Abstract
Purpose
To describe quality and outcomes of patient-reported outcome (PRO) measures (PROMs) used in patients with refractory hormone-producing pituitary adenomas, and to provide an overview of PROs in these challenging pituitary adenomas.
Methods
Three databases were searched for studies reporting on refractory pituitary adenomas. For the purpose of this review, refractory adenomas were defined as tumors resistant to primary therapy. General risk of bias was assessed using a component approach and the quality of PROM reporting was assessed using the International Society for Quality of Life Research (ISOQOL) criteria.
Results
20 studies reported on PROMs in refractory pituitary adenomas, using 14 different PROMs, of which 4 were disease specific (median general risk of bias score: 33.5% (range 6–50%) and ISOQOL score: 46% (range 29–62%)). SF-36/RAND-36 and AcroQoL were most frequently used. Health-related quality of life in refractory patients (measured by AcroQoL, SF-36/Rand-36, Tuebingen CD-25, and EQ-5D-5L) varied greatly across studies, and was not always impaired compared to patients in remission.
Conclusion
There is a scarcity of data on PROs in the subset of pituitary adenomas that is more difficult to treat, e.g., refractory and these patients are difficult to isolate from the total cohort. The patients' perspective on quality of life, therefore, remains largely unknown in refractory patients. Thus, PROs in refractory pituitary adenomas require adequate analysis using properly reported disease specific PROMs in large cohorts to enable appropriate interpretation for use in clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11102-023-01309-4.
Keywords: Pituitary adenomas, Refractory adenomas, Functioning adenomas, Patient-reported outcome measure, Health-related quality of life, Quality of reporting
Introduction
The definition of refractory hormone-producing pituitary adenomas is ambiguous. Moreover, ‘refractory tumors’ or refractoriness was not defined in the 4th edition of the World Health Organization Guidelines for Classification of Pituitary Tumors [1]. Throughout the current literature, multiple definitions have therefore been used depending on the type of pituitary adenoma: adenomas not responding to conventional doses of dopamine agonists (DAs) in prolactinomas [2–4], failure of pituitary tumor resection or radiotherapy (RT) in Cushing’s Disease (CD) [5], and a combination of (a) Ki-67 index > 3%, (b) > 2% monthly growth, (c) resistance to current treatments and (d) recurrence ≤ 6 months after surgery for all pituitary adenomas [6].
Regardless of the exact definition, refractoriness can theoretically result in prolonged treatment, more interventions, higher disease burden, longer exposure to supraphysiological hormone levels, and higher risk of hypopituitarism. Therefore, refractory patients might be more prone to impaired quality of life (QoL) and functional disability compared to patients with pituitary tumors who are cured by a single intervention [7, 8]. Biochemical and other clinician reported outcomes, however, might be discordant with patient-reported health-related QoL (HR-QoL), and other patient-reported outcomes (PROs) in pituitary tumors [9–11]. Thus, clinician reported outcomes and PROs should be used simultaneously [12, 13].
Various generic, and disease-specific patient-reported outcome measures (PROMs) have been developed, which are increasingly being used in the field of pituitary care and research. Moreover, PROMs are used to classify patients holistically, e.g., using SAGIT and ACRODAT in patients with acromegaly [14, 15]. Despite the increased use of PROMs, no previous systematic review has focused on PROs in refractory pituitary adenomas. In this systematic review, quality and outcomes of PROMS used in patients with refractory hormone-producing pituitary adenomas are described.
Materials and methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) guidelines [16].
Literature search and eligibility criteria
A literature search was conducted on 16-09-2022 (PubMed, Embase and Web of Science). The full search strategy, and in- and exclusion criteria are shown in Supplement 1 and 2, respectively. In brief, articles reporting on PROMs in patients with refractory hormone-producing pituitary adenomas in English were included. Articles were excluded if no full text was available, if they reported on < 5 refractory patients per disease, or on non-original data.
Following consensus amongst the authors, for this review, refractory adenomas were defined as difficult-to-treat adenomas, meeting the following criteria: hormone-producing adenomas not responding to first-line therapy—either pituitary surgery for acromegaly, CD, thyrotrophic adenomas (TSH-oma) and gonadotropinomas, or the maximum tolerated dose of DAs for prolactinomas. Studies on prolactinomas resistant to surgical treatment, and studies on pituitary adenomas for which surgery was the primary treatment option, but not performed in all patients (due to contraindications), were also included. Consequently, due to paucity of data, the definition of refractory adenoma was highly inclusive. Notably, no PRO studies on patients with aggressive pituitary tumors were available.
Data extraction
All identified studies were imported into Endnote X9. Studies were screened by title and abstract and those of interest were reviewed by full-text screening. An overview of extracted data was shown in Supplement 3. If data was only presented in figures without absolute values, numerical values were estimated.
PROMS
Questionnaires were the only type of PROMs used in the included articles, and therefore solely these results were reported. All PROMs were described briefly below and elaborately in Supplement 4.
Disease-specific
The validated Acromegaly Quality of Life Questionnaire (AcroQoL) assesses four domains of HR-QoL (range 0–100, with higher scores indicating better HR-QoL) [17]. Tuebingen Cushing’s Disease quality of life inventory (Tuebingen CD-25) and Cushing Quality of Life Questionnaire (CushingQoL), both validated in patients with CD, describe multiple dimensions of HR-QoL in CD (range 0–100, with higher scores indicating worse HR-QoL for Tuebingen CD-25 and better HR-QoL for CushingQoL) [18, 19]. Discomfort in acromegaly is quantified by Acromegaly Comorbidities & Complaints Questionnaire (ACCQ) (range 0–24, with higher scores indicating more discomfort) [20].
Pituitary specific
Pituitary Quality of Life Questionnaire (PIT QOL) describes HR-QoL in patients with pituitary disease (range 0–371, with higher scores indicating better HR-QoL [21]).
Generic HR-QoL
The 36-item short-form (SF-36) and Research and Development-36 (RAND-36) measure eight domains of HR-QoL and two component scales (range 0–100, with higher scores indicating better HR-QoL) [22, 23]. SF-12 is the shorter, 12-question version of this questionnaire [24]. EQ-5D-5L measures 5 health dimensions and includes a visual analogue score (VAS). Raw values can be transformed into index scores using population specific value sets (index score range 0.446–1.00, with higher scores indicating worse HR-QoL, VAS: 0–100, with higher scores indicating better HR-QoL). 15-Dementional (15-D) measures general HR-QoL (range 0–1, with higher scores indicating better HR-QoL) [25].
Symptom specific
Beck Depression Inventory (BDI) determines signs and intensity of depression (range 0–63, with higher scores indicating worse depression). The Multidimensional Body-Self Relations Questionnaire (MBSRQ) measures body satisfaction (range 0–5, with higher scores indicating more satisfaction)[26, 27]. SCL-90-R assesses nine domains of psychopathology (range 0–100, with higher scores indicating more distress or disturbance) [28]. Cloninger’s Tridimensional Personality Questionnaire (TPQ) measures novelty seeking (range 0–34), harm avoidance (range 0–34) an reward dependence (range 0–30), with higher scores indicating stronger emphasis on the behavior [29, 30]. Hospital Anxiety and Depression Scale (HADS) describes the severity of anxiety and depression in outpatient settings (range 0–21, with higher scores indicating more anxiety and depression) [31].
Risk of bias assessment
The quality of selected articles was assessed using a component approach for the general risk of bias [32], and the quality of reporting on PROs by the modified ISOQOL criteria for non-randomized studies [33, 34] (Supplement 5). The cut-off for sufficient quality of reporting was 69%, as previously published [34, 35].
Data analysis
Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) was used for data collection. The primary study outcome were PROMs. The secondary outcomes were the quality of reporting on PROMs and PRO results. Statistical analysis could not be performed, due to insufficient data to perform a meta-analysis.
Results
Study selection
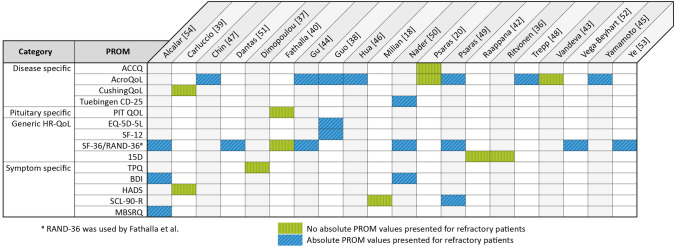
A total of 4554 articles were screened for eligibility, as depicted in the flowchart of article screening and inclusion in Supplement 6. Twenty articles were included in the systematic review, of which 14 were cross-sectional studies, 5 were cohort studies, and 1 article reported on cross-sectional and cohort data (study characteristics: Supplement 7). As some studies reported on multiple types of refractory adenomas, the number of studies reporting on patients with the included pituitary diseases were 14 for refractory acromegaly, 6 for refractory CD, and 4 for refractory prolactinoma. No studies reported on TSH-oma or gonadotropinoma. In total, 14 different PROMs reported on refractory adenomas, of which 4 were disease-specific (overview of PROMs per study: Fig. 1).
Fig. 1.
Patient reported outcome measures for refractory patients per study. AcroQoL Acromegaly Quality of Life Questionnaire, ACCQ Acromegaly Comorbidities & Complaints Questionnaire, BDI Beck Depression Inventory, CushingQoL Cushing Quality of Life Questionnaire, EQ-5D-5L 5-level EuroQoL-5, HADS Hospital Anxiety and Depression Scale, MBSRQ Multidimensional Body-Self Relations Questionnaire, PIT QOL Pituitary Quality of Life, PROM patient reported outcome measure, SCL-90-R Symptom Checklist-90-Revised, SF-36 Short Form-36, RAND-36 Research and Development-36, TPQ Cloninger’s Tridimensional Personality Questionnaire, Tuebingen CD-25 Tuebingen Cushing’s Disease Quality of Life Inventory, 15D 15-Dimentional
Risk of bias assessment
Estimated risk of bias was high in all studies (median score 33.5%, range 6–50%) (Supplement 8). None of the studies defined refractoriness. Two studies explicitly stated that patients with active disease were symptomatic, although none included a definition of the symptoms. Four studies reported on missing PROM data, of which only one reported < 10% missing data [36]. Quality of PROMs reporting was insufficient in all studies (median score 46%, range 29–62%) (Supplement 9). Two studies included a hypothesis specifically for the used PROM [20, 37], whereas only one described the method of statistical analysis for the PROM hypothesis [37]. Solely one study described a statistical approach for missing PRO data [38].
PROMs
AcroQoL and SF-36 were most frequently used. In 8/20 studies, absolute PRO results were not reported, with only conclusions being reported on whether refractory patients scored higher or lower than patients in remission or healthy controls [20, 36, 37, 39–43].
Disease-specific HR-QoL
AcroQoL
AcroQoL was used in nine studies (Fig. 1), of which seven reported absolute values. In these seven studies, scores of refractory acromegaly patients compared to patients in remission varied substantially, described as either comparable in the two patient groups in four studies [44–47], decreased in one study [38], or decreased except for the domain personal relations in another study [48] (Table 1). One study compared refractory acromegaly patients to healthy controls, finding lower scores in all domains for refractory patients [49]. By contrast, two studies did not present absolute values, of which one reported comparable scores in refractory patients compared to patients in remission [20], and the other reported that AcroQoL scores improved less after treatment (TSS/RT/DA) in refractory patients compared to patients in remission [43] (mean follow-up time: 29.6 ± 19.7 months and 29.3 ± 18.8, respectively).
Table 1.
AcroQoL scores per study
| Chin [47] | Gu [44] | Guo [38] | Hua [46] | Psaras [49] | Trepp [48] | Yamamoto [45]f | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Mean (range) N = 36 |
Median [IQR] N = 44 |
Mean ± SD N = 154 |
Mean ± SD N = 11 |
Mean ± SD N = 11 |
Mean ± SD N = 14 |
Mean ± SD N = 6 |
Median [IQR] N = 20 |
Median [IQR] N = 18 |
| Before SMS | Preoperative | SMS ( +)a | SMS (−) | < 65 years-old | ≥ 65 years-old | ||||
| Physical | 67.5 (30.0–97.5) =c | 41.3 ± 25.5 ↓ | 55.1 ± 30.4d | 42.3 ± 33.2d | 67.7 ± 22.4e | 42 ± 32↓ | 70 [31–82] = | 52 [32–80] = | |
| Psychological | 74.3 (27.1–91.4) = c | 40.1 ± 22.9 ↓ | 55.7 ± 24.8d | 56.4 ± 29.4d | 62.5 ± 20.2e | 44 ± 23↓ | 61 [50–76] = | 67 [50–85] = | |
| Appearanceb | 65.7 (22.9–88.6) = c | 33.9 ± 21.5 ↓ | 47.4 ± 26.2d | 43.8 ± 34.5d | 51.8 ± 25.3e | 30 ± 21↓ | |||
| Personal relationsb | 82.9 (31.4–100.0) = c | 46.3 ± 26.9 ↓ | 65.9 ± 24.2d | 69.2 ± 28.7d | 77.3 ± 17.6e | 52 ± 24 = | |||
| Total | 74.6 (33.6–93.6) = c | 64.1 [51.8–71.8] = | 40.5 ± 22.9 ↓ | 56.1 ± 26.4d | 51.3 ± 28.6d | 64.9 ± 17.8e | 43 ± 25↓ | 67 [42–78] = | 61 [43–85] = |
| First follow-up |
12 weeks After starting SMSg |
6 months postoperative |
|||||||
| Physical | 71.3 (40.0–97.5) =c | ||||||||
| Psychological | 78.6 (27.1–97.1) = c | ||||||||
| Appearanceb | 72.3 (22.9–94.3) =c | ||||||||
| Personal relationsb | 80.0 (31.4–100.0) = c | ||||||||
| Total | 74.1 (32.7–99.5) = c | 82.7 [74.1–88.6] = | |||||||
| Second Follow-up |
24 weeks After starting SMSg |
||||||||
| Physical | 71.3 (42.5–97.5) = c | ||||||||
| Psychological | 78.6 (27.1–97.1) = c | ||||||||
| Appearanceb | 77.1 (22.9–97.1) =c | ||||||||
| Personal relationsb | 85.7 (21.4–100.0) = c | ||||||||
| Total | 77.3 (32.7–95.5) = c | ||||||||
AcroQoL scores for refractory patients with acromegaly per study. AcroQoL Acromegaly Quality of Life Questionnaire, IQR interquartile range, SMS( +) on somatostatin analogue treatment, SMS(−) not on somatostatin analogue treatment, ↓ significantly lower compared to acromegaly patients in remission; ↑ significantly higher compared to acromegaly patients in remission, no P-value reported, = tested and no significant difference compared to patients in remission
aPatients received octreotide LAR every two weeks (dose not reported)
bPsychological subscales
cAcroQoL scores did not differ between refractory patients and patients in remission at 24 weeks
dNo significant difference between all refractory patients SMS (+) or SMS (−) and patients in remission. No subgroup analysis performed for SMS(+) and SMS(−) separately
eRefractory patients scored significantly lower than healthy controls
fValues estimated based on figure, absolute values were not presented
gPatients received weekly intramuscular injections of octreotide LAR 20 mg. At 12 weeks a dose escalation to octreotide LAR 30 mg was permitted in case GH > 2.5 ug/L and/or IGF1 above upper limit of normal for age, but this was not obligatory
ACCQ
One study reported on the ACCQ in refractory acromegaly, albeit without presenting absolute values, and concluded the scores were comparable to patients in remission [20].
Tuebingen CD-25
Tuebingen CD-25 scores of refractory CD patients were reported in one study, showing no difference with CD patients in remission [50](Supplement 10).
CushingQoL
The only study reporting on the CushingQoL reported lower (i.e., worse) CushingQoL scores in refractory CD patients compared to patients in remission [39]. Absolute values were not presented.
PIT QOL
PIT QOL scores, solely reported in one study and without presenting absolute values, were comparable in refractory acromegaly patients and patients in remission [40].
Generic HR-QoL
SF 12/36 and RAND-36
SF-12/36 and RAND-36 were reported in nine studies (Table 2), of which eight presented absolute values. The results were inconsistent across studies and between diseases. In acromegaly patients, one study reported comparable results between refractory acromegaly patients and patients in remission [44], one reported lower scores in refractory patients except for physical functioning and general health [38] and another reported lower scores in refractory acromegaly only in the role physical, bodily pain and vitality domains [51]. The study that did not report absolute values found no difference in RAND-36 scores between refractory acromegaly and patients in remission [40].
Table 2.
SF-12 and SF-36 scores per study
| First Author, year of publication | Dantas [51] | Gu [44] | Guo [38] SF-12 |
Psaras [49] | Alcalar [54] | Nader [50]d | Psaras [49] | Vega-Beyhart [52] | Ye [53]d | Vega-Beyhart [19] 52 |
|---|---|---|---|---|---|---|---|---|---|---|
| Disease | AC | AC | AC | AC | CD | CD | CD | CD | CD | PRL |
| Baseline |
Mean N = 14a |
Median [IQR] N = 44 |
Mean ± SD N = 154 |
Mean ± SD N = 14 |
Mean ± SD N = 8 |
Uncleare N = 8 |
Mean ± SD N = 5 |
Median [IQR] N = 7 |
Unclearg N = 7 |
Median [IQR] N = 28 |
| Physical functioning | 54.09 = | 49.1 ± 9.3 = | 51.0 ± 25.8 | 18.63 ± 5.61c | 75 = f | 37.6 ± 32.4 | 45 [20–85]↓ | 55 [52–60] | 82 [48–95]↓ | |
| Role physical | 50.00↓ | 40.9 ± 11.4↓ | 43.2 ± 29.0 | 5.63 ± 1.99 | 75 =f | 25.0 ± 23.1 | 25 [0–50]↓ | 29 [23–32] | 50 [0–100]↓ | |
| Bodily pain | 43.64↓ | 36.7 ± 11.7↓ | 35.7 ± 20.6 | 6.81 ± 3.52c | 25 =f | 44.6 ± 24.2 | 45 [25–67]↓ | 58 [54–62] | 78 [45–90] = | |
| General health | 63.36 = | 32.0 ± 10.7 = | 39.9 ± 29.7b | 11.88 ± 3.68c | 38 =f | 39.7 ± 37.8b | 40 [20–45] = | 34 [30–39] | 48 [26–65]↓ | |
| Social functioning | 60.00 = | 38.3 ± 12.1↓ | 43.4 ± 34.7 | 7.00 ± 2.14 | 62 =f | 31.6 ± 32.9b | 50 [37–62]↓ | 50 [45–55] | 62 [40–75]↓ | |
| Role emotional | 45.27 = | 35.1 ± 12.8↓ | 39.7 ± 31.3b | 4.25 ± 1.28 | 62 =f | 26.0 ± 29.3b | 0 [0–0]↓ | 38 [32–41] | 50 [0–100] ↓ | |
| Mental health | 66.91 = | 37.2 ± 5.6↓ | 38.2 ± 37.2b | 20.00 ± 5.90 | 62 =f | 43.8 ± 42.7b | 44 [16–67]↓ | 56 [50–60] | 56 [36–68]↓ | |
| Vitality | 45.45↓ | 44.7 ± 10.6↓ | 33.8 ± 29.4b | 13.63 ± 5.15 | 50 =f | 47.2 ± 38.0 | 45 [10–60] = | 32 [28–35] | 45 [35–67]↓ | |
| MCS | 38.9 ± 8.0↓ | 29 [22–53]↓ | 57 [31–69]↓ | |||||||
| PCS | 39.6 ± 8.8↓ | 44 [16–70]↓ | 66 [36–81]↓ | |||||||
| Total | 65.4 [63.2–67.7] = | |||||||||
| First follow-up | 6 mos postop | Mean 2.35 mos postop, N = 6 h | ||||||||
| Physical functioning | 49 [42–52] | |||||||||
| Role physical | 23 [19–30] | |||||||||
| Bodily pain | 65 [60–70] | |||||||||
| General health | 28 [22–34] | |||||||||
| Social functioning | 53 [50–69] | |||||||||
| Role emotional | 45 [40–50] | |||||||||
| Mental health | 51 [45–59] | |||||||||
| Vitality | 25 [20–30] | |||||||||
| Total | 75.3 [70.1–82.3] = | |||||||||
| Second follow-up | Mean 7.4 mos postop, N = 4 h | |||||||||
| Physical functioning | 50 [45–54] | |||||||||
| Role physical | 30 [27–34] | |||||||||
| Bodily pain | 60 [66–64] | |||||||||
| General health | 42 [60–47] | |||||||||
| Social functioning | 61 [58–65] | |||||||||
| Role emotional | 58 [52–61] | |||||||||
| Mental health | 55 [50–60] | |||||||||
| Vitality | 39 [35–42] |
SF-12 and SF-36 scores for refractory patients with acromegaly, Cushing’s Disease and prolactinoma per study. AC acromegaly, CD Cushing’s Disease, IQR interquartile range, MCS mental component summary, mos months, PCS physical component summary, postop postoperative, PRL prolactinoma, SD standard deviation, ↓ significantly lower compared to patients in remission; ↑significantly higher compared to patients in remission, no P-value reported, = tested and no significant difference compared to patients in remission
aNumber of patients not reported in article. Author provided information upon request
bRefractory patients scored significantly lower than healthy controls
cRefractory patients scored significantly lower than healthy controls and patients in remission (no post-hoc analysis was performed)
dValues estimated based on figure, absolute values were not presented
eUnclear whether reported numbers concern mean or median values
fAll SF-36 domain scores were lower in refractory patients compared to patients in remission, however not significant
gUnclear what the values indicate. Figure does not include a legenda
hMissing data was not reported in article. Author provided information upon request
In CD patients, one study reported comparable scores in refractory CD compared to patients in remission [50], and one reported lower scores except for the general health and vitality domains [52]. Furthermore, one study concluded no postoperative trend of improvement over time (mean 7.4 months) was observed in refractory CD patients, whereas CD patients in remission did improve postoperatively [53]. Two studies compared refractory CD patients to healthy controls, of which one found lower scores in refractory patients only for physical functioning, bodily pain and general health [54], and the other found lower scores for general health, mental health, social functioning and role emotional [49].
One study reported on SF-36 in refractory prolactinomas, finding lower scores compared to patients in remission except for the bodily pain domain [52].
EQ-5D-5L
EQ-5D-5L scales, reported in solely one study, for pain/discomfort and anxiety/depression were worse in refractory acromegaly patients compared to acromegaly patients in remission [38]. Mean EQ-5D VAS scores were 62.8 ± 21.6 in refractory acromegaly, which was similar compared to acromegaly in remission [38](Supplement 11).
15D
Two studies reported on 15D without presenting absolute values, of which one on refractory acromegaly, CD and prolactinoma patients (without performing a subgroup analysis per disease) [36], and the other reported on refractory prolactinoma and acromegaly [42]. Both studies found comparable results in refractory patients compared to patients in remission.
Symptom-specific
BDI
BDI was reported in two studies, using different cut-off values. In refractory acromegaly, mean BDI scores were 18.9 ± 10.9 (Alcalar et al. used a score of > 17 points to indicate presence of depression) [54] (Supplement 12). In refractory CD, 2/8 patients scored ≥ 18 points (Nader et al. described a score of ≥ 18 points as a severe depression) [50].
SCL-90-R
Two studies reported on SCL-90-R. One found higher hostility scores in refractory AC and CD than in healthy controls, and psychoticism in refractory CD [49] (Supplement 13). The other, without presenting absolute values, reported higher obsessive–compulsive scores in refractory acromegaly patients compared to patients in remission 3 months after surgery, whereas no differences were observed at 12 months [41].
MBSRQ
Refractory CD patients had significantly lower MBSRQ scores for fitness and health evaluation, body areas satisfaction and mean item score compared to those in remission and healthy controls [54] (Supplement 14).
HADS
The only study reporting on HADS found higher anxiety scores in refractory CD patients compared to patients in remission [39]. Absolute values were not presented.
TPQ
TPQ, reported by only one study, without presenting absolute values, found higher fear of uncertainty, fatigability and asthenia, leading to a higher total harm avoidance score in refractory CD patients compared to CD patients in remission [37].
Discussion
An unequivocal definition of refractory is lacking, and data, including patient-reported outcomes, on difficult-to-treat (e.g., refractory) patients is scarce. A plethora of PROMs were used in research and care of pituitary adenomas, of which few were disease specific. The quality of reporting in the available studies was low, with high risk of bias, leading to inconsistent PRO outcomes. Due to the paucity of data, no conclusions on HR-QoL and the contributing factors in refractory patients could be made.
Currently, no consensus on the definition of refractory is available in the literature, resulting in the application of the present definition (i.e., tumors not responding to primary therapy) for data selection. Using this definition, it should be noted that the status of refractoriness is not only dependent on tumor characteristics, but also on the surgical experience within the treating center, as more experienced surgeons may have somewhat better outcomes. However, from a patient's perspective, this definition might implicitly reflect the impact of the disease, due to prolonged absence of disease remission and the need for secondary treatment. Furthermore, the scarcity of data influenced the present, inclusive definition, as studies reporting on PROs in the most challenging patients (persistent disease despite multimodality treatment and aggressive tumors) were lacking. Consequently, these most challenging cases could not be identified at present, and therefore warrant future in-depth systematic investigation.
Nevertheless, there were some studies reported on PROs in patients with persistent disease after primary treatment—the present definition of refractory patients—to address our clinical question. The next challenge was the use of plethora of PROMs, which were mostly generic and sometimes disease-specific. Disease-specific PROMs focus on quality of life domains specifically impaired in the disease of interest, allowing identification of more subtle impairments than generic questionnaires [17, 55, 56].
Despite their better sensitivity, results of the disease specific questionnaires (ACCQ, AcroQoL, Tuebingen-CD25, CushingQoL) were equally ambiguous compared to those of the less sensitive, pituitary-specific (PIT QOL), and generic HQ-QoL questionnaires (EQ-5D-5L, SF12/36, RAND-36, 15D). Surprisingly, independent of the type of questionnaire used, results of refractory patients compared to patients in remission were inconsistent; being lower in some, yet comparable in other studies. A possible explanation may lie in the well-known fact that patients in remission report ongoing impaired quality of life.
Furthermore, there was no clear difference in outcomes between the types of adenomas. HR-QoL measured by SF-12/36 and RAND-36 varied greatly between the studies within same type of adenomas. Previous literature reported the worst HR-QoL in active CD compared to other pituitary adenomas [57], improving partially after remission [11, 58]. However, HR-QoL in refractory CD (measured by SF-12/36 or RAND-36) was not evidently lower than in other adenomas and not always worse compared to CD in remission. The symptom specific PROMS (HADS, TPQ, SCLR-90) found worse scores in varying—mostly psychological—domains, albeit inconsistent across studies. Similarly, in refractory acromegaly, subscales such as bodily pain and physical functioning (SF-12/36, RAND-36) and appearance (AcroQoL), expected to be most affected [7, 59], were not always worse compared to patients in remission. As expected, prolactinomas were the most understudied type of adenoma, with only one study reporting absolute values, thereby impeding proper comparison. Thus, overall results were inconsistent and inconclusive, regardless of questionnaire and adenoma type.
The well-known Wilson and Cleary model (WCM) [60] states that general wellbeing results from a complex interplay of physiological, clinical and social aspects. According to this model, HR-QoL can be influenced, either directly or indirectly, by six factors: biological and psychological factors, symptom status, functional status, general health perceptions and characteristics of the environment and of the individual. In patients with pituitary adenoma, irrespective of whether they are refractory, all these factors might be affected due to prolonged supraphysiological hormone levels, leading to severe symptomatology, decreased functional status, and impaired general health perceptions. Therefore, impaired HR-QoL may be anticipated in all patients with a pituitary tumor and the impact of having a more refractory status may be difficult to distillate from other factors influencing HR-QoL.
In agreement with the WCM, we found HR-QoL in refractory acromegaly and CD was not always worse than in patients in remission. This may be caused by ongoing symptoms in patients in biochemical remission, resulting from permanent complications (e.g., arthropathy in acromegaly and osteoporotic fractures and chronic depression in CD [61–64]) leading to persistently impaired HR-QoL. Contrarily, surgery could have improved symptomatology and functional status, thereby increasing HR-QoL, without achievement of biochemical remission in refractory patients [49]. However, true differences in HR-QoL may have been concealed by biased results, use of small sample sizes and generic questionnaires in the included studies. Furthermore, questionnaires cannot grasp all aspects of life.
The importance of the use of PROs in addition to clinician-reported outcomes is well recognized in care for pituitary disease, as well as other diseases [12, 13]. Ideally, PROs should focus on issues relevant to the specific (refractory) tumor, using a combination of generic, disease-specific and symptom-specific PROMs. Although consensus on which combination of PROMs to use is lacking, our group has gained some experience in selecting PROMs, according to the three-tier Value Based Health Care approach, at each relevant timepoint within the care trajectory [65]. This approach enables individualization of care trajectories. For this purpose, we developed the Leiden Bother and Needs Questionnaire, which is currently used in clinical practice to assess patients' bother related to consequences of the disease and their need for support [66]. An example of prospective PRO research including potentially difficult-to-treat (i.e., refractory) cases is the prolactinoma research project (PRolaCT) [67]. In the future, these care and research strategies should be used in patients with refractory adenomas.
An important limitation to this systematic review was the high risk of bias and low quality of PRO reporting, limiting proper interpretation and comparability. Secondly, isolating the patients who met our definition of refractory was challenging, as information on treatment was not always presented. This led to an inhomogeneous population. Due to the quality of data, no conclusions could be drawn about HR-QoL in refractory patients, compared to those in remission. Lastly, comparison of PROs with biochemical outcomes lay beyond the scope of this review, which would be interesting to place the PRO results in perspective. To adequately treat and support refractory patients, future studies using disease specific PROMs in large cohorts of patients with pituitary adenomas should be performed, with subgroup analyses for patients who are not in remission after primary therapy.
Conclusion
The current systematic review demonstrated a scarcity of high-quality data on PROs in the subset of refractory pituitary adenomas—defined as adenomas being difficult to treat. Additionally, in the current literature, data from refractory patients was difficult to isolate from the rest of the cohort, and the patients' perspective on quality of life therefore remains largely unknown in refractory patients. Thus, PROs in patients with refractory hormone-producing pituitary adenomas require adequate analysis using properly reported disease-specific PROMs in large cohorts to enable appropriate interpretation and use for clinical practice.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The literature search was performed with help of an experienced librarian (P. Gurung). The general component approach for general risk of bias assessment was composed with help of N.A.H. Zamanipoor. The evaluation of risk of bias resulting from hormone determinations was performed with help of an experienced clinical chemist (B.E.P.B. Ballieux).
Author contributions
VRvanT literature search and selection, data analysis, writing draft. ICMP: second reviewer for literature selection, critical revision of draft. NRB: concept for article, critical revision of draft.
Funding
No funding was received.
Data availability
Current manuscript includes no new data, all materials are included in our supplements.
Declarations
Competing interests
The authors have no competing interests to declare.
Ethical approval
No ethical approval was necessary as the manuscript concerns a systematic review of exciting literature.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lopes MBS. The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol. 2017;134(4):521–535. doi: 10.1007/s00401-017-1769-8. [DOI] [PubMed] [Google Scholar]
- 2.Molitch ME. Management of medically refractory prolactinoma. J Neurooncol. 2014;117(3):421–428. doi: 10.1007/s11060-013-1270-8. [DOI] [PubMed] [Google Scholar]
- 3.Tang H, et al. Case Report: temozolomide treatment of refractory prolactinoma resistant to dopamine agonists. Front Endocrinol. 2021;12:616339. doi: 10.3389/fendo.2021.616339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karatas S, Hacioglu Y, Rakicioglu T. Prolactinoma—which patients react favorably to cabergoline medication? Endocr Regul. 2022;56(4):279–283. doi: 10.2478/enr-2022-0030. [DOI] [PubMed] [Google Scholar]
- 5.Dong D, Ji Z, Li H. Autologous adrenal transplantation for the treatment of refractory cushing’s disease. Urol Int. 2019;103(3):344–349. doi: 10.1159/000502345. [DOI] [PubMed] [Google Scholar]
- 6.Dai C, et al. Refractory pituitary adenoma: a novel classification for pituitary tumors. Oncotarget. 2016;7(50):83657–83668. doi: 10.18632/oncotarget.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb SM, Badia X. Quality of life in acromegaly. Neuroendocrinology. 2016;103(1):106–111. doi: 10.1159/000375451. [DOI] [PubMed] [Google Scholar]
- 8.Pivonello R, et al. Cushing’s disease: the burden of illness. Endocrine. 2017;56(1):10–18. doi: 10.1007/s12020-016-0984-8. [DOI] [PubMed] [Google Scholar]
- 9.Geraedts VJ, et al. Predictors of quality of life in acromegaly: no consensus on biochemical parameters. Front Endocrinol (Lausanne) 2017;8:40. doi: 10.3389/fendo.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyriakakis N, et al. Impaired quality of life in patients with treated acromegaly despite long-term biochemically stable disease: Results from a 5-years prospective study. Clin Endocrinol. 2017;86(6):806–815. doi: 10.1111/cen.13331. [DOI] [PubMed] [Google Scholar]
- 11.Lindsay JR, et al. Long-term impaired quality of life in Cushing’s syndrome despite initial improvement after surgical remission. J Clin Endocrinol Metab. 2006;91(2):447–453. doi: 10.1210/jc.2005-1058. [DOI] [PubMed] [Google Scholar]
- 12.Giustina A, et al. A consensus on the diagnosis and treatment of acromegaly comorbidities: an update. J Clin Endocrinol Metabol. 2020;105(4):e937–e946. doi: 10.1210/clinem/dgz096. [DOI] [PubMed] [Google Scholar]
- 13.Melmed S, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary. 2013;16(3):294–302. doi: 10.1007/s11102-012-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Lely AJ, et al. Development of ACRODAT(®), a new software medical device to assess disease activity in patients with acromegaly. Pituitary. 2017;20(6):692–701. doi: 10.1007/s11102-017-0835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giustina A, et al. SAGIT®: clinician-reported outcome instrument for managing acromegaly in clinical practice–development and results from a pilot study. Pituitary. 2016;19(1):39–49. doi: 10.1007/s11102-015-0681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webb SM, et al. Acromegaly Quality of Life Questionnaire (ACROQOL) a new health-related quality of life questionnaire for patients with acromegaly: development and psychometric properties. Clin Endocrinol. 2002;57(2):251–258. doi: 10.1046/j.1365-2265.2002.01597.x. [DOI] [PubMed] [Google Scholar]
- 18.Milian M, et al. Erratum: the development of the Tuebingen Cushing’s disease quality of life inventory Tuebingen CD-25. Part II: Normative data from 1784 healthy people (Clinical Endocrinology (2012) 76 (861-867)). Clinical Endocrinology. 2013;79(6):901–903. doi: 10.1111/cen.12342. [DOI] [PubMed] [Google Scholar]
- 19.Milian M, et al. The development of the Tuebingen Cushing’s disease quality of life inventory (Tuebingen CD-25). Part I: construction and psychometric properties. Clin Endocrinol. 2012;76(6):851–60. doi: 10.1111/j.1365-2265.2011.04191.x. [DOI] [PubMed] [Google Scholar]
- 20.Psaras T, et al. Are there gender-specific differences concerning quality of life in treated acromegalic patients? Exp Clin Endocrinol Diabetes. 2011;119(5):300–305. doi: 10.1055/s-0030-1267912. [DOI] [PubMed] [Google Scholar]
- 21.Kan P, Cusimano M. Validation of a quality-of-life questionnaire for patients with pituitary adenoma. Can J Neurol Sci. 2006;33(1):80–85. doi: 10.1017/S0317167100004741. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item health survey 1.0. Health Econ. 1993;2(3):217–27. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 24.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33(5):328–336. doi: 10.3109/07853890109002086. [DOI] [PubMed] [Google Scholar]
- 26.Raich RM, Clarasó JT, Figueras M. Estudio de la imagen corporal y su relación con el deporte en una muestra de estudiantes universitarios. Análisis Modificación Conducta. 1996;22(85):603–626. [Google Scholar]
- 27.Cash TF. User’s manual for the multidimensional body-self relations questionnaire. Norfolk, VA: Old Dominion University; 2000. [Google Scholar]
- 28.Derogatis LR. SCL-90-R: administration, scoring and procedures: manual II. Psychol Health. 1983;7(06):297–334. [Google Scholar]
- 29.Clonninger, C.R., The Tridimensional Personality Questionnaire, Version iu. St. Louis. 1987.
- 30.Weyers P, Krebs H, Janke W. Reliability and construct validity of the German version of Cloninger’s Tridimensional Personality Questionnaire. Personality Individ Differ. 1995;19(6):854–861. doi: 10.1016/S0191-8869(95)00128-X. [DOI] [Google Scholar]
- 31.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 32.van der Meulen M, et al. State of the art of patient-reported outcomes in acromegaly or GH deficiency: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2022;107(5):1225–1238. doi: 10.1210/clinem/dgab874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brundage M, et al. Patient-reported outcomes in randomized clinical trials: development of ISOQOL reporting standards. Qual Life Res. 2013;22(6):1161–1175. doi: 10.1007/s11136-012-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zamanipoor Najafabadi AH, et al. Impaired health-related quality of life in meningioma patients-a systematic review. Neuro Oncol. 2017;19(7):897–907. doi: 10.1093/neuonc/now250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dirven L, et al. The level of patient-reported outcome reporting in randomised controlled trials of brain tumour patients: a systematic review. Eur J Cancer. 2014;50(14):2432–2448. doi: 10.1016/j.ejca.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Ritvonen E, et al. Normal long-term health-related quality of life can be achieved in patients with functional pituitary adenomas having surgery as primary treatment. Clin Endocrinol. 2015;82(3):412–421. doi: 10.1111/cen.12550. [DOI] [PubMed] [Google Scholar]
- 37.Dimopoulou C, et al. Increased prevalence of anxiety-associated personality traits in patients with cushing's disease: a cross-sectional study. Neuroendocrinology. 2013;97(2):139–145. doi: 10.1159/000338408. [DOI] [PubMed] [Google Scholar]
- 38.Guo XP, et al. Quality of life and its determinants in patients with treated acromegaly: a cross-sectional nationwide study in China. J Clin Endocrinol Metab. 2021;106(1):211–225. doi: 10.1210/clinem/dgaa750. [DOI] [PubMed] [Google Scholar]
- 39.Carluccio A, et al. Predictors of quality of life in 102 patients with treated Cushing’s disease. Clin Endocrinol (Oxf) 2015;82(3):404–411. doi: 10.1111/cen.12521. [DOI] [PubMed] [Google Scholar]
- 40.Fathalla H, et al. Endoscopic transphenoidal surgery for acromegaly improves quality of life. Can J Neurol Sci. 2014;41(6):735–741. doi: 10.1017/cjn.2014.106. [DOI] [PubMed] [Google Scholar]
- 41.Milian M, et al. Health-related quality of life and psychiatric symptoms improve effectively within a short time in patients surgically treated for pituitary tumors–a longitudinal study of 106 patients. Acta Neurochir. 2013;155(9):1637–45. doi: 10.1007/s00701-013-1809-7. [DOI] [PubMed] [Google Scholar]
- 42.Raappana A, et al. Long-term health-related quality of life of surgically treated pituitary adenoma patients: a descriptive study. ISRN Endocrinol. 2012;2012:675310. doi: 10.5402/2012/675310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandeva S, et al. Disease control and treatment modalities have impact on quality of life in acromegaly evaluated by acromegaly quality of life (AcroQoL) questionnaire. Endocrine. 2015;49(3):774–782. doi: 10.1007/s12020-014-0521-6. [DOI] [PubMed] [Google Scholar]
- 44.Gu J, et al. Quality of life in patients with acromegaly before and after transsphenoidal surgical resection. Int J Endocrinol. 2020;2020:5363849. doi: 10.1155/2020/5363849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto N, et al. The effect of aging on quality of life in acromegaly patients under treatment. Front Endocrinol. 2022 doi: 10.3389/fendo.2022.819330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hua SC, Yan YH, Chang TC. Associations of remission status and lanreotide treatment with quality of life in patients with treated acromegaly. Eur J Endocrinol. 2006;155(6):831–837. doi: 10.1530/eje.1.02292. [DOI] [PubMed] [Google Scholar]
- 47.Chin SO, et al. Change in quality of life in patients with acromegaly after treatment with octreotide LAR: first application of AcroQoL in Korea. BMJ Open. 2015;5(6):e006898. doi: 10.1136/bmjopen-2014-006898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trepp R, et al. Assessment of quality of life in patients with uncontrolled vs. Controlled acromegaly using the acromegaly quality of life questionnaire (AcroQoL) Clin Endocrinol. 2005;63(1):103–110. doi: 10.1111/j.1365-2265.2005.02307.x. [DOI] [PubMed] [Google Scholar]
- 49.Psaras T, et al. Predictive factors for neurocognitive function and Quality of Life after surgical treatment for Cushing’s disease and acromegaly. J Endocrinol Investig. 2011;34(7):E168–E177. doi: 10.3275/7333. [DOI] [PubMed] [Google Scholar]
- 50.Nader S, et al. Health-related quality of life in patients after treatment of Cushing's disease. Exp Clin Endocrinol Diabetes. 2016;124(3):187–191. doi: 10.1055/s-0035-1569340. [DOI] [PubMed] [Google Scholar]
- 51.Elias Dantas RA, et al. Physical activities in daily life and functional capacity compared to disease activity control in acromegalic patients: impact in self-reported quality of life. Arq Bras Endocrinol Metabol. 2013;57(7):550–557. doi: 10.1590/s0004-27302013000700009. [DOI] [PubMed] [Google Scholar]
- 52.Vega-Beyhart A, et al. Quality of life is significantly impaired in both secretory and non-functioning pituitary adenomas. Clin Endocrinol. 2019;90(3):457–467. doi: 10.1111/cen.13915. [DOI] [PubMed] [Google Scholar]
- 53.Ye VC, Akagami R. Perioperative quality of life in Cushing’s disease. Can J Neurol Sci. 2017;44(1):69–77. doi: 10.1017/cjn.2016.295. [DOI] [PubMed] [Google Scholar]
- 54.Alcalar N, et al. Evaluation of depression, quality of life and body image in patients with Cushing’s disease. Pituitary. 2013;16(3):333–340. doi: 10.1007/s11102-012-0425-5. [DOI] [PubMed] [Google Scholar]
- 55.Webb SM. Quality of life in acromegaly. Neuroendocrinology. 2006;83(3–4):224–229. doi: 10.1159/000095532. [DOI] [PubMed] [Google Scholar]
- 56.Webb SM, et al. Evaluation of health-related quality of life in patients with Cushing’s syndrome with a new questionnaire. Eur J Endocrinol. 2008;158(5):623–630. doi: 10.1530/EJE-07-0762. [DOI] [PubMed] [Google Scholar]
- 57.Johnson MD, Woodburn CJ, Lee Vance M. Quality of life in patients with a pituitary adenoma. Pituitary. 2003;6(2):81–87. doi: 10.1023/B:PITU.0000004798.27230.ed. [DOI] [PubMed] [Google Scholar]
- 58.Santos A, et al. Psychometric performance of the CushingQoL questionnaire in conditions of real clinical practice. Eur J Endocrinol. 2012;167(3):337–342. doi: 10.1530/EJE-12-0325. [DOI] [PubMed] [Google Scholar]
- 59.Coopmans EC, et al. Evaluating the impact of acromegaly on quality of life. Endocrinol Metab Clin North Am. 2022;51(4):709–725. doi: 10.1016/j.ecl.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. doi: 10.1001/jama.1995.03520250075037. [DOI] [PubMed] [Google Scholar]
- 61.Vermalle M, et al. Lack of functional remission in Cushing’s syndrome. Endocrine. 2018;61(3):518–525. doi: 10.1007/s12020-018-1664-7. [DOI] [PubMed] [Google Scholar]
- 62.Pikkarainen L, Sane T, Reunanen A. The survival and well-being of patients treated for Cushing’s syndrome. J Intern Med. 1999;245(5):463–468. doi: 10.1046/j.1365-2796.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 63.Biermasz NR, et al. Decreased quality of life in patients with acromegaly despite long-term cure of growth hormone excess. J Clin Endocrinol Metab. 2004;89(11):5369–5376. doi: 10.1210/jc.2004-0669. [DOI] [PubMed] [Google Scholar]
- 64.Pelsma ICM, et al. Progression of acromegalic arthropathy in long-term controlled acromegaly patients: 9 years of longitudinal follow-up. J Clin Endocrinol Metab. 2021;106(1):188–200. doi: 10.1210/clinem/dgaa747. [DOI] [PubMed] [Google Scholar]
- 65.Lobatto DJ, et al. Toward value based health care in pituitary surgery: application of a comprehensive outcome set in perioperative care. Eur J Endocrinol. 2019;181(4):375–387. doi: 10.1530/EJE-19-0344. [DOI] [PubMed] [Google Scholar]
- 66.Andela CD, et al. The development and validation of the Leiden Bother and needs questionnaire for patients with pituitary disease: the LBNQ-pituitary. Pituitary. 2016;19(3):293–302. doi: 10.1007/s11102-016-0707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zandbergen IM, et al. The PRolaCT studies—A study protocol for a combined randomised clinical trial and observational cohort study design in prolactinoma. Trials. 2021;22(1):653. doi: 10.1186/s13063-021-05604-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Current manuscript includes no new data, all materials are included in our supplements.