Abstract
Introduction
The primary aim of the study was to identify risk factors associated with fetal or neonatal loss, neonatal morbidity, and the need for surgery in fetuses diagnosed with an abdominal cyst. The secondary aim was to compare the characteristics of the cyst according to trimester at diagnosis.
Material and methods
This was an observational retrospective study performed at Vall d'Hebron University Hospital. The study included pregnant women aged 18 years or older with diagnosis of a fetal abdominal cyst from 2008 to 2021.
Results
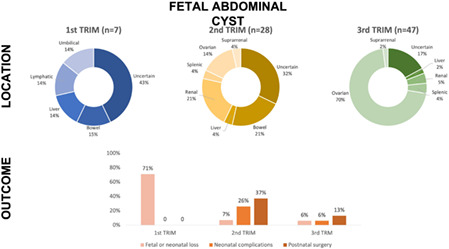
A total of 82 women with a median gestational age of 31+1 weeks (12+0–39+4) were included in the analysis. Seven (8.5%) cases were diagnosed in the first trimester, 28 (34.1%) in the second trimester, and 47 (57.3%) in the third trimester. Fetal or neonatal loss occurred in 10 (12.2%) cases; significant predictors were diagnosis in the first trimester (OR 36.67, 95% CI: 4.89–274.79), male gender (OR 4.75, 95% CI: 1.13–19.9), and associated abnormalities (OR 15.2, 95% CI: 2.92–79.19). A total of 10 of 75 (13.3%) neonates showed at least one neonatal complication, and the only predictor was occurrence of associated abnormalities (OR 7.36, 95% CI: 1.78–30.51). A total of 16 of 75 (21.3%) neonates required postnatal surgery, and the predictors were second‐trimester diagnosis (OR 3.92, 95% CI: 1.23–12.51), associated abnormalities (OR 3.81, 95% CI: 1.15–12.64), and bowel location (OR 10.0, 95% CI: 1.48–67.55).
Conclusions
Factors associated with adverse outcomes in fetuses diagnosed with abdominal cysts are first‐trimester diagnosis and associated abnormalities. Cysts detected in the second trimester and those of intestinal origin are more likely to require surgery.
Keywords: abdominal, cyst, outcome, perinatal, prenatal, ultrasound
Factors associated with adverse outcomes in fetuses diagnosed with abdominal cysts are first‐trimester diagnosis and associated abnormalities. Cysts detected in the second trimester and those of intestinal origin are more likely to require surgery.
Abbreviations
- CI
confidence interval
- MRI
magnetic resonance imaging
- OR
odds ratio
Key message.
First‐ and second‐trimester abdominal cysts are more likely to be associated with other abnormalities and are more likely to result in an adverse perinatal outcome. Cysts diagnosed in the third trimester are more likely to resolve without surgery.
1. INTRODUCTION
Intra‐abdominal cysts are reported as a relative common ultrasound finding during pregnancy, mostly in the second and third trimesters, with a prevalence of 1 in 1000 pregnancies, 1 although cases diagnosed in the first trimester have also been reported. 2 , 3 , 4 During fetal development, intra‐abdominal fetal cysts present as a single anechogenic mass with no vascularization. 1 Intra‐abdominal cysts are usually classified according to fetal gender, gestational age at the time of diagnosis and anatomical location. 1 , 2 , 3 , 5 , 6 Cystic formations are generally found on the ovaries, 6 , 7 gastrointestinal tract, 5 , 7 liver, and mesentery or urinary tract. 2 , 3 , 6 , 7 , 8 Of the abdominal abnormalities detected in female fetuses, ovarian cysts are the most common. 6 , 7 For this reason, gastrointestinal, 9 renal and genital abnormalities in female fetuses are often misdiagnosed as ovarian cysts. 6 Ultrasound features of the cyst, association with other abnormalities, its location, and its evolution during pregnancy may help to establish the anatomical and functional origin of the cyst, and to predict its postnatal outcome and prognosis. 1 , 2 , 3 , 4 , 5 , 6 , 8 , 9 , 10 , 11 , 12 , 13
The main concerns for parents and physicians providing medical care for these women are the chances of adverse perinatal outcomes, such as fetal or neonatal loss, neonatal complications, and the need for surgical treatment. 2 Thus, providing information about fetal and neonatal prognosis is key for a good management of these patients. 1 , 2 , 3 , 4
The primary aim of this study was to identify predictors of fetal or neonatal loss, neonatal morbidity, and the need for surgery in fetuses diagnosed with an abdominal cyst. The secondary aim was to compare the characteristics of the cyst according to trimester at the time of diagnosis.
2. MATERIAL AND METHODS
This was an observational retrospective study performed at the Fetal Medicine Unit in Vall d'Hebron Hospital Campus, a tertiary‐care site in Barcelona, Spain, between 2008 and 2021. The study included pregnant women ≥18 years of age with a diagnosis of a fetal abdominal cyst.
2.1. Clinical protocol
In Catalonia, three ultrasound scans are routinely performed during a normal pregnancy: the first scan is performed between 11 and 13 weeks, the second scan is performed between 19 and 22 weeks, and the third scan is performed between 34 and 36 weeks. Fetal anatomy is assessed in all three ultrasound scans, which are performed by obstetricians. When a fetal abnormality is detected, pregnant women are referred to the Fetal Medicine Unit at one of the referral hospitals. At Vall d'Hebron Hospital's Fetal Medicine Unit, when a fetus having a fetal abnormality is referred, a detailed ultrasound examination is conducted by a Fetal Medicine consultant. In cases with an abdominal cyst, ultrasound features of the cyst, fetal growth and Doppler parameters are recorded, and an amniocentesis is offered for genetic testing. The policy in our site has evolved during the study period. In the first few years, a quantitative fluorescence polymerase chain reaction (QF‐PCR) and karyotyping were routinely performed, and array comparative genomic hybridization (array‐CGH) was offered only to selected cases of invasive testing. However, since 2017, a QF‐PCR and array‐CGH were performed in all cases of invasive testing.
Prenatal magnetic resonance imaging (MRI) was offered when ultrasound diagnosis was inconclusive or to investigate associated abnormalities.
A prenatal counseling visit was routinely scheduled with the neonatal surgery team. Pregnancies were followed up every 4 weeks at the Fetal Medicine Unit. Delivery was performed according to standard obstetric care. A postmortem examination was offered to cases of stillbirth or termination of pregnancy.
After birth, an abdominal ultrasound was performed to assess the abdominal cyst. Pregnancy outcomes, which were obtained from the maternal medical records, were available for those cases with delivery in a public hospital. Neonatal outcomes were only available for neonates born in our hospital.
All data obtained at the ultrasound examination were recorded using the Viewpoint software, and all data were subsequently exported into a database. For the study, a query was run, and data for all cases with a diagnosis of an abdominal cyst during the study period were extracted.
2.2. Outcome measures
Primary outcomes were fetal or neonatal loss, neonatal morbidity, and the need for surgery within the first 6 months of life. Fetal or neonatal loss included intrauterine death, termination of pregnancy, neonatal death and infant death. Neonatal morbidity included admission to the neonatal intensive care unit, bronchopulmonary dysplasia, sepsis, anemia requiring transfusion, necrotizing enterocolitis, and retinopathy of prematurity.
Predictors of the primary outcomes included: anatomical location of the cyst, size of the cyst >40 mm, number of cysts, trimester at diagnosis, associated abnormalities, gender, and in utero resolution.
2.3. Statistical analyses
Categorical variables were reported as absolute and relative frequencies (percentages), and quantitative variables as the median and range. Univariate logistic regression analysis was used to study factors associated with fetal and neonatal loss, neonatal morbidity, and the need for surgical treatment.
The chi‐squared or Fisher's exact tests were used to compare categorical variables across trimesters and the Kruskal–Wallis test was used for continuous variables.
The kappa index (κ) with its 95% confidence interval (CI) was used to evaluate the agreement between pre‐ and postnatal anatomical location of the cyst. A κ value of 0–0.20 was considered a slight agreement, a value of 0.21–0.40 was considered a fair agreement, a value of 0.41–0.60 was considered a moderate agreement, a value of 0.61–0.80 was considered a substantial agreement, and a value of 0.81–1.00 was considered an almost perfect agreement. 16
All statistical analyses were performed with the R Software, version 4.0.5. The two‐sided statistical significance level was set at p < 0.05.
2.4. Ethics statement
The Ethics Committee, Comité de Ética de Investigación con Medicamentos y Comisión de Proyectos de Investigación del Hospital Universitari Vall d'Hebron (CEIm‐VHIR), approved the study (PR[AMI] 476‐2019) on November 29, 2019.
3. RESULTS
During the study period, 98 fetuses with intra‐abdominal cysts were identified. A total of 16 (16.3%) cases were lost to follow‐up and were excluded. Thus, 82 cases were included in the analysis. The ethics committee approved a waiver of the requirement to obtain informed consent.
3.1. Demographic characteristics
The median maternal age was 32.1 years (range 19.7–44.9), the median body mass index was 24.6 kg/m2 (range 16.9–42.4), 73 (89.0%) women were Caucasian, two (2.4%) were African, three (3.7%) were Asian, and four (4.9%) were of mixed race. A total of 79 (96.3%) women conceived spontaneously, and three (3.7%) conceived by assisted reproduction techniques. A total of 10 (12.2%) women reported smoking and one (1.2%) woman reported alcohol consumption during pregnancy. A total of 79 (96.3%) cases were singleton pregnancies, and three (3.2%) cases were twin pregnancies.
3.2. Gestational age and cyst characteristics
The median gestational age at diagnosis was 31+1 weeks (range 12+0–39+4). Seven (8.5%) cases were diagnosed in the first trimester, 28 (34.1%) cases were diagnosed in the second trimester and 47 (57.3%) cases were diagnosed in the third trimester. Abdominal cyst characteristics according to trimester at diagnosis are shown in Table 1. In the first trimester, the male gender was more prevalent (57.1%), while in the third trimester 93.6% were female fetuses. A cyst size >40 mm was more frequent in the third trimester, associated abnormalities were more frequent in first trimester diagnosed cysts, and the anatomical location differed according to trimester at diagnosis, more frequently being of an uncertain location in the first and second trimesters, and more frequently being located in the ovaries in the third trimester. Associated abnormalities were found in 23 (28.0%) cases. Details about associated abnormalities for each trimester are shown in Table 1.
TABLE 1.
Abdominal cyst characteristics according to trimester at diagnosis.
| First trimester (n = 7) | Second trimester (n = 28) | Third trimester (n = 47) | p‐value | |
|---|---|---|---|---|
| Gender | <0.001 | |||
| Female | 2 (28.6%) | 15 (53.6%) | 44 (93.6%) | |
| Male | 4 (57.1%) | 13 (46.4%) | 3 (6.4%) | |
| Not determined | 1 (14.3%) | 0 | 0 | |
| Cyst size >40 mm | 1 (14.3%) | 3 (10.7%) | 19 (40.4%) | 0.011 |
| Associated abnormalities | 5 (71.4%) | 9 (32.1%) | 9 (19.1%) | 0.016 |
| Number of cysts | 0.462 | |||
| One cyst | 7 (100%) | 26 (96.3%) | 43 (95.6%) | |
| Two cysts | 0 | 0 | 2 (4.4%) | |
| Three cysts | 0 | 1 (3.7%) | 0 | |
| In utero resolution | 1 (16.7%) | 4 (16.7%) | 7 (31.8%) | 0.598 |
| Anatomical location | <0.001 | |||
| Ovarian | 0 | 4 (10.7%) | 33 (70.2%) | |
| Renal | 0 | 6 (21.4%) | 2 (4.3%) | |
| Intestinal | 1 (14.3%) | 6 (21.4%) | 0 | |
| Hepatic | 1 (14.3%) | 1 (3.6%) | 1 (2.1%) | |
| Suprarenal | 0 | 1 (3.6%) | 1 (2.1%) | |
| Mesenteric | 0 | 1 (3.6%) | 0 | |
| Lymphatic | 1 (14.3%) | 0 | 0 | |
| Umbilical | 1 (14.3%) | 0 | 0 | |
| Splenic | 0 | 1 (3.6%) | 2 (4.3%) | |
| Uncertain | 3 (42.9%) | 9 (32.1%) | 8 (17.0%) |
Note: Data are reported as absolute and relative (percentage) values. Comparison amongst groups was performed by the chi‐squared test or Fisher's exact test, as appropriate.
An invasive test was performed in 22 cases. A chorionic villous sampling was performed in two fetuses with an abdominal cyst, detected at 13 and 24 weeks of gestation, respectively. In the latter case, the indication for the invasive test was not the abdominal cyst. In 20 cases, an amniocentesis was performed: five had been diagnosed in the first trimester, 12 had been diagnosed in the second trimester, and three had been diagnosed in the third trimester. No chromosomal abnormalities were found in 12 microarray studies and 16 karyotypes (in six cases, both karyotyping and microarray analysis were performed).
An MRI was performed in 10 cases. In four cases, the primary indication for the MRI were associated abnormalities (brain abnormalities in three cases and pulmonary sequestration in one case) and in six cases the MRI was indicated to identify the cyst location. In eight cases, there was an agreement between the ultrasound‐based location and the MRI‐based location of the cyst (three ovarian, two renal, one suprarenal, one intestinal, one uncertain location). In two cases, the MRI did not provide information about cyst location.
3.3. Primary outcomes
During pregnancy, four cases opted for termination of pregnancy and three resulted in stillbirths. A total of 75 (91.4%) pregnancies resulted in live births, from which one resulted in neonatal death and two in infant deaths.
Thus, fetal or neonatal loss occurred in 10 (12.2%) cases, including four terminations of pregnancy, three stillbirths, one neonatal death and two infant deaths. Five (5/7, 71.4%) cases diagnosed during the first trimester, two (2/28, 7.1%) cases diagnosed in the second trimester and three (3/47, 6.4%) cases diagnosed in the third trimester resulted in termination of pregnancy or perinatal death (p < 0.001). Univariate logistic regression analysis showed that significant predictors for fetal or neonatal loss were: first‐trimester diagnosis, male gender, and associated abnormalities (Table 2).
TABLE 2.
Univariate logistic regression analysis to predict fetal or neonatal loss, neonatal morbidity and need for surgical treatment.
| Univariate analysis | Fetal or neonatal loss | Neonatal morbidity | Surgical treatment | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p‐value | OR (95% CI) | p‐value | OR (95% CI) | p‐value | |
| Trimester at diagnosis | <0.001 | 0.055 | 0.038 | |||
| First trimester | 36.67 (4.89–274.79) | 0 (0, Inf) | 0 (0–Inf) | |||
| Second trimester | 1.13 (0.18–7.2) | 5.02 (1.17–21.45) | 3.92 (1.23–12.51) | |||
| Third trimester | Reference | Reference | Reference | |||
| Male gender | 4.75 (1.13–19.9) | 0.012 | 1.89 (0.43–8.4) | 0.416 | 2.23 (0.63–7.83) | 0.223 |
| Cyst size >40 mm | 1.86 (0.47–7.31) | 0.384 | 0.61 (0.12–3.12) | 0.533 | 2.5 (0.79–7.94) | 0.125 |
| Associated abnormalities | 15.2 (2.92–79.19) | <0.001 | 7.36 (1.78–30.51) | 0.005 | 3.81 (1.15–12.64) | 0.031 |
| Number of cysts | 0.661 | 0.632 | 0.134 | |||
| One cyst | Reference | Reference | Reference | |||
| Two cysts | 0 (0–Inf) | 0 (0–Inf) | 0 (0, Inf) | |||
| Three cysts | 0 (0–Inf) | 0 (0–Inf) | 56 344 898.83 (0–Inf) | |||
| In utero resolution | 0.5 (0.05–4.62) | 0.699 | 0.77 (0.14–4.36) | 0.771 | 0.19 (0.02–1.66) | 0.156 |
| Anatomical location | 0.023 | 0.25 | 0.254 | |||
| Ovarian | Reference | Reference | Reference | |||
| Intestinal | 142 422 577.12 (0–Inf) | 11 (1.5–80.43) | 10 (1.48–67.55) | |||
| Renal | 122 076 494.67 (0–Inf) | 0 (0–Inf) | 0.83 (0.08–8.24) | |||
| Suprarenal | 1 (0, Inf) | 0 (0–Inf) | 0 (0–Inf) | |||
| Hepatic | 427 267 731.36 (0–Inf) | 0 (0–Inf) | 0 (0–Inf) | |||
| Mesenteric | 1 (0–Inf) | 0 (0–Inf) | 0 (0–Inf) | |||
| Splenic | 1 (0–Inf) | 0 (0–Inf) | 0 (0–Inf) | |||
| Lymphatic | 730 230 830 059 592 064 (0–Inf) | 0 (0–Inf) | ||||
| Uncertain | 366 229 484.02 (0–Inf) | 3.38 (0.66–17.25) | 2.08 (0.53–8.14) | |||
Note: Neonatal morbidity: composite outcome of respiratory distress syndrome, phototherapy, intraventricular hemorrhage, neonatal anemia, necrotizing enterocolitis, retinopathy, sepsis, admission to the neonatal intensive care unit.
Abbreviations: CI, confidence interval; Inf, infinity; OR, odds ratio.
A total of 10 (10/75, 13.3%) neonates showed at least one neonatal complication: none of the two were diagnosed in the first trimester, seven (7/27, 25.9%) were diagnosed in the second trimester, and three (3/46, 6.5%) were diagnosed in the third trimester. Univariate logistic regression analysis showed that the only significant predictor associated with neonatal morbidity was the presence of associated abnormalities (Table 2).
Details of cases with adverse outcomes are shown in Table 3.
TABLE 3.
Description of cases with adverse pregnancy outcomes.
| Number | Outcome | Trimester and weeks of gestation at diagnosis | Anatomical location according to the fetal ultrasound | Associated abnormalities | Postmortem examination/postnatal clinical findings |
|---|---|---|---|---|---|
| 1 | Termination of pregnancy | First, 12 | Intestinal | Bilateral renal agenesis | Bilateral cystic dysplastic kidneys, pulmonary hypoplasia, rectal atresia, small ventricular septal defect |
| 2 | Termination of pregnancy | First, 13 | Liver | Bilateral polycystic kidneys | Renal–hepatic–pancreatic dysplasia |
| 3 | Termination of pregnancy | First, 13 | Lymphatic | Hydrops at 15 weeks | Massive hydrothorax, lymphedema |
| 4 | Termination of pregnancy | Second, 20 | Uncertain | Genital mass and subcutaneous tumors | Multiple lymphangiomas in neck, thorax, abdomen and genitalia |
| 5 | Stillbirth at 33 weeks | First, 12 | Uncertain, spontaneous resolution of the cyst | Arthrogryposis, growth restriction and tetralogy of Fallot | Declined |
| 6 | Stillbirth at 19 weeks | First, 12 | Uncertain | None | Anorectal atresia |
| 7 | Stillbirth at 36 weeks | Third, 28 | Renal | Brain abnormal sulcation | Declined |
| 8 | Live birth at 37 weeks, neonatal death at 2 days | Third, 34 | Uncertain | Hepatomegaly | Intestinal necrosis and meconium peritonitis |
| 9 | Live birth at 27 weeks, infant death at 46 days | Second, 24 | Uncertain | Isolated | Intestinal subocclusion, meconium peritonitis |
| 10 | Live birth at 39 weeks, infant death at 10 months | Third, 31 | Uncertain | Hypoplastic right heart, severe pulmonary stenosis, ectrodactyly | Abdominal cyst of uncertain location confirmed at 2 months of life. No further follow‐up of the cyst |
3.4. Postnatal surgery
A total of 16 (16/75, 21.3%) neonates required postnatal surgery: none (0/2, 0%) were diagnosed in the first trimester, 10 (10/27, 37%) were diagnosed in the second trimester, and six (6/46, 13%) were diagnosed in the third trimester. Univariate logistic regression analysis showed that significant predictors for the need of postnatal surgery were second‐trimester diagnosis of the cyst, associated abnormalities, and intestinal location of the cyst (Table 2). Details about cases that underwent surgery are shown in Table 4.
TABLE 4.
Description of cases that underwent postnatal surgery.
| Case number | Gestational age at diagnosis (weeks) | Location | Age at surgery | Indication |
|---|---|---|---|---|
| 1 | 21 | Bowel | 5 days | Meckel's diverticulum |
| 2 | 20 | Bowel | 2 days | Intestinal occlusion due to bowel atresia |
| 3 | 20 | Bowel | 1 day | Gastroschisis |
| 4 | 20 | Bowel | 14 months | Meckel's diverticulum |
| 5 | 24 | Bowel | 2 days | Intestinal occlusion due to bowel atresia |
| 6 | 20 | Bowel | 12 months | Transverse mesocolon cystic malformation |
| 7 | 21 | Bowel | 8 days | Intestinal occlusion due to bowel duplication |
| 8 | 23 | Bowel | 3 months | Duplication cyst |
| 9 | 24 | Kidney | 15 days | Urinoma enlargement |
| 10 | 21 | Kidney | 2 months | Recurrent pyelonephritis |
| 11 | 37 | Bowel | 14 months | Gastric duplication cyst |
| 12 | 31 | Bowel | 2 months | Pyloric stenosis + bowel perforation |
| 13 | 34 | Bowel | 0 days | Bowel perforation |
| 14 | 34 | Ovary | 21 months | Ovarian cyst enlargement |
| 15 | 34 | Ovary | 7 months | Ovarian cyst torsion |
| 16 | 34 | Ovary | 5 months | Ovarian cyst enlargement |
3.5. Regression of the cyst
A total of 12 (12/82, 14.6%) cases had a spontaneous intrauterine regression of the cyst: three in the first trimester, four in the second trimester, and five in the third trimester. Of these, 11 cases resulted in a live birth and one case resulted in an intrauterine death.
Amongst the 75 live births, 11 had a spontaneous intrauterine regression of the cyst. From the remaining 64, 17 had no neonatal follow‐up, as they were born in a different hospital. Amongst the 47 neonates with postnatal follow‐up, eight had had a regression of the cyst according to the first postnatal scan (16.7%).
3.6. Postnatal follow‐up
Amongst the seven cases diagnosed with a cyst during the first trimester, one case had an intrauterine regression of the cyst with no abnormalities at birth, two cases had an anorectal atresia (one of them showed an intrauterine regression of the cyst), one case had multiple lymphangiomas, one case had a hepatic cyst with renal–hepatic–pancreatic dysplasia, one case showed regression of the cyst, and one case had two small calcifications in the liver capsule.
Amongst the 28 cases in the second trimester, four had an intrauterine regression of the cyst and no neonatal scans were performed, and five did not have neonatal follow‐up information. Amongst the remaining 19 cases, three had a postnatal regression of the cyst and the distribution of the cyst anatomical location according to the neonatal abdominal ultrasound for the rest was as follows: seven were intestinal, five were renal, one was hepatic, one was lymphatic, one was splenic, and one was of uncertain location.
Amongst the 48 cases diagnosed with a cyst in the third trimester, four cases had an intrauterine regression of the cyst and no neonatal scans were performed, one case resulted in an intrauterine death without postmortem examination, and 12 cases did not have neonatal follow‐up information. Amongst the remaining 30 cases, six had a postnatal regression of the cyst and the distribution of the cyst anatomical location according to the neonatal abdominal ultrasound for the rest was as follows: 18 ovarian, three intestinal, two splenic, and one hepatic.
3.7. Pre‐ and postnatal diagnosis agreement
For the agreement study, we excluded 12 cases with intrauterine regression of the cyst, eight cases with neonatal regression of the cyst, 17 cases with no follow‐up, and one case with stillbirth and no postmortem examination. For the remaining 44 cases, the prenatal diagnosis of 33 cases (75%) was consistent with the postnatal diagnosis. There was also a substantial agreement between the pre‐ and postnatal anatomical location of the cyst (κ = 0.67, 95% CI: 0.52–0.82, p < 0.001).
4. DISCUSSION
We found that, in the event of diagnosis of a fetal abdominal cyst, the presence of associated abnormalities increases the risk of fetal or neonatal loss, neonatal complications, and the need for surgical treatment. Diagnosis of an abdominal cyst in the first trimester increases the risk of fetal or neonatal loss, whereas diagnosis in the second trimester increases the risk of neonatal morbidity and the need for surgery. Male gender is also associated with a higher risk of fetal and neonatal loss, while intestinal location is associated with a higher risk of need for surgery.
Our results showed that fetal abdominal cysts are more frequently diagnosed in the third trimester (57%) as a unilocular anechogenic mass in female fetuses in the ovaries. Cysts diagnosed in the first trimester are more frequent in male fetuses, and 71% of them present with other abnormalities. 4 Furthermore, in almost half of the cases, cyst location cannot be determined. We also found that all cases of termination of pregnancy in our cohort had associated abnormalities.
In our data, male gender appears as a factor associated with an increased risk of fetal or neonatal loss. However, this should be interpreted with caution, as it is most likely a confounding factor. According to data reported by larger studies by Bascietto et al., 2 Husen et al., 5 Ozyuncu et al., 12 Catania et al., 14 and Diguisto et al., 15 the most frequent abdominal cysts are of ovarian origin, generally found in the third trimester and exclusively detected in female fetuses. These cysts are usually treated conservatively and most of them resolve spontaneously within 6 months after birth. By contrast, cysts diagnosed in the first trimester are associated with a poorer prognosis and a greater need for surgical treatment, and in our case series, the proportion of male fetuses was slightly higher than that of female fetuses.
As for intestinal duplication cysts, which are more frequently diagnosed in the second trimester, treatment is surgical, their appearance is not related to gender, and they do not have a poor prognosis, but will require surgery. 7
For cysts diagnosed in the first trimester, it is more difficult to identify the location, although lymphatic or vascular origin is more frequent, which in turn may indicate an association with other pathologies. Interestingly, in two cases diagnosed with a cyst in the first trimester, anorectal atresia was diagnosed postnatally and at the postmortem examination, respectively.
In the first trimester, 71% of the fetuses had associated abnormalities. This might be the main reason for a higher rate of adverse outcomes in the first trimester, especially termination of pregnancy (three cases) and stillbirth (two cases).
According to Sanna et al., 1 the most common presentation of fetal abdominal cysts is an unilocular anechogenic mass with no vascularity, and are most frequently diagnosed in the third trimester, 1 , 2 , 5 , 7 which is consistent with our findings (97% were unilocular cysts, 29% were larger than 40 mm, and 59% were detected in the third trimester of pregnancy).
Lv et al. 13 reported that MRI increased the diagnostic rate to 65%. In our case, MRI helped to confirm the suspected cyst location for eight (80%) cases, although for two (20%) cases MRI was unable to provide accurate information about cyst location.
Gender distribution of fetal abdominal cysts changes throughout pregnancy: they are more frequent in male fetuses in the second trimester and in female fetuses in the third trimester, which is consistent with other larger studies, such as that by Ozkose et al. 7
Husen et al. 5 showed cyst regression in the third trimester for 67.9% of cases, which is higher than the figures reported by Bascietto et al. 2 (53.8%) or Sanna et al. (56%), and significantly higher than the figures reported in our study (14.6%). This can be due to a higher proportion of ovarian cysts, which have a more favorable prognosis. Nevertheless, we found that this proportion can be quite variable, as we see in other studies, such as that conducted by Ozkose et al., 7 which reports an intrauterine resolution of the cysts in 16.8% of cases, similar to the percentage reported in our study.
According to Khalil et al. 3 and Erculiani et al., 9 abdominal cysts detected in the first trimester are more frequent in male fetuses and more frequently associated with anorectal malformations, even if the cysts resolved during pregnancy as shown by Sepulveda et al., 4 which is consistent with our findings (two out of eight fetuses were diagnosed in the first trimester). Overall prognosis of these fetuses is poorer than that of those diagnosed in the third trimester, 3 especially if there are other associated abnormalities, 9 which is consistent with our findings (first‐trimester diagnosis, male fetuses, and association with other abnormalities as markers of poor prognosis).
Agreement between pre‐ and postnatal diagnosis is around 65%–70% according to other authors, 13 which is consistent with our results.
No cases of neonatal malignancies were found, which is consistent with the literature, where the majority of studies report no findings 1 or findings are extremely rare (1/12 500–27 500 live births), accounting for 2% of all childhood cancers, with neuroblastoma as a main finding. 5
According to Thakkar et al. 6 and Ozyunku et al., 12 our results, there are predictors of a poorer prognosis depending on the anatomic location of the cyst: ovarian cysts over 40 mm in size without spontaneous in utero resolution have a higher risk of postnatal surgery; and intestinal cysts, including choledochal cysts, duplication cysts and bowel cysts, which tend to be diagnosed in the second trimester, 1 have a poorer prognosis, which is consistent with our findings.
The main limitation of this study is the retrospective design and the small number of cases in the first and second trimesters, which makes it difficult to accurately estimate the association amongst risk factors due to the wide confidence intervals. Moreover, given the reduced number of adverse outcomes, a multivariate regression analysis could not be performed to account for confounding factors.
The high number (16%) of cases lost to follow‐up was due to patients being referred from other hospitals, and in those cases from more remote hospitals, where it was expected that the neonate would not require immediate neonatal treatment, the parents decided to deliver there. This may have introduced a bias, causing the number of cases with a good prognosis and without neonatal complications to be under‐represented.
In prenatal counseling, we suggest conducting an assessment based on trimester at the time of diagnosis, the most common pathology in each trimester, and the presence or absence of associated abnormalities.
Thus, for abdominal cysts diagnosed in the third trimester, the most common pathology is the ovarian cyst and they are more likely to resolve without surgery, and the prognosis is excellent. Prenatal counseling should be addressed to provide ultrasound follow‐up from diagnosis to delivery. For second‐trimester cysts, the most common pathology is intestinal duplication cysts, which will require resection in the first year of life regardless of gender. In the first trimester, amongst the most common are lymphatic malformations, which may require surgery depending on the course. First‐ and second‐trimester abdominal cysts are associated with other abnormalities in up to 71% and 32% of cases, respectively, and are more likely to result in an adverse perinatal outcome. Hence, in these cases, follow‐up and delivery in a tertiary care setting having a neonatal care unit and pediatric surgery department is advised.
5. CONCLUSION
In fetuses with one or more abdominal cysts, factors associated with an adverse fetal or neonatal outcome are diagnosis of the cyst in the first trimester and associated abnormalities. Abdominal cysts diagnosed in the second trimester and those of intestinal origin are more likely to require surgical treatment.
AUTHOR CONTRIBUTIONS
PGA, NM, CR: Protocol development, data collection or management, data analysis, manuscript writing/editing. PGM, SA, JAM, GG: Data collection or management, manuscript writing/editing. EC: Manuscript writing/editing.
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest.
ACKNOWLEDGMENTS
We thank María del Mar Jiménez Quesada for medical writing support. One or more of the authors is a member of the European Reference Network on Rare Congenital Malformations and Rare Intellectual Disability ERN‐ITHACA (EU Framework Partnership Agreement ID: 3HP‐HP‐FPA ERN‐01‐2016/739516).
Garcia‐Aguilar P, Maiz N, Rodó C, et al. Fetal abdominal cysts: Predicting adverse outcomes. Acta Obstet Gynecol Scand. 2023;102:883‐890. doi: 10.1111/aogs.14584
REFERENCES
- 1. Sanna E, Loukogeorgakis S, Prior T, et al. Fetal abdominal cysts: antenatal course and postnatal outcomes. J Perinat Med. 2019;47:418‐421. [DOI] [PubMed] [Google Scholar]
- 2. Bascietto F, Liberati M, Marrone L, et al. Outcome of fetal ovarian cysts diagnosed on prenatal ultrasound examination: systematic review and meta‐analysis: fetal ovarian cysts. Ultrasound Obstet Gynecol. 2017;50:20‐31. [DOI] [PubMed] [Google Scholar]
- 3. Khalil A, Cooke PC, Mantovani E, Bhide A, Papageorghiou AT, Thilaganathan B. Outcome of first‐trimester fetal abdominal cysts: cohort study and review of the literature: first‐trimester fetal abdominal cyst. Ultrasound Obstet Gynecol. 2014;43:413‐419. [DOI] [PubMed] [Google Scholar]
- 4. Sepulveda W, Dickens K, Casasbuenas A, Gutierrez J, Dezerega V. Fetal abdominal cysts in the first trimester: prenatal detection and clinical significance. Ultrasound Obstet Gynecol. 2008;32:860‐864. [DOI] [PubMed] [Google Scholar]
- 5. Husen M, Schut PC, Neven ACH, et al. Differences in origin and outcome of intra‐abdominal cysts in male and female fetuses. Fetal Diagn Ther. 2019;46:166‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thakkar HS, Bradshaw C, Impey L, Lakhoo K. Post‐natal outcomes of antenatally diagnosed intra‐abdominal cysts: a 22‐year single‐institution series. Pediatr Surg Int. 2015;31:187‐190. [DOI] [PubMed] [Google Scholar]
- 7. Ozkose ZG, Suzen Caypinar S, Bestel A, Ozdemir O. Predictive value of prenatal ultrasound in foetal intraabdominal cystic lesions and evaluation of perinatal outcomes: a single‐centre study results. J Obstet Gynaecol. 2022;42:2659‐2664. [DOI] [PubMed] [Google Scholar]
- 8. Marchitelli G, Stirnemann J, Acanfora MM, Rousseau V, Salomon LJ, Ville Y. Prenatal diagnosis of intra‐abdominal cystic lesions by fetal ultrasonography: diagnostic agreement between prenatal and postnatal diagnosis: fetal abdominal cystic lesions. Prenat Diagn. 2015;35:848‐852. [DOI] [PubMed] [Google Scholar]
- 9. Erculiani M, Trovalusci E, Zanatta C, et al. First trimester lower abdominal cysts as early predictor of anorectal malformations. J Ultrasound. 2022. Online ahead of print. doi: 10.1007/s40477-022-00744-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sherwood W, Boyd P, Lakhoo K. Postnatal outcome of antenatally diagnosed intra‐abdominal cysts. Pediatr Surg Int. 2008;24:763‐765. [DOI] [PubMed] [Google Scholar]
- 11. Sauvageot C, Faure JM, Mousty E, et al. Prenatal and postnatal evolution of isolated fetal splenic cysts. Prenat Diagn. 2018;38:390‐394. [DOI] [PubMed] [Google Scholar]
- 12. Ozyuncu O, Canpolat FE, Ciftci AO, Yurdakok M, Onderoglu LS, Deren O. Perinatal outcomes of fetal abdominal cysts and comparison of prenatal and postnatal diagnoses. Fetal Diagn Ther. 2010;28:153‐159. [DOI] [PubMed] [Google Scholar]
- 13. Lv M, Zhao B, Luo Q. Prenatal diagnosis and prognosis assessment of fetal intra‐abdominal cystic lesions: a retrospective study in 264 cases. J Obstet Gynaecol. 2019;39:922‐927. [DOI] [PubMed] [Google Scholar]
- 14. Catania VD, Briganti V, Di Giacomo V, et al. Fetal intra‐abdominal cysts: accuracy and predictive value of prenatal ultrasound. J Matern Fetal Neonatal Med. 2016;29:1691‐1699. [DOI] [PubMed] [Google Scholar]
- 15. Diguisto C, Winer N, Benoist G, et al. In‐utero aspiration vs expectant management of anechoic fetal ovarian cysts: open randomized controlled trial: IUA of fetal ovarian cysts. Ultrasound Obstet Gynecol. 2018;52:159‐164. [DOI] [PubMed] [Google Scholar]
- 16. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159. [PubMed] [Google Scholar]


