Abstract
Tegoprazan is a novel potassium-competitive acid blocker that treats gastric acid-related diseases. Clarithromycin was widely used as one of various regimens for eradicating Helicobacter pylori. This study compared the pharmacokinetic and safety profile of tegoprazan and clarithromycin between combination therapy and monotherapy to evaluate the potential drug-drug interaction. An open-label, randomized, 6-sequence, 3-period crossover study was conducted in 24 healthy subjects. According to the assigned sequence, the subject was administered the assigned treatment during 5 days in each period. PK parameters of tegoprazan and clarithromycin administered in combination were compared with those of the respective monotherapies. The co-administration of tegoprazan with clarithromycin increased maximum steady-state plasma concentration (Css,max) and area under the plasma concentration-time curve in dosing interval at steady-state (AUCss,tau) of tegoprazan (1.6-fold in Css,max and 2.5-fold in AUCss,tau) and M1 (2.0-fold in Css,max, 2.5-fold in AUCss,tau) than tegoprazan alone. The Css,max and AUCss,tau of 14-hydroxyclarithromycin increased 1.8- and 2.0-fold in co-administration, respectively. The AUCss.tau of clarithromycin was slightly increased in co-administration, but Css,max was not changed. Combination of tegoprazan and clarithromycin and those of the respective monotherapies were tolerated in 24 healthy subjects. There may exist drug interaction that lead to reciprocal increase in plasma drug concentrations when tegoprazan and clarithromycin were administrated in combination and no safety concerns were raised. It is suggested that an in-depth analysis of the concentration-response relationship is necessary to determine whether these concentration changes warrant clinical action.
Trial Registration
ClinicalTrials.gov Identifier: NCT02052336
Keywords: Tegoprazan, Clarithromycin, Drug Interactions
INTRODUCTION
Helicobacter pylori infection is a common bacterial infection in human by causing gastrointestinal diseases such as gastritis, gastric and duodenal ulcers, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma [1]. The various treatments were proposed for H. pylori eradication including dual therapy, triple therapy, concomitant therapy, sequential therapy, hybrid therapy and bismuth based therapy [2]. However, the eradication treatments have been challenged due to insufficient inhibition of intragastric acid [3]. The some antibiotics in eradication treatment for H. pylori are unstable and degradable in acid state and its state is favorable to growth of H. pylori, which suggests that sufficient acid inhibition in eradication treatment is important [4]. However, because proton pump inhibitors (PPIs) which are widely used in the eradication treatment of H. pylori show slow onset by transformation to active form and has the short half-lives of about 1 to 2 hours, acid inhibition of PPIs seems to be insufficient. Furthermore, because PPIs are mainly metabolized by CYP2C19, the acid inhibition of PPIs in the extensive metabolizer (EM) genotype patients were more insufficient than in the poor metabolizer (PM) genotype patients, which leads to lower eradication rate in EM patients [4]. Therefore, development of potent acid suppression agents is desired to improve the eradication rate of H. pylori.
The potassium competitive-acid blocker (P-CAB) is a novel class of inhibiting H+/K+ ATPase and modulates intragastric acid. The P-CAB reversibly inhibits H+/K+ ATPase and shows the rapid onset. Half-life of P-CAB is known to be longer than the PPI and it could be administered without food intake. Tegoprazan is one of the P-CABs and was approved from the Ministry of Food and Drug Safety at Korea (2018). Tegoprazan showed the dose proportional pharmacokinetic (PK) profile in range of 50 to 400 mg. The plasma tegoprazan concentration rapidly reached the maximum plasma concentration (Cmax) within 2 hours and its half-life was of 5–7 hours [5]. Furthermore, tegoprazan attained sufficient acid inhibition with a rapid onset and sustained antisecretory effect after the single and multiple administration of it once daily [5]. These PK and pharmacodynamic properties of tegoprazan suggested that tegoprazan based eradication regimen could be potential as an eradication treatment of H. pylori.
The first-line therapy of H. pylori eradication recommended in several gastroenterological societies [6] or Korea [7] included mainly PPI and 2 antibiotics of amoxicillin and clarithromycin. Clarithromycin is a well-known inhibitor to increase an exposure of substrates of CYP3A4, but amoxicillin might inhibit CYP2C8 activity [8,9]. Metabolism of tegoprazan into the active metabolite, M1, is thought to be mainly due to the action of CYP3A4. When the tegoprazan and clarithromycin is co-administered for eradication of H. pylori, their exposure would be altered by the same metabolic pathway of CYP3A4. According to altered exposure level, unexpected adverse effect may be occurred or the treatment effect may be lower than expectation. Therefore, the aim of this study was to assess the potential drug-drug interaction in co-administration of tegoprazan and clarithromycin in healthy subjects.
METHODS
This clinical trial (ClinicalTrials.gov Identifier: NCT02052336) was conducted in accordance with the Korean Good Clinical Practices and the ethical principles of the Declaration of Helsinki. This study protocol was reviewed and approved by the Ministry of Food and Drug Safety of South Korea and the Institutional Review Board of Busan Paik Hospital of South Korea. Written informed consent was obtained from all subjects before any study procedure.
Study design
The study was an open-label, randomized, 6-sequence, 3-period crossover study to evaluate a PK drug interaction between tegoprazan and clarithromycin in healthy male subjects. The healthy male subjects aged 19–45 years with a body mass index (BMI) of 19–28 kg/m2 were eligible. The major exclusion criteria were:
The eligible subjects were randomized in 1 of 6 sequences (Fig. 1). Each sequence included 3 periods with 3-week washout between periods. In each period, the subjects were received one treatment of 3 treatments (T, R1, R2) according to the assigned sequence. The T represents co-administration of tegoprazan 200 mg once daily and clarithromycin 500 mg twice daily for 5 days. The R1 and R2 represents administration of tegoprazan 200 mg once daily for 5 days and clarithromycin 500 mg twice daily for 5 days, respectively. The study drug was administered with water of 150 mL on day 1 to day 4 or 240 mL on day 5. The morning dose and the evening dose were given after at least 8 hours and 2 hours fasting, respectively. The consumption of food was restricted 4 hours and 2 hours after the morning dose and evening dose, respectively.
Figure 1. Subject disposition. Sequence A with intervention T-R1-R2, sequence B with intervention R1-R2-T, sequence C with intervention R2-T-R1, sequence D with intervention T-R2-R1, sequence E with intervention R1-T-R2, sequence F with intervention R2-R1-T.
T, tegoprazan 200 mg once daily and clarithromycin 500 mg twice daily for 5 days; R1, tegoprazan 200 mg once daily for 5 days; R2, clarithromycin 500 mg twice daily for 5 days.
The blood samples of PK parameters of tegoprazan were collected at pre-dose on day 4 and 5 and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24 hours after dose on day 5 at each period. The blood samples of PK parameters of clarithromycin were collected at pre-dose on day 4 and 5 and 1, 2, 3, 4, 6, 8 and 12 hours after dose on day 5 at each period. The blood samples were collected in heparinized vacutainer tube and centrifuged at 2,000 g for 10 minutes. The supernatant was stored at ≤ −70°C until the assay was performed.
Bioanalytical methods
The plasma concentrations of tegoprazan, M1, clarithromycin and 14-hydroxyclarithromycin were determined by high-performance liquid chromatography (Agilent 1200 series HPLC system; Agilent Technologies, Inc., Wilmington, DE, USA) coupled with mass spectrometry (API 6410 Triple Quad; Agilent Technologies, Inc.) based on validated analytical procedures adopted by the Korean Ministry of Food and Drug safety. The calibration curves were linear in the range of 10–10,000 ng/mL for tegoprazan and 5–5,000 ng/mL for M1 with the lower limit of quantification (LLOQ) values of 10 ng/mL and 5 ng/mL, respectively. The intra-batch precision and the inter-batch precision for tegoprazan were 2.14–4.65% and 1.78–7.36%, respectively, and they for M1 were 2.26–4.12% and 1.48–9.43%, respectively. The intra-batch accuracy and the inter-batch accuracy for tegoprazan were 104.31–111.26% and 108.34–109.54%, respectively, and they for M1 were 108.30–114.91% and 107.11–110.36%, respectively.
The calibration curves were linear in the range of 100–15,000 ng/mL for clarithromycin and 25–5,000 ng/mL for 14-hydroxy clarithromycin with the LLOQ values of 100 ng/mL and 25 ng/mL, respectively. The intra-batch precision and the inter-batch precision for the assay of clarithromycin were 1.67–5.73% and 4.52–8.13%, respectively, and they for the assay of 14-hydroxy clarithromycin were 1.86–4.57% and 3.63–9.29%, respectively. The intra-batch precision and the inter-batch accuracy for the assay of clarithromycin were 105.46–108.60% and 94.75–106.51%, respectively, and they for the assay of 14-hydroxy clarithromycin were 101.21–108.70% and 91.25–98.58%, respectively. The overall intra- and inter-batch accuracy and coefficient of variation values for all analytes were within the acceptance criteria of < 15% (< 20% at LLOQ level).
PK analysis
The PK parameters of tegoprazan, M1, clarithromycin and 14-hydroxyclarithromycin were calculated using noncompartmental analysis with Phoenix® WinNonlin® (version 6.3.0; Certara, Inc., Princeton, NJ, USA): the maximum steady-state plasma concentration (Css,max) and the time to reach Css,max (Tss,max), the area under the plasma concentration-time curve in dosing interval at steady-state (AUCss,tau), terminal elimination half-life (t1/2), and metabolic ratio. Css,max and Tss,max were obtained from the observation values. t1/2 was calculated as 0.693/terminal elimination slope. Metabolic ratio was calculated as AUCss,tau of M1/AUCss,tau of tegoprazan and AUCss,tau of 4-hydroxyclarithromycin/AUCss,tau of clarithromycin.
Safety assessment
The safety and tolerability were assessed for the subjects who received at least one investigational product throughout the study. All adverse events (AEs) were recorded from subjects’ spontaneous reports or investigators’ questionnaires. The severity, course, outcome, seriousness, and relationship to the investigational product of all AEs were assessed by investigators. Physical examinations, vital sign measurements, electrocardiograms (ECGs), laboratory test diagnostic tests were performed according to predefined schedule.
Statistical analysis
To evaluate PK interaction between tegoprazan and clarithromycin, point estimation difference between group and its 90% confidential interval (CI) of log-converted primary PK parameters (Css,max, AUCss,tau) were calculated by linear mixed effect model about each treatment groups using the SAS® software version 9.3 (SAS Institue, Cary, NC, USA). The point estimates and 90% CIs for differences on the log scale will be exponentiated to obtain estimates for ratios of geometric means on the original scale. The secondary PK parameters (t1/2, metabolic ratio) were presented as the arithmetic mean with its corresponding standard deviation. Otherwise, Tss,max were presented as the median value with its corresponding minimum and maximum values.
RESULTS
Subjects
Twenty-four subjects were enrolled in the study. One subject withdraw consent, and then total 23 subjects completed the study. The mean age, weight, and BMI of 24 subjects were 24.79 ± 3.16 years, 68.92 ± 6.18 kg, and 22.82 ± 1.92 kg/m2, respectively. The baseline characteristics of subjects in 6 sequences were summarized in Table 1 and the characteristics were comparable among sequences.
Table 1. The baseline characteristics of subjects in 6 sequences.
| Sequence | Age (yr) | Height (cm) | Body weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|
| A (T-R1-R2, n = 4) | 27.00 ± 2.16 | 173.50 ± 4.04 | 69.13 ± 2.18 | 23.00 ± 1.47 |
| B (R1-R2-T, n = 4) | 27.75 ± 4.35 | 170.75 ± 7.14 | 69.73 ± 6.21 | 23.89 ± 0.84 |
| C (R2-T-R1, n = 4) | 24.25 ± 1.50 | 176.00 ± 1.83 | 75.68 ± 8.21 | 24.40 ± 2.25 |
| D (T-R2-R1, n = 4) | 23.50 ± 3.11 | 173.00 ± 5.72 | 68.33 ± 3.77 | 22.86 ± 1.52 |
| E (R1-T-R2, n = 4) | 23.50 ± 3.11 | 175.00 ± 5.60 | 67.35 ± 6.62 | 21.95 ± 1.09 |
| F (R2-R1-T, n = 4) | 22.75 ± 1.71 | 174.75 ± 7.68 | 63.33 ± 4.13 | 20.84 ± 2.44 |
| Total (n = 24) | 24.79 ± 3.16 | 173.83 ± 5.31 | 68.92 ± 6.18 | 22.82 ± 1.93 |
BMI, body mass index; T, tegoprazan 200 mg once daily and clarithromycin 500 mg twice daily for 5 days; R1, tegoprazan 200 mg once daily for 5 days; R2, clarithromycin 500 mg twice daily for 5 days.
PKs of tegoprazan and M1
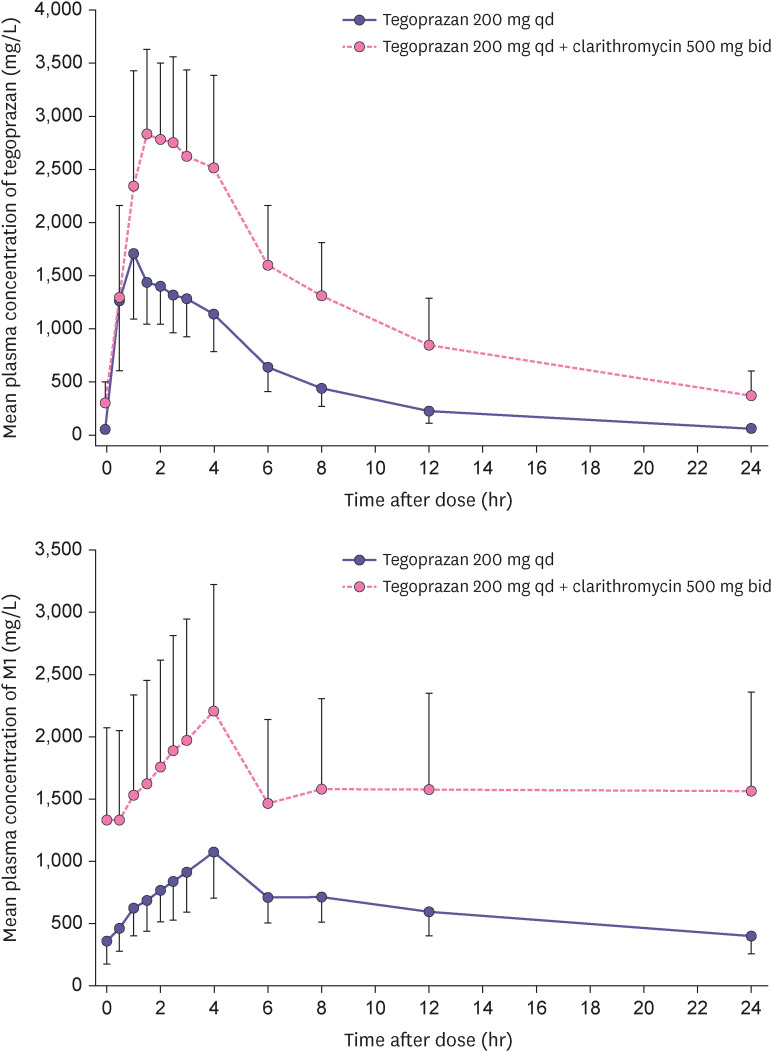
The mean plasma concentration profile of tegoprazan and M1 were increased after the co-administration of tegoprazan and clarithromycin compared with the single administration of tegoprazan (Fig. 2). The PK parameters of tegoprazan and M1 in 2 treatments were summarized in Table 2. The Css,max and AUCss,tau of tegoprazan were increased in the co-administration compared with single administration and their geometric ratio (GMR) with 90% CI were 1.6474 (1.5062–1.8019) and 2.4986 (2.3525–2.6538), respectively. Furthermore, the Css,max and AUCss,tau of M1 were also increased in the co-administration and their GMR (90% CI) were 2.0044 (1.8274–2.1987) and 2.5016 (2.3145–2.7038), respectively. The Tss,max of tegoprazan and M1 in 2 treatments were similar, whereas the co-administration led to increase the t1/2 of them.
Figure 2. Mean plasma concentration-time profiles of tegoprazan and M1 after oral administration of co-administered with clarithromycin for 5 days and tegoprazan alone for 5 days (upper: tegoprazan, lower: M1).
Table 2. Summary table of pharmacokinetic parameters of tegoprazan and M1 following multiple administration.
| Parameter | Co-administration* (n = 23) | Alone† (n = 23) | GMR (90% CI) | |
|---|---|---|---|---|
| Tegoprazan | ||||
| Css,max (µg/L) | 3,095.95 ± 812.65 | 1,868.63 ± 409.68 | 1.6474 (1.5062–1.8019) | |
| AUCss,tau (µg∙hr/L) | 27,796.43 ± 10,513.59 | 10,817.59 ± 2,783.29 | 2.4986 (2.3525–2.6538) | |
| Tmax (hr)‡ | 1.5 (1.00–4.00) | 1.00 (0.50–4.00) | - | |
| t1/2 (hr) | 7.97 ± 2.24 | 4.89 ± 1.02 | - | |
| M1 | ||||
| Css,max (µg/L) | 2,249.80 ± 1,027.45 | 1,085.28 ± 360.34 | 2.0044 (1.8274–2.1987) | |
| AUCss,tau (µg∙hr/L) | 38,714.57 ± 18,127.33 | 14,726.31 ± 4,285.58 | 2.5016 (2.3145–2.7038) | |
| Tmax (hr)‡ | 4.00 (2.00–24.00) | 4.00 (1.00–4.00) | ||
| t1/2 (hr) | 128.83 ± 106.62 | 22.27 ± 9.99 | ||
| Metabolic ratio§ | 1.55 ± 0.61 | 1.48 ± 0.45 | ||
Data were shown as arithmetic mean ± standard deviation and geometric mean (90% confidence interval).
GMR, geometric ratio; CI, confidential interval; Css, max, maximum plasma concentration at steady state; AUCss,tau, area under the plasma concentration-time curves during dosing interval at steady state; tss, max, time to reach Css, max; t1/2, apparent terminal elimination half-life.
*Tegoprazan 200 mg once daily and clarithromycin 500 mg twice daily for 5 days; †Tegoprazan 200 mg once daily for 5 days; ‡Median (minimum - maximum); §Metabolic ratio was calculated as AUCM1 divided by AUCtegoprazan.
PKs of clarithromycin and 14-OH-clarithromycin
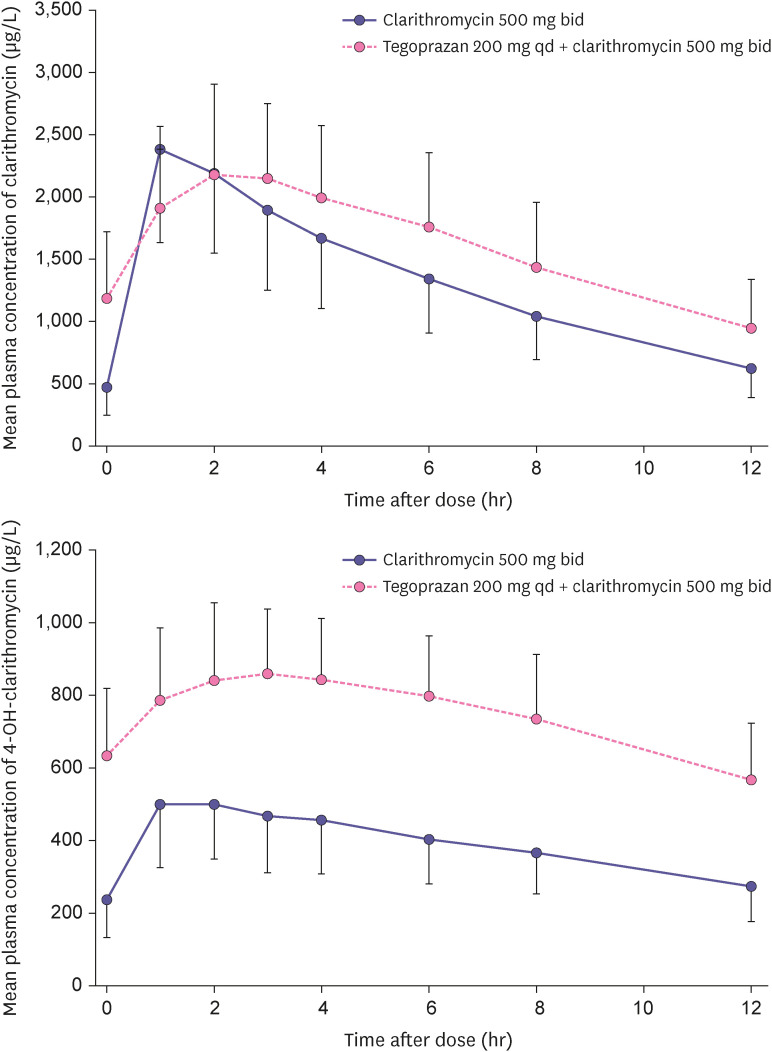
Fig. 3 showed the mean plasma concentration profile of clarithromycin and 14-OH-clarithromcin following single administration of clarithromycin and co-administration of tegoprazan and clarithromycin. The Css,max of clarithromycin were similar in two treatments, whereas the Css,max of 14-OH-clarithromycin were increased in co-administration. The GMR (90% CI) of Css,max of clarithromycin and 14-OH-clarithromycin were 1.0353 (0.8741–1.2262) and 1.8366 (1.6377–2.0596), respectively. The co-administration with tegoprazan and clarithromycin increased AUCtau of clarithromycin and 14-OH-clarithromycin compared with single administration and their GMR (90% CI) were 1.2531 (1.0801–1.4539) and 2.0071 (1.7912–2.2369), respectively. The Tss,max of clarithromycin and 14-OH-clarithromycin were not altered in two treatments. The t1/2 of clarithromycin and 14-OH-clarithromycin were increased in co-administration than single administration. The PK parameters of clarithromycin and 14-OH-clarithromycin were summarized in Table 3.
Figure 3. Mean plasma concentration-time profiles of clarithromycin and 14-OH-clarithromycin after oral administration of co-administered with clarithromycin for 5 days and tegoprazan alone for 5 days (upper: clarithromycin, lower: 14-OH-clarithromycin).
Table 3. Summary table of pharmacokinetic parameters of clarithromycin and 14-OH-clarithromycin following multiple administration.
| Parameter | Co-administration* (n = 23) | Alone† (n = 23) | GMR (90% CI) | |
|---|---|---|---|---|
| Clarithromycin | ||||
| Css,max (µg/L) | 2,293.89 ± 630.53 | 2,326.55 ± 808.19 | 1.0353 (0.8741–1.2262) | |
| AUCtau (µg∙hr/L) | 19,485.56 ± 6,202.88 | 15,924.01 ± 5,173.24 | 1.2531 (1.0801–1.4539) | |
| Tmax (hr)‡ | 2.00 (1.00–3.00) | 1.00 (0.00–3.00) | - | |
| t1/2 (hr) | 7.15 ± 2.42 | 5.90 ± 4.32 | - | |
| 14-OH-clarithromycin | ||||
| Css,max (µg/L) | 887.23 ± 173.30 | 503.85 ± 174.69 | 1.8366 (1.6377–2.0596) | |
| AUCtau (µg∙hr/L) | 8,971.47 ± 2,003.82 | 4,621.12 ± 1,469.63 | 2.0017 (1.7912–2.2369) | |
| Tmax (hr)‡ | 2.00 (1.00–3.00) | 2.00 (0.00–3.00) | - | |
| t1/2 (hr) | 13.03 ± 4.34 | 11.55 ± 6.09 | - | |
| Metabolic ratio§ | 0.47 ± 0.11 | 0.30 ± 0.09 | - | |
Data were shown as arithmetic mean ± standard deviation and geometric mean (90% confidence interval).
GMR, geometric ratio; CI, confidential interval; Css, max, maximum plasma concentration at steady state; AUCss,tau, area under the plasma concentration-time curves during dosing interval at steady state; tss, max, time to reach Css, max; t1/2, apparent terminal elimination half-life.
*Tegoprazan 200 mg once daily and clarithromycin 500 mg twice daily for 5 days; †Clarithromycin 500 mg twice daily for 5 days; ‡Median (minimum–maximum); §Metabolic ratio was calculated as AUC14-OH-clarithromycin divided by AUCclarithromycin.
Safety profile
The safety analysis set included 24 subjects who received at least one dose of investigational product. Following the co-administration of tegoprazan and clarithromycin, 11 AEs form 9 subjects were reported. Following the single administration of tegoprazan or clarithromycin, 2 AEs from 2 subjects and 7 AEs from 6 subjects were reported, respectively. Four AEs from 3 subjects were evaluated as moderate severity and other AEs were as mild severity. The symptoms of AEs were nausea, diarrhea, abdominal discomfort, headache, nasopharyngitis, and nail injury. All AEs were recovered without any intervention. There were no clinically significant changes in physical examination, vital sign, 12-lead ECG test and laboratory test.
DISCUSSION
This study evaluated drug-drug interaction potential between tegoprazan and clarithromycin following single administration and co-administration of both drugs during 5 days. The co-administration of tegoprazan and clarithromycin led to an increase in the exposures of tegoprazan and its metabolite (M1). Furthermore, the exposure of clarithromycin and its metabolite (14-OH-clarithromycin) were increased in co-administration.
Co-administration of clarithromycin increased the mean Css,max of tegoprazan and M1 by 1.6- and 2.0-fold and the mean AUCss,tau of them by 2.5- and 2.5-fold compared with alone, respectively. This result was comparable with it of the tegoprazan based triple regimen in healthy subjects [10]. Clarithromycin has been a well-known CYP3A4 inhibitor [8] and tegoprazan was classified as a substrate of CYP3A4. Clarithromycin would inhibit CYP3A4 in intestine and liver, which results in increasing absorption and reducing metabolism of tegoprazan followed by the increased exposure and half-life of tegoprazan. Furthermore, exposure of M1 rather increased, although the inhibition of CYP3A4 by clarithromycin in the metabolism of tegoprazan after co-administration was expected to reduce exposure of M1. Considering the extended half-life and the increased exposure of M1, clarithromycin, which may be involved in the metabolism of M1, is a potent inhibitor for CYP3A4 or p-glycoprotein (p-gp), but the further studies are need to evaluate it. Clarithromycin is known to have pKa of 8.99 and be degradable in the low pH [11]. When the gastric pH is elevated, the non-ionized form of clarithromycin increases in the gastrointestinal tract, which enhances its bioavailability. Because tegoprazan rapidly elevates the gastric pH by directly inhibiting H+/K+ pump, bioavailability increase of clarithromycin increased exposures of it and its metabolite (14-OH-clarithromycin) in co-administration with tegoprazan. Furthermore, because 14-OH-clarithromycin was also known to be metabolized by CYP3A4 [8], competition with tegoprazan or M1 as a CYP3A4 substrate may explain its exposure increase. The above increases were comparable with the study results in vonoprazan [12] and YH4808 [13] based triple regimen in healthy subjects, which are a class of P-CAB and metabolized by CYP3A4 or expected to inhibit CYP3A4 [14,15].
In conclusion, there may exist drug interaction that lead to reciprocal increase in plasma drug concentrations when tegoprazan and clarithromycin were administrated in combination and no safety concerns were raised. It is suggested that an in-depth analysis of the concentration-response relationship is necessary to determine whether these concentration changes warrant clinical action.
Footnotes
Funding: This study was sponsored by HK inno.N Corp., Seoul, Republic of Korea.
Conflict of Interest: - Author: Heechan Lee, Seokuee Kim, Bongtae Kim and Geun Seog Song are employees of HK inno.N Corp. Jong-Lyul Ghim declare no conflicts of interest
- Reviewers: Nothing to declare
- Editors: Nothing to declare
Reviewer: This article was reviewed by peer experts who are not TCP editors.
- Conceptualization: Kim B, Song GS, Shin JG, Ghim JL.
- Data curation: Oh M, Ghim JL.
- Formal analysis: Oh M, Ghim JL.
- Investigation: Oh M, Ghim JL.
- Writing - original draft: Lee H, Kim S.
- Writing - review & editing: Lee H, Kim S.
References
- 1.Saleem N, Howden CW. Update on the management of Helicobacter pylori infection. Curr Treat Options Gastroenterol. 2020;18:476–487. doi: 10.1007/s11938-020-00300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Connor A, Furuta T, Gisbert JP, O’Morain C. Review - Treatment of Helicobacter pylori infection 2020. Helicobacter. 2020;25(Suppl 1):e12743. doi: 10.1111/hel.12743. [DOI] [PubMed] [Google Scholar]
- 3.Ke H, Li J, Lu B, Yang C, Wang J, Wang Z, et al. The appropriate cutoff gastric pH value for Helicobacter pylori eradication with bismuth-based quadruple therapy. Helicobacter. 2021;26:e12768. doi: 10.1111/hel.12768. [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Ikuma M, et al. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007;12:317–323. doi: 10.1111/j.1523-5378.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 5.Han S, Choi HY, Kim YH, Nam JY, Kim B, Song GS, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple oral doses of tegoprazan (CJ-12420), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2019;50:751–759. doi: 10.1111/apt.15438. [DOI] [PubMed] [Google Scholar]
- 6.Miftahussurur M, Pratama Putra B, Yamaoka Y. The potential benefits of vonoprazan as Helicobacter pylori infection therapy. Pharmaceuticals (Basel) 2020;13:276. doi: 10.3390/ph13100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung HK, Kang SJ, Lee YC, Yang HJ, Park SY, Shin CM, et al. Evidence-based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Gut Liver. 2021;15:168–195. doi: 10.5009/gnl20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto F, Harada S, Mitsuyama T, Harada Y, Kitahara Y, Yoshida M, et al. Concentration of clarithromycin and 14-R-hydroxy-clarithromycin in plasma of patients with Mycobacterium avium complex infection, before and after the addition of rifampicin. Jpn J Antibiot. 2004;57:124–133. [PubMed] [Google Scholar]
- 9.Niwa T, Morimoto M, Hirai T, Hata T, Hayashi M, Imagawa Y. Effect of penicillin-based antibiotics, amoxicillin, ampicillin, and piperacillin, on drug-metabolizing activities of human hepatic cytochromes P450. J Toxicol Sci. 2021;61:913–922. doi: 10.2131/jts.41.143. [DOI] [PubMed] [Google Scholar]
- 10.Ghim JL, Chin MC, Jung J, et al. Pharmacokinetics and pharmacodynamics of tegoprazan coadministered with amoxicillin and clarithromycin in healthy subjects. J Clin Pharmacol. 2016;41:143–146. doi: 10.1002/jcph.1805. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa Y, Itai S, Yoshida T, Nagai T. Physicochemical properties and stability in the acidic solution of a new macrolide antibiotic, clarithromycin, in comparison with erythromycin. Chem Pharm Bull (Tokyo) 1992;40:725–728. doi: 10.1248/cpb.40.725. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai Y, Shiino M, Okamoto H, Nishimura A, Nakamura K, Hasegawa S. Pharmacokinetics and safety of triple therapy with vonoprazan, amoxicillin, and clarithromycin or metronidazole: a phase 1, open-label, randomized, crossover study. Adv Ther. 2016;33:1519–1535. doi: 10.1007/s12325-016-0374-x. [DOI] [PubMed] [Google Scholar]
- 13.Lee WY, Oh E, Cui M, Kim CO, Lim Y, Kim H, et al. Evaluation of pharmacokinetic interactions between amoxicillin, clarithromycin, and the potassium-competitive acid blocker YH4808 in healthy subjects. Transl Clin Pharmacol. 2020;28:55–65. doi: 10.12793/tcp.2020.28.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westblom TU, Duriex DE. Enhancement of antibiotic concentrations in gastric mucosa by H2-receptor antagonist. Implications for treatment of Helicobacter pylori infections. Dig Dis Sci. 1991;36:25–28. doi: 10.1007/BF01300082. [DOI] [PubMed] [Google Scholar]
- 15.Gustavson LE, Kaiser JF, Edmonds AL, Locke CS, DeBartolo ML, Schneck DW. Effect of omeprazole on concentrations of clarithromycin in plasma and gastric tissue at steady state. Antimicrob Agents Chemother. 1995;39:2078–2083. doi: 10.1128/aac.39.9.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]