterior pole, similar changes were noted in the peripheral of omentum's proliferative membrane. After full dialation of the left pupil, examination of the vitreous body revealed 4+ turbidity and a grayish-white, dense, cotton-like appearance. Due to the density and cloudiness of the vitreous body, fundus visualization was unclear. The eye B-ultrasound examination indicated retinal detachment (Figure 2B). For further treatment, the patient's health was evaluated through various of methods including laboratory evaluations, electrocardiogram (ECG) and echocardiography, as well as other related examinations. The results indicated that the patient is in good health. Meanwhile, we collaborated with neurologists to conduct physical examinations and found no evidence of peripheral nerve involvement. Due to the financial difficulties of the patient's family, they declined further full-body examination and biopsy of other tissues or organs. Much to our regret, her left eye underwent a standard three-channel vitrectomy via the flat part of the ciliary body for the second time. After removal of diseased vitreous, total retinal detachment was observed in the affected eye, and a retinal tear appeared in the peripheral omentum at 10 o'clock direction.
During the PPV procedure, certain yellow-white deposits that were hard, free and movable in nature were identified beneath the peripheral retina and within the posterior pole region (Figure 3A).
Figure 3. Subretinal deposits in the patient.
A: During the pars plana vitrectomy (PPV) procedure, hard, yellow-white deposits were observed in the posterior pole and beneath the peripheral retina that were also mobile; B: A retinal stoma was created in the posterior pole to facilitate removal of these subretinal deposits; C: The excised subretinal deposits were obtained from the affected eye.
To account for the impact of the deposit on the posterior retina detachment, a retinal stoma operation was performed wherein the intraocular foreign body forceps forceps were utilized to extract the deposit (Figure 3B), followed by omentum resetting and silicone oil filling of the eyes. After the surgery, the right eye's BCVA improved to the anterior index while subretinal deposits were observed beneath the peripheral retina (Figure 3C). Positive Congo red staining was observed in the vitreous and subretinal deposit specimens, with apple green dots and flaky birefringence visible under polarized light microscopy (Figure 4A and 4B). Simultaneous immunohistochemical detection revealed positive cytoplasmic expression of vascular endothelial growth factor (VEGF) in subretinal deposit tissues, specifically within the endothelial cells (Figure 4C and 4D).
Figure 4. Pathological analysis of vitreous and subretinal deposits of FVA patient.
The Congo-red staining of both retina (A) and subretinal deposit (B) showed deposits with apple-green deposits birefringence under the polarized light microscope. HE staining (C) of subretinal (200×) showed lots of eosinophilic homogeneous substance. Immunohistochemical detection (D) of VEGF protein expression in subretinal deposit tissue (200×) showed positive VEGF expression in vascular endothelial cells. VEGF: Vascular edothelial growth factor.
After obtaining informed consent from both patients and their family members, peripheral blood samples of the patients were collected for WES. The results revealed a TTR c.307G>C (p.Gly103Arg) mutation, a point mutation of Gly103Arg in the 103rd amino acid of exon 3 of the TTR gene, which was mutated from glycine (Gly) to arginine (Arg) (Figure 5A). SIFT and PolyPhen-2 software were used to predict the function of the mutant genes, SIFT indicated that the mutation was deleterious, while PolyPhen-2 suggested a likely harmful effect. The population frequency is absent in the gnomAD database, and similarly, the East Asian population frequency is also absent in the gnomAD-EAS population database. The population frequency is not observed in the local internal database. First-generation sequencing results indicate that the patient carries a heterozygous variant known to be pathogenic and associated with vitreous amyloidosis as listed in the HGMD database (PMID: 18709962).
Figure 5. Mutation of TTR gene detected by whole exome sequencing.
The arrow indicates the mutation at the nucleotide position c.G103>C in TTR gene. A: The patient under study; B: Their female cousin are depicted. TTR: Transthyretin.
The patient reported a familial history of similar symptoms, which both her father and cousins experiencing them. However, her father had never been subjected to examination due to traffic inconvenience. All of her three male cousins and one female cousin underwent bilateral vitrectomy at our hospital. The average postoperative logMAR BCVA remained stable at 0.21±0.11. A mutation in the TTR gene c.307G>C (p.Gly103Arg) was also detected in her female cousin, as shown in Figure 5B. Based on this information, it is highly likely that the patient has early-onset familial amyloidosis with vitreous involvement.
DISCUSSION
The clinical manifestation of FVA is characterized by progressive vision loss, while vitreous changes that are grayish-white, dense and thick like cotton serve as key diagnostic indicators for FVA. Diagnosis of this condition relies on laboratory tests such as vitreous histopathological examination and genetic testing. The primary therapeutic approach for FVA is PPV[1],[10].
To the best of our knowledge, the combination of FVA and total retinal detachment appears to be infrequent[11]–[13]. We have previously conducted PPV surgery on 17 patients with familial exudative vitreoretinopathy (PEVR), representing 3 families and a total of 31 eyes. During the procedure, only 2 eyes were found to have retinal detachment. In this case, the retinal hiatus located in the peripheral retina was identified during PPV operation, and the degenerated vitreous adhered firmly to the peripheral retina and blood vessels, while it did not adhered tightly to most of the retinas, particularly the liquefied vitreous in the posterior pole that exerted minimal traction on the retina. We hypothesized that the inability of the vitreous body to detach and exert traction on the retina after complete formation may be attributed to retinal tissue degeneration and thinning. With the progression of the disease, there may be secondary occurrence of retinal hiatus or retinal detachment. The affected eye was filled with silicone oil during surgery and is currently under follow-up.
It has been reported that liver is the main place of TTR synthesis, however, TTR can also be synthesized in retinal pigment epithelial cells[14], previous studies have confirmed that FAP patients may still experience ocular complications even after undergoing liver transplantation[15]–[16]. Research evidence indicate a direct correlation between the various characteristics of TTR gene mutation and the prevalence of vitreous opacity. They found that patients with amyloid transthyretin (ATTR) Thr60Ala or ATTR Val112Ile did not exhibit any ocular manifestations, while those with ATTR Val30Met were more prone to vitreous opacities. In contrast, individuals with ATTR Tyr114Cys had a higher incidence and a earlier onset age compared with the ATTR Val30Met group[17]–[18]. Meanwhile, the investigation revealed that patients carrying Glu89Lys mutation presented with bilateral neurotrophic keratitis, those harboring Ala36Pro mutation exhibited bilateral secondary open angle glaucoma, and individuals possessing Val30Met had bilateral exfoliative glaucoma[18].
To the best of our knowledge, there have been no previous reports of subretinal sediments. The pathological examination revealed positive Congo red staining. Based on our analysis, it is believe that the subretinal deposits are primarily caused by the continuous production of intraocular amyloid via retinal tears. On the other hand, it is possible that the retinal detachment occurred one year ago given the patient's prolonged history of the disease. However, due to the economic constraints, the patient was unable to receive appropriate treatment. Eventually, the continuous accumulation of amyloid fibers beneath the retina and subsequent deposits formation have a detrimental effect on both retinal reattachment and visual prognosis. Amongst this family (comprising 5 individuals with 9 eyes), only the patient in question experienced secondary retinal detachment, which was total in nature. Therefore, while it remains uncertain whether there exists a direct correlation between this ocular phenomenon and TTR gene mutation, we cannot discount its possibility.
The immunohistochemical expression of VEGF in vascular endothelial cells within sediment specimens exhibited a robustly positive result. According to our analysis, it is likely that the deposit was introduced into the vascular tissue during scleral puncture and retinostomy removal procedures. Despite this patient, we previously encountered an FVA patient whose experienced vitreous hemorrhage for 8 and 9y following PPV surgery, respectively. In the second PPV operation, a significant amount of flocculent degeneration within the vitreous cavity and residual dense vitreous were observed, concomitant with the retinal neovascularization (Figure 6). The occurrence of neovascular glaucoma (NVG) followed subsequently. After the combination of anti-VEGF drug and anti-glaucoma treatments, visual acuity still remains at finger counting and light perception levels.
Figure 6. Fundus photography of patients with FVA after PPV.

Eight years post-surgery, fundus photography of the left eye revealed a plethora of neovascularization around the optic disc, peripheral omental white line-like vessels, extensive perivascular opacification except for the macula and large subretinal hemorrhages in the inferior hemisphere. FVA: Familial vitreous amyloidosis; PPV: Pars plana vitrectomy.
Retinovascular disease is a recognized ocular manifestation of inherited amyloidosis[19]–[22]. Tripathy et al[23] found that patients afflicted with Tyr114Cys experience neovascular glaucoma. O'Hearn et al[24] found a significant increase in the level of VEGF within the vitreous cavity among patients with FAP who experienced complicated involing the vitreous; Combined with the immunohistochemical analysis, we believed that the VEGF in the eyes of FVA patients might be overexpressed.
There are several possibilities worth considering, such as damage to the retinal blood vessels during separation of the vitreous body from the retina adhesion in PPV surgery, increased degeneration of vitreous tissues during and after surgery, ongoing accumulation of amyloid substances in the vitreous cavity, and depositon of some of these substances in peripheral small retinal vessels leading to mechanical compression, which mechanically compress and damage the vascular endothelial cells. Alternatively, insoluble small-molecule proteins may deposit in blood vessels, leading to vascular obstruction and subsequent retinal ischemia and hypoxia that result in non-perfusion areas. Other factors may also contribute to the development of retinal angiopathy-related diseases by increasing VEGF expression. Hence, it is imperative to completely remove the vitreous body, particularly the degenerative vitreous tissue attached to retinal blood vessels during PPV operation. Patients should be regularly followed up and receive FFA examinations when necessary. Timely retinal laser photocoagulation treatment can prevent the formation of retinal neovascularization and safeguard postoperative vision.
In our study, we identified the c.307G>C (p.Gly103Arg) mutation in the TTR gene of both the patient and her cousin. Notably, this particular mutation has been previously documented in 6 Chinese families affiliated with vitreous amyloidosis[25]–[30]. It should be noted that in references[26]–[27], the p.G103R mutation was referred to as p.G83R. Similar to our study, no other clinical features were observed among the probands in Chinese families reported above, except for decreased vision and vitreous amyloidosis[25]–[30]. Thus, this mutation may be a suitable candidate for clinical diagnosis of vitreous amyloidosis in Chinese Han population, and might represent a common and unique gene mutation site among Chinese FVA patients.
Acknowledgments
Foundations: Supported by Zhejiang Provincial Health Science and Technology Program of Traditional Chinese Medicine (No.2021ZB284; No.2023ZR053); Science and Technology Bureau of Jiaxing City (No.2021AY30007; No.2021AY30008); Jiaxing Key Laboratory of Diabetic Angiopathy Research (No.2019ZDSYS).
Conflicts of Interest: Feng YB, None; Shi YB, None; He YY, None; Ma ZY, None; Zhu YX, None; Weng WQ, None.
REFERENCES
- 1.Choi KJ, Son KY, Kang SW, et al. Ocular manifestations of asp38ala and thr59lys familial transthyretin amyloidosis. Retina. 2022;42(2):396–403. doi: 10.1097/IAE.0000000000003296. [DOI] [PubMed] [Google Scholar]
- 2.Li ZX, Du K, Chu XJ, Lv H, Zhang W, Wang ZX, Yuan Y, Meng LC. TTR Gly83Arg mutation: beyond familial vitreous amyloidosis. Front Neurol. 2022;12:821003. doi: 10.3389/fneur.2021.821003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowczenio DM, Noor I, Gillmore JD, et al. Online registry for mutations in hereditary amyloidosis including nomenclature recommendations. Hum Mutat. 2014;35(9):E2403–E2412. doi: 10.1002/humu.22619. [DOI] [PubMed] [Google Scholar]
- 4.Minnella AM, Rissotto R, Antoniazzi E, Di Girolamo M, Luigetti M, Maceroni M, Bacherini D, Falsini B, Rizzo S, Obici L. Ocular involvement in hereditary amyloidosis. Genes. 2021;12(7):955. doi: 10.3390/genes12070955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dammacco R, Merlini G, Lisch W, Kivelä TT, Giancipoli E, Vacca A, Dammacco F. Amyloidosis and ocular involvement: an overview. Semin Ophthalmol. 2020;35(1):7–26. doi: 10.1080/08820538.2019.1687738. [DOI] [PubMed] [Google Scholar]
- 6.Iakovleva I, Hall M, Oelker M, Sandblad L, Anan I, Sauer-Eriksson AE. Structural basis for transthyretin amyloid formation in vitreous body of the eye. Nat Commun. 2021;12(1):7141. doi: 10.1038/s41467-021-27481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minnella AM, Rissotto R, Maceroni M, Romano A, Fasciani R, Luigetti M, Sabatelli M, Rizzo S, Falsini B. Ocular involvement in hereditary transthyretin amyloidosis: a case series describing novel potential biomarkers. Genes. 2021;12(6):927. doi: 10.3390/genes12060927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds MM, Veverka KK, Gertz MA, Dispenzieri A, Zeldenrust SR, Leung N, Pulido JS. Ocular manifestations of familial transthyretin amyloidosis. Am J Ophthalmol. 2017;183:156–162. doi: 10.1016/j.ajo.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Marques JH, Coelho J, Menéres MJ, Beirão JM. Progressive vitreous deposits during treatment with inotersen for hereditary ATTR amyloidosis. Amyloid. 2021;28(4):275–276. doi: 10.1080/13506129.2021.1975673. [DOI] [PubMed] [Google Scholar]
- 10.Kakihara S, Hirano T, Imai A, Miyahara T, Murata T. Small gauge vitrectomy for vitreous amyloidosis and subsequent management of secondary glaucoma in patients with hereditary transthyretin amyloidosis. Sci Rep. 2020;10(1):5574. doi: 10.1038/s41598-020-62559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patil NS, Iqbal MM, Bursztyn LLCD. Conjunctival lymphangiectasia and retinal angiopathy in hereditary transthyretin amyloidosis. Int J Retina Vitreous. 2022;8(1):4. doi: 10.1186/s40942-021-00357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marques JH, Malheiro L, Malheiro J, Oliveira L, Menéres MJ, Beirão JM. Pupillometry: an objective test to assess endocular hereditary transthyretin amyloidosis. Eur J Ophthalmol. 2022;32(1):637–642. doi: 10.1177/1120672121997294. [DOI] [PubMed] [Google Scholar]
- 13.Blandford AD, Yordi S, Kapoor S, Yeaney G, Cotta CV, Valent J, Perry JD, Singh AD. Ocular adnexal amyloidosis: a mass spectrometric analysis. Am J Ophthalmol. 2018;193:28–32. doi: 10.1016/j.ajo.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 14.Kawaji T, Ando Y, Nakamura M, Yamamoto K, Ando E, Takano A, Inomata Y, Hirata A, Tanihara H. Transthyretin synthesis in rabbit ciliary pigment epithelium. Exp Eye Res. 2005;81(3):306–312. doi: 10.1016/j.exer.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Beirão JM, Malheiro J, Lemos C, et al. Impact of liver transplantation on the natural history of oculopathy in Portuguese patients with transthyretin (V30M) amyloidosis. Amyloid. 2015;22(1):31–35. doi: 10.3109/13506129.2014.989318. [DOI] [PubMed] [Google Scholar]
- 16.Monteiro C, Martins da Silva A, Ferreira N, Mesgarzadeh J, Novais M, Coelho T, Kelly JW. Cerebrospinal fluid and vitreous body exposure to orally administered tafamidis in hereditary ATTRV30M (p.TTRV50M) amyloidosis patients. Amyloid. 2018;25(2):120–128. doi: 10.1080/13506129.2018.1479249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koga T, Ando E, Hirata A, et al. Vitreous opacities and outcome of vitreous surgery in patients with familial amyloidotic polyneuropathy. Am J Ophthalmol. 2003;135(2):188–193. doi: 10.1016/s0002-9394(02)01838-x. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds MM, Veverka KK, Gertz MA, Dispenzieri A, Zeldenrust SR, Leung N, Pulido JS. Ocular manifestations of familial transthyretin amyloidosis. Am J Ophthalmol. 2017;183:156–162. doi: 10.1016/j.ajo.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Rousseau A, Terrada C, Touhami S, et al. Angiographic signatures of the predominant form of familial transthyretin amyloidosis (Val30Met mutation) Am J Ophthalmol. 2018;192:169–177. doi: 10.1016/j.ajo.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Kakihara S, Hirano T, Matsuda Y, Takano D, Imai A, Miyahara T, Murata T. Deposits on retinal surface seen on OCT in ocular amyloidosis. Ophthalmol Retina. 2021;5(10):1005–1008. doi: 10.1016/j.oret.2020.12.028. [DOI] [PubMed] [Google Scholar]
- 21.Marques JH, Coelho J, Malheiro J, Pessoa B, Beirão JM. Subclinical retinal angiopathy associated with hereditary transthyretin amyloidosis-assessed with optical coherence tomography angiography. Amyloid. 2021;28(1):66–71. doi: 10.1080/13506129.2020.1827381. [DOI] [PubMed] [Google Scholar]
- 22.Mano F, Dispenzieri A, Kusaka S, Pavesio C, Khalid H, Keane PA, Pulido JS. Association between choroidal characteristics and systemic severity in amyloidosis. Retina. 2021;41(5):1037–1046. doi: 10.1097/IAE.0000000000002961. [DOI] [PubMed] [Google Scholar]
- 23.Tripathy K, Chawla R, Selvan H, Venkatesh P. Ocular manifestations of familial transthyretin amyloidosis. Am J Ophthalmol. 2018;186:169–170. doi: 10.1016/j.ajo.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 24.O'Hearn TM, Fawzi A, He S, Rao NA, Lim JI. Early onset vitreous amyloidosis in familial amyloidotic polyneuropathy with a transthyretin Glu54Gly mutation is associated with elevated vitreous VEGF. Br J Ophthalmol. 2007;91(12):1607–1609. doi: 10.1136/bjo.2007.119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Y, Zhao Y, Zhou JJ, Wang X. Identification of a TTR gene mutation in a family with hereditary vitreous amyloidosis. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2012;29(1):13–15. doi: 10.3760/cma.j.issn.1003-9406.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Zhang AM, Wang H, Sun P, Hu QX, He YQ, Yao YG. Mutation p.G83R in the transthyretin gene is associated with hereditary vitreous amyloidosis in Han Chinese families. Mol Vis. 2013;19:1631–1638. [PMC free article] [PubMed] [Google Scholar]
- 27.Chen LY, Lu L, Li YH, et al. Transthyretin Arg-83 mutation in vitreous amyloidosis. Int J Ophthalmol. 2011;4(3):329–331. doi: 10.3980/j.issn.2222-3959.2011.03.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu T, Zhang B, Jin X, et al. Ophthalmic manifestations in a Chinese family with familial amyloid polyneuropathy due to a TTR Gly83Arg mutation. Eye (Lond) 2014;28(1):26–33. doi: 10.1038/eye.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin J, Xia XY, Shi Y, Lu Y, Zhao CL, Huang ZP, Tian N. Chinese familial transthyretin amyloidosis with vitreous involvement is associated with the transthyretin mutation Gly83Arg: a case report and literature review. Amyloid. 2014;21(2):140–142. doi: 10.3109/13506129.2014.892871. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Chen XW, Su G, Ren HX, Gou Y, Jiang M, Nie XM, Xie B, Cai SJ. Long-term follow-up observation after vitrectomy in a family with vitreous amyloidosis due to transthyretin gene Gly83Arg mutation. Chin J Ocul Fundus Dis. 2021;37(6):418–422. [Google Scholar]