Abstract
AIM
To evaluate corneal astigmatic outcomes of femtosecond laser-assisted arcuate keratotomies (FAKs) combined with femtosecond-laser assisted cataract surgery (FLACS) over 12mo follow-up.
METHODS
Totally 145 patients with bilateral cataracts and no ocular co-morbidities were recruited to a single-centre, single-masked, prospective randomized controlled trial (RCT) comparing two monofocal hydrophobic acrylic intraocular lenses. Eyes with corneal astigmatism (CA) of >0.8 dioptres (D) received unpaired, unopened, surface penetrating FAKs at the time of FLACS. Visual acuity, subjective refraction and Scheimpflug tomography were recorded at 1, 6, and 12mo. Alpins vectoral analyses were performed.
RESULTS
Fifty-one patients (61 eyes), mean age 68.2±9.6y [standard deviation (SD)], received FAKs. Sixty eyes were available for analysis, except at 12mo when 59 attended. There were no complications due to FAKs. Mean pre-operative CA was 1.13±0.20 D. There was a reduction of astigmatism at all post-operative visits (residual CA 1mo: 0.85±0.42 D, P=0.0001; 6mo: 0.86±0.35 D, P=0001; and 12mo: 0.90±0.39, P=0.0001). Alpins indices remained stable over 12mo. Overall, the cohort was under-corrected at all time points. At 12mo, 61% of eyes were within ±15 degrees of pre-operative astigmatic meridian.
CONCLUSION
Unpaired unopened penetrating FAKs combined with on-axis phacoemulsification are safe but minimally effective. CA is largely under-corrected in this cohort using an existing unmodified nomogram. The effect of arcuate keratotomies on CA remained stable over 12mo.
Keywords: femtosecond, cataract surgery, astigmatic keratotomy, laser cataract surgery, femtosecond-laser assisted cataract surgery, arcuate keratotomy
INTRODUCTION
The excellent refractive and visual outcomes of modern, small incision cataract surgery over recent decades have been associated with increasing patient and surgeon expectations[1]. As such the aim of modern cataract surgery is not just confined to the restoration of vision, but also to the neutralization of spherical refractive error, and corneal astigmatism (CA) to diminish postoperative dependence on spectacles[2]–[5].
Peripheral corneal relaxing incisions (PCRIs) have been employed for decades to reduce CA at the time of cataract surgery and have been accompanied by the development of multiple nomograms and methodologies to optimize their efficacy[6]–[11]. With the advent of femtosecond laser, femtosecond laser-assisted arcuate keratotomies (FAKs) have been advocated to reduce CA at the time of cataract surgery and optimize unaided visual acuity[6],[12]–[16]. It has been shown that FAKs may be more effective, reliable, and reproducible compared to manually performed PCRI[13],[15]. FAKs can be surface penetrating (pFAKs)[12]–[13] or intrastromal (iFAKs)[6],[15]–[17]. It is generally accepted that opening of pFAKs at the time of, or after the surgery increases their efficacy and is relatively straight forward undertaking in the presence of pFAKs[18]. Although largely safe, complications with pFAKs such as epithelial down-growth, wound gaping and infection have been reported[18]–[21], which has not been the case with iFAKs.
Although a number of authors have evaluated FAKs over a follow-up period of up to 6mo[6],[8],[15]–[16],[22], there are a paucity of prospective, or indeed retrospective studies that have evaluated FAKs over a longer follow-up. Visco et al[14] reported a retrospective case series, but unfortunately their 12-month follow up rate was only 41%. Chan et al[7],[23] published the results of a retrospective case series of 44 patients (44 eyes) who had undergone unopened paired pFAKs at 2mo, 2y, and more recently at 5y and showed relative stability of correction. To address this lack of data concerning FAKs, we conducted this prospective evaluation of unpaired, unopened pFAKs with a follow-up period of 12mo in a setting of a large safety and efficacy randomized controlled trial (RCT), to further evaluate their efficacy, safety, and the stability of astigmatic correction.
SUBJECTS AND METHODS
Ethical Approval
The analysis of refractive and astigmatic outcomes of patients treated with pFAKs was performed as secondary outcome of a prospective, single-center single-masked (Guy's and St Thomas' Hospital NHS Foundation Trust, London, United Kingdom), RCT (ClinicalTrials.gov, NCT02825693), approved by West-Midlands Solihull Research Ethics Committee (reference 17/WM/0414), which adhered to the tenets of the Declaration of Helsinki[12]. Full informed consent was obtained from all participants. This was a safety and efficacy trial, comparing two monofocal aspheric hydrophobic acrylic intraocular lenses (IOL). We hypothesized that unopened pFAKs combined with on-axis cataract surgery are safe and effective way of correcting low levels of CA at the time of cataract surgery.
Subjects
All patients with anterior CA greater than 0.8 dioptres (D) on corneal tomography as measured with Scheimpflug corneal tomography (Pentacam HD, Oculus Optikgeräte GmbH, Germany) and meeting the inclusion criteria of the trial underwent pFAKs[12]. Scheimpflug imaging (Pentacam HD, Oculus Optikgeräte GmbH, Germany) was used for all pre- and post-operative visits and the following “anterior” measurements were always used: “K1” “K2” and “Astig”. The technicians performing tomography ensured that each scan had a quality specification (QS) of “OK” or otherwise the scan would be repeated. Hence, at each visit, each patient had between 1 and 6 scans. As per the RCT criteria all patients had between 0.8 and 1.5 D of CA.
Inclusion Criteria
Bilateral cataracts requiring surgical intervention; good visual potential in both eyes; aged 18–100y; ability to understand informed consent and the objectives of the trial; not pregnant, not breastfeeding; no previous eye surgery; corneal astigmatism less than 1.5 D in both eyes.
Exclusion Criteria
Any patient with coexisting ocular condition that might reduce visual acuity and, hence, confound the results such as: age-related macular degeneration, glaucoma, previous retinal vascular disorders, previous retinal detachment or tear, any neuro-ophthalmological condition, any inherited retinal disorder or pathology, previous strabismus surgery or record of amblyopia, previous transient ischemic attack, cerebrovascular accident, or other vaso-occlusive disease; already enrolled in another study; exclusion criteria related to clinical contraindications for femtosecond laser assisted cataract surgery (FLACS), such as: significant corneal opacities, small pupils after pharmacological dilation, patients unable to lie sufficiently flat to be positioned underneath the laser machine.
Methods
All operations were performed under topical anaesthesia. All FAKs were performed with the LensX platform (Alcon Inc.), were transepithelial and based on Donnenfeld's nomogram via an online software program (LRI calculator, Abbott Medical Optics Inc.; available at: https://www.lricalculator.com), using Pentacam HD corneal tomography measurements (Oculus Optikgeräte GmbH, Germany). Target induced astigmatism (TIA) was always 100% correction. On-axis surgery with 2.2 mm phacoemulsification probe (placed on the steep-meridian as calculated by Pentacam) with unpaired (180 degrees away from main corneal incision) FAKs was always performed. All surgeons used a stepped corneal incision. The online LRI calculator (https://www.lricalculator.com) used the individual surgeon's surgically induced astigmatism (SIA) value [or surgically induced cylinder (SIC)], provided by each surgeon and based on personal audit results. The online calculator (https://www.lricalculator.com) used this SIC, to calculate the size (in degrees) of opposite-side limbal relaxing incision (LRI), which is then input into the femtosecond laser platform.
All pFAKs were 8.0 mm diameter unpaired arcs and were limbal centred. The arcs were programmed to be 80% penetrating based on corneal pachymetry as measured by the femtosecond laser platform integral optical coherence tomography (OCT). Other pFAK parameters were as follows: 90-degree side-cut angle; horizontal and vertical spot spacing of 5 µm and 10 µm, respectively; pulse energy of 5 mJ.
The femtosecond laser platform was utilized for creation of capsulorrhexis and nucleus division and was always performed by one surgeon (Stanojcic N). Phacoemulsification was performed in all eyes using an active-fluidics torsional phacoemulsification system (Centurion, Alcon Inc.) by six surgeons who had completed at least 30 FLACS procedures (Stanojcic N, Azan E, O'Brart D, Wagh V, Bhogal M, and Robbie S) before study commencement.
The default IOLs for in-the-bag placement were either Clareon (Alcon Inc.) or Tecnis PCB00 (Johnson & Johnson Inc.). Both are aspheric, monofocal, hydrophobic, acrylic IOLs, similar in design and performance[12]. The MA60 lens (Alcon Inc.) was used as a sulcus lens if surgically indicated.
The results were presented as standard graphs for reporting outcomes for refractive surgery[24]. Analyses of astigmatic outcomes were based on pre-operative and post-operative CA (as per Pentacam measurements) and were performed using the Alpins method[25] at all time points. We also performed sub-analysis of results for with-the-rule (WTR) astigmatism and against-the-rule (ATR) astigmatism and compared them over the same time points.
WTR astigmatism and ATR astigmatism were defined as having steep corneal meridians at 67.5° to 112.5° and either 0 to 22.5° or 157.5° to 180° as per recently published nomenclature[26].
Statistical Analysis and Power Calculations
The RCT aimed to recruit 145 patients (290 eyes). This sample size was calculated to have a 95% chance of detecting a 0.25 D difference in refraction based on a 0.4 D standard deviation (SD), a 0.06 difference logarithm of the minimum angle of resolution (logMAR) in visual acuity based on a 0.1 SD.
As vectoral analyses of FAKs were sub-group analyses, no separate prior power calculations were undertaken. Continuous data were reported using mean±SD if the data was Gaussian. Kolmogorov-Smirnov test was used to test the data for normality. Binary data were reported as frequencies and percentages and evaluated with Fisher's exact test. Student's t-tests were used for parametric data with non-parametric equivalent tests used when data failed the parametric test assumptions. Statistical analysis was performed by one investigator (Stanojcic N) who, because of the nature of intervention, could not be masked to the participants' treatment arm. Data organization and descriptive statistics were handled with Excel 2013 (Microsoft Corporation, WA, USA) and further statistical analyses with GraphPad (version 8.0; GraphPad Software, CA, USA). All statistical tests were two-sided with a significance level of 5% (P<0.05).
RESULTS
A total of 145 patients were recruited into the RCT. Of which, 5 withdrew before the surgery date and 140 continued with the trial. Seventy-one patients (142 eyes) were randomized to the Clareon IOL arm, with 140 eyes treated. Sixty-eight patients (136 eyes) were randomized to the Tecnis (PCB00) IOL group. Of these, 134 eyes (68 patients) were treated[12].
All patients underwent bilateral sequential cataract surgery with or without pFAKs between Feb 2018 and Aug 2018. Fifty-one patients (61 eyes) received pFAKs (30 eyes of 26 patients in the Tecnis group and 31 eyes of 26 patients in the Clareon group). One patient (one eye) in the Tecnis group who received a sulcus lens and corneal sutures because of surgical complication was excluded from the analysis. Hence 60 eyes of 51 were included in this analysis. Nine patients had bilateral pFAKs (5 in the Clareon IOL group and 4 in the Tecnis PCB00 group). One patient failed to attend their 12-month follow up. Mean age was 68.2y (SD 9.6). Thirteen were male and 38 were female (74%). There were 29 right eyes (48%). Mean pre-operative corrected distance visual acuity (CDVA; logMAR) was 0.32 (SD 0.29). Mean pre-operative spherical equivalent refractive error was: -0.30±1.81 (arithmetic mean±SD); 1.48±1.10 (absolute mean±SD). Mean pre-operative cylindrical (negative cylinder) refractive error was 1.14±0.71. Mean pre-operative axial length was 23.62±1.03 mm and mean pre-operative steep-axis keratometry was 43.64±5.31 D.
No complications arose due to pFAKs over the 12-month follow-up.
CA reduced in the eyes that underwent pFAKs from 1.13±0.20 D preoperatively to 0.85±0.42 D at 1mo, which was statistically significant [P=0.0001; 95% confidence interval (CI) 0.16, 0.4]. The statistically significant reduction in CA was maintained at 6mo (0.86±0.35 D, P=0.0001) and at 12mo (0.90±0.39 D, P=0.0001; 95%CI 0.11, 0.34).
Refractive cylinder (negative) in the eyes that underwent pFAKs reduced from 1.14±0.72 D preoperatively to -0.89±0.47 D at 1mo (P=0.03, 95%CI -0.47, -0.03). This significant reduction was maintained at 6mo (-0.85±0.40 D, P=0.007; 95%CI -0.5, -0.08) and at 12mo postoperatively (-0.80±0.48 D, P=0.003; 95%CI -0.56, -0.12).
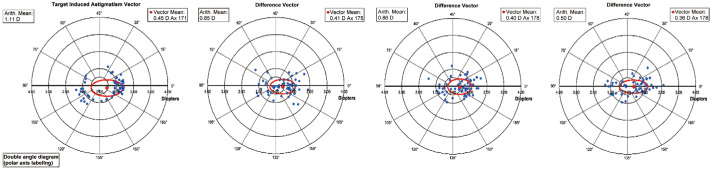
The pre-operative CA TIA and postoperative CA difference vector (DV) at 1, 6, and 12mo are displayed with double-angle plots in Figure 1 (all calculations were based on CA only).
Figure 1. The target induced astigmatism (TIA) and post-operative difference vector at 1, 6, and 12mo are displayed with double-angle plots (all calculations were based on corneal astigmatism).
Figure 2 shows TIA vs SIA relationship at each of the follow up visits (based on CA measurements only), indicating an overall under-correction of CA in our cohort.
Figure 2. Target induced astigmatism (TIA) vs surgically induced astigmatism (SIA) relationship at each of the follow up visits (1, 6, and 12mo; all calculations were based on corneal astigmatism measurements).
Alpins' vectoral indices showed no significant change between 1 and 12mo postoperatively (Table 1). At 12mo the correction index was 0.46±0.66 and 61% of treated patients were within ±15 degrees of the pre-operative astigmatic meridian. The distribution of angle of error at each of the follow up visits (1, 6, and 12mo) is shown in Figure 3.
Table 1. Postoperative astigmatic vectoral analyses.
| Vectoral analyses parameters | 1mo (n=60) | 6mo (n=60) | 12mo (n=59) |
P (95%CI) |
||
| 1mo vs 6mo | 6mo vs 12mo | 1mo vs 12mo | ||||
| TIA | ||||||
| Arithmetic (D) | 1.13±0.20 | - | ||||
| Summated vector mean | 0.46 Ax 170 | - | ||||
| SIA | ||||||
| Arithmetic | 0.68±0.43 | 0.71±0.56 | 0.72±0.60 | 0.74 (-0.21, 0.15) | 0.93 (-0.22, 0.20) | 0.68 (-0.23, 0.15) |
| Summated vector mean | 0.09 Ax 146 | 0.13 Ax 142 | 0.13 Ax 148 | - | - | - |
| Correction index | ||||||
| Geometric | 0.50±0.40 | 0.46±0.51 | 0.46±0.66 | 0.63 (-0.13, 0.21) | 1 (-0.21, 0.21) | 0.69 (-0.16, 0.24) |
| Difference vector | ||||||
| Arithmetic (D) | 0.85±0.42 | 0.86±0.35 | 0.90±0.40 | 0.89 (-0.15, 0.13) | 0.56 (-0.18, 0.10) | 0.51 (-0.20, 0.10) |
| Summated vector mean | 0.41 Ax 175 | 0.40 Ax 178 | 0.36 Ax 178 | - | - | - |
| Index of success | ||||||
| Geometric | 0.64±0.33 | 0.71±0.28 | 0.74±0.43 | 0.21 (-0.18, 0.04) | 0.65 (-0.16, 0.10) | 0.16 (-0.24, 0.04) |
| Angle of error (degree) | ||||||
| Arithmetic | 0.26±32.43 | 0.04±32.00 | 1.84±31.69 | 0.97 (-11.43, 11.87) | 0.76 (-13.36, 9.76) | 0.79 (-13.22, 10.06) |
| Absolute | 24.31±21.24 | 24.04±20.89 | 24.46±19.98 | 0.94 (-7.35, 7.89) | 0.91 (-7.84, 7.00) | 0.97 (-7.64, 7.34) |
All measurements were based on corneal astigmatism only. SD: Standard deviation; TIA: Target induced astigmatism; SIA: Surgically induced astigmatism; CI: Confidence interval.
mean±SD
Figure 3. Distribution of angle of error at each of the follow up visits (1, 6, and 12mo; all calculations were based on corneal astigmatism measurements).
WTR vs ATR sub-analysis (Table 2) showed that statistically significantly greater SIA and higher correction index were achieved in the ATR group compared to the WTR subgroup, at 1 and 6mo but not at 12mo postoperatively. At 6mo postoperatively the absolute angle of error (AE) was statistically significantly lower in the ATR group but this was not the case at 1 and 12mo.
Table 2. With-the-rule and against-the-rule subgroups astigmatic vectoral analyses over 12mo.
| Vectoral analyses parameters | WTR |
ATR |
WTR vs ATR, P (95%CI) |
||||||
| 1mo (n=35) | 6mo (n=35) | 12mo (n=34) | 1mo (n=15) | 6mo (n=15) | 12mo (n=15) | 1mo | 6mo | 12mo | |
| TIA | |||||||||
| Arithmetic (D) | 1.17±0.20 | 1.09±0.18 | 0.19 (-0.04, 0.20) | ||||||
| Summated vector mean | 1.13 Ax 179 | 1.01 Ax 99 | - | ||||||
| SIA | |||||||||
| Arithmetic | 0.53±0.33 | 0.54±0.39 | 0.58±0.51 | 0.90 ±0.47 | 1.01±0.71 | 0.96±0.80 | 0.003 (-0.60, -0.14) | 0.004 (-0.78, -0.16) | 0.05 (-0.76, 0.001) |
| Summated vector mean | 0.27 Ax 2 | 0.34 Ax 3 | 0.36 Ax 1 | 0.55 Ax 99 | 0.79 Ax 100 | 0.70 Ax 101 | |||
| Correction index | |||||||||
| Geometric | 0.37±0.30 | 0.34±0.34 | 0.34±0.48 | 0.74±0.39 | 0.74±0.70 | 0.63±0.78 | 0.001 (-0.57, 0.17) | 0.01 (-0.69, -0.11) | 0.12 (-0.66, 0.08) |
| Difference vector | |||||||||
| Arithmetic (D) | 0.97±0.42 | 0.93±0.31 | 0.98±0.33 | 0.72±0.38 | 0.76±0.42 | 0.85±0.48 | 0.05 (-0.004, 0.50) | 0.12 (-0.05, 0.39) | 0.28 (-0.11, 0.37) |
| Summated vector mean | 0.86 Ax 178 | 0.79 Ax 177 | 0.77 Ax 179 | 0.46 Ax 100 | 0.22 Ax 97 | 0.31 Ax 96 | |||
| Index of Success | |||||||||
| Geometric | 0.73±0.31 | 0.76±0.23 | 0.80±0.25 | 0.57±0.35 | 0.60±0.39 | 0.64±0.44 | 0.11 (-0.04, 0.36) | 0.08 (-0.02, 0.34) | 0.11 (-0.04, 0.36) |
| Angle of error (degree) | |||||||||
| Arithmetic | -0.61±37.58 | -4.50±35.04 | 1.60±32.65 | -1.32±25.84 | -0.32±16.29 | -4.12±28.96 | 0.95 (-20.74, 22.16) | 0.66 (-23.28, 14.92) | 0.56 (-13.98, 25.42) |
| Absolute | 28.48±24.03 | 27.56±21.60 | 25.95±19.35 | 19.23±16.54 | 11.89±10.67 | 19.46±21.24 | 0.18 (-4.47, 22.97) | 0.01 (3.84, 27.50) | 0.30 (-5.94, 18.92) |
All measurements were based on corneal astigmatism only. SD: Standard deviation; TIA: Target induced astigmatism; SIA: Surgically induced astigmatism; CI: Confidence interval; WTR: With-the-rule; ATR: Against-the-rule.
mean±SD
From standard graphs (Figure 4; difference of UDVA and CDVA), 80% of eyes that were treated with pFAKs attained their visual potential without needing correction at 12mo. Seventy six percent of the treated eyes had ≤1.00 D of residual refractive astigmatism and 76% had residual refractive spherical equivalent of ±0.50 D at 12mo. Figure 5 shows the distribution of pre-operative CA and postoperative CA at each of the follow of visits. At 1mo 71% of patients had ≤1.00 D of CA. This remained stable at 12mo (68%).
Figure 4. Distribution of postoperative UDVA and CDVA, UDVA vs CDVA (Snellen lines), spherical equivalent refractive accuracy and refractive cylinder.
UDVA: uncorrected distance visual acuity; CDVA: Best-corrected distance visual acuity.
Figure 5. Distribution of pre-operative corneal astigmatism (CA) and postoperative CA at each of the follow of visits (1, 6, and 12mo).
Mean posterior CA did not significantly change from pre-operatively compared to any post-operative point: 1mo (-0.07±0.05, 95%CI -0.16, 0.02, P=0.133), 6mo (-0.01±0.06, 95%CI -0.13, 0.12, P=0.867) and 12mo (0.04±0.04, 95%CI -0.03, 0.11, P=0.278).
DISCUSSION
To our knowledge, this is the first study that performed pFAKs in a prospective interventional trial setting using unmodified Donnenfeld nomogram on patients with low levels of CA and followed up patients with Scheimpflug tomography at 1, 6, and 12mo post-operatively with high attendance rate (100% at 1 and 6mo and 98% at 12mo).
The few studies concerning FAKs that have been published differ in terms of femtosecond laser platform, corneal tomographers used for pre-operative planning, length of follow up and surgical technique (paired vs unpaired arcs; intrastromal vs penetrating; opened vs unopened pFAKs). In addition, a variety of differing astigmatic nomograms have been employed in these studies with differing optical properties of IOLs implanted, all of which will influence post-operative visual and refractive outcomes. Indeed, in one recent study the results of both monofocal and multifocal IOLs were reported together[14].
In this study we used unmodified Donnenfeld nomogram designed for LRIs. This nomogram has previously been employed for FAKs, but no results have yet been published[18]. It has been suggested that such unmodified Donnenfeld nomograms may be relatively ineffective in reducing astigmatism with FAKs[18] with the author of the nomogram suggesting that unopened FAKs are unlikely to result in any significant astigmatism reduction[27]. However, a study by Baharozian et al[9] found that a slightly modified Donnenfeld nomogram was relatively effective in their cohort of patients undergoing FAKs. We therefore postulated in this study that pFAKs using such nomograms offered a safe and controlled method of CA correction, with the option of opening of the pFAKs post-operatively if indicated, avoiding any unpredictable refractive outcomes associated with over-correction which can result in astigmatic axis reversal and possible astigmatic anisometropia[18]. Additionally, our RCT was a safety and efficacy study and hence we aimed to use the theoretically safest possible technique. Although rare, complications associated with pFAKs have been reported[18]–[21]. Therefore, we opted for initially unpaired, unopened pFAKs to negate such rare documented problems as infection, epithelial down growth or wound gape[18]–[21]. Arcuate keratotomies have also been shown to result in increase of higher order aberrations and by not opening them we aimed to avoid issues associated with this[28]. Although we did not perform post-operative aberrometry, which is a limitation of this study, our visual and patient reported outcome measures[12] did not raise any concerns to suspect any significant effect from higher order aberrations. Akin to previous studies[13],[15],[23], we achieved with our conservative approach a statistically significant reduction in CA and our astigmatic correction was stable over 12mo with no complications associated with our pFAKs. However, despite using full 8-mm arcs the total reduction of CA by arcuate keratotomies was relatively small (Figure 1), which has previously been suggested[18],[27]. Nevertheless, when combined with on axis-surgery the pFAKs were albeit minimally, effective in reducing low amounts of CA in this cohort (Figure 5).
As expected, the change in posterior CA over 12mo was not statistically significant at any time point (compared to preoperatively) and was minimal compared to anterior CA. Additionally, these changes may contain the test-retest variability, which may be important[29], but this was not formally evaluated in this study.
The strength of our study was that we were able to achieve standardization by using only 2 different IOLs (similar in design and performance) and we were able to follow up almost all patients over 12mo. In addition, our RCT was a single-site study using the same equipment for pre-operative planning and results analysis (Pentacam HD, Oculus Optikgeräte GmbH, Germany) and the same surgical equipment in terms of the femtosecond laser platform (LensX, Alcon Inc.) and phacoemulsification machine (Centurion, Alcon Inc.).
Different research groups have reported different levels of effectiveness of FAKs over time. Day and Stevens[16] found a relatively low correction index of 0.59±0.31 in a large retrospective series of iFAKs using their own nomogram, but the follow up was limited to 1mo. Visco et al[14] in a retrospective case series of 189 eyes reported a correction index of 0.91 at 3mo, using a personalized Nichamin-Woodcock nomogram. Although the same authors[14] reported their 12-month results, the recall rate was relatively low to allow full elucidation of the stability of such astigmatic corrections [only 77/189 eyes (41%) were available for 12mo follow up]. Chan et al[7],[23] reported results from a retrospective case study, at 2mo, 2y and at 5y, having used the author's own nomogram, modified from the Wallace Limbal Relaxing Incision Nomogram. They achieved a relatively high correction index (0.85) in a cohort of 44 patients (44 eyes) and found that the effect of their arcuate keratotomies remained stable over the studied period with a tendency for increasing overcorrection of pre-operative WTR astigmatism and under-correction of ATR over the same time[22]. However, although their SIA was significantly higher in the WTR group, this difference did not translate into difference in DV at 2y, but only at 5y follow up.
Similar to the findings of Chan et al[23], we found a small increase in the effect of FAKs in the WTR group over 12mo (SIA). Although there was an initial increase in effect in ATR group from 1 to 6mo (SIA), this decreased between 6 and 12mo (SIA). However, these minimal effects were based on a relatively narrow range of mild pre-operative CA and contain the test-retest variability, which may also be important[29]. Interestingly, we found a slightly greater SIA (and correspondingly greater correction index) in the ATR sub-group compared to the WTR sub-group at 1 and 6mo postoperatively, although this statistically significant difference disappeared at 12mo. In addition, neither the DV nor the index of success (IOS) at any time point was statistically different between the WTR and ATR sub-groups. The only significant difference in angle of error was at 6mo and favoured the ATR group when only absolute angle of error was considered. This may be a result of fluctuation of corneal re-modelling or intra-individual variation in measurements. We hypothesize that this difference reverses after a longer period of time and that the effect of pFAKs in WTR group becomes greater at 2 or 5y post-operatively, which was documented in the study by Chan et al[23]. In summary, the overall effect of pFAKs was minimal in both sub-groups and the eyes were on average left with ATR astigmatism.
We only used Scheimpflug-method based tomography for corneal measurements (Pentacam HD, Oculus Optikgeräte GmbH, Germany). Both Chan et al[23] and Visco et al[14] used placido-disc based topographers (OPD-Scan III, NIDEK Co., Ltd.) for evaluation of their patients. In addition, Visco et al[14] used the LED-based corneal analyser (Cassini color LED, Cassini Technologies, B.V.) although it is not clear how these measurements were combined in the analysis. Day et al[6] and Day and Stevens[16] used a different placedo disc-based tomographer/topographer–autorefractor (KR8100PA Topcon Europe Medical BV) and their results are most closely related to ours in terms of overall under-correction as expressed by correction index (0.63±0.32 and 0.59±0.31 respectively).
We demonstrated relative stability of FAKs with minor fluctuations at different postoperative time points. Overall, our refractive and visual outcomes were good with 80% of participants attaining their visual potential without correction at 12mo (Figure 5). However, overall, our cohort was under-corrected as demonstrated by a relatively low correction index. Importantly, we encountered no intraoperative or postoperative arcuate keratotomy-related events such as perforation, wound gape, or infection.
We conclude that unpaired unopened pFAK combined with on-axis FAKs using unmodified Donnenfeld nomogram is a safe albeit minimally effective method for correcting low levels of CA at the time of cataract surgery. Larger studies are needed to compare different methods (including astigmatic nomograms) for administering FAKs and to evaluate their effectiveness over a long period of time. It will be of interest to ascertain the degree of any increase in astigmatic correction (and its long-term stability) that may be achieved by opening the pFAK incisions.
Acknowledgments
Foundation: Supported by independent research grant from Alcon (IIT #34114517).
Conflicts of Interest: Professor O'Brart D holds non-commercial research grants from Rayner Ltd. and Johnson and Johnson Inc for intraocular lens research; Stanojcic N, None; Hull C, None; Wagh V, None; Azan E, None; Bhogal M, None; Robbie S, None; Li JPO, None.
REFERENCES
- 1.Pager CK. Expectations and outcomes in cataract surgery: a prospective test of 2 models of satisfaction. Arch Ophthalmol. 2004;122(12):1788–1792. doi: 10.1001/archopht.122.12.1788. [DOI] [PubMed] [Google Scholar]
- 2.Chang DH, Hu J, Miller KM, Vilupuru S, Zhao W. Post-market evaluation of rotational stability and visual performance of a new toric intraocular lens with frosted haptics. Clin Ophthalmol. 2022;16:4055–4064. doi: 10.2147/OPTH.S389304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poll JT, Wang L, Koch DD, Weikert MP. Correction of astigmatism during cataract surgery: toric intraocular lens compared to peripheral corneal relaxing incisions. J Refract Surg. 2011;27(3):165–171. doi: 10.3928/1081597X-20100526-01. [DOI] [PubMed] [Google Scholar]
- 4.Titiyal JS, Khatik M, Sharma N, et al. Toric intraocular lens implantation versus astigmatic keratotomy to correct astigmatism during phacoemulsification. J Cataract Refract Surg. 2014;40(5):741–747. doi: 10.1016/j.jcrs.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 5.Lam DK, Chow VW, Ye C, Ng PK, Wang Z, Jhanji V. Comparative evaluation of aspheric toric intraocular lens implantation and limbal relaxing incisions in eyes with cataracts and ≤3 dioptres of astigmatism. Br J Ophthalmol. 2016;100(2):258–262. doi: 10.1136/bjophthalmol-2014-306587. [DOI] [PubMed] [Google Scholar]
- 6.Day AC, Lau NM, Stevens JD. Nonpenetrating femtosecond laser intrastromal astigmatic keratotomy in eyes having cataract surgery. J Cataract Refract Surg. 2016;42(1):102–109. doi: 10.1016/j.jcrs.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 7.Chan TC, Ng AL, Cheng GP, Wang Z, Woo VC, Jhanji V. Corneal astigmatism and aberrations after combined femtosecond-assisted phacoemulsification and arcuate keratotomy: two-year results. Am J Ophthalmol. 2016;170:83–90. doi: 10.1016/j.ajo.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Ji M, Wu J, Wang Y, Zhou J, Zhu RR, Lu H, Guan HJ. Effect of femtosecond laser-assisted steepest-meridian clear corneal incisions on preexisting corneal regular astigmatism at the time of cataract surgery. Int J Ophthalmol. 2020;13(12):1895–1900. doi: 10.18240/ijo.2020.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baharozian CJ, Song C, Hatch KM, Talamo JH. A novel nomogram for the treatment of astigmatism with femtosecond-laser arcuate incisions at the time of cataract surgery. Clin Ophthalmol. 2017;11:1841–1848. doi: 10.2147/OPTH.S141255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zukaite I, Bedi KK, Ali S, Nanavaty MA. Influence of peripheral corneal relaxing incisions during cataract surgery for corneal astigmatism up to 2.5 dioptres on corneal densitometry. Eye (Lond) 2019;33(5):804–811. doi: 10.1038/s41433-018-0327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noh H, Yoo YS, Shin KY, Lim DH, Chung TY. Comparison of penetrating femtosecond laser-assisted astigmatic keratotomy and toric intraocular lens implantation for correction of astigmatism in cataract surgery. Sci Rep. 2021;11(1):7340. doi: 10.1038/s41598-021-86763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanojcic N, O'Brart D, Hull C, Wagh V, Azan E, Bhogal M, Robbie S, Li JO. Visual and refractive outcomes and glistenings occurrence after implantation of 2 hydrophobic acrylic aspheric monofocal IOLs. J Cataract Refract Surg. 2020;46(7):986–994. doi: 10.1097/j.jcrs.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 13.Löffler F, Böhm M, Herzog M, Petermann K, Kohnen T. Tomographic analysis of anterior and posterior and total corneal refractive power changes after femtosecond laser-assisted keratotomy. Am J Ophthalmol. 2017;180:102–109. doi: 10.1016/j.ajo.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Visco DM, Bedi R, Packer M. Femtosecond laser-assisted arcuate keratotomy at the time of cataract surgery for the management of preexisting astigmatism. J Cataract Refract Surg. 2019;45(12):1762–1769. doi: 10.1016/j.jcrs.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Roberts HW, Wagh VK, Sullivan DL, Archer TJ, O'Brart DPS. Refractive outcomes after limbal relaxing incisions or femtosecond laser arcuate keratotomy to manage corneal astigmatism at the time of cataract surgery. J Cataract Refract Surg. 2018;44(8):955–963. doi: 10.1016/j.jcrs.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Day AC, Stevens JD. Stability of keratometric astigmatism after non-penetrating femtosecond laser intrastromal astigmatic keratotomy performed during laser cataract surgery. J Refract Surg. 2016;32(3):152–155. doi: 10.3928/1081597X-20160204-01. [DOI] [PubMed] [Google Scholar]
- 17.Stanojcic N, Roberts HW, Wagh VK, Li JO, Naderi K, O'Brart DP. A randomised controlled trial comparing femtosecond laser-assisted cataract surgery versus conventional phacoemulsification surgery: 12-month results. Br J Ophthalmol. 2021;105(5):631–638. doi: 10.1136/bjophthalmol-2020-316311. [DOI] [PubMed] [Google Scholar]
- 18.Vickers LA, Gupta PK. Femtosecond laser-assisted keratotomy. Curr Opin Ophthalmol. 2016;27(4):277–284. doi: 10.1097/ICU.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 19.Biswas P, Chatterjee S, Batra S, Ginodia A, Biswas P. Arcuate keratotomy infiltration following uneventful femtosecond laser assisted cataract surgery. Indian J Ophthalmol. 2019;67(10):1742–1744. doi: 10.4103/ijo.IJO_72_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou TY, Abazari A, Barash A, Shah S, Kaplowitz K. Early-onset methicillin-resistant Staphylococcus aureus keratitis and late-onset infectious keratitis in astigmatic keratotomy incision following femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2015;41(8):1772–1777. doi: 10.1016/j.jcrs.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Grillo LM, Epstein IJ, Donnenfeld ED, Perry HD. Late-onset microsporidial keratitis in femtosecond astigmatic keratotomy after laser-assisted phacoemulsification. Cornea. 2018;37(11):1471–1473. doi: 10.1097/ICO.0000000000001743. [DOI] [PubMed] [Google Scholar]
- 22.Byun YS, Kim S, Lazo MZ, Choi MH, Kang MJ, Lee JH, Yoo YS, Whang WJ, Joo CK. Astigmatic correction by intrastromal astigmatic keratotomy during femtosecond laser–assisted cataract surgery: factors in outcomes. J Cataract Refract Surg. 2018;44(2):202–208. doi: 10.1016/j.jcrs.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Chan TCY, Ng ALK, Wang Z, Chang JSM, Cheng GPM. Five-year changes in corneal astigmatism after combined femtosecond-assisted phacoemulsification and arcuate keratotomy. Am J Ophthalmol. 2020;217:232–239. doi: 10.1016/j.ajo.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Reinstein DZ, Archer TJ, Srinivasan S, Mamalis N, Kohnen T, Dupps WJ, Jr, Randleman JB. Standard for reporting refractive outcomes of intraocular lens-based refractive surgery. J Cataract Refract Surg. 2017;43(4):435–439. doi: 10.1016/j.jcrs.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Alpins N. Astigmatism analysis by the Alpins method 2. J Cataract Refract Surg. 2001;27(1):31–49. doi: 10.1016/s0886-3350(00)00798-7. [DOI] [PubMed] [Google Scholar]
- 26.Abulafia A, Koch DD, Holladay JT, Wang L, Hill W. Pursuing perfection in intraocular lens calculations. J Cataract Refract Surg. 2018;44(10):1169–1174. doi: 10.1016/j.jcrs.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg ED, Nattis AS, Donnenfeld ED. Astigmatic keratotomy and limbal relaxing incisions: principles, indications, nomograms. https://www.sightmd.com/wp-content/uploads/2019/11/LRI-PAPERFINAL-1.pdf; accessed on 2021.1.30.
- 28.Kim BK, Chung YT. Two-year outcomes after full-thickness astigmatic keratotomy combined with small-incision lenticule extraction for high astigmatism. BMC Ophthalmol. 2021;21(1):23. doi: 10.1186/s12886-020-01756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klijn S, van der Sommen CM, Sicam VADP, Reus NJ. Value of posterior keratometry in the assessment of surgically induced astigmatic change in cataract surgery. Acta Ophthalmol. 2016;94(5):494–498. doi: 10.1111/aos.13003. [DOI] [PubMed] [Google Scholar]