Abstract
Introduction
This study examined obstetric outcomes in patients diagnosed with uterine adenomyosis.
Material and methods
This historical cohort study queried the Healthcare Cost and Utilization Project's National Inpatient Sample. The study population was all hospital deliveries in women aged 15–54 years between January 2016 and December 2019. The exposure was a diagnosis of uterine adenomyosis. The main outcome measures were obstetric characteristics, including placenta previa, placenta accreta spectrum, and placental abruption. Secondary outcomes were delivery complications including severe maternal morbidity. Analytic steps to assess these outcomes included (i) a 1‐to‐N propensity score matching to mitigate and balance prepregnancy confounders to assess obstetric characteristics, followed by (ii) an adjusting model with preselected pregnancy and delivery factors to assess maternal morbidity. Sensitivity analyses were also performed with restricted cohorts to account for prior uterine scar, uterine myoma, and extra‐uterine endometriosis.
Results
After propensity score matching, 5430 patients with adenomyosis were compared to 21 720 patients without adenomyosis. Adenomyosis was associated with an increased odds of placenta accreta spectrum (adjusted‐odds ratio [aOR] 3.07, 95% confidence interval [CI] 2.01–4.70), placenta abruption (aOR 3.21, 95% CI: 2.60–3.98), and placenta previa (aOR 5.08, 95% CI: 4.25–6.06). Delivery at <32 weeks of gestation (aOR 1.48, 95% CI: 1.24–1.77) and cesarean delivery (aOR 7.72, 95% CI: 7.04–8.47) were both increased in women with adenomyosis. Patients in the adenomyosis group were more likely to experience severe maternal morbidity at delivery compared to those in the nonadenomyosis group (aOR 1.86, 95% CI: 1.59–2.16). Results remained robust in the aforementioned several sensitivity analyses.
Conclusions
This national‐level analysis suggests that a diagnosis of uterine adenomyosis is associated with an increased risk of placental pathology (placenta accreta spectrum, placenta abruption, and placental previa) and adverse maternal outcomes at delivery.
Keywords: adenomyosis, placenta accreta spectrum, placenta previa, placental abruption, pregnancy, preterm delivery, severe maternal morbidity
The results of this nationwide assessment suggest that pregnant patient with a diagnosis of adenomyosis may be associated with increased risk of placental pathology (placenta accreta spectrum, placenta abruption, and placental previa) and adverse maternal outcomes at delivery.
Abbreviations
- aOR
adjusted odds ratio
- ART
assisted reproductive technology
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- ICD‐10
International Classification of Disease 10th edition
- NIS
National Inpatient Sample
Key message.
The results of this nationwide assessment suggest that pregnant patients with a diagnosis of adenomyosis may be at increased risk of placental pathology (placenta accreta spectrum, placenta abruption, and placental previa) and adverse maternal outcomes at delivery.
1. INTRODUCTION
Uterine adenomyosis is a condition characterized by the heterotopic presence of endometrial glands and stroma within the myometrial layer. 1 , 2 Many patients present with dysmenorrhea and pelvic pain, abnormal uterine bleeding, and/or subfertility. While the true pathogenesis is unknown, the disease is posited to arise from the invagination of endometrium basalis via an altered or disrupted endometrial‐myometrial interface. 2 Adenomyosis historically was a histopathological diagnosis, but advances in imaging modalities, namely in 2D and 3D transvaginal ultrasonography and magnetic resonance imaging, have led to improved clinical diagnosis in reproductive‐aged women. 2 , 3
The true prevalence of adenomyosis is unknown due to varying histopathological and radiological criteria used to define adenomyosis, as well as the fact that there may be a considerable number of asymptomatic patients with undiagnosed disease. 4 However, as clinical and radiological diagnosis of adenomyosis has improved over the past two decades, the impact of clinically significant adenomyosis on fertility and obstetric outcomes warrants careful study to counsel patients with adenomyosis who are considering pregnancy. This is particularly relevant for older patients who are at highest risk of symptomatic adenomyosis, and for whom assisted reproductive technology (ART) has dramatically increased the potential for pregnancy. 5
Existing literature has suggested that adenomyosis negatively impacts pregnancy outcomes. 6 , 7 Prior studies, however, have generally been limited by sample size, and populational‐level data is sparse. 8 , 9 , 10 Of particular interest is the risk of placental pathology given the disruption of the endometrial‐myometrial interface and impact on maternal morbidity. The objective of this study was to evaluate obstetric risks and maternal morbidity at delivery in patients diagnosed with adenomyosis on a populational scale.
2. MATERIAL AND METHODS
2.1. Data source
This historical cohort study utilized the National Inpatient Sample (NIS). The NIS is distributed as part of the Healthcare Cost and Utilization Project by the Agency for Healthcare Research and Quality. It represents hospital discharge data for >90% of the United States population using survey weights based on a random sampling of 20% of hospitalized patients annually. The dataset is both publicly available and deidentified. 11
2.2. Sample selection and exposure allocation
All hospital deliveries, both vaginal deliveries and cesarean sections, in pregnant patients aged 15–54 years between January 2016 and December 2019 were abstracted from the dataset. The age cutoffs were based on recent studies. 12 , 13 The World Health Organization's International Classification of Disease 10th edition (ICD‐10) Clinical Modification codes were used to define the presence of adenomyosis as well as delineate additional diagnoses and procedures performed within the index hospital admission. The exposure was based on the presence or absence of adenomyosis (ICD‐10 clinical modification code N80.0).
2.3. Clinical information
The following clinical information was abstracted from the NIS for analysis: (i) patient baseline demographics, (ii) obstetric outcomes, (iii) hospital factors, and (iv) delivery information. Covariate selections were based on a prior analysis in which ICD‐10 Clinical Modification and Procedure Classification System codes and Diagnosis‐Related Group codes are shown in Table S1. 14
Patient baseline demographics included age, year, race and ethnicity, primary expected payer, census‐level median household income, tobacco use, grand multiparity, prior uterine scar including cesarean section, uterine myoma, and region. Prepregnancy medical comorbidities included pregestational hypertension, pregestational diabetes mellitus, and obesity.
Obstetric outcomes included placental previa, placenta accreta spectrum, placenta abruption, gestational hypertension, pre‐eclampsia, gestational diabetes mellitus, multifetal gestation, breech presentation, fetal growth restriction, large for gestational age, preterm labor, premature rupture of membrane, and uterine rupture.
Hospital factors examined in the analysis included relative hospital bed capacity, and hospital teaching and location setting.
Delivery information included route of delivery, labor dystocia, hemorrhage, and Centers for Disease Control and Prevention (CDC)‐defined severe maternal morbidity. 15 A total of 21 indicators for severe maternal morbidity were evaluated. Length of hospital admission and total charge for the index admission were also examined.
2.4. Outcome measures
The primary outcomes of interest were obstetric complications associated with adenomyosis, with particular attention to placental pathology (placenta previa, placenta accreta spectrum, and placenta abruption). The secondary outcomes of interest were delivery complications and maternal morbidity in patients with adenomyosis.
2.5. Statistical analyses
In the first step of the analysis, propensity score matching was performed to balance differences in baseline characteristics between patients with adenomyosis and those without adenomyosis with the aim of minimizing confounding variables. 16 A binary logistic regression model was used to compute the propensity score. With the assumption that adenomyosis is a pre‐existing condition prior to the index pregnancy, other prepregnancy baseline patient demographics and clinical factors as shown in Table 1 were included in the model.
TABLE 1.
Proportional balance statistics after propensity score match.
| Characteristic | Adenomyosis (−) | Adenomyosis (+) | SD* |
|---|---|---|---|
| Number | n = 21 720 | n = 5430 | |
| Age (y) | 33.4 (5.7) | 33.4 (5.4) | 0.010 |
| Year | 0.016 | ||
| 2016 | 22.2 | 22.1 | |
| 2017 | 25.1 | 24.5 | |
| 2018 | 25.3 | 25.4 | |
| 2019 | 27.4 | 28.0 | |
| Race/ethnicity | 0.026 | ||
| White | 43.3 | 43.2 | |
| Black | 15.7 | 16.5 | |
| Hispanic | 17.4 | 17.0 | |
| Asian | 13.2 | 12.6 | |
| Other | 6.0 | 6.1 | |
| Unknown | 4.5 | 4.6 | |
| Primary payer | 0.032 | ||
| Private | 65.1 | 65.7 | |
| Medicaid | 28.0 | 27.9 | |
| Medicare | 0.9 | 1.0 | |
| Self‐pay | 3.3 | 2.9 | |
| Other | 2.5 | 2.5 | |
| Unknown | 0.1 | ** | |
| Household income | 0.037 | ||
| QT1 (lowest) | 20.0 | 20.9 | |
| QT2 | 22.1 | 21.5 | |
| QT3 | 24.8 | 24.5 | |
| QT4 (highest) | 32.1 | 31.9 | |
| Unknown | 1.0 | 1.3 | |
| Region | 0.031 | ||
| Northeast | 23.6 | 22.8 | |
| Midwest | 16.5 | 16.0 | |
| South | 32.8 | 34.2 | |
| West | 27.0 | 27.0 | |
| Obesity | 0.010 | ||
| No | 85.3 | 85.0 | |
| Yes | 14.7 | 15.0 | |
| Pre‐existing HTN | 0.012 | ||
| No | 92.9 | 92.5 | |
| Yes | 7.1 | 7.5 | |
| Pre‐existing DM | 0.021 | ||
| No | 97.7 | 98.0 | |
| Yes | 2.3 | 2.0 | |
| Tobacco use | 0.015 | ||
| No | 96.8 | 97.1 | |
| Yes | 3.2 | 2.9 | |
| Grand multiparity | <0.001 | ||
| No | 99.4 | 99.4 | |
| Yes | 0.6 | 0.6 | |
| Prior uterine scar | 0.013 | ||
| No | 61.5 | 60.9 | |
| Yes | 38.5 | 39.1 | |
| Uterine myoma | 0.019 | ||
| No | 83.5 | 82.8 | |
| Yes | 16.5 | 17.2 |
Note: Percentage per group or mean (standard deviation) in the 1‐to‐N propensity score matched data is shown.
Abbreviations: DM, diabetes mellitus; HTN, hypertension; QT, quartile; SD, standardized difference.
A standardized difference of <0.10 indicates statistically well‐balanced cohorts, and a value of >0.20 indicates clinical imbalance between the two groups.
Small number suppressed.
Then, an automated algorithm was used to perform 1‐to‐1 propensity score‐based matching with the optimal caliper width for estimating differences of equal to 0.2 of the standard deviation for the logit of the propensity score (0.00015). 17 This iteration of matching was repeated for 1‐to‐N ratio analysis as long as the 1‐to‐1 matching ratio between the exposure groups continued. Balance statistics were assessed with standardized difference in the propensity score matched model, and the value of >0.20 was interpreted as clinical imbalance.
To assess the primary outcome of placental complications, a multivariable analysis was performed in the propensity score‐matched model. The preselected pregnancy factors as described above were entered in the initial model, and parsimonious, conditional backward selection was performed with the stopping rule of p < 0.05 in the final model. The effect size for adenomyosis was estimated with adjusted odds ratio (aOR) and a corresponding 95% confidence interval (CI).
Delivery complications and CDC‐defined severe maternal morbidity in pregnant patients with adenomyosis were then analyzed. Obstetric conditions that occur during pregnancy prior to delivery that were identified as independent factors associated with adenomyosis that were also risk factors for severe maternal morbidity were chosen a priori as adjusting factors in the analysis. Based on this logic, six adjusting factors were selected for assessing the exposure‐outcome relationship: placenta previa, placenta accreta spectrum, placenta abruption, pre‐eclampsia, gestational age, and cesarean delivery. 14 , 15 , 18 , 19 , 20 , 21 Effect size of the exposure group (adenomyosis compared to nonadenomyosis) on outcome (severe maternal morbidity) was estimated with aOR with corresponding 95% CI.
Several sensitivity analyses were performed to assess the robustness of the study results. First, the study cohort was restricted to only those without a prior uterine scar or uterine myoma, based on the observation that uterine scarring is a well‐known risk factor for placental pathology, particularly placenta accreta spectrum, and that uterine myomas often coexist with adenomyosis and may also affect placentation. 22 Second, severe maternal morbidity was examined again after excluding blood products transfusion and/or hysterectomy as performed previously in CDC analyses. 15 Third, characteristics and outcomes were evaluated by excluding patients with extra‐uterine endometriosis in addition to adenomyosis. In addition, individual morbidity indicators were examined with exposure‐outcome analysis. Lastly, obstetric hemorrhage, total charges for the index admission, and prolonged hospital stay of ≥7 days were assessed.
All statistical analyses were based on two‐tailed hypotheses, and a p < 0.05 was considered statistically significant. The weighted values for national estimates provided by the NIS program were used for the analysis. Missing values in each covariate were grouped for analysis. Statistical Package for Social Sciences (IBM SPSS, version 28.0.) and R statistics (version 3.5.3, R foundation for Statistical Computing) were used for the analysis. The STROBE guidelines were consulted for the performance of this observational cohort study. 23
2.6. Ethics statement
The National Inpatient Sample is publicly available on the Healthcare Cost and Utilization Project website for access by researchers (available on request at https://www.hcup‐us.ahrq.gov), hence the current study was exempt for ethics approval (ethical committee exemption: HS‐16‐00481). Informed consent from patients was not required.
The corresponding author (K.M.) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. The National Inpatient Sample is developed for the Healthcare Cost and Utilization Project that is sponsored by the Agency for Healthcare Research and Quality, and the program is the source of the deidentified data used; race/ethnicity was grouped by the program; and the program has not verified and is not responsible for the statistical validity of the data analysis or the conclusions derived by the study team.
3. RESULTS
3.1. Propensity score matching
The iteration of propensity score matching resulted in 1‐to‐4 ratio such that 5430 patients with a diagnosis of adenomyosis were compared to 21 720 patients without adenomyosis. Prior to propensity score matching, nearly half (6 of 13) of the prepregnancy baseline covariates that were included exhibited clinical imbalance between the two exposure groups (standardized difference >0.20), including age, uterine myoma, prior uterine scar, race/ethnicity, household income, and hospital region (Figure 1). Of those, age (standardized difference 0.807) and uterine myoma (standardized difference 0.574) exhibited moderate to large imbalance (standardized difference >0.50 and >0.80, respectively). However, after propensity score matching, all 13 prepregnancy baseline covariates were well‐balanced (all, standardized difference ≤0.037; Table 1).
FIGURE 1.
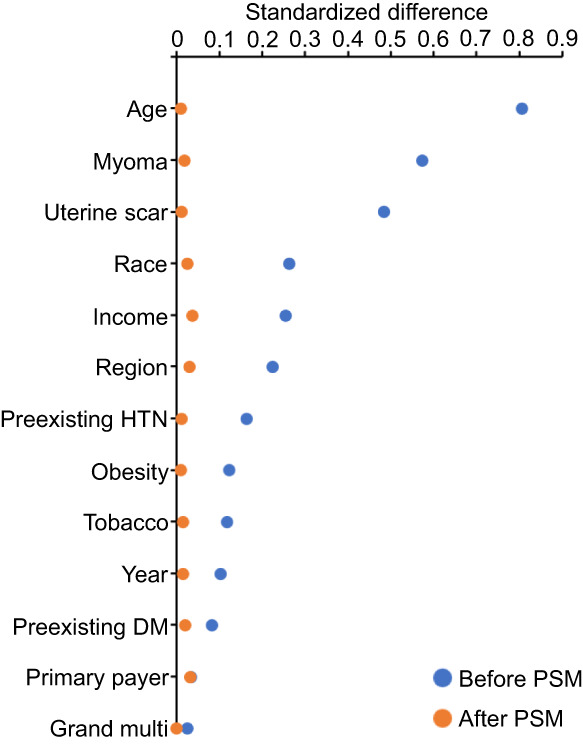
Standardized differences before and after propensity score matching. Standardized differences of >0.20, >0.50, and >0.80 indicate small, moderate, and large clinical imbalance between the two exposure groups (adenomyosis and nonadenomyosis), respectively. DM, diabetes; HTN, hypertension; multi, multiparity; PSM, propensity score matching.
3.2. Cohort‐level characteristics
The mean age was 33.4 years for both exposure groups (Table 1). In the adenomyosis and nonadenomyosis groups, respectively, the most common race was White (43.2% vs. 43.3%), and the majority were privately insured (65.7% vs. 65.1%), nonobese (85.0% vs. 85.3%), and had neither pregestational hypertension nor diabetes mellitus (92.5% vs. 92.9%, and 98.0% vs. 97.7%, respectively).
Approximately 40% of patients in both groups had a prior uterine scar (39.1% and 38.5%). Uterine myomas were present in 17.2% and 16.5% of patients in each group, respectively. Among 5430 patients with uterine adenomyosis, 18.3% had extra‐uterine endometriosis, of which the ovary was the most common anatomical site (13.4%) followed by the fallopian tube (4.6%) and pelvic peritoneum (3.9%). The prevalence of extra‐uterine endometriosis in the nonadenomyosis group was very low (<0.2%).
3.3. Obstetric characteristics related to adenomyosis
A multivariable analysis identified nine obstetric outcomes related to adenomyosis (Table 2). Patients with adenomyosis had an increased risk of placental pathology, including placenta accreta spectrum (aOR 3.07, 95% CI: 2.01–4.70), placenta abruption (aOR 3.21, 95% CI: 2.60–3.98), and placenta previa (aOR 5.08, 95% CI: 4.25–6.06). This observed association remained robust in restricted subcohorts of patients with no prior uterine scar, no uterine myoma, and neither prior uterine scar nor uterine myoma (Table 3) as well as patients without extra‐uterine endometriosis (Table S2).
TABLE 2.
Pregnancy characteristics related to adenomyosis.
| Characteristic | aOR (95% CI) | p‐value |
|---|---|---|
| Placenta previa | ||
| No | 1.00 (reference) | |
| Yes | 5.08 (4.25–6.06) | <0.001 |
| Placenta accreta spectrum | ||
| No | 1.00 (reference) | |
| Yes | 3.07 (2.01–4.70) | <0.001 |
| Placenta abruption | ||
| No | 1.00 (reference) | |
| Yes | 3.21 (2.60–3.98) | <0.001 |
| Pre‐eclampsia | ||
| No | 1.00 (reference) | |
| Yes | 1.19 (1.07–1.32) | 0.001 |
| PROM | <0.001* | |
| No | 1.00 (reference) | |
| Preterm | 1.45 (1.25–1.69) | <0.001 |
| Term | 1.11 (0.97–1.26) | 0.136 |
| Multifetal gestation | ||
| No | 1.00 (reference) | |
| Yes | 1.36 (1.15–1.61) | <0.001 |
| Large for gestational age | ||
| No | 1.00 (reference) | |
| Yes | 2.55 (2.22–2.93) | <0.001 |
| Fetal growth restriction | ||
| No | 1.00 (reference) | |
| Yes | 1.43 (1.23–1.65) | <0.001 |
| Breech presentation | ||
| No | 1.00 (reference) | |
| Yes | 1.61 (1.44–1.80) | <0.001 |
Note: Multivariable analysis of pregnancy characteristics in the propensity score‐matched model. Parsimonious, conditional backward selection method was used with a final stopping rule of p < 0.05.
Abbreviations: aOR, adjusted‐odds ratio; CI, confidence interval; PROM, premature rupture of membrane.
Overall p‐value.
TABLE 3.
Sensitivity analysis for pregnancy characteristics.
| Uterine scar (−) | Myoma (−) | Uterine scar/myoma (−) | |
|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Placenta previa | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 12.4 (9.75–15.7) | 5.57 (4.57–6.78) | 15.4 (11.7–20.4) |
| Placenta accreta spectrum | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 13.9 (4.97–38.7) | 2.70 (1.63–4.49) | 13.2 (4.62–37.8) |
| Placenta abruption | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 4.96 (3.84–6.41) | 3.66 (2.89–4.65) | 5.83 (4.34–7.82) |
| Pre‐eclampsia | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 1.37 (1.20–1.56) | 1.39 (1.23–1.57) | 1.61 (1.38–1.87) |
| PROM | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Preterm | 1.66 (1.39–1.99) | 1.57 (1.32–1.88) | 1.80 (1.46–2.22) |
| Term | 1.20 (1.04–1.40) | 1.17 (1.02–1.35) | 1.28 (1.09–1.50) |
| Multifetal gestation | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 2.13 (1.73–2.62) | 1.29 (1.07–1.57) | 1.91 (1.49–2.43) |
| Large for gestational age | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 3.16 (2.68–3.74) | 2.48 (2.13–2.88) | 3.18 (2.65–3.82) |
| Fetal growth restriction | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 1.69 (1.42–2.01) | 1.61 (1.37–1.90) | 2.09 (1.72–2.53) |
| Breech presentation | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 2.29 (2.01–2.62) | 1.89 (1.66–2.14) | 2.87 (2.46–3.35) |
Note: Independent characteristics in the whole cohort analysis were tested in three restricted cohorts: no uterine scar, no uterine myoma or neither of the two diagnoses.
Abbreviations: aOR, adjusted‐odds ratio; CI, confidence interval; PROM, premature rupture of membranes.
In addition to the increased risk of placenta pathology, pregnant patients with adenomyosis were more likely to have pre‐eclampsia, preterm prelabor rupture of membranes, multifetal gestation, large for gestational age, intrauterine growth restriction, and breech presentation (Tables 2, 3 and Table S2).
3.4. Delivery complications related to adenomyosis
Pregnant patients with adenomyosis were more likely to undergo cesarean delivery compared to those without adenomyosis (aOR 7.72, 95% CI: 7.04–8.47; Table 4). Adenomyosis was also associated with modestly increased risk of labor dystocia (aOR 1.23, 95% CI: 1.08–1.40). In addition, adenomyosis was associated with nearly 50% increased risk of delivery at less than 32 weeks gestational age (aOR 1.48, 95% CI: 1.24–1.77).
TABLE 4.
Delivery characteristics in women with adenomyosis.
| Characteristic | aOR (95% CI) | p‐value |
|---|---|---|
| Gestational age (w) | <0.001* | |
| ≥37 | 1.00 (reference) | |
| 32–36 | 0.97 (0.86–1.09) | <0.603 |
| <32 | 1.48 (1.24–1.77) | <0.001 |
| Unknown | 1.58 (1.19–2.09) | 0.002 |
| Labor dystocia | ||
| No | 1.00 (reference) | |
| Yes | 1.23 (1.08–1.40) | 0.002 |
| Delivery mode | ||
| Vaginal | 1.00 (reference) | |
| Cesarean | 7.72 (7.04–8.47) | <0.001 |
| Hospital bed capacity | 0.679* | |
| Small | 1.00 (reference) | |
| Mid | 1.03 (0.93–1.14) | 0.551 |
| Large | 1.04 (0.95–1.14) | 0.379 |
| Hospital setting | <0.001* | |
| Rural | 1.30 (1.11–1.51) | <0.001 |
| Urban nonteaching | 1.00 (reference) | |
| Urban teaching | 1.35 (1.23–1.47) | <0.001 |
Note: Delivery characteristics in women with adenomyosis compared to those without adenomyosis, adjusting for the obstetric factors in Table 2.
Abbreviations: aOR, adjusted‐odds ratio; CI, confidence interval.
Overall p‐value.
Patients with adenomyosis were more likely to experience severe maternal morbidity (aOR 1.86, 95% CI: 1.59–2.16) compared to those without adenomyosis after controlling for pregnancy and obstetric factors between adenomyosis and adverse outcomes including placental pathology, pre‐eclampsia, gestational age at delivery, and cesarean delivery (Table 5). This association remained robust when examining only patients who did not require transfusion of blood products (aOR 2.00, 1.61–2.48) as well as in those who did not require blood products nor hysterectomy (aOR 2.13, 1.67–2.72). The association of adenomyosis and severe maternal morbidity also persisted in the sensitivity cohorts who lacked a prior uterine scar and/or uterine myomas (Table 6) as well as who did not have extra‐uterine endometriosis (Table S3).
TABLE 5.
Severe maternal morbidity in pregnancy with adenomyosis.
| Adenomyosis (−) | Adenomyosis (+) | aOR (95% CI) a | |
|---|---|---|---|
| Severe maternal morbidity (composite) | |||
| Any b | 2.2 | 6.3 | 1.86 (1.59–2.16)* |
| Except for blood transfusion | 1.1 | 3.4 | 2.00 (1.61–2.48)* |
| Except for blood transfusion/hysterectomy | 0.8 | 2.4 | 2.13 (1.67–2.72)* |
| Individual morbidity indicator c | |||
| Blood products transfusion | 1.5 | 3.9 | 1.73 (1.43–2.09)* |
| Hysterectomy | 0.3 | 1.4 | 2.59 (1.73–3.87)* |
| Coagulopathy | 0.2 | 1.3 | 4.46 (2.97–6.69)* |
| Acute renal failure | 0.3 | 0.5 | 0.95 (0.56–1.54) |
| Sepsis | 0.1 | 0.5 | 4.19 (2.19–8.04)* |
| Shock | 0.1 | 0.5 | 3.59 (1.84–7.03)* |
| Adult respiratory distress syndrome | 0.1 | 0.5 | 2.50 (1.37–4.57)* |
| Other outcome measures | |||
| Postpartum hemorrhage | 4.5 | 8.7 | 1.78 (1.58–2.00)* |
| Total charge ($) | 19 835 | 26 515 | ** |
| Hospital stay ≥7 days | 2.6 | 6.6 | 1.52 (1.29–1.78)* |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
Effect size of the exposure group (adenomyosis compared to nonadenomyosis) on outcome measures (severe maternal morbidity). The nonadenomyosis group served as the referent group. The exposure‐outcome association was adjusted for pregnancy factors (placenta previa, placenta accreta spectrum, placenta abruption, and pre‐eclampsia) and delivery factors (gestational age and cesarean delivery).
Included any one of the 21 indicators for severe maternal morbidity per the CDC definition.
Selected indicators are listed.
p < 0.05
p < 0.001 (Mann–Whitney U test).
TABLE 6.
Sensitivity analysis for maternal morbidity outcomes.
| Uterine scar (−) | Myoma (−) | Uterine scar/myoma (−) | |
|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Severe maternal morbidity (composite) | |||
| Any | 2.01 (1.60–2.52)* | 2.21 (1.85–2.66)* | 3.89 (2.84–5.32)* |
| Excluded blood transfusion | 1.96 (1.42–2.70)* | 1.98 (1.52–2.57)* | 3.26 (2.10–5.08)* |
| Excluded blood transfusion/hysterectomy | 2.23 (1.57–3.16)* | 2.34 (1.74–3.15)* | 3.61 (2.24–5.81)* |
| Individual morbidity indicator | |||
| Blood products transfusion | 1.68 (1.28–2.21)* | 2.26 (1.80–2.83)* | 3.50 (2.40–5.10)* |
| Hysterectomy | 2.56 (1.33–4.91)* | 1.88 (1.11–3.21)* | 6.78 (2.49–18.5)* |
| Coagulopathy | 1.91 (1.04–3.52)* | 5.69 (3.35–9.66)* | 2.46 (1.03–5.86)* |
| Acute renal failure | 1.36 (0.69–2.71) | 0.83 (0.45–1.51) | 3.24 (1.15–9.09)* |
| Sepsis | 4.52 (2.09–9.75)* | 3.37 (1.70–6.68)* | 3.04 (1.34–6.91)* |
| Shock | 2.67 (1.12–6.34)* | 3.13 (1.56–6.30)* | 2.26 (0.94–5.47) |
| Adult respiratory distress syndrome | 2.00 (0.88–4.54) | 1.97 (0.95–4.08) | 2.21 (0.73–6.71) |
| Other outcome measures | |||
| Postpartum hemorrhage | 1.52 (1.29–1.78)* | 1.65 (1.44–1.90)* | 1.53 (1.26–1.87)* |
| Hospital stay ≥7 days | 1.28 (1.05–1.57)* | 1.62 (1.33–1.96)* | 1.65 (1.27–2.15)* |
Note: The exposure‐outcome association was examined in the three restricted cohorts: (i) no prior uterine scar, (ii) no uterine myoma, and (iii) neither of the two diagnoses. Adjusting factors were identical to the analysis performed for the main cohort analysis: pregnancy factors (placenta previa, placenta accreta spectrum, placenta abruption, and pre‐eclampsia) and delivery factors (gestational age and cesarean delivery).
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
p < 0.05.
When examining individual indicators of severe maternal morbidity at the time of delivery, the effect size of adenomyosis was particularly high for coagulopathy (aOR 4.46, 95% CI: 2.97–6.69), sepsis (aOR 4.19, 95% CI: 2.19–8.04), shock (aOR 3.59, 95% CI: 1.84–7.03), hysterectomy (aOR 2.59, 95% CI: 1.73–3.87), and adult respiratory distress syndrome (aOR 2.50, 95% CI: 1.37–4.57) (Table 5). Finally, patients with a diagnosis of adenomyosis were more likely to require prolonged hospital admission for delivery compared to those without adenomyosis (≥7 days, aOR 1.52, 95% CI: 1.29–1.78).
4. DISCUSSION
In this nationwide assessment, adenomyosis was associated with significantly increased risk of several adverse obstetric outcomes, most notably placenta accreta, previa, and abruption, in addition to significant maternal morbidity at the time of delivery.
Major strengths of the current study include the examination of a large sample size of patients with adenomyosis, a comprehensive analysis of obstetric outcomes, and exhaustive sensitivity analyses. Propensity score matching also helped to minimize confounding variables, which often affect the results of studies with smaller sample sizes.
However, this study also has important limitations that warrant consideration. Most importantly, the diagnosis of adenomyosis is controversial and lacks consensus. 24 Due to the lack of actual medical record review, it is unclear if patients were diagnosed with adenomyosis, histopathologically, clinically based on symptomatology, or radiographically in this study. The severity of adenomyosis was also unknown, that is, whether adenomyosis was diffuse or focal, and there may be missed cases of asymptomatic adenomyosis in the control group resulting in possible misclassification. Prior treatments for adenomyosis were not also captured in this dataset, and it has been suggested that treatments such as GnRH‐agonists may increase the risk for placental pathology. 25 Prior uterine curettage was also not assessable due to lack of specific codes, but this could be a possible unmeasured confounder for abnormal placentation.
An increased risk for several adverse obstetric and delivery outcomes in patients with adenomyosis has been shown previously. 6 , 7 , 10 , 26 However, the results of current study regarding placental complications were particularly striking. While another study also showed that placental previa and low‐lying placenta were nearly five times more common in patients with adenomyosis, our study also reports on the increased risk of placenta accreta spectrum, which is not only highly morbid for the index pregnancy but also compromises a woman's future reproductive potential. 7
The increased risk of placental pathology in patients with adenomyosis is likely mediated by alterations at the endometrial‐myometrial interface, also called the junctional zone, which is abnormally thickened and asymmetric in adenomyotic uteri. 2 , 27 The junctional zone plays a critical role in sperm transport, implantation, and angiogenesis. Thus, aberrations in the junctional zone may lead to altered endometrial receptivity and defective trophoblast invasion or migration. Indeed, one prior study found that odds of pregnancy were inversely proportional to the junctional zone thickness. 28
On a molecular level, abnormal levels of endometrial growth factors, cytokines, and adhesion molecules in adenomyotic uteri may decrease chances of implantation and negatively impact placentation. 29 , 30 In particular, the HOX family of homeobox transcription factors have been implicated in endometrial receptivity and embryo implantation in humans. 31 In one study, HOX10 levels were significantly lower in patients with adenomyosis compared to controls. 29
Physical microtrauma at the junctional zone may also be responsible for the increased risk of placental pathology in patients with adenomyosis. In a mouse model, adenomyosis was induced following iatrogenic mechanical or thermal trauma to the endometrial‐myometrial interface. 32 , 33 Placenta accreta spectrum is strongly associated with decidual defects and uterine scarring from prior cesarean section, myomectomy, or other uterine procedures. 34 It is unclear whether microtrauma may cause adenomyosis or vice versa; however, this interplay may explain the excess risk of placenta accreta spectrum in patients with adenomyosis as well as the rare occurrence of placenta accreta spectrum in nulliparous patients or those without a history of a true uterine scar. 33 , 34 , 35 , 36
Conception with ART is also associated with an increased risk of placental pathology during the ensuing pregnancy. 37 The mechanism for this association is not clear, but it is tempting to speculate that adenomyosis may be more prevalent among patients undergoing in vitro fertilization due to its association with subfertility. Therefore, it is possible that our results may partly explain the observed association between ART and placental pathology. This topic, however, merits further investigation and was beyond the scope of the current study.
Higher morbidity at the time of delivery in patients with adenomyosis may be multifactorial. Surely, placental pathologies, which often necessitate cesarean section and increase risk of bleeding, may contribute to complications. However, adenomyosis may also lead to altered uterine contractility and hemostatic mechanisms independent of the placenta. 38 , 39 One study found an increased concentration of potassium channels in adenomyotic myometrium compared to controls, which may promote excessive uterine smooth muscle relaxation. 40 This may increase the risk of labor dystocia leading to cesarean section as well as uterine atony and postpartum hemorrhage following delivery.
Postpartum hemorrhage may also be increased in patients with adenomyosis due to the same mechanisms that underly the heavy uterine bleeding with adenomyosis in the nonpregnant state. In adenomyosis, there is a proangiogenic environment with elevated levels of vascular endothelial growth factor, tissue factor, and endothelial nitric oxide synthase, leading to higher microvessel density and surface area of capillaries. 33 This may also lead to abnormal activation of the coagulation pathway, with a predisposition to disseminated intravascular coagulation as was observed in this study. 33 , 41 , 42 , 43
5. CONCLUSION
Pregnant patients with adenomyosis may be considered a high‐risk population due to excess risk of obstetric and delivery complications. This data can be helpful in the preconception counseling of patients with adenomyosis prior to pregnancy as well as antepartum management during pregnancy.
AUTHOR CONTRIBUTIONS
RSM generated the study concept, initiated the collaborations, accessed the data source, generated/cleaned the dataset, interpreted the results and drafted the manuscript. SJFM, CJV, JZG, KAD, SM, and MMQ contributed to the study discussion and intellectual inputs, performed literature review, interpreted the results, and edited the manuscript. JGO and RJP interpreted the results, supervised the study team, and revised the manuscript. KM designed the study, initiated the collaborations, cleaned and analyzed the data, created the figures and tables, interpreted the results, and drafted and revised the manuscript with others. He is the corresponding author of the study.
FUNDING INFORMATION
Ensign Endowment for Gynecologic Cancer Research (K.M.).
CONFLICT OF INTEREST STATEMENT
Consultant, Ferring (R.J.P.), research grant, Merck (S.M.); Rest of authors declare no conflicts of interest.
Supporting information
Table S1.
Table S2.
Table S3.
Mandelbaum RS, Melville SJF, Violette CJ, et al. The association between uterine adenomyosis and adverse obstetric outcomes: A propensity score‐matched analysis. Acta Obstet Gynecol Scand. 2023;102:833‐842. doi: 10.1111/aogs.14581
Rachel S. Mandelbaum and Koji Matsuo contributed equally.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality at https://www.hcup‐us.ahrq.gov/nassoverview.jsp (reference number 11).
REFERENCES
- 1. Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril. 2012;98:572‐579. [DOI] [PubMed] [Google Scholar]
- 2. Chapron C, Vannuccini S, Santulli P, et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update. 2020;26:392‐411. [DOI] [PubMed] [Google Scholar]
- 3. Dueholm M, Lundorf E, Hansen ES, Sørensen JS, Ledertoug S, Olesen F. Magnetic resonance imaging and transvaginal ultrasonography for the diagnosis of adenomyosis. Fertil Steril. 2001;76:588‐594. [DOI] [PubMed] [Google Scholar]
- 4. Upson K, Missmer SA. Epidemiology of adenomyosis. Semin Reprod Med. 2020;38:89‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu O, Schulze‐Rath R, Grafton J, Hansen K, Scholes D, Reed SD. Adenomyosis incidence, prevalence and treatment: United States population‐based study 2006‐2015. Am J Obstet Gynecol. 2020;223:94.e91‐94.e10. [DOI] [PubMed] [Google Scholar]
- 6. Sharma S, Bathwal S, Agarwal N, Chattopadhyay R, Saha I, Chakravarty B. Does presence of adenomyosis affect reproductive outcome in IVF cycles? A retrospective analysis of 973 patients. Reprod Biomed Online. 2019;38:13‐21. [DOI] [PubMed] [Google Scholar]
- 7. Hashimoto A, Iriyama T, Sayama S, et al. Adenomyosis and adverse perinatal outcomes: increased risk of second trimester miscarriage, preeclampsia, and placental malposition. J Matern Fetal Neonatal Med. 2018;31:364‐369. [DOI] [PubMed] [Google Scholar]
- 8. Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a meta‐analysis. Fertil Steril. 2017;108:483‐490.e483. [DOI] [PubMed] [Google Scholar]
- 9. Vercellini P, Consonni D, Dridi D, Bracco B, Frattaruolo MP, Somigliana E. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta‐analysis. Hum Reprod. 2014;29:964‐977. [DOI] [PubMed] [Google Scholar]
- 10. Nirgianakis K, Kalaitzopoulos DR, Schwartz ASK, et al. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: a systematic review and meta‐analysis. Reprod Biomed Online. 2021;42:185‐206. [DOI] [PubMed] [Google Scholar]
- 11. Overview of the National (Nationwide) Inpatient Sample (NIS) . Agency for Healthcare Research and Quality. Accessed March 6, 2022. https://www.hcup‐us.ahrq.gov/nisoverview.jsp
- 12. Logue TC, Wen T, Monk C, et al. Trends in and complications associated with mental health condition diagnoses during delivery hospitalizations. Am J Obstet Gynecol. 2022;226:405.e401‐405.e416. [DOI] [PubMed] [Google Scholar]
- 13. Youssefzadeh AC, Tavakoli A, Panchal VR, Mandelbaum RS, Ouzounian JG, Matsuo K. Incidence trends of shoulder dystocia and associated risk factors: a nationwide analysis in the United States. Int J Gynaecol Obstet. 2023. doi: 10.1002/ijgo.14699 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14. Matsuzaki S, Mandelbaum RS, Sangara RN, et al. Trends, characteristics, and outcomes of placenta accreta spectrum: a national study in the United States. Am J Obstet Gynecol. 2021;225:534.e531‐534.e538. [DOI] [PubMed] [Google Scholar]
- 15. Severe maternal morbidity in the United States . Centers for Disease Control and Prevention. Accessed March 6, 2022. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html
- 16. Austin PC. An Introduction to propensity score Methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hitti J, Sienas L, Walker S, Benedetti TJ, Easterling T. Contribution of hypertension to severe maternal morbidity. Am J Obstet Gynecol. 2018;219(4):405.e1‐405.e7. [DOI] [PubMed] [Google Scholar]
- 19. Ananth CV, Lavery JA, Vintzileos AM, et al. Severe placental abruption: clinical definition and associations with maternal complications. Am J Obstet Gynecol. 2016;214:272.e1‐272.e9. [DOI] [PubMed] [Google Scholar]
- 20. Kilpatrick SJ, Abreo A, Gould J, Greene N, Main EK. Confirmed severe maternal morbidity is associated with high rate of preterm delivery. Am J Obstet Gynecol. 2016;215(233):e231‐e237. [DOI] [PubMed] [Google Scholar]
- 21. Silver RM. Abnormal placentation: placenta previa, vasa previa, and placenta accreta. Obstet Gynecol. 2015;126:654‐668. [DOI] [PubMed] [Google Scholar]
- 22. Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107:1226‐1232. [DOI] [PubMed] [Google Scholar]
- 23. Ghaferi AA, Schwartz TA, Pawlik TM. STROBE reporting guidelines for observational studies. JAMA Surg. 2021;156:577‐578. [DOI] [PubMed] [Google Scholar]
- 24. Gordts S, Brosens JJ, Fusi L, Benagiano G, Brosens I. Uterine adenomyosis: a need for uniform terminology and consensus classification. Reprod Biomed Online. 2008;17:244‐248. [DOI] [PubMed] [Google Scholar]
- 25. Agrawala S, Patil J, Campbell S, Woodard TL. A rare case of extensive placenta accreta in twin pregnancy after GnRH agonist treatment of adenomyosis. Fertil Res Pract. 2021;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shinohara S, Okuda Y, Hirata S, Suzuki K. Adenomyosis as a potential risk factor for adverse pregnancy outcomes: a multicenter case‐control study. Tohoku J Exp Med. 2020;251:231‐239. [DOI] [PubMed] [Google Scholar]
- 27. Tanos V, Lingwood L, Balami S. The importance of the junctional zone of the endometrium in human reproduction. Hum Fertil (Camb). 2022;25:4‐12. [DOI] [PubMed] [Google Scholar]
- 28. Maged AM, Ramzy AM, Ghar MA, et al. 3D ultrasound assessment of endometrial junctional zone anatomy as a predictor of the outcome of ICSI cycles. Eur J Obstet Gynecol Reprod Biol. 2017;212:160‐165. [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Huang C, Jiang R, et al. Decreased endometrial IL‐10 impairs endometrial receptivity by downregulating HOXA10 expression in women with adenomyosis. Biomed Res Int. 2018;2018:2549789‐2549789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhihong N, Yun F, Pinggui Z, Sulian Z, Zhang A. Cytokine profiling in the Eutopic endometrium of adenomyosis during the implantation window after ovarian stimulation. Reprod Sci. 2016;23:124‐133. [DOI] [PubMed] [Google Scholar]
- 31. Xu B, Geerts D, Bu Z, et al. Regulation of endometrial receptivity by the highly expressed HOXA9, HOXA11 and HOXD10 HOX‐class homeobox genes. Hum Reprod. 2014;29:781‐790. [DOI] [PubMed] [Google Scholar]
- 32. Hao M, Liu X, Guo SW. Adenomyosis in mice resulting from mechanically or thermally induced endometrial‐myometrial interface disruption and its possible prevention. Reprod Biomed Online. 2020;41:925‐942. [DOI] [PubMed] [Google Scholar]
- 33. Zhai J, Vannuccini S, Petraglia F, Giudice LC. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. 2020;38:129‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cahill AG, Beigi R, Heine RP, Silver RM, Wax JR. Placenta Accreta Spectrum. Am J Obstet Gynecol. 2018;219:B2‐b16. [DOI] [PubMed] [Google Scholar]
- 35. Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence‐based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75‐87. [DOI] [PubMed] [Google Scholar]
- 36. Li N, Hou R, Liu C, Yang T, Qiao C, Wei J. Integration of transcriptome and proteome profiles in placenta accreta reveals trophoblast over‐migration as the underlying pathogenesis. Clin Proteomics. 2021;18:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sacha CR, Mortimer RM, James K, et al. Placental pathology of term singleton live births conceived with fresh embryo transfer compared with those conceived without assisted reproductive technology. Fertil Steril. 2022;117:758‐768. [DOI] [PubMed] [Google Scholar]
- 38. Brosens I, Derwig I, Brosens J, Fusi L, Benagiano G, Pijnenborg R. The enigmatic uterine junctional zone: the missing link between reproductive disorders and major obstetrical disorders? Hum Reprod. 2010;25:569‐574. [DOI] [PubMed] [Google Scholar]
- 39. Ono Y, Ota H, Takimoto K, et al. Perinatal outcomes associated with the positional relationship between the placenta and the adenomyosis lesion. J Gynecol Obstet Hum Reprod. 2021;50:102114. [DOI] [PubMed] [Google Scholar]
- 40. Shi JH, Jin L, Leng JH, Lang JH. Expression of potassium channels in uterine smooth muscle cells from patients with adenomyosis. Chin Med J (Engl). 2016;129:200‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lockwood CJ, Paidas M, Murk WK, et al. Involvement of human decidual cell‐expressed tissue factor in uterine hemostasis and abruption. Thromb Res. 2009;124:516‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu X, Nie J, Guo SW. Elevated immunoreactivity to tissue factor and its association with dysmenorrhea severity and the amount of menses in adenomyosis. Hum Reprod. 2011;26:337‐345. [DOI] [PubMed] [Google Scholar]
- 43. Erez O, Othman M, Rabinovich A, Leron E, Gotsch F, Thachil J. DIC in pregnancy–pathophysiology, clinical characteristics, diagnostic scores, and treatments. J Blood Med. 2022;13:21‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S3.
Data Availability Statement
The data that support the findings of this study are openly available in Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality at https://www.hcup‐us.ahrq.gov/nassoverview.jsp (reference number 11).


