Abstract
Introduction
Despite the considerable progress made in assisted reproductive technologies (ART), the implantation rate of transferred embryos remains low and in many cases, the reasons for failure remain unclear. We aimed to determine the potential impact of female and male partners’ reproductive tract microbiome composition on ART outcome.
Material and methods
The ART couples (n = 97) and healthy couples (n = 12) were recruited into the study. The smaller healthy group underwent a careful selection according to their reproductive and general health criteria. Both vaginal and semen samples were subjected to 16S rDNA sequencing to reveal the bacterial diversity and identify distinct microbial community types.
Ethics statement
The study was approved by the Ethics Review Committee on Human Research of Tartu University, Tartu, Estonia (protocol no. 193/T‐16) on 31 May 2010. Participation in the research was voluntary. Written informed consent was obtained from all study participants.
Results
The men with Acinetobacter‐associated community who had children in the past, had the highest ART success rate (P < 0.05). The women with bacterial vaginosis vaginal microbiome community and with L. iners‐predominant and L. gasseri‐predominant microbiome had a lower ART success rate than women with the L. crispatus‐predominant or the mixed lactic‐acid‐bacteria‐predominant type (P < 0.05). The 15 couples where both partners had beneficial microbiome types had a superior ART success rate of 53%, when compared with the rest of the couples (25%; P = 0.023).
Conclusions
Microbiome disturbances in the genital tract of both partners tend to be associated with couple's infertility as well as lower ART success levels and may thus need attention before the ART procedure. The incorporation of genitourinary microbial screening as a part of the diagnostic evaluation process may become routine for ART patients if our results are confirmed by other studies.
Keywords: assisted reproductive technologies (ART), couple, infertility, microbiome, reproductive tract, semen
Disturbed microbial communities in the reproductive tract in both partners may be one of the reasons for assisted reproductive technologies (ART) failure. In our study, Lactobacillus crispatus‐dominated vaginal microbiome and Acinetobacter‐dominated seminal microbiome supported beneficial ART outcome.
Abbreviations
- ART
assisted reproductive technologies
- BV
bacterial vaginosis
- ICSI
intracytoplasmic sperm injection
- IVF
in vitro fertilization
- OTU
operational taxonomic unit
- WBC
white blood cells
Key message.
Disturbed microbiome in the reproductive tract in both partners may be one of the reasons for ART failure.
1. INTRODUCTION
Microorganisms living in the genital tract form a complex and dynamic ecosystem where different bacteria coexist and interact with each other and with host and environmental factors. 1 , 2 Healthy female microbial communities are dominated by the genus Lactobacillus, which plays an important role first in maintaining the optimum environment for pH retention and preventing urogenital diseases and secondly in encouraging embryo implantation and ensuring normal pregnancy. 3 , 4 , 5 A frequent disturbance of genital tract microbiome, bacterial vaginosis (BV), has been associated with reproductive failure via several mechanisms – BV‐associated bacteria may ascend and cause pelvic inflammatory disease‐related closure of fallopian tubes, implantation failure and increased miscarriage rates. 6 , 7 , 8
In comparison, the male reproductive tract microbiome is less studied. Lower genital tract (urethra, coronal sulcus) is a suitable place for aerobic, microaerophilic and anaerobic bacteria. 1 In the urethra, mostly lactic acid bacteria such as lactobacilli and streptococci predominate, whereas in the coronal sulcus, a large number of skin bacteria (corynebacteria, staphylococci) can be found, but also anaerobic bacteria. 9 Seminal fluid reflects microbial communities in the upper genital tract and therefore it is researched mostly in infertility or prostatitis patients. 10 , 11 , 12 Both conditions are significantly associated with imbalanced microbial communities and can affect male fertility in different ways, damaging spermatogenesis, causing obstructions and excessive oxidative stress. 1 , 13
Infertility affects nearly 10%–15% of couples. Around 35%–40% of couples’ infertility cases are related to female problems, 35%–40% to male problems and 20%–30% to both partners’ problems. 14 Female causes include tuboperitoneal abnormalities, endometriosis, uterine anatomical abnormalities, as well as autoimmune, genetic and endocrine disorders. 14 , 15 Male causes may include anatomical, congenital, genetic and endocrine disorders, but also reproductive system inflammation and trauma. 16 , 17 Sexually transmitted infections and their complications are considered to be an important cause of both female and male infertility. 14 However, in many cases the cause of infertility still remains unknown; an imbalanced genital tract microbiome can be among the possible reasons. 12 , 18 During sexual intercourse, partners share their genital tract microorganisms. This bidirectional transfer can affect the microbial composition of one or both partners. 11 As BV‐related microorganisms and inflammatory prostatitis have been shown to be frequently co‐occurring, 19 the testing of partners’ microbiome could be justified.
Assisted reproductive technologies (ART) are the cornerstone of contemporary infertility treatment. Despite the considerable progress made in ART, the implantation rate of transferred embryos remains low and has been shown to depend on numerous clinical and laboratory factors. The success and failure in ART have been attributed to variables such as the patient's age, weight, endometrial receptivity, embryo quality and the transfer technique used. 20 , 21 However, in many cases, the reasons for failure remain unclear. Again, imbalanced genital tract microbiome can be involved. The impact of BV on ART outcome has long been discussed, with a lack of final consensus in the scientific and medical communities. 5 Also, there are very few data available about the associations between male genital tract microbiome and ART outcome. We aimed to determine the potential impact of female and male partners’ genital tract microbiome composition on ART outcome.
2. MATERIAL AND METHODS
2.1. Study group
The study enrolled 97 couples going to an ART procedure and 12 healthy couples. The ART couples were recruited in the Nova Vita fertility clinic (Tallinn, Estonia) as consecutive couples who were willing to participate and thereafter gave an informed consent for study‐related procedures. The patients had been suffering from infertility for at least 1 year. Before the procedure, the couples were tested for sexually transmitted infections and treated when appropriate. The clinical data of ART couples are presented in the Table S1. Additional information about the study group and ART procedures was published previously. 21 Of the 97 couples undergoing ART, 39% were allocated to the in vitro fertilization (IVF) and 61% to the intracytoplasmic sperm injection (ICSI) procedure.
The fertile couples were recruited at the Tartu University Hospital (Tartu, Estonia) and the Competence Center on Health Technologies (Tartu, Estonia) in 2010–2012. Inclusion and exclusion criteria for healthy couples are presented in the Table S2.
2.2. Collection and characterization of the samples
Vaginal and semen samples from the ART group were collected on follicle puncture day. Vaginal fluid was collected from the upper third of posterior fornix with two swabs. One swab was quickly frozen for molecular studies; the second swab was used for Gram‐stained smear. Vaginal smears were Gram stained and evaluated by microscopy. BV was evaluated on the basis of the Nugent score (NS). Different bacterial morphotypes (Lactobacillus spp., Gardnerella vaginalis/Bacteroides spp., Mobiluncus spp.) were quantified and the NSs were calculated. A score of 0–3 indicated normal/healthy, 4–6 intermediate/transition, and ≥7 BV. 22
Semen samples were collected after 2–7 days of abstinence. Semen was obtained by masturbation and ejaculated into a sterile collection tube, after post‐urination washing of the glans penis with soap and water. The collected sperm sample was shortly (<10 minutes) incubated at 37°C and left for 25–45 minutes in room temperature for liquefaction. The semen analysis was performed according to WHO guidelines. 23 Inflammatory prostatitis was assessed by the neutrophil concentration in semen as described. 24
2.3. Molecular methods and data analysis
DNA extraction from samples was performed with a QIAamp® DNA Blood Mini Kit (Qiagen) according to the manufacturer's instructions. Extracted DNA was stored at −80°C. The relation between different sub‐regions based on the geodesic distance indicates that V4–V6 are the most reliable regions for representing the full‐length 16S rRNA sequences in the phylogenetic analysis of most bacterial phyla. 25 Therefore, separated DNA samples were amplified by PCR using the V6 hypervariable region of the 16S rDNA. Samples were characterized by profiling the microbial communities based on the 16S rDNA by using the Illumina HiSeq2000 sequencing combinatorial sequence‐tagged PCR products. Details of molecular methods, bioinformatics and statistical analysis are provided in the Table S3.
2.4. Ethics statement
The study was approved by the Ethics Review Committee on Human Research of Tartu University, Tartu, Estonia (protocol no. 193/T‐16) on May 31, 2010. Participation in the research was voluntary. Written informed consent was obtained from all study participants.
3. RESULTS
3.1. Clinical data
In the ART group, the mean age of women was 34.1 years (25–46 years) and that of men 37.4 years (25–58 years). In total, 23.7% of the women and 65% of the men were overweight. BV was diagnosed in 15.5% of women by Gram stain. Leukocytospermia according to WHO criteria (≥1 × 106 white blood cells [WBC]/mL) was found in 7.3% of men; according to reduced criteria (≥0.2 × 106 WBC/mL) leukocytospermia was found in in 39.6% of men. Primary infertility was diagnosed in 66% of women and secondary infertility in 34% of women. In all, 48.5% of the men had children. Of the 97 couples, an embryo was transferred to 93 women; in 4 cases the fertilization of oocytes failed. Of these 93 women, clinically detected pregnancy was achieved in 28 women (30.1%). Higher ART success rate was associated with lower age and lower BMI in women and presence of children in men as previously published. 21
In the control group, the mean age of the women was 32.2 years (25–42 years) and that of the men 34.1 years (22–42 years). All study participants in the control group had normal BMI, the women had an NS of 0 and the men had insignificant leukocyte count in their semen (<0.2 × 106 WBC/mL). Additional clinical data are presented in Tables S1 and S2.
3.2. Overall sequence statistics and diversity analysis
From the initial 36 108 978 trimmed 75‐bp sequencing reads, 1 556 084 sequences in 666 operational taxonomic units were obtained after normalization. The largest average number of sequences was identified in healthy women (216 767 ± 167 651), followed by ART women (158 270 ± 83 568), though the difference was not statistically significant. An average of 140 549 ± 112 506 sequences were detected in healthy men, whereas ART men had significantly lower number of sequences (80 170 ± 48 788, P < 0.05). Both groups of men differed from both groups of women (P < 0.05). Combining Greengene database queries with additional BLAST analysis significantly improved the classification over all taxonomic levels (P < 0.05), with a total of 84.53% taxonomic units on phylum, 63.36% on genus and 24.92% on species level. As expected, a higher number of taxonomic units (OTUs) was found in men (ART men 340 ± 49; healthy men 336 ± 36) than in women (ART women 111 ± 34; healthy women 106 ± 77). Among females, whereas healthy women had 0.6% unique OTUs, the ART women had 33% (P = 0.00001). Among males, healthy men had 0.1%, whereas ART men had 6% of unique OTUs (P = 0.00001). Partners in healthy couples shared 65% of the OTUs, whereas partners in ART couples shared 96% of the OTUs (P = 0.00001).
Based on the alpha‐diversity analysis that estimates the species diversity in the single sample and that was performed applying Chao, Shannon and inverse Simpson indices, men had larger microbiome diversity compared with women. Both women and men of ART couples had a trend for higher microbiome diversity compared with healthy men and women (Figure 1). A statistically significant difference was found in inverse Simpson diversity index between healthy women and ART men and between ART men and women (P < 0.05). Chao diversity showed a statistically significant difference between all groups (P < 0.05) except healthy and ART women.
FIGURE 1.
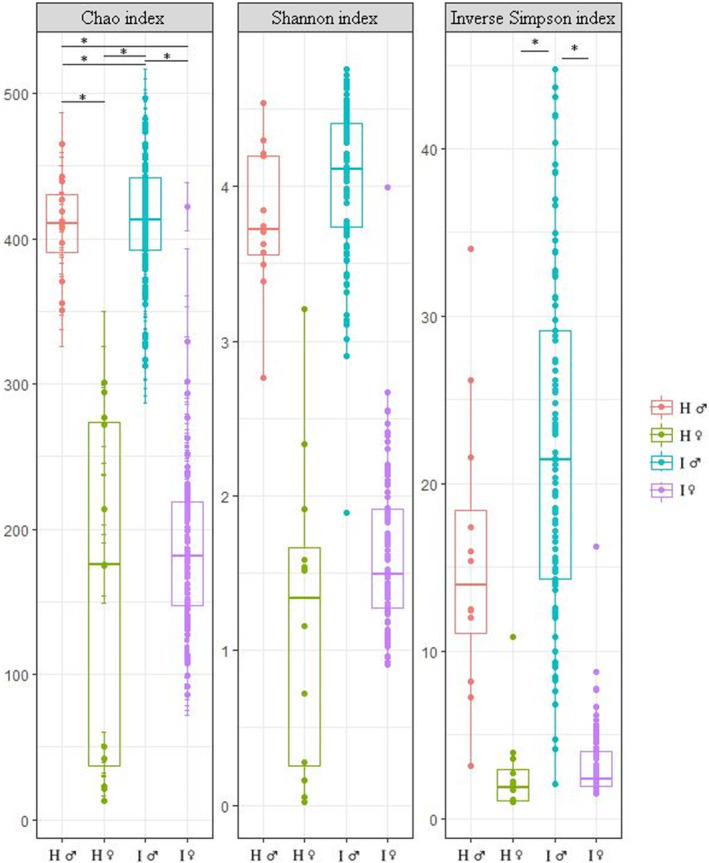
Boxplot of sample groups with different alpha diversity indices. Asterisks indicate differences between the groups (P < 0.05). H, healthy; I, infertile; ♀, woman; ♂, man.
Beta‐diversity analysis, which compares similarities between the bacterial communities, clearly showed distinct differences between seminal and vaginal samples (Figure S1). Samples of healthy women formed two different subgroups, according to predominant Lactobacillus species. The semen samples of ART men deviating from the main cluster consisted mostly of Gram‐negative anaerobic/microaerophilic bacteria (Prevotella, Porphyromonas, Dialister, Campylobacter, referred to below as community group II).
3.3. Structure of vaginal microbial communities
Predominant phylum in vaginal samples was Firmicutes; however, in ART women it was significantly decreased, whereas Actinobacteria was increased (both P < 0.05) (Figure 2). At family level, Lactobacillaceae was predominant in women within both healthy and infertile couples. However, ART women had proportionally less Lactobacillaceae (slightly over significance level), whereas more Bifidobacteriaceae, Coriobacteriaceae, Enterobacteriaceae and Clostridiaceae (P < 0.05 for all) were found in their vagina compared with healthy women. On the genus level, Lactobacillus dominated in vaginal samples, but in ART women their counts were reduced and the Gardnerella, Atopobium, Bifidobacterium and Clostridium counts were increased (P < 0.05 for all).
FIGURE 2.
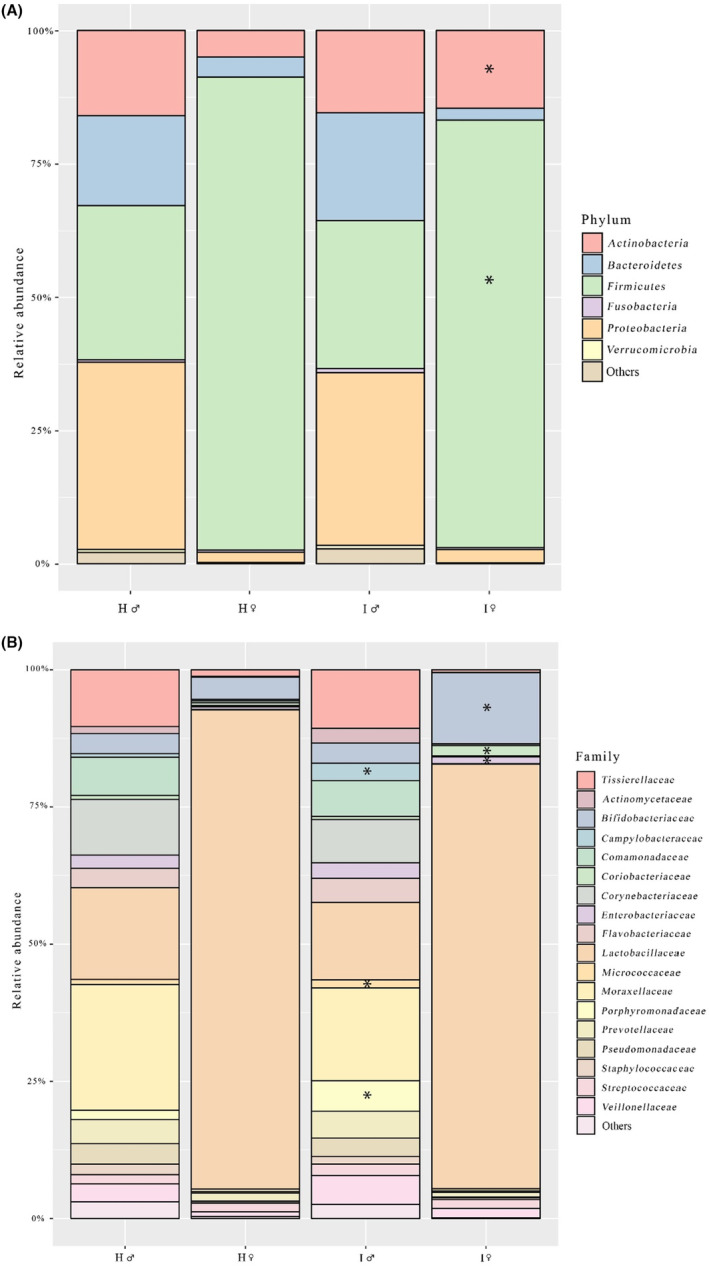
Predominant phyla (A) and families (B) in vaginal and semen samples. Asterisks indicate differences in comparison with healthy controls (P < 0.05). H, healthy; I, infertile; ♀, woman; ♂, man.
Hierarchical clustering of vaginal samples revealed eight community types (Figure 3, Table 1). Most of them were dominated by different lactobacilli species. BV community (III, dominated by G. vaginalis, Shuttleworthia, Atopobium, Prevotella, Megasphaera and others) was found only among ART women, due to the exclusion criteria for healthy couples.
FIGURE 3.
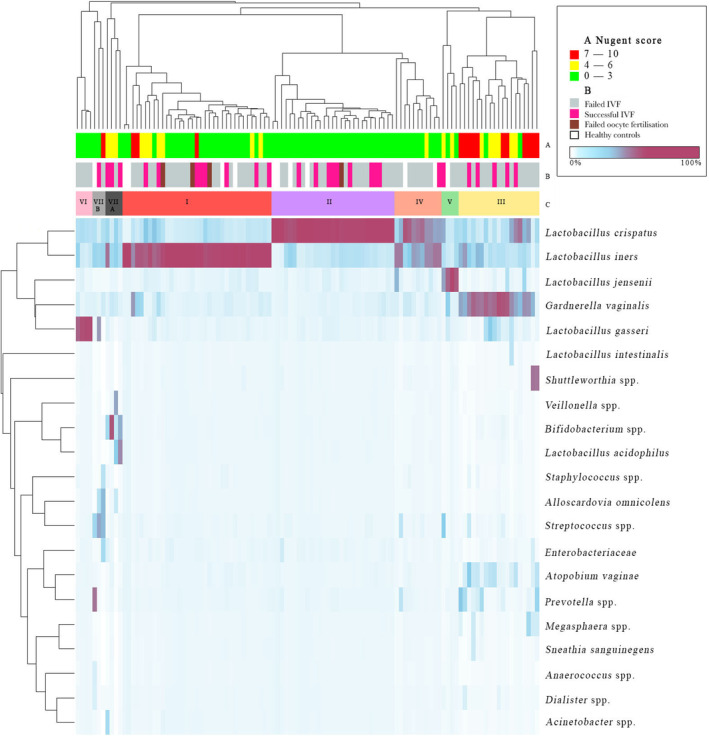
Hierarchical clustering of vaginal samples. Columns represent female vaginal microbiome and rows represent identified bacterial clusters. A: Nugent score; B: health status and ART result; C: microbiome community type. ART, assisted reproductive technologies; IVF, in vitro fertilization.
TABLE 1.
ART procedure success according to vaginal and semen community type.
| Community type | Healthy group, n (%) | ART group, n (%) | ART success rate, n (%) a | |
|---|---|---|---|---|
| Vaginal communities | ||||
| I | Lactobacillus iners | 4 (33.3) | 31 (31.9) | 8 (25.8) |
| II | Lactobacillus crispatus | 4 (33.3) | 25 (25.8) | 9 (36.0) |
| III | BV community | 0 | 19 (19.6) | 5 (26.3) |
| IV | Two lactobacilli (L. iners + L. crispatus) | 2 (16.7) | 9 (9.3) | 1 (11.1) |
| V | Lactobacillus jensenii | 1 (8.3) | 3 (3.1) | 1 (33.3) |
| VI | Lactobacillus gasseri | 0 | 4 (4.1) | 0 |
| VII A | Diverse community A (Lactobacillus + Bifidobacterium) | 0 | 4 (4.1) | 3 (75.0) |
| VII B | Diverse community B (Lactobacillus + Streptococcus) | 1 (8.3) | 2 (2.1) | 1 (50.0) |
| Women with community types with higher success rate (II, V, VII), mean age 34.6 ± SD 4.3 | 14 (41.2)** | |||
| Women with community types with lower success rate (I, III, IV, VI), mean age 34.0 ± SD 4.9 | 14 (22.2)** | |||
| Semen communities | ||||
| I | Acinetobacter (plus L. iners, Corynebacterium, Flavobacterium, Prevotella) | 7 (58.3) | 48 (49.5) | 17 (35.4)* |
| II | Gram‐negative anaerobic/ microaerophilic (Prevotella, Porphyromonas, Dialister or Campylobacter) | 1 (8.3) | 14 (14.4) | 1 (7.1)* |
| III | Gram‐negative + Gram‐positive (Prevotella, Acinetobacter, Porphyromonas, Dialister, Campylobacter, L. iners, L. crispatus, Corynebacterium, Gardnerella vaginalis, Finegoldia, Fenollaria) | 1 (8.3) | 14 (14.4) | 4 (28.6) |
| IV | L. iners + vaginal bacteria (L. crispatus, G. vaginalis, Sneathia, Corynebacterium) | 2 (16.7) | 13 (13.4) | 2 (15.4) |
| V | Corynebacterium | 1 (8.3) | 4 (4.1) | 2 (50) |
| VI | L. iners | 0 | 2 (2.1) | 1 (50) |
| Veillonella‐predominated community | 0 | 1 (1.0) | 1 (7.1) | |
| Haemophilus parainfluenzae‐ predominated community | 0 | 1 (1.0) | 0 | |
| Men with community type with higher success rate (I), mean age 38.6 ± SD 7.6 | 17 (35.4) | |||
| Men with community types with lower success rate or scarce types (II–VI), mean age 36.4 ± SD 5.8 | 11 (22.4) | |||
ART (assisted reproductive technologies) procedure success as clinical pregnancy rate.
P < 0.05 (Chi‐square test)
P < 0.05 (Chi‐square test).
Correlogram illustrates the co‐occurrence of taxa in the communities (Figure S2A). As expected, BV‐related bacteria were associated positively with each other and negatively with lactobacilli. Two predominant lactobacilli species (L. iners, L. crispatus) were in negative correlation with each other (r = −0,50, P < 0.05). Positive correlation was noted between the aerobic Gram‐negative Proteobacteria (Acinetobacter, Pseudomonas, Flavobacterium, Acidovorax), three of them being numerous in semen samples. Two oral bacteria (Aggregatibacter, Haemophilus) were also positively correlated with each other.
3.4. Structure of seminal microbial communities
In semen samples, Proteobacteria, Firmicutes, Actinobacteria and Bacteroidetes were the dominant phyla, and Lactobacillus, Acinetobacter, Prevotella and Corynebacterium were the most frequent genera. Prevalence of genus Lactobacillus tended to be lower and Porphyromonas and Campylobacter higher in ART men compared with healthy controls (P < 0.05 for both). At family level, ART men had decreased numbers of Lactobacillaceae, Moraxellaceae and Corynebacteriaceae and increased numbers of Porphyromonadaceae*, Campylobacteraceae*, Micrococcaceae*, Flavobacteriaceae, Veillonellaceae and Prevotellaceae compared with healthy men (*P < 0.05) (Figure 2). The most frequent lactobacilli species were L. iners and L. crispatus (present in 92% and 83% of semen samples, respectively), L. jensenii (25%) and L. gasseri (17%) being less frequent. There were three dominant Acinetobacter species in ART men: Acinetobacter junii, Acinetobacter lwoffii and Acinetobacter schindleri.
Hierarchical clustering of semen samples revealed six community types that were significantly more diverse in comparison with that in women (Figure 4, Table 1). The most common type was Acinetobacter in different combinations with other bacteria (I), followed by Gram‐negative anaerobes/microaerophiles (II), mixed communities containing different Gram‐positive and Gram‐negative bacteria (III), and L. iners together with vaginal bacteria (IV).
FIGURE 4.
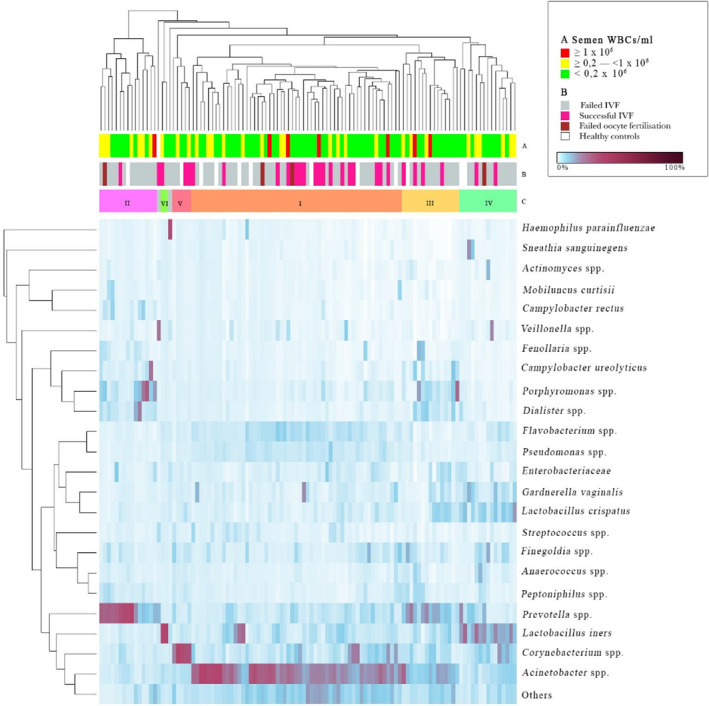
Hierarchical clustering of semen samples. Columns represent semen microbiome and rows represent identified bacterial clusters. A: WBC concentration in semen, B: health status and ART result, C: microbiome community type. Two samples did not cluster into the groups – in one of them, Veillonella predominated and in another, Haemophilus parainfluenzae predominated. ART, assisted reproductive technologies; IVF, in vitro fertilization.
Correlogram illustrates the co‐occurrence of taxa in the communities (Figure S2B). Several anaerobic bacteria were positively associated with each other, and several aerobic bacteria were clustered together, too, but these two groups were negatively correlated with each other. In contrast to women, lactobacilli and BV‐bacteria in men were positively correlated.
3.5. Associations between clinical and microbiological data
We evaluated the associations between single bacteria and clinical data (Figure S3). Bifidobacterium in vaginal samples of ART women was positively associated with successful previous deliveries (P < 0.01). Higher BMI in ART women was associated with Aerococcus urinae (P < 0.01). As expected, a significant positive association was found between an increased NS and various BV‐related bacteria such as G. vaginalis, Atopobium vaginae and Megasphaera (P < 0.001 for all), and also Dialister (P < 0.001), Prevotella and Shuttleworthia (P < 0.01 for both), whereas there was a negative association with L. crispatus (P < 0.001). Gram‐negative anaerobes (Prevotella, Porphyromonas, Dialister) and microaerophiles (Campylobacter) tended to be negatively associated with ART success in both men and women; however, these correlations remained below the significance level.
The women with BV, with L. iners‐predominant and with L. gasseri‐predominant microbiome (communities I, III, IV and VI), had a lower ART success level, as clinical pregnancy rate, than the women with L. crispatus‐dominant or other lactic‐acid‐bacteria‐predominant microbiome types (communities II, V, VIIA and VIIB). Taken together, a statistically significant difference was detected between these clusters (22.2% vs 41.2%, P < 0.05) (Table 1).
The men with community type I (Acinetobacter in different combinations with other bacteria) had the highest ART clinical pregnancy rate (35.4%); the same men had also the highest number of previous children (27% with previous partner and 33% with current partner). The men with community type II (dominated by Gram‐negative anaerobic and/or microaerophilic bacteria such as Prevotella, Porphyromonas, Dialister, Campylobacter) had a poor ART outcome result (one of 14 succeeded only), a significantly different result when compared with men with community type I (35.4% vs 7.1%, P < 0.05) (Table 1).
There were 15 couples in which both partners had the above‐described beneficial microbiome types (communities II, V, VII in women and community I in men). These couples had an ART success rate of 53%, whereas the rest of the couples had a success rate of 25% (P = 0.023).
When looking at the confounding factors for these associations, we applied two different methods. permutational multivariate ANOVA (PERMANOVA) analysis enabled us to illustrate the possible confounders, using both the OTU composition and the community type (Figures 5 and 6, Figures S4 and S5). Previous pregnancy loss status was significantly associated with vaginal community type (Pr[>F] 0.0417), and primary infertility was significantly associated with elevated BMI (Pr[>F] 0.0201). Fertilization procedure (IVF vs ICSI) was significantly associated with microbial community type (Pr[>F] 0.0332) and BMI (Pr[>F] 0.0228); women undergoing ICSI had higher BMI.
FIGURE 5.
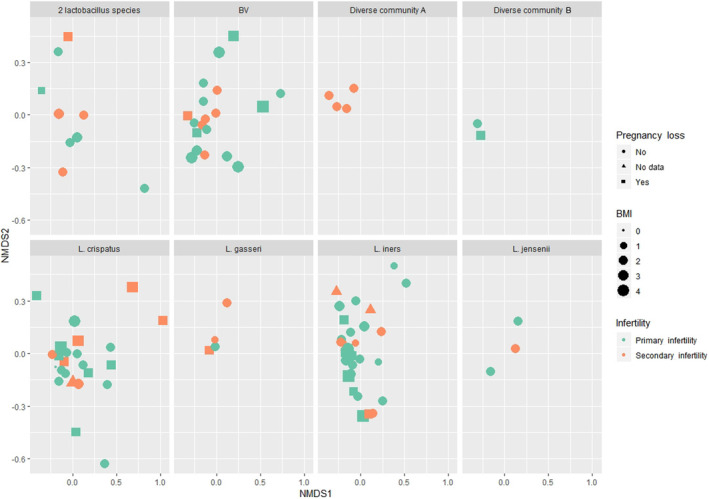
Previous pregnancy loss, body mass index (BMI), primary or secondary infertility, and reproductive tract microbial community types (eight panels) in infertile women undergoing an ART procedure. The figure matrix (NMDS1 vs NMDS2) displays the OTU composition of the individual women on two‐dimensional plane, each woman being presented as an individual datapoint. Women experiencing primary (symbols in green) or secondary (symbols in orange) infertility according to previous pregnancy loss (circle – no previous pregnancy loss; triangle – no data; square – previous pregnancy loss) and their BMI (the bigger the symbol, the heavier the woman). BMI categories: 0, no data; 1, <18.5 kg/m2 (underweight); 2, 18.5–24.9 kg/m2 (normal weight); 3, 25–29.9 kg/m2 (overweight); 4, ≥30 (class I & II obesity). PERMANOVA analysis revealed several associations: microbial community type was significantly associated with women's previous pregnancy loss status (Pr[>F] 0.0417) and primary infertility type was significantly associated with elevated BMI (Pr[>F] 0.0201). ART, assisted reproductive technologies; BMI, body mass index; OTU, operational taxonomic unit.
FIGURE 6.
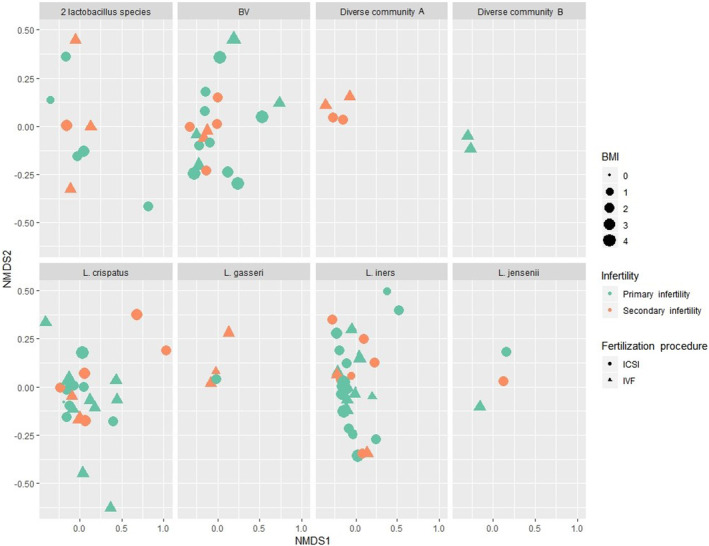
Body mass index (BMI), primary or secondary infertility, fertilization type (IVF vs ICSI), and reproductive tract microbial community types (eight panels) in infertile women undergoing an ART procedure. The figure matrix (NMDSI1 vs NMDS2) displays the OTU composition of the individual women on a two‐dimensional plane, each woman being presented as an individual datapoint. Women experiencing primary (symbols in green) or secondary (symbols in orange) infertility according to the type of fertilization (circle – ICSI; triangle – IVF) and BMI (the bigger the symbol, the heavier the woman). BMI categories: 0, no data; 1, <18.5 kg/m2 (underweight); 2, 18.5–24.9 kg/m2 (normal weight); 3, 25–29.9 kg/m2 (overweight); 4, ≥30 (class I & II obesity). PERMANOVA analysis revealed several associations: fertilization type was significantly associated with women's BMI (Pr[>F] 0.0228; women undergoing ICSI had higher BMI) and microbial community type (Pr[>F] 0.0332). ART, assisted reproductive technologies; BMI, body mass index; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; OTU, operational taxonomic unit.
When adjusting the ANOVA model on microbial community type and clinical pregnancy, we found that the quality of the transferred embryos alone increased the chance of positive clinical pregnancy outcome, as expected (Pr[<F] 0.0223) (Table S4). The association between community type and clinical pregnancy also remained significant when embryo quality and female BMI (Pr[<F] 0.0292), and embryo quality, female age and BMI (Pr[<F] 0.0461) were taken into account. There remained a statistically significant three‐way interaction in case of the number of transferred embryos (1–3 embryos) and female age (Pr[<F] 0.0112), number of transferred embryos and female BMI (Pr[<F] 0.0271), and number of transferred embryos and embryo quality (Pr[<F] 0.0211), but transferring more embryos without taking into account the other confounders did not increase the clinical pregnancy rate (Pr[<F] 0.3593). Besides the community type, the male partners’ factors did not influence the ART pregnancy rate.
4. DISCUSSION
Our study revealed that the microbial communities within the reproductive tract of couples undergoing ART procedure were significantly more diverse and with different predominance patterns in comparison with healthy fertile couples. Women with BV and with L. iners‐predominant and L. gasseri‐predominant microbiome had a lower ART success rate than women with L. crispatus‐predominant or mixed lactic‐acid‐bacteria‐predominant type. The men whose microbial community was dominated by Gram‐negative anaerobic and/or microaerophilic bacteria (Prevotella, Porphyromonas, Dialister, Campylobacter) had a poor ART outcome result. The couples with beneficial microbiome types had a significantly higher ART success rate compared with other couples.
Consecutive couples attending the fertility clinic for the ART procedure that consented to the participation in the microbiome study were recruited. The healthy couples were enrolled on the basis of good reproductive and general health according to the strict study criteria (Table S2). Both partners were at a fertile age (20–45 years old), had at least one joint pregnancy during past 5 years, were in a regular monogamous sexual relationship, with female partners reporting regular menstruations. Exclusion criteria included signs, symptoms and diagnoses of reproductive tract diseases, any diagnosed general disease, repeated spontaneous abortions, infertility treatment, pregnancy and lactation, surgery, tumor or trauma in the urogenital tract, using medication, intravaginal over‐the‐counter devices, intrauterine devices, condoms and spermicides. All control subjects had normal BMI, the women had an NS of 0 and the men had an insignificant leukocyte count in their semen (<0.2 × 106 WBC/mL). Thus, we ruled out the majority of possible health concerns in order to have as healthy a control group as possible.
The list of main lactobacilli species found from vaginal samples in this study (L. crispatus, L. iners, L. gasseri and L. jensenii) coincided with the species list found in former studies performed in Estonia 4 , 19 , 26 as well as other countries. 3 , 27 The microbiome profiles of women could be grouped into eight types, the majority of which were dominated by lactobacilli; one type represented the BV‐community.
While comparing the two groups of women in our current study we found that in both groups the predominant phylum was Firmicutes and the predominant genus was Lactobacillus; however, in ART women, their counts were significantly decreased. Instead, the ART women displayed increased phylum Actinobacteria and genera Gardnerella, Atopobium, Bifidobacterium, Clostridium and family Enterobacteriaceae. In addition, the ART women showed greater bacterial richness and diversity compared with healthy controls, as noted before. 27 This was the case despite the fact that the samples from ART women were collected on the follicle puncture day when the women had a high estradiol level, which supports growth of lactobacilli. Unfortunately, we do not have data about oral sex practices among the participants that may also have an impact on vaginal communities. In contrast to the intestinal tract, the higher diversity in the reproductive tract (mostly accompanied by decreased lactobacilli counts) is not beneficial to health.
The four main phyla in the semen samples were Proteobacteria, Firmicutes, Bacteroidetes and Actinobacteria. Similar results were obtained in our past studies 10 as well as in other studies. 9 , 12 , 13 , 25 In general, the seminal microbiome was more diverse but with lower bacterial concentration than the vaginal microbiome, which coincides with our previous report. 19 Six community types were revealed in men that were also more diverse than that of women; therefore, not just one predominant bacterium but a cluster of bacteria characterized most of the types.
Although lactobacilli and BV‐associated bacteria were negatively associated in vaginal microbiome, they were positively correlated in semen samples, as was also noted by our previous study on prostatitis patients. 10 Both microbial groups are transmitted between partners. 11 , 19 Our study also revealed that ART couples shared more bacterial OTUs than healthy couples, which may indicate higher sexual activity in the couples wishing to have an offspring.
Similarly to vaginal samples, the semen samples of ART men showed greater bacterial richness and diversity compared with healthy controls, as reported previously. 28 Just like in vaginal samples, the prevalence of Lactobacillus was lower in ART men's semen samples than in those of healthy controls. In addition, ART men displayed more numerous counts of several Gram‐negative and anaerobic bacteria compared with the men of healthy couples.
The most common community type constituent in men was Acinetobacter in different combinations with other bacteria. Our data correspond to the data of Yang et al. 29 and Kiessling et al.,30 who found significant abundance of this genus in semen samples, and partly with our previous studies that have revealed this genus in semen in lower counts. 10 The prevailing species in the present study were A. junii, A. lwoffii and A. schindleri. Acinetobacter junii has been previously isolated from semen samples in men undergoing fertility treatment. 30 Acinetobacter lwoffii has been found to inhabit the human oropharynx, skin and perineum in up to a quarter of the population. 31 Acinetobacter schindleri has been isolated from vagina and urine. 32 Conversely, Garcia‐Segura et al. 13 and Lundy et al. 28 did not mention Acinetobacter among the most numerous seminal bacteria. Acinetobacter is a Gram‐negative non‐fermenting aerobic rod that can well adapt to different environments. Therefore, it is widely distributed in nature and it has a common habitat on skin but can also cause various infections. Some previous studies have suggested that lab contamination cannot be fully ruled out in the case of this genus. 33 However, in our study, vaginal samples were handled in the same lab with the same tools and they did not contain high numbers of this genus. In addition, the reference databases used for microbiome analyses have been vigorously updated during last decade, thus the spectrum of microorganisms in the same samples analyzed in different years can vary.
The majority of balanced vaginal communities is composed of lactobacilli, where they play an important role in maintaining a healthy environment. In seminal communities, their proportions are significantly lower and their functions are less clear. Some recent studies have shown contradictory results in their proportions and species composition. In Chinese men, lactobacilli had the highest proportion among the seminal bacteria. 29 Similarly, in a Nigerian IVF cohort, Lactobacillus was the most abundant genus in semen and, among this genus, L. iners was the most abundant species. 34 In a study of Garcia‐Segura et al., 13 Lactobacillus belonged to top 10 in a western Mediterranean population but was not the most abundant genus. In an Italian cohort, the proportion of lactobacilli in the seminal communities was highly biased, ranging from 0% to 4% in 15 men, 10% to 24% in four men, and 30% to 66% in four men. 27 At the same time, Lundy et al. 28 did not find lactobacilli in semen of Cleveland (USA) cohort containing fertile and infertile men of three races. We have previously observed a decrease in Lactobacillus abundance in the case of prostatitis. 10 In next generation sequencing studies, method of DNA extraction can affect the results, especially the proportion of Gram‐positive bacteria. On the other hand, geographic differences are possible.
Association between lactobacilli and male fertility status remains controversial, too. A study of Weng et al. 12 revealed that the majority of high‐quality semen samples clustered into the Lactobacillus‐predominant group, suggesting that this genus could be a potential probiotic for semen quality maintenance. Also, Baud et al. 35 observed an increased relative abundance of Lactobacillus in samples with normal sperm morphology, Monteiro et al. 18 showed reduction of lactobacilli in semen in the case of male infertility and Okwelogu et al. 34 in the case of negative ART outcome. Surprisingly, some contrary studies have also been published. It has been demonstrated that some lactobacilli species may reduce sperm motility in vitro. 36 Yang et al. 25 found an increased proportion of Lactobacillus in asthenospermic men and proposed that the bacillus may lower the pH of semen, with an adverse effect on male fertility. These conflicting results need more thorough studies as, again, methodical and geographic differences are possible. To date, an inhibitory effect of a reasonable proportion of seminal lactobacilli on anaerobic, microaerophilic and aerobic Gram‐negative bacteria should be considered.
Our study revealed the association between the vaginal microbiome community type and the ART outcome. The women with BV and the women with a L. iners‐dominant community had a lower success rates in comparison with women with a L. crispatus‐dominant vaginal community. Lactobacillus crispatus has also associated with successful artificial insemination procedures in the past. 27 , 37 This species has been isolated mainly in healthy women, and several probiotic strains have been derived from this species. Also, L. crispatus was significantly negatively associated with NS in the current study, which is consistent with previous results. 38
Latobacillus iners is one of the most commonly described species in the vaginal microbiome. It has been described as the most common species in Nigerian women undergoing ART. 34 However, the L. iners‐dominant community has been considered to be a transitional/intermediate community type. 39 Recently, Kindinger et al. 40 demonstrated an association between L. iners dominance in vaginal microbiome and preterm delivery. It is possible that the small genome of L. iners (compared with other lactobacilli species) is the reason for the lower protective function of the vaginal microbiome in this species. Therefore, the presence of L. iners may not reflect the good status of the female vaginal microbiome, and this species needs further research to clarify its role in the reproductive tract. 39 , 41
Lactobacillus jensenii and L. gasseri dominated in two small community groups; therefore no far‐reaching conclusions can be drawn for these species. Successful ART outcome was noted in one woman dominated by L. jensenii. It has been found that high levels of L. gasseri in the follicular fluid may cause fragmentation of oocyte DNA, which may have affected the results of artificial insemination. 42
In two small subgroups, the lactobacilli were combined with other lactic acid bacteria (Bifidobacterium, Streptococcus). Taken together, four of six cases resulted in successful ART, thus confirming the importance of lactic acid bacteria in the vagina.
BV is a polymicrobial vaginal microbiome condition characterized by a significant decrease in protective lactobacilli and an increase in anaerobic bacteria. 38 BV may be overlooked because its symptoms are often absent, but previous studies have shown an association of BV with the presence of several cytokines and interleukins in the reproductive tract that can interfere with embryo implantation and cause tissue damage; 43 , 44 therefore, the clinical consequences in reproductive medicine tend to be significant. In our study, BV was more frequent among women with a higher average BMI and age. Diverse and L. gasseri‐dominated communities were also more frequent in older women in our study. As revealed in our previous study, 21 older women, regardless of their BMI, are more likely to have unsuccessful ART compared with younger, normal‐weight women. 14
The men in our study with Acinetobacter‐associated community type had the highest ART success rate and reported the highest number of children prior to the ART procedure. Such a correlation has not been reported before.
At the same time, a group of men with community type dominated by Gram‐negative anaerobic and/or microaerophilic bacteria such as Prevotella, Porphyromonas, Dialister and Campylobacter had a poor ART outcome, as most partners of these males failed to conceive (13/14). Prevotella has previously been associated with poorer sperm parameters and inflammation of the upper genital tract 12 , 19 and Porphyromonas was previously found in samples with reduced sperm concentration and sperm motility. 12 Prevotella, Porphyromonas and Dialister have also been associated with BV in women 12 , 38 and increased levels of Campylobacter were found in samples from men with prostatitis. 10 Some species of Campylobacter belong to the intestinal pathogens; the others can cause urethritis and periodontitis. 45
There are few studies describing the microbiome of both partners undergoing ART procedure and comparing the ART outcome with bacteria. 27 , 34 In the Nigerian cohort, in the case of a positive IVF outcome, the mean proportions of L. jensenii and L. iners were increased and those of Proteobacteria and Gram‐negative anaerobes decreased in semen samples, whereas in vaginal samples, the mean proportion of L. gasseri was increased, whereas that of Bacteroides and other lactobacilli was decreased. 34 In the Italian cohort, positive outcome of intrauterine insemination was associated with increased proportion of L. crispatus in vaginal samples, whereas no statistically significant difference was revealed in semen samples. 27
The impact of microbiome on reproductive functions is further complicated because of the fluctuation of the communities due to several factors such as sexual activity, hormonal changes, antimicrobial treatment and many other reasons. This has been more investigated in women;46 very little is known about dynamics of the male reproductive tract microbiome. In men, it can be more stable, which that may explain higher number of previous children in our study group with beneficial community type and better ART success rate, but it may fluctuate in time as well. The microbiome should be ideally tested approximately 1 month before the ART procedure to ensure a suitable time frame for microbiome modulation.
Genital tract microorganisms can affect fertility in different ways, one of the mechanisms being infection‐related or dysbiosis‐related oxidative stress in both partners, as revealed by our previous studies. 47 , 48 , 49 It is interesting that the highest oxidative stress levels were found in both partners when biochemically detectable pregnancy with a positive HCG value did not develop into clinically detectable pregnancy. 48 Thus, in addition to microbiome‐balancing therapy, the ART patients may also need antioxidant therapy. In our current study, there were 15 couples in which both partners had the beneficial microbiome types (communities II, V or VII in women and I in men). These couples had a significantly a higher ART success rate (53% vs 25%) than the rest of the couples, indicating a need for a routine test‐and‐treat approach for both partners prior to ART.
One limitation of the study was the size of our control group; however, our controls were selected according to very strict criteria that included good reproductive and general health. Regardless, due to the size of the control group, several differences between the groups did not show statistical significance. However, enrolling couples rather than individuals to the study group allowed for the assessment of the potential microbial community composition effect of the couple on the ART procedure outcome.
5. CONCLUSION
Microbiome disturbances in the genital tract of both partners tend to be associated with a couple's infertility as well as lower ART success levels and may thus need attention before the ART procedure. The incorporation of genitourinary microbial screening as a part of the diagnostic evaluation process may become routine for infertile couples and ART patients if our results are confirmed by prospective randomized controlled trials. To correct the balance of disturbed microbiome communities, targeted genital tract probiotics can be used, and individualized treatment regimens may also be needed.
AUTHOR CONTRIBUTIONS
KK and KS – analysis and interpretation of data, drafting the article. ST – analysis and interpretation of data, revising the article critically. EL and MJ – acquisition of data, analysis and interpretation of data, revising the article critically. ET, PKarits, Karin Rosenstein, AS, DS and KH –acquisition of data, revising the article critically. P Korrovits—conception and design, acquisition of data, revising the article critically. HK, AS and RM – conception and design, revising the article critically. All authors gave final approval of the version to be published and have agreed to be accountable for all aspects of the work.
FUNDING INFORMATION
This study was supported by Enterprise Estonia (grant no. EU48695); Estonian Research Council (grant nos. IUT34‐19 and PRG1076), Horizon 2020 innovation grant (ERIN, grant no. EU952516), Eureka Eurostars ReadUteru project and Estonian Ministry of Education and Research (grant no. KOGU‐HUMB).
CONFLICT OF INTEREST STATEMENT
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Supporting information
Table S1.
Table S2.
Table S3.
Table S4.
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Figure S5.
ACKNOWLEDGMENTS
The authors than Ms. Merlin Pajuva for excellent technical help.
Koort K, Sõsa K, Türk S, et al. Lactobacillus crispatus‐dominated vaginal microbiome and Acinetobacter‐dominated seminal microbiome support beneficial ART outcome. Acta Obstet Gynecol Scand. 2023;102:921‐934. doi: 10.1111/aogs.14598
REFERENCES
- 1. Altmäe S, Franasiak JM, Mändar R. The seminal microbiome in health and disease. Nat Rev Urol. 2019;16:703‐721. [DOI] [PubMed] [Google Scholar]
- 2. Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive‐age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680‐4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drell T, Lillsaar T, Tummeleht L, et al. Characterization of the vaginal micro‐ and mycobiome in asymptomatic reproductive‐age Estonian women. PLoS ONE. 2013;8:e54379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Punzón‐Jiménez P, Labarta E. The impact of the female genital tract microbiome in women health and reproduction: a review. J Assist Reprod Genet. 2021;38:2519‐2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Oostrum N, De Sutter P, Meys J, Verstraelen H. Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta‐analysis. Hum Reprod. 2013;28:1809‐1815. [DOI] [PubMed] [Google Scholar]
- 7. Ravel J, Moreno I, Simón C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am J Obstet Gynecol. 2021;224:251‐257. [DOI] [PubMed] [Google Scholar]
- 8. Venneri MA, Franceschini E, Sciarra F, Rosato E, D’Ettorre G, Lenzi A. Human genital tracts microbiota: dysbiosis crucial for infertility. J Endocrinol Invest. 2022;45:1151‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nelson DE, Dong Q, Van der Pol B, et al. Bacterial communities of the coronal sulcus and distal urethra of adolescent males. PLoS ONE. 2012;7:e36298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mändar R, Punab M, Korrovits P, et al. Seminal microbiome in men with and without prostatitis. Int J Urol. 2017;24:211‐216. [DOI] [PubMed] [Google Scholar]
- 11. Hou D, Zhou X, Zhong X, et al. Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril. 2013;100:1261‐1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weng SL, Chiu CM, Lin FM, et al. Bacterial communities in semen from men of infertile couples: metagenomic sequencing reveals relationships of seminal microbiota to semen quality. PLoS ONE. 2014;9:e110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia‐Segura S, Del Rey J, Closa L, et al. Seminal microbiota of idiopathic infertile patients and its relationship with sperm DNA integrity. Front Cell Dev Biol. 2022;10:937157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deyhoul N, Mohamaddoost T, Hosseini M. Infertility‐related risk factors: a systematic review. Int J Women's Health Reprod Sci. 2017;5:24‐29. [Google Scholar]
- 15. Haller‐Kikkatalo K, Salumets A, Uibo R. Review on autoimmune reactions in female infertility: antibodies to follicle stimulating hormone. Clin Dev Immunol. 2012;2012:762541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Punab M, Poolamets O, Paju P, et al. Causes of male infertility: a 9‐year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum Reprod. 2017;32:18‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minhas S, Bettocchi C, Boeri L, et al. European Association of Urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur Urol. 2021;80:603‐620. [DOI] [PubMed] [Google Scholar]
- 18. Monteiro C, Marques PI, Cavadas B, et al. Characterization of microbiota in male infertility cases uncovers differences in seminal hyperviscosity and oligoasthenoteratozoospermia possibly correlated with increased prevalence of infectious bacteria. Am J Reprod Immunol. 2018;79:e12838. [DOI] [PubMed] [Google Scholar]
- 19. Mändar R, Punab M, Borovkova N, et al. Complementary seminovaginal microbiome in couples. Res Microbiol. 2015;166:440‐447. [DOI] [PubMed] [Google Scholar]
- 20. Farquhar C, Marjoribanks J. Assisted reproductive technology: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2018;8:CD010537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Korrovits P, Lapp E, Mändar R. Couple‐related factors of ART outcome. Clin Exp Obstet Gynecol. 2016;43:747‐750. [PubMed] [Google Scholar]
- 22. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization . WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. World Health Organization; 2010. [Google Scholar]
- 24. Punab M, Lõivukene K, Kermes K, Mändar R. The limit of leucocytospermia from the microbiological viewpoint. Andrologia. 2003;35:271‐278. [PubMed] [Google Scholar]
- 25. Yang B, Wang Y, Qian PY. Sensitivity and correlation of hypervariable regions in 16S rRNA genes in phylogenetic analysis. BMC Bioinformatics. 2016;17:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smidt I, Kiiker R, Oopkaup H, et al. Comparison of detection methods for vaginal lactobacilli. Benef Microbes. 2015;6:747‐751. [DOI] [PubMed] [Google Scholar]
- 27. Amato V, Papaleo E, Pasciuta R, et al. Differential composition of vaginal microbiome, but not of seminal microbiome, is associated with successful intrauterine insemination in couples with idiopathic infertility: a prospective observational study. Open Forum Infect Dis. 2019;7:ofz525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lundy SD, Sangwan N, Parekh NV, et al. Functional and taxonomic dysbiosis of the gut, urine, and semen microbiomes in male infertility. Eur Urol. 2021;79:826‐836. [DOI] [PubMed] [Google Scholar]
- 29. Yang H, Zhang J, Xue Z, et al. Potential pathogenic bacteria in seminal microbiota of patients with different types of dysspermatism. Sci Rep. 2020;10:6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kiessling AA, Desmarais BM, Yin HZ, Loverde J, Eyre RC. Detection and identification of bacterial DNA in semen. Fertil Steril. 2008;90:1744‐1756. [DOI] [PubMed] [Google Scholar]
- 31. Ku SC, Hsueh PR, Yang PC, Luh KT. Clinical and microbiological characteristics of bacteremia caused by Acinetobacter lwoffii . Eur J Clin Microbiol Infect Dis. 2000;19:501‐505. [DOI] [PubMed] [Google Scholar]
- 32. Nemec A, De Baere T, Tjernberg I, Vaneechoutte M, van der Reijden TJ, Dijkshoorn L. Acinetobacter ursingii sp. nov. and Acinetobacter schindleri sp. nov. isolated from human clinical specimens. Int J Syst Evol Microbiol. 2001;51:1891‐1899. [DOI] [PubMed] [Google Scholar]
- 33. Salter SJ, Cox MJ, Turek EM, et al. Reagent and laboratory contamination can critically impact sequence‐based microbiome analyses. BMC Biol. 2014;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Okwelogu SI, Ikechebelu JI, Agbakoba NR, Anukam KC. Microbiome compositions from infertile couples seeking in vitro fertilization, using 16S rRNA gene sequencing methods: any correlation to clinical outcomes? Front Cell Infect Microbiol. 2021;11:709372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baud D, Pattaroni C, Vulliemoz N, Castella V, Marsland BJ, Stojanov M. Sperm microbiota and its impact on semen parameters. Front Microbiol. 2019;10:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang H, Chen T, Chen Y, et al. Evaluation of the inhibitory effects of vaginal microorganisms on sperm motility in vitro . Exp Ther Med. 2020;19:535‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koedooder R, Singer M, Schoenmakers S, et al. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum Reprod. 2019;34:1042‐1054. [DOI] [PubMed] [Google Scholar]
- 38. Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE. 2012;7:e37818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Petrova MI, Lievens E, Malik S, Imholz N, Lebeer S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol. 2015;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kindinger LM, Bennett PR, Lee YS, et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. 2017;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Macklaim JM, Gloor GB, Anukam KC, Cribby S, Reid G. At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB‐1. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4688‐4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pelzer ES, Harris JE, Allan JA, et al. TUNEL analysis of DNA fragmentation in mouse unfertilized oocytes: the effect of microorganisms within human follicular fluid collected during IVF cycles. J Reprod Immunol. 2013;99:69‐79. [DOI] [PubMed] [Google Scholar]
- 43. Anahtar MN, Byrne EH, Doherty KE, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42:965‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boomsma CM, Kavelaars A, Bozkurt N, et al. Is bacterial vaginosis associated with a pro‐inflammatory cytokine profile in endometrial secretions of women undergoing IVF? Reprod Biomed Online. 2010;21:133‐141. [DOI] [PubMed] [Google Scholar]
- 45. Liu F, Ma R, Wang Y, Zhang L. The clinical importance of Campylobacter concisus and other human hosted Campylobacter species. Front Cell Infect Microbiol. 2018;8:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ma B, Forney LJ, Ravel J. Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol. 2012;66:371‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mändar R, Kullisaar T, Borovkova N, Punab M. Sexual intercourse with leukocytospermic men may be a possible booster of oxidative stress in female partners of infertile couples. Andrology. 2013;1:464‐468. [DOI] [PubMed] [Google Scholar]
- 48. Ahelik A, Mändar R, Korrovits P, et al. Systemic oxidative stress could predict assisted reproductive technique outcome. J Assist Reprod Genet. 2015;32:699‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Altmäe S, Kullisaar T. Genitourinary microbial screening for all infertile men? Nat Rev Urol. 2022;19:199‐200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S3.
Table S4.
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Figure S5.


