Abstract
Aims
To assess time to, cumulative incidence of, and functional benefit of achieving sustained ≥2-step Diabetic Retinopathy Severity Scale (DRSS) improvement in diabetic macular oedema (DMO).
Methods
Post hoc analysis of VISTA/VIVID including eyes with DMO treated with intravitreal aflibercept injections (IAI), 2 mg q4 weeks (2q4, n = 250) or q8 weeks after 5 monthly doses (2q8, n = 249), or laser control (n = 249). Changes from baseline in best-corrected visual acuity (BCVA) and central subfield thickness (CST) were evaluated in sustained (≥2 consecutive visits) DRSS subgroups (≥1-step worsening, no change, ≥2-step improvement).
Results
Time to sustained ≥2-step DRSS improvement was shorter for both the IAI 2q4 and IAI 2q8 groups versus laser (both log-rank p < 0.001). Cumulative incidences of sustained ≥2-step DRSS improvement with IAI 2q4 and IAI 2q8 versus laser were 40.0% and 42.8% versus 15.5% (both p < 0.001) through week 100. Mean differences (95% CI) in BCVA gains from baseline at weeks 52 and 100 between eyes with sustained ≥2-step DRSS improvement versus sustained ≥1-step DRSS worsening were –3.0 (–8.9, 2.9) and 6.2 (0.2, 12.2) letters with laser, and 4.2 (0.8, 7.6) and 4.9 (1.3, 8.4) letters with IAI combined, respectively. Difference (95% CI) in CST reduction was significantly greater only with IAI combined at week 100 (–83.0 [–140.8, –25.3]). Correlations between BCVA and CST changes were weak.
Conclusions
DMO eyes treated with IAI achieved sustained ≥2-step DRSS improvement significantly earlier and more frequently versus laser. This improvement was associated with greater BCVA gains, independent of CST reductions.
Trial registration
ClinicalTrials.gov (https://clinicaltrials.gov/) identifiers: NCT01363440 and NCT01331681.
Subject terms: Retinal diseases, Eye abnormalities
Introduction
Diabetic retinopathy (DR) is a progressive microvascular complication of diabetes that, when left untreated, can result in loss of vision and blindness. The risk of progressing from nonproliferative DR (NPDR) to proliferative DR (PDR) increases with the severity of NPDR. Approximately 50% of patients with severe NPDR (Diabetic Retinopathy Severity Scale [DRSS] score of 53) develop PDR after 1 year as compared with 26% of patients with moderately severe NPDR (DRSS score of 47) or up to 12% of those with moderate NPDR or better (DRSS score of ≤43) [1]. Worsening DR is associated with lower vision-related quality of life and increasing vision-related functional burden [2–4]. Diabetic macular oedema (DMO) may occur at any stage of DR but shows higher prevalence with increasing severity of DR [5]. The hallmark of PDR is angiogenesis in the retina and anterior segment. In addition to the higher prevalence of DMO, eyes with PDR have an increased risk for other vision-threatening complications such as vitreous haemorrhage, macular ischaemia, and tractional retinal detachment [1].
Anti-vascular endothelial growth factor (anti-VEGF) agents, such as intravitreal aflibercept injection (IAI), ranibizumab, and off-label bevacizumab significantly reduce DMO and improve vision and are the standard-of-care for treatment of DMO. In the VISTA and VIVID trials, IAI improved DR severity by ≥2 steps on DRSS in approximately 1/3 of eyes with DMO at both weeks 52 and 100 [6–8]. A recent post hoc analysis of the RIDE and RISE trials suggested that eyes with DRSS improvement may have greater visual outcomes [9]. The relationship between DRSS improvement and visual outcomes in eyes treated for DMO, however, is not fully understood. In clinical practice when treating eyes with DR, and to successfully manage patient expectations, it is important to know the time to achieving clinically meaningful DRSS improvements, sustainability of the DRSS improvement, and potential functional benefit thereof. Here, we report the findings of a post hoc analysis evaluating the time to onset of sustained DRSS improvement and the potential functional benefit of such improvement in eyes with DR treated for DMO with IAI or laser in the VISTA and VIVID trials.
Materials and methods
VISTA and VIVID study design
VISTA (NCT01363440) and VIVID (NCT01331681) were 2 similarly designed, double-blind, randomised, active-controlled, phase 3 trials as described previously [8]. Briefly, VISTA was conducted across 54 sites in the United States and VIVID was conducted in 73 sites across Australia, Europe, and Japan. Each clinical site’s respective institutional review board/ethics committee approved the study protocol. All patients provided written informed consent. Both studies were conducted in compliance with the International Conference on Harmonisation guidelines and the Health Insurance Portability and Accountability Act.
Adult patients with type 1 or 2 diabetes mellitus who presented with central-involved DMO, defined as retinal thickening involving the 1-mm central optical coherence tomography (OCT) subfield thickness, were eligible for enrolment if best-corrected visual acuity (BCVA) was between 73 and 24 letters (20/40–20/320 Snellen equivalent) in the study eye. Only one eye per patient was enrolled in the study. Eyes were randomised in a 1:1:1 ratio to receive IAI 2 mg every 4 weeks (2q4), IAI 2 mg every 8 weeks after 5 initial monthly doses (2q8), or macular laser photocoagulation (laser control group). Eyes were treated through week 96.
In VISTA and VIVID, BCVA was measured at baseline and every 4 weeks using the Early Treatment Diabetic Retinopathy Study protocol [10]. Central subfield thickness (CST) was assessed at baseline and every 4 weeks using spectral-domain OCT. DRSS scores were assessed at baseline and at weeks 24, 52, 72, and 100 using fundus photography. Masked graders at independent central reading centres evaluated OCT images for CST (Duke Reading Center, Durham, NC, USA, for VISTA; Vienna Reading Center, Vienna, Austria, for VIVID) and fundus images for DRSS score assessments (Digital Angiography Reading Center, Great Neck, NY, USA, for VISTA; Vienna Reading Center, Vienna, Austria, for VIVID).
Post hoc analysis
This post hoc analysis included eyes that received study medication and had a baseline and ≥1 postbaseline BCVA assessment (full analysis set) with gradable baseline fundus images. Three DRSS subgroups of sustained ≥1-step worsening, sustained no change, and sustained ≥2-step improvement were analysed within each treatment group. Sustained was defined as the indicated changes at both weeks 24 and 52 (for the week 52 analysis) or at both weeks 72 and 100 (for the week 100 analysis). For eyes that received rescue treatment, data were censored from the time rescue treatment was given. All analyses were performed using observed data.
Outcome measures
Cumulative incidence of sustained ≥2-step DRSS improvement was evaluated from baseline through week 100 for the laser control and IAI treatment groups. For each of the 3 sustained DRSS subgroups, mean changes from baseline in BCVA and CST were evaluated at weeks 52 and 100 in the laser control and IAI combined (2q4 + 2q8 after 5 initial monthly doses) treatment groups. The correlation between BCVA gains and CST reductions within each DRSS subgroup was evaluated at weeks 52 and 100. PDR events were defined as the incidence of PDR, panretinal photocoagulation, or vitrectomy.
Statistical methods
The time to an event was evaluated by Kaplan–Meier analysis. The log-rank test was used to test the difference between the cumulative incidence curves of the treatment groups. Hazard ratios comparing the IAI groups with the laser control group were estimated by Cox proportional hazards analysis stratified by study (VISTA vs VIVID). The time at risk for each eye was defined as the minimum time from randomisation to whichever of the following occurred first: (a) the date a patient discontinued the study, (b) the date of the episode of the first evaluated event, or (c) the end of the study. Time at risk was expressed as 100 person-years at risk (PYR), and the rate was expressed as the number of events/PYR. The relative hazard was defined as the ratio of the hazard rate in each IAI group to that of the laser control group. The correlations between BCVA gain and CST reduction were evaluated by Pearson correlation analysis. The absolute value of the correlation coefficient (r) ≤0.4 was considered a weak correlation. The difference in BCVA gain between the 2 groups was evaluated by an unpaired t-test. The Cochran–Mantel–Haenszel test was used to examine the difference in the incidence of PDR events between treatment groups. All p values were considered nominal. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Patients
Of 872 eyes in VISTA and VIVID, 10 eyes were excluded from the post hoc analysis due to a missing BCVA assessment (either at baseline or during the study), and an additional 114 eyes were excluded due to nongradable fundus images at baseline (Fig. 1). Hence, 748 eyes were included in this post hoc analysis (Table 1). Baseline characteristics of these patients were similar across treatment groups with respect to age, gender, duration of diabetes mellitus, haemoglobin A1c status, and DRSS severity (Table 1).
Fig. 1. Flow diagram.
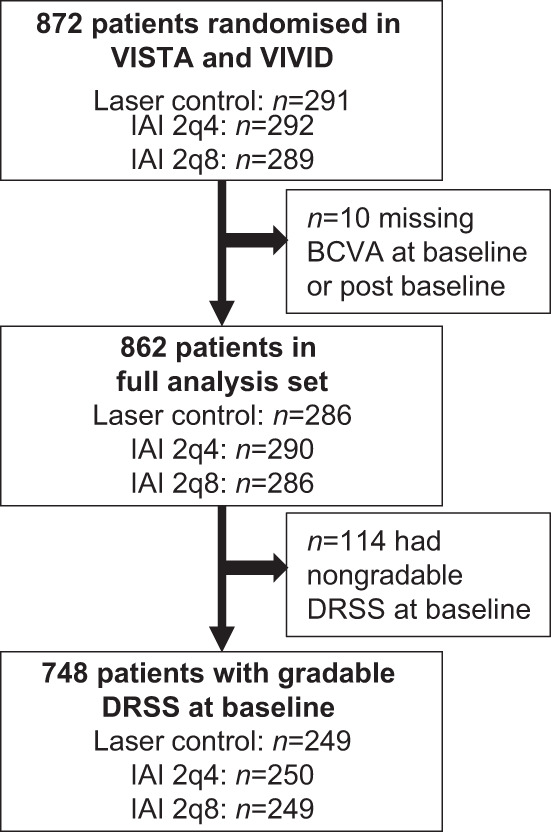
IAI intravitreal aflibercept injection, 2q4 2 mg q4 weeks, 2q8 2 mg q8 weeks after 5 initial monthly doses, BCVA best-corrected visual acuity, DRSS Diabetic Retinopathy Severity Scale.
Table 1.
Baseline characteristics of patients with gradable baseline DRSS.
| Laser control (n = 249) | IAI 2q4 (n = 250) | IAI 2q8 (n = 249) | IAI combined (n = 499) | Total (N = 748) | |
|---|---|---|---|---|---|
| Age, mean (SE), years | 62.6 (0.6) | 61.9 (0.6) | 63.4 (0.6) | 62.7 (0.4) | 62.7 (0.3) |
| Female, n (%) | 107 (43.0) | 103 (41.2) | 104 (41.8) | 207 (41.5) | 314 (42.0) |
| Duration of diabetes mellitus | |||||
| N | 247 | 248 | 248 | 496 | 743 |
| Mean (SE), years | 16.5 (0.6) | 15.6 (0.6) | 16.3 (0.7) | 15.9 (0.5) | 16.1 (0.4) |
| Haemoglobin A1c | |||||
| N | 248 | 246 | 249 | 495 | 743 |
| >8%, n (%) | 75 (30.1) | 95 (38.0) | 88 (35.3) | 183 (36.7) | 258 (34.5) |
| DRSS score, n (%) | |||||
| ≤43 | 108 (43.4) | 96 (38.4) | 97 (39.0) | 193 (38.7) | 301 (40.2) |
| 47 | 50 (20.1) | 44 (17.6) | 59 (23.7) | 103 (20.6) | 153 (20.5) |
| ≥53 | 91 (36.5) | 110 (44.0) | 93 (37.3) | 203 (40.7) | 294 (39.3) |
IAI intravitreal aflibercept injection, 2q4 2 mg q4 weeks, 2q8 2 mg q8 weeks after 5 initial monthly doses, SE standard error, DRSS Diabetic Retinopathy Severity Scale.
At baseline, 710 of 748 eyes had NPDR (DRSS score ≤53). Among these eyes, 11.1% (26/235) of eyes in the laser control group and 4.4% (21/475) of eyes in the IAI combined group subsequently experienced a PDR event through week 100 (difference –6.7% [95% CI –11.7, –1.6]; p = 0.0008). In these eyes, mean BCVA at baseline and weeks 52 and 100 were, respectively, 60.5, 60.3, and 60.6 letters in the laser control group, and 57.0, 66.9, and 63.6 letters in the IAI combined group. In contrast to eyes experiencing a PDR event, 35.3% (88/249) of eyes in the laser control group and 66.3% (331/499) of eyes in the IAI combined group subsequently experienced an improvement in DRSS score (≥1 step) through week 100.
Sustained ≥2-step DRSS improvement
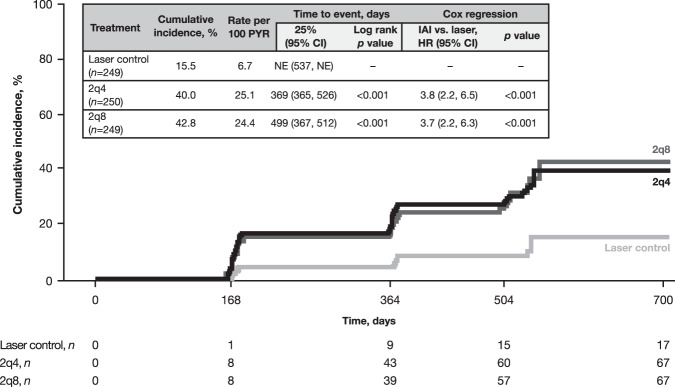
Time to first sustained ≥2-step DRSS improvement was shorter with IAI 2q4 and IAI 2q8 versus laser (both log-rank p < 0.001; Fig. 2). The cumulative incidence of first sustained ≥2-step DRSS improvement from baseline to week 100 was higher with IAI 2q4 and IAI 2q8 compared with eyes treated with laser control (40.0% and 42.8% vs 15.5%, respectively; both p < 0.001; Fig. 2). Overall, compared with laser control, eyes treated with IAI 2q4 and 2q8 were 3.8-fold and 3.7-fold more likely to achieve a sustained ≥2-step DRSS improvement, respectively (Fig. 2).
Fig. 2. Cumulative incidence of sustained ≥2-step DRSS improvement from baseline through week 100.
DRSS was assessed at baseline and at weeks 24, 52, 72, and 100. DRSS Diabetic Retinopathy Severity Scale, PYR patient-years at risk, HR hazard ratio, IAI intravitreal aflibercept injection, NE not estimable, 2q4 2 mg q4 weeks, 2q8 2 mg q8 weeks after 5 initial monthly doses.
BCVA outcomes in sustained DRSS subgroups
Visual benefits of achieving a sustained DRSS improvement were assessed across DRSS subgroups within both the laser control and IAI combined treatment groups at weeks 52 and 100. Changes in CST were also evaluated to examine the correlation between BCVA gain and CST reduction within each DRSS subgroup. Baseline characteristics of patients in the sustained DRSS subgroups of ≥1-step worsening, no change, and ≥2-step improvement in each treatment group at weeks 52 and 100 are shown in Supplementary Tables 1 and 2, respectively. Mean baseline BCVA and CST among eyes assessed at weeks 52 and 100 were similar across DRSS subgroups in both the laser control and IAI combined treatment groups.
Changes from baseline to week 52
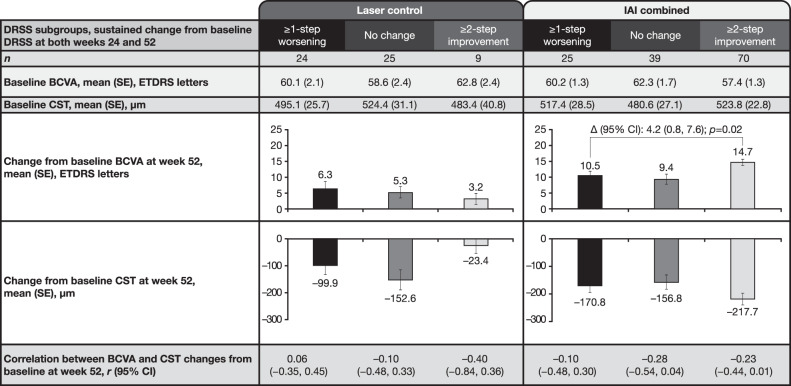
In the laser control group, mean BCVA gains from baseline were 6.3, 5.3, and 3.2 letters at week 52 in eyes with sustained ≥1-step worsening, no change, and ≥2-step DRSS improvement, respectively (Fig. 3). The corresponding CST reductions from baseline were –99.9, –152.6 and –23.4 µm at week 52, respectively. Across the sustained DRSS subgroups, the differences in BCVA gains and CST reductions were not statistically significant within the laser control group. The correlation (r) between BCVA gain and CST reduction within each sustained DRSS subgroup was also weak, ranging from –0.40 to 0.06 (Fig. 3).
Fig. 3. Change from baseline in BCVA and CST by treatment and sustained DRSS subgroups at week 52.
Observed cases. Full analysis set. Data from rescued patients were censored after rescue. Patients who did not have available BCVA at week 52 were excluded. BCVA best-corrected visual acuity, CST central subfield thickness, DRSS Diabetic Retinopathy Severity Scale, IAI intravitreal aflibercept injection, ETDRS Early Treatment Diabetic Retinopathy Study, Δ difference.
In the IAI combined group, mean BCVA gains from baseline were 10.5, 9.4, and 14.7 letters at week 52 in eyes with sustained ≥1-step worsening, no change, and ≥2-step DRSS improvement, respectively. Mean BCVA gain in eyes with ≥2-step DRSS improvement was greater than in eyes with sustained ≥1-step worsening (difference 4.2 [95% CI 0.8–7.6]; p = 0.02). The corresponding CST reductions from baseline were –170.8, –156.8, and –217.7 µm for eyes with sustained ≥1-step worsening, no change, and ≥2-step DRSS improvement, respectively, and were not statistically different from each other. The correlation (r) between BCVA gain and CST reduction within each sustained DRSS subgroup was also weak, ranging from –0.28 to –0.10 (Fig. 3).
Changes from baseline to week 100
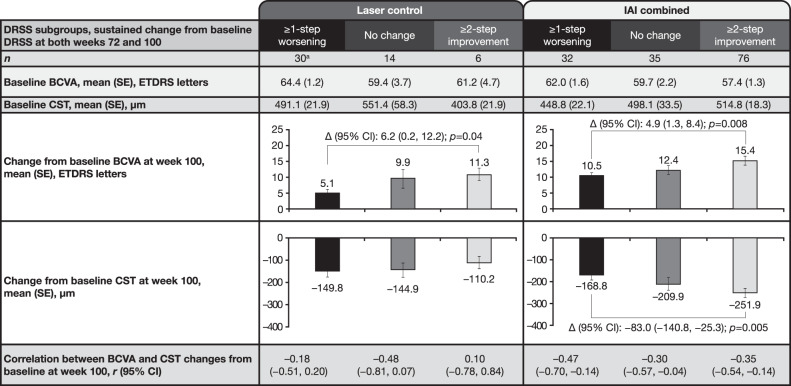
In the laser control group, mean BCVA gains from baseline were 5.1, 9.9, and 11.3 letters at week 100 in eyes with sustained ≥1-step worsening, no change, and ≥2-step DRSS improvement, respectively. Mean BCVA gain in eyes with sustained ≥2-step DRSS improvement was greater than in eyes with sustained ≥1-step worsening (difference 6.2 [95% CI 0.2, 12.2]; p = 0.04). The corresponding CST reductions from baseline were –149.8, –144.9, and –110.2 µm for eyes with sustained ≥1-step worsening, no change, and ≥2-step DRSS improvement, respectively. Differences in CST reductions were not significant across the DRSS subgroups. The correlation (r) between BCVA gain and CST reduction within each sustained DRSS subgroup was also weak, ranging from –0.48 to 0.10 (Fig. 4).
Fig. 4. Change from baseline in BCVA and CST by treatment and sustained DRSS subgroups at week 100.
aOne patient did not have CST data at week 100. Observed cases. Full analysis set. Data from rescued patients were censored after rescue. Patients who did not have available BCVA at week 100 were excluded. BCVA best-corrected visual acuity, CST central subfield thickness, DRSS Diabetic Retinopathy Severity Scale, IAI intravitreal aflibercept injection, ETDRS Early Treatment Diabetic Retinopathy Study, Δ difference.
In the IAI combined group, mean BCVA gains from baseline were 10.5, 12.4, and 15.4 letters at week 100 in eyes with sustained ≥1-step worsening, no change, and ≥2-step DRSS improvement, respectively. Mean BCVA gain in eyes with sustained ≥2-step DRSS improvement was greater than in eyes with sustained ≥1-step worsening (difference 4.9 [95% CI 1.3, 8.4]; p = 0.008). The corresponding CST reductions from baseline were –168.8, –209.9, and –251.9 µm for eyes with sustained ≥1-step worsening, no change, and ≥2-step DRSS improvement, respectively. Mean CST reduction in eyes with sustained ≥2-step DRSS improvement was greater than in those with sustained ≥1-step worsening (difference –83.0 [95% CI –140.8, –25.3]; p = 0.005). The correlation (r) between BCVA gain and CST reduction within each sustained DRSS subgroup was relatively weak, ranging from –0.47 to –0.30 (Fig. 4).
Discussion
The findings from the current analysis of the VISTA and VIVID trials demonstrate that with continued IAI treatment approximately 40% of patients achieved and maintained ≥2-step DRSS improvement for ≥1 year. In this patient population, time to achieving sustained ≥2-step DRSS improvement was shorter with IAI 2q4 and IAI 2q8 than with laser treatment over the 100-week study. Compared with laser control, nearly 4 times as many eyes treated with IAI achieved such improvement and did so significantly earlier.
These findings suggest that in patients with DR and DMO, achieving and sustaining a ≥2-step improvement in DRSS score requires a long-term commitment to the therapy, which is expected given the chronic nature of the underlying disease. We also further explored whether there is a functional benefit associated with such anatomic improvement. Our findings demonstrate that a sustained ≥2-step DRSS improvement with IAI was associated with greater visual acuity improvements compared with a sustained ≥1-step DRSS worsening, at both weeks 52 and 100. The association between sustained ≥2-step DRSS improvement and greater visual acuity gain was independent of reductions in CST, as shown by no or weak correlation between changes in BCVA and CST from baseline. This finding is consistent with the preponderance of data to date, suggesting that there is at best a moderate correlation between changes in visual acuity and changes in CST in DMO [11, 12]. A recent post hoc analysis of the Protocol T trial data found that there was a broad range of changes in BCVA from baseline for any given change in CST from baseline (and vice versa) at 1 and 2 years, suggesting that CST changes are not a reliable guide to inform about changes in BCVA with anti-VEGF treatment [12]. Our findings up to 100 weeks confirm the results of a past post hoc analysis of the RISE and RIDE trials that looked at the relationship between DRSS changes and visual outcomes over 24 weeks, suggesting that DRSS improvement may be associated with better visual outcomes, and provide further evidence that such benefit may be independent of CST reduction in patients with DMO [9].
Although the dosing regimen in VIVID and VISTA for IAI was either 2q4 or 2q8 (following 5 initial monthly doses), recent data from the PANORAMA trial suggest that a longer dosing interval can also lead to long-term ≥2-step DRSS improvements in patients with moderately severe or severe NPDR without central-involved DMO at baseline [13]. These data provide additional evidence to help inform physicians regarding appropriate treatment options and injection intervals for patients with DR.
Despite treatment, some patients with DMO may still experience progression to PDR [14]. In the current study, 2.5-fold higher proportion of eyes treated with laser control experienced a PDR event compared with those treated with IAI. Vision did not appear to be clinically affected by the occurrence of PDR events in this subpopulation of eyes treated with laser control or IAI.
A strength of the current analysis is the use of data from large, controlled clinical trials using fixed dosing schedules. BCVA measurements and OCT evaluations were performed by independent, masked examiners and reading centres, respectively, according to a standardised protocol. The results, however, must be interpreted with caution due to the post hoc nature of this analysis. There were a small and unbalanced number of patients, particularly in the laser group among those who achieved sustained ≥2-step DRSS improvement. This was due to the strict criteria for this analysis, which included only patients who had not received rescue treatment, had available BCVA at weeks 52 or 100, and had sustained ≥2-step DRSS improvement over 2 consecutive visits.
In conclusion, the data reported here suggest that a substantial proportion of patients treated with IAI for DMO achieve sustained DRSS improvement, which may confer an additional benefit of a greater magnitude of visual acuity improvements, over 2 years of continued treatment. These findings may help clinicians establish an optimal anti-VEGF treatment strategy and appropriately manage patient expectations.
Summary
What was known before
In clinical practice, for managing expectations of patients with diabetic retinopathy, it is important to know the time to achieving clinically meaningful DRSS improvements as well as the sustainability and potential functional benefit of these improvements. However, the relationship between DRSS improvement and visual outcomes in patients treated for DMO is not fully understood.
What this study adds
Approximately 40% of patients treated with IAI for DMO achieved sustained DRSS improvement for over 1 year, which was accompanied by a greater magnitude of vision gains. These findings on the clinical importance of the sustained DRSS improvements may help clinicians establish an optimal anti-VEGF treatment strategy and appropriately manage patient expectations.
Supplementary information
Acknowledgements
Medical writing support was provided by Melissa Purves, PhD, and Rob Campbell, PhD, of Core (London, UK) according to Good Publication Practice guidelines (link) and was funded by Regeneron Pharmaceuticals, Inc.
Author contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the concept and design of the study; acquisition, analysis, and interpretation of the data; drafting of the manuscript; and critical revision of the manuscript for important intellectual content. WD provided the statistical analysis.
Funding
This study was funded by Regeneron Pharmaceuticals, Inc.
Data availability
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymised participant data will be considered for sharing once the indication has been approved by major health authorities, if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.
Competing interests
DSD is a consultant for Bayer, Novartis, Regeneron Pharmaceuticals, Inc., Genentech, Allergan, Alimera, Santen, Allegro, Notal Vision, and Eyepoint Pharmaceuticals. HM, KR, WD, RV and AJB are employees of and hold equity in Regeneron Pharmaceuticals, Inc. RPS is a consultant for Genentech/Roche, Regeneron Pharmaceuticals, Inc., Novartis, Zeiss, Bausch and Lomb, Asclepix, Alcon, and Gyroscope and has received research funding from Apellis and NGM Biopharma.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-022-02058-7.
References
- 1.Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98:823–33. doi: 10.1016/S0161-6420(13)38014-2. [DOI] [PubMed] [Google Scholar]
- 2.Coyne KS, Margolis MK, Kennedy-Martin T, Baker TM, Klein R, Paul MD, et al. The impact of diabetic retinopathy: perspectives from patient focus groups. Fam Pract. 2004;21:447–53. doi: 10.1093/fampra/cmh417. [DOI] [PubMed] [Google Scholar]
- 3.Mazhar K, Varma R, Choudhury F, McKean-Cowdin R, Shtir CJ, Azen SP, et al. Severity of diabetic retinopathy and health-related quality of life: the Los Angeles Latino Eye Study. Ophthalmology. 2011;118:649–55. doi: 10.1016/j.ophtha.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willis JR, Doan QV, Gleeson M, Haskova Z, Ramulu P, Morse L, et al. Vision-related functional burden of diabetic retinopathy across severity levels in the United States. JAMA Ophthalmol. 2017;135:926–32. doi: 10.1001/jamaophthalmol.2017.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102:7–16. doi: 10.1016/S0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell P, McAllister I, Larsen M, Staurenghi G, Korobelnik JF, Boyer DS, et al. Evaluating the impact of intravitreal aflibercept on diabetic retinopathy progression in the VIVID-DME and VISTA-DME studies. Ophthalmol Retin. 2018;2:988–96. doi: 10.1016/j.oret.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–54. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122:2044–52. doi: 10.1016/j.ophtha.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Ip MS, Zhang J, Ehrlich JS. The clinical importance of changes in diabetic retinopathy severity score. Ophthalmology. 2017;124:596–603. doi: 10.1016/j.ophtha.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. doi: 10.1001/archopht.1985.01050120030015. [DOI] [PubMed] [Google Scholar]
- 11.Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, Fong DS, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–36. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bressler SB, Odia I, Maguire MG, Dhoot DS, Glassman AR, Jampol LM, et al. Factors associated with visual acuity and central subfield thickness changes when treating diabetic macular edema with anti-vascular endothelial growth factor therapy: an exploratory analysis of the Protocol T randomized clinical trial. JAMA Ophthalmol. 2019;137:382–9. doi: 10.1001/jamaophthalmol.2018.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DM, Wykoff CC, Boyer D, Heier JS, Clark WL, Emanuelli A, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139:946–55. doi: 10.1001/jamaophthalmol.2021.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymised participant data will be considered for sharing once the indication has been approved by major health authorities, if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.