Abstract
This study aimed to compare the pharmacokinetic (PK) and safety profiles of 2 fenofibric acid formulations under fasting and fed conditions. The reference was a 135 mg capsule, while the test was a 110 mg enteric-coated tablet. This randomized, open-label, two-sequence, two-period crossover phase 1 clinical trial was conducted in healthy Korean men. Sixty participants were enrolled in each of the fasting and feeding groups. Blood samples were collected 72 hours after drug administration. PK parameters were calculated using a non-compartmental method with Phoenix WinNonlin®. A total of 53 and 51 participants from the fasting and feeding groups, respectively, completed the study. The geometric mean ratio and 90% confidence intervals of the maximum concentration (Cmax) and area under the concentration-time curve to the last measurable plasma concentration were 0.9195 (0.8795–0.9614) and 0.8630 (0.8472–0.8791) in the fasting study and 1.0926 (1.0102–1.1818) and 0.9998 (0.9675–1.0332) in the fed study, respectively. The time to reach Cmax of the enteric-coated tablet compared to that of the capsule was extended by 1 and 3 hours under fasting and fed conditions, respectively. In conclusion, enteric-coated tablets have a higher bioavailability than capsules. In addition, the enteric-coated tablet was smaller than the capsule, making it easier for patients to swallow.
Trial Registration
Clinical Research Information Service Identifier: KCT0007177, KCT0003304
Keywords: Dyslipidemias; Pharmacokinetics; Drug Compounding; Biological Availability; Clinical Trials, Phase I as Topic
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death among noncommunicable diseases globally. Early detection and pharmacotherapeutic management are important to lower the mortality rate associated with CVD [1]. The major risk factors for CVD include elevated concentrations of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), and low high-density lipoprotein cholesterol (HDL-C) [2]. Maintaining normal lipid levels is important to prevent CVD [3,4].
Statins are specialized in LDL-lowering and have a weaker effect on other lipid profiles, such as TG. According to clinical studies, approximately 10–22% of patients experience myalgia as an adverse event (AE) according to the clinical studies [5,6]. In addition, even if patients maintain an LDL-C target level, reduction of LDL-C alone does not adequately reduce the risk of CVD [6,7,8].
Fenofibrate effectively lowers TG, TC, and LDL-C levels and increases HDL-C levels in patients with dyslipidemia. The effects of fenofibrate on plasma lipid profiles were sustained during long-term (2–7 years) treatment [9,10]. The existing fenofibrate formulation has a low absorption rate when taken under fasting conditions and has poor water solubility; therefore, it is recommended to be taken after a meal [11]. As fenofibrate bioavailability is low, it is difficult to absorb it quickly and completely from the gastrointestinal tract [12].
Several improved fenofibrate formulations have been developed to compensate for low bioavailability. Fenofibric acid is a newly approved form of fenofibrate with improved bioavailability [7,11]. Fenofibrate is a prodrug hydrolyzed by liver carboxylesterase 1 to the active metabolite, fenofibric acid [12,13]. Fenofibric acid activates peroxisome proliferator receptor alpha, thereby activating lipoprotein lipase and reducing apoprotein C-III production, increasing lipolysis, and removing TG from plasma [14]. Fenofibric acid has a higher solubility at intestinal pH than at gastric pH because its solubility increases as pH increases [12]. Therefore, fenofibric acid does not function properly in the stomach and is chemically modified due to the low pH of gastric juice.
Korea United Pharm. Inc. (Seoul, Korea) attempted to improve fenofibric acid bioavailability using enteric-coated tablets. Enteric-coated tablets are made with a coating that dissolves only at pH 5.5 or higher. This prevents the active ingredient from decomposing in the stomach, avoiding the loss of the main ingredient in the stomach, and releasing it into the small intestine, to improve bioavailability [15]. As bioavailability improves, the administered dose can be lowered, which can improve medication compliance by reducing the drug size [16]. The objective of this study was to compare the pharmacokinetic (PK) and safety profiles of fenofibric acid after oral administration of 135 mg capsules or 110 mg enteric-coated tablets under fasting and fed conditions.
METHODS
Study design
This randomized, open-label, two-sequence, two-period crossover phase 1 clinical trial was conducted under fasting and fed conditions at the Chungnam National University Hospital Clinical Trials Center. The patients were enrolled in fasting and fed studies, and 60 participants per study were randomly assigned to sequences A and B in a 1:1 ratio. During each study period, the participants received either the test or reference drug. The original capsule formulation containing 135 mg of fenofibric acid (Hanmi Pharm. Co., Ltd., Seoul, Korea) was used as the reference drug. The new enteric-coated tablet formulation containing 110 mg of fenofibric acid (Korea United Pharm. Inc.) was used as the test drug. The participants were alternately administered two treatments during each period (Sequence A: reference drug → test drug, Sequence B: test drug → reference drug). The period in each treatment sequence was separated by a 14-days washout period. In the fasting study, all participants received their investigational products (IPs) orally under fasting conditions. In the fed study, the participants received IPs after being fed a high-fat diet (904.4 kcal; 41.80% from fat).
Both studies were conducted in accordance with the International Council for Harmonization (ICH) guidelines and the Korean Good Clinical Practice (KGCP) guidelines. Both studies were conducted according to the protocols approved by the Korean Ministry of Food and Drug Safety (MFDS) and the Chungnam National University Hospital Institutional Review Board (IRB). All studies were registered with the Clinical Research Information Service (Fasting study: KCT0007177, Fed study: KCT0003304).
In each study, PK blood samples were collected before administration and at 1, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 12, 24, 48, and 72 hours after administration. They were centrifuged at 1,910–1,970 × g for 10 minutes at 4°C. Plasma was transferred to Eppendorf tubes and stored at −70°C until plasma fenofibric acid concentrations could be determined.
Participants
Each study recruited 60 healthy adult men between the ages of 19 and 45 years, weighing 55 kg or more, also within ±20% of ideal body weight (IBW). IBW was calculated using the following equation: IBW (kg) = (Height [cm] − 100) × 0.9. The participants were screened based on medical history, vital signs, physical examination, clinical laboratory tests, and 12-lead electrocardiogram (ECG). The inclusion and exclusion criteria for both studies were identical. The major exclusion criteria were an alanine transaminase level of 100 IU/L and renal creatinine clearance calculated by the Cockcroft-Gault formula of less than 30 mL/min.
All participants provided written informed consent before the procedure. The consent form was approved by the MFDS and the Chungnam National University IRB (fasting study: IRB No. CNUH 2017-03-027; fed study: IRB No. CNUH 2017-03-028).
Determination of plasma concentration
Both studies analyzed the concentration of fenofibric acid in the plasma using the same method. The plasma concentration of fenofibric acid was determined by liquid chromatography with tandem mass spectrometry (LC-MS/MS) (HPLC system: Agilent 1200 series USA [Mass spectrometer] 6460 triple quadrupole MS/MS system; Agilent Technologies, Santa Clara, CA, USA). For the column, Zorbax XDB-C18 (3.0 mm × 100 mm, 3.5 µm; Agilent Technologies) was used for the stationary phase. The mobile phase was 0.1% formic acid in distilled water:acetonitrile = 35:65 (v/v). The flow rate was 0.35 mL/min, and the injection volume was 5 µL. Electrospray positive ionization (ESI+) and multiple reaction monitoring (MRM) were used for concentration measurements. The mass-to-charge ratio (m/z) of fenofibric acid was 319–232.9, and that of clarithromycin was 7,485.5–158.1.
The fenofibric acid standard was dissolved in acetonitrile to prepare a 100 µg/mL stock solution. This solution was diluted with blank plasma to prepare standard solutions for the calibration curves with fenofibric acid concentrations of 100, 200, 1,000, 2,000, 10,000, and 20,000 ng/mL. Clarithromycin, an internal standard, was dissolved in acetonitrile to prepare an internal standard solution with a concentration of 1,000 ng/mL. Fifty microliters of internal standard solution and 1 mL of acetonitrile were added to 50 µL of each standard solution for the calibration curve, followed by mixing and shaking for 5 minutes. After centrifuging the mixture at 14,000 rpm for 5 minutes, 5 µL was injected into LC-MS/MS. A standard calibration curve was constructed using the peak area ratio of fenofibric acid to that of the internal standard. The plasma samples were thawed at room temperature, mixed, and shaken for 1 minute. A 50-µL sample of plasma was pretreated in the same way as the standard calibration curve preparation method and injected into LC-MS/MS. A calibration curve was obtained using the peak area ratio of the analyte to the internal standard, expressed by the following regression equation: y = a(x) + b (x: nominal concentration of analyte; y: peak area ratio of analyte to internal standard; a: slope of the regression line; b: intercept of the regression line).
PK analysis
PK parameters of both studies were calculated using a non-compartmental method using Phoenix™ WinNonlin® (version 8.1; Pharsight, Sunnyvale, CA, USA). The primary PK parameters were the area under the concentration-time curve to the last measured concentration over the limit of quantitation (AUClast) and maximum plasma concentration (Cmax) of fenofibric acid. The secondary PK parameters included the area under the concentration-time curve from dosing to time infinity (AUCinf), time to reach Cmax (Tmax), terminal half-life (T1/2), and apparent clearance (CL/F) of fenofibric acid. The area under the concentration-time curve (AUC) was calculated using the linear trapezoidal method for the rising interval and the log-linear trapezoidal method for the decreasing interval. Cmax and Tmax were directly determined from the observed concentration-time data. T1/2 was calculated as terminal elimination constant (λz) and ln(2)/λz.
Statistical analysis
The statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics are presented as mean ± standard deviation (SD) or median (range). PK parameters were analyzed descriptively and statistically for each dose group. For each AUClast and Cmax of fenofibric acid, the geometric mean ratios (GMRs) of the test drug compared with the reference drug were obtained, and the 90% confidence intervals (CIs) within the range of 0.8–1.25 were evaluated. Analysis of variance was performed by correcting for the effects of sequence and period using a linear mixed-effect model.
Safety assessment
Study participants who were administered at least one dose during the study period were included in the safety analysis. Safety and tolerability were evaluated based on vital signs, 12-lead ECG, and clinical laboratory tests. All AEs were documented regarding signs and symptoms and coded with system organ classes and preferred terms by the Medical Dictionary for Regulatory Authorities version 19.1.
RESULTS
Participant demographics
Table 1 summarizes the baseline demographic characteristics of study participants in the fasting and feeding studies. In the fasting study, 60 male participants (Sequence A: 30, Sequence B: 30) were randomized, and the mean age, height, and weight of randomized participants were 24.0 years, 174.4 cm, and 69.9 kg, respectively. In the fed study, 60 male participants (Sequence A: 30, Sequence B: 30) were randomized, and the mean age, height, and weight were 24.8 years, 175.3 cm, and 71.6 kg, respectively. In both studies, none of the demographic variables differed significantly between sequences A and B.
Table 1. Participant demographics.
| Variables | Fasting study | Fed study | |||||
|---|---|---|---|---|---|---|---|
| Sequence A RT† (n = 30) | Sequence B TR† (n = 30) | Total (n = 60) | Sequence A RT† (n = 30) | Sequence B TR† (n = 30) | Total (n = 60) | ||
| Age (yr) | |||||||
| Mean ± SD | 24.5 ± 3.9 | 23.4 ± 2.7 | 24.0 ± 3.4 | 25.3 ± 4.1 | 24.2 ± 4.2 | 24.8 ± 4.2 | |
| Range | 19.0–35.0 | 19.0–31.0 | 19.0–35.0 | 19.0–34.0 | 19.0–36.0 | 19.0–36.0 | |
| p-value* | 0.5905 | 0.2256 | |||||
| Height (cm) | |||||||
| Mean ± SD | 174.2 ± 5.4 | 174.6 ± 5.9 | 174.4 ± 5.6 | 175.1 ± 6.5 | 175.6 ± 5.3 | 175.3 ± 5.9 | |
| Range | 160.1–187.4 | 162.4–185.7 | 160.1–187.4 | 163.3–186.0 | 165.2–190.6 | 163.3–190.6 | |
| p-value* | 0.7824 | 0.7204 | |||||
| Weight (kg) | |||||||
| Mean ± SD | 70.6 ± 8.1 | 69.2 ± 7.7 | 69.9 ± 7.9 | 70.5 ± 8.7 | 72.7 ± 7.4 | 71.6 ± 8.0 | |
| Range | 55.1–92.9 | 55.7–85.0 | 55.1–92.9 | 55.4–87 | 57.8–87.1 | 55.4–87.1 | |
| p-value* | 0.4758 | 0.2968 | |||||
SD, standard deviation.
*p-value, two-sample t-test or Wilcoxon rank-sum test (continuous variables).
†R: Reference drug (fenofibric acid 135 mg capsule); T: Test drug (fenofibric acid 110 mg enteric-coated tablet).
PKs
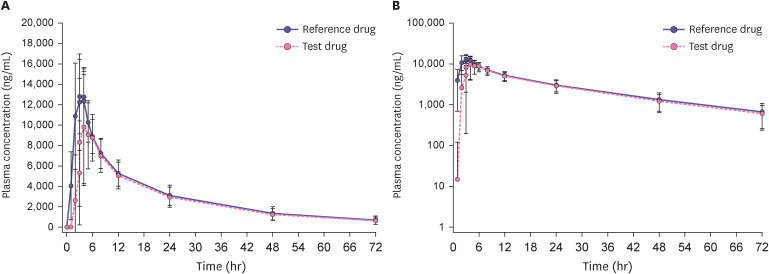
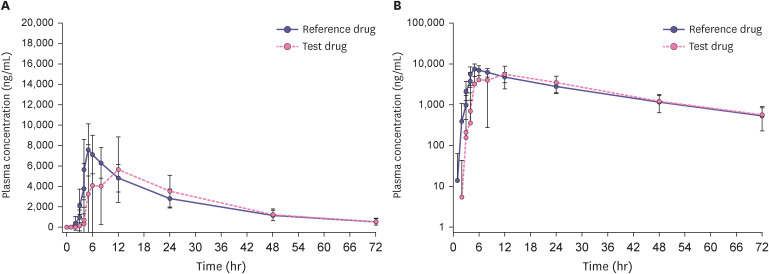
Participants who completed all study procedures were included in the PK analysis set, with 53 participants in the fasting study and 51 in the fed study. The mean concentration-time profile of fenofibric acid after a single dose under fasting conditions is shown in Fig. 1, and the profile under fed conditions is shown in Fig. 2. The PK parameters of fenofibric acid are listed in Table 2. The Cmax and AUClast 110 mg enteric-coated tablet of fenofibric acid were lower under the fed conditions 28.7% and 13.4%, respectively. As the CL/F increased after a high-fat meal, the T1/2 decreased.
Figure 1. Mean plasma fenofibric acid concentration-time profiles under 72 hours fasting conditions after single-dose oral administration of the 135 mg capsule (reference drug) and the 110 mg enteric-coated tablet (test drug) in healthy Korean men (n = 53). The error bar represents the standard deviation. (A) Linear scale and (B) Semi-log scale.
Figure 2. Mean plasma fenofibric acid concentration-time profiles under 72 hours fed conditions after single-dose oral administration of the 135 mg capsule (reference drug) and the 110 mg enteric-coated tablet (test drug) in healthy Korean men (n = 51). The error bar represents the standard deviation. (A) Linear scale and (B) Semi-log scale.
Table 2. Pharmacokinetic parameters of fenofibric acid after the single-dose administration of the 135 mg capsule and the 110 mg enteric-coated tablet under fasting and fed conditions.
| Parameter | Fasting study (n = 53) | Fed study (n = 51) | ||
|---|---|---|---|---|
| 135 mg capsule | 110 mg enteric-coated tablet | 135 mg capsule | 110 mg enteric-coated tablet | |
| Cmax (ng/mL) | 14,598.4 ± 2,285.6 (15.7) | 13,532.7 ± 2,590.5 (19.1) | 8,525.2 ± 2,236.8 (26.2) | 9,650.7 ± 2,596.0 (26.9) |
| Cmax/Dose (ng/mL/mg) | 108.1 ± 16.9 | 123.0 ± 23.6 | 63.1 ± 16.6 | 87.7 ± 23.6 |
| AUClast (h∙ng/mL) | 223,397.1 ± 56,822.1 (25.4) | 193,548.8 ± 50,858.7 (26.3) | 164,757.3 ± 49,494.7 (30.0) | 167,595.3 ± 40,274.9 (24.0) |
| AUClast/Dose (ng/mL/mg) | 1,654.8 ± 420.9 | 1,759.5 ± 46.2 | 1,220.4 ± 366.6 | 1,523.6 ± 366.1 |
| AUCinf (h∙ng/mL) | 245,345.8 ± 73,141.7 (29.8) | 213,019.2 ± 65,346.1 (30.7) | 184,174.4 ± 55,092.0 (29.9) | 181,393.0 ± 48,733.9 (26.9) |
| T1/2 (h) | 20.4 ± 4.2 (20.4) | 19.9 ± 4.2 (21.2) | 19.1 ± 4.0 (21.1) | 17.8 ± 3.5 (19.9) |
| CL/F (mL/h) | 594.7 ± 155.7 (26.2) | 564.1 ± 163.7 (29.0) | 793.4 ± 226.8 (28.6) | 650.0 ± 176.4 (27.1) |
| Tmax (h) | 2.5 (2.0–5.0) | 3.5 (2.0–12.0) | 5.0 (0.0–12.0) | 8.0 (3.0–24.12) |
Values are expressed as arithmetic mean ± standard deviation (coefficient of variation), except for Tmax, which is shown as median (minimum-maximum).
Cmax, maximum plasma concentration; AUClast, area under the concentration-time curve to the last measured concentration over the limit of quantitation; AUCinf, area under the concentration-time curve from dosing to time infinity; T1/2, terminal half-life; CL/F, apparent clearance; Tmax, time to reach Cmax.
The GMRs and 90% CIs of the test drug to the reference drug under fasting conditions for the Cmax and AUClast were 0.9195 (0.8795–0.9614) and 0.8630 (0.8472–0.8791), respectively. The GMRs and 90% CIs of the test drug against the reference drug under fed conditions for the Cmax and AUClast were 1.0926 (1.0102–1.1818) and 0.9998 (0.9675–1.0332), respectively (Table 3).
Table 3. Point estimate (test drug/reference drug) and 90% CIs of the pharmacokinetic parameters of fenofibric acid after single-dose administration of the 135 mg capsule (reference drug) and the 110 mg enteric-coated tablet (test drug) under fasting and fed conditions.
| Variable | Point estimate | 90% CI | |
|---|---|---|---|
| Fasting study (n = 53) | |||
| Cmax | 0.9195 | 0.8795–0.9614 | |
| AUClast | 0.8630 | 0.8472–0.8791 | |
| Fed study (n = 51) | |||
| Cmax | 1.0926 | 1.0102–1.1818 | |
| AUClast | 0.9998 | 0.9675–1.0332 | |
CI, confidence interval; Cmax, maximum plasma concentration; AUClast, area under the concentration-time curve of the last measured concentration over the limit of quantitation.
Safety
The safety evaluation was conducted on participants who were administered IP at least once.
In the fasting study, 53 participants were included in the safety analysis, excluding 1 participant who was uncooperative and 6 participants who were dropped by the judgment of investigator. A total of three adverse drug reactions (ADRs) were reported from three participants, among which one ADR (dizziness) was reported in the reference drug group, while two ADRs (headache, epigastric discomfort) were reported from two participants in the test drug group. All the AEs were mild and resolved without medication or sequelae. No serious AEs were reported after the IP administration.
In the fed study, 58 participants were included in the safety analysis set. Six ADRs were reported in five participants, among which three ADRs (urticaria and headache) were reported in two participants in the reference drug group, while three ADRs (urticaria, headache, and dizziness) were reported in three participants in the test drug group. All the AEs were mild and resolved without medication or sequelae. No SAEs occurred during the study period in both studies, there were no clinically significant changes in vital signs, 12-lead ECG, physical examinations, or clinical laboratory test results.
DISCUSSION
This study was conducted in two stages. It was compared the PKs and safety of fenofibric acid 135 mg capsule (reference drug) and fenofibric acid 110 mg enteric-coated tablet (test drug) under fasting and fed conditions.
In the case of the fasting study, the GMRs and 90% CIs of the test drug to the reference drug for the Cmax and AUClast were 0.9195 (0.8795–0.9614) and 0.8630 (0.8472–0.8791), respectively. And in the fed study, they were 1.0926 (1.0102–1.1818) and 0.9998 (0.9675–1.0332), respectively. The GMRs and 90% CIs of the test drug to the reference drug for the Cmax and AUClast of fenofibric acid under fasting and fed conditions were within the traditional bioequivalence range of 0.8–1.25.
In both studies, the sample size was determined based on the largest value of the intra-subject variability (12.6%) of fenofibric acid AUC and Cmax reported in previous study [11]. However, the largest calculated intra-subject variability value of fenofibric acid AUClast and Cmax was 23.86% according to this clinical trial. When the sample size was re-calculated under the same conditions using 23.86% in this study, the sample size was 26. As a total of 53 and 51 participants from the fasting and fed groups, respectively, had completed the study, this participant number was appropriate for comparative PK assessment.
In the fasting study, Tmax of the test drug was extended by 1 hour compared with that of the reference drug. In the fed study, Tmax of the test drug was extended by 3 hours compared with that of the reference drug. This significant difference in Tmax can be explained by delayed drug absorption in the duodenum and small intestine, where pH is higher than the stomach [17]. According to the individual time-concentration graph (Supplementary Fig. 1), the onset of absorption of the test drug was clearly delayed compared to that of the reference drug. Since a high-fat diet slow down gastrointestinal motility, the difference in median Tmax between capsule and enteric-coated tablet in the fed study is larger. However, about three hour delayed absorption in fenofibric acid is not clinically important as this drug is treatment for dyslipidemia, a chronic disease.
The capsule formulation of fenofibric acid was found to dissolve its drug components even in low pH conditions such as gastric juice. However, the strong acidity of gastric juice may prevent the drug from exerting its intended effect, and increase the likelihood of drug loss during the approximate 2-hour residence time in the stomach. As a result, the bioavailability of fenofibric acid may be reduced [18]. To address this issue, enteric-coated tablet formulation was improved to minimize drug loss in the stomach (pH 1.2) and enable drug release in the small intestine. This formulation allows for higher bioavailability of fenofibric acid with less doses compared to the capsule formulation.
In a previous study, it was reported that one-third of patients experienced difficulty in swallowing pills due to the size of the drug [19]. The dosage of the enteric-coated tablets was reduced by approximately 20% compared to capsule formulations (135 mg → 110 mg), with a size reduction of approximately 20% compared to capsule formulations (127.3 mm2 → 104.04 mm2). These formulation improvements can be evaluated as superior to the previous formulation in terms of dose-normalized and size-normalized measures. Given that dyslipidemia is a chronic disease, such formulation improvements in fenofibric acid may provide sufficient therapeutic benefits to improve patient adherence to drug. No clinically significant changes were observed in the vital signs, 12-lead ECGs, physical examinations, or clinical laboratory test results between the reference and test drugs, and the fasting and fed studies were the same. Therefore, there was no significant difference in safety or tolerability between the enteric-coated tablet formulation (fenofibric acid 110 mg) and the existing capsule formulation (fenofibric acid 135 mg). The test drug was bioequivalent with the reference drug at lower administered dose than the reference.
In conclusion, this study showed that the capsule (fenofibric acid 135 mg) and enteric-coated tablet (fenofibric acid 110 mg) had similar PK and safety profiles under both fasting and fed conditions. This indicates that the enteric-coated tablet formulation has higher bioavailability than the existing formulation. In addition, the enteric-coated tablet was smaller than the capsule, making it easier for patients to swallow.
ACKNOWLEDGMENTS
We thank all the study participants and investigators for their involvement in this study. This study was sponsored by Korea United Pharm. Inc., Seoul, Republic of Korea.
Footnotes
Conflict of Interest: - Authors: WT.J., K-Y.N., N.K., Y-W.C., SM.C., and D-H.K are employees of Korea United Pharm. Inc. H.J.L., JH.M., SS.L., and JH.K. are employees of Caleb MultiLab. Inc. All other authors declare no conflicts of interest.
- Reviewers: Nothing to declare
- Editors: Nothing to declare
Reviewer: This article was reviewed by peer experts who are not TCP editors.
- Conceptualization: Kim JH, Hong JH, Sunwoo J, Jung JG.
- Data curation: Kim JH, Lee HJ, Moon J, Lee S, Kim J.
- Formal analysis: Kim JH.
- Project administration: Hong JH.
- Supervision: Hong JH, Jung JG.
- Visualization: Seo YB, Song JH.
- Writing - original draft: Seo YB, Kim JH.
- Writing - review & editing: Kim JH, Sunwoo J, Jung WT, Nam KY, Kim N, Choi YW, Cho SM, Ki DH.
SUPPLEMENTARY MATERIAL
Individual fenofibric acid plasma concentration versus time graph, linear scale. Solid line represents the test drug. Dotted line represents the reference drug. The characters after the underscore in legends indicate the group (A or B) and subject number.
References
- 1.World Health Organization. Top 10 causes of death worldwide in 2019. Geneva: World Health Organization; 2020. [Google Scholar]
- 2.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 3.Francula-Zaninovic S, Nola IA. Management of measurable variable cardiovascular disease’ risk factors. Curr Cardiol Rev. 2018;14:153–163. doi: 10.2174/1573403X14666180222102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:393–403. doi: 10.3949/ccjm.78a.10073. [DOI] [PubMed] [Google Scholar]
- 7.Saurav A, Kaushik M, Mohiuddin SM. Fenofibric acid for hyperlipidemia. Expert Opin Pharmacother. 2012;13:717–722. doi: 10.1517/14656566.2012.658774. [DOI] [PubMed] [Google Scholar]
- 8.Jones PH, Davidson MH, Goldberg AC, Pepine CJ, Kelly MT, Buttler SM, et al. Efficacy and safety of fenofibric acid in combination with a statin in patients with mixed dyslipidemia: pooled analysis of three phase 3, 12-week randomized, controlled studies. J Clin Lipidol. 2009;3:125–137. doi: 10.1016/j.jacl.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Najib J. Fenofibrate in the treatment of dyslipidemia: a review of the data as they relate to the new suprabioavailable tablet formulation. Clin Ther. 2002;24:2022–2050. doi: 10.1016/s0149-2918(02)80095-9. [DOI] [PubMed] [Google Scholar]
- 10.Balfour JA, McTavish D, Heel RC. Fenofibrate. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in dyslipidaemia. Drugs. 1990;40:260–290. doi: 10.2165/00003495-199040020-00007. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey AR, Digiacinto J, Davis MW. Single-dose bioequivalence of 105-mg fenofibric acid tablets versus 145-mg fenofibrate tablets under fasting and fed conditions: a report of two phase I, open-label, single-dose, randomized, crossover clinical trials. Clin Ther. 2011;33:766–775. doi: 10.1016/j.clinthera.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 12.Zhu T, Ansquer JC, Kelly MT, Sleep DJ, Pradhan RS. Comparison of the gastrointestinal absorption and bioavailability of fenofibrate and fenofibric acid in humans. J Clin Pharmacol. 2010;50:914–921. doi: 10.1177/0091270009354995. [DOI] [PubMed] [Google Scholar]
- 13.Laizure SC, Herring V, Hu Z, Witbrodt K, Parker RB. The role of human carboxylesterases in drug metabolism: have we overlooked their importance? Pharmacotherapy. 2013;33:210–222. doi: 10.1002/phar.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DrugBank. Fenofibric acid. Alberta: University of Alberta and The Metabolomics Innovation Centre; 2021. [Google Scholar]
- 15.Shin KH. Process improvement and effect for enteric tablet coating using aqueous system [dissertation] Cheongju: Chungbuk National University; 2004. [Google Scholar]
- 16.Kabeya K, Satoh H, Hori S, Miura Y, Sawada Y. Threshold size of medical tablets and capsules: based on information collected by Japanese medical wholesaler. Patient Prefer Adherence. 2020;14:1251–1258. doi: 10.2147/PPA.S253663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel H, Gohel M. A review on enteric coated pellets composed of core pellets prepared by extrusion-spheronization. Recent Pat Drug Deliv Formul. 2019;13:83–90. doi: 10.2174/1872211313666190212115139. [DOI] [PubMed] [Google Scholar]
- 18.WIPO. WO2019208967 - Enteric coated tablet comprising fenofibric acid or pharmaceutically acceptable salt thereof. Seoul: Korea United Pharm. Inc.; 2019. [Google Scholar]
- 19.Fields J, Go JT, Schulze KS. Pill properties that cause dysphagia and treatment failure. Curr Ther Res Clin Exp. 2015;77:79–82. doi: 10.1016/j.curtheres.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual fenofibric acid plasma concentration versus time graph, linear scale. Solid line represents the test drug. Dotted line represents the reference drug. The characters after the underscore in legends indicate the group (A or B) and subject number.