Abstract
Rationale and objectives
To investigate the predictive value of lipid metabolism in predicting the recurrence of hypertriglyceridemic acute pancreatitis (HTG-AP).
Materials and methods
A total of 892 patients were admitted to our hospital for acute pancreatitis (AP) from January 2017 to December 2020, of whom 198 diagnosed with HTG-AP were enrolled in this retrospective study. Demographic information, length of stay, smoking index, alcohol abuse, necrosis, severity, baseline lipid metabolism and other blood biochemical indicators were recorded. The risk factors of recurrence were evaluated using univariate and multivariate Cox proportional risk analyses, and the cumulative recurrence-free survival rate of patients were calculated using Kaplan Meier method and the differences between groups were compared using the log-rank test.
Results
Univariate and multivariate analysis showed that triglyceride (hazard ratio, 2.421; 95% CI, 1.152–5.076; P = 0.020), non high-density lipoprotein (hazard ratio, 4.630; 95% CI, 1.692–12.658; P = 0.003) and apolipoprotein A1 (hazard ratio, 1.735; 95% CI, 1.093–2.754; P = 0.019) were important predictors for recurrence of HTG-AP. Subsequently, patients were divided into four groups according to the cut off values of triglyceride, non high-density lipoprotein and apolipoprotein A1. It was found that the cumulative recurrence-free survival rate of patients in highest-risk group or high-risk group was significantly lower than that of medium-risk group (P < 0.001, P = 0.003) or low risk group (P < 0.001).
Conclusion
Serum triglycerides, non high-density lipoprotein and apolipoprotein A1 are independent predictors of recurrence in HTG-AP patients, which can provide reference for individualized treatment and prevention of recurrence in HTG-AP patients.
Keywords: Acute pancreatitis, Hypertriglyceridemia, Recurrence, Lipid metabolism
Abbreviations
- AP
acute pancreatitis
- HTG-AP
hypertriglyceridemic acute pancreatitis
- RAP
recurrent acute pancreatitis
- BMI
body mass index
- LOS
length of hospital stay
- TG
triglycerides
- TC
total cholesterol
- VLDL
very low density lipoprotein
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- NHDL
non high-density lipoprotein
- FBG
fasting blood glucose
- AST
aspartate transaminase
- ALT
alanine transaminase
- CRP
C-reactive protein
- NEUT
percentage of neutrophils
- IQR
interquartile range
1. Introduction
Acute pancreatitis (AP) is an inflammatory disease characterized by edema and necrosis of glandular tissue, and it has become one of the most common acute abdominal conditions clinically [1]. In recent years, with the improvement of the living standard and the change of diet structure, the incidence rate of hypertriglyceridemic acute pancreatitis (HTG-AP) is gradually increasing, with a tendency to surpass alcoholic pancreatitis as the second common cause of AP [2]. Compared with pancreatitis caused by other causes, HTG-AP has more complications, longer course and higher recurrence rate, with reported recurrence rates of approximately 16.0%–49.2% [[3], [4], [5]]. Some studies [[6], [7], [8]] have suggested that recurrent acute pancreatitis (RAP) is a high-risk group for chronic pancreatitis and pancreatic cancer. Therefore, it is of great clinical significance to analyze the relevant risk factors affecting the recurrence of HTG-AP, so as to provide active treatment and prevention measures for patients with potential recurrence, and prevent the disease from evolving.
The previous studies [3,4] have shown that smoking, high levels of triglycerides (TG), and substandard control of triglycerides are risk factors for recurrence of HTG-AP. At the same time, the core of lipid-lowering drugs in clinical treatment of HTG-AP is to rapidly reduce serum TG (the most ideal state is to reduce TG to below 5.65 mmol/L) [9,10]. However, in addition to TG, other blood lipid indicators of HTG-AP patients characterized by lipid metabolism disorder often have abnormalities to varying degrees [[11], [12], [13], [14]]. Therefore, understanding the differences in various basic lipid levels may have a certain guiding role in the individualized treatment of HTG-AP and the prevention of recurrence. Unfortunately, the impact of other lipid indicators in the lipid metabolism on the recurrence of HTG-AP was ignored in the previous studies.
To solve this problem, our study was to explore the influence of the baseline value of lipid metabolism on the recurrence of patients with an initial attack of HTG-AP, so as to provide scientific reference for clinical intervention measures to effectively prevent the recurrence of HTG-AP.
2. Materials and methods
2.1. Patients
Permission for this investigation was given by the Medical Ethics Committee of our hospital. Because this was a retrospective study design, the ethics committee waived signed informed consent forms.
We conducted a continuous retrospective analysis of 892 AP patients diagnosed and treated in our hospital from January 2017 to December 2020, and enrolled them according to the inclusion and exclusion criteria and the recurrence results during follow-up. The inclusion criteria were as follows: (1) all patients met the diagnostic standard of HTG-AP, (2) and patients in the recurrence group met the diagnostic criteria of RAP. The exclusion criteria were as follows: (1) more than 72 h from onset to admission or had been treated in other hospitals (119 cases, 13.3%); (2) acute pancreatitis caused by biliary, alcoholic or other causes (460 cases, 51.6%); (3) acute attack of chronic pancreatitis (36 cases, 4.0%); (4) complicated with pancreatic tumor, bile duct tumor, ampullary tumor or severe chronic wasting disease (58 cases, 6.5%); (5) incomplete clinical medical records, or loss of follow-up (21 cases, 2.4%).
2.2. Definition of AP, HTG-AP and RAP
The diagnosis of AP shall at least meet 2 of the following 3 items [15]: (1) sudden, severe and persistent abdominal pain, which may be accompanied by back radiation pain; (2) serum amylase and/or lipase shall be at least 3 times higher than the upper limit of normal value; (3) characteristic abdominal imaging findings consistent with AP. The diagnosis of HTG-AP [9] was made if patients had AP in the presence of serum TG ≥ 11.3 mmol/L, or had a serum TG level of 5.65–11.3 mmol/L accompanied by chylous serum after excluding other known risk factors for AP. The diagnostic criteria of RAP [16] includes two or more AP attacks and an interval between the two episodes of AP of at least 3 months, during which the patients reached the standard of recovery or near recovery. In this study, patients were graded as having non-severe and severe HTG-AP based on the Atlanta 2012 classification (including whether there are local complications and organ failure, as well as the duration of organ failure). Moreover, an alcohol abuser was defined as one who has drunk at least 40 g/d (20 g/d for female) for over 5 years in our study [4].
2.3. Treatment and follow-up
All patients received conventional comprehensive treatment, including fasting, acid inhibition, enzyme inhibition, fluid infusion, parenteral nutrition, analgesia, antibiotics, early enteral nutrition and plasma exchange. Follow-up occurred through telephone or admission notes until October 2022 to record recurrence results. In order to avoid recurrence as much as possible, all patients were recommended to continue to limit fat diet after treatment, and take lipid lowering drugs for patients when necessary. If there was no recurrence of HTG-AP during the follow-up period, patients will be classified as the non-recurrence group (n = 112); If recurrence occurred during the period, patients shall be classified as recurrence group (n = 86).
2.4. Blood lipid measurement
The venous blood samples before treatment were collected for examination within 12 h after admission. The blood lipids measurements were performed via a homogeneous enzymatic assay method, including serum TG, total cholesterol (TC), very low density lipoprotein (VLDL), low density lipoprotein (LDL), high density lipoprotein (HDL), non high density lipoprotein (NHDL), lipoprotein a, apolipoprotein A1 (apo A1) and apolipoprotein B100 (apo B100). NHDL [17] refers to the sum of cholesterol contained in other lipoproteins except HDL. In addition to LDL, it also includes VLDL and cholesterol contained in residual lipoproteins.
2.5. Data collection
We viewed the medical records of each enrolled patient from a hospital-based electronic database. The following baseline clinical data were collected: (1) general characteristics, including age, gender, body mass index, length of stay, smoking index, alcohol abuse, and comorbidity (hypertension, fatty liver, diabetes); (2) laboratory data, including fasting blood glucose (FBG), aspartate transaminase (AST); alanine transaminase (ALT), total bilirubin, direct bilirubin, indirect bilirubin, uric acid, urea, creatinine, white blood cells, percentage of neutrophils, albumin, C-reactive protein (CRP), serum pancreatic amylase, serum amylase, serum lipase; (3) necrosis and severity of HTG-AP.
3. Statistics
Statistical analyses were conducted by using SPSS 27.0 and X-tile 3.6.1. Continuous variables were presented as mean (standard deviation [SD]) or median and interquartile range (IQR) based on their distributions, and categorical variables were presented as numbers and percentages. The Kolmogorov–Smirnov method was applied to test the normality of the data. Independent sample t-tests and Mann–Whitney U tests were used for continuous variables, when appropriate. The chi-square test was used for categorical variables. Obtaining the best cut off value through the X-tile software. Univariate and multivariate Cox proportional hazard analysis were used to evaluate the risk factors for recurrence of HTG-AP. Variables with a P value less than 0.1 in the univariate analysis were tested in the multivariate analysis; The hazard ratio (HR) and its corresponding 95% confidence interval (CI) were estimated by the Cox proportional hazard regression model. Finally, Kaplan Meier method was used to calculate the cumulative recurrence-free survival rate (referred to the proportion of patients who did not experience recurrence during the follow-up period) of HTG-AP, and log rank test was used to evaluate the differences among the four subgroups divided by independent predictors (see below). The difference was considered statistically significant at P < 0.05.
4. Result
4.1. Patient characteristics
The clinical manifestations of the cases in both the non-recurrence group and recurrence group are listed in Table 1. The proportion of alcohol abuse in non-recurrence group and recurrence group was 26.8% and 45.3% respectively (P = 0.007). The median value of TG in patients with recurrence (16.53 mmol/L; IQR, 12.90–19.47 mmol/L) was significantly higher than that in patients without recurrence (11.60 mmol/L; IQR, 8.11–18.97 mmol/L; P < 0.001); The median value of TC in patients with recurrence (8.74 mmol/L; IQR, 6.93–11.59 mmol/L) was higher than that in patients without recurrence (7.58 mmol/L; IQR, 5.52–10.86 mmol/L; P = 0.025); In addition, the median value of NHDL in patients with recurrence (7.97 mmol/L; IQR, 5.98–10.39 mmol/L) was also higher than that in patients without recurrence (6.95 mmol/L; IQR, 4.63–9.81 mmol/L; P = 0.012). However, the mean value of apo A1 (0.85 ± 0.33 mmol/L) in the recurrence group was lower than that in the non-recurrence group (0.98 ± 0.29 mmol/L; P = 0.006), which was consistent with the difference in CRP between the two groups. There was no significant difference in the median values of VLDL, LDL and HDL between the two groups.
Table 1.
Patient characteristics.
| non-recurrence group(n = 112) | recurrence group(n = 86) | Statistics value | P value | |
|---|---|---|---|---|
| Age (years) | 42 (34–48) | 41 (35–48) | −0.263 | 0.793 |
| Sex (male/female) | 89/23 | 62/24 | 1.460 | 0.227 |
| BMI (kg/m2) | 25.67 ± 2.94 | 26.16 ± 3.33 | −1.085 | 0.279 |
| LOS (day) | 10 (8–15) | 9.5 (8–16) | −0.348 | 0.728 |
| Smoking index (pack-years) | 1.75 (0–10) | 5 (0–20) | −0.944 | 0.345 |
| Alcohol abuse (n/%) | 30/26.8 | 39/45.3 | 7.383 | 0.007 |
| Hypertension (n/%) | 19/17.0 | 11/12.9 | 0.606 | 0.436 |
| Fatty liver (n/%) | 85/77.3 | 64/74.4 | 0.216 | 0.642 |
| Diabetes mellitus (n/%) | 54/48.2 | 41/47.7 | 0.006 | 0.940 |
| TG (mmol/L) | 11.60 (8.11–18.97) | 16.53 (12.90–19.47) | −3.562 | <0.001 |
| TC (mmol/L) | 7.58 (5.52–10.86) | 8.74 (6.93–11.59) | −2.242 | 0.025 |
| VLDL (mmol/L) | 4.68 (2.64–7.07) | 4.76 (2.78–7.00) | −0.579 | 0.562 |
| LDL (mmol/L) | 1.33 (0.84–2.90) | 1.57 (0.81–5.36) | −1.337 | 0.181 |
| HDL (mmol/L) | 0.71 (0.55–0.93) | 0.75 (0.53–1.08) | −0.737 | 0.461 |
| NHDL (mmol/L) | 6.95 (4.63–9.81) | 7.97 (5.98–10.39) | −2.505 | 0.012 |
| Lipoprotein a (mg/L) | 22.20 (11.10–72.30) | 24.95 (9.85–109.98) | −0.120 | 0.904 |
| Apolipoprotein A1 (g/L) | 0.98 ± 0.29 | 0.85 ± 0.33 | 2.799 | 0.006 |
| Apolipoprotein B (g/L) | 0.61 (0.38–0.79) | 0.52 (0.24–0.76) | −1.273 | 0.203 |
| FBG (mmol/L) | 11.85 (7.01–14.94) | 10.71 (7.50–15.89) | −0.081 | 0.935 |
| AST (U/L) | 33.00 (25.00–41.20) | 27.50 (21.00–45.03) | −0.870 | 0.384 |
| ALT (U/L) | 27.00 (17.00–42.56) | 21.00 (13.23–36.50) | −1.764 | 0.078 |
| Total bilirubin (umol/L) | 18.75 (13.00–24.08) | 15.60 (12.40–24.05) | −1.136 | 0.256 |
| Direct bilirubin (umol/L) | 4.65 (2.70–5.75) | 3.90 (2.08–7.31) | −0.264 | 0.792 |
| Indirect bilirubin (umol/L) | 14.90 (9.90–18.78) | 11.80 (8.48–16.63) | −1.872 | 0.061 |
| Uric acid (umol/L) | 379.65 (294.63–484.58) | 368.45 (306.08–476.30) | −0.100 | 0.920 |
| Urea (mmol/L) | 4.25 (3.20–5.38) | 4.74 (3.90–5.45) | −1.538 | 0.124 |
| Creatinine (umol/L) | 56.30 (43.33–65.35) | 53.50 (33.13–66.33) | −1.549 | 0.121 |
| WBC ( × 109/L) | 15.63 ± 5.60 | 14.47 ± 4.24 | 1.655 | 0.100 |
| Neut | 85.30 (82.50–88.08) | 86.30 (82.88–89.63) | −1.514 | 0.130 |
| Albumin (g/L) | 44.20 (40.50–47.78) | 43.60 (38.98–46.40) | −1.405 | 0.160 |
| CRP (mg/L) | 59.95 (16.17–119.42) | 39.68 (15.11–79.29) | −2.550 | 0.011 |
| Amylopsin (U/L) | 204 (125–420.23) | 184.50 (85.20–401.78) | −1.083 | 0.279 |
| Amylase (U/L) | 294.85 (167.10–527.00) | 244.05 (117.75–485.68) | −1.255 | 0.210 |
| Lipase (U/L) | 458.25 (237.00–979.43) | 467.15 (154.00–740.20) | −1.496 | 0.135 |
| Severity (non-severe/severe) | 81/31 | 68/18 | 1.190 | 0.275 |
| Necrosis (n/%) | 25/22.3 | 24/27.9 | 0.815 | 0.367 |
Bold values are statistically significant.
BMI,body mass index; LOS,length of hospital stay; TG,Triglycerides; TC,total cholesterol; VLDL,very low density lipoprotein; LDL,low density lipoprotein; HDL,high density lipoprotein; NHDL,non high-density lipoprotein; FBG,fasting blood glucose; AST,aspartate transaminase; ALT,alanine transaminase; CRP,C-reactive protein; NEUT,percentage of neutrophils.
4.2. Risk factors for recurrence of HTG-AP by univariate and multivariate analysis
By comparing the characteristics of patients, the following indicators were found to have statistically significant differences between the two groups: alcohol abuse, serum TG, TC, NHDL, apo A1, CRP. Then, these indicators were included in univariate and multivariate Cox proportional hazard analysis to assess the risk factors of HTG-AP recurrence. In addition, other blood lipid indicators discussed in this study were also included in univariate and multivariate analysis. Before that, we described the continuous variables as categorical variables according to the cut off values. The results of univariate and multivariate analysis were shown in Table 2. In this study, only the P values of TG, NHDL and apo A1 were lower than 0.05, and the corresponding HR values were 2.421 (95% CI, 1.152–5.076), 4.630 (95% CI, 1.692–12.658) and 1.735 (95% CI, 1.093–2.754), respectively. High serum TG and NHDL and low apo A1 were independent risk factors for recurrence of HTG-AP.
Table 2.
Univariate and multivariate Cox proportional hazards analyses.
| Variables | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Alcohol abuse | 1.681 (1.098–2.571) | 0.017 | 1.531 (0.970–2.421) | 0.068 |
| TG, ≥11.70vs < 11.70 mmol/L | 4.975 (2.639–9.346) | <0.001 | 2.421 (1.152–5.076) | 0.020 |
| TC, ≥6.80vs < 6.80 mmol/L | 2.283 (1.381–3.774) | 0.001 | 1.629 (0.834–3.183) | 0.153 |
| VLDL, ≥1.98vs < 1.98 mmol/L | 4.310 (1.736–10.753) | 0.002 | 1.795 (0.602–5.348) | 0.294 |
| LDL, ≥3.69vs < 3.69 mmol/L | 1.575 (1.021–2.427) | 0.040 | 1.632 (0.894–2.977) | 0.111 |
| HDL,≥1.30vs < 1.30 mmol/L | 1.739 (1.020–2.967) | 0.042 | 1.629 (0.826–3.205) | 0.158 |
| NHDL, ≥4.90vs < 4.90 mmol/L | 6.289 (2.732–14.493) | <0.001 | 4.630 (1.692–12.658) | 0.003 |
| Lipoprotein a, ≥5.40vs < 5.40 mg/L | 1.672 (0.906–3.085) | 0.100 | ||
| Apolipoprotein A1, ≥0.76vs < 0.76 g/L | 2.279 (1.488–3.492) | <0.001 | 1.735 (1.093–2.754) | 0.019 |
| Apolipoprotein B100, ≥0.20vs < 0.20 g/L | 1.530 (0.899–2.604) | 0.117 | ||
| CRP, ≥55.20vs < 55.20 mg/L | 1.486 (0.945–2.335) | 0.086 | 1.602 (0.975–2.632) | 0.063 |
Bold values in the univariate analysis are further analyzed in the multivariate analysis and bold values in the multivariate analysis are statistically significant.
4.3. Association of TG, NHDL and apo A1 with HTG-AP cumulative recurrence-free survival
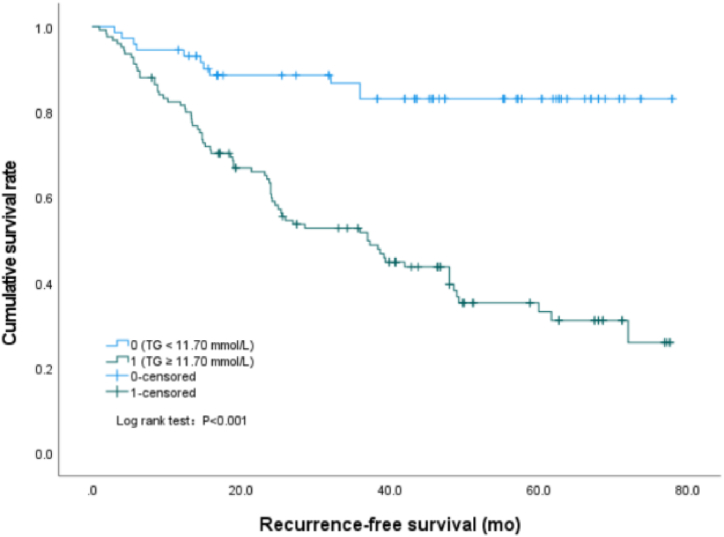
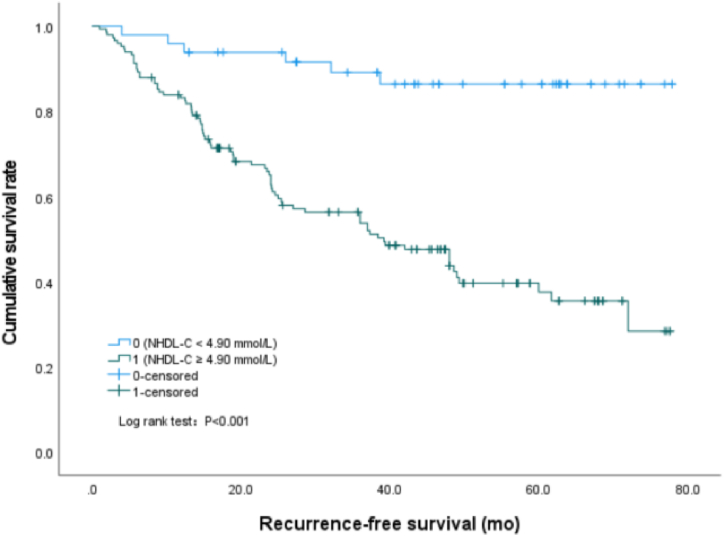
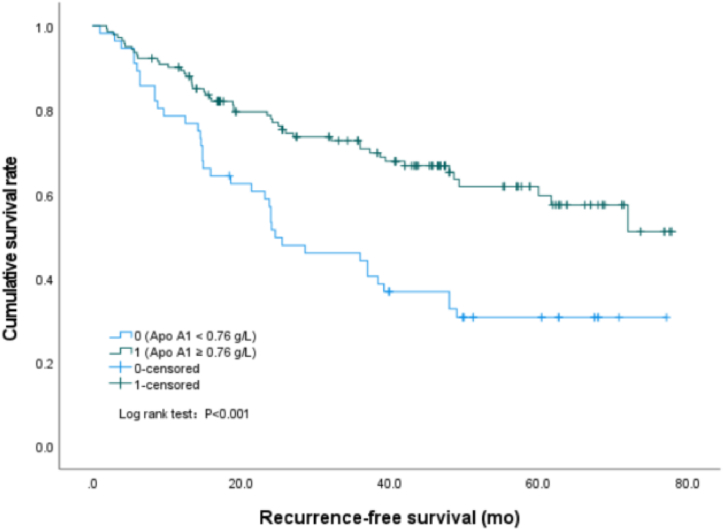
In this study, we found that the cumulative recurrence-free survival rate of patients with serum TG value equal to or greater than 11.70 mmol/L was significantly lower than that of patients with serum TG value less than 11.70 mmol/L (P < 0.001) (Fig. 1), the median survival time was 40.7 (95%CI: 35.5–45.9) mo and 67.7 (95%CI: 62.2–73.2) mo, respectively. Similarly, the survival rate of patients with NHDL values of 4.90 mmol/L or above was significantly lower than that of patients with NHDL values of less than 4.90 mmol/L (P < 0.001) (Fig. 2), the median survival time was 43.0 (95%CI: 38.0–48.0) mo and 70.3 (95%CI: 64.6–76.0) mo, respectively. However, the cumulative recurrence-free survival rate of patients with apo A1 values less than 0.76 g/L was significantly lower than that of patients with apo A1 values of 0.76 g/L or above (P < 0.001) (Fig. 3), the median survival time was 37.8 (95%CI: 30.2–45.3) mo and 55.6 (95%CI: 50.5–60.6) mo, respectively.
Fig. 1.
Survival curves by Kaplan-Meier analyses, comparing the cumulative recurrence-free survival rate between patients with TG ≥ 11.70 mmol/L and TG < 11.70 mmol/L.
Fig. 2.
Survival curves by Kaplan-Meier analyses, comparing the cumulative recurrence-free survival rate between patients with NHDL ≥4.90 mmol/L and NHDL <4.90 mmol/L.
Fig. 3.
Survival curves by Kaplan-Meier analyses, comparing the cumulative recurrence-free survival rate between patients with Apo A1 ≥0.76 g/L and Apo A1 < 0.76 g/L.
4.4. Combination of TG, NHDL, and apo A1 to predict the recurrence of HTG-AP
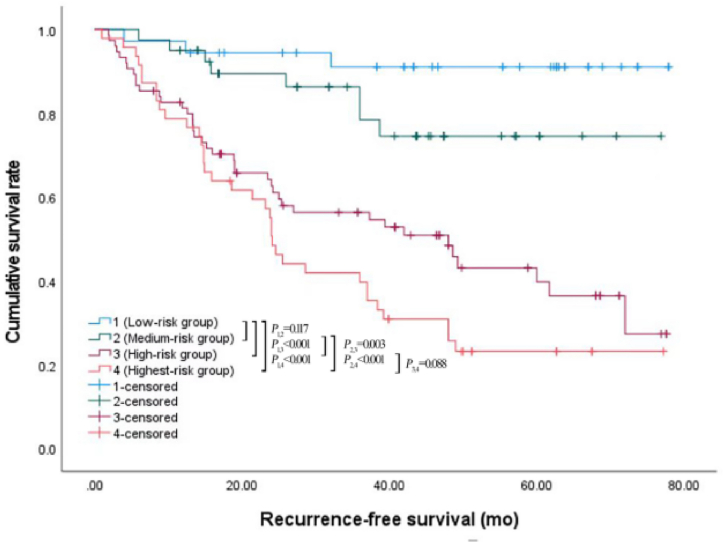
According to the above results, TG, NHDL and apo A1 were effective factors to predict the recurrence of HTG-AP. Therefore, we further divided HTG-AP patients into four subgroups according to the cut off values of TG, NHDL and apo A1, as follows: low-risk group (TG < 11.70 mmol/L, NHDL<4.90mmoL/L, apo A1 ≥ 0.76 g/L), medium-risk group (met any one of the following three indicators: TG ≥ 11.70 mmol/L, NHDL ≥4.90 mmol/L, apo A1<0.76 g/L), high-risk group (met any two of the following three indicators: TG ≥ 11.70 mmol/L, NHDL ≥4.90 mmol/L, apo A1<0.76 g/L) and highest-risk group (TG ≥ 11.70 mmol/L, NHDL ≥4.90 mmol/L, apo A1<0.76 g/L). The recurrence rates of the four subgroups were 8.3%, 20%, 53.3%, and 74.5%, respectively. Then, Kaplan Meier analysis and log rank test were used to compare the difference of cumulative recurrence-free survival rate among the four subgroups (Fig. 4). The analysis results showed that the cumulative recurrence-free survival rate of highest-risk group or high-risk group was significantly lower than that of medium-risk group (P < 0.001, P = 0.003) or low risk group (P < 0.001), and there were no significant difference between highest-risk group and high-risk group (P = 0.088), medium-risk group and low risk group (P = 0.117). Furthermore, similar results that showed TG, NHDL, and apo A1 are independent predictors of recurrence could also be found when using them as continuous variables, and the combination of the three indicators had moderate value in predicting the recurrence of HTG-AP. The regression equation was: Y = 0.048 × X1+ 0.107 × X2 -1.042 × X3 -0.898(Y represented the risk of recurrence, X1 represented TG, X2 represented NHDL, X3 represented apo A1), the OR were 1.049 (95% CI: 1.002–1.098), 1.113 (95% CI: 1.006–1.232), and 0.353 (95% CI: 0.131–0.947), respectively.
Fig. 4.
Survival curves by Kaplan-Meier analyses, comparing the cumulative recurrence-free survival rate among the highest-risk group, high-risk group, medium-risk group, and low-risk group.
5. Discussion
In this study, univariate and multivariate Cox proportional hazard analysis confirmed that serum TG, NHDL and apo A1 were independent predictors for recurrence of HTG-AP. To the best of our knowledge, this was the first study to explore the value of lipid metabolism in early prediction the recurrence of HTG-AP. In the previous study [3], 171 patients with acute pancreatitis caused by severe hyperlipidemia were analyzed. It was found that the peak level of serum TG higher than 3000 mg/dL was an independent predictor of HTG-AP recurrence, and the decrease of TG value level had certain correlation with the reduction of recurrence risk. Unfortunately, Zafrir et al. [3] did not evaluate the impact of other blood lipid indicators in the lipid metabolism on the recurrence of HTG-AP, which was the most significant difference between our study and theirs.
In our study, serum TG is an independent risk factor for recurrence of HTG-AP patients. Wang et al. [18] also supported this view in the meta-analysis, that is, the increase of serum TG is also related to the severity and worse prognosis of AP. The exact mechanism of high TG causing acute pancreatitis is still unclear. Lipoprotein rich in TG (especially chyle particles) may damage the circulating blood flow in the pancreatic capillary bed, leading to local ischemia. Excessive TG could be degraded by pancreatic lipase to produce excess proinflammatory free fatty acids, which could cause pancreatic acinar cell necrosis by inhibiting mitochondrial complexes I and V, leading to necrotizing pancreatitis [10]. Free fatty acids could also directly damage pancreatic capillaries, leading to local ischemia and acidic environment, and increase the toxicity of free fatty acids [19].
NHDL refers to the sum of all cholesterol except HDL, mainly including LDL and VLDL. It is well known that HDL is a protective factor in cardiovascular disease, while LDL and VLDL are risk factors [20]. Some studies [21,22] proposed that NHDL is simpler and more convenient than LDL, and even the strongest blood lipid index to predict coronary heart disease risk. International guidelines have suggested that NHDL should be listed as the capital goal of primary and secondary prevention of atherosclerotic cardiovascular disease [23]. Therefore, our study included NHDL into the analysis, and found that NHDL is an independent risk factor for the recurrence of HTG-AP, which may affect the occurrence and development of pancreatitis from the following aspects: It could cause the increase of blood viscosity, promote the formation of microthrombosis, and lead to pancreatic microcirculation disorder; It directly damages the vascular endothelial cells, and causes the decrease in the number of endothelial progenitor cells and dysfunction, thereby affecting angiogenesis and the repair of vascular endothelium after injury; It could cause the increase of intracellular calcium concentration, which could accelerate the activation of trypsinogen and damage pancreatic cells [24]; LDL and VLDL can promote the production of reactive oxygen species in islet B cells and cause damage to islet B cells due to the lack of enzymes to remove reactive oxygen species in islet B cells.
Previous studies [12,25,26] showed that apo A1 has a good predictive value for the severity of HTG-AP and the occurrence of organ failure. Our results also confirmed the value of apo A1 in HTG-AP patients. We found that the lower apo A1 value was an independent risk factor for recurrence of HTG-AP, which had a good clinical warning effect for patients with high risk of recurrence. We speculated that the possible mechanisms are as follows. Apo A1 is the main component of HDL. Apo A1 has anti-inflammatory effect by inhibiting the trans endothelial migration of immunocompetent cells, inhibiting the activation of monocytes and the production of cytokines contacted by T lymphocytes, inhibiting lipid peroxidation and regulating innate immune receptors. At the same time, apo A1 could reduce the secretion of inflammatory cytokines induced by lipopolysaccharide endotoxin [27,28]. Therefore, the reduction of apo A1 will increase the risk of recurrence.
At the same time, we combined TG, NHDL and apo A1 to early predict the recurrence of HTG-AP. The result showed that the combination of the three can stratify the risk of HTG-AP recurrence, which can provide a reference for clinicians to individualized treatment and prevention of HTG-AP, as well as early warning for high risk initial HTG-AP patients from the perspective of lipid metabolism, so as to prevent and reduce the recurrence.
In addition, our study found that the proportion of alcohol abuse patients in recurrence group was higher than that in non-recurrence group, which was consistent with the previous study [3]. Alcohol can increase TG levels. In clinical practice, long-term heavy drinking will lead to a significant increase in TG and the synthesis of VLDL, reduce the activity of LPL, as well as reduce fat production and glucose oxidation in adipose tissue, thus inducing HTG-AP. The smoking index in our study had no statistically significant difference between the two groups, which was inconsistent with the results of previous study [4]. A single center and the random allocation of patients in our study may be the reason for the different results. In any case, patients who smoked and drank excessively should be encouraged to give up these bad habits.
Several limitations existed in this research. First, the sample size is relatively small, and due to retrospective study and the unavailability of data, factors such as hypothyroidism and chronic kidney disease, which can lead to significantly increased TG levels, were not included in the analysis. Secondly, this study did not discuss the influence of the dynamic changes of lipid metabolism during hospitalization, which may affect the comprehensiveness of the results to a certain extent. Therefore, our future research will increase the sample size or conduct multicenter research, and incorporate more potential influencing factors for comprehensive analysis as far as possible.
In summary, indicators in lipid metabolism (serum TG, NHDL and apo A1) are independent predictors for recurrence of HTG-AP. The combination of TG, NHDL and apo A1 can stratify the risk for recurrence of HTG-AP. Early warning of potential recurrence patients from the perspective of lipid metabolism can guide clinicians to carry out individualized precise diagnosis, treatment and prevention of HTG-AP patients.
Author contribution statement
Lingling Tang: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Qing Jia: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Nian Liu: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Qianqian Liu; Ke Pan; Lixing Lei: Performed the experiments; Analyzed and interpreted the data.
Xiaohua Huang: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This study was funded by the Science and Technology Project of the Health Planning Committee of Sichuan (grant number 19PJ203, to Nian Liu), Bureau of Science & Technology and Intellectual Property Nanchong City (grant numbers 20SXQT0303, to Xiaohua Huang), Scientific Research and Development Plan Project of North Sichuan Medical College (grant number CBY22-QNA30, to Lingling Tang), Scientific Research and Development Plan Project of Affiliated Hospital of North Sichuan Medical College (grant number 2023JC047, to Lingling Tang).
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Mederos M.A., Reber H.A., Girgis M.D. Acute pancreatitis: a review. JAMA. 2021;325:382–390. doi: 10.1001/jama.2020.20317. [DOI] [PubMed] [Google Scholar]
- 2.Chen W.J., et al. Hypertriglyceridemic acute pancreatitis in emergency department: typical clinical features and genetic variants. J. Dig. Dis. 2017;18:359–368. doi: 10.1111/1751-2980.12490. [DOI] [PubMed] [Google Scholar]
- 3.Zafrir B., et al. Severe hypertriglyceridemia-related pancreatitis: characteristics and predictors of recurrence. Pancreas. 2019;48:182–186. doi: 10.1097/MPA.0000000000001235. [DOI] [PubMed] [Google Scholar]
- 4.Xiang J.X., et al. Impact of cigarette smoking on recurrence of hyperlipidemic acute pancreatitis. World J. Gastroenterol. 2017;23:8387–8394. doi: 10.3748/wjg.v23.i47.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin M., et al. A 16-year trend of etiology in acute pancreatitis: the increasing proportion of hypertriglyceridemia-associated acute pancreatitis and its adverse effect on prognosis. J. Clin. Lipidol. 2019;13:947–953. doi: 10.1016/j.jacl.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Seppänen H., Puolakkainen P. Classification, severity assessment, and prevention of recurrences in acute pancreatitis. Scand. J. Surg. 2020;109:53–58. doi: 10.1177/1457496920910007. [DOI] [PubMed] [Google Scholar]
- 7.Coté G.A., et al. Recurrent acute pancreatitis significantly reduces quality of life even in the absence of overt chronic pancreatitis. Am. J. Gastroenterol. 2018;113:906–912. doi: 10.1038/s41395-018-0087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu X., et al. Individualized prediction of acute pancreatitis recurrence using a nomogram. Pancreas. 2021;50:873–878. doi: 10.1097/MPA.0000000000001839. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary A., et al. Acute pancreatitis secondary to severe hypertriglyceridemia: management of severe hypertriglyceridemia in emergency setting. Gastroenterol. Res. 2017;10:190–192. doi: 10.14740/gr762e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherer J., et al. Issues in hypertriglyceridemic pancreatitis: an update. J. Clin. Gastroenterol. 2014;48:195–203. doi: 10.1097/01.mcg.0000436438.60145.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen Z., et al. Metabolic syndrome components and acute pancreatitis: a case-control study in China. BMC Gastroenterol. 2021;21:17. doi: 10.1186/s12876-020-01579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J., et al. Serum apolipoprotein B-to-apolipoprotein A1 ratio is independently associated with disease severity in patients with acute pancreatitis. Sci. Rep. 2019;9:7764. doi: 10.1038/s41598-019-44244-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berglund L., et al. Treatment options for hypertriglyceridemia: from risk reduction to pancreatitis. Best Pract. Res. Clin. Endocrinol. Metabol. 2014;28:423–437. doi: 10.1016/j.beem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y., et al. Radiomics model of contrast-enhanced computed tomography for predicting the recurrence of acute pancreatitis. Eur. Radiol. 2019;29:4408–4417. doi: 10.1007/s00330-018-5824-1. [DOI] [PubMed] [Google Scholar]
- 15.Banks P.A., et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 16.Guda N.M., et al. Recurrent acute pancreatitis: international state-of-the-science conference with recommendations. Pancreas. 2018;47:653–666. doi: 10.1097/MPA.0000000000001053. [DOI] [PubMed] [Google Scholar]
- 17.Sniderman A.D., Navar A.M., Thanassoulis G. Apolipoprotein B vs low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol as the primary measure of apolipoprotein B lipoprotein-related risk: the debate is over. Jama Cardiol. 2022;7:257–258. doi: 10.1001/jamacardio.2021.5080. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q., et al. Elevated serum triglycerides in the prognostic assessment of acute pancreatitis: a systematic review and meta-analysis of observational studies. J. Clin. Gastroenterol. 2017;51:586–593. doi: 10.1097/MCG.0000000000000846. [DOI] [PubMed] [Google Scholar]
- 19.Bosques-Padilla F.J., et al. Hypertriglyceridemia-induced pancreatitis and risk of persistent systemic inflammatory response syndrome. Am. J. Med. Sci. 2015;349:206–211. doi: 10.1097/MAJ.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 20.Mortensen M.B., Nordestgaard B.G. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70-100 years: a contemporary primary prevention cohort. Lancet. 2020;396:1644–1652. doi: 10.1016/S0140-6736(20)32233-9. [DOI] [PubMed] [Google Scholar]
- 21.Masson W., et al. Impact of lipid-lowering therapy on mortality according to the baseline non-HDL cholesterol level: a meta-analysis. High Blood Pres. Cardiovasc. Prev. 2019;26:263–272. doi: 10.1007/s40292-019-00330-8. [DOI] [PubMed] [Google Scholar]
- 22.Sunil B., Ashraf A.P. Childhood hypertriglyceridemia: is it time for a new approach? Curr. Atherosclerosis Rep. 2022;24:265–275. doi: 10.1007/s11883-022-01000-2. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson T.A., et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1--full report. J. Clin. Lipidol. 2015;9:129–169. doi: 10.1016/j.jacl.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Gerasimenko J.V., Gerasimenko O.V., Petersen O.H. The role of Ca2+ in the pathophysiology of pancreatitis. J. Physiol. 2014;592:269–280. doi: 10.1113/jphysiol.2013.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huh J.H., et al. Predictive value of apolipoprotein B and A-I ratio in severe acute pancreatitis. J. Gastroenterol. Hepatol. 2018;33:548–553. doi: 10.1111/jgh.13860. [DOI] [PubMed] [Google Scholar]
- 26.Zhou C.L., et al. Early prediction of persistent organ failure by serum apolipoprotein A-I and high-density lipoprotein cholesterol in patients with acute pancreatitis. Clin. Chim. Acta. 2018;476:139–145. doi: 10.1016/j.cca.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Vuilleumier N., et al. Pro- or anti-inflammatory role of apolipoprotein A-1 in high-density lipoproteins? Swiss Med. Wkly. 2013;143 doi: 10.4414/smw.2013.13781. [DOI] [PubMed] [Google Scholar]
- 28.Sirniö P., et al. Decreased serum apolipoprotein A1 levels are associated with poor survival and systemic inflammatory response in colorectal cancer. Sci. Rep. 2017;7:5374. doi: 10.1038/s41598-017-05415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.