Abstract
Carnosine is a naturally occurring endogenous dipeptide composed by the ligation of β-alanine and L-histidine performed particularly by tissues with an increased oxidative metabolism such as muscles and brain. In the last 50 years different studies have assessed the role and function of carnosine through numerous in vitro, in vivo, and clinical studies, demonstrating the multimodal mechanism of action of this dipeptide that includes anti-aggregant, antioxidant, and anti-inflammatory activities. In particular its activity has been investigated in experimental models of cardiovascular disease (CVD), type 2 diabetes mellitus (T2DM), and neurodegenerative disorders, such as cerebral ischemia and Alzheimer's disease (AD). In the present review, we examined the protective role that carnosine could exert in the context of T2DM, CVD, and AD, which show common pathogenic mechanisms including oxidative stress, inflammation, and aggregation phenomena. Carnosine's pharmacodynamic profile is multimodal and combines the systemic anti-inflammatory and antioxidant activities with its anti-aggregant and neuroprotective efficacy in the central nervous system. This enlarged pharmacological activity opens a new path to explore the therapeutic potential of carnosine in all the three diseases, and in particular in patients with T2DM, who often show a history of CVD and also have an increased risk to develop mild cognitive impairment and AD.
Keywords: Carnosine, Oxidative stress, Inflammation, Protein aggregation, Neuroprotection
Graphical abstract
Highlights
-
•
Carnosine is an endogenous dipeptide with a multimodal pharmacodynamic profile.
-
•
Carnosine possesses anti-aggregant, antioxidant, and anti-inflammatory activities.
-
•
CVD, T2DM, and AD show common pathogenic mechanisms (e.g., oxidative stress).
-
•
Carnosine has shown a promising therapeutic potential against CVD, T2DM, and AD.
1. Introduction
Carnosine was discovered more than 100 years ago in Ukraine at the Charkow University by Gulewitsch and Amiradžibi while they were analyzing a meat extract (Gulewitsch and Amiradžibi, 1900). It is a natural occurring dipeptide made of β-alanine and L-histidine and widely distributed in mammalian tissues, where it can be found in a very high concentration in cardiac and skeletal muscles (Mannion et al., 1992), as well as in the brain (Hipkiss et al., 1998a). Some invertebrate species also contain carnosine (Drozak et al., 2010).
Many recent studies have identified the different pharmacological activities exerted by carnosine, among which the anti-aggregant, the antioxidant, and the anti-inflammatory activities are definitely noteworthy.
Both reactive oxygen (ROS) and nitrogen (RNS) species are normally produced by organisms and involved in signaling mechanisms and physiological functions, while their dyshomeostasis has been associated with numerous pathological conditions, and in particular neurodegenerative disorders (Mainz et al., 2012; de Campos et al., 2015). An imbalance between their production and neutralization, that can depend on both the accumulation of ROS/RNS or to an impairment of the antioxidant system, leads to a condition of oxidative and nitrosative stress (Birben et al., 2012; Gunasekara et al., 2014).
Our organism responds to biological, chemical, or physical stimuli by increasing the concentrations of pro-inflammatory mediators, in order to suppress the triggering condition and/or to prevent damages on itself. Among the pro-inflammatory cytokines, three are the most studied: tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 (Stevens et al., 1992; Popko et al., 2010; Umare et al., 2014), and are the main responsible for the state of inflammation that follows (Beesu et al., 2016).
Aggregation phenomena occur when proteins that are normally soluble become insoluble due to an alteration of their secondary or tertiary structure, this being caused by mutations or chemical and environmental modifications that act on the conformational stability of the protein. The conformational changes and aggregation that follow can lead to detrimental consequences in direct (by exerting a toxic activity) or indirect (impairing the physiological role of the native protein) ways (Thomas et al., 1995; Kelly, 1996; Carrell and Lomas, 1997; Carrell and Gooptu, 1998; Soto, 1999; Gaggelli et al., 2006). Several diseases have been demonstrated linked to protein misfolding and are grouped under the name of protein conformational disorders, such as Alzheimer's disease (AD).
Numerous neurodegenerative disorders are known to be linked to the three detrimental mechanisms discussed above (oxidative stress, inflammation, and protein aggregation), such as brain ischemia (Hu and Chen, 2012), cardiovascular disease (CVD), type 2 diabetes mellitus (T2DM), and the already mentioned AD (Iqbal and Grundke-Iqbal, 2010); carnosine, therefore, through its multimodal mechanism of action (Caruso et al., 2019b), could play an important role in counteracting and/or, in the best scenario, completely preventing the molecular alterations underlying neuronal death in these pathologies.
The present review aims to be a deep analysis of the current state of the art in the knowledge of the protective role that carnosine could exert in the context of neurodegenerative diseases related to oxidative stress, inflammation, and aggregation phenomena. In particular, we will examine the cellular and molecular mechanisms exerted by carnosine, underlying the therapeutic potential of this natural occurring dipeptide, towards which numerous evidence justifies the growing interest documented in recent years. The final aim is to translate its already well-known preclinical efficacy into the clinical applications to improve patient's quality life.
2. Carnosine metabolism and biological activities
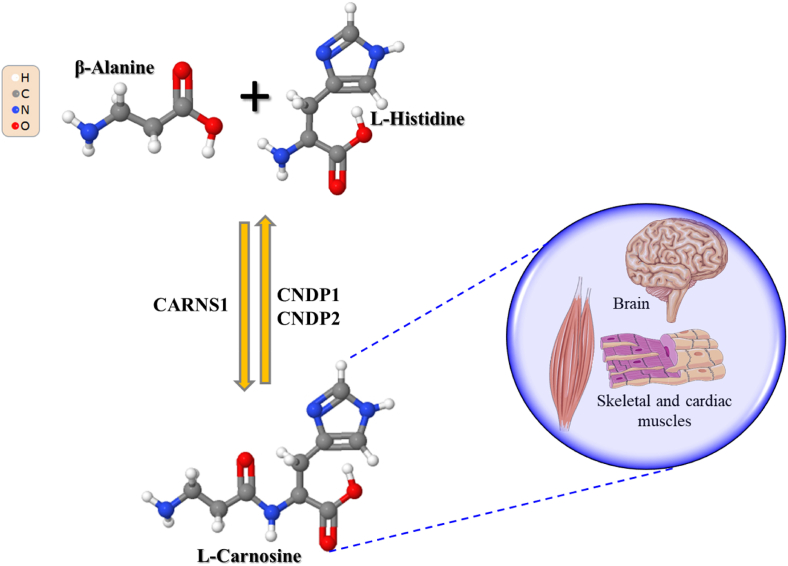
Carnosine synthesis is on charge of carnosine synthetase 1 (CARNS1) (Kalyankar and Meister, 1959; Winnick and Winnick, 1959), able to form, through an ATP-dependent reaction, the dipeptide (β-alanyl-L-histidine) starting from its two component amino acids β-alanine and L-histidine. Carnosine degradation, the main mechanism regulating its levels in the tissues as well as in biological fluids, is instead due to the activity of two different dipeptidase belonging to the M20 metalloprotease family: carnosine dipeptidase 1 (CNDP1) in serum (Lenney et al., 1982), and carnosine dipeptidase 2 (CNDP2) in cytosol (Lenney et al., 1985; Teufel et al., 2003) (Fig. 1).
Fig. 1.
Carnosine metabolism (synthesis and degradation) and localization (tissues with highest concentration). Part of this figure was created with https://smart.servier.com.
As previously mentioned, the levels of carnosine are very high in cardiac and skeletal muscles, containing ∼99% of total carnosine of the body (Gariballa and Sinclair, 2000; Boldyrev et al., 2013), and at brain level (Hipkiss et al., 1998a). Different additional histidine-containing dipeptides including anserine, the methylated analogue of carnosine, can also be found in the tissues of animal species (Turner et al., 2021).
Carnosine possesses numerous and worth of mention biological activities. In fact, it is able to exert its biological activities not only at muscle and brain levels, where it is very high-concentrated, but also in other organs and tissues. During the last decades a plethora of publications have been dedicated to the description of both carnosine structure and biological activity in different body areas even though, probably because its predominant localization, numerous studies have initially considered the main physiological role exerted by this dipeptide in the context of muscle (Severin et al., 1953a; Boldyrev and Petukhov, 1978; Stvolinskii et al., 1992; Kramarenko et al., 2001). In fact, it has been shown the ability of carnosine to strongly contribute to “Severin's phenomenon” (Severin et al., 1953b), prevent intramuscular acidification by decreasing lactate accumulation (Stvolinskiĭ et al., 1992), regulate muscles energy metabolism (Rubtsov, 2001), and enhance physical performance and executive functions (Glenn et al., 2015; de Andrade Kratz et al., 2017; Brisola et al., 2018; Furst et al., 2018), just to summarize the most relevant pharmacological activities. As a consequence of its numerous activities, that includes the ability to amiliorate intramuscular buffering capacity, carnosine has already been considered as a “natural endogenous antidote” (Caruso et al., 2022b).
Moving out from the muscle context, carnosine has shown to act as neurotransmitter (Tiedje et al., 2010), ameliorate cell energy metabolism (Caruso et al., 2019c; Fresta et al., 2020), enhance immune response (Mal'tseva et al., 1992), regulate the metabolism of RNS (including nitric oxide (NO)) (Caruso et al., 2017b, 2021a; Fresta et al., 2018), exert anti-glycation and anti-aging activities (Boldyrev et al., 1999; Pepper et al., 2010), and sequestrate and/or chelate heavy metals (Brown and Antholine, 1979; Hasanein and Felegari, 2017). Carnosine also possesses a well-demonstrated ability to modulate the glutamatergic system by up-regulating the activity of glutamate transporter 1 (GLT-1), leading to the reduction of glutamate levels in the central nervous system (CNS) and therefore preventing excitotoxicity (Ouyang et al., 2016).
With specific regard to carnosine metabolism, it is accountable to eight different proteins: CARNS1 is responsible for its synthesis, CNDP1 and CNDP2 are responsible for carnosine degradation, and carnosine N-methyltransferase (CARNMT1) regulates carnosine methylation. Four additional proteins are carnosine transporters (Caruso et al., 2019b).
In human brain, CARNS1 is only expressed in oligodendrocytes (while in mice it is expressed only in newly formed and myelinating oligodendrocytes) which are also accountable for most of its degradation, occurring through CNDP1 activity (Caruso et al., 2019b). Astrocytes possess the transporters essential for the uptake of carnosine, meaning that these cells somehow use this dipeptide (Bauer, 2005), even though they are not able to synthesize it, probably because β-alanine would interfere with gamma-aminobutyric acid (GABA) reuptake (Shimizu et al., 2007). Another cell type able to manage carnosine is microglia (Caruso et al., 2019b).
The first evidence making a correlation between carnosine and CNS physiology is represented by the deficits due its insufficiency. Carnosinaemia is a pathology characterized by a degenerative alteration of the CNS, that often emerges contemporary to carnosinuria, a condition due to an excess of carnosine in the urine, and limited or, in the worst scenario, absent serum carnosinase activity (Perry et al., 1968). Autopsy in a post-mortem brain of a patient with the above-mentioned deficiencies also shows axonal degeneration and “sferoids” in grey matter, demyelination, and fibrosis, along with Purkinje cells’ loss (Caruso et al., 2019b). Several other pathologies have been linked to a deficit of carnosine such as age-related macular degeneration (Chao de la Barca et al., 2020) and AD (Fonteh et al., 2007; Caruso et al., 2021b).
Different pharmacological activities, including the anti-aggregant, antioxidant, and anti-inflammatory ones, have been demonstrated for carnosine, reason why its therapeutic potential has been considered in numerous diseases including cerebral ischemia (Hu and Chen, 2012), CVD (Caruso et al., 2020a), T2DM, depression and dementia (Hipkiss, 2017; Caruso et al., 2019a), cancer (Turner et al., 2021), Parkinson's disease (PD) (Hipkiss, 2018), AD (Hipkiss, 2007; Iqbal and Grundke-Iqbal, 2010), and, very recently, COVID-19 including long-term complications of this infection (Hipkiss, 2020).
3. Carnosine and diseases
As mentioned above, the therapeutic potential of carnosine has been investigated in several diseases and the following sections will explore in more detail the state of the art of carnosine involvement in the pathophysiology of AD, T2DM, and CVD, all pathologies characterized by oxidative and nitrosative stress, inflammation, and aberrant protein aggregation.
3.1. Alzheimer's disease (AD)
AD represents the most common kind of primary dementia and it places a heavy social and financial cost worldwide. The main clinical characteristics of AD are represented by memory deficit, cognitive deterioration, and neuropsychiatric symptoms (Ballard et al., 2009; Caruso et al., 2022c).
The two hallmark of AD, intracellular aggregates of tau protein (neurofibrillary tangles) and senile plaques containing amyloid-β (Aβ), have been studied for both their potential interactions and their synergistic effects in inducing neuronal death in AD brain (Caruso et al., 2019f).
According to the revised amyloid hypothesis, the 42-amino acid form of Aβ (Aβ1-42) oligomers represent the main toxic molecules that trigger a complex pathogenic cascade that results, among all, in the hyperphosphorylation of tau protein and synaptic dysfunction, ending with neuronal cell death (Giacobini and Gold, 2013; Musiek and Holtzman, 2015; Selkoe and Hardy, 2016). Moreover, it has been shown that monomeric Aβ1-42 exerts a key function in neuronal survival, neuronal glucose homeostasis, as well as in learning and memory processes (Giuffrida et al., 2015; Caruso et al., 2022a). Thus, it has been proposed that in the early stages of AD, the occurrence of Aβ1-42 aggregation may consume monomers (that are converted into oligomers) and decrease trophic support given to neuronal cells, leading to the onset of cognitive deficits and contributing to AD pathology (Caruso et al., 2017a; Copani, 2017).
Carnosine has been shown to inhibit the aggregation of Aβ in the CNS of transgenic mice models of AD (Corona et al., 2011) and it reduces the in vitro fibrillogenesis of Aβ1-42 (Aloisi et al., 2013; Attanasio et al., 2013). It has been hypothesized that carnosine interferes with both the elongation phase of the fibrils as well as the globular oligomeric species in the assembling phase.
Neuroinflammation is commonly recognized as a core factor in the pathophysiology of AD (Sastre et al., 2006), with a reciprocal interaction between microglia and astrocytes promoted by Aβ oligomers (Schwab and McGeer, 2008). The over-production of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, has been shown to be deeply involved in AD, while the levels of the anti-inflammatory mediator transforming growth factor (TGF)-β1 are reduced in AD (Caruso et al., 2019e). Furthermore, Aβ aggregates have been demonstrated to disrupt Ca2+ homeostasis, raising oxidative stress levels, thus promoting synaptotoxicity and neuronal death (Pritam et al., 2022). Moreover, metabolic alterations have been linked to AD pathogenesis since advanced glycation end products (AGEs) were also found in Aβ plaques (Fernàndez-Busquets et al., 2010).
It is well-established that a lack of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) (Iulita and Cuello, 2014) contributes significantly to neurodegeneration in the AD brain (Cuello, 1996; Iulita and Cuello, 2014; Song et al., 2015b) and a deficit of BDNF, and its receptor tropomyosin receptor kinase B (TrkB), occuring early in AD (Cotman, 2005; Song et al., 2015a). Interestingly, in recent studies, carnosine has been proven to cross the blood brain barrier and promote the release of neurotrophins such as BDNF and NGF from glial cells (Yamashita et al., 2018).
We have previously hypothesized (Caruso et al., 2019b, 2019e) and we have preliminary data suggesting the ability of carnosine to preserve the levels of Aβ monomers by inhibiting their aggregation into Aβ globular oligomers, or to dissociate the aggregates that are already formed. A main goal for our future studies is to validate the preclinical efficacy of carnosine in different experimental and animal models of AD to then plan Phase I/II clinical studies in patients with mild cognitive impairment and early AD.
3.2. Type 2 Diabetes Mellitus (T2DM)
Diabetes mellitus is a metabolic condition characterized by excessively high blood glucose levels (hyperglycemia). Type 1 diabetes (no insulin production) and T2DM (impaired insulin responsiveness and pancreatic cell failure) are the two most frequent types of diabetes. Insulin insensitivity due to insulin resistance, decreased insulin production, and pancreatic failure are the main characteristics of T2DM (Kahn, 1994; Robertson, 1995; Chen et al., 2012). Diabetes complications include ketoacidosis, neuropathy, retinopathy, nephropathy, nerve damage, and atherosclerosis, all contributing to the severity and death of diabetic patients.
It is well-recognized that hyperglycemia and hyperlipidemia occurring in T2DM are strictly correlated with oxidative stress and inflammation, and they are involved in the development of diabetic complications (Lee et al., 2005; Baye et al., 2016; Han et al., 2018) and in pancreatic β-cell disruption (Miceli et al., 2018). Moreover, it is well-known the occurrence of amylin aggregation in T2DM pancreatic beta cells (Jaikaran and Clark, 2001; Caruso et al., 2017a, 2018), with different proposed cytotoxic molecular mechanisms (Betsholtz et al., 1989; Johnson et al., 1992), including oxidative stress due to ROS generation (Mattson and Goodman, 1995; Lazzarino et al., 2018).
Different studies have investigated the neurobiological link between carnosine metabolism and T2DM. From a genetic point of view, carnosinase 1 gene polymorphisms (Qiu et al., 2022) and CNDP1 gene (Janssen et al., 2005; Riedl et al., 2007) have been linked to diabetic kidney disease. Moreover, it has been shown the protective effect of carnosine on human kidney cells under hyperglycemic conditions (Hipkiss et al., 1998a; Janssen et al., 2005), and the metabolic control it exerts in diabetes (Riedl et al., 2011; Peters et al., 2014), as well as the correlation between serum carnosinase levels and nephropathy susceptibility in animal models (Albrecht et al., 2017), suggesting a kidney-protective effect of carnosine (Kurata et al., 2006; Albrecht et al., 2017).
Several studies have suggested the therapeutic potential of carnosine in preventing complications in T2DM (Hipkiss, 2017). Clinical evidence shows the efficacy of carnosine supplementation on glucose, triglycerides, and TNF-α levels in T2DM patients as an add-on to antidiabetic agents (Houjeghani et al., 2018).
Diabetes microvascular complications are due to the excessive production of AGEs (Babizhayev et al., 2013). Carnosine in this context has been demonstrated able to react with reactive carbonyl species (RCS) and inhibit their formation (Aldini et al., 2014).
To summarize, many evidence suggests the potential therapeutic role that carnosine could exert against T2DM being an antioxidant, anti-glycation, and anti-nitrating compound, also able to affect glycemic control, and to prevent diabetic complications (Lee et al., 2005; Baye et al., 2016).
3.3. Cardiovascular disease (CVD)
The term “cardiovascular disease” (CVD) takes into account different pathologies (Stewart et al., 2017), including atherosclerosis, representing the main leading cause of CVD. Atherosclerosis is characterized by both hardening and narrowing of arteries as well as by plaques formation in the inner coronary artery walls (Mehta et al., 1998). The formation of these plaques is associated to the release of hormones, growth factors, and pro-inflammatory and pro-oxidant mediators, as a consequence of the interaction between endothelial cells, low-density lipoproteins (LDLs), and immune cells such as macrophages (Zwaka et al., 2001).
The therapeutic potential of carnosine in the context of CVD and in the prevention of cardiometabolic risk factors has been considered (Menon et al., 2021; Feehan et al., 2022), with most of the studies focusing on atherosclerosis. Barski et al., by employing an atherosclerosis model consisting of ApoE−/− mice fed with a high fat diet, demonstrated the ability of dietary carnosine supplementation to prevent the formation of early atherosclerotic lesions, that was mainly mediated by its positive modulation of oxidative stress-related phenomena (Barski et al., 2013; Caruso et al., 2020a). The same animal model was used by other research groups obtaining similar results, i.e. attenuation of atherosclerosis and renal disease through the reduction of oxidative stress and inflammation (Menini et al., 2012). An additional study by Brown and collaborators, employing a streptozotocin-induced diabetic ApoE−/− mice subjected to prolonged oral supplementation with carnosine showed a significant decrease of triglycerides and plaques formation paralleled by an increased recruitment of macrophages (Brown et al., 2014). Carnosine treatment has also shown to provide macro- and microvascular protection, attenuating the progression of macroangiopathy and favoring the development of more stable lesions, in a study evaluating aortic and renal lesions as well as protein and gene expression of disease markers in diabetic mice (Menini et al., 2015).
Carnosine supplementation (pre-treatment) has shown the ability to decrease the size of ischemic lesions in the heart, brain, liver, and kidney of animal models of ischemia (Fujii et al., 2003; Dobrota et al., 2005; Fouad et al., 2007; Rajanikant et al., 2007; Shen et al., 2008; Dursun et al., 2011). Carnosine, administered intraperitoneally, protected the heart, also improving its function, by counteracting cardiac ischemia in rats; the protective effects exerted by carnosine were also related to its ability to decrease the lipid peroxidation of cell membranes (Dursun et al., 2011).
Carnosine has also demonstrated to protect against cerebral ischemia in rats (Rajanikant et al., 2007), probably as a consequence of penumbra reduction. In addition to the above, carnosine administration significantly decreased ischemia/reperfusion-induced renal dysfunction (Fujii et al., 2003) as well as liver damage in rats (Fouad et al., 2007). The ability of carnosine to protect mast cells against the ischemic damage due to oxygen glucose deprivation has also been demonstrated (Shen et al., 2008).
Carnosine is able to prevent the formation of AGEs and advanced lipoxydation endproducts through RCS detoxification, a mechanism probably responsible for the protective effects of carnosine exerted in the context of pathologies such as atherosclerosis and related diabetic complications (Ghodsi and Kheirouri, 2018; Gilardoni et al., 2020; Aldini et al., 2021). The neuroprotective effects of carnosine combined with its antioxidant and cardioprotective activity suggests that this dipeptide might represent an ideal pharmacological tool for future clinical studies in patients with diabetes where both cerebrovascular and cardiovascular complications often coexist.
4. Cellular and molecular mechanisms modulated by carnosine
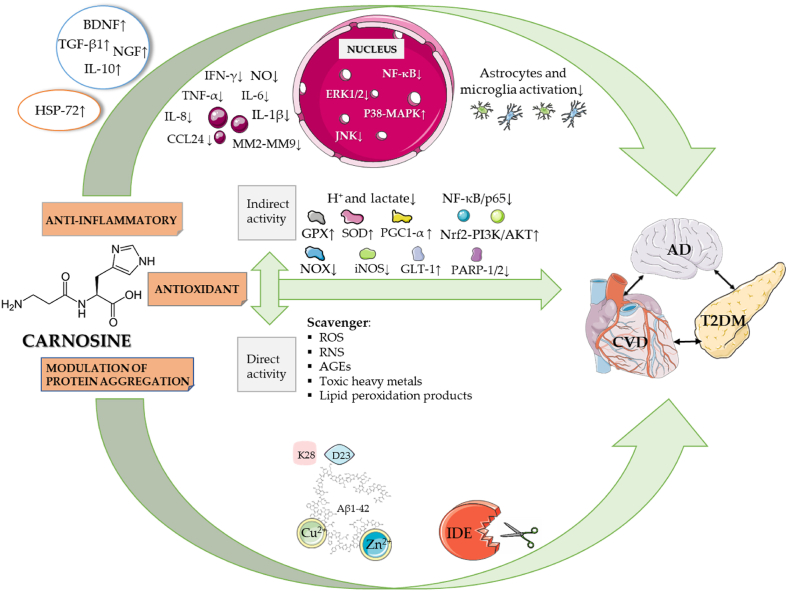
Several studies have shown the different activities that carnosine could exert, among which the antioxidant, anti-inflammatory, and antiaggregant ones are under our attention in the context of this review, them being the most useful in the perspective of a therapeutical approach, for our present knowledge (Fig. 2).
Fig. 2.
Multimodal mechanism of action of carnosine. Part of this figure was created with https://smart.servier.com.
This starting from the well-established theory according to which oxidative stress and pro-inflammatory phenomena (occurring because of it) have a central role in numerous detrimental conditions, including T2DM, cancer, CVD, and AD (Gella and Durany, 2009; Cervantes Gracia et al., 2017; Oguntibeju, 2019; Alhakamy et al., 2020; Hayes et al., 2020) as previously discussed. The three phenomena which are below presented as separated are indeed interconnected in a unique pharmacodynamic profile and the modulation exerted by carnosine should be reconsidered in the perspective of a systemic protective effect towards the organism.
4.1. Direct and indirect antioxidant activity
The aerobic metabolism leads to ROS and RNS physiological production (Mainz et al., 2012; de Campos et al., 2015) and, because of this, cells have developed a molecular machinery to neutralize it, in order to avoid the damages they can cause to proteins and membranes. The equilibrium between production and neutralization is strictly regulated so that, when an unbalance occurs, it activates a signaling process that alerts the whole organism of the excessive production of those species or of the deficit in the antioxidant mechanisms leading to a condition of oxidative or nitrosative stress (Birben et al., 2012; Gunasekara et al., 2014).
In vitro and in vivo studies have assessed the antioxidant activity of carnosine, both in a direct way, with carnosine acting as a scavenger, and indirectly, with an enhancement of the antioxidant machinery, concurrent to a decrease in the pro-oxidant/pro-inflammatory enzymes (Feehan et al., 2022).
Carnosine exerts its direct antioxidant activity acting as a non-enzymatic free-radical scavenger: it interacts with products of lipid peroxidation (Gorbunov and Erin, 1991), neutralizes toxic heavy metals (Baran, 2000; Reddy et al., 2005), and decreases the intracellular levels of numerous ROS/RNS among which superoxide and hydroxyl radicals (Gorbunov and Erin, 1991; Caruso et al., 2017c), NO (Caruso et al., 2017b), and carbonyl species (Aldini et al., 2005). In particular, concerning this last, a relevant pharmacological activity of carnosine has been demonstrated against aldehydes-induced toxicity, both in vitro (Hipkiss et al., 1997, 1998a) and in vivo (Nagasawa et al., 2001). The dipeptide is able, thanks to its histidine imidazole moiety (Guiotto et al., 2005a), to scavenge molecules of different nature, from glucose to the highly toxic malondialdehyde (Hipkiss et al., 1998a; Decker et al., 2000; Guiotto et al., 2005b). Carnosine has also shown to react with low molecular weight aldehydes (like AGEs), α,β-unsaturated aldehydes deriving from unsaturated fatty acids under oxidative stress (e.g., 4-hydroxynonenal (HNE) and trans-2-hexenal (Zhou and Decker, 1999)), and catecholaldehydes (products of the metabolism of dopamine and norepinephrine by monoamine oxidase (MAO) whose activity is strongly impaired by oxidative stress) (Jinsmaa et al., 2009; Nelson et al., 2019). Thanks to this scavenger activity carnosine is able to impede the consequences of aldehyde-induced protein-protein and DNA-protein cross-linking as well as the formation of protein carbonyl groups and glycated proteins (Vinson and Howard, 1996; Hipkiss et al., 1998a; Hipkiss et al., 1998b; Swearengin et al., 1999; Guiotto et al., 2005b) by forming “carnosinylated” proteins (Tanaka and Kawahara, 2020) through a reaction of trans-glycation.
Carnosine has also shown to exert an antioxidant activity, even stronger than the one of glutathione (which is involved in one of the most important detoxifying systems) (Lazzarino et al., 2019), when, in presence of hydrogen peroxide (H2O2), its imidazole ring is oxidized. The derived 2-oxo-carnosine was found in neuroblastoma cells SH-SY5Y, which stably express CARNS1 (Dias et al., 2013; Ihara et al., 2019). In addition, the imidazole ring of carnosine can react with hypochlorous acid , leading to the formation of an imidazole chloramine and then protecting cells from the toxicity of the compound (Pattison and Davies, 2006; Boldyrev et al., 2013; Aldini et al., 2021).
The indirect antioxidant activity of carnosine is instead related to an enhancement of the endogenous antioxidant system (Choi et al., 1999; Ukeda et al., 2002), with a clear example represented by the rescue of nuclear factor erythroid 2–related factor 2 (Nrf2) pathway by increasing its nuclear translocation (Alsheblak et al., 2016). This pharmacological activity of carnosine can become clinically-relevant in the prevention of drug-induced neuro- and cardiotoxicity (Caruso et al., 2022b); in fact, Nrf2 regulates the expression of numerous genes, including but not limited to thioredoxin 1, superoxide dismutase-1 (SOD1), or catalase (Mou et al., 2020; Aldini et al., 2021), together with enzymes able to extinguish carbonyl groups reactivity by reducing them to alcohol or other less reactive compounds and so being implicated in the anti-glycating activity above described, reducing toxicity of methylglyoxal and AGEs (Aldini et al., 2021; Solana-Manrique et al., 2022). According to this evidence, it has been hypothesized that Nrf2 plays a pivotal role in mediating the neuroprotective and antioxidant activity of carnosine. The proposed mechanism that leads carnosine to modulate the Nrf2 pathway involves the activation of phosphatidylinositol-3-kinase (PI3K)/AKT signaling pathway (Zhao et al., 2019).
The opposite mechanism (with the same final aim) was demonstrated for carnosine in preventing the translocation into the nucleus induced by H2O2 of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)/p65, which is an important redox-sensitive transcription factor in pancreatic islet cells, protecting cells and consequently preserving insulin synthesis (Miceli et al., 2018).
In this context, the activity of carnosine has been deeply investigated in microglial murine cultures (BV-2) where carnosine was able to prevent cell death in cells treated with Aβ oligomers through different mechanisms (Caruso et al., 2019d); carnosine diminished both NO and superoxide production as well as the expression of NADPH oxidase and inducible nitric oxide synthase pro-oxidant enzymes, responsible for their production. In addition to the above, carnosine is also able to modulate the activity of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), a transcription coactivator involved in biological pathways among which glucose and fatty acid metabolism, lowering downstream oxidative stress level and potentiating the expression of anti-inflammatory cytokines, endowed with an antioxidant activity such as IL-10 (Liang and Ward, 2006). Other studies conducted both in vivo and in vitro have demonstrated that carnosine is able to increase the activity of SOD and glutathione peroxidase (GPX) (Aydin et al., 2010; Alpsoy et al., 2011).
Carnosine is also able to reduce the expression of poly (ADP-ribose) polymerase-1 (PARP-1) and −2 (PARP-2) in primary rat astrocytes and oligodendrocytes (Spina-Purrello et al., 2010) and instead to maintain the expression of GLT-1 in astrocytes exposed to ischemia both in vitro and in vivo (Shen et al., 2010), decreasing the excitotoxicity that would otherwise follow the uncontrolled release of this neurotransmitter (Rodrigo et al., 2013; Chamorro et al., 2016), which contributes to oxidative stress damages in different neurodegenerative disorders including stroke (Coyle and Puttfarcken, 1993). Moreover, directly binding Cu2+ and Zn2+, known to be direct inhibitors of the N-methyl-D-aspartate (NMDA) glutamate receptor, carnosine might also act as a negative allosteric modulator of the glutamatergic system (Boldyrev et al., 2013; Kawahara et al., 2018).
Carnosine is also able to prevent lactate accumulation and excessive acidification acting as a proton buffer, not only at a muscle level, but also in the oligodendrocytes, where it has been demonstrated that the alteration of the lactate shuttle (which functions depends on the consumption of a proton (Broer et al., 1997)) promotes axonal degeneration (Keytsman et al., 2018).
According to all the evidence discussed above, it has been shown that under stress conditions macrophages have a 3-fold increased uptake of carnosine (Fresta et al., 2017), and, then not surprisingly, different studies have shown a good preclinical efficacy of this dipeptide in preventing brain function integrity and neuronal homeostasis against oxidative damage (Gallant et al., 2000; Min et al., 2008; Tsai et al., 2010).
4.2. Anti-inflammatory activity
An inflammation state derives from an increased production of pro-inflammatory mediators (e.g., TNF-α, IL-1β, and IL-6) and a lowered release of anti-inflammatory ones (e.g., TGF-β1 and IL-10) (Kitaura-Inenaga et al., 2003), and involves almost all the cells of the different interested tissues. Focusing on neuroinflammation, it has been demonstrated that neurons and glial cells, including microglia, play a key role in these events (Shastri et al., 2013; Caruso et al., 2020b).
T2DM, AD, and CVD are the ideal examples of the pathological conditions with a high incidence and prevalence that can lead to systemic inflammation, which is the physiological body response to damage. As every physiological response, it becomes deleterious when persistent, ending in failure of the control mechanisms responsible for its shutting down (Singh et al., 2019).
The anti-inflammatory activity of carnosine has firstly been described by Nagai's group, in the context of the inhibition of edema formation and allergic responses (Nagai et al., 1970, 1971; Nagai, 1971). From that study, different applications have been found and tested in different experimental models (Suzuki and Nagai, 1974).
The molecular mechanisms underlying these anti-inflammatory responses are however different, displaying carnosine as a dipeptide exerting a multimodal activity. Focusing on an example related to CNS and neuroinflammation, carnosine has shown to exert anti-inflammatory activity by decreasing astrocytic activation and interferon (IFN)-γ release in a mouse model of subcortical ischemic vascular dementia (Ma et al., 2015). In addition to this, carnosine was also demonstrated able to decrease the secretion of pro-inflammatory cytokines, when supplemented in BV-2 cells, simultaneously increasing the synthesis and the release of TGF-β1 (Caruso et al., 2019e). The release of TGF-β1 has also been demonstrated to be diminished by carnosine at a kidney level thus diminishing matrix accumulation and the related pathologies (such as diabetic nephropathy) (Köppel et al., 2011). According to this evidence, carnosine is able to exert a stabilizing pharmacological activity on the TGF-β1 pathway, reducing, where necessary, its overactivation in tissues such as liver and lung (e.g. during fibrosis), and rescuing its levels and promoting neuroprotection in neurodegenerative disorders such as stroke and AD.
Carnosine supplementation, together with anserine (in an anserine/carnosine ratio of 3:1) for 3 months reduced the expression of the C–C motif inflammatory cytokine ligand 24 (CCL24) (Katakura et al., 2017). The dipeptide object of our review was also capable to diminish the expression and activity of matrix metalloproteinase-2 (MMP-2) (human fibrosarcoma HT1080 cells (Kim et al., 2014)) contemporary inhibiting its upstream process linked to the pro-urokinase plasminogen activator signaling pathway, and MMP-9 (endothelial SK-Hep-1 cells (Chuang and Hu, 2008) and human fibrosarcoma HT1080 cells (Kim et al., 2014)), which are physiologically involved in the modulation of the extracellular matrix and pathologically related to CVD (Death et al., 2003).
Carnosine in the CNS acts on microglial cells, which are the monocyte-macrophage line specialization in the tissue, representing the immune system. Because of this, not only carnosine, but also histamine (precursor of histidine, one of the two components of the dipeptide) has been linked to the motility and structural plasticity of microglial cells as well as to the regulation of IL-1β release (O'Mahony et al., 2011; Ferreira et al., 2012). The regulation of these mechanisms involves the modulation of a specific potassium channel named two-pore domain potassium channel (THIK-1) (Madry et al., 2018).
A particular form of carnosine, the one chelating the zinc ion, also called Zinc L-carnosine (or polaprezinc) is able to downregulate the activation of NF-κB as well as the expression of IL-8 in gastric epithelial cells (Shimada et al., 1999), also inducing heat shock protein-72 (Hsp-72) (Odashima et al., 2006; Watari et al., 2013). The same compound acts on macrophages inhibiting the LPS-stimulated expression of pro-inflammatory mediators (Ooi et al., 2016). Even more interestingly, zinc and copper ions are able to inhibit NMDA receptors activity, an additive mechanism of neuroprotection towards glutamate excitotoxicity given by carnosine (as discussed above, with the increase of GLT-1 expression) (Boldyrev et al., 2013; Kawahara et al., 2018; Solana-Manrique et al., 2022).
Moreover, carnosine has been shown to reduce the phosphorylation level of extracellular regulated kinases 1 and 2 (ERK1/2) and p38 mitogen-activated protein kinase (MAPK) in mesangial cells (with promising effects for diabetic nephropathy therapy (Jia et al., 2009)). This suppressive effect on ERK1/2 with a downstream regulation of the cell cycle was also demonstrated in rat neuronal cells (Kulebyakin et al., 2012), with all of these pharmacological activities of carnosine significantly contributing to the systemic anti-inflammatory and protective effects of the dipeptide.
4.3. Modulation of protein aggregation
The first demonstrations of carnosine acting as an anti-protein-cross-linking agent came in 1995, with the work of Hipkiss and colleagues (Hipkiss et al., 1995). This activity has been accounted to the presence of imidazole functional group part of histidine (Hobart et al., 2004) and well-described in vitro for α-crystallin, both preventing its aggregation and disassembling the already formed aggregates, then helping to maintain the physiological chaperone function of the protein (Seidler et al., 2004; Attanasio et al., 2009; Javadi et al., 2017).
Other studies have investigated the activity of carnosine towards the inhibition of Aβ1-42 fibrils/aggregates formation (Aloisi et al., 2013), which has been hypothesized being related to its ability to perturb the hydrogen bonds around residues that are pivotal for fibrillogenesis. One study in particular evidenced the amino acids comprised between the 17th and the 21st (17LVFFA21) as the main target for carnosine activity (Attanasio et al., 2013), while in another study conducted through an in silico docking approach the 23rd (D) and 28th (K) amino acids emerged as fundamental. This last cited study has analyzed 89 different compounds, including homocarnosine (gamma-aminobutyryl-histidine), anserine , balenine (N(beta)-Alanyl-1-methyl-histidine), and 86 inhibitors of the Aβ1-42 aggregation, predicting carnosine as the best ligand among the natural histidine-containing dipeptides tested, with a very good preclinical efficacy compared to the other inhibiting compounds. The specific activity against the aggregation, according to this study, involves the arrangement of carnosine in a coiled region localized between the two β-sheet portion of Aβ peptide under the folded conformational state that is acquired by the peptide during the process of fibril polymerization (Lührs et al., 2005)), where D23 and K28 are located, playing a fundamental role in the process of aggregation (Lührs et al., 2005). In particular, it has been demonstrated that the imidazole ring of L-histidine part of carnosine is able to bind the D23 residue of Aβ1-42, while β-alanine interacts with the K28 residue, counteracting the binding that would otherwise occur between the D23 of an Aβ residue and the K28 of the adjacent one, finally inhibiting the process of fibrillogenesis. These studies confirm the results that had recently been obtained from in vitro experiments (Grasso et al., 2017; Zhang et al., 2018).
Together with this deeply demonstrated mechanism, a more general one has been proposed to explain the anti-aggregant activity of carnosine, being this dipeptide a well-characterized chelator of Cu2+ and Zn2+ (Baran, 2000), which are well-known enhancers of Aβ aggregation (Miller et al., 2010; Bin et al., 2013); carnosine can slow down the process of aggregation through sequestering the above cited metals. A different mechanism by which carnosine inhibits the aggregation is stimulating the degradation exerted by the insulin degrading enzyme (IDE), which is an enzyme secreted by microglial cells. Carnosine has been shown to stimulate its activity towards not only Aβ but also insulin, both of which are the so called long-substrates of the enzyme. One of the mechanisms proposed is the modulation that carnosine may have in favoring the oligomerization and therefore the activation of the IDE, finally preventing Aβ-induced toxicity in neuronal cultures (Distefano et al., 2022).
5. Conclusions
Several studies have been carried out in the last 70 years to assess the structure, role, function, and biological activities of carnosine, and many of them have led to evidence of the multimodal mechanism of action of carnosine, that passes through its anti-aggregant, antioxidant, and anti-inflammatory properties, which are of main interest in the case of T2DM, CVD, and AD as well as in the case of many others systemic and neurodegenerative disorders.
In the present review we have examined the protective role that carnosine could exert in the context of different diseases, such as T2DM, CVD, and AD, which show common pathogenic mechanisms including oxidative stress, inflammation, and aggregation phenomena. The impact of these mechanisms can change in the pathophysiology of these three diseases, with a more prevalent role of one mechanism in one disease compared to the others (e.g. Aβ aggregation in AD pathogenesis compared to systemic inflammation in CVD). The multimodal pharmacodynamic profile of carnosine combines the systemic anti-inflammatory and antioxidant activities with its anti-aggregant and neuroprotective efficacy in the CNS. This enlarged pharmacological activity offers the possibility to explore the therapeutic potential of carnosine in all the three diseases, in particular in patients with T2DM, who often show an history of comorbidity with CVD and also have an increased risk to develop mild cognitive impairment and AD.
Funding
This research was funded by the Italian Ministry of University and Research, PRIN2017 - Program of Relevant National interest - 2017AY8BP4004, to F.C., and by the Italian Ministry of Health Research Program, RC2022-N4, to G.C.
CRediT authorship contribution statement
Giuseppe Caruso: Conceptualization, Writing – review & editing, Supervision. Lucia Di Pietro: Writing – original draft. Vincenzo Cardaci: Writing – original draft. Salvatore Maugeri: Writing – original draft. Filippo Caraci: Conceptualization, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare no conflict of interest.
Acknowledgments
L.D. and V.C. would like to thank the Scuola Superiore di Catania at the University of Catania. Part of Fig. 1, Fig. 2 has been generated by using Servier Medical Art available at smart.servier.com.
Contributor Information
Giuseppe Caruso, Email: giuseppe.caruso2@unict.it.
Lucia Di Pietro, Email: dptlcu00@gmail.com.
Vincenzo Cardaci, Email: vincenzocardaci9@gmail.com.
Salvatore Maugeri, Email: salvo-maugeri@hotmail.com.
Filippo Caraci, Email: fcaraci@unict.it.
Data availability
No data was used for the research described in the article.
References
- Albrecht T., Schilperoort M., Zhang S., Braun J.D., Qiu J., Rodriguez A., Pastene D.O., Krämer B.K., Köppel H., Baelde H., de Heer E., Anna Altomare A., Regazzoni L., Denisi A., Aldini G., van den Born J., Yard B.A., Hauske S.J. Carnosine attenuates the development of both type 2 diabetes and diabetic nephropathy in BTBR ob/ob mice. Sci. Rep. 2017;7 doi: 10.1038/srep44492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldini G., Carini M., Yeum K.J., Vistoli G. Novel molecular approaches for improving enzymatic and nonenzymatic detoxification of 4-hydroxynonenal: toward the discovery of a novel class of bioactive compounds. Free Radic. Biol. Med. 2014;69:145–156. doi: 10.1016/j.freeradbiomed.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Aldini G., de Courten B., Regazzoni L., Gilardoni E., Ferrario G., Baron G., Altomare A., D'Amato A., Vistoli G., Carini M. Understanding the antioxidant and carbonyl sequestering activity of carnosine: direct and indirect mechanisms. Free Radic. Res. 2021;55(4):321–330. doi: 10.1080/10715762.2020.1856830. [DOI] [PubMed] [Google Scholar]
- Aldini G., Facino R.M., Beretta G., Carini M. Carnosine and related dipeptides as quenchers of reactive carbonyl species: from structural studies to therapeutic perspectives. Biofactors. 2005;24(1–4):77–87. doi: 10.1002/biof.5520240109. [DOI] [PubMed] [Google Scholar]
- Alhakamy N.A., Badr-Eldin S.M., U A.F., Alruwaili N.K., Awan Z.A., Caruso G., Alfaleh M.A., Alaofi A.L., Arif F.O., Ahmed O.A.A., Alghaith A.F. Thymoquinone-loaded soy-phospholipid-based phytosomes exhibit anticancer potential against human lung cancer cells. Pharmaceutics. 2020;12(8) doi: 10.3390/pharmaceutics12080761. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Aloisi A., Barca A., Romano A., Guerrieri S., Storelli C., Rinaldi R., Verri T. Anti-aggregating effect of the naturally occurring dipeptide carnosine on abeta1-42 fibril formation. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpsoy L., Akcayoglu G., Sahin H. Anti-oxidative and anti-genotoxic effects of carnosine on human lymphocyte culture. Hum. Exp. Toxicol. 2011;30(12):1979–1985. doi: 10.1177/0960327111404908. [DOI] [PubMed] [Google Scholar]
- Alsheblak M.M., Elsherbiny N.M., El-Karef A., El-Shishtawy M.M. Protective effects of L-carnosine on CCl4 -induced hepatic injury in rats. Eur. Cytokine Netw. 2016;27(1):6–15. doi: 10.1684/ecn.2016.0372. [DOI] [PubMed] [Google Scholar]
- Attanasio F., Cataldo S., Fisichella S., Nicoletti S., Nicoletti V.G., Pignataro B., Savarino A., Rizzarelli E. Protective effects of L- and D-carnosine on alpha-crystallin amyloid fibril formation: implications for cataract disease. Biochemistry. 2009;48(27):6522–6531. doi: 10.1021/bi900343n. [DOI] [PubMed] [Google Scholar]
- Attanasio F., Convertino M., Magno A., Caflisch A., Corazza A., Haridas H., Esposito G., Cataldo S., Pignataro B., Milardi D., Rizzarelli E. Carnosine inhibits Abeta(42) aggregation by perturbing the H-bond network in and around the central hydrophobic cluster. Chembiochem. 2013;14(5):583–592. doi: 10.1002/cbic.201200704. [DOI] [PubMed] [Google Scholar]
- Aydin A.F., Küskü-Kiraz Z., Doğru-Abbasoğlu S., Güllüoğlu M., Uysal M., Koçak-Toker N. Effect of carnosine against thioacetamide-induced liver cirrhosis in rat. Peptides. 2010;31(1):67–71. doi: 10.1016/j.peptides.2009.11.028. [DOI] [PubMed] [Google Scholar]
- Babizhayev M.A., Lankin V.Z., Savel'Yeva E.L., Deyev A.I., Yegorov Y.E. Diabetes mellitus: novel insights, analysis and interpretation of pathophysiology and complications management with imidazole-containing peptidomimetic antioxidants. Recent Pat. Drug Deliv. Formulation. 2013;7(3):216–256. doi: 10.2174/1872211307666131117121058. [DOI] [PubMed] [Google Scholar]
- Ballard C.G., Gauthier S., Cummings J.L., Brodaty H., Grossberg G.T., Robert P., Lyketsos C.G. Management of agitation and aggression associated with Alzheimer disease. Nat. Rev. Neurol. 2009;5(5):245–255. doi: 10.1038/nrneurol.2009.39. [DOI] [PubMed] [Google Scholar]
- Baran E.J. Metal complexes of carnosine. Biochemistry (Mosc.) 2000;65(7):789–797. [PubMed] [Google Scholar]
- Barski O.A., Xie Z., Baba S.P., Sithu S.D., Agarwal A., Cai J., Bhatnagar A., Srivastava S. Dietary carnosine prevents early atherosclerotic lesion formation in apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 2013;33(6):1162–1170. doi: 10.1161/ATVBAHA.112.300572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer K. Carnosine and homocarnosine, the forgotten, enigmatic peptides of the brain. Neurochem. Res. 2005;30(10):1339–1345. doi: 10.1007/s11064-005-8806-z. [DOI] [PubMed] [Google Scholar]
- Baye E., Ukropcova B., Ukropec J., Hipkiss A., Aldini G., de Courten B. Physiological and therapeutic effects of carnosine on cardiometabolic risk and disease. Amino Acids. 2016;48(5):1131–1149. doi: 10.1007/s00726-016-2208-1. [DOI] [PubMed] [Google Scholar]
- Beesu M., Caruso G., Salyer A.C., Shukla N.M., Khetani K.K., Smith L.J., Fox L.M., Tanji H., Ohto U., Shimizu T., David S.A. Identification of a human toll-like receptor (TLR) 8-specific agonist and a functional pan-TLR inhibitor in 2-aminoimidazoles. J. Med. Chem. 2016;59(7):3311–3330. doi: 10.1021/acs.jmedchem.6b00023. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Christmansson L., Engström U., Rorsman F., Svensson V., Johnson K.H., Westermark P. Sequence divergence in a specific region of islet amyloid polypeptide (IAPP) explains differences in islet amyloid formation between species. FEBS Lett. 1989;251(1–2):261–264. doi: 10.1016/0014-5793(89)81467-x. [DOI] [PubMed] [Google Scholar]
- Bin Y., Li X., He Y., Chen S., Xiang J. Amyloid-beta peptide (1-42) aggregation induced by copper ions under acidic conditions. Acta Biochim. Biophys. Sin. 2013;45(7):570–577. doi: 10.1093/abbs/gmt044. [DOI] [PubMed] [Google Scholar]
- Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldyrev A.A., Aldini G., Derave W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013;93(4):1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- Boldyrev A.A., Gallant S.C., Sukhich G.T. Carnosine, the protective, anti-aging peptide. Biosci. Rep. 1999;19(6):581–587. doi: 10.1023/a:1020271013277. [DOI] [PubMed] [Google Scholar]
- Boldyrev A.A., Petukhov V.B. Localization of carnosine effect on the fatigued muscle preparation. Gen. Pharmacol. Vasc. Syst. 1978;9(1):17–20. doi: 10.1016/0306-3623(78)90051-4. [DOI] [PubMed] [Google Scholar]
- Brisola G.M.P., de Souza Malta E., Santiago P.R.P., Vieira L.H.P., Zagatto A.M. β-Alanine supplementation's improvement of high-intensity game activities in water polo. Int. J. Sports Physiol. Perform. 2018;13(9):1208–1214. doi: 10.1123/ijspp.2017-0636. [DOI] [PubMed] [Google Scholar]
- Broer S., Rahman B., Pellegri G., Pellerin L., Martin J.L., Verleysdonk S., Hamprecht B., Magistretti P.J. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem. 1997;272(48):30096–30102. doi: 10.1074/jbc.272.48.30096. [DOI] [PubMed] [Google Scholar]
- Brown B.E., Kim C.H., Torpy F.R., Bursill C.A., McRobb L.S., Heather A.K., Davies M.J., van Reyk D.M. Supplementation with carnosine decreases plasma triglycerides and modulates atherosclerotic plaque composition in diabetic apo E(-/-) mice. Atherosclerosis. 2014;232(2):403–409. doi: 10.1016/j.atherosclerosis.2013.11.068. [DOI] [PubMed] [Google Scholar]
- Brown C.E., Antholine W.E. Chelation chemistry of carnosine. Evidence that mixed complexes may occur in vivo. J. Phys. Chem. 1979;83(26):3314–3319. [Google Scholar]
- Carrell R.W., Gooptu B. Conformational changes and disease--serpins, prions and Alzheimer's. Curr. Opin. Struct. Biol. 1998;8(6):799–809. doi: 10.1016/s0959-440x(98)80101-2. [DOI] [PubMed] [Google Scholar]
- Carrell R.W., Lomas D.A. Conformational disease. Lancet. 1997;350(9071):134–138. doi: 10.1016/S0140-6736(97)02073-4. [DOI] [PubMed] [Google Scholar]
- Caruso G., Benatti C., Blom J.M.C., Caraci F., Tascedda F. The many faces of mitochondrial dysfunction in depression: from pathology to treatment. Front. Pharmacol. 2019;10:995. doi: 10.3389/fphar.2019.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Benatti C., Musso N., Fresta C.G., Fidilio A., Spampinato G., Brunello N., Bucolo C., Drago F., Lunte S.M., Peterson B.R., Tascedda F., Caraci F. Carnosine protects macrophages against the toxicity of aβ1-42 oligomers by decreasing oxidative stress. Biomedicines. 2021;9(5) doi: 10.3390/biomedicines9050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Caraci F., Jolivet R.B. Pivotal role of carnosine in the modulation of brain cells activity: multimodal mechanism of action and therapeutic potential in neurodegenerative disorders. Prog. Neurobiol. 2019;175:35–53. doi: 10.1016/j.pneurobio.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Caruso G., Distefano D.A., Parlascino P., Fresta C.G., Lazzarino G., Lunte S.M., Nicoletti V.G. Receptor-mediated toxicity of human amylin fragment aggregated by short- and long-term incubations with copper ions. Mol. Cell. Biochem. 2017;425(1–2):85–93. doi: 10.1007/s11010-016-2864-1. [DOI] [PubMed] [Google Scholar]
- Caruso G., Fresta C.G., Fidilio A., O'Donnell F., Musso N., Lazzarino G., Grasso M., Amorini A.M., Tascedda F., Bucolo C., Drago F., Tavazzi B., Lazzarino G., Lunte S.M., Caraci F. Carnosine decreases PMA-induced oxidative stress and inflammation in murine macrophages. Antioxidants. 2019;8(8) doi: 10.3390/antiox8080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Fresta C.G., Grasso M., Santangelo R., Lazzarino G., Lunte S.M., Caraci F. Inflammation as the common biological link between depression and cardiovascular diseases: can carnosine exert a protective role? Curr. Med. Chem. 2020;27(11):1782–1800. doi: 10.2174/0929867326666190712091515. [DOI] [PubMed] [Google Scholar]
- Caruso G., Fresta C.G., Lazzarino G., Distefano D.A., Parlascino P., Lunte S.M., Lazzarino G., Caraci F. Sub-toxic human amylin fragment concentrations promote the survival and proliferation of SH-SY5Y cells via the release of VEGF and HspB5 from endothelial RBE4 cells. Int. J. Mol. Sci. 2018;19(11) doi: 10.3390/ijms19113659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Fresta C.G., Martinez-Becerra F., Antonio L., Johnson R.T., de Campos R.P.S., Siegel J.M., Wijesinghe M.B., Lazzarino G., Lunte S.M. Carnosine modulates nitric oxide in stimulated murine RAW 264.7 macrophages. Mol. Cell. Biochem. 2017;431(1–2):197–210. doi: 10.1007/s11010-017-2991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Fresta C.G., Musso N., Giambirtone M., Grasso M., Spampinato S.F., Merlo S., Drago F., Lazzarino G., Sortino M.A., Lunte S.M., Caraci F. Carnosine prevents abeta-induced oxidative stress and inflammation in microglial cells: a key role of TGF-beta1. Cells. 2019;8(1) doi: 10.3390/cells8010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Fresta C.G., Musso N., Giambirtone M., Grasso M., Spampinato S.F., Merlo S., Drago F., Lazzarino G., Sortino M.A., Lunte S.M., Caraci F. Carnosine prevents aβ-induced oxidative stress and inflammation in microglial cells: a key role of TGF-β1. Cells. 2019;8(1) doi: 10.3390/cells8010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Fresta C.G., Siegel J.M., Wijesinghe M.B., Lunte S.M. Microchip electrophoresis with laser-induced fluorescence detection for the determination of the ratio of nitric oxide to superoxide production in macrophages during inflammation. Anal. Bioanal. Chem. 2017;409(19):4529–4538. doi: 10.1007/s00216-017-0401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Godos J., Castellano S., Micek A., Murabito P., Galvano F., Ferri R., Grosso G., Caraci F. The therapeutic potential of carnosine/anserine supplementation against cognitive decline: a systematic review with meta-analysis. Biomedicines. 2021;9(3) doi: 10.3390/biomedicines9030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Godos J., Privitera A., Lanza G., Castellano S., Chillemi A., Bruni O., Ferri R., Caraci F., Grosso G. Phenolic acids and prevention of cognitive decline: polyphenols with a neuroprotective role in cognitive disorders and alzheimer's disease. Nutrients. 2022;14(4) doi: 10.3390/nu14040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Grasso M., Fidilio A., Tascedda F., Drago F., Caraci F. Antioxidant properties of second-generation antipsychotics: focus on microglia. Pharmaceuticals. 2020;13(12) doi: 10.3390/ph13120457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Privitera A., Antunes B.M., Lazzarino G., Lunte S.M., Aldini G., Caraci F. The therapeutic potential of carnosine as an antidote against drug-induced cardiotoxicity and neurotoxicity: focus on Nrf2 pathway. Molecules. 2022;27(14) doi: 10.3390/molecules27144452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Spampinato S.F., Cardaci V., Caraci F., Sortino M.A., Merlo S. β-Amyloid and oxidative stress: perspectives in drug development. Curr. Pharmaceut. Des. 2019;25(45):4771–4781. doi: 10.2174/1381612825666191209115431. [DOI] [PubMed] [Google Scholar]
- Caruso G., Torrisi S.A., Mogavero M.P., Currenti W., Castellano S., Godos J., Ferri R., Galvano F., Leggio G.M., Grosso G., Caraci F. Polyphenols and neuroprotection: therapeutic implications for cognitive decline. Pharmacol. Ther. 2022;232 doi: 10.1016/j.pharmthera.2021.108013. [DOI] [PubMed] [Google Scholar]
- Cervantes Gracia K., Llanas-Cornejo D., Husi H. CVD and oxidative stress. J. Clin. Med. 2017;6(2) doi: 10.3390/jcm6020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A., Dirnagl U., Urra X., Planas A.M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15(8):869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- Chao de la Barca J.M., Rondet-Courbis B., Ferré M., Muller J., Buisset A., Leruez S., Plubeau G., Macé T., Moureauzeau L., Chupin S., Tessier L., Blanchet O., Lenaers G., Procaccio V., Mirebeau-Prunier D., Simard G., Gohier P., Miléa D., Reynier P. A plasma metabolomic profiling of exudative age-related macular degeneration showing carnosine and mitochondrial deficiencies. J. Clin. Med. 2020;9(3) doi: 10.3390/jcm9030631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Magliano D.J., Zimmet P.Z. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat. Rev. Endocrinol. 2012;8(4):228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- Choi S.Y., Kwon H.Y., Kwon O.B., Kang J.H. Hydrogen peroxide-mediated Cu,Zn-superoxide dismutase fragmentation: protection by carnosine, homocarnosine and anserine. Biochim. Biophys. Acta. 1999;1472(3):651–657. doi: 10.1016/s0304-4165(99)00189-0. [DOI] [PubMed] [Google Scholar]
- Chuang C.H., Hu M.L. L-carnosine inhibits metastasis of SK-Hep-1 cells by inhibition of matrix metaoproteinase-9 expression and induction of an antimetastatic gene, nm23-H1. Nutr. Cancer. 2008;60(4):526–533. doi: 10.1080/01635580801911787. [DOI] [PubMed] [Google Scholar]
- Copani A. The underexplored question of beta-amyloid monomers. Eur. J. Pharmacol. 2017;817:71–75. doi: 10.1016/j.ejphar.2017.05.057. [DOI] [PubMed] [Google Scholar]
- Corona C., Frazzini V., Silvestri E., Lattanzio R., La Sorda R., Piantelli M., Canzoniero L.M., Ciavardelli D., Rizzarelli E., Sensi S.L. Effects of dietary supplementation of carnosine on mitochondrial dysfunction, amyloid pathology, and cognitive deficits in 3xTg-AD mice. PLoS One. 2011;6(3) doi: 10.1371/journal.pone.0017971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C.W. The role of neurotrophins in brain aging: a perspective in honor of Regino Perez-Polo. Neurochem. Res. 2005;30(6–7):877–881. doi: 10.1007/s11064-005-6960-y. [DOI] [PubMed] [Google Scholar]
- Coyle J.T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Cuello A.C. Effects of trophic factors on the CNS cholinergic phenotype. Prog. Brain Res. 1996;109:347–358. doi: 10.1016/s0079-6123(08)62117-2. [DOI] [PubMed] [Google Scholar]
- de Andrade Kratz C., de Salles Painelli V., de Andrade Nemezio K.M., da Silva R.P., Franchini E., Zagatto A.M., Gualano B., Artioli G.G. Beta-alanine supplementation enhances judo-related performance in highly-trained athletes. J. Sci. Med. Sport. 2017;20(4):403–408. doi: 10.1016/j.jsams.2016.08.014. [DOI] [PubMed] [Google Scholar]
- de Campos R.P., Siegel J.M., Fresta C.G., Caruso G., da Silva J.A., Lunte S.M. Indirect detection of superoxide in RAW 264.7 macrophage cells using microchip electrophoresis coupled to laser-induced fluorescence. Anal. Bioanal. Chem. 2015;407(23):7003–7012. doi: 10.1007/s00216-015-8865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Death A.K., Fisher E.J., McGrath K.C., Yue D.K. High glucose alters matrix metalloproteinase expression in two key vascular cells: potential impact on atherosclerosis in diabetes. Atherosclerosis. 2003;168(2):263–269. doi: 10.1016/s0021-9150(03)00140-0. [DOI] [PubMed] [Google Scholar]
- Decker E.A., Livisay S.A., Zhou S. A re-evaluation of the antioxidant activity of purified carnosine. Biochemistry (Mosc.) 2000;65(7):766–770. [PubMed] [Google Scholar]
- Dias V., Junn E., Mouradian M.M. The role of oxidative stress in Parkinson's disease. J. Parkinsons Dis. 2013;3(4):461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distefano A., Caruso G., Oliveri V., Bellia F., Sbardella D., Zingale G.A., Caraci F., Grasso G. Neuroprotective effect of carnosine is mediated by insulin-degrading enzyme. ACS Chem. Neurosci. 2022;13(10):1588–1593. doi: 10.1021/acschemneuro.2c00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrota D., Fedorova T., Stvolinsky S., Babusikova E., Likavcanova K., Drgova A., Strapkova A., Boldyrev A. Carnosine protects the brain of rats and Mongolian gerbils against ischemic injury: after-stroke-effect. Neurochem. Res. 2005;30(10):1283–1288. doi: 10.1007/s11064-005-8799-7. [DOI] [PubMed] [Google Scholar]
- Drozak J., Veiga-da-Cunha M., Vertommen D., Stroobant V., Van Schaftingen E. Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1) J. Biol. Chem. 2010;285(13):9346–9356. doi: 10.1074/jbc.M109.095505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun N., Taşkın E., Oztürk F. Protection against adriamycin-induced cardiomyopathy by carnosine in rats: role of endogenous antioxidants. Biol. Trace Elem. Res. 2011;143(1):412–424. doi: 10.1007/s12011-010-8875-y. [DOI] [PubMed] [Google Scholar]
- Feehan J., Hariharan R., Buckenham T., Handley C., Bhatnagar A., Baba S.P., de Courten B. Carnosine as a potential therapeutic for the management of peripheral vascular disease. Nutr. Metabol. Cardiovasc. Dis. 2022;32(10):2289–2296. doi: 10.1016/j.numecd.2022.07.006. [DOI] [PubMed] [Google Scholar]
- Fernàndez-Busquets X., Ponce J., Bravo R., Arimon M., Martiáñez T., Gella A., Cladera J., Durany N. Modulation of amyloid beta peptide(1-42) cytotoxicity and aggregation in vitro by glucose and chondroitin sulfate. Curr. Alzheimer Res. 2010;7(5):428–438. doi: 10.2174/156720510791383787. [DOI] [PubMed] [Google Scholar]
- Ferreira R., Santos T., Goncalves J., Baltazar G., Ferreira L., Agasse F., Bernardino L. Histamine modulates microglia function. J. Neuroinflammation. 2012;9:90. doi: 10.1186/1742-2094-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteh A.N., Harrington R.J., Tsai A., Liao P., Harrington M.G. Free amino acid and dipeptide changes in the body fluids from Alzheimer's disease subjects. Amino Acids. 2007;32(2):213–224. doi: 10.1007/s00726-006-0409-8. [DOI] [PubMed] [Google Scholar]
- Fouad A.A., El-Rehany M.A., Maghraby H.K. The hepatoprotective effect of carnosine against ischemia/reperfusion liver injury in rats. Eur. J. Pharmacol. 2007;572(1):61–68. doi: 10.1016/j.ejphar.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Fresta C.G., Chakraborty A., Wijesinghe M.B., Amorini A.M., Lazzarino G., Lazzarino G., Tavazzi B., Lunte S.M., Caraci F., Dhar P., Caruso G. Non-toxic engineered carbon nanodiamond concentrations induce oxidative/nitrosative stress, imbalance of energy metabolism, and mitochondrial dysfunction in microglial and alveolar basal epithelial cells. Cell Death Dis. 2018;9(2):245. doi: 10.1038/s41419-018-0280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresta C.G., Fidilio A., Lazzarino G., Musso N., Grasso M., Merlo S., Amorini A.M., Bucolo C., Tavazzi B., Lazzarino G., Lunte S.M., Caraci F., Caruso G. Modulation of pro-oxidant and pro-inflammatory activities of M1 macrophages by the natural dipeptide carnosine. Int. J. Mol. Sci. 2020;21(3) doi: 10.3390/ijms21030776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresta C.G., Hogard M.L., Caruso G., Melo Costa E.E., Lazzarino G., Lunte S.M. Monitoring carnosine uptake by RAW 264.7 macrophage cells using microchip electrophoresis with fluorescence detection. Anal. Methods. 2017;9(3):402–408. doi: 10.1039/C6AY03009B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Takaoka M., Muraoka T., Kurata H., Tsuruoka N., Ono H., Kiso Y., Tanaka T., Matsumura Y. Preventive effect of L-carnosine on ischemia/reperfusion-induced acute renal failure in rats. Eur. J. Pharmacol. 2003;474(2–3):261–267. doi: 10.1016/s0014-2999(03)02079-x. [DOI] [PubMed] [Google Scholar]
- Furst T., Massaro A., Miller C., Williams B.T., LaMacchia Z.M., Horvath P.J. β-Alanine supplementation increased physical performance and improved executive function following endurance exercise in middle aged individuals. J. Int. Soc. Sports Nutr. 2018;15(1):32. doi: 10.1186/s12970-018-0238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggelli E., Kozlowski H., Valensin D., Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer's, prion, and Parkinson's diseases and amyotrophic lateral sclerosis) Chem. Rev. 2006;106(6):1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- Gallant S., Kukley M., Stvolinsky S., Bulygina E., Boldyrev A. Effect of carnosine on rats under experimental brain ischemia. Tohoku J. Exp. Med. 2000;191(2):85–99. doi: 10.1620/tjem.191.85. [DOI] [PubMed] [Google Scholar]
- Gariballa S.E., Sinclair A.J. Carnosine: physiological properties and therapeutic potential. Age Ageing. 2000;29(3):207–210. doi: 10.1093/ageing/29.3.207. [DOI] [PubMed] [Google Scholar]
- Gella A., Durany N. Oxidative stress in Alzheimer disease. Cell Adhes. Migrat. 2009;3(1):88–93. doi: 10.4161/cam.3.1.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghodsi R., Kheirouri S. Carnosine and advanced glycation end products: a systematic review. Amino Acids. 2018;50(9):1177–1186. doi: 10.1007/s00726-018-2592-9. [DOI] [PubMed] [Google Scholar]
- Giacobini E., Gold G. Alzheimer disease therapy--moving from amyloid-beta to tau. Nat. Rev. Neurol. 2013;9(12):677–686. doi: 10.1038/nrneurol.2013.223. [DOI] [PubMed] [Google Scholar]
- Gilardoni E., Baron G., Altomare A., Carini M., Aldini G., Regazzoni L. The disposal of reactive carbonyl species through carnosine conjugation: what we know now. Curr. Med. Chem. 2020;27(11):1726–1743. doi: 10.2174/0929867326666190624094813. [DOI] [PubMed] [Google Scholar]
- Giuffrida M.L., Tomasello M.F., Pandini G., Caraci F., Battaglia G., Busceti C., Di Pietro P., Pappalardo G., Attanasio F., Chiechio S., Bagnoli S., Nacmias B., Sorbi S., Vigneri R., Rizzarelli E., Nicoletti F., Copani A. Monomeric ss-amyloid interacts with type-1 insulin-like growth factor receptors to provide energy supply to neurons. Front. Cell. Neurosci. 2015;9:297. doi: 10.3389/fncel.2015.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J.M., Smith K., Moyen N.E., Binns A., Gray M. Effects of acute beta-alanine supplementation on anaerobic performance in trained female cyclists. J. Nutr. Sci. Vitaminol. 2015;61(2):161–166. doi: 10.3177/jnsv.61.161. [DOI] [PubMed] [Google Scholar]
- Gorbunov N.V., Erin A.N. [Mechanism of antioxidant action of carnosine] Biull. Eksp Biol. Med. 1991;111(5):477–478. [PubMed] [Google Scholar]
- Grasso G.I., Bellia F., Arena G., Satriano C., Vecchio G., Rizzarelli E. Multitarget trehalose-carnosine conjugates inhibit Abeta aggregation, tune copper(II) activity and decrease acrolein toxicity. Eur. J. Med. Chem. 2017;135:447–457. doi: 10.1016/j.ejmech.2017.04.060. [DOI] [PubMed] [Google Scholar]
- Guiotto A., Calderan A., Ruzza P., Borin G. Carnosine and carnosine-related antioxidants: a review. Curr. Med. Chem. 2005;12(20):2293–2315. doi: 10.2174/0929867054864796. [DOI] [PubMed] [Google Scholar]
- Guiotto A., Calderan A., Ruzza P., Borin G. Carnosine and carnosine-related antioxidants: a review. Curr. Med. Chem. 2005;12(20):2293–2315. doi: 10.2174/0929867054864796. [DOI] [PubMed] [Google Scholar]
- Gulewitsch W., Amiradžibi S. Ueber das Carnosin, eine neue organische Base des Fleischextractes. Ber. Dtsch. Chem. Ges. 1900;33(2):1902–1903. [Google Scholar]
- Gunasekara D.B., Siegel J.M., Caruso G., Hulvey M.K., Lunte S.M. Microchip electrophoresis with amperometric detection method for profiling cellular nitrosative stress markers. Analyst. 2014;139(13):3265–3273. doi: 10.1039/c4an00185k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Xu X., Tang C., Gao P., Chen X., Xiong X., Yang M., Yang S., Zhu X., Yuan S., Liu F., Xiao L., Kanwar Y.S., Sun L. Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018;16:32–46. doi: 10.1016/j.redox.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanein P., Felegari Z. Chelating effects of carnosine in ameliorating nickel-induced nephrotoxicity in rats. Can. J. Physiol. Pharmacol. 2017;95(12):1426–1432. doi: 10.1139/cjpp-2016-0647. [DOI] [PubMed] [Google Scholar]
- Hayes J.D., Dinkova-Kostova A.T., Tew K.D. Oxidative stress in cancer. Cancer Cell. 2020;38(2):167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkiss A.R. Could carnosine or related structures suppress Alzheimer's disease? J. Alzheimers Dis. 2007;11(2):229–240. doi: 10.3233/jad-2007-11210. [DOI] [PubMed] [Google Scholar]
- Hipkiss A.R. Depression, diabetes and dementia: formaldehyde may Be a common causal agent; could carnosine, a pluripotent peptide, Be protective? Aging Dis. 2017;8(2):128–130. doi: 10.14336/AD.2017.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkiss A.R. Glycotoxins: dietary and metabolic origins; possible amelioration of neurotoxicity by carnosine, with special reference to Parkinson's disease. Neurotox. Res. 2018;34(1):164–172. doi: 10.1007/s12640-018-9867-5. [DOI] [PubMed] [Google Scholar]
- Hipkiss A.R. COVID-19 and senotherapeutics: any role for the naturally-occurring dipeptide carnosine? Aging Dis. 2020;11(4):737–741. doi: 10.14336/AD.2020.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkiss A.R., Michaelis J., Syrris P. Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett. 1995;371(1):81–85. doi: 10.1016/0014-5793(95)00849-5. [DOI] [PubMed] [Google Scholar]
- Hipkiss A.R., Preston J.E., Himsworth D.T., Worthington V.C., Keown M., Michaelis J., Lawrence J., Mateen A., Allende L., Eagles P.A., Abbott N.J. Pluripotent protective effects of carnosine, a naturally occurring dipeptide. Ann. N. Y. Acad. Sci. 1998;854:37–53. doi: 10.1111/j.1749-6632.1998.tb09890.x. [DOI] [PubMed] [Google Scholar]
- Hipkiss A.R., Preston J.E., Himswoth D.T., Worthington V.C., Abbot N.J. Protective effects of carnosine against malondialdehyde-induced toxicity towards cultured rat brain endothelial cells. Neurosci. Lett. 1997;238(3):135–138. doi: 10.1016/s0304-3940(97)00873-2. [DOI] [PubMed] [Google Scholar]
- Hipkiss A.R., Worthington V.C., Himsworth D.T., Herwig W. Protective effects of carnosine against protein modification mediated by malondialdehyde and hypochlorite. Biochim. Biophys. Acta. 1998;1380(1):46–54. doi: 10.1016/s0304-4165(97)00123-2. [DOI] [PubMed] [Google Scholar]
- Hobart L.J., Seibel I., Yeargans G.S., Seidler N.W. Anti-crosslinking properties of carnosine: significance of histidine. Life Sci. 2004;75(11):1379–1389. doi: 10.1016/j.lfs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Houjeghani S., Kheirouri S., Faraji E., Jafarabadi M.A. l-Carnosine supplementation attenuated fasting glucose, triglycerides, advanced glycation end products, and tumor necrosis factor-α levels in patients with type 2 diabetes: a double-blind placebo-controlled randomized clinical trial. Nutr. Res. 2018;49:96–106. doi: 10.1016/j.nutres.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Hu W.W., Chen Z. Role of histamine and its receptors in cerebral ischemia. ACS Chem. Neurosci. 2012;3(4):238–247. doi: 10.1021/cn200126p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara H., Kakihana Y., Yamakage A., Kai K., Shibata T., Nishida M., Yamada K.I., Uchida K. 2-Oxo-histidine-containing dipeptides are functional oxidation products. J. Biol. Chem. 2019;294(4):1279–1289. doi: 10.1074/jbc.RA118.006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K., Grundke-Iqbal I. Alzheimer's disease, a multifactorial disorder seeking multitherapies. Alzheimers Dement. 2010;6(5):420–424. doi: 10.1016/j.jalz.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iulita M.F., Cuello A.C. Nerve growth factor metabolic dysfunction in Alzheimer's disease and Down syndrome. Trends Pharmacol. Sci. 2014;35(7):338–348. doi: 10.1016/j.tips.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Jaikaran E.T., Clark A. Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology. Biochim. Biophys. Acta. 2001;1537(3):179–203. doi: 10.1016/s0925-4439(01)00078-3. [DOI] [PubMed] [Google Scholar]
- Janssen B., Hohenadel D., Brinkkoetter P., Peters V., Rind N., Fischer C., Rychlik I., Cerna M., Romzova M., de Heer E., Baelde H., Bakker S.J., Zirie M., Rondeau E., Mathieson P., Saleem M.A., Meyer J., Köppel H., Sauerhoefer S., Bartram C.R., Nawroth P., Hammes H.P., Yard B.A., Zschocke J., van der Woude F.J. Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes. 2005;54(8):2320–2327. doi: 10.2337/diabetes.54.8.2320. [DOI] [PubMed] [Google Scholar]
- Javadi S., Yousefi R., Hosseinkhani S., Tamaddon A.M., Uversky V.N. Protective effects of carnosine on dehydroascorbate-induced structural alteration and opacity of lens crystallins: important implications of carnosine pleiotropic functions to combat cataractogenesis. J. Biomol. Struct. Dyn. 2017;35(8):1766–1784. doi: 10.1080/07391102.2016.1194230. [DOI] [PubMed] [Google Scholar]
- Jia H., Qi X., Fang S., Jin Y., Han X., Wang Y., Wang A., Zhou H. Carnosine inhibits high glucose-induced mesangial cell proliferation through mediating cell cycle progression. Regul. Pept. 2009;154(1–3):69–76. doi: 10.1016/j.regpep.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Jinsmaa Y., Florang V.R., Rees J.N., Anderson D.G., Strack S., Doorn J.A. Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem. Res. Toxicol. 2009;22(5):835–841. doi: 10.1021/tx800405v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.H., O'Brien T.D., Betsholtz C., Westermark P. Islet amyloid polypeptide: mechanisms of amyloidogenesis in the pancreatic islets and potential roles in diabetes mellitus. Lab. Invest. 1992;66(5):522–535. [PubMed] [Google Scholar]
- Kahn C.R. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43(8):1066–1085. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- Kalyankar G.D., Meister A. Enzymatic synthesis of carnosine and related beta-alanyl and gamma-aminobutyryl peptides. J. Biol. Chem. 1959;234:3210–3218. [PubMed] [Google Scholar]
- Katakura Y., Totsuka M., Imabayashi E., Matsuda H., Hisatsune T. Anserine/carnosine supplementation suppresses the expression of the inflammatory chemokine CCL24 in peripheral blood mononuclear cells from elderly people. Nutrients. 2017;9(11) doi: 10.3390/nu9111199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara M., Tanaka K.I., Kato-Negishi M. Zinc, carnosine, and neurodegenerative diseases. Nutrients. 2018;10(2) doi: 10.3390/nu10020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J.W. Alternative conformations of amyloidogenic proteins govern their behavior. Curr. Opin. Struct. Biol. 1996;6(1):11–17. doi: 10.1016/s0959-440x(96)80089-3. [DOI] [PubMed] [Google Scholar]
- Keytsman C., Blancquaert L., Wens I., Missine M., Noten P.V., Vandenabeele F., Derave W., Eijnde B.O. Muscle carnosine in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult. Scler. Relat. Disord. 2018;21:24–29. doi: 10.1016/j.msard.2018.02.013. [DOI] [PubMed] [Google Scholar]
- Kim S.R., Eom T.K., Byun H.G. Inhibitory effect of the carnosine-gallic acid synthetic peptide on MMP-2 and MMP-9 in human fibrosarcoma HT1080 cells. J. Pept. Sci. 2014;20(9):716–724. doi: 10.1002/psc.2658. [DOI] [PubMed] [Google Scholar]
- Kitaura-Inenaga K., Hara M., Higuchi K., Yamamoto K., Yamaki A., Ono K., Nakano A., Kinoshita M., Sasayama S., Matsumori A. Gene expression of cardiac mast cell chymase and tryptase in a murine model of heart failure caused by viral myocarditis. Circ. J. 2003;67(10):881–884. doi: 10.1253/circj.67.881. [DOI] [PubMed] [Google Scholar]
- Köppel H., Riedl E., Braunagel M., Sauerhoefer S., Ehnert S., Godoy P., Sternik P., Dooley S., Yard B.A. L-carnosine inhibits high-glucose-mediated matrix accumulation in human mesangial cells by interfering with TGF-β production and signalling. Nephrol. Dial. Transplant. 2011;26(12):3852–3858. doi: 10.1093/ndt/gfr324. [DOI] [PubMed] [Google Scholar]
- Kramarenko G.G., Markova E.D., Ivanova-Smolenskaya I.A., Boldyrev A.A. Peculiarities of carnosine metabolism in a patient with pronounced homocarnosinemia. Bull. Exp. Biol. Med. 2001;132(4):996–999. doi: 10.1023/a:1013687832424. [DOI] [PubMed] [Google Scholar]
- Kulebyakin K., Karpova L., Lakonsteva E., Krasavin M., Boldyrev A. Carnosine protects neurons against oxidative stress and modulates the time profile of MAPK cascade signaling. Amino Acids. 2012;43(1):91–96. doi: 10.1007/s00726-011-1135-4. [DOI] [PubMed] [Google Scholar]
- Kurata H., Fujii T., Tsutsui H., Katayama T., Ohkita M., Takaoka M., Tsuruoka N., Kiso Y., Ohno Y., Fujisawa Y., Shokoji T., Nishiyama A., Abe Y., Matsumura Y. Renoprotective effects of l-carnosine on ischemia/reperfusion-induced renal injury in rats. J. Pharmacol. Exp. Therapeut. 2006;319(2):640–647. doi: 10.1124/jpet.106.110122. [DOI] [PubMed] [Google Scholar]
- Lazzarino G., Listorti I., Bilotta G., Capozzolo T., Amorini A.M., Longo S., Caruso G., Lazzarino G., Tavazzi B., Bilotta P. Water- and fat-soluble antioxidants in human seminal plasma and serum of fertile males. Antioxidants. 2019;8(4) doi: 10.3390/antiox8040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarino G., Listorti I., Muzii L., Amorini A.M., Longo S., Di Stasio E., Caruso G., D'Urso S., Puglia I., Pisani G., Lazzarino G., Tavazzi B., Bilotta P. Low-molecular weight compounds in human seminal plasma as potential biomarkers of male infertility. Hum. Reprod. 2018;33(10):1817–1828. doi: 10.1093/humrep/dey279. [DOI] [PubMed] [Google Scholar]
- Lee Y.T., Hsu C.C., Lin M.H., Liu K.S., Yin M.C. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur. J. Pharmacol. 2005;513(1–2):145–150. doi: 10.1016/j.ejphar.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Lenney J.F., George R.P., Weiss A.M., Kucera C.M., Chan P.W., Rinzler G.S. Human serum carnosinase: characterization, distinction from cellular carnosinase, and activation by cadmium. Clin. Chim. Acta. 1982;123(3):221–231. doi: 10.1016/0009-8981(82)90166-8. [DOI] [PubMed] [Google Scholar]
- Lenney J.F., Peppers S.C., Kucera-Orallo C.M., George R.P. Characterization of human tissue carnosinase. Biochem. J. 1985;228(3):653–660. doi: 10.1042/bj2280653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Ward W.F. PGC-1alpha: a key regulator of energy metabolism. Adv. Physiol. Educ. 2006;30(4):145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- Lührs T., Ritter C., Adrian M., Riek-Loher D., Bohrmann B., Döbeli H., Schubert D., Riek R. 3D structure of Alzheimer's amyloid-beta(1-42) fibrils. Proc. Natl. Acad. Sci. U. S. A. 2005;102(48):17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Chen J., Bo S., Lu X., Zhang J. Protective effect of carnosine after chronic cerebral hypoperfusion possibly through suppressing astrocyte activation. Am. J. Transl. Res. 2015;7(12):2706–2715. [PMC free article] [PubMed] [Google Scholar]
- Madry C., Kyrargyri V., Arancibia-Carcamo I.L., Jolivet R., Kohsaka S., Bryan R.M., Attwell D. Microglial ramification, surveillance, and interleukin-1beta release are regulated by the two-pore domain K(+) channel THIK-1. Neuron. 2018;97(2):299–312 e296. doi: 10.1016/j.neuron.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainz E.R., Gunasekara D.B., Caruso G., Jensen D.T., Hulvey M.K., da Silva J.A.F., Metto E.C., Culbertson A.H., Culbertson C.T., Lunte S.M. Monitoring intracellular nitric oxide production using microchip electrophoresis and laser-induced fluorescence detection. Anal. Methods. 2012;4(2):414–420. [Google Scholar]
- Mal'tseva V.V., Sergienko V.V., Stvolinskii S.L. [The effect of carnosine on hematopoietic stem cell activity in irradiated animals] Biokhimiia. 1992;57(9):1378–1382. [PubMed] [Google Scholar]
- Mannion A.F., Jakeman P.M., Dunnett M., Harris R.C., Willan P.L. Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans. Eur. J. Appl. Physiol. Occup. Physiol. 1992;64(1):47–50. doi: 10.1007/BF00376439. [DOI] [PubMed] [Google Scholar]
- Mattson M.P., Goodman Y. Different amyloidogenic peptides share a similar mechanism of neurotoxicity involving reactive oxygen species and calcium. Brain Res. 1995;676(1):219–224. doi: 10.1016/0006-8993(95)00148-j. [DOI] [PubMed] [Google Scholar]
- Mehta J.L., Saldeen T.G., Rand K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J. Am. Coll. Cardiol. 1998;31(6):1217–1225. doi: 10.1016/s0735-1097(98)00093-x. [DOI] [PubMed] [Google Scholar]
- Menini S., Iacobini C., Ricci C., Blasetti Fantauzzi C., Pugliese G. Protection from diabetes-induced atherosclerosis and renal disease by D-carnosine-octylester: effects of early vs late inhibition of advanced glycation end-products in Apoe-null mice. Diabetologia. 2015;58(4):845–853. doi: 10.1007/s00125-014-3467-6. [DOI] [PubMed] [Google Scholar]
- Menini S., Iacobini C., Ricci C., Scipioni A., Blasetti Fantauzzi C., Giaccari A., Salomone E., Canevotti R., Lapolla A., Orioli M., Aldini G., Pugliese G. D-Carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation. Br. J. Pharmacol. 2012;166(4):1344–1356. doi: 10.1111/j.1476-5381.2012.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon K., Cameron J.D., de Courten M., de Courten B. Use of carnosine in the prevention of cardiometabolic risk factors in overweight and obese individuals: study protocol for a randomised, double-blind placebo-controlled trial. BMJ Open. 2021;11(5) doi: 10.1136/bmjopen-2020-043680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli V., Pampalone M., Frazziano G., Grasso G., Rizzarelli E., Ricordi C., Casu A., Iannolo G., Conaldi P.G. Carnosine protects pancreatic beta cells and islets against oxidative stress damage. Mol. Cell. Endocrinol. 2018;474:105–118. doi: 10.1016/j.mce.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Miller Y., Ma B., Nussinov R. Zinc ions promote Alzheimer Abeta aggregation via population shift of polymorphic states. Proc. Natl. Acad. Sci. U. S. A. 2010;107(21):9490–9495. doi: 10.1073/pnas.0913114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J., Senut M.C., Rajanikant K., Greenberg E., Bandagi R., Zemke D., Mousa A., Kassab M., Farooq M.U., Gupta R., Majid A. Differential neuroprotective effects of carnosine, anserine, and N-acetyl carnosine against permanent focal ischemia. J. Neurosci. Res. 2008;86(13):2984–2991. doi: 10.1002/jnr.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou Y., Wen S., Li Y.X., Gao X.X., Zhang X., Jiang Z.Y. Recent progress in Keap1-Nrf2 protein-protein interaction inhibitors. Eur. J. Med. Chem. 2020;202 doi: 10.1016/j.ejmech.2020.112532. [DOI] [PubMed] [Google Scholar]
- Musiek E.S., Holtzman D.M. Three dimensions of the amyloid hypothesis: time, space and 'wingmen. Nat. Neurosci. 2015;18(6):800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K. Physiological implications of carnosine on the inflammation with reference to its inhibitory action of allergy and the vital defense mechanism. J. Nihon Univ. Sch. Dent. 1971;13(1):1–12. [PubMed] [Google Scholar]
- Nagai K., Murakami H., Sano A., Kabutake H. [Physiological role of carnosine and homocarnosine in inflammation] Arzneimittelforschung. 1970;20(12):1876–1878. [PubMed] [Google Scholar]
- Nagai K., Murakami H., Sano H., Kakishita T., Takano M. [Inhibitory effect of carnosine on various allergic reactions] Arzneimittelforschung. 1971;21(8):1222–1225. [PubMed] [Google Scholar]
- Nagasawa T., Yonekura T., Nishizawa N., Kitts D.D. In vitro and in vivo inhibition of muscle lipid and protein oxidation by carnosine. Mol. Cell. Biochem. 2001;225(1-):29–34. doi: 10.1023/a:1012256521840. [DOI] [PubMed] [Google Scholar]
- Nelson M.M., Builta Z.J., Monroe T.B., Doorn J.A., Anderson E.J. Biochemical characterization of the catecholaldehyde reactivity of L-carnosine and its therapeutic potential in human myocardium. Amino Acids. 2019;51(1):97–102. doi: 10.1007/s00726-018-2647-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony L., Akdis M., Akdis C.A. Regulation of the immune response and inflammation by histamine and histamine receptors. J. Allergy Clin. Immunol. 2011;128(6):1153–1162. doi: 10.1016/j.jaci.2011.06.051. [DOI] [PubMed] [Google Scholar]
- Odashima M., Otaka M., Jin M., Wada I., Horikawa Y., Matsuhashi T., Ohba R., Hatakeyama N., Oyake J., Watanabe S. Zinc L-carnosine protects colonic mucosal injury through induction of heat shock protein 72 and suppression of NF-kappaB activation. Life Sci. 2006;79(24):2245–2250. doi: 10.1016/j.lfs.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Oguntibeju O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019;11(3):45–63. [PMC free article] [PubMed] [Google Scholar]
- Ooi T.C., Chan K.M., Sharif R. Zinc carnosine inhibits lipopolysaccharide-induced inflammatory mediators by suppressing NF-kappab activation in raw 264.7 macrophages, independent of the MAPKs signaling pathway. Biol. Trace Elem. Res. 2016;172(2):458–464. doi: 10.1007/s12011-015-0615-x. [DOI] [PubMed] [Google Scholar]
- Ouyang L., Tian Y., Bao Y., Xu H., Cheng J., Wang B., Shen Y., Chen Z., Lyu J. Carnosine decreased neuronal cell death through targeting glutamate system and astrocyte mitochondrial bioenergetics in cultured neuron/astrocyte exposed to OGD/recovery. Brain Res. Bull. 2016;124:76–84. doi: 10.1016/j.brainresbull.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Pattison D.I., Davies M.J. Evidence for rapid inter- and intramolecular chlorine transfer reactions of histamine and carnosine chloramines: implications for the prevention of hypochlorous-acid-mediated damage. Biochemistry. 2006;45(26):8152–8162. doi: 10.1021/bi060348s. [DOI] [PubMed] [Google Scholar]
- Pepper E.D., Farrell M.J., Nord G., Finkel S.E. Antiglycation effects of carnosine and other compounds on the long-term survival of Escherichia coli. Appl. Environ. Microbiol. 2010;76(24):7925–7930. doi: 10.1128/AEM.01369-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry T., Hansen S., Love D. Serum-Carnosinase deficiency in carnosinæmia. Lancet. 1968;291(7554):1229–1230. doi: 10.1016/s0140-6736(68)91924-7. [DOI] [PubMed] [Google Scholar]
- Peters V., Riedl E., Braunagel M., Höger S., Hauske S., Pfister F., Zschocke J., Lanthaler B., Benck U., Hammes H.P., Krämer B.K., Schmitt C.P., Yard B.A., Köppel H. Carnosine treatment in combination with ACE inhibition in diabetic rats. Regul. Pept. 2014;194–195:36–40. doi: 10.1016/j.regpep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Popko K., Gorska E., Stelmaszczyk-Emmel A., Plywaczewski R., Stoklosa A., Gorecka D., Pyrzak B., Demkow U. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010;15:120–122. doi: 10.1186/2047-783X-15-S2-120. Suppl 2(Suppl 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritam P., Deka R., Bhardwaj A., Srivastava R., Kumar D., Jha A.K., Jha N.K., Villa C., Jha S.K. Antioxidants in alzheimer's disease: current therapeutic significance and future prospects. Biology. 2022;11(2) doi: 10.3390/biology11020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Yard B.A., Krämer B.K., van Goor H., van Dijk P., Kannt A. Association between serum carnosinase concentration and activity and renal function impairment in a type-2 diabetes cohort. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.899057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajanikant G.K., Zemke D., Senut M.C., Frenkel M.B., Chen A.F., Gupta R., Majid A. Carnosine is neuroprotective against permanent focal cerebral ischemia in mice. Stroke. 2007;38(11):3023–3031. doi: 10.1161/STROKEAHA.107.488502. [DOI] [PubMed] [Google Scholar]
- Reddy V.P., Garrett M.R., Perry G., Smith M.A. Carnosine: a versatile antioxidant and antiglycating agent. Sci. Aging Knowl. Environ. 2005;2005(18):pe12. doi: 10.1126/sageke.2005.18.pe12. [DOI] [PubMed] [Google Scholar]
- Riedl E., Koeppel H., Brinkkoetter P., Sternik P., Steinbeisser H., Sauerhoefer S., Janssen B., van der Woude F.J., Yard B.A. A CTG polymorphism in the CNDP1 gene determines the secretion of serum carnosinase in Cos-7 transfected cells. Diabetes. 2007;56(9):2410–2413. doi: 10.2337/db07-0128. [DOI] [PubMed] [Google Scholar]
- Riedl E., Pfister F., Braunagel M., Brinkkötter P., Sternik P., Deinzer M., Bakker S.J., Henning R.H., van den Born J., Krämer B.K., Navis G., Hammes H.P., Yard B., Koeppel H. Carnosine prevents apoptosis of glomerular cells and podocyte loss in STZ diabetic rats. Cell. Physiol. Biochem. 2011;28(2):279–288. doi: 10.1159/000331740. [DOI] [PubMed] [Google Scholar]
- Robertson R.P. Antagonist: diabetes and insulin resistance--philosophy, science, and the multiplier hypothesis. J. Lab. Clin. Med. 1995;125(5):560–564. ; discussion 565. [PubMed] [Google Scholar]
- Rodrigo R., Fernandez-Gajardo R., Gutierrez R., Matamala J.M., Carrasco R., Miranda-Merchak A., Feuerhake W. Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol. Disord.: Drug Targets. 2013;12(5):698–714. doi: 10.2174/1871527311312050015. [DOI] [PubMed] [Google Scholar]
- Rubtsov A.M. Molecular mechanisms of regulation of the activity of sarcoplasmic reticulum Ca-release channels (ryanodine receptors), muscle fatigue, and Severin's phenomenon. Biochemistry (Mosc.) 2001;66(10):1132–1143. doi: 10.1023/a:1012485030527. [DOI] [PubMed] [Google Scholar]
- Sastre M., Klockgether T., Heneka M.T. Contribution of inflammatory processes to Alzheimer's disease: molecular mechanisms. Int. J. Dev. Neurosci. 2006;24(2–3):167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Schwab C., McGeer P.L. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J. Alzheimers Dis. 2008;13(4):359–369. doi: 10.3233/jad-2008-13402. [DOI] [PubMed] [Google Scholar]
- Seidler N.W., Yeargans G.S., Morgan T.G. Carnosine disaggregates glycated alpha-crystallin: an in vitro study. Arch. Biochem. Biophys. 2004;427(1):110–115. doi: 10.1016/j.abb.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severin S.E., Kirzon M.V., Kaftanova T.M. Effect of carnosine and anserine on action of isolated frog muscles. Dokl. Akad. Nauk SSSR. 1953;91(3):691–694. [PubMed] [Google Scholar]
- Severin S.E., Kirzon M.V., Kaftanova T.M. [Effect of carnosine and anserine on action of isolated frog muscles] Dokl. Akad. Nauk SSSR. 1953;91(3):691–694. [PubMed] [Google Scholar]
- Shastri A., Bonifati D.M., Kishore U. Innate immunity and neuroinflammation. Mediat. Inflamm. 2013;2013 doi: 10.1155/2013/342931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., He P., Fan Y.Y., Zhang J.X., Yan H.J., Hu W.W., Ohtsu H., Chen Z. Carnosine protects against permanent cerebral ischemia in histidine decarboxylase knockout mice by reducing glutamate excitotoxicity. Free Radic. Biol. Med. 2010;48(5):727–735. doi: 10.1016/j.freeradbiomed.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Shen Y., Zhang S., Fu L., Hu W., Chen Z. Carnosine attenuates mast cell degranulation and histamine release induced by oxygen-glucose deprivation. Cell Biochem. Funct. 2008;26(3):334–338. doi: 10.1002/cbf.1447. [DOI] [PubMed] [Google Scholar]
- Shimada T., Watanabe N., Ohtsuka Y., Endoh M., Kojima K., Hiraishi H., Terano A. Polaprezinc down-regulates proinflammatory cytokine-induced nuclear factor-kappaB activiation and interleukin-8 expression in gastric epithelial cells. J. Pharmacol. Exp. Therapeut. 1999;291(1):345–352. [PubMed] [Google Scholar]
- Shimizu H., Watanabe E., Hiyama T.Y., Nagakura A., Fujikawa A., Okado H., Yanagawa Y., Obata K., Noda M. Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron. 2007;54(1):59–72. doi: 10.1016/j.neuron.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Singh N., Baby D., Rajguru J.P., Patil P.B., Thakkannavar S.S., Pujari V.B. Inflammation and cancer. Ann. Afr. Med. 2019;18(3):121–126. doi: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana-Manrique C., Sanz F.J., Martínez-Carrión G., Paricio N. Antioxidant and neuroprotective effects of carnosine: therapeutic implications in neurodegenerative diseases. Antioxidants. 2022;11(5) doi: 10.3390/antiox11050848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.H., Yu J.T., Tan L. Brain-derived neurotrophic factor in alzheimer's disease: risk, mechanisms, and therapy. Mol. Neurobiol. 2015;52(3):1477–1493. doi: 10.1007/s12035-014-8958-4. [DOI] [PubMed] [Google Scholar]
- Song M.S., Learman C.R., Ahn K.C., Baker G.B., Kippe J., Field E.M., Dunbar G.L. In vitro validation of effects of BDNF-expressing mesenchymal stem cells on neurodegeneration in primary cultured neurons of APP/PS1 mice. Neuroscience. 2015;307:37–50. doi: 10.1016/j.neuroscience.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Soto C. Alzheimer's and prion disease as disorders of protein conformation: implications for the design of novel therapeutic approaches. J. Mol. Med. 1999;77(5):412–418. doi: 10.1007/s001090050371. [DOI] [PubMed] [Google Scholar]
- Spina-Purrello V., Giliberto S., Barresi V., Nicoletti V.G., Giuffrida Stella A.M., Rizzarelli E. Modulation of PARP-1 and PARP-2 expression by L-carnosine and trehalose after LPS and INFgamma-induced oxidative stress. Neurochem. Res. 2010;35(12):2144–2153. doi: 10.1007/s11064-010-0297-x. [DOI] [PubMed] [Google Scholar]
- Stevens C., Walz G., Singaram C., Lipman M.L., Zanker B., Muggia A., Antonioli D., Peppercorn M.A., Strom T.B. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig. Dis. Sci. 1992;37(6):818–826. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- Stewart J., Manmathan G., Wilkinson P. Primary prevention of cardiovascular disease: a review of contemporary guidance and literature. JRSM Cardiovasc. Dis. 2017;6 doi: 10.1177/2048004016687211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stvolinskii S.L., Dobrota D., Mezeshova V., Liptai T., Pronaiova N., Zalibera L., Boldyrev A.A. Carnosine and anserine in working muscles--study using proton NMR spectroscopy. Biokhimiia (Moscow, Russia) 1992;57(9):1317–1323. [PubMed] [Google Scholar]
- Stvolinskiĭ S.L., Dobrota D., Mezeshova V., Liptaĭ T., Pronaĭova N., Zalibera L., Boldyrev A.A. [Carnosine and anserine in working muscles--study using proton NMR spectroscopy] Biokhimiia. 1992;57(9):1317–1323. [PubMed] [Google Scholar]
- Suzuki T., Nagai K. Local antiinflammatory effect of L-carnosine (βalanyl 1-histidine) associated with pains of post dental extraction. J. Nihon Univ. Sch. Dent. 1974;16(1):18–22. [Google Scholar]
- Swearengin T.A., Fitzgerald C., Seidler N.W. Carnosine prevents glyceraldehyde 3-phosphate-mediated inhibition of aspartate aminotransferase. Arch. Toxicol. 1999;73(6):307–309. doi: 10.1007/s002040050623. [DOI] [PubMed] [Google Scholar]
- Tanaka K.-I., Kawahara M. Carnosine and lung disease. Curr. Med. Chem. 2020;27(11):1714–1725. doi: 10.2174/0929867326666190712140545. [DOI] [PubMed] [Google Scholar]
- Teufel M., Saudek V., Ledig J.P., Bernhardt A., Boularand S., Carreau A., Cairns N.J., Carter C., Cowley D.J., Duverger D., Ganzhorn A.J., Guenet C., Heintzelmann B., Laucher V., Sauvage C., Smirnova T. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J. Biol. Chem. 2003;278(8):6521–6531. doi: 10.1074/jbc.M209764200. [DOI] [PubMed] [Google Scholar]
- Thomas P.J., Qu B.-H., Pedersen P.L. Defective protein folding as a basis of human disease. Trends Biochem. Sci. 1995;20(11):456–459. doi: 10.1016/s0968-0004(00)89100-8. [DOI] [PubMed] [Google Scholar]
- Tiedje K., Stevens K., Barnes S., Weaver D. β-Alanine as a small molecule neurotransmitter. Neurochem. Int. 2010;57(3):177–188. doi: 10.1016/j.neuint.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Tsai S.J., Kuo W.W., Liu W.H., Yin M.C. Antioxidative and anti-inflammatory protection from carnosine in the striatum of MPTP-treated mice. J. Agric. Food Chem. 2010;58(21):11510–11516. doi: 10.1021/jf103258p. [DOI] [PubMed] [Google Scholar]
- Turner M.D., Sale C., Garner A.C., Hipkiss A.R. Anti-cancer actions of carnosine and the restoration of normal cellular homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2021;1868(11) doi: 10.1016/j.bbamcr.2021.119117. [DOI] [PubMed] [Google Scholar]
- Ukeda H., Hasegawa Y., Harada Y., Sawamura M. Effect of carnosine and related compounds on the inactivation of human Cu,Zn-superoxide dismutase by modification of fructose and glycolaldehyde. Biosci. Biotechnol. Biochem. 2002;66(1):36–43. doi: 10.1271/bbb.66.36. [DOI] [PubMed] [Google Scholar]
- Umare V., Pradhan V., Nadkar M., Rajadhyaksha A., Patwardhan M., Ghosh K.K., Nadkarni A.H. Effect of proinflammatory cytokines (IL-6, TNF-α, and IL-1β) on clinical manifestations in Indian SLE patients. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/385297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson J.A., Howard T.B., III Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J. Nutr. Biochem. 1996;7(12):659–663. [Google Scholar]
- Watari I., Oka S., Tanaka S., Aoyama T., Imagawa H., Shishido T., Yoshida S., Chayama K. Effectiveness of polaprezinc for low-dose aspirin-induced small-bowel mucosal injuries as evaluated by capsule endoscopy: a pilot randomized controlled study. BMC Gastroenterol. 2013;13:108. doi: 10.1186/1471-230X-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnick R.E., Winnick T. Carnosineanserine synthetase of muscle. I. Preparation and properties of soluble enzyme from chick muscle. Biochim. Biophys. Acta. 1959;31(1):47–55. doi: 10.1016/0006-3002(59)90437-8. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Sato M., Matsumoto T., Kadooka K., Hasegawa T., Fujimura T., Katakura Y. Mechanisms of carnosine-induced activation of neuronal cells. Biosci. Biotechnol. Biochem. 2018;82(4):683–688. doi: 10.1080/09168451.2017.1413325. [DOI] [PubMed] [Google Scholar]
- Zhang H., Dong X., Sun Y. Carnosine-LVFFARK-NH2 conjugate: a moderate chelator but potent inhibitor of Cu(2+)-mediated amyloid beta-protein aggregation. ACS Chem. Neurosci. 2018;9(11):2689–2700. doi: 10.1021/acschemneuro.8b00133. [DOI] [PubMed] [Google Scholar]
- Zhao K., Li Y., Wang Z., Han N., Wang Y. Carnosine protects mouse podocytes from high glucose induced apoptosis through PI3K/AKT and Nrf2 pathways. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/4348973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Decker E.A. Ability of carnosine and other skeletal muscle components to quench unsaturated aldehydic lipid oxidation products. J. Agric. Food Chem. 1999;47(1):51–55. doi: 10.1021/jf980780j. [DOI] [PubMed] [Google Scholar]
- Zwaka T.P., Hombach V., Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103(9):1194–1197. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.