Abstract
As plants are sessile organisms, they are inevitably exposed to a variety of environmental stimuli that trigger rapid changes in the generation and disposal of reactive oxygen species such as hydrogen peroxide (H2O2). A major H2O2 scavenging system in plant cells is the ascorbate-glutathione cycle, in which ascorbate peroxidase (APX) catalyzes the conversion of H2O2 into water employing ascorbate as specific electron donor. In higher plants, distinct APX isoforms can occur in multiple subcellular compartments, including chloroplasts, mitochondria, and peroxisomes and the cytosol, to modulate organellar and cellular levels of H2O2. It is well established that APX plays crucial roles in protecting plant cells against diverse environmental stresses, as well as in plant growth and development. Apart from ascorbate, recently, APXs have been found to have a broader substrate specificity and possess chaperone activity, hence participating various biological processes. In this review, we describe the antioxidant properties of APXs and highlight their novel roles beyond ‘ascorbate peroxidases’.
Keywords: APX, H2O2, Antioxidant activity, Substrate, Chaperone
1. Introduction
Reactive oxygen species (ROS), such as, singlet oxygen, superoxide anion, hydroxyl radical, and hydrogen peroxide (H2O2), are inevitable components of aerobic metabolism in all living organisms [1]. When plants are exposed to harsh environmental conditions, ROS levels can increase excessively and cause marked oxidative damage to DNA, RNA, proteins, lipids, and other redox-sensitive molecules. Therefore, plants must develop a sophisticated antioxidant system to modulate cellular ROS concentrations [[2], [3], [4]]. Among the ROS compounds, H2O2 is fairly stable and can transport between cellular compartments through aquaporins, which function as a cellular signaling molecule to myriad of biological processes such as stress responses, growth and development [5,6]. Accumulating evidence suggests that H2O2 largely signals via oxidative post-translational modifications (OxiPTMs) of proteins, enabling proteins to fine-tune their conformational states and activities [1,7].
In plants, H2O2 can be scavenged by several antioxidant enzymes, including catalases (CATs), ascorbate peroxidases (APXs), peroxiredoxins (PRXs) and glutathione S-transferases (GSTs) with different mechanisms [8]. Among them, the heme-containing CAT and APX are two key enzymes associated with H2O2 metabolism. CATs are mainly localized in peroxisomes and degrade H2O2 without reductants. APX has a higher affinity for H2O2 than CAT and catalyzes the reduction of H2O2 to water using ascorbate (ASC) as an electron donor in various subcellular compartments (Fig. 1) [[9], [10], [11], [12]]. In Arabidopsis thaliana, eight AtAPX genes were reported previously, which encode three cytosolic (cytAPXs: AtAPX1, 2, and 6), three peroxisomal (perAPXs: AtAPX3, 4 and 5), and two chloroplastic (chlAPXs: soluble stromal AtsAPX, and thylakoid membrane-bound AttAPX) isoforms [13]. However, AtAPX4 and AtAPX6 are unlikely to encode classical APXs. Due to lack of essential catalytic residues, ASC-binding and heme-binding sites [13], AtAPX4 (also termed TL29) has been renamed APX-like (APX-L) [14]. However, the Arabidopsis apx4 null mutants exhibit decreased soluble APX activity and increased H2O2 accumulation, suggesting a H2O2-scavenging role of APX-L [15]. AtAPX6 is a heme peroxidase that does not utilize ASC as substrate to reduce H2O2 [16], and has been considered to belong to APX-related (APX-R) family [16,17]. Moreover, AtAPX6 was demonstrated as a chloroplast targeted protein [16], though it was previously reported as a cytosolic protein. In recent years, APX-L and APX-R have been reclassified as two novel families of class I peroxidases [14]. Therefore, Arabidopsis harbors only six functional APXs. In contrast to APXs encoded by multiple genes [18,19], both APX-L and APX-R are generally encoded by a single gene in plants [20].
Fig. 1.
Main sources of ROS formation in the plant cell. 1O2 is mostly produced at PSII in thylakoid membranes of chloroplasts whereas both O2•− and H2O2 are generated at several subcellular and extracellular sites. The O2•− is converted to H2O2 either via spontaneous dismutation or by the action of SODs. The levels of H2O2 are controlled by ASC-GSH cycle, which operates in various cellular compartments, including the cytosol, chloroplasts, mitochondria and peroxisomes [82,83]. H2O2 might cross biological membranes via aquaporins. In the reaction with H2O2, ASC is oxidized to MDHA, which can rapidly disproportionate to produce DHA and ASC. Using GSH as the reducing agent, DHA is reduced to ASC either chemically or via the action of DHAR. Abbreviations: ASC, ascorbate; APX, ascorbate peroxidase; CAT, catalase; DHA(R), dehydroascorbate (reductase); GOX, glycolate oxidase; GR, glutathione reductase; GSH, reduced glutathione; GSSG, glutathione disulphide; MDHA(R), monodehydroascorbate (reductase); 2 PG, 2-phosphoglycolate; PGA, 3-phosphoglycerate; PSI/II, photosystem I/II, RETC, respiratory electron transport chain; RBOH, respiratory burst oxidase homolog; RuBisCO, ribulose 1,5-bisphosphate carboxylase/oxygenase; RuBP, ribulose 1,5-bisphosphate; SOD, superoxide dismutase; XO, xanthine oxidase.
As an essential enzyme of the ascorbate-glutathione (ASC-GSH) cycle [21], APX has been showed to participate in many plant growth and development processes, such as lateral root formation [22], nodule development [23], leaf senescence [24], seed germination [25], and programmed cell death (PCD) [26], and play a pivotal role in plant responses to environmental stimuli that have been extensively reviewed previously [18,27]. For example, ZmAPX1-overexpressing transgenic maize plants display enhanced resistance against Bipolaris maydis by decreasing H2O2 levels and activating the jasmonic acid signaling pathway [28]. Arabidopsis plants overexpressing AttAPX exhibit enhanced resistance to paraquat-induced stress [29]. Often, stacking of APX and other antioxidant enzymes makes transgenic plants more tolerant to various stresses [[30], [31], [32]]. Although these data indicate the importance of APX in maintaining the H2O2 homeostasis under adverse environmental conditions, its functional role may be more complicated. For instance, Arabidopsis apx1 mutants showed enhanced tolerance to selenium and lead [33,34]. Arabidopsis mutants deficient in APX2 exhibited reduced tolerance to heat stress during the seedling stage, but enhanced thermotolerance during the reproductive stage [35]. Simultaneous deficiency of APX and CAT are more resistant to oxidative stress than the respective single mutants [[36], [37], [38]].
Our following discussion is not to repeat the importance of APX in plant stress response, growth and development, but rather focus on recent advances, highlighting the OxiPTMs of APX, and new facets of APX including their, newly-discovered substrates in vivo, and chaperone activity.
1.1. OxiPTMs of cytAPX
Although APX is the key regulator in sustaining the steady-state levels of H2O2 in plant cells, it is subjected to multiple OxiPTMs (Fig. 2), and the cytAPX is the best-studied isoform. The cytAPX was identified as a target of thioredoxins (TRXs) by proteomic approaches [[39], [40], [41]]. Treatment of recombinant cytAPX with TRX or GSH inhibited its activity dramatically, which suggests that activation of cytAPX is associated with cysteine (Cys) oxidation or glutathionylation [42]. The Cys thiols (-SH) are particularly susceptible to oxidation by H2O2, and their reaction leads to the formation of sulfenic acid (-SOH, sulfenylation). In addition to H2O2, Cys thiols of APX can undergo a range of other OxiPTMs. Nitric oxide (NO) and hydrogen sulfide (H2S), the most studied reactive nitrogen species (RNS) and reactive sulfur species (RSS), can react with Cys thiols to generate S-nitrosated and persulfidated residues, respectively [43]. The S-nitrosation of Arabidopsis APX1 at Cys32 elevates its enzymatic activity of removing H2O2, leading to enhanced resistance to oxidative stress [44]. By contrast, a rapid reduction in cytAPX activity due to S-nitrosation has also been reported in tobacco cells during PCD [26]. Persulfidation by H2S has been observed to occur at Cys residue that stimulates cytAPX activity in both Arabidopsis and tomato [45,46]. Furthermore, Cys thiols of APX also can react with other cellular compounds such as fatty acids (FAs) or cyanide (HCN), referred to as S-acylation or S-cyanylation [47,48], and their effects on cytAPX need to be explored. During conditions of strong oxidative stress, competing OxiPTMs might take place at Cys residues simultaneously, which makes the modifications of APX more complicated to be analyzed though exciting.
Fig. 2.
Main OxiPTMs of APX in plants. The main PTMs that can affect the of Cys thiols of APX, including S-sulfenylation, persulfidation, S-nitrosation, S-glutathionylation, S-cyanylation and S-acylation. OxiPTMs also occur at Met and Tyr residues of APX. Furthermore, actitity of APX can be inhibited by the reversible binding of NO to the heme prosthetic group. Besides specific sites, H2O2 can attact romdom sites of APX, such as carboxylation. Solid arrows denote activation, whereas the T-shaped lines indicate inhibition. The solid arrow and question mark symbolize contradictory data about the way that S-nitrosation affects APX activity. Dash arrows represent the regulatory effect remains to be elucidated.
Similar to Cys, the sulfur-containing methionine (Met) residues are susceptible to H2O2. In banana, Met oxidation (sulfoxidation) in cytAPX inactivates its activity, which can be reversed partially by Met sulfoxide reductase B2 [49]. Moreover, RNS have been described to regulate APX function through tyrosine (Tyr) nitration and metal nitrosylation. In citrus plants exposed to salinity stress, cytAPX gets nitrated in roots but not in shoots [50]. Interestingly, cytAPX from pea is modulated by RNS in a dual fashion. While nitration at Tyr5 and Tyr235 residues by peroxynitrate (ONOO−) inhibits APX activity, S-nitrosation at Cys32 residue enhances its activity [51]. In tobacco, NO interacts with the iron atom of the heme prosthetic group of APX, leading to reversible inhibition of enzyme activity [52].
Apart from specific site modifications (e.g., Cys and Met oxidation), OxiPTMs also occur at more random sites. Carbonylation at arginine (Arg), lysine (Lys), proline (Pro) or threonine (Thr) residues irreversibly inhibits APX activity, during seed dehydration of Antiaris toxicaria [53].
1.2. APX participates in lignin biosynthesis
Lignin is the second most plentiful biopolymers on earth after by cellulose, accounting for ca. 30% of the organic carbon in the biosphere [54]. It primarily consists of three monolignols, p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol. Upon their production in the cytoplasm, monolignols are transported to the apoplastic space. Once secreted, monolignols are oxidized by class III peroxidases (utilizing H2O2) and/or laccases (using molecular oxygen) to form p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) units of lignin, respectively. In the lignin biosynthesis pathway, the conversion of coumarate to caffeate was previously proposed to be catalyzed by a complex of two membrane-bound cytochrome P450 enzymes, viz. the cinnamate 4-hydroxylase and coumaroyl shikimate 3′-hydroxylase [54]. Recently, a soluble coumarate 3-hydroxylase (C3H) has been identified as a bifunctional APX (C3H/APX) that can oxidize both ASC and 4-coumarate at comparable rates in the cytosol of Brachypodium distachyon and Arabidopsis [55] (Fig. 3). Structural modeling of the of C3H/APX showed that 4-coumarate and ASC bind at the δ- and γ-edges of the heme cofactor, respectively [55]. The cytAPX (SbAPX) from sorghum (Sorghum bicolor) was also demonstrated to catalyze the 3-hydroxylation of 4-coumarate in the presence of ASC and H2O2. However, most caffeate was produced from a nonenzymatic reaction and hence the C3H activity by SbAPX might be negligible in sorghum [19]. Therefore, to what extent the bio-functionality of APX/C3H is conserved among higher plants remains to be clarified.
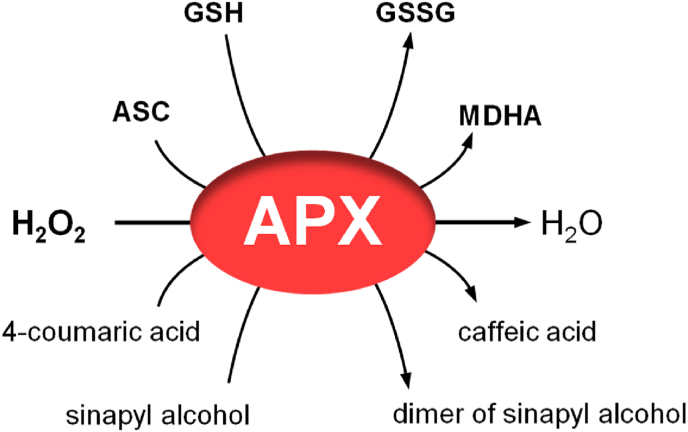
Fig. 3.
Multiple substrates of APX identified in plants. In addition to ASC, APXs have been found to accept other substrates, such as glutathione, 4-coumarate and sinapyl alcohol in plants [56,60,84].
In addition, Zhang et al. (2022) has reported that mitochondrial APX (PtomitAPX) of Chinese white poplar (Populus tomentosa) detoxifies H2O2 through the ASC-GSH cycle in mitochondria in living cells, while in fibres and tracheary elements, PtomitAPX is translocated to cell walls during PCD and recruits monolignols as substrates to catalyze monolignol polymerization during the early stage of lignification. In the late stage of lignification, however, lignin polymers may be catalyzed by class III peroxidases and laccases rather than PtomtAPX [56]. Enzymatic activities tests showed that PtomtAPX could catalyze all three monolignols, with the fastest reaction rates for sinapyl alcohol, followed by p-coumaryl alcohol and coniferyl alcohol, however, the catalytic efficiency (Kcat/Km) was pronouncedly lower for the monolignols than for ASC [56]. Similar to PtomtAPX, SbAPX was also suggested to participate in lignin polymerization because it could catalyze oxidative polymerization of multiple phenylpropanoid intermediates in the monolignol pathway [19]. Nevertheless, whether SbAPX is translocated to the cell wall was not conducted.
Furthermore, the availability of H2O2 is a restricting factor in lignification [57]. Simultaneous overexpression of APX and CuZn-superoxide dismutase in potato resulted in enhanced expression of genes and transcription factors associated with lignin biosynthesis under salinity stress [58]. It suggests that enhancement of APX activity plays a vital role in maintaining an optimal level of H2O2, which might serve as a signal to stimulate lignification under saline conditions. As far as the bifunctionality of APX is concerned, which activity is the causative factor in lignin synthesis under external stimuli remains to be explored.
1.3. GSH oxidation activity of APX
ASC and GSH often are considered to work together to scavenge ROS. In the ASC-GSH cycle, GSH can regenerate ASC by reducing dehydroascorbate (DHA), either via non-enzymic reduction or through DHA reductase (DHAR) activity [59]. Recently, Chin et al. (2019) has reported that a cytAPX of orchid Oncidium (OgAPX1) can utilize not only ASC but also GSH as a substrate at different active sites [60] (Fig. 3). Structural modeling and site-directed mutagenesis demonstrate that Pro63, aspartate (Asp)75 and Tyr97 are necessary for GSH oxidation in OgAPX1, whereas the corresponding site in AtAPX1 is comprised of Asp63, histidine (His)75 and His97 without GSH-binding activity. The dual catalytic activity of OgAPX1 confers greater protection against salt and heat tolerances when overexpressed in Arabidopsis compared with overexpression of AtAPX1. Aside from OgAPX1, recombinant cytAPXs from rice, maize and soybean also possess GSH oxidation activity that refers to glutathione peroxidase (GPX) [60].
It is worth noting that most plant GPXs preferentially utilize TRX rather than GSH as a reducing agent [[61], [62], [63]], and therefore sometimes are called GPX-like proteins [64]. In plants, the oxidation of GSH to glutathione disulfide (GSSG) can be also attributed to several other enzymes, including DHAR, lambda class of GSTs with peroxidase activity and certain PRXs that are coupled GSH oxidation via glutaredoxin (GRX) action [65]. Notably, Arabidopsis DHAR has been identified as a crucial player in ensuring GSH oxidation in response to intracellular oxidative stress [66,67]. To fully elucidate the GSH oxidation mechanism of OgAPX1, it is necessary to determine the substrate specificity of GPX in orchid Ocidium, and the dual catalytic activity of OgAPX1 would be redefined.
1.4. APX functions as a molecular chaperone
Besides their primary antioxidant properties, APXs have been identified as chaperone molecules in Arabidopsis (AtAPX1) [68] and rice (Oryza sativa, OsAPX2) [69]. The dual functions of AtAPX1 and OsAPX2 correlate closely with their structural conformations. The low-molecular-weight (LMW) forms of the AtAPX1 and OsAPX2 predominantly exhibit peroxidase activity, whereas the high-molecular-weight (HMW) complexes display chaperone activity. Moreover, structural status of the AtAPX1 and OsAPX2 proteins could be modulated by heat and salt stresses through association and disassociation, respectively [68,69]. Whether a peroxidase-chaperone functional switch of APX is conserved in land plants will require further exploration.
As with AtAPX1, 2-Cys PRX changes its structure from LMW to HMW structures by oxidative stress concomitantly leading to a functional switching from peroxidase to molecular chaperone [70]. Intriguingly, several other redox proteins in Arabidopsis, including thioredoxin reductase type C (NTRC) [71], GRXS17 [72], tetratricoredoxin (TDX) [73], and TRX-h3 [74], which play essential roles in oxidative and heat tolerance and exhibit a chaperone function, undergo conformational changes from LMW to HMW structures (Table 1). Further research is needed to determine how plants orchestrate APX and other redox-dependent chaperones (e.g., NTRC, 2-Cys PRX, TDX or TRXh3) to combat oxidative stress.
Table 1.
Structural and functional switching of Arabidopsis redox enzymes in response to redox state.
| Protein | Protein structures and functions |
Ref | |
|---|---|---|---|
| Reduction state | Oxidation state | ||
| APX1 | LMW forms. Peroxidase activity. |
HMW forms. Molecular chaperone. |
[68] |
| 2-Cys PRX | LMW forms. Peroxidase activity. |
HMW forms. Chaperone. |
[70] |
| NTRC | LMW forms. Disulfide reductase and foldase chaperone. |
HMW forms. Holdase chaperone. |
[71] |
| GRXS17 | LMW forms. Involvement in the maturation of iron-sulfur proteins. |
HMW forms. Holdase chaperone. |
[72] |
| TDX | LMW forms. Disulfide reductase and foldase chaperone. |
HMW forms. Holdase chaperone. |
[73] |
| TRX-h3 | LMW forms. Disulfide reductase activity. |
HMW forms. Molecular chaperone. |
[74] |
2. Conclusions and outlook
Although much progress has been made in understanding the basic characteristics of APX isoenzymes, such as their distribution, structure and enzymological properties, there are still many unanswered questions. For example, the relative contribution of APXs in different cellular compartment is not entirely clear, and the functional crosstalk and overlap between APXs and other antioxidant enzymes also remain elusive. Besides its canonical H2O2 scavenging role, APX is likely to act as a H2O2 signaling regulator [75]. Chloroplastic APXs (tAPX and sAPX) are suggested to play a role in regulating retrograde H2O2 signal to modulate plant stress responses [76]. However, it is still unclear how much H2O2 is controlled by APXs. In order to further understand the H2O2 signaling, it is required to measure accurately H2O2 levels in different compartments using incisive redox sensors [77,78].
In addition to redox-based PTMs, nonoxidative PTMs also make contributions to altered APX activity in plants. Upon pathogen challenge, the wheat kinase start 1.1 is translocated to chloroplasts where it binds and phosphorylates tAPX, reducing its activity and ability to remove H2O2 [79]. Complete crotonylation at Lys136 of APX in chrysanthemum increases its enzymatic activity and further reduce the oxidative damage caused by low-temperature stress [80]. Notably, many of oxidative and nonoxidative PTMs are transient making comprehensive analysis under varied environmental stimuli difficult.
Despite their specificity towards ASC, APXs can also oxidize non-physiological aromatic substrates in vitro, such as p-cresol, o-dianisidine and guaiacol, at rates comparable to ASC [81]. Recent studies discussed in this review have demonstrated that APX activity also towards other substrates in plants, such as 4-coumarate, sinapyl alcohol, and GSH. It is not difficult to foresee that more potential substrates of APXs would be discovered in the future. The story of the multifaceted functions of APXs may have just begun!
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32071477, 31700227), Innovation Base for Introducing Talents of Discipline of Hubei Province (2021EJD025, 2019BJH021), and Key Research and Development Program of Hubei Province (2021BBA224).
Data availability
No data was used for the research described in the article.
References
- 1.Waszczak C., Carmody M., Kangasjarvi J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018;69:209–236. doi: 10.1146/annurev-arplant-042817-040322. [DOI] [PubMed] [Google Scholar]
- 2.Foyer C., Noctor G. Redox regulation in photosynthetic organisms signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- 3.Castro B., Citterico M., Kimura S., Stevens D., Wrzaczek M., et al. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants. 2021;7:403–412. doi: 10.1038/s41477-021-00887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittler R., Zandalinas S., Fichman Y., Breusegem F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022;23:663–679. doi: 10.1038/s41580-022-00499-2. [DOI] [PubMed] [Google Scholar]
- 5.Petrov V., Van Breusegem F. Hydrogen peroxide-a central hub for information flow in plant cells. AoB Plants. 2012;2012:pls014. doi: 10.1093/aobpla/pls014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smirnoff N., Arnaud D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019;221:1197–1214. doi: 10.1111/nph.15488. [DOI] [PubMed] [Google Scholar]
- 7.Corpas F.J., Gonzalez-Gordo S., Rodriguez-Ruiz M., Munoz-Vargas M.A., Palma J.M. Thiol-based oxidative posttranslational modifications (OxiPTMs) of plant proteins. Plant Cell Physiol. 2022;63:889–900. doi: 10.1093/pcp/pcac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foyer C., Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anjum N.A., Sharma P., Gill S.S., Hasanuzzaman M., Khan E.A., et al. Catalase and ascorbate peroxidase-representative H2O2-detoxifying heme enzymes in plants. Environ. Sci. Pollut. Res. Int. 2016;23:19002–19029. doi: 10.1007/s11356-016-7309-6. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi K., Mori H., Nishimura M. A novel isoenzyme of ascorbate peroxidase localized on glyoxysomal and leaf peroxisomal membranes in pumpkin. Plant Cell Physiol. 1995;36:1157–1162. doi: 10.1093/oxfordjournals.pcp.a078862. [DOI] [PubMed] [Google Scholar]
- 11.Bunkelmann J.R., Trelease R.N. Ascorbate peroxidase. A prominent membrane protein in oilseed glyoxysomes. Plant Physiol. 1996;110:589–598. doi: 10.1104/pp.110.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura K., Yabuta Y., Tamoi M., Ishikawa T., Shigeoka S. Alternatively spliced mRNA variants of chloroplast ascorbate peroxidase isoenzymes in spinach leaves. Biochem. J. 1999;338:41–48. [PMC free article] [PubMed] [Google Scholar]
- 13.Granlund I., Storm P., Schubert M., Garcia-Cerdan J.G., Funk C., et al. The TL29 protein is lumen located, associated with PSII and not an ascorbate peroxidase. Plant Cell Physiol. 2009;50:1898–1910. doi: 10.1093/pcp/pcp134. [DOI] [PubMed] [Google Scholar]
- 14.Lazzarotto F., Turchetto-Zolet A.C., Margis-Pinheiro M. Revisiting the non-animal peroxidase superfamily. Trends Plant Sci. 2015;20:807–813. doi: 10.1016/j.tplants.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y.Y., Hecker A.G., Hauser B.A. The APX4 locus regulates seed vigor and seedling growth in Arabidopsis thaliana. Planta. 2014;239:909–919. doi: 10.1007/s00425-014-2025-2. [DOI] [PubMed] [Google Scholar]
- 16.Lazzarotto F., Wahni K., Piovesana M., Maraschin F., Messens J., et al. Arabidopsis APx-R is a plastidial ascorbate-independent peroxidase regulated by photomorphogenesis. Antioxidants. 2021;10:65. doi: 10.3390/antiox10010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazzarotto F., Teixeira F.K., Rosa S.B., Dunand C., Fernandes C.L., et al. Ascorbate peroxidase-related (APx-R) is a new heme-containing protein functionally associated with ascorbate peroxidase but evolutionarily divergent. New Phytol. 2011;191:234–250. doi: 10.1111/j.1469-8137.2011.03659.x. [DOI] [PubMed] [Google Scholar]
- 18.Pandey S., Fartyal D., Agarwal A., Shukla T., James D., et al. Abiotic stress tolerance in plants: myriad roles of ascorbate peroxidase. Front. Plant Sci. 2017;8:581. doi: 10.3389/fpls.2017.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang B., Lewis J.A., Vermerris W., Sattler S.E., Kang C. A sorghum ascorbate peroxidase with four binding sites has activity against ascorbate and phenylpropanoids. Plant Physiol. 2023;192:102–118. doi: 10.1093/plphys/kiac604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jardim-Messeder D., Caverzan A., Bastos G.A., Galhego V., Souza-Vieira Y., et al. Genome-wide, evolutionary, and functional analyses of ascorbate peroxidase (APX) family in Poaceae species. Genet. Mol. Biol. 2022;46 doi: 10.1590/1678-4685-GMB-2022-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noctor G., Foyer C. Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 22.Correa-Aragunde N., Foresi N., Delledonne M., Lamattina L. Auxin induces redox regulation of ascorbate peroxidase 1 activity by S-nitrosylation/denitrosylation balance resulting in changes of root growth pattern in Arabidopsis. J. Exp. Bot. 2013;64:3339–3349. doi: 10.1093/jxb/ert172. [DOI] [PubMed] [Google Scholar]
- 23.Keyster M., Klein A., Egbichi I., Jacobs A., Ludidi N. Nitric oxide increases the enzymatic activity of three ascorbate peroxidase isoforms in soybean root nodules. Plant Signal. Behav. 2011;6:956–961. doi: 10.4161/psb.6.7.14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro C.W., Korbes A.P., Garighan J.A., Jardim-Messeder D., Carvalho F.E.L., et al. Rice peroxisomal ascorbate peroxidase knockdown affects ROS signaling and triggers early leaf senescence. Plant Sci. 2017;263:55–65. doi: 10.1016/j.plantsci.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Huang L., Jia J., Zhao X., Zhang M., Huang X., et al. The ascorbate peroxidase APX1 is a direct target of a zinc finger transcription factor ZFP36 and a late embryogenesis abundant protein OsLEA5 interacts with ZFP36 to co-regulate OsAPX1 in seed germination in rice. Biochem. Biophys. Res. Commun. 2018;495:339–345. doi: 10.1016/j.bbrc.2017.10.128. [DOI] [PubMed] [Google Scholar]
- 26.de Pinto M.C., Locato V., Sgobba A., Romero-Puertas Mdel C., Gadaleta C., et al. S-nitrosylation of ascorbate peroxidase is part of programmed cell death signaling in tobacco Bright Yellow-2 cells. Plant Physiol. 2013;163:1766–1775. doi: 10.1104/pp.113.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caverzan A., Passaia G., Rosa S., Ribeiro C., Lazzarotto F., et al. Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012;35:1011–1019. doi: 10.1590/s1415-47572012000600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J., Jia X., Wang G.F., Ma S., Wang S., et al. Ascorbate peroxidase 1 confers resistance to southern corn leaf blight in maize. J. Integr. Plant Biol. 2022;64:1196–1211. doi: 10.1111/jipb.13254. [DOI] [PubMed] [Google Scholar]
- 29.Murgia I D.T., Vannini C., Bracale M., Carravieri S., et al. Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J. 2004;38:940–953. doi: 10.1111/j.1365-313X.2004.02092.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim M.D., Kim Y.H., Kwon S.Y., Yun D.J., Kwak S.S., et al. Enhanced tolerance to methyl viologen-induced oxidative stress and high temperature in transgenic potato plants overexpressing the CuZnSOD, APX and NDPK2 genes. Physiol. Plantarum. 2010;140:153–162. doi: 10.1111/j.1399-3054.2010.01392.x. [DOI] [PubMed] [Google Scholar]
- 31.Diaz-Vivancos P., Faize M., Barba-Espin G., Faize L., Petri C., et al. Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums. Plant Biotechnol. J. 2013;11:976–985. doi: 10.1111/pbi.12090. [DOI] [PubMed] [Google Scholar]
- 32.Xu J., Yang J., Duan X., Jiang Y., Zhang P. Increased expression of native cytosolic Cu/Zn superoxide dismutase and ascorbate peroxidase improves tolerance to oxidative and chilling stresses in cassava (Manihot esculenta Crantz) BMC Plant Biol. 2014;14:208. doi: 10.1186/s12870-014-0208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang L., Wang W., Chen Z., Gao Q., Xu Q., et al. A role for APX1 gene in lead tolerance in Arabidopsis thaliana. Plant Sci. 2017;256:94–102. doi: 10.1016/j.plantsci.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Jiang L., Chen Z., Gao Q., Ci L., Cao S., et al. Loss-of-function mutations in the APX1 gene result in enhanced selenium tolerance in Arabidopsis thaliana. Plant Cell Environ. 2016;39:2133–2144. doi: 10.1111/pce.12762. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki N., Miller R., Sejima H., Harper J., Mittler R. Enhanced seed production under prolonged heat stress conditions in cytosolic ascorbate peroxidase 2. J. Exp. Bot. 2013;64:253–263. doi: 10.1093/jxb/ers335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanderauwera S., Suzuki N., Miller G., van de Cotte B., Morsa S., et al. Extranuclear protection of chromosomal DNA from oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 2011;108:1711–1716. doi: 10.1073/pnas.1018359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sousa R.H.V., Carvalho F.E.L., Lima-Melo Y., Alencar V., Daloso D.M., et al. Impairment of peroxisomal APX and CAT activities increases protection of photosynthesis under oxidative stress. J. Exp. Bot. 2019;70:627–639. doi: 10.1093/jxb/ery354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizhsky L., Hallak-Herr E., Van Breusegem F., Rachmilevitch S., Barr J.E., et al. Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J. 2002;32:329–342. doi: 10.1046/j.1365-313x.2002.01427.x. [DOI] [PubMed] [Google Scholar]
- 39.Marchand C., Le Marechal P., Meyer Y., Miginiac-Maslow M., Issakidis-Bourguet E., et al. New targets of Arabidopsis thioredoxins revealed by proteomic analysis. Proteomics. 2004;4:2696–2706. doi: 10.1002/pmic.200400805. [DOI] [PubMed] [Google Scholar]
- 40.Yamazaki D., Motohashi K., Kasama T., Hara Y., Hisabori T. Target proteins of the cytosolic thioredoxins in Arabidopsis thaliana. Plant Cell Physiol. 2004;45:18–27. doi: 10.1093/pcp/pch019. [DOI] [PubMed] [Google Scholar]
- 41.Wong J.H., Cai N., Balmer Y., Tanaka C.K., Vensel W.H., et al. Thioredoxin targets of developing wheat seeds identified by complementary proteomic approaches. Phytochemistry. 2004;65:1629–1640. doi: 10.1016/j.phytochem.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Gelhaye E., Navrot N., Macdonald I.K., Rouhier N., Raven E.L., et al. Ascorbate peroxidase-thioredoxin interaction. Photosynth. Res. 2006;89:193–200. doi: 10.1007/s11120-006-9100-x. [DOI] [PubMed] [Google Scholar]
- 43.Zhou H., Huang J., Willems P., Van Breusegem F., Xie Y. Cysteine thiol-based post-translational modification: what do we know about transcription factors? Trends Plant Sci. 2022;28:415–428. doi: 10.1016/j.tplants.2022.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Yang H., Mu J., Chen L., Feng J., Hu J., et al. S-nitrosylation positively regulates ascorbate peroxidase activity during plant stress responses. Plant Physiol. 2015;167:1604–1615. doi: 10.1104/pp.114.255216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aroca A., Serna A., Gotor C., Romero L.C., S-sulfhydration A cysteine posttranslational modification in plant systems. Plant Physiol. 2015;168:334–342. doi: 10.1104/pp.15.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J., Shi C., Wang X., Liu C., Ding X., et al. Hydrogen sulfide regulates the activity of antioxidant enzymes through persulfidation and improves the resistance of tomato seedling to Copper Oxide nanoparticles (CuO NPs)-induced oxidative stress. Plant Physiol. Biochem. 2020;156:257–266. doi: 10.1016/j.plaphy.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Garcia I., Arenas-Alfonseca L., Moreno I., Gotor C., Romero L.C. HCN regulates cellular processes through posttranslational modification of proteins by S-cyanylation. Plant Physiol. 2019;179:107–123. doi: 10.1104/pp.18.01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar M., Carr P., Turner S. An atlas of Arabidopsis protein S-acylation reveals its widespread role in plant cell organization and function. Native Plants. 2022;8:670–681. doi: 10.1038/s41477-022-01164-4. [DOI] [PubMed] [Google Scholar]
- 49.Xiao L., Jiang G., Yan H., Lai H., Su X., et al. Methionine sulfoxide reductase B regulates the activity of ascorbate peroxidase of banana fruit. Antioxidants. 2021;10:310. doi: 10.3390/antiox10020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanou G., Filippou P., Belghazi M., Job D., Diamantidis G., et al. Oxidative and nitrosative-based signaling and associated post-translational modifications orchestrate the acclimation of citrus plants to salinity stress. Plant J. 2012;72:585–599. doi: 10.1111/j.1365-313X.2012.05100.x. [DOI] [PubMed] [Google Scholar]
- 51.Begara-Morales J.C., Sanchez-Calvo B., Chaki M., Valderrama R., Mata-Perez C., et al. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. J. Exp. Bot. 2014;65:527–538. doi: 10.1093/jxb/ert396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark D., Durner J., Navarre D., Df K. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol. Plant Microbe Interact. 2000;13:1380–1384. doi: 10.1094/MPMI.2000.13.12.1380. [DOI] [PubMed] [Google Scholar]
- 53.Bai X., Yang L., Tian M., Chen J., Shi J., et al. Nitric oxide enhances desiccation tolerance of recalcitrant Antiaris toxicaria seeds via protein S-nitrosylation and carbonylation. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoon J., Choi H., An G. Roles of lignin biosynthesis and regulatory genes in plant development. J. Integr. Plant Biol. 2015;57:902–912. doi: 10.1111/jipb.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barros J., Escamilla-Trevino L., Song L.H., Rao X.L., Serrani-Yarce J.C., et al. 4-Coumarate 3-hydroxylase in the lignin biosynthesis pathway is a cytosolic ascorbate peroxidase. Nat. Commun. 2019;10:1994. doi: 10.1038/s41467-019-10082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J., Liu Y., Li C., Yin B., Xiatong Liu X., et al. PtomtAPX is an autonomous lignification peroxidase during the earliest stage of secondary wall formation in Populus tomentosa Carr. Native Plants. 2022;8:828–839. doi: 10.1038/s41477-022-01181-3. [DOI] [PubMed] [Google Scholar]
- 57.Marjamaa K., Kukkola E.M., Fagerstedt K.V. The role of xylem class III peroxidases in lignification. J. Exp. Bot. 2009;60:367–376. doi: 10.1093/jxb/ern278. [DOI] [PubMed] [Google Scholar]
- 58.Shafi A., Pal A.K., Sharma V., Kalia S., Kumar S., et al. Transgenic potato plants overexpressing SOD and APX exhibit enhanced lignification and starch biosynthesis with improved salt stress tolerance. Plant Mol. Biol. Rep. 2017;35:504–518. [Google Scholar]
- 59.Terai Y., Ueno H., Ogawa T., Sawa Y., Miyagi A., et al. Dehydroascorbate reductases and glutathione set a threshold for high-light-induced ascorbate accumulation. Plant Physiol. 2020;183:112–122. doi: 10.1104/pp.19.01556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chin D.C., Rajendran S.K., Suen C.S., Chien C.Y., Hwang M.J., et al. Plant cytosolic ascorbate peroxidase with dual catalytic activity modulates abiotic stress tolerances. iScience. 2019;16:31–49. doi: 10.1016/j.isci.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iqbal A., Yabuta Y., Takeda T., Nakano Y., Shigeoka S. Hydroperoxide reduction by thioredoxin-specific glutathione peroxidase isoenzymes of Arabidopsis thaliana. FEBS J. 2006;273:5589–5597. doi: 10.1111/j.1742-4658.2006.05548.x. [DOI] [PubMed] [Google Scholar]
- 62.Navrot N., Collin V., Gualberto J., Gelhaye E., Hirasawa M., et al. Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses. Plant Physiol. 2006;142:1364–1379. doi: 10.1104/pp.106.089458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bela K., Riyazuddin R., Csiszar J. Plant glutathione peroxidases: non-heme peroxidases with large functional flexibility as a core component of ROS-processing mechanisms and signalling. Antioxidants. 2022;11:1624. doi: 10.3390/antiox11081624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Attacha S., Solbach D., Bela K., Moseler A., Wagner S., et al. Glutathione peroxidase-like enzymes cover five distinct cell compartments and membrane surfaces in Arabidopsis thaliana. Plant Cell Environ. 2017;40:1281–1295. doi: 10.1111/pce.12919. [DOI] [PubMed] [Google Scholar]
- 65.Rahantaniaina M., Tuzet A., Mhamdi A., Noctor G. Missing links in understanding redox signaling via thioldisulfide modulation how is glutathione oxidized in plants. Front. Plant Sci. 2013;4:477. doi: 10.3389/fpls.2013.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rahantaniaina M.S., Li S., Chatel-Innocenti G., Tuzet A., Issakidis-Bourguet E., et al. Cytosolic and chloroplastic DHARs cooperate in oxidative stress-driven activation of the salicylic acid pathway. Plant Physiol. 2017;174:956–971. doi: 10.1104/pp.17.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rahantaniaina M.S., Li S., Chatel-Innocenti G., Tuzet A., Mhamdi A., et al. Glutathione oxidation in response to intracellular H2O2: key but overlapping roles for dehydroascorbate reductases. Plant Signal. Behav. 2017;12 doi: 10.1080/15592324.2017.1356531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaur S., Prakash P., Bak D.H., Hong S.H., Cho C., et al. Regulation of dual activity of ascorbate peroxidase 1 from Arabidopsis thaliana by conformational changes and posttranslational modifications. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.678111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hong S.H., Tripathi B.N., Chung M.S., Cho C., Lee S., et al. Functional switching of ascorbate peroxidase 2 of rice (OsAPX2) between peroxidase and molecular chaperone. Sci. Rep. 2018;8:9171. doi: 10.1038/s41598-018-27459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.König J., Galliardt H., Jutte P., Schaper S., Dittmann L., et al. The conformational bases for the two functionalities of 2-cysteine peroxiredoxins as peroxidase and chaperone. J. Exp. Bot. 2013;64:3483–3497. doi: 10.1093/jxb/ert184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chae H.B., Moon J.C., Shin M.R., Chi Y.H., Jung Y.J., et al. Thioredoxin reductase type C (NTRC) orchestrates enhanced thermotolerance to Arabidopsis by its redox-dependent holdase chaperone function. Mol. Plant. 2013;6:323–336. doi: 10.1093/mp/sss105. [DOI] [PubMed] [Google Scholar]
- 72.Martins L., Knuesting J., Bariat L., Dard A., Freibert S.A., et al. Redox modification of the iron-sulfur glutaredoxin GRXS17 activates holdase activity and protects plants from heat stress. Plant Physiol. 2020;184:676–692. doi: 10.1104/pp.20.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee J., Lee S., Jang H., Lee Y., Park J., et al. Heat-shock dependent oligomeric status alters the function of a plant-specific thioredoxin-like protein. AtTDX. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5978–5983. doi: 10.1073/pnas.0811231106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park S.K., Jung Y.J., Lee J.R., Lee Y.M., Jang H.H., et al. Heat-shock and redox-dependent functional switching of an h-type Arabidopsis thioredoxin from a disulfide reductase to a molecular chaperone. Plant Physiol. 2009;150:552–561. doi: 10.1104/pp.109.135426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maruta T., Sawa Y., Shigeoka S., Ishikawa T. Diversity and evolution of ascorbate peroxidase functions in chloroplasts: more than just a classical antioxidant enzyme. Plant Cell Physiol. 2016;57:1377–1386. doi: 10.1093/pcp/pcv203. [DOI] [PubMed] [Google Scholar]
- 76.Maruta T., Noshi M., Tanouchi A., Tamoi M., Yabuta Y., et al. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J. Biol. Chem. 2012;287:11717–11729. doi: 10.1074/jbc.M111.292847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niemeyer J., Scheuring D., Oestreicher J., Morgan B., Schroda M. Real-time monitoring of subcellular H2O2 distribution in Chlamydomonas reinhardtii. Plant Cell. 2021;33:2935–2949. doi: 10.1093/plcell/koab176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nietzel T., Elsasser M., Ruberti C., Steinbeck J., Ugalde J.M., et al. The fluorescent protein sensor roGFP2-Orp1 monitors in vivo H2O2 and thiol redox integration and elucidates intracellular H2O2 dynamics during elicitor-induced oxidative burst in Arabidopsis. New Phytol. 2019;221:1649–1664. doi: 10.1111/nph.15550. [DOI] [PubMed] [Google Scholar]
- 79.Gou J.Y., Li K., Wu K., Wang X., Lin H., et al. Wheat stripe rust resistance protein WKS1 reduces the ability of the thylakoid-associated ascorbate peroxidase to detoxify reactive oxygen species. Plant Cell. 2015;27:1755–1770. doi: 10.1105/tpc.114.134296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin P., Bai H., He L., Huang Q-x, Zeng Q-h, et al. Proteome-wide and lysine crotonylation profiling reveals the importance of crotonylation in chrysanthemum (Dendranthema grandiforum) under low-temperature. BMC Genom. 2021;22:51. doi: 10.1186/s12864-020-07365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raven E.L. Understanding functional diversity and substrate specificity in haem peroxidases: what can we learn from ascorbate peroxidase? Nat. Prod. Rep. 2003;20:367–381. doi: 10.1039/b210426c. [DOI] [PubMed] [Google Scholar]
- 82.Chew O., Whelan J., Millar A.H. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J. Biol. Chem. 2003;278:46869–46877. doi: 10.1074/jbc.M307525200. [DOI] [PubMed] [Google Scholar]
- 83.Jimenez A., Hernandez J.A., Del Rio L.A., Sevilla F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 1997;114:275–284. doi: 10.1104/pp.114.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dixon R.A., Barros J. Lignin biosynthesis: old roads revisited and new roads explored. Open Biol. 2019;9 doi: 10.1098/rsob.190215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.