Abstract
Bacillus coagulans has a potential role in improving intestinal injury. However, the specific mechanism is still unclear. In this study, the protective effect of B. coagulans MZY531 on intestinal mucosa injury in cyclophosphamide (CYP)-induced immunosuppressed mice were investigated. The results indicated that the immune organ (thymus and spleen) indices of B. coagulans MZY531 treatment groups were significantly increased compared to the CYP group. B. coagulans MZY531 administration promotes the expression of immune proteins (IgA, IgE, IgG, and IgM). B. coagulans MZY531 could upregulate the ileum levels of IFN-γ, IL-2, IL-4, and IL-10 in immunosuppressed mice. Moreover, B. coagulans MZY531 restores the villus height and crypt depth of the jejunum and alleviates injury of intestinal endothelial cells caused by CYP. Furthermore, the western blotting results showed that B. coagulans MZY531 ameliorated CYP-induced intestinal mucosal injury and inflammatory via up-regulates the ZO-1 pathway and down-regulates the expression of the TLR4/MyD88/NF-κB pathway. After treatment with B. coagulans MZY531, the relative abundance of Firmicutes phylum was dramatically increased, as well as the genera of Prevotella and Bifidobacterium, and reducing harmful bacteria. These findings suggested that B. coagulans MZY531 has a potential immunomodulatory activity on chemotherapy-induced immunosuppression.
Subject terms: Microbiology, Zoology, Gastroenterology
Introduction
Normal gastrointestinal function is essential for the absorption of nutrients, while gastrointestinal changes can lead to serious defects in the intestinal barrier and gastrointestinal diseases1,2. Tight junction proteins, including ZO-1, occludin, and claudin-1, constitute intestinal epithelial cells as a physical barrier, and their complex interactions maintain the integrity of the intestinal barrier and reduce intestinal permeability3. Intestinal leakage can activate immune cells to secrete inflammatory cytokines, which in turn increases intestinal permeability and causes systemic inflammation4. In addition, microorganisms in the gastrointestinal tract can also damage the intestinal barrier function by promoting mucosal reaction, reducing the expression of tight junction proteins. After reaching the blood, bacteria can trigger inflammation by binding TLR4 receptors expressed on the liver through the portal vein5,6.
Cyclophosphamide (CYP) was an alkylated anticancer agent which was widely used in the treatment of various cancers. However, long-term treatment of CYP may could result various side effects such as acute cytotoxicity, immunosuppression, and gastrointestinal mucosal barrier damage. To improve this situation, some protective agents are increasingly being used to alleviate adverse side effects in chemotherapy patients. Certain anti-parasitics have also been reported to have an immunomodulatory activity such as levamisole. Levamisole is a drug widely used to enhance the immunity of various human diseases, including leprosy, rheumatoid arthritis, and in adjuvanted therapy of colorectal cancer. In recent years, more and more attention has been paid to alleviating CYP induced immunosuppression based on gut microbiota regulation. In 2013, Viaud et al. demonstrated that CYP alters microbiota composition in the small intestine and induces the translocation of selected species of Gram-positive bacteria into secondary lymphoid organs7. A few years later, Xie et al. discovered that Lactobacillus plantarum intervention could protect the intestinal mucosal injury and intestinal barrier function in mice induced by CYP by regulating intestinal flora imbalance8. Therefore, regulating intestinal flora, inflammation, and intestinal barrier may be a novel potential therapeutic strategy for the treatment of the intestinal injury.
Probiotics are considered a novel choice to reduce the side effects of chemotherapy on patients. Probiotics can maintain the balance of intestinal microorganisms and protect the intestinal integrity of epithelial cells by increasing the mucus layer and the expression of TJ9. Bacillus coagulans, as a probiotic, can stay in the gut for a longer time; it also has a strong adhesive ability and significant immunomodulatory effect10. It has been reported that some active components in the fermentation supernatant of B. coagulans can form a biological protective barrier in the human intestinal tract, promote the immune response of the digestive tract mucosa, and thus improve intestinal immunity11. In addition, B. coagulans 13002 can stimulate the growth of Bifidobacterium and Lactobacillus and reduce the intestinal side effects caused by cyclophosphamide12. Furthermore, B. coagulans TL3 can protect rats from inflammation caused by endotoxin and inhibit the reproduction of harmful bacteria by blocking the expression of the TLR4 pathway so as to enhance intestinal immunity13. Moreover, previous studies have shown that B. coagulans 13002 and fructooligosaccharides significantly reduce CYP-induced intestinal mucosal damage and improve immune function by regulating intestinal microflora12. In this study, we examined the effects of B. coagulans MZY531 (MZY531) on intestinal mucosal injury, inflammation, and intestinal microflora induced by CYP in mice and explored its protective mechanism. These data provide a theoretical basis for the development and utilization of B. coagulans and support its addition to functional foods to improve intestinal health.
Materials and methods
Preparation of bacterial strain
B. coagulans MZY531 is a probiotic strain isolated from naturally fermented kimchi and stored in China Center for Type Culture Collection (CCTCC, accession M2021622, Wuhan, China). B. coagulans MZY531 was inoculated in LB liquid medium and cultured in a constant temperature vibration incubator for 48 h (180 r/min, 50 °C). Then the culture medium was centrifuged (2000×g, 10 min) and washed three times with aseptic phosphate-buffered saline (PBS, pH 7.4) to remove the residual medium and collect bacteria. Next, the bacteria were resuspended in saline solution, and the concentration was adjusted to 1.0 × 109 CFU/mL, which was stored at 4 °C for subsequent intragastric administration of mice.
Animals and experimental design
Immunosuppressive model was induced by CYP according to previous study14. A total of 40 7-week-old female BALB/C mice were purchased from Changchun Yisi Experimental Animal Technology Co., Ltd. (Changchun, China). All mice were kept in a suitable environment with a temperature of 22 ± 1 °C, relative humidity of 50 ± 1%, and a light/dark cycle of 12 h, and had free access to water and food. All animal studies (including the mice euthanasia procedure) were done in compliance with the regulations and guidelines of the Jilin Academy of Agricultural Sciences institutional animal care and conducted according to the AAALAC and the IACUC guidelines.
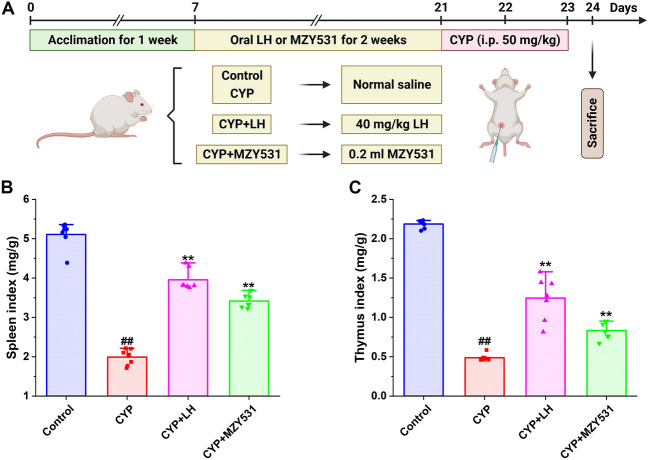
The experimental animal protocol is shown in Fig. 1A. After 1 week of adaptation, the mice were randomly divided into four groups (n = 10 in each group): Control group, CYP group, CYP + LH group, and CYP + MZY531 group. The body weights of the mice were measured twice every week. The CYP + LH group was given 40 mg/kg levamisole hydrochloride (LH), the CYP + MZY531 group was given B. coagulans MZY531, and the Control and CYP groups were given the same dose of normal saline. All mice were given oral administration according to the volume of 0.1 mL/10 g for 14 days, once daily. The immunosuppression mouse model induced by CTX was established according to the previous method. CYP (50 mg/kg/days) was intraperitoneally injected into the mice in CYP, CYP + LH, and CYP + MZY531 groups on days 15 and 16. Control group was administered intraperitoneally with the same volume of physiological saline. After the last injection, the mice were starved for 24 h but given free access to water. The mice were sacrificed by cervical dislocation, and the jejunum, ileum, spleen, and feces were collected.
Figure 1.
A schedule of experimental procedures (A) and effects of B. coagulans MZY531 on spleen (B) and thymus (C) index in immunosuppressed mice. All data were statistically analyzed using a one-way analysis of variance and Tukey multiple comparison. #P < 0.05 and ##P < 0.01 vs. control group; *P < 0.05 and **P < 0.01 vs. CYP group.
Determination of immune organ index
Before the mice were killed, the final weight of the mice was recorded. Then, the thymus and spleen tissues were immediately dissected, washed in precooled normal saline at 4 °C, dried using filter paper, and weighed. The spleen and thymus index were calculated according to the following formula: spleen and thymus index = spleen and thymus weight (mg)/final weight (g)15.
Determination of immune and inflammatory factors in the ileum
The ileum of mice was quickly collected and placed in an ice bath. An appropriate amount of ileum tissue was then selected and mixed with normal saline according to the proportion of 1:9 to prepare 10% tissue homogenate. Next, the homogenate was centrifuged (4000×g, 10 min) at 4 °C, and the supernatant was collected. The immunoglobulin (IgA, IgE, IgG, and IgM) and inflammatory factors (IL-2, IFN-γ, IL-4, and IL-10) concentrations were detected using ELISA kits according to the instructions of Jiangsu Enzymatic Biology Co., Ltd. (Jiangsu, China). The optical density (OD) value of the solution was measured at 450 nm using an automatic microplate reader.
Pathological observation of jejunum
The fresh jejunum tissue was washed with normal saline, fixed in 4% paraformaldehyde for 48 h, embedded in paraffin, and prepared into 8-μm slices. Then, samples were stained with hematoxylin–eosin (H&E) for 5 min, dehydrated, and sealed with neutral glue. The pathological changes in the jejunum of each group were observed under light microscope (Nikon Corporation, Tokyo, Japan), and the villus length (V) and crypt depth (C) were recorded16. Moreover, the images were acquired at a magnification of × 25 and × 200 (Supplementary Information).
Western blotting
The ileal tissue was lysed by RIPA kit, and the supernatant was collected. The BCA kit determined the total protein concentration in the supernatant. Samples were then mixed with the protein sample buffer at 1:1 and boiled in a water bath for 8 min to collect the proteins, which were then separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membrane. The membrane was blocked for 60 min in TBST solution containing 3% bovine serum albumin (BSA) and then incubated with rabbit anti-ZO-1, Occludin, Claudin-1, TLR4, MyD88, NF-κB, IKBα, and β-actin overnight at 4 °C. Samples were then incubated with horseradish peroxidase (HRP) labeled secondary antibody for 60 min at room temperature. Samples were then washed with TBST three times, and the protein expression was detected by an enhanced chemiluminescence reagent. The gray values of the bands were detected by Image Quant LAS 4000 (Shanghai, China) and standardized by β-actin.
Gut microbial analysis
Fresh fecal samples of the cecum were immediately frozen in liquid nitrogen and stored at − 80 °C. QIAamp Fast DNA kit was used to extract total DNA from feces. According to the previous study17, the V3 and V4 regions of 16S rDNA were amplified by universal primers using polymerase chain reaction (PCR). Then the amplified products were sequenced by Illumina MiSeq, and the sequences of high quality with 97% similarity were incorporated into a taxon on QIIME software, and the diversity of gut microbiota was analyzed. The Chao1, Shannon, Simpson, and Pielou-e indices were used to investigate α diversity. The principal coordinate method of weighted UniFrac phylogenetic distance matrix was used to analyze β diversity; the relative abundance at the gate level was used to indicate the difference in bacterial colony structure among groups, and the heat map analysis showed the difference in different microorganisms at the genus level. In addition, Spearman’s analysis revealed the correlation between gut microbiota and immune and inflammatory levels in mice. The original data and sequencing sample data obtained in this study can be obtained from the National Center for Biotechnology Information (NCBI) database with the registration number: PRJNA884309.
Statistical analysis
All the experimental data were expressed as mean ± standard deviation (SD). SPSS20.0 and Origin8.0 were used for data processing and analysis. The overall significant difference was evaluated by single factor analysis of variance (ANOVA) and Tukey multiple comparisons. A P value < 0.05 was considered to be statistically significant.
Institutional review board statement
The animal experiment procedures were approved by the Committee of Animal Experimental Ethical Inspection of Laboratory Animal Centre, Yanbian University (approved number: SCXK-2020-0001).
ARRIVE guidelines
All the research methods contained in the manuscript are carried out in accordance with the requirements of ARRIVE.
Results
B. coagulans MZY531 increases the immune organ index of intestinal injury mice
As shown in Fig. 1, the intervention of CYP significantly decreased the spleen (Fig. 1B) and thymus (Fig. 1C) index of mice compared with the control group, while the treatment of LH and B. coagulans MZY531 significantly increased the spleen and thymus index (all P < 0.01). After B. coagulans MZY531 treatment, the spleen and thymus index increased by 71.36% and 69.39%, respectively, compared with the CYP group (all P < 0.01). These results indicate that B. coagulans MZY531 could effectively alleviate immune organ atrophy induced by CYP. Additionally, the spleen and thymus indexes of the B. coagulans MZY531 group were higher than in the CYP group, with the indexes being close to those of the LH group. These findings suggest that B. coagulans MZY531 plays a crucial role in preventing the atrophy of immune organs.
B. coagulans MZY531 increases the level of immune protein in the ileum of intestinal injury mice
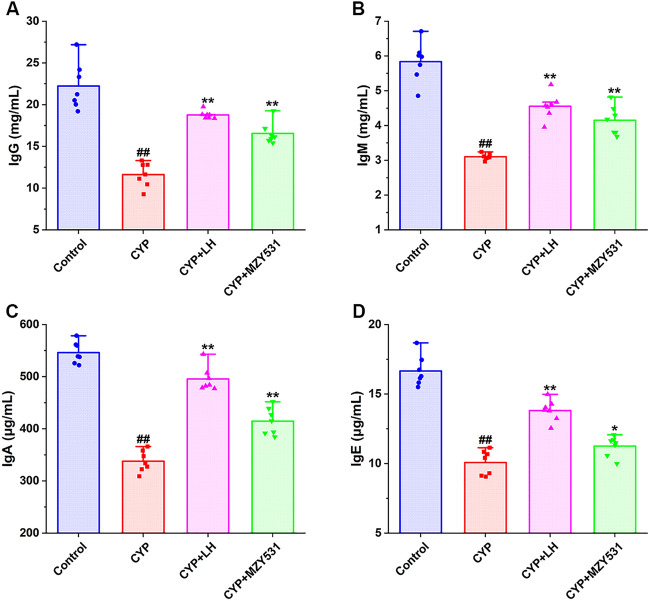
The results showed that (Fig. 2A–D), the induction of CYP significantly decreased the levels of IgG, IgM, IgA, and IgE by 47.75%, 46.92%, 38.15%, and 39.50%, respectively, compared with the control group (all P < 0.01). The levels of IgG, IgM, IgA, and IgE in the B. coagulans MZY531 treatment were significantly higher than those in the CYP group (P < 0.05), approaching the values of the control group. After the B. coagulans MZY531 treatment, the levels of IgG and IgM were similar to those of the positive control LH group (P > 0.05). These results showed that the treatment of B. coagulans MZY531 could reverse the decrease of immune protein level induced by CYP and improve the immunity of mice.
Figure 2.
Effects of B. coagulans MZY531 on the levels of immune proteins level in ileum of mice with intestinal injury induced by CYP. IgG (A); IgM (B); IgA (C) and IgE (D). All data were statistically analyzed using a one-way analysis of variance and Tukey multiple comparison. #P < 0.05 and ##P < 0.01 vs. control group; *P < 0.05 and **P < 0.01 vs. CYP group.
B. coagulans MZY531 increases the level of anti-inflammatory cytokines in the ileum of intestinal injury mice
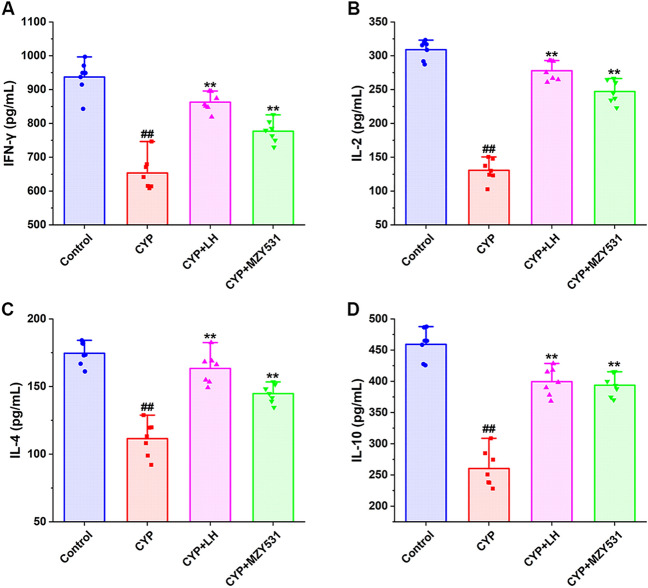
As shown in Fig. 3A–D, the levels of IFN-γ, IL-2, IL-4, and IL-10 in the CYP group were significantly lower than those in the control group (P < 0.05), indicating that CYP could significantly inhibit the production of anti-inflammatory cytokines. Compared with the CYP group, the treatment of B. coagulans MZY531 significantly increased the levels of IFN-γ, IL-2, IL-4, and IL-10 by 18.86%, 89.03%, 29.81%, and 51.14%, respectively (P < 0.05). The above results also showed that B. coagulans MZY531 could improve the anti-inflammatory ability of CYP-induced intestinal injury model mice. Furthermore, B. coagulans MZY531, in particular, significantly improved the secretion of IL-10. These results indicated that B. coagulans MZY531 could improve inflammatory responses by increasing the secretion of anti-inflammatory cytokines in ileum of CYP-induced immunosuppressed mice.
Figure 3.
Effects of B. coagulans MZY531 on the levels of anti-inflammatory factors in ileum of immunosuppressed mice. IFN-γ (A); IL-2 (B); IL-4 (C) and IL-10 (D). All data were statistically analyzed using a one-way analysis of variance and Tukey multiple comparison. #P < 0.05 and ##P < 0.01 vs. control group; *P < 0.05 and **P < 0.01 vs. CYP group.
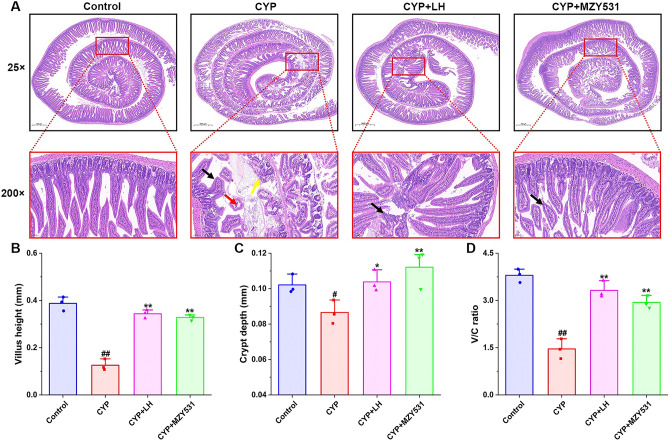
B. coagulans MZY531 improves the histomorphological changes of jejunum in intestinal injury mice
The results of HE staining of the jejunum of mice are shown in Fig. 4A, while no obvious pathological changes in jejunum were found in the blank group. In the CYP group, the jejunal villi were shortened and exfoliated (black arrow), the intestinal epithelium of the local mucous layer was missing, the lamina propria was exposed (yellow arrow), slight edema could be seen locally, and the gap between the intestinal epithelium and lamina propria was seen (red arrow). However, the villi length increased, and the intestinal epithelial structure was significantly recovered in LH and B. coagulans MZY531 groups. In addition, the treatment of MZY531 significantly increased villus length (Fig. 4B), crypt depth (Fig. 4C), and the V/C ratio (Fig. 4D), which were 153.85%, 26.44%, and 101.37%, respectively, higher than those of the CYP group (all P < 0.01). These results also suggested that B. coagulans MZY531 could improve the pathological intestinal damage in CYP-induced intestinal injury model mice.
Figure 4.
Effects of B. coagulans MZY531 on the histomorphological changes of jejunum in immunosuppressed mice. The pathological changes of jejunum (magnification × 25 and × 200) (A). Black arrows indicated the shorterning of intestinal villi, yellow arrows indicated exposed lamina propria, and black arrows indicated mild edema and enlarged spaces. Villus height (B), Crypt depth (C). The ratio of villus height to crypt depth (D). All data were statistically analyzed using a one-way analysis of variance and Tukey multiple comparison. #P < 0.05 and ##P < 0.01 vs. control group; *P < 0.05 and **P < 0.01 vs. CYP group.
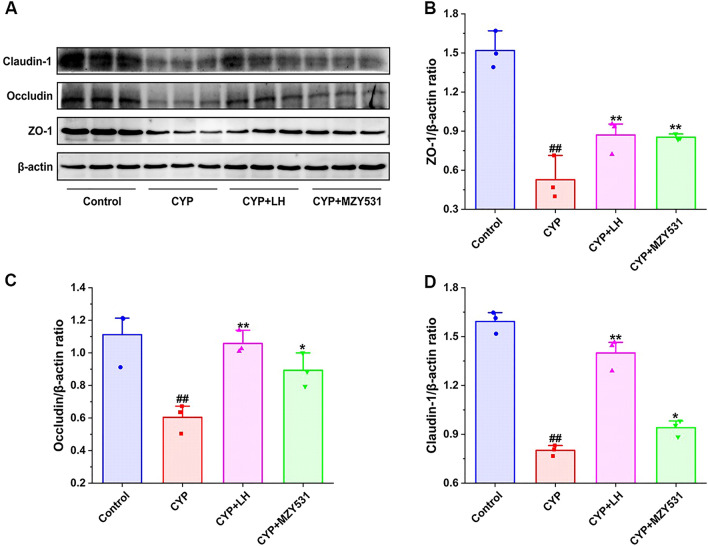
B. coagulans MZY531 improves intestinal barrier function in intestinal injury mice
The effect of B. coagulans MZY531 on proteins (ZO-1, occludin, and claudin-1) of the intestinal barrier pathway in the ileum of mice was evaluated by Western blotting. As shown in Fig. 5, compared with the blank group, the induction of CYP significantly decreased the protein levels of ZO-1, occludin, and claudin-1 (all P < 0.05). Compared with CYP group, Intervention with B. coagulans MZY531 significantly increased the protein expression of ZO-1, occluding, and claudin-1 by 61.87% (P < 0.01), 47.80% (P < 0.05), and 17.32% (P < 0.05), respectively. Based on this result, B. coagulans MZY531 could repair the intestinal barrier damage induced by CYP in mice.
Figure 5.
Effects of B. coagulans MZY531 on ZO-1 intestinal barrier pathway in ileum of immunosuppressed mice. The protein expression of Claudin-1, Occludin and ZO-1 in the ileal were detected by western blot (A). The ratios of ZO-1/β-actin (B), Occludin/β-actin (C) and Claudin-1/β-actin (D) protein bands for each region were quantified using densitometry and presented in the graph. All data were statistically analyzed using a one-way analysis of variance and Tukey multiple comparison. #P < 0.05 and ##P < 0.01 vs. control group; *P < 0.05 and **P < 0.01 vs. CYP group.
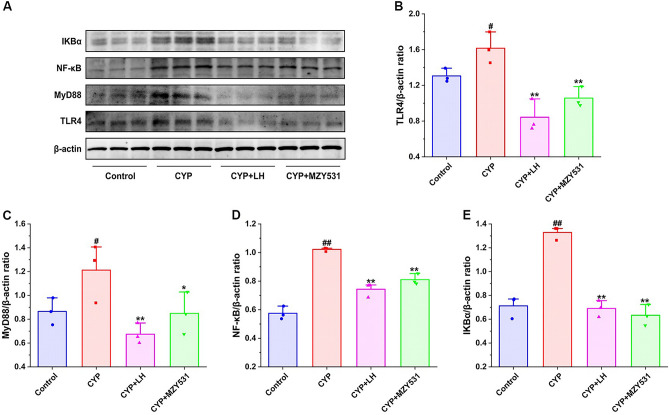
B. coagulans MZY531 inhibits the level of inflammation in intestinal injury mice
The results presented in Fig. 6 shows that the expression of TLR4 inflammatory pathway protein in mouse jejunum. The induction of CYP significantly upregulated the levels of TLR4, MyD88, NF-κB, and IKBα. On the contrary, the treatment of LH and B. coagulans MZ531 significantly inhibited the expression of TLR4, MyD88, NF-κB and IKBα, and the level of MZY531 group was down-regulated by 34.55% (P < 0.01), 29.95% (P < 0.05), 20.71% (P < 0.01), and 52.36% (P < 0.01) compared with CYP group, respectively. Thus, these results revealed that the treatment of B. coagulans MZY531 markedly resisted the expression of intestinal inflammation induced by CYP through downregulation of TLR4/MyD88/NF-κB inflammatory signaling pathways.
Figure 6.
Effects of B. coagulans MZY531 on TLR4/MyD88 inflammatory pathway in ileum of immunosuppressed mice. The protein expression of IKBα, NF-κB, MyD88 and TLR4 in the ileal were detected by western blot (A). The ratios of TLR4/β-actin (B), MyD88/β-actin (C), NF-κB/β-actin (D) and IKBα/β-actin (E) protein bands for each region were quantified using densitometry and presented in the graph. All data were statistically analyzed using a one-way analysis of variance and Tukey multiple comparison. #P < 0.05 and ##P < 0.01 vs. control group; *P < 0.05 and **P < 0.01 vs. CYP group.
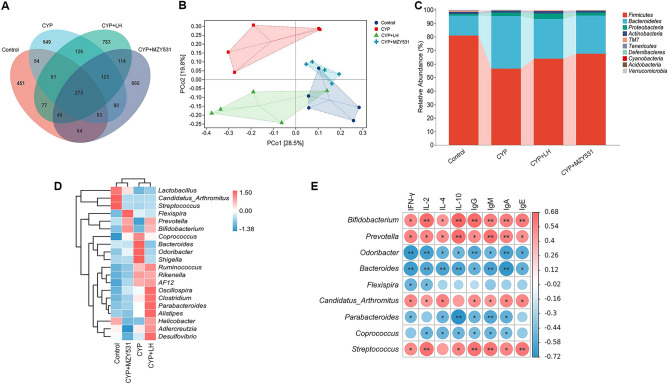
B. coagulans MZY531 remodels the intestinal microflora of intestinal injury mice
We evaluated the effect of B. coagulans MZY531 on gut microbiota by 16S rRNA high-throughput sequencing. The results of α diversity showed that (Table 1), the indexes of Chao1, Shannon, Simpson, and Pielou-e in the B. coagulans MZY531 group were significantly higher than those in the CYP group (all P < 0.01), which suggested that the intervention of B. coagulans MZY531 increased the richness and diversity of gut microbiota. Venn diagram (Fig. 7A) further showed that the four groups shared 273 OTU, while the number of unique OTU in the blank group, CYP group, LH group, and MZY531 group was 451, 549, 783, and 666, respectively, indicating that the treatment of B. coagulans MZY531 increases the number of OTU induced by CYP. In addition, in PCoA analysis (Fig. 7B), the CYP group was far away from the blank group, LH group, and MZY531 group, while the treatment of LH and B. coagulans MZY531 made the diversity of gut microbiota of mice more inclined to the blank group. In order to further evaluate the specific changes in gut microbiota, we investigated the relative abundance of gut microbiota (Fig. 7C) at the gate level. We found that B. coagulans MZY531 treatment increased Firmicutes but decreased Bacteroidetes abundance. In genus-level thermographic analysis (Fig. 7D), the intervention of B. coagulans MZY531 increased the abundance of probiotic, including Lactobacillus, Prevotella and Bifidobacterium, and decreased the level of harmful bacteria Odoribacter and Shigella. These results showed that the intervention of B. coagulans MZY531 could reshape the structure of gut microbiota, increase the abundance of probiotics, and reduce the level of pathogenic bacteria.
Table 1.
Effects of MZY531 on α-diversity of gut microbiota in mice with intestinal injury induced by CYP.
| Groups | Chao1 | Shannon | Simpson | Pielou_e |
|---|---|---|---|---|
| Control | 516.75 ± 112.82** | 5.57 ± 0.66** | 0.9326 ± 0.027** | 0.6286 ± 0.05* |
| CYP | 341.79 ± 82.56## | 4.33 ± 0.52## | 0.8397 ± 0.04## | 0.5254 ± 0.05# |
| CYP + LH | 548.66 ± 39.71** | 5.83 ± 0.49** | 0.9488 ± 0.024** | 0.651 ± 0.05** |
| CYP + MZY531 | 615.62 ± 72.18** | 6.00 ± 0.41** | 0.9516 ± 0.02** | 0.658 ± 0.04** |
All data were statistically analyzed using a one-way analysis of variance and Tukey multiple comparison.
#P < 0.05 and ##P < 0.01 vs. control group; *P < 0.05 and **P < 0.01 vs. CYP group.
Figure 7.
Effects of B. coagulans MZY531 on the changes of gut microbiota in immunosuppressed mice. Venn diagram (A); PCoA analysis (B). The species compositions analysis at phylum level (C). The heat map at genus level (D). The correlation analysis between gut microbiota and intestinal immune proteins and anti-inflammatory factors at genus level by Spearman analysis (E). #P < 0.05 and ##P < 0.01 vs. control group; *P < 0.05 and **P < 0.01 vs. CYP group.
Next, Spearman analysis was used to analyze the correlation between single strains of gut microbiota and immune and anti-inflammatory proteins in mice (Fig. 7E), revealing that Prevotella and Bifidobacterium were positively correlated with immune proteins, including IgG, IgM, IgA, and IgE, and anti-inflammatory factors, including IFN-γ, IL-2, IL-4, and IL-10, while Bacteroidetes and Odoribacter was negatively correlated with immune proteins and anti-inflammatory factors. These results further reveal that the intervention of B. coagulans MZY531 could increase the immunity and anti-inflammatory ability of mice by increasing the abundance of probiotics.
Discussion
CYP is a widely used chemotherapeutic drug for cancer treatment. Yet, CYP can seriously damage the body’s immunity and induce the disorder of intestinal microflora, thus increasing the risk of immune deficiency and intestinal injury diseases18. It has been found that probiotics can improve the function of intestinal microflora by regulating the value of specific microflora in the intestinal tract, thus having a beneficial effect on the body19. Therefore, we speculate that probiotics may be a new therapeutic way to alleviate CYP-induced intestinal injury. This study focused on the protective effect of B. coagulans MZY531 on intestinal injury induced by CYP.
As important immune organs, spleen and thymus have an important role in regulating systemic immune function20. Our study showed that the intervention of B. coagulans MZY531 significantly increases the spleen and thymus index of mice induced by CYP. Coincidentally, Awad et al. reported that the use of probiotics increases the spleen and thymus index of broilers21; similar results were obtained by Kabir et al.22. In addition, the occurrence of immune dysfunction seems to be regulated by IFN-γ and IL-4 levels, and the decrease of their levels may lead to impaired immune function23. Studies have shown that CYP, as an effective immunosuppressant, can induce the decrease of IFN-γ and IL-4 levels, thus destroying immune homeostasis and leading to immunosuppression24. It is worth noting that B. coagulans MZY531 treatment significantly increases the levels of IFN-γ and IL-4 and increases the expression of immune-related cytokines, including IgG, IgM, IgA, and IgE. Furthermore, Bomko et al. found that B. coagulans had normalised both the quantitative parameters of the immune system and the cells’ functional activity by decreasing the level of immune-related cytokines25. This study further suggests that treating B. coagulans MZY531 can resist the immune function damage induced by CYP by improving the function of immune organs and the level of immune protein.
As the main food digestive organ most easily affected by foreign antigens or microorganisms, the intestinal tract is the first line of defense against pathogenic microorganisms26. A harmful environment may induce oxidation and inflammation in the intestinal tract, damaging intestinal mucosal. Studies have shown that CYP can induce the shortening and shedding of intestinal villi and, in turn, lead to intestinal mucosal damage27. In this study, the pathological results showed that the treatment of B. coagulans MZY531 significantly reduces the shedding of intestinal villi, increases the crypt depth, and reduces the edema of intestinal endothelial cells, which indicates that the intervention of B. coagulans MZY531 could alleviate the intestinal mucosal injury induced by CYP. It is worth noting that the impairment of intestinal mucosal barrier function seems to be the main inducing factor of intestinal mucosal injury28. Therefore, increasing intestinal barrier function seems to be a good means to prevent or treat intestinal injury. As intestinal tight junction proteins, ZO-1, occludin, and claudin-1 have an important role in maintaining intestinal barrier permeability and constitute intestinal mucosal barrier with intestinal epithelial cells29,30. According to previous studies, B. coagulans SCC-19 improves the intestinal barrier of carp induced by heavy metal cadmium (Cd) by up-regulating the mRNA expression of ZO-1, occluding, and claudin-131. In addition, Zhou et al. confirmed that NCU116 extracellular polysaccharides of Lactobacillus plantarum could increase the expression of the ZO-1 tight junction protein pathway by promoting the binding of STAT3 to occludin and ZO-1 promoters, thus repairing the intestinal mechanical barrier function induced by dextransodiumsulfate (DSS)32. Importantly, our study also found that MZY531 activates the expression of ZO-1, occluding, and claudin-1 proteins, thus restoring intestinal mucosal barrier function. Therefore, we speculate that MZY531 may improve intestinal permeability by activating the expression of intestinal tight junction protein, thus resisting intestinal mucosal injury induced by CYP.
In recent years, increasing evidence has shown that intestinal injury can also induce intestinal leakage (leaky gut), causing bacteria and their metabolites to translocate to the blood, releasing inflammatory factors, including LPS and TNF-α33. In addition, the accumulation of pro-inflammatory mediators can break the balance of anti-inflammatory and pro-inflammatory factors and further aggravate the inflammatory cascade and intestinal injury34. As a specific anti-inflammatory factor, IL-10 has an important role in enhancing the anti-inflammatory ability of the body35. It has been reported that probiotics can reduce advocated inflammatory expression by activating IL-10-mediated immune pathways36. Interestingly, similar results were obtained in this study. Therefore, enhancing the expression of the anti-inflammatory factor IL-10 may be one of the keys to controlling intestinal inflammation. Nevertheless, some studies have reported that the expression of IL-10 is affected by the TLR4 pathway. As a type I transmembrane protein expressed on the cell membrane, TLR4 has an important role in regulating the balance of inflammation37. The NF-κB pathway is a downstream signal transduction pathway dependent on TLR4/MyD88 pathway. When the body is in normal homeostasis, NF-κB will bind to I-κB and remain static38. However, external stimulation can activate the expression of the TLR4/MyD88 pathway and further induce the activation of the I-κB complex, thus regulating the expression of target genes, including TNF-α, IL-1β, IL-6, and IL-1039. It is reported that B. coagulans TL3 inhibits intestinal inflammation induced by LPS through TLR4/MyD88, which suggests that the TLR4/MyD88 signal pathway may be the signal transduction mechanism of B. coagulans inhibiting intestinal inflammation expression13. In addition, some scholars have reported that the combined treatment of several probiotics, including B. coagulans, suppresses DSS-induced colitis by up-regulating the level of IL-1040. Interestingly, it has been reported that E5564, an antagonist of TLR4, can competitively bind to TLR4-MD2, further inhibit the activation of downstream NF-κB and promote the release of anti-inflammatory factor IL-1041. In this study, we found that MZY531 down-regulates the expression of the TLR4/MyD88 pathway and increases the level of IL-10. This further suggests that B. coagulans MZY531 may be an inhibitor of TLR4, i.e., it increases the level of IL-10 in the intestine of mice by inhibiting the TLR4/MyD88 pathway, thus improving the anti-inflammatory ability of mice and finally improving the intestinal inflammatory injury induced by CYP.
B. coagulans can regulate the disorder of intestinal microflora, which has a beneficial effect on the host42. Studies have shown increased abundance and diversity of probiotics in feces collected from elderly taking B. coagulans GBI-30 and 6086 for 28 days43. Our study also obtained consistent results; we found that the intervention of B. coagulans MZY531 increases the total number of bacteria in the feces of mice with intestinal injury induced by CYP, which also indicates that B. coagulans MZY531 restores the abundance of intestinal flora. In addition, we also found that B. coagulans MZY531 can increase the abundance of probiotics (Bifidobacterium, Prevotella and Firmicutes) and reduce the number of bacteria causing inflammation (Bacteroides and Shigella). Of note, it has also been reported that taking B. coagulans 13002 increases intestinal damage induced by cyclophosphamide by increasing the abundance of probiotics12. In addition, Xie et al. reported that taking L. plantarum NCU116 may increase the number of Bifidobacteria in the feces of mice, and further reduce the disorder of gut microbiota, thus improving the intestinal mucosal damage induced by CYP8. This indirectly confirms that B. coagulans MZY531 has an important role in improving the intestinal injury induced by CYP. Interestingly, we examined the correlation between intestinal flora and intestinal immune function and anti-inflammatory ability by Spearman analysis, finding that Bifidobacterium and Prevotella were positively correlated with immune proteins and anti-inflammatory factors in the intestinal tract; similar conclusions were reached by Li et al.44. This further suggests that the intervention of B. coagulans MZY531 can improve the immune and anti-inflammatory ability of the intestine by increasing the abundance of probiotics in the intestine (Fig. 8), thus reducing the intestinal injury caused by intestinal inflammation and immunosuppression caused by CYP.
Figure 8.
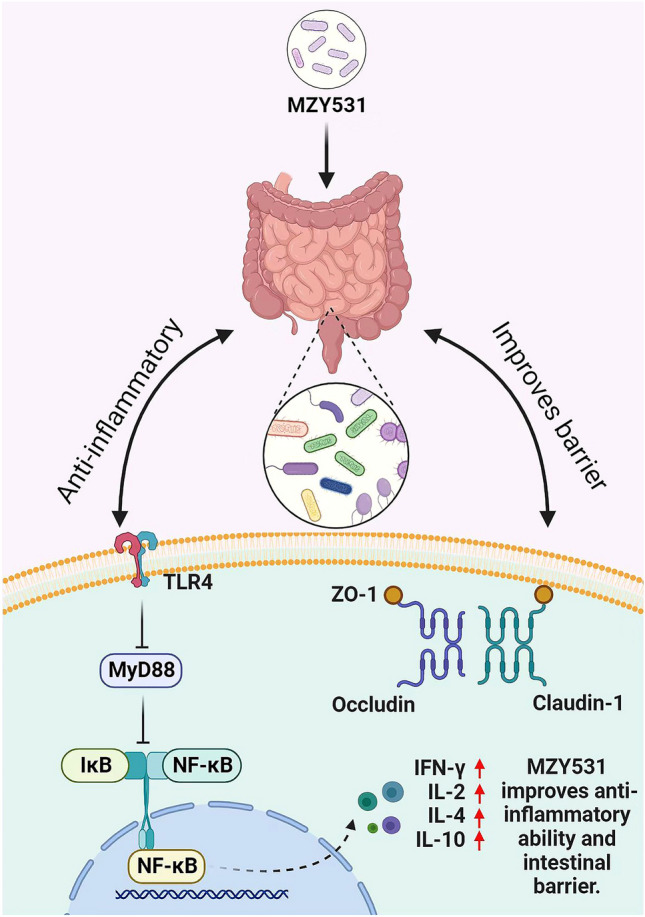
Schematic illustration showing the mechanisms of B. coagulans MZY531 alleviates intestinal mucosal injury in immunosuppressive mice.
Conclusion
To sum up, B. coagulans MZY531 treatment improves intestinal barrier function and inflammatory expression in CYP-induced immunosuppressive mice, and its possible mechanism is related to the ZO-1 intestinal barrier pathway and TLR4/MyD88 inflammatory pathway. In addition, B. coagulans MZY531 also improves the disorder of intestinal microflora by increasing the abundance of probiotics in the intestine and further improving the immune function and anti-inflammatory ability of mice. Therefore, this study provides a new research idea for treating intestinal injury in CYP-induced immunosuppressive mice and a solid theoretical basis for the development and utilization of B. coagulans.
Supplementary Information
Acknowledgements
This work was sponsored by 2022 Jilin Province Science and Technology Development Plan, Natural Science Foundation of Jilin Province (20220101310JC).
Author contributions
Methodology, Software, Formal analysis, Investigation, Writing—original draft, Writing—review & editing, Visualization, Z.Z.; Methodology, Supervision, Writing—review & editing, M.S.; Project administration, Data curation, Writing-review & editing, X.C.; Writing—review & editing, J.C.; Conceptualization, Methodology, C.L.; Project administration, Resources, Formal analysis, Investigation, Writing—original draft, Writing—review & editing, Funding acquisition, Supervision, X.Z. All authors have read and agreed to the published version of the manuscript.
Data availability
The data presented in this study are available on request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-38379-0.
References
- 1.McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm. Bowel Dis. 2009;15:100–113. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso-Silva D, et al. Intestinal barrier function in gluten-related disorders. Nutrients. 2019;11:2325. doi: 10.3390/nu11102325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odenwald MA, Turner JR. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teshima CW, Dieleman LA, Meddings JB. Abnormal intestinal permeability in Crohn's disease pathogenesis. Ann. N. Y. Acad. Sci. 2012;1258:159–165. doi: 10.1111/j.1749-6632.2012.06612.x. [DOI] [PubMed] [Google Scholar]
- 5.Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020;20:40–54. doi: 10.1038/s41577-019-0198-4. [DOI] [PubMed] [Google Scholar]
- 6.Allam-Ndoul B, Castonguay-Paradis S, Veilleux A. Gut microbiota and intestinal trans-epithelial permeability. Int. J. Mol. Sci. 2020;21:6402. doi: 10.3390/ijms21176402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viaud S, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie JH, et al. Lactobacillus plantarum NCU116 attenuates cyclophosphamide-induced intestinal mucosal injury, metabolism and intestinal microbiota disorders in mice. Food Funct. 2016;7:1584–1592. doi: 10.1039/c5fo01516b. [DOI] [PubMed] [Google Scholar]
- 9.Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G807–G819. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- 10.Shinde T, et al. Probiotic Bacillus coagulans MTCC 5856 spores exhibit excellent in-vitro functional efficacy in simulated gastric survival, mucosal adhesion and immunomodulation. J. Funct. Foods. 2019;52:100–108. doi: 10.1016/j.jff.2018.10.031. [DOI] [Google Scholar]
- 11.Liu JJ, Reid G, Jiang Y, Turner MS, Tsai CC. Activity of HIV entry and fusion inhibitors expressed by the human vaginal colonizing probiotic Lactobacillus reuteri RC-14. Cell. Microbiol. 2007;9:120–130. doi: 10.1111/j.1462-5822.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhao S, et al. Bacillus coagulans 13002 and fructo-oligosaccharides improve the immunity of mice with immunosuppression induced by cyclophosphamide through modulating intestinal-derived and fecal microbiota. Food Res. Int. 2021;140:109793. doi: 10.1016/j.foodres.2020.109793. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, et al. Bacillus coagulans TL3 inhibits LPS-induced caecum damage in rat by regulating the TLR4/MyD88/NF-κB and Nrf2 signal pathways and modulating intestinal microflora. Oxid. Med. Cell. Longev. 2022;2022:5463290. doi: 10.1155/2022/5463290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ou Z, et al. Betulinic acid attenuates cyclophosphamide-induced intestinal mucosa injury by inhibiting the NF-κB/MAPK signalling pathways and activating the Nrf2 signalling pathway. Ecotoxicol. Environ. Saf. 2021;225:112746. doi: 10.1016/j.ecoenv.2021.112746. [DOI] [PubMed] [Google Scholar]
- 15.Guo MZ, Meng M, Feng CC, Wang X, Wang CL. A novel polysaccharide obtained from Craterellus cornucopioides enhances immunomodulatory activity in immunosuppressive mice models via regulation of the TLR4-NF-κB pathway. Food Funct. 2019;10:4792–4801. doi: 10.1039/c9fo00201d. [DOI] [PubMed] [Google Scholar]
- 16.Salva S, Marranzino G, Villena J, Agüero G, Alvarez S. Probiotic Lactobacillus strains protect against myelosuppression and immunosuppression in cyclophosphamide-treated mice. Int. Immunopharmacol. 2014;22:209–221. doi: 10.1016/j.intimp.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, et al. Lactobacillus plantarum DP189 reduces α-SYN aggravation in MPTP-induced Parkinson's disease mice via regulating oxidative damage, inflammation, and gut microbiota disorder. J. Agric. Food Chem. 2022;70:1163–1173. doi: 10.1021/acs.jafc.1c07711. [DOI] [PubMed] [Google Scholar]
- 18.Yu Q, et al. Molecular mechanism underlying chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced immunosuppressed mice. J. Funct. Foods. 2015;15:52–60. doi: 10.1016/j.jff.2015.03.015. [DOI] [Google Scholar]
- 19.Lahtinen SJ, et al. Probiotics modulate the Bifidobacterium microbiota of elderly nursing home residents. Age. 2009;31:59–66. doi: 10.1007/s11357-008-9081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Zhu CP, Zhang Y, Li Y, Sun JR. Immunomodulatory and antioxidant effects of pomegranate peel polysaccharides on immunosuppressed mice. Int. J. Biol. Macromol. 2019;137:504–511. doi: 10.1016/j.ijbiomac.2019.06.139. [DOI] [PubMed] [Google Scholar]
- 21.Awad WA, Ghareeb K, Abdel-Raheem S, Böhm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009;88:49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- 22.Emami NK, Dalloul RA. Centennial review: Recent developments in host-pathogen interactions during necrotic enteritis in poultry. Poult. Sci. 2021;100:101330. doi: 10.1016/j.psj.2021.101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, et al. Ameliorative effects of Antrodia cinnamomea polysaccharides against cyclophosphamide-induced immunosuppression related to Nrf2/HO-1 signaling in BALB/c mice. Int. J. Biol. Macromol. 2018;116:8–15. doi: 10.1016/j.ijbiomac.2018.04.178. [DOI] [PubMed] [Google Scholar]
- 24.Lis M, Obmińska-Mrukowicz B. Modulatory effects of bestatin on T and B lymphocyte subsets and the concentration of cytokines released by Th1/Th2 lymphocytes in cyclophosphamide-treated mice. Central Eur. J. Immunol. 2013;38:42–53. doi: 10.5114/ceji.2013.34357. [DOI] [Google Scholar]
- 25.Bomko TV, Nosalskaya TN, Kabluchko TV, Lisnyak YV, Martynov AV. Immunotropic aspect of the Bacillus coagulans probiotic action. J. Pharm. Pharmacol. 2017;69:1033–1040. doi: 10.1111/jphp.12726. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, et al. The role of autophagy in maintaining intestinal mucosal barrier. J. Cell. Physiol. 2019;234:19406–19419. doi: 10.1002/jcp.28722. [DOI] [PubMed] [Google Scholar]
- 27.Ying M, et al. Cultured Cordyceps sinensis polysaccharides attenuate cyclophosphamide-induced intestinal barrier injury in mice. J. Funct. Foods. 2019;62:103523. doi: 10.1016/j.jff.2019.103523. [DOI] [Google Scholar]
- 28.Park CM, Song YS. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 2647 cells. Nutr. Res. Pract. 2013;7:423–429. doi: 10.4162/nrp.2013.7.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krug SM, Schulzke JD, Fromm M. Tight junction, selective permeability, and related diseases. Semin. Cell Dev. Biol. 2014;36:166–176. doi: 10.1016/j.semcdb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhuang Y, et al. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway. Oxid. Med. Cell. Longev. 2019;2019:7591840. doi: 10.1155/2019/7591840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang X, et al. Bacillus coagulans SCC-19 maintains intestinal health in cadmium-exposed common carp (Cyprinus carpio L.) by strengthening the gut barriers, relieving oxidative stress and modulating the intestinal microflora. Ecotoxicol. Environ. Saf. 2021;228:112977. doi: 10.1016/j.ecoenv.2021.112977. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X, et al. Exopolysaccharides from Lactobacillus plantarum NCU116 regulate intestinal barrier function via STAT3 signaling pathway. J. Agric. Food Chem. 2018;66:9719–9727. doi: 10.1021/acs.jafc.8b03340. [DOI] [PubMed] [Google Scholar]
- 33.Liu B, et al. Raw bowl tea (Tuocha) polyphenol prevention of nonalcoholic fatty liver disease by regulating intestinal function in mice. Biomolecules. 2019;9:435. doi: 10.3390/biom9090435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 35.Neurath MF. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 36.Sun M, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018;9:3555. doi: 10.1038/s41467-018-05901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H, et al. In vitro immune toxicity of ochratoxin A in porcine alveolar macrophages: A role for the ROS-relative TLR4/MyD88 signaling pathway. Chem. Biol. Interact. 2017;272:107–116. doi: 10.1016/j.cbi.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Parlato M, Yeretssian G. NOD-like receptors in intestinal homeostasis and epithelial tissue repair. Int. J. Mol. Sci. 2014;15:9594–9627. doi: 10.3390/ijms15069594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun L, et al. A novel role of OS-9 in the maintenance of intestinal barrier function from hypoxia-induced injury via p38-dependent pathway. Int. J. Biol. Sci. 2015;11:664–671. doi: 10.7150/ijbs.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, et al. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl. Microbiol. Biotechnol. 2020;104:335–349. doi: 10.1007/s00253-019-10259-6. [DOI] [PubMed] [Google Scholar]
- 41.Mullarkey M, et al. Inhibition of endotoxin response by e5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J. Pharmacol. Exp. Ther. 2003;304:1093–1102. doi: 10.1124/jpet.102.044487. [DOI] [PubMed] [Google Scholar]
- 42.Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015;12:303–310. doi: 10.1038/nrgastro.2015.47. [DOI] [PubMed] [Google Scholar]
- 43.Nyangale EP, Farmer S, Keller D, Chernoff D, Gibson GR. Effect of prebiotics on the fecal microbiota of elderly volunteers after dietary supplementation of Bacillus coagulans GBI-30, 6086. Anaerobe. 2014;30:75–81. doi: 10.1016/j.anaerobe.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Li S, Wang L, Liu B, He N. Unsaturated alginate oligosaccharides attenuated obesity-related metabolic abnormalities by modulating gut microbiota in high-fat-diet mice. Food Funct. 2020;11:4773–4784. doi: 10.1039/c9fo02857a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.