Abstract
Strontium (Sr) and strontium ranelate (SR) are commonly used therapeutic drugs for patients suffering from osteoporosis. Researches have showed that Sr can significantly improve the biological activity and physicochemical properties of materials in vitro and in vivo. Therefore, a large number of strontium containing biomaterials have been developed for repairing bone defects and promoting osseointegration. In this review, we provide a comprehensive overview of Sr-containing biomaterials along with the current state of their clinical use. For this purpose, the different types of biomaterials including calcium phosphate, bioactive glass, and polymers are discussed and provided future outlook on the fabrication of the next-generation multifunctional and smart biomaterials.
Keywords: Strontium, Biomaterials, Bone tissue engineering, Osteoporosis
Graphical abstract
Highlights
-
•
Reviewed the different mechanisms of strontium promoting osteogenesis
-
•
Different strontium containing biomaterials in bone tissue engineering
-
•
Application potential of co-doping of strontium with other therapeutic ions materials
-
•
Future research directions regarding strontium-containing biomaterials are prospected.
1. Introduction
The skeleton is a vital organ in the human body that protects important organs, maintains human movement, regulates the endocrine system, and maintains mineral as well as nutrient stability [1,2]. Bone tissues are formed during the growth process and the stability of bone mass is maintained via continuous matrix renewal [3]. Bone formation is achieved via interactions of two cell types: osteoclasts that absorb the calcified bone matrix and osteoblasts that are responsible for new bone formation [4]. Before adulthood, osteoblast-mediated bone formation exceeds bone resorption, leading to bone growth [5]. In adulthood, bone absorption and bone formation exhibit a dynamic balance to maintain a certain amount of bone mass [6]. In old age, this balance is broken: bone absorption is stronger than bone formation, resulting in negative bone balance [4,7,8]. At this stage, there is faster bone remodeling, fracture of bone trabeculae, and changes in bone trabecular structure, resulting in reduced bone strength [9]. The persistence of this state leads to osteoporosis [10]. Osteoporosis is a metabolic bone disorder that is characterized by low bone mass and micro-architectural bone tissue deterioration [11]. Fracture risk for osteoporosis patients is much higher than that of normal people [12]. With the increase in the aging global population, osteoporosis has become an important cause of fractures [13]. In the United States, more than half of individuals aged 50 years of age have osteoporosis or low bone mass [14]. Given that osteoporosis is closely associated with estrogen levels and age, in postmenopausal women and elderly people, osteoporotic fracture prevention and treatment are essential to reducing the risks of osteoporotic fractures [15,16].
There are different types of chemotherapies for osteoporosis treatment, which include (i) bisphosphonates, (ii) denosumab, (iii) intermittent parathyroid hormone, (iv) sclerostin, and (iv) strontium ranelate (SR) [17]. The molecular structure of SR consists of two non-radioactive Sr2+ and a ranelate ion. The absorption of ranelate ion is low due to its high polarity and thus quickly excreted from the body through the kidney. Therefore, Sr2+ is the main component of SR that plays a pharmacological role [18]. Compared to other osteoporosis drugs, SR has two pharmacological effects [19]. First, SR activates OPG/RANKL/RANK, NFκB, and other signaling pathways to inhibit osteoclast activities and survival [[20], [21], [22], [23]]. Second, SR enhances alkaline phosphatase (ALP) activities, collagen synthesis, and expressions of osteoblast markers such as bone sialoprotein (BSP) and osteocalcin (OCN), leading to increased osteogenesis [24]. However, long-term systemic use of SR is associated with serious adverse reactions. In 2013, the European Drug Administration limited the indication of SR to the treatment of severe osteoporosis as its increased use enhances the risk of myocardial infarction, thromboembolic events, serious skin reactivity, and other diseases [[25], [26], [27]]. In addition, pharmacokinetic studies have shown that the oral bioavailability of SR is very low, and oral calcium (Ca) or a high Ca diet can significantly reduce the bioavailability of SR [18]. Therefore, studies on bone tissue engineering have proposed a new strategy for the treatment of bone defects during osteoporosis i.e., implanting strontium (Sr)-containing biomaterials (SrBMs) to continuously release Sr2+ locally. A series of SrCMs have been extensively investigated [25,[28], [29], [30]]. These materials are of great significance in the treatment of osteoporosis-associated critical bone defects and compression fractures.
This review summarizes the applications of SrBMs in bone tissue repair. We comprehensively elucidated on structural characteristics and biological effects of these advanced biomaterials (BMs). Based on published experimental studies, we analyzed the focus of research in this field and predicted the development trend of SrBMs in the future.
2. Mechanisms of Sr at cellular and molecular levels
2.1. Effects of Sr on osteocytes
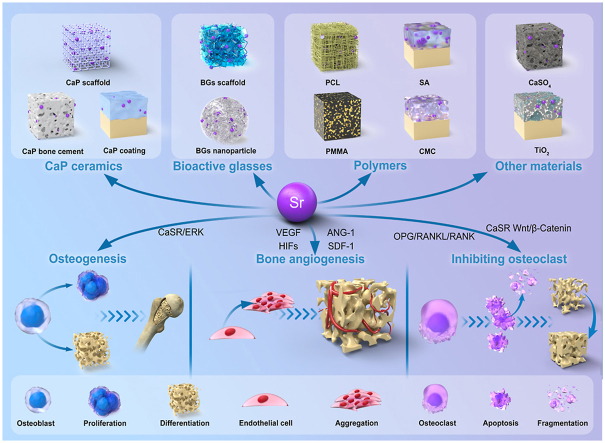
Osteoporosis is a common orthopedic disease that often leads to pathological fractures and has a serious impact on the daily life of patients. As a common therapeutic drug for osteoporosis, Sr activates various signaling pathways in bone cells, promotes osteoblast proliferation as well as differentiation, and inhibits osteoclast activities (Fig. 1) [10,31]. In this chapter, we summarized the positive effects of Sr on bone cells at molecular and cellular levels.
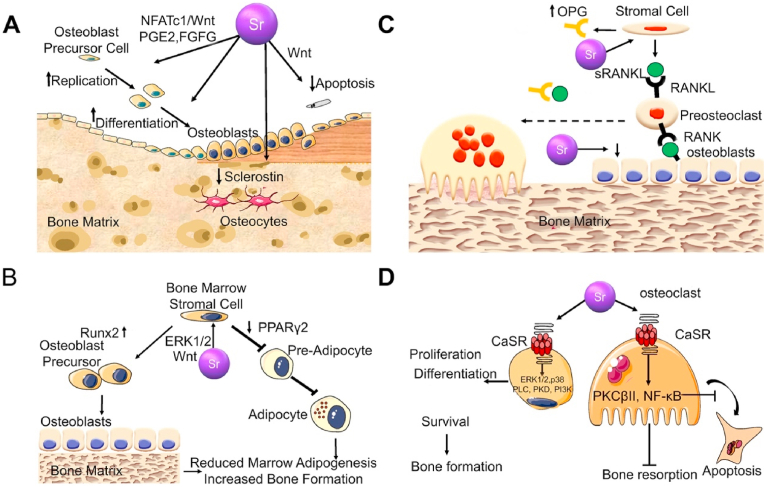
Fig. 1.
Sr mediated signal pathway in bone cells [10]. (A) Pharmacological actions of Sr on osteoblastogenesis. (B) Pharmacological effects of Sr2+ on mesenchymal cell lineage. (C) Pharmacological actions of Sr2+ on osteoclast differentiation. (D) Implications of the Ca sensing receptor in the pharmacological actions of Sr2+ in bone cells.
Sr2+ stimulates the extracellular calcium-sensitive receptor (CaSR) to activate various signaling pathways. The ERK1/2-MAPK, Wnt/β-Catenin, and Akt signaling pathways rely on CaSR to play their regulatory roles on osteoblasts [10]. Physiologically, CaSR is a member of the G protein-coupled receptor superfamily that plays a key role in regulating Ca2+ concentrations in the extracellular fluid [32]. The binding of Ca2+ and CaSR activates the receptor, which undergoes structural changes, thereby triggering cascade reactions of phospholipase C and cAMP-dependent signal transduction pathways [33]. Moreover, CaSR is activated by other divalent/trivalent metal cations, which are less effective than Ca2+ [34]. Compared to the other divalent metal cations, Sr2+ is a complete activator of CaSR, and its efficacy is close to that of Ca2+ [35]. This is because the ionic properties of Sr2+ and Ca2+ are quite comparable [36]. They are both spherical, double-charged alkaline earth metal cations with similar physicochemical properties, and have a significant affinity for oxygen-containing ligands [18]. In vivo, they exhibit analogous osteogenic characteristics [18].
Physiologically, Sr2+ activates extracellular signal-regulated kinase (ERK) 1/2 phosphorylation to regulate the biological behaviors of osteoblasts [37]. Sr induces cyclooxygenase (COX)-2 and prostaglandin E2 (PGE2) expressions by activating the ERK signaling pathway [38,39]. Prostaglandins (PGs) are potent regulators of bone cell functions [40]. They act on osteoblastic as well as osteoclastic lineages to stimulate and inhibit bone resorption and formation [41]. In vivo, PGs are produced by actions of COX on the arachidonic acid that is released from membrane phospholipids by phospholipase [42]. COX-2 is rapidly and transiently induced in response to various stimuli (cytokines, growth factors, and hormones), which are key regulators of bone cell metabolism [43,44]. In a previous study, Sr2+ was used to reverse the inhibitory effects of glucocorticoids on bone marrow mesenchymal stem cells (BMSCs). It was found that dexamethasone-induced ERK phosphorylation in BMSCs was reduced and the ERK signaling pathway was reactivated by Sr2+ treatment. Moreover, ERK signal pathway activation by Sr was alleviated by treatment with the ERK signal pathway inhibitor (U0126). These results suggest that Sr enhances osteogenesis and matrix mineralization via the ERK signaling pathway [45].
The Sr2+ promotes osteoclast apoptosis via the OPG/RANKL/RANK signaling pathway [46]. The OPG/RANKL/RAN signaling pathway has been extensively studied. Identification of the OPG/RANKL/RANK system as the dominant, final mediator of osteoclastogenesis represents major advances in bone biology [47,48]. Initially, osteoprotegerin (OPG) was found to be synthesized as a 401 amino acid peptide, with a 21-amino acid propeptide that was cleaved, resulting in a mature protein of 380 amino acids [49]. As a soluble decoy receptor belonging to the tumor necrosis factor (TNF) receptor superfamily, preliminary cloning and identification of OPG is the first step leading to the disintegration of this system [50]. The OPG mRNA is expressed in many tissues, including the lungs, heart, kidneys, liver, stomach, gastrointestinal tract, brain and spinal cord, thyroid, and bone [51]. Functionally, OPG inhibits osteoclast differentiation and activity [52]. It interferes with interactions between receptor activators of nuclear factor kappa-Β ligand (RANKL) and RANK, which further inhibits osteoclast differentiation [53]. Shortly after its discovery, researchers used OPG as a probe for expression cloning and identified its ligand to be OPG-L/ODF [53]. Moreover, RANKL and OPG-L/ODF have been established to be identical in terms of function [53]. Human RANKL is a polypeptide composed of 317 amino acids [54]. It exists in two forms: the 40–45 kDa cell membrane binding form and the 31 kDa soluble form, which are split from 140 or 145 full-length forms [54]. RANKL mRNA is expressed at the highest levels in the bone, bone marrow, and lymphoid tissues [52]. Its main roles in the bone are to promote osteoclast differentiation, and activities and inhibit their apoptosis [55]. With the discovery of OPG/RANKL, the RANK receptor of RANKL was also identified [56]. The RANK receptor on osteoclast cells is the only receptor of RANKL on these cells [56]. This was revealed by the fact that RANK gene knockout mice suffered from severe ossification due to a lack of osteoclasts [57]. Sr2+ enhances OPG expressions and inhibits RANKL expressions [58]. Even though Sr2+ has no real effects on RANKL proteins in cells, it indirectly affects RANKL membrane localization [59]. With increasing OPG protein production, membrane-related RANKL levels decrease [59]. This explains why Sr2+ enhances the proliferation and differentiation of osteoblasts and inhibits osteoclast activities.
The Wnt signaling path is divided into dependent β-Catenin proteins and independent β-Catenin proteins, which can regulate late bone formation and bone resorption [60]. Mature osteoblast β-analysis of mice with increased and loss of catenin function mutations showed that Wnt/β-Catenin signaling significantly increased bone mass due to decreased bone resorption, but did not affect bone formation [61]. Moreover, Wnt in mature osteoblasts/β-catenin signal promotes OPG expressions while inhibiting osteoclast formation [62]. These findings indicate that the Wnt/β-Catenin signaling pathway plays a key role in bone anabolism. Independent β-Catenin signaling path, Wnt5a, as a Wnt ligand, binds the Ror2 receptor on osteoclast precursors to promote RANK expressions and increase bone resorption activities [63]. The Sr2+ can enhance classical and non-classical Wnt signaling pathways [64]. Sr2+ upregulates β-catenin to activate transcription factors such as Runx2 [65]. Sr2+ inhibits the expressions of Wnt pathway inhibitors, prevents β-catenin degradation, and promotes osteogenic differentiation [65]. Moreover, Sr2+ activates the CaR of mesenchymal stem cells (MSCs) to stimulate Wnt secretion and upregulate the expressions of β-catenin and FZD8 receptors to enhance osteogenesis [66].
2.2. Effects of Sr on osteoimmunomodulation
The immune microenvironment of the bone defect is one of the important factors affecting bone reconstruction and repair. The design of orthopedic BMs is gradually transitioning from biological inertia to immunomodulation [67]. Studies have shown that Sr2+ can regulate the immune microenvironment in bone tissue to promote bone and vascular regeneration [68,69]. First, Sr2+ can affect the polarization direction of macrophages [70]. Macrophages are important regulators of innate and adaptive immunity, often derived from monocytes. When the body is injured, macrophages not only clear dead cells and cell debris through phagocytosis and activate specific inflammatory factors but also secrete proteins or cytokines that stimulate wound healing through paracrine signaling [71]. The different functions of macrophages are related to their polarization direction. As an important defense line for human immunity, macrophages must respond quickly to pathogens or foreign damage signals to remove foreign objects or damaged and apoptotic cells [72]. At this point, macrophages polarize in the M1 direction. M1 macrophages (proinflammatory macrophages) mainly secrete proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6, attracting other immune cells (e.g., CD4+ T cells, CD8+ T cells, and dendritic cells) to participate in the recruitment of cells at the wound site, the clearance of pathogens, and the initiation of acute inflammatory reactions, thereby laying the foundation for subsequent bone tissue repair [73]. Generally, the powerful proinflammatory effect of M1 macrophages determines the destroyed destiny of substantial bone tissues. However, several recent studies have generally believed that moderately activated M1 macrophages are beneficial for osteogenic differentiation of MSCs mediated by oncostatin M (OSM) or bone morphogenetic protein 2 (BMP2) [74,75]. Contrary to M1 macrophages, M2 macrophages (anti-inflammatory macrophages) are associated with tissue repair, remodeling, and healing. The inhibitory cytokines used by M2 macrophages to resist inflammation mainly include interleukin-1 receptor antagonist (IL-1RA), IL-10, and transforming growth factor beta (TGF-β). IL-10 and IL-1RA are known to promote osteogenesis and inhibit osteoclast activity [76]. Research has demonstrated that Sr2+ promotes macrophage polarization in the M2 direction, resulting in a beneficial osteogenic microenvironment that supports bone regeneration [70]. However, this osteogenic effect has a strong dose dependence. Some studies have shown that excessive M2 macrophages may lead to pathological osteogenesis, which is because M2 macrophages can also secrete a large number of fibroblast cytokines, leading to the formation of fibrosis [77]. Moreover, an excessive polarization of macrophages towards the M2 phenotype causes a reduction in M1 macrophages. Studies have shown that M1 macrophages play a positive role in regulating osteogenesis, and very few M1 macrophages are not conducive to the construction of an osteogenic microenvironment [78,79]. Therefore, how to generate the optimal M1/M2 ratio is an important factor in determining the bone repair ability of BMs. Confirming the optimal Sr2+ range and regulating macrophages to promote bone healing will be one of the issues that Sr-containing bone repair materials must overcome.
T lymphocytes constitute the primary constituent of the adaptive immune system, and when activated, they can stimulate osteoclast activity by activating RANKL, thereby increasing bone resorption. T lymphocytes can also release interferon-γ to interfere with TNF receptor-related factor 6 (TRAF6), a key signaling pathway for RANK/RANKL, which can inhibit osteoclast formation by blocking the activation of this signaling pathway [71]. Currently, research on the direct impact of Sr2+ on T lymphocytes are very limited. The impact of Sr2+ on lymphocytes may be another direction for studying the bone immune environment in the future.
2.3. Effects of Sr on angiogenesis
Blood vessels play a crucial role in promoting the regeneration and repair of segmental bone defects. Blood vessel deficiency can lead to severe necrosis at fracture sites [80]. Insufficient blood supply after the bone injury is a major cause of poor fracture healing, affecting the recovery of approximately 10% of bone fracture patients [81]. Excellent bone tissue engineering materials should be able to promote local vascularization. It has been reported that Sr activates multiple signaling pathways and enhances the expressions of angiogenesis-related genes [[82], [83], [84]]. Vascular endothelial growth factor (VEGF), as one of the most potent mediators of bone formation, is expressed by hypertrophic chondrocytes of the growth plate [85]. The VEGF protein exerts its angiogenic functions in ATDC5 chondrocytes in an autocrine/paracrine manner [86]. Zhu et al. [87] found that Sr2+ enhances VEGF expressions in rat chondrocytes, and the higher the concentration of Sr2+ within a certain range, the higher the VEGF levels. In vitro, SR was shown to enhance VEGF expressions in the femoral head, thereby improving the pathological damage caused by femoral head avascular necrosis [88]. Angiotensin-1 (ANG-1) is an agonist that is required for endothelial cell survival, proliferation, and vascular maturation [89]. Ang-1 and VEGF regulate angiogenesis to varying degrees, VEGF induces vascular germination and growth while Ang-1 mediates vascular remodeling and maturation [90]. Yan et al. [91] and Yu et al. [39] confirmed that Sr2+ enhances VEGF and Ang-1 expressions. Hypoxia-inducible factors (HIFs) are transcriptional complexes that are heterodimers consisting of HIF-α and HIF-β subunits [92]. The hypoxia-inducible factor 1-alpha (HIF-1α) regulates VEGF's transcriptional activation of hypoxia and insulin-like growth factor-I (IGF-I) to stimulate vascular growth [93,94]. In a previous study, Sr-modified titanium (Ti) alloy implants significantly increased HIF gene expressions in human umbilical vein endothelial cells. Compared with the untreated group, Sr-modified implants exhibited a stronger ability to induce angiogenesis [95]. Moreover, Sr activates the PI3K/AKT/mTOR signaling pathway to induce angiogenesis [96]. The PI3K/AKT pathway is involved in various cellular processes, including cell proliferation, migration, and survival, and also contributes to vascularization [96]. mTOR, a key kinase downstream of this pathway, is involved in angiogenesis [97]. Guo et al. [98] found that SR-induced AKT and mTOR protein expressions in HUVECs were markedly increased. However, after PI3K/AKT inhibitor treatment, AKT and mTOR protein expressions were inhibited while the in vitro angiogenesis abilities of HUVECs were alleviated [98]. These results confirm that SR promotes angiogenesis via the PI3K/AKT/mTOR pathway.
3. SrBMs
After decades of development, BMs are widely used in the clinical treatment of bone defects [99]. The development of BMs in bone tissue engineering has gone through three stages. The first stage was the generation of bio-inert materials with excellent mechanical properties, which are used to treat injury- or disease-related bone defects. The second stage was the generation of biocompatible second-generation materials with a high affinity for surrounding tissue structures to support bone growth (bone conductivity). The third stage was the generation of bioactive materials with super bone conductivity, bone induction, bone integration, and significant anti-inflammatory/anti-infection abilities. In the future, the Sr element will play a great role in bone tissue engineering. First, SrBMs greatly enhance the biological activities of materials [25]. BMs that slowly release Sr in the body can also result in good bone integration under the conditions of osteoporosis [19]. Herein, we summarize SrBMs, including calcium phosphate (CaP) ceramics, bioactive glasses (BGs) and polymers. These materials can be used to make bone scaffolds, cement, and coatings. The osteogenic and angiogenic effects of these materials at the cellular level and in vivo were also discussed.Table 1
Table 1.
Summary of Sr-containing materials in the bone tissue engineering.
| Material | Chemical compound | Implant type | In vitro result | In vivo result | Reference |
|---|---|---|---|---|---|
| CaPs | Sr, CaP | Scaffold | Promote cell adhesion and induce (Ca + Sr)/P = 1.64 in the HA layer close to natural bone tissue | None | [114] |
| Sr, CCP | Scaffold | Promote the growth of bone cells in the high calcium environment and inhibit the growth of bone cells in the low calcium environment | Promote rabbit's bone formation | [115] | |
| Gelatin, Sr, CaP | Scaffold | Promote the proliferation of human osteoblasts | None | [119] | |
| SrPG, β-TCP | Scaffold | Promote osteoblast growth and inhibit osteoclast activity | Promote bone growth and angiogenesis | [120] | |
| Gelatin, Sr, BCP-PVA | Scaffold | Promote osteoblast proliferation and differentiation | None | [121] | |
| PCA, citric acid, potassium citrate, Sr, CaP | Bone cement | None | Nove | [132] | |
| Sr, HA, α-TCP, DCPD, | Bone cement | Enhance osteoprogenitor cell proliferation and osteogenic differentiation | None | [133] | |
| Sr, DCPD, BaSO4 α-TCP |
Bone cement | Promote the expression of osteogenic and angiogenic genes | Promote the formation of bones and blood vessels | [134] | |
| Sr, HA | Coating | None | Improve bone integration in osteoporosis rats | [144] | |
| BGs | Sr, BGs | Scaffold | None | Promote bone growth | [156] |
| Sr, Mg, BGs, Chitosan | Scaffold | Promote apatite formation | None | [160] | |
| Sr, BGs | Nanoparticles | Promote osteogenic differentiation of MSC | None | [164] | |
| Sr, BGs | Nanoparticles | Promote the polarization of macrophages toward the M2 direction | None | [165] | |
| Sr, BGs | Nanoparticles | Promote osteoblast phenotype and inhibit osteoclast growth | None | [166] | |
| Sr, BGs | Bone cement | Promote MSC osteogenic differentiation and HUVEC tubular formation | Promote bone regeneration at bone defects | [167] | |
| Polymer | SrCO3, MgHPO4⋅3H2O, Mg(OH)2, PCL | Scaffold | Promote osteoblast proliferation and differentiation | Promote hip bone regeneration in horses | [181] |
| Sr, CPP, PMMA | Bone cement | Promote cell growth and mineralization deposition | None | [194] | |
| Sr, PMMA | Bone cement | None | None | [195] | |
| Sr, BGs, PMMA | Bone cement | Promote cell proliferation | Stimulate the new bone formation | ||
| Sr, SA | Hydrogel | Promote differentiation of osteoblast | None | [205] | |
| Sr, SA, BGs | Hydrogel | Promote chondrogenic differentiation of cells and affect the polarization of macrophages | Promote cartilage regeneration | [207] | |
| Sr, CMC | Hydrogel | Promote the proliferation and mineralization of osteoblasts | None | [214] | |
| Other BMs | Sr, CaSiO3, CaSO4·2H2O | Bone cement | Promote osteoblast activity and proliferation | None | [220] |
| Sr, CaSiO3, CaSO4·2H2O, Chitosan | Bone cement | None | Promote bone and blood vessel regeneration | [221] | |
| Sr, TiO2 | Coating | Promote the activity of osteoblasts under the condition of osteoporosis | Enhance bone integration in the osteoporosis model | [230] |
3.1. Sr-containing CaP ceramics
Bone tissue is composed of ∼70 wt% CaP nanocrystals with apatitic structures that are heterogeneously nucleated on a three-dimensional (3D) collagenous matrix and includes various bioactive ions. The disordered nature of this apatite (Apt) phase is conducive to regulating the dynamic biological processes of bone metabolism, making this abiotic mineral phase a “living inorganic crystal” [100]. Inspired by bionics, CaP materials have been widely used in orthopedics and dentistry, including synthetic bone graft substitutes and coatings on the surface of metal implants. Research and use of CaP materials have achieved impressive success, such as improving the clinical survival rate of femoral components in total hip implants [101]. Currently, CaP materials that have been extensively studied include hydroxyapatite (HA), tricalcium phosphate (TCP), octacalcium phosphate (OCP), and biphasic calcium phosphate (BCP), composed of HA/TCP [[102], [103], [104]]. HA is the main component of bone's inorganic phase and was first used to prepare bioactive materials. However, unmodified HA has several serious drawbacks. For example, large particles may cause local aseptic inflammation, poor mechanical properties make it difficult to replace the load-bearing site, and the slow degradation rate requires the patient to bear the pain of removal surgery [[105], [106], [107]]. Additionally, a very rapid biodegradation rate means that this material cannot match the growth rate of a new bone. To solve this problem, researchers mixed HA and TCP at different ratios to prepare BCP, which combines the advantages of TCP and has a controllable degradation rate [108] However, too fast a biodegradation rate means that it cannot match the growth rate of new bones. To solve this problem, researchers mixed HA and TCP at different ratios to prepare BCP, which combines the advantages of HA and TCP and has a controllable degradation rate [109]. OCP is an important phosphate component in natural bones, which is a precursor of HA, and converts to HA during bone formation. Unlike TCP, the degradation rate of the OCP material in vivo matches the rate of bone formation, enhancing its potential to become a new generation of bone tissue replacement materials in the future [110]. It is noteworthy that CaP alone cannot achieve good bone integration under osteoporotic conditions. Therefore, Sr2+ — with the ability to inhibit osteoclast activity and promote osteoblast differentiation — is bound to CaP materials. In the current stage of research, Sr-containing CaP materials have been developed into bone scaffolds, bone cements, and implant surface coatings, and have achieved excellent results.
It is well known that Ca2+ in the lattice structure of HA can usually be replaced to some extent by divalent cations. The substitution amount changes the crystal structure of Ca and also leads to structural deformation and changes in the phase stability, solubility, and reactivity, as well as in the mechanical properties of the material [111]. One study showed that doping Sr2+ into the lattice of HA can affect the physicochemical properties of materials, including improving their mechanical strength [112]. These results are also applicable to other CaP materials. For instance, Xu et al. [113] found that after adding 30 and 50% of Sr to β-TCP, the elastic modulus and hardness of the sample were significantly improved. The reason behind this is complex. Some people believe that because Ca in HA is replaced by Sr2+ with a larger radius, its lattice parameters will elongate and the basic crystal structure will deform [111]. However, the specific reason why Sr improves CaP materials remains unclear and warrants further exploration. Nonetheless, the most important role of Sr/Sr2+ is not to improve the mechanical properties of the material. Sr containing CaP materials are believed to continuously release Sr2+ locally, thereby improving bone mineral density and mechanical properties in osteoporosis.
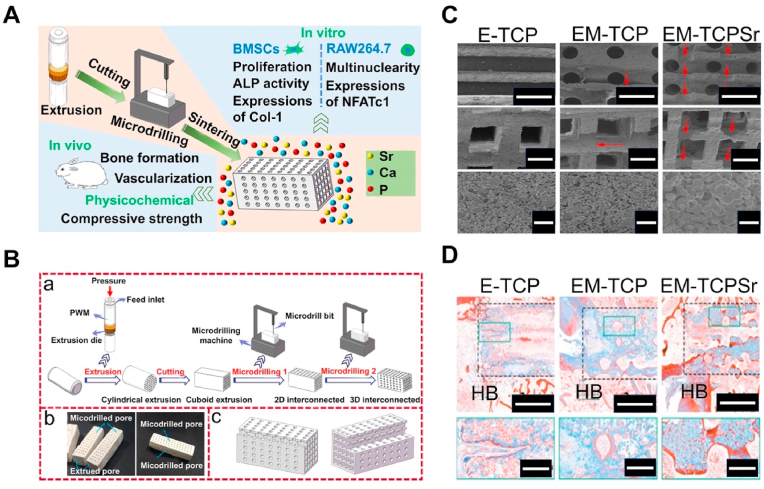
When Sr-containing CaP materials are developed into bone scaffolds, they can effectively improve bone integration at the bone scaffold interface and promote bone regeneration. A study has shown that as an additive to CaP, Sr has a stronger osteogenic activity than some commonly used therapeutic ions. Li et al. [114] compared the abilities of CaP scaffolds doped with different therapeutic elements (magnesium (Mg), Sr, and zinc (Zn)) to induce HA. The Sr doped scaffolds were the only ones that exhibited the ability to induce the Apt layer. Although we believe that the addition of Sr/Sr2+ has a positive impact on the osteogenic ability of CaP scaffolds, this osteogenic effect may be influenced by the internal environment. One study found that Sr2+ in different concentrations of Ca2+ environments had completely different effects on cells. Sr2+ can inhibit osteoblast functions by suppressing ALP activities and inhibiting osteopontin as well as osteocalcin absorption under standard Ca2+ concentrations (1.8 mM). Conversely, elevated Ca2+ concentrations (9 mM) enhance the bone regeneration effects of Sr2+ [115]. Thus, proper Ca/Sr should be selected when preparing Sr-containing CaP scaffolds to ensure their effects on osteoblasts are promoting rather than inhibiting. Although Sr2+ can improve the mechanical properties of CaP materials to a certain extent, it is not sufficient to change the physicochemical properties of CaP materials themselves. For instance, Sr containing HA materials still have shortcomings such as fragility and poor plasticity [116,117]. More importantly, CaP materials only simulate natural bone in composition and significantly differ from the bone in structure. Therefore, modifying CaP materials with poor plasticity is an effective strategy to change their mechanical properties. The composite CaP material obtained by adding natural or synthetic polymer particles to the CaP material exhibits good mechanical properties and biocompatibility [118]. The modified CaP material is processable, and precise simulation of bone tissue can be achieved through some special manufacturing techniques. Wu et al. [119] developed a gelatin Sr–CaP scaffold with directional osteogenesis. The scaffold is manufactured via the freeze-drying method, which can simulate the bone tissue by introducing a unidirectional temperature gradient to control the ice crystal network to form axial holes. This manufacturing method can adjust the structure of the scaffold hole to make it close to the natural bone. As an adhesive, gelatin improves the plasticity of CaP scaffolds. He et al. [120] combined Sr-containing phosphate-based glass (SrPG) with β-TCP and used the extrusion micro drilling technology to prepare three-dimensional interconnects scaffolds (Fig. 2). The scaffolds exhibit a channel-shaped square large hole (≥650 μm) formed by extrusion, a channel-shaped circular large hole (≥570 μm) formed via micro drilling and abundant micropores. This structural design is more suitable for cell adhesion on scaffold surfaces. The addition of BGS has improved the compressive strength of β-TCP. In vivo and in vitro, the release of Sr2+ inhibited osteoclast activities and improved osteogenic activities. A considerable number of new blood vessels and new bone tissues were formed in scaffold holes implanted in rabbit femoral defects. Mohapatra and Rautray [121] mixed gelatin, Sr containing HA, and β-TCP to prepare a novel BCP scaffold. Compared with the traditional CaP scaffold, this new scaffold can control the degradation rate of the scaffold in vivo by adjusting the proportion between components and adapting better to the dynamic bone defect healing process.
Fig. 2.
Fabrication of three-dimensional (3D) interconnected bioceramic scaffolds using extrusion-microdrilling [120]. (A) Schematic diagram of manufacturing bioceramic scaffold and its effect in vivo and in vitro. (B) a) Schematic diagram of the extrusion-microdrilling process for preparing 3D interconnected green bodies. b) Digital photographs of the extrusion-microdrilling samples. c) Schematic diagram of the configuration of extruded pores and microdrilled pores in the extrusion-microdrilling samples. (C) X-ray diffraction (XRD) patterns. (D) Photographs of Masson's trichrome staining in femoral defects implanted with E-TCP, EM-TCP, and EM-TCP/SrPG scaffolds at the 6th week postoperatively.
The CaP cement (CPC) was first proposed in the 1980s by Brown and Chow in the United States [122]. In the beginning, CPC was only used to treat maxillofacial defects and fractures [123]. Currently, various formulas of CPC have been developed to meet the treatment conditions of specific diseases [124]. Compared with polymethyl methacrylate (PMMA), the main advantage of CPC is that it can solidify in vivo [125]. The orthophosphate powder of various components is mixed with a liquid to form a plastic paste, which solidifies and hardens when implanted [126]. Compared to polymerization-solidified PMMA, CPC is the result of dissolution and precipitation, and precipitation crystal entanglement is the cause of cement solidification [127]. The coagulation reaction of CPC is nonexothermic; thus, it is often used as a carrier for protein drugs [128]. Moreover, CPC has excellent biological activities. It can directly combine with the bone, thus, there is no need to worry about poor bone integration [129]. However, poor mechanical strength limits the further application of CPC [130]. In recent years, polymer-modified CPC has significantly improved its mechanical properties and is considered to have the potential to replace PMMA bone cement [131]. Sr/Sr2+ can improve the mechanical properties of CPC to a certain extent. More importantly, CPC-containing Sr can achieve better bone bonding under conditions of osteoporosis. Sun et al. [132] added polycarboxylic acid (PCA), citric acid, potassium citrate, and Sr to CPC to develop a high-strength bone cement. It has not been determined whether the mechanical strength of this bone cement is superior to that of PMMA. But, the Sr-containing CPC can compensate for the difference in mechanical strength between it and PMMA by enhancing peripheral bone integration in osteoporosis. To further verify this supposition, Lode et al. [133] performed balloon kyphoplasty of human cadavers and revealed that the mechanical properties of Sr-containing CPC are completely suitable for this type of surgery. Furthermore, Wu et al. [134] documented that Sr-containing CPC has significant angiogenic capacities. Compared with CPC, the number of new blood vessels formed by Sr-containing CPC after implantation in rats was significantly increased (Fig. 3).
Fig. 3.
Sr modified hybrid calcium phosphate cement [134]. (A) Schematic illustration. (B) Injectability and anti-collapsibility of cement. (C) 3D reconstruction by Micro-CT 12 weeks after implantation.
In recent years, some modification technologies have significantly improved the mechanical properties of CaP scaffolds; but these scaffolds still have the disadvantage of poor mechanical properties. The preparation of CaP coatings on different metal substrates has attracted widespread attention in the past few decades. CaP and its composite coatings have been proven to improve osteogenic activity, corrosion resistance, and even antibacterial properties of the surface of metal prostheses [135]. Studies have shown that the biological effects of CaP coatings in vivo are related to the type of CaP material, Ca-phosphorus ratio, surface morphology, and additives [[136], [137], [138]]. Currently, researchers have proposed various strategies for functionalizing CaP coatings, including the addition of proteins, growth factors, and therapeutic ions to cope with complex internal environments [[139], [140], [141]]. Among them, Sr-containing CaP coatings are considered to be an effective strategy for inhibiting bone resorption at the implant interface and promoting bone integration. Because Sr is a high-temperature-resistant metal element, the method of coating Sr-containing CaP mainly depends on the type of the CaP material and the base material. For high-temperature phase CaP (HA, TCP), plasma spraying is the most commonly used coating method in clinical practice. However, the potential for coating cracking during heating and cooling is a serious disadvantage of this coating method, especially in long-term clinical applications, which may cause inflammatory reactions and even osteolysis [135]. To overcome this shortcoming, Boyd et al. [142] used magnetron sputtering (MS) to combine Sr into an HA lattice to form SrHA coating and found that surface morphologies of the coating changed significantly with an increase in SrHA amounts. Christensen et al. [143] found that Sr in the SrHA coating prepared by MS can be effectively released into the surrounding bone tissue. In addition, some chemical methods for preparing Sr–CaP-containing coatings have achieved good results. For instance, Li et al. [144] prepared SrHA coating on Ti implant surfaces via sol-gel and showed good bone integration effects in osteoporosis models. This proved the feasibility of preparing SrHA coatings by sol-gel to improve implant fixation in osteoporotic bones. Ding et al. [145] developed a SrHA-containing CS coating and investigated the effects of SrHA with different degrees of substitution on bone tissue. This coating can be loaded with 100% SrHA and is expected to be widely used in orthopedics, dentistry, and craniofacial surgery to promote bone regeneration.
3.2. Sr-containing BGs (SrBGs)
BGs is a type of medical BM with good biocompatibility and can regenerate and repair bone defects. In 1969, Professor Larry L. Hench of the University of Florida decided to prepare a degradable glass with high Ca content and near ternary eutectic in Na2O–CaO–SiO2 in the Na2O–CaO–SiO2–P2O5 system and named Bioglass or 45S5 [146]. The new proposed definition of a bioactive glass is “a non-equilibrium, non-crystalline material that has been designed to induce specific biological activity” [147]. When BGs is implanted in the body, chemical reactions mediated by ion exchange occur between BGs surfaces and the surrounding biological fluids, resulting in the formation of a layer of hydroxycarbonate apatite (HCA) at the bone/implant interface [148]. This stage is very comparable to the CaP mineral stage of mammalian bones. Furthermore, the ions and degradation products released from BGs can activate the expression of genes related to osteogenesis and angiogenesis, which has led to BGs becoming a popular material for bone defect repair [146]. The manufacturing process of BGs includes the traditional melting method and the sol-gel method. Compared with the traditional melting method, the sol-gel method has been proven to prepare highly uniform and nanosized BGs (nBGs) [149]. Since doping glass by introducing therapeutic ions into the sol is relatively easier than traditional melt quenching routes, various functionalized BGs have been developed in succession [150]. Among different therapeutic elements, adding Sr2+ to BGs to improve bone remodeling in osteoporosis has received considerable attention. In SrBGs, some Ca is replaced by Sr, resulting in changes in its physicochemical properties and reactivity in contact with biological fluids. Kargozar et al. [150] provided a detailed overview of the changes in physicochemical properties caused by Sr replacing Ca2+ or Na+ in BGs. In this chapter, we focus on the repair effects of different SrBGs materials on bone defects in an osteoporotic environment.
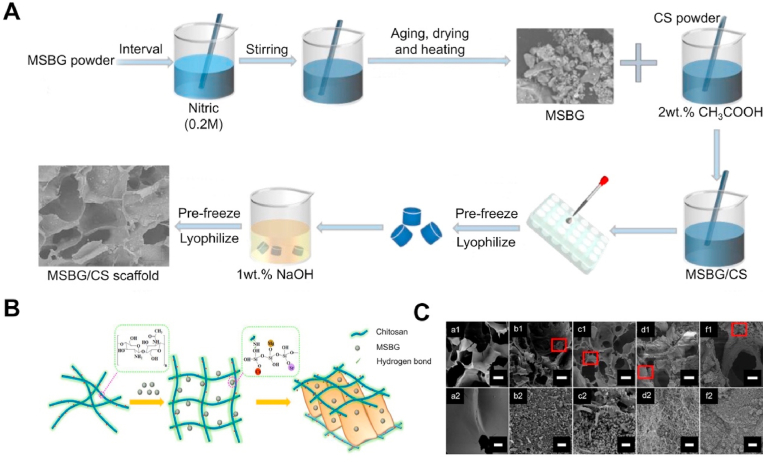
As a new strategy for the treatment of bone defects in osteoporosis, SrBGs scaffolds have been extensively studied for performance and in vivo evaluation. Currently, the preparation methods of porous BGs scaffolds include foaming, template, freeze-drying, and 3D printing [[151], [152], [153], [154]]. The principle of foaming is to introduce gas into the reaction system through physical or chemical means during the process of material molding, and as the bubbles stabilize, the material has a porous structure [155]. Erol et al. [156] prepared 3D SrBGs scaffolds using a melt polymer foaming process. They demonstrated that such scaffolds can continuously release Sr2+ in a synthetic body fluid (SBF) for a long time. However, excessive temperature can lead to the loss of biological activity of the material. Nommeots-Nomm et al. [157] introduced a new foaming technology, a gel-casting foaming process. The advantage of this technology is that the gelatin temperature-controlled gel is used instead of the original polypropylene polymerization to prevent the reduction of the biological activity caused by the crystallization of glass particles during sintering due to excessive temperature difference. In rabbit models, SrBGs scaffolds exhibited the ability to support and maintain bone growth for a long time. The template method is a common method for preparing porous scaffolds. Its principle is to create a space for the material reaction using a template designed in advance. After the reaction is completed, the template is removed to obtain a porous scaffold. A template-prepared Sr-based mesoporous bioactive glass (MBGs) scaffold has been proven to effectively stimulate the proliferation and differentiation of BMSCs and promote new bone formation in bone defects under osteoporotic conditions [158]. However, the main limitation of this method is its high brittleness, which limits its clinical application. Freeze-drying is a porous scaffold manufacturing technology that is performed at low temperatures and low pressure. The main principle is to process aqueous raw materials into specific shapes, freeze the water into ice, and then dry the raw materials under a vacuum. The space occupied by the original ice can form a pore structure [159,160]. This method preserves the physical structure and appearance of the raw material and prevents a decrease in the biological activity caused by high-temperature crystallization. Guo et al. [161] prepared an Mg and Sr co-doped bioactive glass/chitosan (MSBG/CS) composite scaffold using the freeze-drying method and demonstrated that this composite scaffold has good mechanical properties and Apt inducing ability (Fig. 4). Among the manufacturing technologies of porous BGs scaffolds, 3D printing technology has the most potential for clinical application. Compared with other manufacturing technologies, 3D printing technology can simultaneously control the macro and microstructure of the scaffold to achieve dual matching with natural bone tissue [162]. One study showed that the compressive strength of the Sr-based MBGs scaffold manufactured using 3D printing technology is 170 times higher than that manufactured using the template method [163]. This type of high-strength scaffold can be used in load-bearing areas such as the hip and knee.
Fig. 4.
The freeze-drying method was used to synthesize the Mg and Sr co-doped bioactive glass/chitosan (MSBG/CS) composite scaffolds [161]. (A) The fabrication process of MSBG/CS composite scaffold. (B) The cross-linking of CS. (C) SEM images of MSBG/CS composite scaffolds immersed in SBF solution for 14 days.
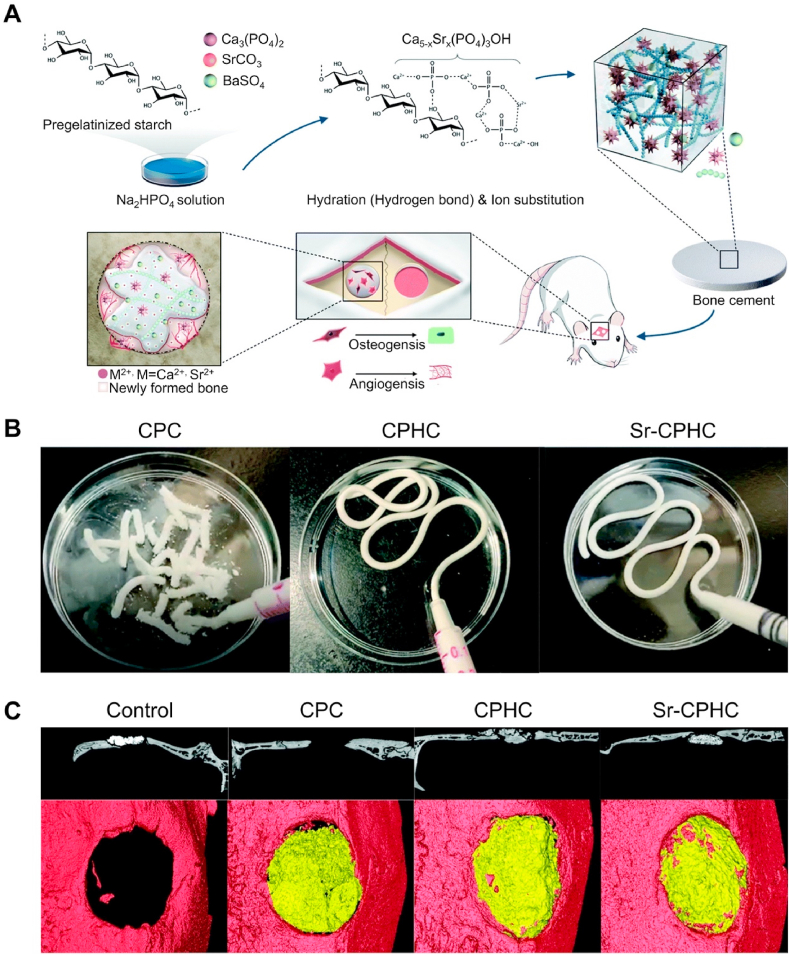
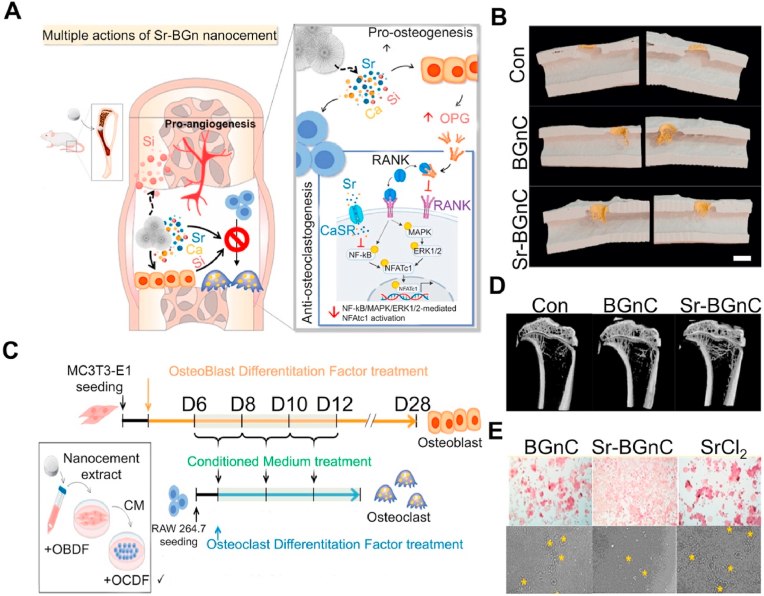
In the past few decades, the development of nanotechnology has revolutionized BGs materials. BGs nanoparticles (NPs) are capable of producing higher mechanical strength and bioactive materials [164]. At present, the main preparation method for biological NPs is the sol-gel method. To obtain NPs with better dispersion, the traditional sol-gel method is divided into two steps: (i) hydrolyze alkoxy organometallic compounds in the precursor to hydroxyl compounds under acidic conditions, and (ii) condense hydroxyl compounds to produce almost monodisperse NPs under alkaline conditions. To further prevent the polymerization of NPs, it is necessary to freeze-dry and calcine the samples prepared using the sol-gel method [164]. SrBGs NPs can effectively promote the osteogenic differentiation of stem cells. The main mechanism is for the NPs to enter the vesicles of human MSCs through a mixed endocytosis mechanism and maintain continuous degradation to release ions [165]. The mechanism of intracellular degradation has also been observed in macrophages, and the presence of SrBGs NPs in the macrophages’ vesicles does not cause a decrease in cell viability. When SrBGs NPs are cocultured with RAW264.7 cells, the cells polarize towards the M2 population, which promotes regeneration, rather than the M1 population, which promotes inflammation [166]. These results demonstrate the good immune induction of SrBGs NPs. In addition, SrBGs NPs have been shown to selectively affect osteoblasts. For example, SrBGs NPs enhance the mineralization ability of osteoblasts while interfering with osteoclast formation in vitro [167]. In addition, another new type of bone cement composed of SrBGs NPs has attracted significant attention from researchers. This nano bone cement has a unique solidification method: the ions released by nBGs in the disodium hydrogen phosphate (Na2HPO4) solution precipitate on the surface of the NPs together with the ions in the solution. The precipitate is composed of Ca – Sr – Si oxides and is in an amorphous phase, enabling the NPs to form a network and harden. This nanoparticle bone cement exhibits a higher surface area, approximately 9 times higher than that of traditional CPC [168]. Moreover, when nano bone cement is immersed in SBF, ultrafine Apt nanocrystals are produced, resulting in a considerable protein molecular adsorption level (approximately 160 times that of CPC). This new type of self-hardening Sr-containing nBGs bone cement exhibits excellent bone defect repair capabilities in vivo and in vitro (under osteoporosis). Through the release of various therapeutic ions, it is expected to become a substitute for PMMA and CPC (Fig. 5) [169].
Fig. 5.
Sr-containing nano bioactive glass bone cement [169]. (A) Schematic summarizing the multiple-actions of Sr-BGsnC in the regeneration of osteoporotic bone defect. (B) μCT images showing neo-bone formation pseudo-colored in yellow according to the morphometric analysis (left, scale bar = 2 mm), and 3D reconstructed images of the neo-bone tissue (right). (C) Schematic timeline of the conditioned medium treatment. (D) μCT analysis of trabecular bone near the tibia condyle. (E) TRAP staining and pit formation assay.
3.3. Sr-containing polymer
Polymer is a material with good biocompatibility, controllable degradation rate, and good flexibility [170]. Its excellent properties make it a popular choice in bone tissue engineering [171]. The first batch of biodegradable scaffolds for clinical use is made of natural polymer materials (sodium alginate (SA), chitosan, collagen, etc.) because they interact well with various cell types and do not induce any immune rejection [172]. However, the mechanical strength of natural polymers is poor and they cannot be used as bone scaffolds for load-bearing sites [173]. Therefore, synthetic polymer scaffolds came into being. Compared with natural polymers, most synthetic polymer scaffolds are cheap [174]. Synthetic polymers have high strength, high elasticity, and high biodegradability, are lightweight, and perfectly match the 3D printing manufacturing process [175]. Although synthetic polymer materials have excellent performance, their low biological activities, and hydrophobic surfaces inhibit the attachment of osteoblasts, leading to low bone induction and bone conduction capacities [176]. Thus, some bioactive materials have been mixed with polymer materials [177]. Sr-containing polymer materials have been extensively studied in the past decade. Sr/Sr2+ has been contained in polymer powder to form a highly bioactive material.
3.3.1. Sr-containing poly(ε-caprolactone) (PCL)
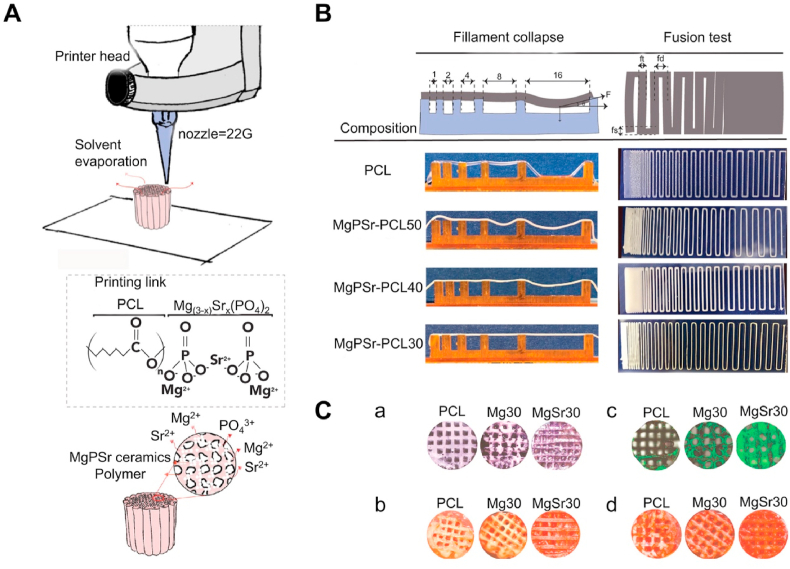
PCL has good biodegradability, chemical stability, thermal stability, histocompatibility, and solute permeability [178]. Compared with other polymers, PCL has a higher compressive strength; thus, it is widely used in fields with higher requirements for mechanical properties [179]. PCL has a relatively slow degradation rate in vivo, which ultimately degrades into water and carbon dioxide under the catalysis of body fluids [180]. However, this degradation rate is not fixed and is affected by factors such as processing technology and geometric shape [181]. Based on this property, PCL materials can be used as carriers for various drugs. It is well known that PCL materials can either be used as additives to improve their mechanical properties by mixing with Sr-based CaPs or BGs or can be used alone as carriers for Sr/Sr2+ to be developed into scaffolds or coatings to exert effects. For instance, Golafshan et al. [182] developed an MgPSr-PCL composite scaffold with high mechanical strength. This support has been proven to have a compressive strength of 4.3 MPa and support up to 50 cycles without plastic deformation. In addition, the scaffold has ultra-high bone inductivity and can induce osteogenic differentiation of stem cells without the addition of bone inducers (Fig. 6) [182]. Such Sr/Sr2+-containing PCL materials can replace unstable and expensive growth factors to impart biological activity to materials.
Fig. 6.
Sr-containing 3D printing MgP/PCL biological scaffold [182]. (A) Schematic illustration of the low-temperature printing process and the composition of the ink. (B) Printability evaluation. Filament test: different compositions extruded over pillar support with different spacings. (C) a) Alkaline phosphatase images of the printed samples. b) and d) Formation of the calcified matrix by eMSCs investigated using Alizarin Red S staining after 30 days of culture. c) the live-dead staining assay during 14 days of culturing of eMSCs in basal media.
3.3.2. Sr-containing PMMA bone cement
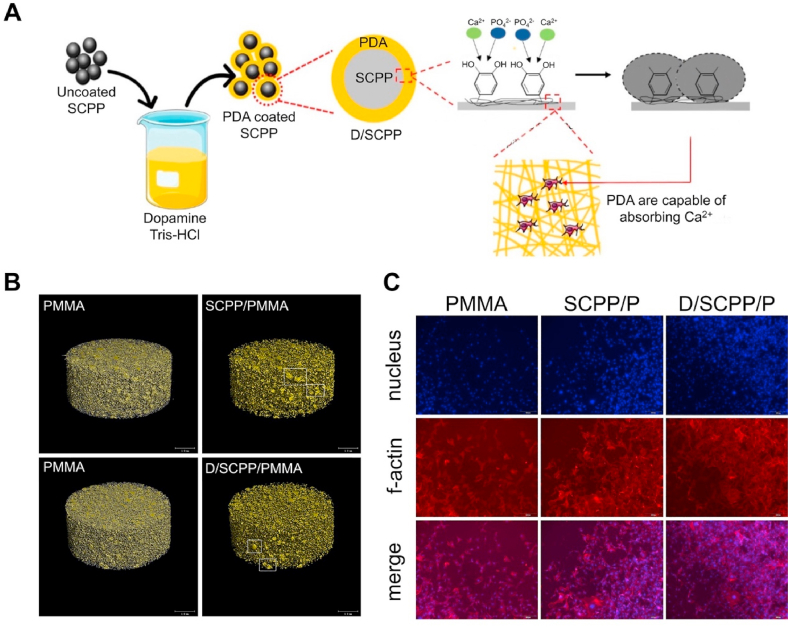
The first-generation bone cement in the world was PMMA bone cement, which was developed by Charnley in 1960. It is the most widely used bone cement today [183]. The PMMA bone cement is composed of a powdered PMMA copolymer and a liquid methyl methacrylate (MMA) monomer [183]. During operation, the liquid mixture can be converted into high-strength solid bone cement after the above two substances are mixed and stirred [184]. The polymer can reach 90% of its ultimate strength within 1 h of mixing [185]. Galibert et al. first proposed the reinforcement of vertebral compression fractures with PMMA bone cement via percutaneous vertebroplasty (PVP) in 1987 [186]. They injected the PMMA cement into the compressed cone to mechanically stabilize the fracture [187]. This method has a revolutionary significance in the treatment of bone compression fractures [188]. PMMA is a biologically inert material that is not reabsorbed after being injected into the human body [189]. However, PMMA is highly associated with bone cement leakage, which can be as high as 65% in osteoporosis patients [190]. Cement leakage may result in serious complications, including life-threatening pulmonary embolism, cerebral embolism, and acute respiratory distress syndrome [191]. Apart from avoiding improper operations, the prevention of osteoporosis reduces bone cement leakage risks [192]. This is because osteoporotic fractures form cavities that are easy to leak the bone cement after injection [193]. Another disadvantage of PMMA is that when two monomers polymerize, they release a lot of heat, which may cause damage to the body [194]. Due to these limitations, various biological modifications have been performed on PMMA to improve their osteogenic activities and reduce bone cement leakage risks. A variety of Sr-containing PMMA bone cements have proven to have better mechanical properties and biological activity, Such as Liu et al. [195] developed a Sr-containing calcium polyphosphate (SCPP) modified PMMA. Compared with ordinary PMMA, this bone cement has stronger biological activities and low reaction temperatures (Fig. 7). In another study, Goñi et al. [196] prepared a Sr-containing bioactive glass-modified PMMA (SrBGs/PMMA) bone cement. This type of mixed bone cement has low maximum exothermic temperatures, slow setting time, and high injectability. Cui et al. [197] proved that the SrBGs/PMMA bone cement has better performance and stronger osteogenic capacities. They found that 12 weeks after implantation in rats, a new bone was formed around the SrBGs/PMMA bone cement, while the connective tissue was only formed in the PMMA bone cement. These bone cements reduce the heat released by mixing the bone cement by reducing the proportions of PMMA in bone cement, and bone cement bioactivities are further improved by Sr modifications. However, they only revealed that the Sr-modified PMMA bone cement promotes bone integration under physiological conditions, and more studies should aim at elucidating their significance in treating bone defects in osteoporosis models.
Fig. 7.
Sr-containing calcium polyphosphate and polyamine-modified PMMA bone cement [195]. (A) Preparation, characterization, physicochemical properties, and bioactivity of composite bone cements. (B) Micro-CT of PMMA bone cement composite. (C) The confocal microscopy images of adhesion and growth of MG63 cells on PMMA composite bone cement.
3.3.3. Sr-containing hydrogel
Hydrogels are excellent carrier materials that have gained wide attention in the past decade [198]. They are hydrophilic three-position network structure gels [199]. As drug carriers, they can control the speed and time of drug release, and maintain a certain drug concentration in damaged parts to achieve therapeutic effects [200]. Their physicochemical properties determine the drug release rate and duration [200]. Compared with other coating technologies, hydrogel coatings can control Sr/Sr2+ release, which can avoid the negative impact of excess or insufficient Sr/Sr2+.
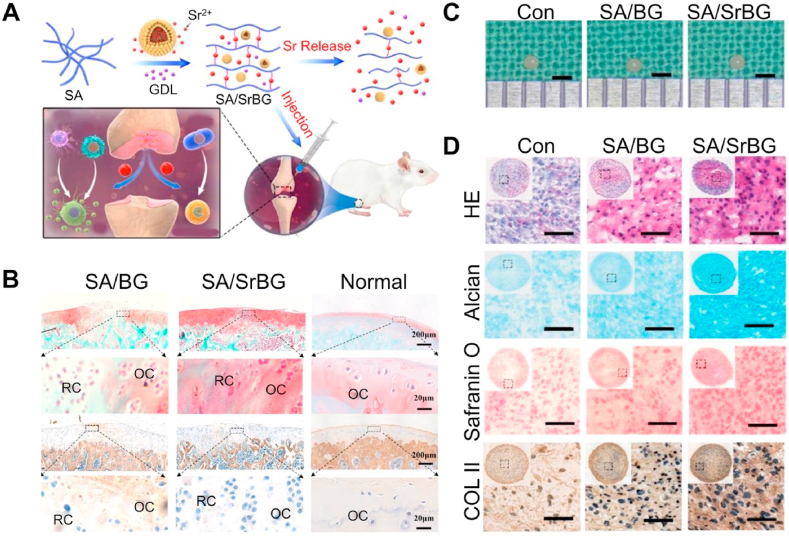
SA, a natural polysaccharide, is a by-product of iodine and mannitol and is extracted from kelp or sargassum alginate [201]. Under mild conditions, SA can crosslink with Sr2+ to form hydrogels [202]. The gelling environment of SA is mild, which avoids the inactivation of active substances such as proteins, cells, and enzymes [203]. Due to these characteristics, SA has a wide range of applications in the medical field. As a carrier of Sr2+, SA hydrogel has natural advantages. The sodium ion on the SA G unit can exchange with Sr2+, and the G unit stacks to form a cross-linked network structure, forming a hydrogel. As the concentration of strontium ions increases, the crosslinking degree and elastic modulus of SA increase, and the swelling and degradation rate decrease. Therefore, the physicochemical properties of the SA hydrogel can be adjusted based on Sr2+ concentration [204]. Sr-containing SA can deliver Sr2+ to bone defects in the form of a coating or scaffold. However, there may be some worrying issues. First, the binding force between the SA hydrogel and base material is not strong enough, which may cause the coating to fall off too quickly and release the drug in undesignated areas [205]. To solve this problem, Yuan et al. [206] used dopamine as an adhesive to enhance adhesion between the coating and implant and achieved good results. In addition, SA as a carrier may have an early explosive release, especially for unmodified SA [207] According to the current research, most of the Sr2+-crosslinked SAs have not exhibited significant cytotoxicity; however, this issue has been largely considered by researchers. Cai et al. [208] reported a new type of Sr-containing BGs-crosslinked SA hydrogel. Sr2+ is first released from BGs, then cross-linked with SA, and finally released to osteochondral defects through the degradation of SA. This model prevents the short-term burst release of Sr2+ directly from the hydrogel. When this composite hydrogel is injected into the cartilage defect model, it stimulates macrophages and polarizes to the M2 phenotype through the local release of Sr2+, and then promotes the chondrogenic differentiation of BMSCs (Fig. 8).
Fig. 8.
Sr crosslinked sodium alginate bioactive glass hydrogel [208]. (A) Schematic diagram of Sr cross-linked sodium alginate bioactive glass hydrogel. (B) Safranin-O/fast green staining and IHC for COL I. (C) The gross appearance of pellets cultured in different extracts. (D) HE staining, AB staining, Safranin-O staining, and IHC for COL II of pellets cultured in different extracts.
Carboxymethyl cellulose (CMC), a white fibrous or granular powder, is an anionic polymer compound with a molecular weight of several thousand to several million Da [209]. Interestingly, the dispersion of CMC in water can form a transparent colloidal solution, a CMC gel [210]. Due to its low toxicity and immunogenicity, good responsiveness, biodegradability, and biocompatibility, the CMC gel has become one of the most promising materials to be used as drug carriers [211]. With increasing studies on hydrogels, researchers have shifted their attention from ordinary hydrogels to intelligent response hydrogels. Various intelligent hydrogels that can respond to changes in environmental conditions, including temperature, electric field, pH, or other conditions have been developed [212,213]. The CMC can be cross-linked in different environments, including metal ions, radiation, and natural polymers [214]. An Sr2+ crosslinked CMC hydrogel coating can promote bone regeneration on implant surfaces. Lopa et al. [215] coated CMC hydrogel loaded with Sr2+ and BMSCs on the surface of macroporous Ti. They found that when the SrCl2 concentration in the hydrogel was 5 μg/ml, it significantly improved the osteogenic differentiation abilities of BMSCs, and increased the expressions of the calcified matrix, type I collagen, and ALP activities. Lovati et al. [216] investigated the carboxymethyl cellulose hydrogel loaded with Sr2+ and human BMSCs. The bioactive hydrogel and BMSCs exerted synergistic effects in enhancing bone deposition. Therefore, they postulated that using Sr2+ loaded CMC hydrogels and seeding BMSC prosthesis implants is an effective strategy for improving the bone integration abilities of implants.
3.4. Other SrBMs
As a BM that is widely used in clinical practice, calcium sulfate (CaSO4) has various advantages, including good biocompatibility and degradability, sufficient sources, convenient sterilization, and other characteristics [217]. CaSO4 cement has been approved by the US Food and Drug Administration (FDA) to be used in clinical bone defect treatment [218,219]. In the body, CaSO4 can be completely degraded and absorbed through humoral-mediated dissolution and cell-mediated phagocytosis and does not have any significant impacts on Ca levels in blood [218]. However, if the CaSO4 degrades too fast, the biological support of the material is lost, the bone conduction activity is reduced, and the fibrous tissue is formed in the filling area, which is not conducive for bone matrix mineralization and affects new bone remodeling [220]. Sr/Sr2+ plays a crucial role in the modification of CaSO4 bone cement by improving osteogenic activity and slowing down the degradation rate. Wang et al. [221] reported a two-phase bone cement composed of Sr-containing Ca silicate (Sr–CSi), and CaSO4 in vitro experiments have shown that the addition of Sr–CSi improves the biological activity of the composite and delays the release of Si4+ and Sr2+, as well as the biodegradation of the cement. However, the specific mechanism by which Sr2+ affects the degradation of CaSO4 bone cement is yet to be elucidated. Miao et al. [222] also proved that the Sr-containing CaSO4 bone cement has good biodegradability. In vivo and in vitro, the Sr-containing CaSO4 cement effectively promoted bone regeneration.
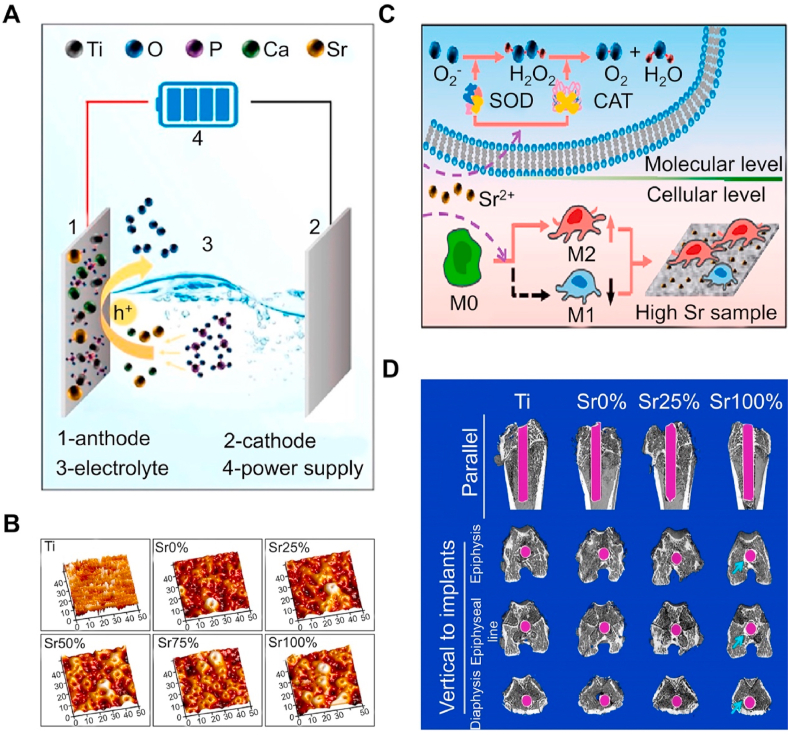
Titanium dioxide (TiO2) coatings are usually prepared on Ti alloy surfaces by electrochemical techniques or hydrothermal methods. TiO2 coating with micro and nanostructures prepared by chemical reaction not only endows the Ti alloy with certain biological activity but also improves the wear and corrosion resistance of the Ti alloy surface. The TiO2 nanotube structure formed on the surface of Ti by anodic oxidation is considered to be a good carrier, and the local release of drugs can be achieved by storing Sr/Sr2+ in the nanotube to promote osteogenesis [[223], [224], [225], [226]]. However, anodic oxidation is limited by the fact that it cannot control the drug release rate in TiO2, which may cause large amounts of Sr2+ to be released around the implant, leading to cytotoxicity and affecting bone regeneration [227]. Therefore, a new coating technology, namely micro-arc oxidation, has been developed by increasing the external electric field voltage and enhancing arc discharge on material surfaces [228]. Compared with anodic oxidation, the coating prepared by micro-arc oxidation has higher wear and corrosion resistance [229]. The micro-nano surface produced by micro-arc oxidation has higher hydrophilicity and can better immobilize the coating materials to achieve sustained drug release [230]. Shen et al. [231] prepared Sr–TiO2 coating by micro-arc oxidation and proved that it has excellent biocompatibility. The coating — when containing with high concentrations of Sr2+ — does not only cause cytotoxicity but also effectively promotes the regeneration of bone defects in osteoporosis models (Fig. 9).
Fig. 9.
Preparation of Sr-containing TiO2 coatings by micro-arc oxidation [231]. (A) Schematic diagram of micro-arc oxidation treatment. (B) AFM images of different samples. (C) Schematic diagram of phenotypic regulation by peroxidase pathway in high Sr group. (D) Representative micro-CT scan images of different groups.
4. Co-doping of Sr with therapeutic ions
In recent years, the strategy of using therapeutic ions as additives has achieved significant success in the field of BMs (see Table 2). Currently, researchers have sought to promote tissue regeneration, increase antibacterial activity, and improve bone integration by doping different therapeutic ions in some BMs such as HA and BGs. Although the impact of each therapeutic ion combination is currently being studied, the potential for binary, ternary, and mixed element doping is considered to have greater practical applications. Synergistic effects between different elements can produce more complex effects, possibly endowing BMs with more and stronger therapeutic functions (Table 3). In this section, we discussed the physicochemical properties of BMs which co-doping Sr with other therapeutic ions and their effects on cell behavior, providing a new reference for the future development of multifunctional SrBMs.
Table 2.
Summary of effects of Sr/Sr2+ addition on physicochemical properties of BMs.
| Materials | Physicochemical properties | Reference |
|---|---|---|
| HA | Improved mechanical strength, increased solubility, decreased crystallinity, and increased d-spacing and crystal cell unit parameters. | [111,112] |
| β-TCP | Increase elastic modulus and mechanical strength | [113] |
| BGs | Increase crystallinity, crystallization starting point, crystallization peak value, and degradation rate | [150] |
| SA | As the concentration of strontium ions increases, the crosslinking degree and elastic modulus of SA increase, and the swelling and degradation rate decrease | [204] |
| CaSO4 | Decrease the degradation rate | [221] |
Table 3.
Summary of co-doping Sr with other therapeutic ions.
| Ions | Function | Reference |
|---|---|---|
| Sr–Fe | Promote osteogenesis and endow materials with magnetism | [112] [232,233] |
| Sr–Ag | Improve antibacterial properties | [[234], [238], [237], [236], [235]] |
| Sr–Zn | Improve antibacterial properties, osteogenesis, and antioxidant properties | [[239], [240], [241], [242]] |
| Sr–Co | Promote angiogenesis and antibacterial properties and endow materials with magnetism | [[243], [244], [245], [246]] |
| Sr–Cu | Improve antibacterial properties and osteogenesis | [247] |
| Sr–F | Improved antibacterial, mechanical properties and osteogenesis | [[248], [249], [250], [251]] |
| Sr–Mn | Improve corrosion resistance and osteogenesis | [252,253] |
4.1. Iron (Fe)–Sr co-doping
Fe is an essential element in human life activities. It is involved in several complex metabolic processes, including hemoglobin synthesis, the conversion of blood sugar into energy, and the production of enzymes [117]. During bone growth, a Fe-deficient diet at an early age may result in the inhibition of osteoblasts, which can greatly affect bone development. Conversely, excessive Fe in the body can inhibit osteogenic activity and lead to osteoporosis in children, adults, and postmenopausal women [232]. In recent years, research on Fe–Sr co-doped materials has mainly focused on the field of HA materials. HA lattices have 10 known Ca sites that can be occupied by external ions and alter their physicochemical and biomechanical characteristics [232]. Studies have shown that different ions have different substitution positions in the HA lattice. In Sr-doped HA materials, a low Sr content (<1%) is conducive to the substitution of Ca–I sites, while a high Sr content is conducive to the substitution of Ca-II sites. For Fe, the Ca-II site (6-fold coordination) is favorable for Fe2+ substitution, while the Ca–I site (4-fold coordination) allows for Fe3+ substitution. The Fe–Sr co-doped HA material developed based on the above theory has been proven to have good biocompatibility [232]. As scaffold materials, the addition of Fe and Sr improves the mechanical properties of HA and accelerates angiogenesis and bone regeneration after implantation in vivo [112]. As a nanocarrier, the addition of Fe endows HA materials with a certain degree of magnetism, which provides potential advantages for their application in high-temperature and targeted drug delivery systems through external magnetic fields. A study has shown that Sr–Fe co-doped HA NPs can load more chemotherapy drugs and enhance efficacy. Furthermore, Sr–Fe-HA exhibits superparamagnetism, enabling it to be directed to specific locations through the application of an external magnetic field [233]. However, some findings are also worth our attention. Firstly, in materials containing high doses of Sr–Fe-HA, research on the release and controllability of Sr and Fe ions still has significant limitations. Moreover, studies have shown that when Fe3+ replaces Ca in HA, the thermal stability of the material significantly decreases [232]. Therefore, replacement with large doses of Fe3+ may affect the storage of drugs.
4.2. Silver (Ag)–Sr co-doping
Although SrBMs have been proven to have good osteogenic activity, their poor mechanical properties and antibacterial properties limit their clinical application. As a long history of antibacterial agents, Ag and Ag+ have strong antibacterial effects and a broad spectrum of antimicrobial activity [234]. Ag silver preparations possess excellent antibacterial properties; however, if their concentration surpasses a particular threshold, they can have harmful effects on human cells [235]. Some researchers have proposed that the addition of secondary chemicals is an effective strategy to eliminate the side effects of Ag preparations and maintain their antibacterial properties. A previous study has shown that the addition of Sr to Ag-doped CaP powder can effectively counteract the negative effects of Ag and improve the mechanical properties and osteogenic activity of the material [236]. Although Sr can counteract the cytotoxic effects of Ag, it does not inherently impede the release of Ag, and the cytotoxicity of Ag might resurface once the Sr has been released entirely. Therefore, Huang et al. innovatively adopted graphene oxide (GO) to slow down the release of Ag+ and Sr2+ in Ag–Sr co-doped HA materials, effectively reducing the cytotoxicity generated when high concentrations of Ag+ are released [237]. The combination of Ag–Sr co-doped HA and frozen gel composed of silk fibroin and chitosan can also effectively prevent the cytotoxicity caused by ion burst release [238]. These modification strategies could be applied in the treatment of infectious bone defects in the future.
4.3. Zn–Sr co-doping
It is well known that Zn is an essential trace element in the human body and it plays an important role in bone formation. As a metal ion with both antibacterial and osteogenic functions, Zn2+ can not only enhance the expression of osteogenic-related genes but also effectively inhibit the proliferation of Staphylococcus aureus [239]. When Zn and Sr are doped into BGs, they significantly affect mechanical properties, including dissolution behavior and the glass transition temperature (Tg) [240,241]. In addition, the antioxidant properties of BGs show a dose dependence on Sr and Zn. When the release concentrations of Sr and Zn are limited to below 10 and 2 ppm, respectively, BGs can exhibit good antioxidant behavior by enhancing cell viability and eliminating oxidative stress reactions [240]. One study pointed out that in Sr–Zn co-doped BGs, the release of Zn can stimulate osteogenic differentiation and proliferation of stem cells and promote the formation of Apt layers in simulated body fluids, while the release of Sr can only promote the differentiation of stem cells but cannot promote their proliferation, and inhibit the formation of Apt [242]. This antagonistic effect may be related to the dissolution behavior of Sr and Zn in simulated body fluids. However, research on Zn–Sr co-doped CaP materials is limited. Therefore, this antagonistic behavior warrants further investigation in the future.
4.4. Cobalt (Co)–Sr co-doping
Co is an element with therapeutic potential. Although Co has been treated as a toxic element for a long time, researchers have found that at low concentrations, it can pass through HIF-1α pathways to induce cell hypoxia, thereby increasing the expression of vascular endothelial growth factor (VEGF) to promote angiogenesis [243]. Some studies have shown that Co can enter HA through hydroxide (OH) channels or replace Ca sites, endowing HA materials with certain antibacterial and magnetic properties [244]. Therefore, researchers conducted in-depth studies on the angiogenesis ability of Co–Sr co-doped BGs materials. It was found that at a certain concentration, Co–Sr co-doped BGs materials showed no significant cytotoxicity and their excellent ability to promote osteogenesis and angiogenesis in a rabbit bone defect model has been proven [245,246]. These findings provide a new approach for the repair of bone defects and the regeneration of the hard/soft tissue interface. However, whether high-dose Co-doped materials have cytotoxicity and whether Sr and Co have synergistic effects deserve further discussion.
4.5. Other element-Sr co-doping
Copper (Cu) is an important element that participates in the synthesis of various enzymes and proteins. Cu can stimulate vascular regeneration and inhibit bacterial proliferation in vitro. Sr–Cu co-doped MBGs can maintain the continuous release of two metal ions, enhancing the antibacterial and angiogenesis capabilities of the material [247]. Unlike other dopants, fluorine ion (F−) is a non-metallic anion that can replace the hydroxide ions (OH−) in Apt to form fluoroapatite (FA). Compared with pure Apt materials, the addition of F− increases the compressive strength and osteogenic activity of the material [248,249]. Interestingly, the two studies gave opposite conclusions. The experimental results of Ganjali et al. [250] showed that Sr-doped FA coatings increase cellular activity and antibacterial activity of the scaffold surface. Shahrouzifar et al. showed that Sr-doped FA scaffolds have poorer biocompatibility and bioactivity compared with Sr-doped Apt scaffolds and pure FA scaffolds [251]. The reasons for this difference may be related to the manufacturing method of FA and the dosage of the doped ions. Manganese (Mn) is a trace mineral with a radius very close to Ca2+, which allows Mn2+ to enter osteoblasts through Ca channels to regulate the differentiation of osteoblasts [252]. Studies have shown that when Mn2+ is doped into a bioglass, it can effectively stimulate the expression of ALP [253]. In addition, in CaP materials co-doped with Sr, the addition of Mn2+ further increases the osteogenic activity of the material while improving its corrosion resistance [252]. This is because Mn2+ and Sr2+ reduce the unit volume and particle size of HA, thereby increasing the density of HA coating and reducing the corrosion current in physiological body fluids.
In summary, there are numerous types of therapeutic elements co-doped with Sr, and remarkable achievements have been made in increasing antibacterial activity and osteogenic activity. However, it is undeniable that research on such materials is very limited and insufficient, and most studies cannot explain whether there is a synergistic effect between therapeutic elements and Sr, as well as whether doping doses can ensure biosafety. Therefore, future research should focus on the synergy between elements and explore the optimal content ratio to achieve the best therapeutic effect.
5. Expectations
5.1. Long-term Sr delivery composites
The use rate of oral bisphosphonate in osteoporosis treatment is much higher than that of SR. This is not because the therapeutic effect of bisphosphonate is better than SR, but because orally administered SR has many side effects. To ensure patient safety, only patients with severe osteoporosis can use SR. To reduce the side effects of oral SR, there is a need to develop a BM that can release Sr2+ for a long time (in years). Various SrBMs have been developed to address fractures and bone defects in osteoporosis. However, osteoporosis is a systemic disease that does not disappear because local fractures are cured. Long-term oral anti-osteoporotic drugs remain the only approach for patients with osteoporosis to prevent such fractures. We propose a strategy to prevent and treat osteoporosis by replacing oral drugs with BMs, which release Sr2+ over an extended period. Because we cannot predict whether the risk of this invasive implant surgery is lower than the risk borne by long-term oral medication. Therefore, we believe that this strategy is only suitable for patients who have developed fractures or bone defects and need BM implantation for treatment. This material can prevent or treat osteoporosis by locally releasing Sr2+ into the blood, which then reaches the bone tissues throughout the body via blood circulation. It may be the combination of Sr-containing coating and Sr-containing implant. The coating outside the implant can ensure that Sr2+ in the implant is not released. When the coating is slowly degraded, Sr2+ in the implant begins to be slowly released, thereby extending the release time of Sr2+. This material should have a stable Sr2+ release rate as it should load a large amount of Sr2+. When released too fast, it can easily result in serious cytotoxicity. The load and release rate of Sr2+ can be adjusted according to the severity of the patient's condition. If this composite material can be successfully developed, it may be able to avoid the long-term drug use-associated psychological, gastrointestinal, and liver function pressures in osteoporosis patients.
5.2. Treatment of bone tumors using 89Sr-containing materials
Metastatic bone tumors are a serious threat to human health. Lung cancer, breast cancer, thyroid cancer, and prostate cancer are prone to distant bone metastasis [[254], [255], [256], [257]]. Therefore, studies are aimed at establishing appropriate strategies to inhibit the progression of bone malignancies and alleviate the pain caused by bone malignancies. 89Sr, trade name Metatron, is a drug that provides palliative treatment for patients suffering from bone pain due to metastatic bone tumors [258]. When intravenously administered, 89SrCl2 replaces Ca in bone HA. This radioactive drug is excreted through the kidneys and feces, and about 30%–35% of the radioactive drug remains in the bones [258]. Since the absorption rate of 89Sr by bone tumor cells is much higher than that of healthy bones, it can inhibit tumor development and relieve pain [258]. However, large doses of radiopharmaceuticals can result in significant bone marrow suppression after intravenous administration, followed by a decrease in leukocyte and platelet counts. This may result in other complications, such as anemia, infection, or internal bleeding [259]. Therefore, there is a need to develop a BM that can be completely degraded in the human body, fix 89Sr in it, and implant it into the tumor cell aggregation site for continuous release. In this way, the amount of radioactive substances entering the body circulation is far lower than that of intravenous administration, and local high-dose drugs can also effectively inhibit tumor cell proliferation and metastasis.
5.3. Injectable Sr-containing hydrogel for fracture
It is well known that the probability of fractures in patients with osteoporosis is much higher than in healthy individuals. In some cases, fractures associated with osteoporosis are not serious; however, the imbalance in the ratio of osteoblasts to osteoclasts in the body may cause a nonunion of fractures. Clinically, patients are often required to take oral bisphosphonates to treat or prevent this type of disease. Although oral bisphosphonates are effective in preventing and treating osteoporotic fractures, the side effects of long-term oral bisphosphonates, such as gastrointestinal disorders, renal burden, and bone necrosis, cannot be overlooked. Researchers have proposed strategies for the subcutaneous administration of drugs to prevent osteoporosis and fractures [260]. Therefore, we plan to develop a biodegradable Sr-based hydrogel that can be used for nonvertebral fractures. It can be injected subcutaneously into the fracture site to improve the osteogenesis of the fracture site and the immune microenvironment to promote fracture healing. This type of hydrogel is temperature sensitive. When injected into the fracture site, it can respond to the body temperature and become a gel immediately, and can be adsorbed in the fracture or bone defect area. It is noteworthy that this treatment strategy is not suitable for an osteoporotic population with large bone defects because the mechanical strength of this injectable hydrogel may not be comparable to that of bone tissue temporarily. Furthermore, how to accurately inject the hydrogel into a specific site is a challenge that must be solved, especially for deep fractures.
6. Conclusion
SR/Sr are well-known drugs for osteoporosis treatment. Apart from oral administration, Sr is widely used in BMs. As a metal element with osteogenic activities, Sr is doped into various BMs and released locally for a long time to promote new bone formation. Studies on SrBMs are only limited to cell and animal experiments, clinical applications and large-scale production are still far away. These materials should be verified as having the ability to play expected roles in the human body. Moreover, the stability, durability, economic benefits, and repeatability of materials are key factors for their commercialization. Finally, treatment and surgical indications as well as potential complications after implantation of these materials should be elucidated further.
Author contributions
Xiao Sheng: Conceptualization, Investigation, and Writing - Original draft preparation. Chen Li: Data curation and Writing - Reviewing and Editing. Zhonghan Wang: Validation and Methodology. Yu Xu: Data curation and Validation. Yang Sun: Conceptualization, Methodology, and Supervision. Weiming Zhang: Visualization and Software. Jincheng Wang: Conceptualization, Validation, and Methodology. He Liu: Supervision, Funding acquisition, Conceptualization, and Writing — Reviewing and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Science and technology research project of Education Department of Jilin Province (JJKH20211172KJ), Project of “Medical + X″ Interdisciplinary Innovation Team of Norman Bethune Health Science Center of Jilin University (2022JBGS06), the National Natural Science Foundation of China (82001971) Scientific Development Program of Jilin Province (Grant Nos. YDZJ202301ZYTS031), The Science and Technology Development Program of Jilin Province (20210101319JC).
Contributor Information
Xiao Sheng, Email: 943889051@qq.com.
Chen Li, Email: chenliortho@jlu.edu.cn.
Zhonghan Wang, Email: zhonghan19@mails.jlu.edu.cn.
Yu Xu, Email: 1013263162@qq.com.
Yang Sun, Email: sunyang@jlu.edu.cn.
Weimin Zhang, Email: z1933554836@qq.com.
He Liu, Email: heliu@jlu.edu.cn.
Jincheng Wang, Email: jinchengwangjlu@163.com.
Data availability
No data was used for the research described in the article.
References
- 1.Battaglino R. The skeleton as an endocrine organ: metabolic functions of osteocalcin. Actual. Osteol. 2017;13(3):225–232. [Google Scholar]
- 2.Hu C., Ashok D., Nisbet D.R., Gautam V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials. 2019;219 doi: 10.1016/j.biomaterials.2019.119366. [DOI] [PubMed] [Google Scholar]
- 3.Lin X., Patil S., Gao Y.G., Qian A. The bone extracellular matrix in bone formation and regeneration. Front. Pharmacol. 2020;11:757. doi: 10.3389/fphar.2020.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florencio-Silva R., Sasso G.R., Sasso-Cerri E., Simões M.J., Cerri P.S. Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X., Wang Z., Duan N., Zhu G., Schwarz E.M., Xie C. Osteoblast–osteoclast interactions. Connect. Tissue Res. 2018;59(2):99–107. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An J., Yang H., Zhang Q., Liu C., Zhao J., Zhang L., Chen B. Natural products for treatment of osteoporosis: the effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016;147:46–58. doi: 10.1016/j.lfs.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Olsen B.R., Reginato A.M., Wang W. Bone development. Annu. Rev. Cell Dev. Biol. 2000;16(1):191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 8.Langdahl B., Ferrari S., Dempster D.W. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Ther. Adv. Musculoskelet. Dis. 2016;8(6):225–235. doi: 10.1177/1759720X16670154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song S., Guo Y., Yang Y., Fu D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol. Ther. 2022;237 doi: 10.1016/j.pharmthera.2022.108168. [DOI] [PubMed] [Google Scholar]
- 10.Saidak Z., Marie P.J. Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Ther. 2012;136(2):216–226. doi: 10.1016/j.pharmthera.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Black D.M., Rosen C.J. Postmenopausal osteoporosis, N. Engl. J. Med. 2016;374(3):254–262. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 12.Curry S.J., Krist A.H., Owens D.K., Barry M.J., Caughey A.B., Davidson K.W., Doubeni C.A., Epling J.W., Kemper A.R., Kubik M., Landefeld C.S., Mangione C.M., Phipps M.G., Pignone M., Silverstein M., Simon M.A., Tseng C.W., Wong J.B. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(24):2521–2531. doi: 10.1001/jama.2018.7498. [DOI] [PubMed] [Google Scholar]
- 13.Khosla S., Amin S., Orwoll E. Osteoporosis in men. Endocr. Rev. 2008;29(4):441–464. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heffner M.A., Genetos D.C., Christiansen B.A. Bone adaptation to mechanical loading in a mouse model of reduced peripheral sensory nerve function. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0187354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiernan J., Davies J.E., Stanford W.L. Concise review: musculoskeletal stem cells to treat age-related osteoporosis. Stem Cells Transl. Med. 2017;6(10):1930–1939. doi: 10.1002/sctm.17-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snodgrass P., Zou A., Gruntmanis U., Gitajn I.L. Osteoporosis diagnosis, management, and referral practice after fragility fractures. Curr. Osteoporos. Rep. 2022;20:163–169. doi: 10.1007/s11914-022-00730-1. [DOI] [PubMed] [Google Scholar]
- 17.Uebelhar D., Frey D., Frey-Rindova P., Goerres G., Michel B.A. Therapy of osteoporosis: bisphosphonates, SERM's, teriparatide and strontium. Z. Rheumatol. 2003;62(6):512–517. doi: 10.1007/s00393-003-0560-5. [DOI] [PubMed] [Google Scholar]
- 18.Deeks E.D., Dhillon S. Strontium ranelate A review of its use in the treatment of postmenopausal osteoporosis. Drugs. 2010;70(6):733–759. doi: 10.2165/10481900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Kołodziejska B., Stępień N., Kolmas J. The influence of strontium on bone tissue metabolism and its application in osteoporosis treatment. Int. J. Mol. Sci. 2021;22(12):6564. doi: 10.3390/ijms22126564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang D., Zhao F., Gao W., Chen X., Guo Z., Zhang W. Strontium-substituted sub-micron bioactive glasses inhibit ostoclastogenesis through suppression of RANKL-induced signaling pathway. Regen. Biomater. 2020;7(3):303–311. doi: 10.1093/rb/rbaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarins J., Pilmane M., Sidhoma E., Salma I., Locs J. The role of Strontium enriched hydroxyapatite and tricalcium phosphate biomaterials in osteoporotic bone regeneration. Symmetry. 2019;11(2):229. doi: 10.3390/sym11020229. [DOI] [Google Scholar]
- 22.Zarins J., Pilmane M., Sidhoma E., Salma I., Locs J. Immunohistochemical evaluation after Sr-enriched biphasic ceramic implantation in rabbits femoral neck: comparison of seven different bone conditions. J. Mater. Sci. Mater. Med. 2018;29(8):119. doi: 10.1007/s10856-018-6124-7. [DOI] [PubMed] [Google Scholar]
- 23.Rybchyn M.S., Slater M., Conigrave A.D., Mason R.S. An Akt-dependent increase in canonical Wnt signaling and a decrease in sclerostin protein levels are involved in strontium ranelate-induced osteogenic effects in human osteoblasts. J. Biol. Chem. 2011;286(27):23771–23779. doi: 10.1074/jbc.M111.251116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kern C., Quade M., Ray S., Thomas J., Schumacher M., Gemming T., Gelinsky M., Alt V., Rohnke M. Investigation of strontium transport and strontium quantification in cortical rat bone by time-of-flight secondary ion mass spectrometry. J. R. Soc. Interface. 2019;16(151) doi: 10.1098/rsif.2018.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao R., Yang X., Zhu X., Zhang X. Application of trace element strontium-doped biomaterials in the field of bone regeneration. Prog. Chem. 2021;33(4):533–542. doi: 10.7536/PC200537. [DOI] [Google Scholar]
- 26.Rizzoli R., Reginster J.Y., Boonen S., Bréart G., Diez-Perez A., Felsenberg D., Kaufman J.M., Kanis J.A., Cooper C. Adverse reactions and drug–drug interactions in the management of women with postmenopausal osteoporosis. Calcif. Tissue Int. 2011;89(2):91–104. doi: 10.1007/s00223-011-9499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musette P., Brandi M.L., Cacoub P., Kaufman J.M., Rizzoli R., Reginster J.Y. Treatment of osteoporosis: recognizing and managing cutaneous adverse reactions and drug-induced hypersensitivity. Osteoporos. Int. 2010;21(5):723–732. doi: 10.1007/s00198-009-1097-5. [DOI] [PubMed] [Google Scholar]
- 28.Manoochehri H., Ghorbani M., Moosazadeh Moghaddam M., Nourani M.R., Makvandi P., Sharifi E. Strontium doped bioglass incorporated hydrogel-based scaffold for amplified bone tissue regeneration. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-14329-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Dias A.M., do Nascimento Canhas I., Bruziquesi C.G., Speziali M.G., Sinisterra R.D., Cortés M.E. Magnesium (Mg2+), strontium (Sr2+), and zinc (Zn2+) co-substituted bone cements based on nano-hydroxyapatite/monetite for bone regeneration. Biol. Trace Elem. Res. 2022 doi: 10.1007/s12011-022-03382-5. [DOI] [PubMed] [Google Scholar]
- 30.Christensen T.E., Berglund Davidsen M., Van Malderen S., Garrevoet J., Offermanns V., Andersen O.Z., Foss M., Birkedal H. Local release of strontium from sputter-deposited coatings at implants increases the strontium-to-calcium ratio in peri-implant bone. ACS Biomater. Sci. Eng. 2022;8(2):620–625. doi: 10.1021/acsbiomaterials.1c01004. [DOI] [PubMed] [Google Scholar]
- 31.Bonnelye E., Chabadel A., Saltel F., Jurdic P. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. 2008;42(1):129–138. doi: 10.1016/j.bone.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 32.Cheshmedzhieva D., Ilieva S., Permyakov E.A., Permyakov S.E., Dudev T. Ca2+/Sr2+ selectivity in calcium-sensing receptor (CaSR): implications for strontium's anti-osteoporosis effect. Biomolecules. 2021;11(11):1576. doi: 10.3390/biom11111576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W., Sun R., Zhong H., Tang N., Liu Y., Zhao Y., Zhang T., He F. CaSR participates in the regulation of vascular tension in the mesentery of hypertensive rats via the PLC-IP3/AC-V/cAMP/RAS pathway. Mol. Med. Rep. 2019;20(5):4433–4448. doi: 10.3892/mmr.2019.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santa Maria C., Cheng Z., Li A., Wang J., Shoback D., Tu C.L., Chang W. Interplay between CaSR and PTH1R signaling in skeletal development and osteoanabolism. Semin. Cell Dev. Biol. 2016;49:11–23. doi: 10.1016/j.semcdb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren W.H., Xin S., Yang K., Yu Y.B., Li S.M., Zheng J.J., Huang K., Zeng R.C., Yang X.X., Gao L., Li S.Q., Zhi K. Strontium-doped hydroxyapatite promotes osteogenic differentiation of bone marrow mesenchymal stem cells in osteoporotic rats through the CaSR-JAK2/STAT3 signaling pathway. Adv. NanoBiomed Res. 2022;2(9) doi: 10.1002/anbr.202200018. [DOI] [Google Scholar]
- 36.Cheshmedzhieva D., Ilieva S., Permyakov E.A., Permyakov S.E., Dudev T. Ca2+/Sr2+ selectivity in calcium-sensing receptor (CaSR): implications for strontium's anti-osteoporosis effect. Biomolecules. 2021;11(11):1576. doi: 10.3390/biom11111576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aimaiti A., Wahafu T., Keremu A., Li Y., Cao L. Strontium ameliorates glucocorticoid inhibition of osteogenesis via the ERK signaling pathway. Biol. Trace Elem. Res. 2020;197(2):591–598. doi: 10.1007/s12011-019-02009-6. [DOI] [PubMed] [Google Scholar]
- 38.Ghayor C., Caverzasio J. Prostaglandins-dependent activation of ERK mediates cell proliferation induced by TGF beta in MC3T3-E1 osteoblastic cells, Calcif. Tissue Int. 2004;74:S32. doi: 10.1016/j.bone.2004.10.007. S32. [DOI] [PubMed] [Google Scholar]
- 39.Yu X., Wang X., Li D., Sheng R., Qian Y., Zhu R., Wang X., Lin K. Mechanically reinforced injectable bioactive nanocomposite hydrogels for in-situ bone regeneration. Chem. Eng. J. 2022;433 doi: 10.1016/j.cej.2021.132799. [DOI] [Google Scholar]
- 40.Lisowska B., Kosson D., Domaracka K. Lights and shadows of NSAIDs in bone healing: the role of prostaglandins in bone metabolism. Drug Des. Dev. Ther. 2018;12:1753–1758. doi: 10.2147/DDDT.S164562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raisz L.G. Prostaglandins and bone: physiology and pathophysiology. Osteoarthritis Cartilage. 1999;7(4):419–421. doi: 10.1053/joca.1998.0230. [DOI] [PubMed] [Google Scholar]
- 42.Caron M.M., Means P.J., Sanen K., Surtel D.A., Cremers A., Ophelders D., van Rhijn L.W., Welting T.J. The role of prostaglandins and COX-enzymes in chondrogenic differentiation of ATDC5 progenitor cells. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0153162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He B., Wang J. Chitooligosaccharides prevent osteopenia by promoting bone formation and suppressing bone resorption in ovariectomised rats: possible involvement of COX-2. Nat. Prod. Res. 2015;29(4):359–362. doi: 10.1080/14786419.2014.942301. [DOI] [PubMed] [Google Scholar]
- 44.Frick K.K., Krieger N.S., LaPlante K., Smith S.B., Bushinsky D.A. Decreased acid-induced bone resorption in COX-2 knockout mice. J. Bone Miner. Res. 2004;19:S188. S188. [Google Scholar]
- 45.Aimaiti A., Wahafu T., Keremu A., Li Y., Cao L. Strontium ameliorates glucocorticoid inhibition of osteogenesis via the ERK signaling pathway. Biol. Trace Elem. Res. 2020;197(2):591–598. doi: 10.1007/s12011-019-02009-6. [DOI] [PubMed] [Google Scholar]
- 46.Ma B., Zhang Q., Wu D., Wang Y.L., Hu Y.Y., Cheng Y.P., Yang Z.D., Zheng Y.Y., Ying H.J. Strontium fructose 1,6-diphosphate prevents bone loss in a rat model of postmenopausal osteoporosis via the OPG/RANKL/RANK pathway. Acta Pharmacol. Sin. 2012;33(4):479–489. doi: 10.1038/aps.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yasuda H. Discovery of the RANKL/RANK/OPG system. J. Bone Miner. Metabol. 2021;39:2–11. doi: 10.1007/s00774-020-01175-1. [DOI] [PubMed] [Google Scholar]
- 48.Kovács B., Vajda E., Nagy E.E. Regulatory effects and interactions of the Wnt and OPG-RANKL-RANK signaling at the bone-cartilage interface in osteoarthritis. Int. J. Mol. Sci. 2019;20(18):4653. doi: 10.3390/ijms20184653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reid P., Holen I. Pathophysiological roles of osteoprotegerin (OPG) Eur. J. Cell Biol. 2009;88(1):1–7. doi: 10.1016/j.ejcb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Holen I., Shipman C.M. Role of osteoprotegerin (OPG) in cancer. Clin. Sci. 2006;110(3):279–291. doi: 10.1042/CS20050175. [DOI] [PubMed] [Google Scholar]
- 51.Chaudhari S.D., Sharma K.K., Marchetto J.J., Hydren J.R., Burton B.M., Moreno A.P. Modulating OPG and TGF-β1 mRNA expression via bioelectrical stimulation. BoneKEy Rep. 2021;15 doi: 10.1016/j.bonr.2021.101141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giner M., Montoya M.J., Vázquez M.A., Rios M.J., Moruno R., Miranda M.J., Perez-Cano R. Modifying RANKL/OPG mRNA expression in differentiating and growing human primary osteoblasts. Horm. Metab. Res. 2008;40(12):869–874. doi: 10.1055/s-0028-1082083. [DOI] [PubMed] [Google Scholar]
- 53.Olesen M., Skov V., Ledet T., Rasmussen L.M. Osteoprotegerin (OPG) and its ligand RANKL in vascular tissue. Faseb. J. 2009;23:1006.6. 1006.6. [Google Scholar]
- 54.Yasuda H., Furuya Y., Uchida K. From discovery of RANKL to clinical application of anti-human RANKL antibody. Arthritis Res. Ther. 2012;14:O33. doi: 10.1186/ar3588. [DOI] [Google Scholar]
- 55.Cheng M., Li T., Li W., Chen Y., Xu W., Xu L. Leptin can promote mineralization and up-regulate RANKL mRNA expression in osteoblasts from adult female SD rats. Int. J. Clin. Exp. Pathol. 2018;11(3):1610–1619. [PMC free article] [PubMed] [Google Scholar]
- 56.Kurban S., Mehmetoglu I. Osteoprotegerin, rank and rank ligand. Turk. J. Biochem. 2007;32(4):178–184. [Google Scholar]
- 57.Sojod B., Chateau D., Mueller C.G., Babajko S., Berdal A., Lézot F., Castaneda B. RANK/RANKL/OPG signalization implication in periodontitis: new evidence from a RANK transgenic mouse model. Front. Physiol. 2017;8:338. doi: 10.3389/fphys.2017.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stuss M., Rieske P., Cegłowska A., Stêpień-Kłos W., Liberski P.P., Brzeziańska E., Sewerynek E. Assessment of OPG/RANK/RANKL gene expression levels in peripheral blood mononuclear cells (PBMC) after treatment with strontium ranelate and ibandronate in patients with postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 2013;98(5):E1007–E1011. doi: 10.1210/jc.2012-3885. [DOI] [PubMed] [Google Scholar]
- 59.Trouvin A.P., Goëb V. Receptor activator of nuclear factor-kappa B ligand and osteoprotegerin: maintaining the balance to prevent bone loss. Clin. Interv. Aging. 2010;5:345–354. doi: 10.2147/CIA.S10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burgers T.A., Williams B.O. Regulation of Wnt/β-catenin signaling within and from osteocytes. Bone. 2013;54(2):244–249. doi: 10.1016/j.bone.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tu X., Delgado-Calle J., Condon K.W., Maycas M., Zhang H., Carlesso N., Taketo M.M., Burr D.B., Plotkin L.I., Bellido T. Osteocytes mediate the anabolic actions of canonical Wnt/beta-catenin signaling in bone. Proc. Natl. Acad. Sci. U.S.A. 2015;112(5):E478–E486. doi: 10.1073/pnas.1409857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kramer I., Halleux C., Keller H., Pegurri M., Gooi J.H., Weber P.B., Feng J.Q., Bonewald L.F., Kneissel M. Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol. Cell Biol. 2010;30(12):3071–3085. doi: 10.1128/MCB.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okamoto M., Udagawa N., Uehara S., Maeda K., Yamashita T., Nakamichi Y., Kato H., Saito N., Minami Y., Takahashi N., Kobayashi Y. Noncanonical Wnt5a enhances Wnt/β-catenin signaling during osteoblastogenesis. Sci. Rep. 2014;4:4493. doi: 10.1038/srep04493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang F., Yang D., Tu J., Zheng Q., Cai L., Wang L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cell. 2011;29(6):981–991. doi: 10.1002/stem.646. [DOI] [PubMed] [Google Scholar]
- 65.Qi H.H., Bao J., Zhang Q., Ma B., Gu G.Y., Zhang P.L., Ou-Yang G., Wu Z.M., Ying H.J., Ou-Yang P.K. Wnt/β-catenin signaling plays an important role in the protective effects of FDP-Sr against oxidative stress induced apoptosis in MC3T3-E1 cell. Bioorg. Med. Chem. Lett. 2016;26(19):4720–4723. doi: 10.1016/j.bmcl.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 66.Tan S., Zhang B., Zhu X., Ao P., Guo H., Yi W., Zhou G.Q. Deregulation of bone forming cells in bone diseases and anabolic effects of strontium-containing agents and biomaterials. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/814057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.You J., Zhang Y., Zhou Y. Strontium functionalized in biomaterials for bone tissue engineering: a prominent role in osteoimmunomodulation. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.928799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao D.W., Liu C., Zuo K.Q., Su P., Li L.B., Xiao G.Y., Cheng L. Strontium-zinc phosphate chemical conversion coating improves the osseointegration of titanium implants by regulating macrophage polarization. Chem. Eng. J. 2021;408 doi: 10.1016/j.cej.2020.127362. Article 127362. [DOI] [Google Scholar]
- 69.Yang L., Zhou J.G., Yu K.D., Yang S.Y., Sun T.F., Ji Y.H., Xiong Z.K., Guo X.D. Surface modified small intestinal submucosa membrane manipulates sequential immunomodulation coupled with enhanced angio- and osteogenesis towards ameliorative guided bone regeneration. Materials Science & Engineering C-Materials for Biological Applications. 2021;119 doi: 10.1016/j.msec.2020.111641. Article 111641. [DOI] [PubMed] [Google Scholar]
- 70.Shen X.K., Fang K., Yie K.H.R., Zhou Z.X., Shen Y.D., Wu S.Y., Zhu Y., Deng Z.N., Ma P.P., Ma J.F., Liu J.S. High proportion strontium-doped micro-arc oxidation coatings enhance early osseointegration of titanium in osteoporosis by anti-oxidative stress pathway. Bioact. Mater. 2022;10:405–419. doi: 10.1016/j.bioactmat.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao T., Chu Z.Z., Ma J., Ouyang L.P. Immunomodulation effect of biomaterials on bone formation. J. Funct. Biomater. 2022;13(3) doi: 10.3390/jfb13030103. Article 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raziyeva K., Kim Y., Zharkinbekov Z., Kassymbek K., Jimi S., Saparov A. Immunology of acute and chronic wound healing. Biomolecules. 2021;11(5) doi: 10.3390/biom11050700. Article 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.You J.Q., Zhang Y.D., Zhou Y.M. Strontium functionalized in biomaterials for bone tissue engineering: a prominent role in osteoimmunomodulation. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.928799. Article 928799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cho D.I., Kim M.R., Jeong H.Y., Jeong H.C., Jeong M.H., Yoon S.H., Kim Y.S., Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 2014;46 doi: 10.1038/emm.2013.135. Article e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mommert S., Huer M., Schaper-Gerhardt K., Gutzmer R., Werfel T. Histamine up-regulates oncostatin M expression in human M1 macrophages. Br. J. Pharmacol. 2020;177(3):600–613. doi: 10.1111/bph.14796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahon O.R., Browe D.C., Gonzalez-Fernandez T., Pitacco P., Whelan I.T., Von Euw S., Hobbs C., Nicolosi V., Cunningham K.T., Mills K.H.G., Kelly D.J., Dunne A. Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials. 2020;239 doi: 10.1016/j.biomaterials.2020.119833. Article 119833. [DOI] [PubMed] [Google Scholar]
- 77.Tu B., Li J.H., Sun Z.Y., Zhang T.T., Liu H., Yuan F., Fan C.Y. Macrophage-derived TGF-beta and VEGF promote the progression of trauma-induced heterotopic ossification. Inflammation. 2023;46(1):202–216. doi: 10.1007/s10753-022-01723-z. [DOI] [PubMed] [Google Scholar]
- 78.Huang R., Wang X., Zhou Y.H., Xiao Y. RANKL-induced M1 macrophages are involved in bone formation. Bone Research. 2017;5 doi: 10.1038/boneres.2017.19. Article 17019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang, L., Xiao, L., Gao, W. D., Huang, X., Wei, F., Zhang, Q., & Xiao, Y. Macrophages at Low-Inflammatory Status Improved Osteogenesis via Autophagy Regulation. Tissue Eng.. 10.1089/ten.tea.2021.0015. [DOI] [PubMed]
- 80.Sivaraj K.K., Adams R.H. Blood vessel formation and function in bone. Development. 2016;143(15):2706–2715. doi: 10.1242/dev.136861. [DOI] [PubMed] [Google Scholar]
- 81.Korstjens C.M., Rutten S., Nolte P.A., van Duin M.A., Klein Nulend J. Low-intensity pulsed ultrasound increased blood vessel size during fracture healing in patients with a delayed-union of the osteotomized fibula. Histol. Histopathol. 2018;33(7):737–746. doi: 10.14670/HH-11-972. [DOI] [PubMed] [Google Scholar]
- 82.Hoppe A., Güldal N.S., Boccaccini A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 83.Bose S., Fielding G., Tarafder S., Bandyopadhyay A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013;31(10):594–605. doi: 10.1016/j.tibtech.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo X., Wei S., Lu M., Shao Z., Lu J., Xia L., Lin K., Zou D. Dose-dependent effects of strontium ranelate on ovariectomy rat bone marrow mesenchymal stem cells and human umbilical vein endothelial cells. Int. J. Biol. Sci. 2016;12(12):1511–1522. doi: 10.7150/ijbs.16499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kleinheinz J., Stratmann U., Joos U., Wiesmann H.P. VEGF-activated angiogenesis during bone regeneration. J. Oral Maxillofac. Surg. 2005;63(9):1310–1316. doi: 10.1016/j.joms.2005.05.303. [DOI] [PubMed] [Google Scholar]
- 86.Bluteau G., Julien M., Magne D., Mallein-Gerin F., Weiss P., Daculsi G., Guicheux J. VEGF and VEGF receptors are differentially expressed in chondrocytes. Bone. 2007;40(3):568–576. doi: 10.1016/j.bone.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 87.Zhu X., Kong Y., Huang Y., Zhao B., Wang J. Influence of strontium on vascular endothelial growth factor and fibroblast growth factor 2 expression in rat chondrocytes cultured in vitro. Biol. Trace Elem. Res. 2019;190(2):466–471. doi: 10.1007/s12011-018-1564-y. [DOI] [PubMed] [Google Scholar]
- 88.Pan F.Y., Li Z.M., Liu X.W., Luo Y., Ma Z., Feng S.X., Xu N. Effect of strontium ranelate on rabbits with steroid-induced osteonecrosis of femoral head through TGF-β1/BMP2 pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24(3):1000–1006. doi: 10.26355/eurrev_202002_20150. [DOI] [PubMed] [Google Scholar]
- 89.Sampaio W.O., Souza dos Santos R.A., Faria-Silva R., da Mata Machado L.T., Schiffrin E.L., Touyz R.M. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49(1):185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 90.Krishnan B., Smith T.L., Dubey P., Zapadka M.E., Torti F.M., Willingham M.C., Tallant E.A., Gallagher P.E. Angiotensin-(1-7) attenuates metastatic prostate cancer and reduces osteoclastogenesis. Prostate. 2013;73(1):71–82. doi: 10.1002/pros.22542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan R., Li J., Wu Q., Zhang X., Hu L., Deng Y., Jiang R., Wen J., Jiang X. Trace element-augmented titanium implant with targeted angiogenesis and enhanced osseointegration in osteoporotic rats. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.839062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bobek J., Oralova V., Kratochvilova A., Zvackova I., Lesot H., Matalova E. Tuftelin and HIFs expression in osteogenesis. Histochem. Cell Biol. 2019;152(5):355–363. doi: 10.1007/s00418-019-01813-4. [DOI] [PubMed] [Google Scholar]
- 93.Treins C., Murdaca J., Van Obberghen E., Giorgetti-Peraldi S. AMPK activation inhibits the expression of HIF-1α induced by insulin and IGF-1. Biochem. Biophys. Res. Commun. 2006;342(4):1197–1202. doi: 10.1016/j.bbrc.2006.02.088. [DOI] [PubMed] [Google Scholar]
- 94.Araldi E., Schipani E. Hypoxia, HIFs and bone development. Bone. 2010;47(2):190–196. doi: 10.1016/j.bone.2010.04.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu W., Zhou C., Ma Y., Li J., Jiang J., Chen Y., Dong L., He F. Improved osseointegration of strontium-modified titanium implants by regulating angiogenesis and macrophage polarization. Biomater. Sci. 2022;10(9):2198–2214. doi: 10.1039/d1bm01488a. [DOI] [PubMed] [Google Scholar]
- 96.Sun Y., Li Y., Zhang Y., Wang T., Lin K., Liu J. A polydopamine-assisted strontium-substituted apatite coating for titanium promotes osteogenesis and angiogenesis via FAK/MAPK and PI3K/AKT signaling pathways. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;131 doi: 10.1016/j.msec.2021.112482. [DOI] [PubMed] [Google Scholar]
- 97.Cheng Y., Huang L., Wang Y., Huo Q., Shao Y., Bao H., Li Z., Liu Y., Li X. Strontium promotes osteogenic differentiation by activating autophagy via the the AMPK/mTOR signaling pathway in MC3T3-E1 cells. Int. J. Mol. Med. 2019;44(2):652–660. doi: 10.3892/ijmm.2019.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo X., Wei S., Lu M., Shao Z., Lu J., Xia L., Lin K., Zou D. Dose-dependent effects of strontium ranelate on ovariectomy rat bone marrow mesenchymal stem cells and human umbilical vein endothelial cells. Int. J. Biol. Sci. 2016;12(12):1511–1522. doi: 10.7150/ijbs.16499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rehman M., Madni A., Webster T.J. The era of biofunctional biomaterials in orthopedics: what does the future hold? Expet Rev. Med. Dev. 2018;15(3):193–204. doi: 10.1080/17434440.2018.1430569. [DOI] [PubMed] [Google Scholar]
- 100.Ruffini A., Sandri M., Dapporto M., Campodoni E., Tampieri A., Sprio S. Nature-inspired unconventional approaches to develop 3D bioceramic scaffolds with enhanced regenerative ability. Biomedicines. 2021;9(8):916. doi: 10.3390/biomedicines9080916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Geesink R.G.T. Osteoconductive coatings for total joint arthroplasty. Clin. Orthop. Relat. Res. 2002;(395):53–65. doi: 10.1097/00003086-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 102.Kamitakahara M., Ohtsuki C., Miyazaki T. Review paper: behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. J. Biomater. Appl. 2008;23(3):197–212. doi: 10.1177/0885328208096798. [DOI] [PubMed] [Google Scholar]
- 103.Mofakhami S., Salahinejad E. Biphasic calcium phosphate microspheres in biomedical applications. J. Contr. Release. 2021;338:527–536. doi: 10.1016/j.jconrel.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 104.Habraken W., Habibovic P., Epple M., Bohner M. Calcium phosphates in biomedical applications: materials for the future? Mater. Today. 2016;19(2):69–87. doi: 10.1016/j.mattod.2015.10.008. [DOI] [Google Scholar]
- 105.Prakasam M., Locs J., Salma-Ancane K., Loca D., Largeteau A., Berzina-Cimdina L. Fabrication, properties and applications of dense hydroxyapatite: a review. J. Funct. Biomater. 2015;6(4):1099–1140. doi: 10.3390/jfb6041099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu S.C., Kao Y.L., Lu Y.C., Hsu H.C., Ho W.F. Preparation and characterization of microrod hydroxyapatite bundles obtained from oyster shells through microwave irradiation. Journal of the Australian Ceramic Society. 2021;57(5):1541–1551. doi: 10.1007/s41779-021-00657-3. [DOI] [Google Scholar]
- 107.Sabokbar A., Pandey R., Diaz J., Quinn J.M.W., Murray D.W., Athanasou N.A. Hydroxyapatite particles are capable of inducing osteoclast formation. J. Mater. Sci. Mater. Med. 2001;12(8):659–664. doi: 10.1023/a:1011267005465. [DOI] [PubMed] [Google Scholar]
- 108.Liu B., Lun D.-x. Current application of beta-tricalcium phosphate composites in orthopaedics. Orthop. Surg. 2012;4(3):139–144. doi: 10.1111/j.1757-7861.2012.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lobo S.E., Arinzeh T.L. Biphasic calcium phosphate ceramics for bone regeneration and tissue engineering applications. Materials. 2010;3(2):815–826. doi: 10.3390/ma3020815. [DOI] [Google Scholar]
- 110.Suzuki O. Octacalcium phosphate: osteoconductivity and crystal chemistry. Acta Biomater. 2010;6(9):3379–3387. doi: 10.1016/j.actbio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 111.Demirel M., Kaya A.I. Effect of strontium-containing compounds on bone grafts. J. Mater. Sci. 2020;55(15):6305–6329. doi: 10.1007/s10853-020-04451-7. [DOI] [Google Scholar]
- 112.Cui W., Yang L., Ullah I., Yu K., Zhao Z., Gao X., Liu T., Liu M., Li P., Wang J., Guo X. Biomimetic porous scaffolds containing decellularized small intestinal submucosa and Sr(2+)/Fe(3+)co-doped hydroxyapatite accelerate angiogenesis/osteogenesis for bone regeneration. Biomed. Mater. 2022;17(2) doi: 10.1088/1748-605X/ac4b45. [DOI] [PubMed] [Google Scholar]
- 113.Xu Y.M., Geng Z., Gao Z.H., Zhuo X.L., Li B., Cui Z.D., Zhu S.L., Liang Y.Q., Li Z.Y., Yang X.J. Effects of both Sr and Mg substitution on compositions of biphasic calcium phosphate derived from hydrothermal method. Int. J. Appl. Ceram. Technol. 2018;15(1):210–222. doi: 10.1111/ijac.12771. [DOI] [Google Scholar]
- 114.Li J., Cai S., Xu G., Li X., Zhang W., Zhang Z. In vitro biocompatibility study of calcium phosphate glass ceramic scaffolds with different trace element doping. Mater. Sci. Eng. C. 2012;32(2):356–363. doi: 10.1016/j.msec.2011.11.005. [DOI] [Google Scholar]
- 115.Xie H., Gu Z., He Y., Xu J., Xu C., Li L., Ye Q. Microenvironment construction of strontium-calcium-based biomaterials for bone tissue regeneration: the equilibrium effect of calcium to strontium. J. Mater. Chem. B. 2018;6(15):2332–2339. doi: 10.1039/c8tb00306h. [DOI] [PubMed] [Google Scholar]
- 116.Pina S., Ferreira J.M.F. Brushite-forming Mg-, Zn- and Sr-substituted bone cements for clinical applications. Materials. 2010;3(1):519–535. doi: 10.3390/ma3010519. [DOI] [Google Scholar]
- 117.Ratnayake J.T.B., Mucalo M., Dias G.J. Substituted hydroxyapatites for bone regeneration: a review of current trends. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105(5):1285–1299. doi: 10.1002/jbm.b.33651. [DOI] [PubMed] [Google Scholar]
- 118.Habraken W., Zhang Z., Wolke J.G.C., Grijpma D.W., Mikos A.G., Feijen J., Jansen J.A. Introduction of enzymatically degradable poly(trimethylene carbonate) microspheres into an injectable calcium phosphate cement. Biomaterials. 2008;29(16):2464–2476. doi: 10.1016/j.biomaterials.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 119.Wu Y.C., Lin W.Y., Yang C.Y., Lee T.M. Fabrication of gelatin-strontium substituted calcium phosphate scaffolds with unidirectional pores for bone tissue engineering. J. Mater. Sci. Mater. Med. 2015;26(3):152. doi: 10.1007/s10856-015-5490-7. [DOI] [PubMed] [Google Scholar]
- 120.He F., Lu T., Fang X., Feng S., Feng S., Tian Y., Li Y., Zuo F., Deng X., Ye J. Novel extrusion-microdrilling approach to fabricate calcium phosphate-based bioceramic scaffolds enabling fast bone regeneration. ACS Appl. Mater. Interfaces. 2020;12(29):32340–32351. doi: 10.1021/acsami.0c07304. [DOI] [PubMed] [Google Scholar]
- 121.Mohapatra B., Rautray T.R. Strontium-substituted biphasic calcium phosphate scaffold for orthopedic applications. J. Korean Ceram. Soc. 2020;57(4):392–400. doi: 10.1007/s43207-020-00028-x. [DOI] [Google Scholar]
- 122.Wang P., Zhao L., Liu J., Weir M.D., Zhou X., Xu H.H.K. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res. 2014;2 doi: 10.1038/boneres.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barralet J.E., Duncan C.O., Dover M.S., Bassett D.C., Nishikawa H., Monaghan A., Gbureck U. Cortical bone screw fixation in lonically modified apatite cements. J. Biomed. Mater. Res. B Appl. Biomater. 2005;73(2):238–243. doi: 10.1002/jbm.b.30197. [DOI] [PubMed] [Google Scholar]
- 124.Ginebra M.P., Canal C., Espanol M., Pastorino D., Montufar E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012;64(12):1090–1110. doi: 10.1016/j.addr.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 125.Yu T., Ye J., Gao C., Yu L., Wang Y. Effect of biomedical organic compounds on the setting reaction of calcium phosphates. Colloids Surf. B Biointerfaces. 2010;75(1):363–369. doi: 10.1016/j.colsurfb.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 126.Verron E., Khairoun I., Guicheux J., Bouler J.M. Calcium phosphate biomaterials as bone drug delivery systems: a review. Drug Discov. Today. 2010;15(13–14):547–552. doi: 10.1016/j.drudis.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 127.Zhang J., Liu W., Schnitzler V., Tancret F., Bouler J.M. Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater. 2014;10(3):1035–1049. doi: 10.1016/j.actbio.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 128.Wong S.K., Wong Y.H., Chin K.Y., Ima-Nirwana S. A Review on the enhancement of calcium phosphate cement with biological materials in bone defect healing. Polymers. 2021;13(18):3075. doi: 10.3390/polym13183075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jeong J., Kim J.H., Shim J.H., Hwang N.S., Heo C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019;23(1):4. doi: 10.1186/s40824-018-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Demir-Oğuz Ö., Boccaccini A.R., Loca D. Injectable bone cements: what benefits the combination of calcium phosphates and bioactive glasses could bring? Bioact. Mater. 2023;19:217–236. doi: 10.1016/j.bioactmat.2022.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Orshesh Z., Hesaraki S., Khanlarkhani A. Blooming gelatin: an individual additive for enhancing nanoapatite precipitation, physical properties, and osteoblastic responses of nanostructured macroporous calcium phosphate bone cements. Int. J. Nanomed. 2017;12:745–758. doi: 10.2147/ijn.S128368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun Z., Fu Y., Chen D. Effect of phase transitions between a-tricalcium phosphate and b-tricalcium phosphate on the setting properties of novel strontium-containing bioactive bone cement. Asian J. Chem. 2013;25(6):3087–3091. [Google Scholar]
- 133.Lode A., Heiss C., Knapp G., Thomas J., Nies B., Gelinsky M., Schumacher M. Strontium-modified premixed calcium phosphate cements for the therapy of osteoporotic bone defects. Acta Biomater. 2018;65:475–485. doi: 10.1016/j.actbio.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 134.Wu X., Tang Z., Wu K., Bai Y., Lin X., Yang H., Yang Q., Wang Z., Ni X., Liu H., Yang L. Strontium-calcium phosphate hybrid cement with enhanced osteogenic and angiogenic properties for vascularised bone regeneration. J. Mater. Chem. B. 2021;9(30):5982–5997. doi: 10.1039/d1tb00439e. [DOI] [PubMed] [Google Scholar]
- 135.Su Y., Cockerill I., Zheng Y., Tang L., Qin Y.X., Zhu D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019;4:196–206. doi: 10.1016/j.bioactmat.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wen S.F., Liu X.L., Ding J.H., Liu Y.L., Lan Z.T., Zhang Z.M., Chen G.M. Hydrothermal synthesis of hydroxyapatite coating on the surface of medical magnesium alloy and its corrosion resistance. Progress in Natural Science-Materials International. 2021;31(2):324–333. doi: 10.1016/j.pnsc.2020.12.013. [DOI] [Google Scholar]
- 137.Wennerberg A., Albrektsson T. Structural influence from calcium phosphate coatings and its possible effect on enhanced bone integration. Acta Odontol. Scand. 2009;67(6):333–340. doi: 10.1080/00016350903188325. [DOI] [PubMed] [Google Scholar]
- 138.Hou N.Y., Perinpanayagam H., Mozumder M.S., Zhu J. Novel development of biocompatible coatings for bone implants. Coatings. 2015;5(4):737–757. doi: 10.3390/coatings5040737. [DOI] [Google Scholar]
- 139.Lin J.P., Zhang S.N., Chen T., Liu C.S., Lin S.L., Tian X.H. Calcium phosphate cement reinforced by polypeptide copolymers. J. Biomed. Mater. Res. B Appl. Biomater. 2006;76B(2):432–439. doi: 10.1002/jbm.b.30392. [DOI] [PubMed] [Google Scholar]
- 140.Blom E.J., Klein-Nulend J., Yin L., van Waas M.A.J., Burger E.H. Transforming growth factor-beta(I) incorporated in calcium phosphate cement stimulates osteotransductivity in rat calvarial bone defects. Clin. Oral Implants Res. 2001;12(6):609–616. doi: 10.1034/j.1600-0501.2001.120609.x. [DOI] [PubMed] [Google Scholar]
- 141.Ota F., Fujisaki K., Osanai K., Sasagawa K. A novel method for coating calcium phosphate with trace elements extracted from bone using electrical stimulation. J. Mech. Behav. Biomed. Mater. 2022;133 doi: 10.1016/j.jmbbm.2022.105330. Article 105330. [DOI] [PubMed] [Google Scholar]
- 142.Boyd A.R., Rutledge L., Randolph L.D., Meenan B.J. Strontium-substituted hydroxyapatite coatings deposited via a co-deposition sputter technique. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;46:290–300. doi: 10.1016/j.msec.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 143.Christensen T.E.K., Davidsen M.B., Van Malderen S., Garrevoet J., Offermanns V., Andersen O.Z., Foss M., Birkedal H. Local release of strontium from sputter-deposited coatings at implants increases the strontium-to-calcium ratio in peri-implant bone. ACS Biomater. Sci. Eng. 2022;8(2):620–625. doi: 10.1021/acsbiomaterials.1c01004. [DOI] [PubMed] [Google Scholar]
- 144.Li Y., Li Q., Zhu S., Luo E., Li J., Feng G., Liao Y., Hu J. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials. 2010;31(34):9006–9014. doi: 10.1016/j.biomaterials.2010.07.112. [DOI] [PubMed] [Google Scholar]
- 145.Ding X., Li X., Li C., Qi M., Zhang Z., Sun X., Wang L., Zhou Y. Chitosan/dextran hydrogel constructs containing strontium-doped hydroxyapatite with enhanced osteogenic potential in rat cranium. ACS Biomater. Sci. Eng. 2019;5(9):4574–4586. doi: 10.1021/acsbiomaterials.9b00584. [DOI] [PubMed] [Google Scholar]
- 146.Jones J.R. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013;9(1):4457–4486. doi: 10.1016/j.actbio.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 147.Huang C., Yu M., Li H., Wan X., Ding Z., Zeng W., Zhou Z. Research progress of bioactive glass and its application in orthopedics. Adv. Mater. Interfac. 2021;8(22) doi: 10.1002/admi.202100606. [DOI] [Google Scholar]
- 148.Shearer A., Montazerian M., Mauro J.C. Modern definition of bioactive glasses and glass-ceramics. J. Non-Cryst. Solids. 2023;608 doi: 10.1016/j.jnoncrysol.2023.122228. Article 122228. [DOI] [Google Scholar]
- 149.Baino F., Fiume E., Barberi J., Kargozar S., Marchi J., Massera J., Verne E. Processing methods for making porous bioactive glass-based scaffolds-A state-of-the-art review. Int. J. Appl. Ceram. Technol. 2019;16(5):1762–1796. doi: 10.1111/ijac.13195. [DOI] [Google Scholar]
- 150.Kargozar S., Montazerian M., Fiume E., Baino F. Multiple and promising applications of strontium (Sr)-Containing bioactive glasses in bone tissue engineering. Front. Bioeng. Biotechnol. 2019;7 doi: 10.3389/fbioe.2019.00161. Article 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pereira M.M., Jones J.R., Hench L.L. Bioactive glass and hybrid scaffolds prepared by sol-gel method for bone tissue engineering. Adv. Appl. Ceram. 2005;104(1):35–42. doi: 10.1179/174367605225011034. [DOI] [Google Scholar]
- 152.Gupta N., Santhiya D. Role of cellulose functionality in bio-inspired synthesis of nano bioactive glass. Materials Science and Engineering C-Materials for Biological Applications. 2017;75:1206–1213. doi: 10.1016/j.msec.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 153.Bui X.V., Oudadesse H., Le Gal Y., Merdrignac-Conanec O., Cathelineau G. Bioactivity behaviour of biodegradable material comprising bioactive glass. Kor. J. Chem. Eng. 2012;29(2):215–220. doi: 10.1007/s11814-011-0151-0. [DOI] [Google Scholar]
- 154.Kang J.H., Jang K.J., Sakthiabiram K., Jang J.G., Lee S.K., Park S.W. Synthesis and characterization of thermoplastic 45S5 bioactive glasses for 3D printing applied to tissue engineering. Basic Clin. Pharmacol. Toxicol. 2018;124:13–14. [Google Scholar]
- 155.Baino F., Fiume E., Barberi J., Kargozar S., Marchi J., Massera J., Verne E. Processing methods for making porous bioactive glass-based scaffolds-A state-of-the-art review. Int. J. Appl. Ceram. Technol. 2019;16(5):1762–1796. doi: 10.1111/ijac.13195. [DOI] [Google Scholar]
- 156.Erol M., Ozyuguran A., Ozarpat O., Kucukbayrak S. 3D Composite scaffolds using strontium containing bioactive glasses. J. Eur. Ceram. Soc. 2012;32(11):2747–2755. doi: 10.1016/j.jeurceramsoc.2012.01.015. [DOI] [Google Scholar]
- 157.Nommeots-Nomm A., Labbaf S., Devlin A., Todd N., Geng H., Solanki A.K., Tang H.M., Perdika P., Pinna A., Ejeian F., Tsigkou O., Lee P.D., Esfahani M.H.N., Mitchell C.A., Jones J.R. Highly degradable porous melt-derived bioactive glass foam scaffolds for bone regeneration. Acta Biomater. 2017;57:449–461. doi: 10.1016/j.actbio.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 158.Zhang Y., Wei L., Chang J., Miron R.J., Shi B., Yi S., Wu C. Strontium-incorporated mesoporous bioactive glass scaffolds stimulating in vitro proliferation and differentiation of bone marrow stromal cells and in vivo regeneration of osteoporotic bone defects. J. Mater. Chem. B. 2013;1(41):5711–5722. doi: 10.1039/c3tb21047b. [DOI] [PubMed] [Google Scholar]
- 159.Zhang X.M., Zhang L., He X.Y., Wu J.T. Fabrication and application of new polymer-based materials by freeze-drying. Prog. Chem. 2014;26(11):1832–1839. doi: 10.7536/pc140723. [DOI] [Google Scholar]
- 160.Hatton J., Davis G.R., Mourad A.H.I., Cherupurakal N., Hill R.G., Mohsin S. Fabrication of porous bone scaffolds using alginate and bioactive glass. J. Funct. Biomater. 2019;10(1) doi: 10.3390/jfb10010015. Article 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guo R., Hou X., Zhao D., Wang H., Shi C., Zhou Y. Mechanical stability and biological activity of Mg–Sr co-doped bioactive glass/chitosan composite scaffolds. J. Non-Cryst. Solids. 2022;583 doi: 10.1016/j.jnoncrysol.2022.121481. [DOI] [Google Scholar]
- 162.Sheng X., Wang A., Wang Z., Liu H., Wang J., Li C. Advanced surface modification for 3D-printed titanium alloy implant interface functionalization. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.850110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang J., Zhao S., Zhu Y., Huang Y., Zhu M., Tao C., Zhang C. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014;10(5):2269–2281. doi: 10.1016/j.actbio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 164.Capuana E., Lopresti F., Pavia F.C., Brucato V., La Carrubba V. Solution-based processing for scaffold fabrication in tissue engineering applications: a brief review. Polymers. 2021;13(13) doi: 10.3390/polym13132041. Article 2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Naruphontjirakul P., Tsigkou O., Li S.W., Porter A.E., Jones J.R. Human mesenchymal stem cells differentiate into an osteogenic lineage in presence of strontium containing bioactive glass nanoparticles. Acta Biomater. 2019;90:373–392. doi: 10.1016/j.actbio.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 166.Naruphontjirakul P., Li S., Pinna A., Barrak F., Chen S., Redpath A.N., Rankin S.M., Porter A.E., Jones J.R. Interaction of monodispersed strontium containing bioactive glass nanoparticles with macrophages. Biomater Adv. 2022;133 doi: 10.1016/j.msec.2021.112610. [DOI] [PubMed] [Google Scholar]
- 167.Kumar A., Banrjee S., Roy P., Xu H., Mariappan C.R. Osteogenic commitment of strontium nanoparticles doped mesoporous bioactive glass-ceramics. Mater. Sci. Eng., B. 2022;286 doi: 10.1016/j.mseb.2022.116068. [DOI] [Google Scholar]
- 168.Kang M.S., Lee N.H., Singh R.K., Mandakhbayar N., Perez R.A., Lee J.H., Kim H.W. Nanocements produced from mesoporous bioactive glass nanoparticles. Biomaterials. 2018;162:183–199. doi: 10.1016/j.biomaterials.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 169.Lee N.H., Kang M.S., Kim T.H., Yoon D.S., Mandakhbayar N., Jo S.B., Kim H.S., Knowles J.C., Lee J.H., Kim H.W. Dual actions of osteoclastic-inhibition and osteogenic-stimulation through strontium-releasing bioactive nanoscale cement imply biomaterial-enabled osteoporosis therapy. Biomaterials. 2021;276 doi: 10.1016/j.biomaterials.2021.121025. [DOI] [PubMed] [Google Scholar]
- 170.Zhang Y., Liu X., Zeng L., Zhang J., Zuo J., Zou J., Ding J., Chen X. Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv. Funct. Mater. 2019;29(36) doi: 10.1002/adfm.201903279. [DOI] [Google Scholar]
- 171.Basha R.Y., Kumar T.S.S., Doble M. Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;57:452–463. doi: 10.1016/j.msec.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 172.Hsu S.H., Hung K.C., Chen C.W. Biodegradable polymer scaffolds. J. Mater. Chem. B. 2016;4(47):7493–7505. doi: 10.1039/C6TB02176J. [DOI] [PubMed] [Google Scholar]
- 173.Pérez-Madrigal M.M., Shaw J.E., Arno M.C., Hoyland J.A., Richardson S.M., Dove A.P. Robust alginate/hyaluronic acid thiol-yne click-hydrogel scaffolds with superior mechanical performance and stability for load-bearing soft tissue engineering. Biomater. Sci. 2020;8(1):405–412. doi: 10.1039/c9bm01494b. [DOI] [PubMed] [Google Scholar]
- 174.Prabu P., Kim K.W., Dharmaraj N., Park J.H., Khil M.S., Kim H.Y. Antimicrobial drug release scaffolds of natural and synthetic biodegradable polymers. Macromol. Res. 2008;16(4):303–307. doi: 10.1007/BF03218521. [DOI] [Google Scholar]
- 175.Xu Y., Zhang F., Zhai W., Cheng S., Li J., Wang Y. Unraveling of advances in 3D-printed polymer-based bone scaffolds. Polymers. 2022;14(3):566. doi: 10.3390/polym14030566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sengupta P., Prasad B.L. Surface modification of polymeric scaffolds for tissue engineering applications. Regen. Eng. Transl. Med. 2018;4(2):75–91. doi: 10.1007/s40883-018-0050-6. [DOI] [Google Scholar]
- 177.Wang H., Zhao S., Zhou J., Shen Y., Huang W., Zhang C., Rahaman M.N., Wang D. Evaluation of borate bioactive glass scaffolds as a controlled delivery system for copper ions in stimulating osteogenesis and angiogenesis in bone healing. J. Mater. Chem. B. 2014;2(48):8547–8557. doi: 10.1039/c4tb01355g. [DOI] [PubMed] [Google Scholar]
- 178.Patrício T., Glória A., Bártolo P.J. 2nd International Conference on Advanced Materials and Engineering Materials. ICAMEM 2012); Beijing, China: 2012. PCL and PCL/PLA scaffolds for bone tissue regeneration. [Google Scholar]
- 179.Siddiqui N., Asawa S., Birru B., Baadhe R., Rao S. PCL-based composite scaffold matrices for tissue engineering applications. Mol. Biotechnol. 2018;60(7):506–532. doi: 10.1007/s12033-018-0084-5. [DOI] [PubMed] [Google Scholar]
- 180.Sun H.F., Mei L., Song C.X., Cui X.M., Wang P.Y. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006;27(9):1735–1740. doi: 10.1016/j.biomaterials.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 181.Bartnikowski M., Dargaville T.R., Ivanovski S., Hutmacher D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019;96:1–20. doi: 10.1016/j.progpolymsci.2019.05.004. [DOI] [Google Scholar]
- 182.Golafshan N., Vorndran E., Zaharievski S., Brommer H., Kadumudi F.B., Dolatshahi-Pirouz A., Gbureck U., Van Weeren R., Castilho M., Malda J. Tough magnesium phosphate-based 3D-printed implants induce bone regeneration in an equine defect model. Biomaterials. 2020;261 doi: 10.1016/j.biomaterials.2020.120302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Breusch S.J., Kühn K.D. Bone cements based on polymethylmethacrylate. Orthopä. 2003;32(1):41–50. doi: 10.1007/s00132-002-0411-0. [DOI] [PubMed] [Google Scholar]
- 184.Hasandoost L., Rodriguez O., Alhalawani A., Zalzal P., Schemitsch E.H., Waldman S.D., Papini M., Towler M.R. The role of poly(methyl Methacrylate) in management of bone loss and infection in revision total knee arthroplasty: a review. J. Funct. Biomater. 2020;11(2):25. doi: 10.3390/jfb11020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhu J., Jiang G., Qiu Z., Lu J., Shen F., Cui F.Z. Modification of poly (methyl methacrylate) bone cement for vertebroplasty. J. Biomater. Tissue Eng. 2018;8(5):1607–1616. doi: 10.1166/jbt.2018.1800. [DOI] [Google Scholar]
- 186.Martikos K., Greggi T., Vommaro F., Boriani L., Scarale A., Zarantonello P., Carbone G., Stallone S., Zucchini R. Vertebroplasty in the treatment of osteoporotic vertebral compression fractures: patient selection and perspectives. Open Access Rheumatol. 2019;11:157–161. doi: 10.2147/OARRR.S174424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Laredo J.D., Hamze B. Complications of percutaneous vertebroplasty and their prevention. Skeletal Radiol. 2004;33(9):493–505. doi: 10.1007/s00256-004-0776-8. [DOI] [PubMed] [Google Scholar]
- 188.Robinson Y., Heyde C.E., Försth P., Olerud C. Kyphoplasty in osteoporotic vertebral compression fractures - guidelines and technical considerations. J. Orthop. Surg. Res. 2011;6:43. doi: 10.1186/1749-799X-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xie X., Zhang Q., Zhang P., Zhou H., Zhang R., Wang X., Li Y. Particulate poly(methyl methacrylate) could stimulate proinflammatory CD4 T cell responses in a monocyte-dependent manner, and directly mediate cell death. Hum. Immunol. 2019;80(12):1012–1019. doi: 10.1016/j.humimm.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 190.Pleser M., Roth R., Wörsdörfer O., Manke C. Pulmonary embolism caused by PMMA in percutaneous vertebroplasty. Case report and review of the literature. Unfallchirurg. 2004;107(9):807–811. doi: 10.1007/s00113-004-0763-5. [DOI] [PubMed] [Google Scholar]
- 191.Shridhar P., Chen Y., Khalil R., Plakseychuk A., Cho S.K., Tillman B., Kumta P.N., Chun Y. A review of PMMA bone cement and intra-cardiac embolism. Materials. 2016;9(10):821. doi: 10.3390/ma9100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gao S., Lv Y., Yuan L., Ren H., Wu T., Liu B., Zhang Y., Zhou R., Li A., Zhou F. Improved bone ingrowth of tricalcium phosphate filled Poly(methyl methacrylate) (PMMA) bone cements in vivo. Polym. Test. 2019;76:513–521. doi: 10.1016/j.polymertesting.2019.02.015. [DOI] [Google Scholar]
- 193.Zhang T.Y., Zhang P.X., Xue F., Zhang D.Y., Jiang B.G. Risk factors for cement leakage and nomogram for predicting the intradiscal cement leakage after the vertebra augmented surgery. BMC Muscoskel. Disord. 2020;21(1):792. doi: 10.1186/s12891-020-03810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kuhn K.D., Hontzsch D. Augmentation with PMMA cement. Unfallchirurg. 2015;118(9):737–748. doi: 10.1007/s00113-015-0059-y. [DOI] [PubMed] [Google Scholar]
- 195.Liu X., Cheng C., Peng X., Xiao H., Guo C., Wang X., Li L., Yu X. A promising material for bone repair: PMMA bone cement modified by dopamine-coated strontium-doped calcium polyphosphate particles. R. Soc. Open Sci. 2019;6(10) doi: 10.1098/rsos.191028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Goñi I., Rodríguez R., García-Arnáez I., Parra J., Gurruchaga M. Preparation and characterization of injectable PMMA-strontium-substituted bioactive glass bone cement composites. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106(3):1245–1257. doi: 10.1002/jbm.b.33935. [DOI] [PubMed] [Google Scholar]
- 197.Cui X., Huang C., Zhang M., Ruan C., Peng S., Li L., Liu W., Wang T., Li B., Huang W., Rahaman M.N., Lu W.W., Pan H. Enhanced osteointegration of poly(methylmethacrylate) bone cements by incorporating strontium-containing borate bioactive glass. J. R. Soc. Interface. 2017;14(131) doi: 10.1098/rsif.2016.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Agarwal R., García A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015;94:53–62. doi: 10.1016/j.addr.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fu M., Liang Y., Lv X., Li C., Yang Y.Y., Yuan P., Ding X. Recent advances in hydrogel-based anti-infective coatings. J. Mater. Sci. Technol. 2021;85:169–183. doi: 10.1016/j.jmst.2020.12.070. [DOI] [Google Scholar]
- 200.Baroli B. Hydrogels for tissue engineering and delivery of tissue-inducing substances. J. Pharmaceut. Sci. 2007;96(9):2197–2223. doi: 10.1002/jps.20873. [DOI] [PubMed] [Google Scholar]
- 201.Reakasame S., Boccaccini A.R. Oxidized alginate-based hydrogels for tissue engineering applications: a review. Biomacromolecules. 2018;19(1):3–21. doi: 10.1021/acs.biomac.7b01331. [DOI] [PubMed] [Google Scholar]
- 202.Zhang X., Wang L., Weng L., Deng B. Strontium ion substituted alginate-based hydrogel fibers and its coordination binding model. J. Appl. Polym. Sci. 2020;137(16) doi: 10.1002/app.48571. [DOI] [Google Scholar]
- 203.Giri T.K., Thakur D., Alexander A., Ajazuddin, Badwaik H., Tripathi D.K. Alginate based hydrogel as a potential biopolymeric carrier for drug delivery and cell delivery systems: present status and applications. Curr. Drug Deliv. 2012;9(6):539–555. doi: 10.2174/156720112803529800. [DOI] [PubMed] [Google Scholar]
- 204.Zhao D.L., Wang X., Tie C.R., Cheng B., Yang S.S., Sun Z., Yin M.M., Li X.B., Yin M. Bio-functional strontium-containing photocrosslinked alginate hydrogels for promoting the osteogenic behaviors. Materials Science & Engineering C-Materials for Biological Applications. 2021;126 doi: 10.1016/j.msec.2021.112130. Article 112130. [DOI] [PubMed] [Google Scholar]
- 205.Wan F., Ping H., Wang W., Zou Z., Xie H., Su B.L., Liu D., Fu Z. Hydroxyapatite-reinforced alginate fibers with bioinspired dually aligned architectures. Carbohydr. Polym. 2021;267 doi: 10.1016/j.carbpol.2021.118167. [DOI] [PubMed] [Google Scholar]
- 206.Yuan N., Jia L., Geng Z., Wang R., Li Z., Yang X., Cui Z., Zhu S., Liang Y., Liu Y. The incorporation of strontium in a sodium alginate coating on titanium surfaces for improved biological properties. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/9867819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Koopaie M., Nassar D., Shokrolahi M. Three-dimensional bioprinting of mucoadhesive scaffolds for the treatment of oral mucosal lesions; an in vitro study. 3d Printing in Medicine. 2022;8(1) doi: 10.1186/s41205-022-00157-5. Article 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cai Z., Li Y., Song W., He Y., Li H., Liu X. Anti-inflammatory and prochondrogenic in situ-formed injectable hydrogel crosslinked by strontium-doped bioglass for cartilage regeneration. ACS Appl. Mater. Interfaces. 2021;13(50):59772–59786. doi: 10.1021/acsami.1c20565. [DOI] [PubMed] [Google Scholar]
- 209.Janmohammadi M., Nazemi Z., Salehi A.O.M., Seyfoori A., John J.V., Nourbakhsh M.S., Akbari M. Cellulose-based composite scaffolds for bone tissue engineering and localized drug delivery. Bioact. Mater. 2023;20:137–163. doi: 10.1016/j.bioactmat.2022.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rahman M.S., Hasan M.S., Nitai A.S., Nam S., Karmakar A.K., Ahsan M.S., Shiddiky M.J.A., Ahmed M.B. Recent developments of carboxymethyl cellulose. Polymers. 2021;13(8):1345. doi: 10.3390/polym13081345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Halib N., Perrone F., Cemazar M., Dapas B., Farra R., Abrami M., Chiarappa G., Forte G., Zanconati F., Pozzato G., Murena L., Fiotti N., Lapasin R., Cansolino L., Grassi G., Grassi M. Potential applications of nanocellulose-containing materials in the biomedical field. Materials. 2017;10(8):977. doi: 10.3390/ma10080977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu Y., Wang L., Mi Y., Zhao S., Qi S., Sun M., Peng B., Xu Q., Niu Y., Zhou Y. Transparent stretchable hydrogel sensors: materials, design and applications. J. Mater. Chem. C. 2022;10(37):13351–13371. doi: 10.1039/D2TC01104B. [DOI] [Google Scholar]
- 213.Hendi A., Hassan M.U., Elsherif M., Alqattan B., Park S., Yetisen A.K., Butt H. Healthcare applications of pH-sensitive hydrogel-based devices: a review. Int. J. Nanomed. 2020;15:3887–3901. doi: 10.2147/IJN.S245743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang W., Liu Y., Xuan Y., Zhang S. Synthesis and applications of carboxymethyl cellulose hydrogels. Gels. 2022;8(9):529. doi: 10.3390/gels8090529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lopa S., Mercuri D., Colombini A., De Conti G., Segatti F., Zagra L., Moretti M. Orthopedic bioactive implants: hydrogel enrichment of macroporous titanium for the delivery of mesenchymal stem cells and strontium. J. Biomed. Mater. Res. 2013;101(12):3396–3403. doi: 10.1002/jbm.a.34649. [DOI] [PubMed] [Google Scholar]
- 216.Lovati A.B., Lopa S., Talò G., Previdi S., Recordati C., Mercuri D., Segatti F., Zagra L., Moretti M. In vivo evaluation of bone deposition in macroporous titanium implants loaded with mesenchymal stem cells and strontium-enriched hydrogel. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103(2):448–456. doi: 10.1002/jbm.b.33228. [DOI] [PubMed] [Google Scholar]
- 217.Zhang F., Zhu H., Wang G., Xie J., Tao Y., Xia W., Yang H. Preparation and characterization of a silk fibroin/calcium sulfate bone cement. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106(2):512–519. doi: 10.1002/jbm.b.33855. [DOI] [PubMed] [Google Scholar]
- 218.Tay B.K., Patel V.V., Bradford D.S. Calcium sulfate–and calcium phosphate–based bone substitutes: mimicry of the mineral phase of bone. Orthop. Clin. N. Am. 1999;30(4):615–623. doi: 10.1016/s0030-5898(05)70114-0. [DOI] [PubMed] [Google Scholar]
- 219.Sandhya S., Mohanan P.V., Sabareeswaran A., Varma H.K., Komath M. Preclinical safety and efficacy evaluation of 'BioCaS' bioactive calcium sulfate bone cement. Biomed. Mater. 2017;12(1) doi: 10.1088/1748-605X/aa5522. [DOI] [PubMed] [Google Scholar]
- 220.Shams M., Nezafati N., Poormoghadam D., Zavareh S., Zamanian A., Salimi A. Synthesis and characterization of electrospun bioactive glass nanofibers-reinforced calcium sulfate bone cement and its cell biological response. Ceram. Int. 2020;46(8):10029–10039. doi: 10.1016/j.ceramint.2019.12.270. [DOI] [Google Scholar]
- 221.Wang J., Zhang L., Sun X., Chen X., Xie K., Lin M., Yang G., Xu S., Xia W., Gou Z. Preparation and in vitro evaluation of strontium-doped calcium silicate/gypsum bioactive bone cement. Biomed. Mater. 2014;9(4) doi: 10.1088/1748-6041/9/4/045002. [DOI] [PubMed] [Google Scholar]
- 222.Miao Q., Yang S., Ding H., Liu J. Controlled degradation of chitosan-coated strontium-doped calcium sulfate hemihydrate composite cement promotes bone defect repair in osteoporosis rats. Biomed. Mater. 2020;15(5) doi: 10.1088/1748-605X/ab9fcf. [DOI] [PubMed] [Google Scholar]
- 223.Zhang Y.Y., Zhu Y., Lu D.Z., Dong W., Bi W.J., Feng X.J., Wen L.M., Sun H., Qi M.C. Evaluation of osteogenic and antibacterial properties of strontium/silver-containing porous TiO2 coatings prepared by micro-arc oxidation. J. Biomed. Mater. Res. B Appl. Biomater. 2021;109(4):505–516. doi: 10.1002/jbm.b.34719. [DOI] [PubMed] [Google Scholar]
- 224.Frank M.J., Walter M.S., Tiainen H., Rubert M., Monjo M., Lyngstadaas S.P., Haugen H.J. Coating of metal implant materials with strontium. J. Mater. Sci. Mater. Med. 2013;24(11):2537–2548. doi: 10.1007/s10856-013-5007-1. [DOI] [PubMed] [Google Scholar]
- 225.Zhu Y., Zheng T., Wen L.M., Li R., Zhang Y.B., Bi W.J., Feng X.J., Qi M.C. Osteogenic capability of strontium and icariin-loaded TiO2 nanotube coatings in vitro and in osteoporotic rats. J. Biomater. Appl. 2021;35(9):1119–1131. doi: 10.1177/0885328221997998. [DOI] [PubMed] [Google Scholar]
- 226.Nguyen T.D.T., Jang Y.S., Lee M.H., Bae T.S. Effect of strontium doping on the biocompatibility of calcium phosphate-coated titanium substrates. J. Appl. Biomater. Funct. Mater. 2019;17(1) doi: 10.1177/2280800019826517. [DOI] [PubMed] [Google Scholar]
- 227.Alipal J., Lee T.C., Koshy P., Abdullah H.Z., Idris M.I. Evolution of anodised titanium for implant applications. Heliyon. 2021;7(7) doi: 10.1016/j.heliyon.2021.e07408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gao A., Hang R., Bai L., Tang B., Chu P.K. Electrochemical surface engineering of titanium-based alloys for biomedical application. Electrochim. Acta. 2018;271:699–718. doi: 10.1016/j.electacta.2018.03.180. [DOI] [Google Scholar]
- 229.Tang G.X., Zhang R.J., Yan Y.N. Preparation of biocompatible structural gradient coatings on pure titanium. Trans. Nonferrous Metals Soc. China. 2004;14:323–327. [Google Scholar]
- 230.Jin L., Cui W., Song X., Zhou L., Liu X. 12th World Conference on Titanium (Ti-2011) Nonferrous Met Soc China; Beijing, China: 2011. Preparation and electrochemical behavior of Ca-P containing porous titania coating on TiNbZrFe alloy by micro-arc oxidation. [Google Scholar]
- 231.Shen X., Fang K., Yie K.H.R., Zhou Z., Shen Y., Wu S., Zhu Y., Deng Z., Ma P., Ma J., Liu J. High proportion strontium-doped micro-arc oxidation coatings enhance early osseointegration of titanium in osteoporosis by anti-oxidative stress pathway. Bioact. Mater. 2022;10:405–419. doi: 10.1016/j.bioactmat.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ullah I., Zhang W., Yang L., Ullah M.W., Atta O.M., Khan S., Wu B., Wu T., Zhang X. Impact of structural features of Sr/Fe co-doped HAp on the osteoblast proliferation and osteogenic differentiation for its application as a bone substitute. Mater Sci Eng C Mater Biol Appl. 2020;110 doi: 10.1016/j.msec.2020.110633. [DOI] [PubMed] [Google Scholar]
- 233.Ramasamy S., Dhamecha D., Kaliyamoorthi K., Pillai A.S., Alexander A., Dhanaraj P., Menon J.U., Enoch I.V.M.V. Magnetic hydroxyapatite nanomaterial–cyclodextrin tethered polymer hybrids as anticancer drug carriers. Materials Advances. 2021;2(10):3315–3327. doi: 10.1039/d1ma00142f. [DOI] [Google Scholar]
- 234.Sivolella S., Stellini E., Brunello G., Gardin C., Ferroni L., Bressan E., Zavan B. Silver nanoparticles in alveolar bone surgery devices. J. Nanomater. 2012;2012 doi: 10.1155/2012/975842. Article 975842. [DOI] [Google Scholar]
- 235.Liu X.J., He W., Fang Z., Kienzle A., Feng Q.L. Influence of silver nanoparticles on osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Nanotechnol. 2014;10(7):1277–1285. doi: 10.1166/jbn.2014.1824. [DOI] [PubMed] [Google Scholar]
- 236.Marques C.F., Perera F.H., Marote A., Ferreira S., Vieira S.I., Olhero S., Miranda P., Ferreira J.M.F. Biphasic calcium phosphate scaffolds fabricated by direct write assembly: mechanical, anti-microbial and osteoblastic properties. J. Eur. Ceram. Soc. 2017;37(1):359–368. doi: 10.1016/j.jeurceramsoc.2016.08.018. [DOI] [Google Scholar]
- 237.Huang Y., Zhang Y., Li M., Yang H., Liang J., Chen Y., Zhang Y., Huang X., Xie L., Lin H., Qiao H., Lan J. Physicochemical, osteogenic and antimicrobial properties of graphene oxide reinforced silver/strontium-doped hydroxyapatite on titanium for potential orthopedic applications. Surf. Coating. Technol. 2022;446 doi: 10.1016/j.surfcoat.2022.128788. [DOI] [Google Scholar]
- 238.Li P., Jia Z., Wang Q., Tang P., Wang M., Wang K., Fang J., Zhao C., Ren F., Ge X., Lu X. A resilient and flexible chitosan/silk cryogel incorporated Ag/Sr co-doped nanoscale hydroxyapatite for osteoinductivity and antibacterial properties. J. Mater. Chem. B. 2018;6(45):7427–7438. doi: 10.1039/c8tb01672k. [DOI] [PubMed] [Google Scholar]
- 239.Chen Y., Zhou C., Xie Y., Xu A., Guan Y., Lu W., Wang X., He F. Zinc- and strontium- co-incorporated nanorods on titanium surfaces with favorable material property, osteogenesis, and enhanced antibacterial activity. J. Biomed. Mater. Res. B Appl. Biomater. 2021;109(11):1754–1767. doi: 10.1002/jbm.b.34834. [DOI] [PubMed] [Google Scholar]
- 240.Kapoor S., Goel A., Tilocca A., Dhuna V., Bhatia G., Dhuna K., Ferreira J.M. Role of glass structure in defining the chemical dissolution behavior, bioactivity and antioxidant properties of zinc and strontium co-doped alkali-free phosphosilicate glasses. Acta Biomater. 2014;10(7):3264–3278. doi: 10.1016/j.actbio.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 241.Kapoor S., Goel A., Pascual M.J., Ferreira J.M.F. Thermo-mechanical behaviour of alkali free bioactive glass-ceramics co-doped with strontium and zinc. J. Non-Cryst. Solids. 2013;375:74–82. doi: 10.1016/j.jnoncrysol.2013.05.007. [DOI] [Google Scholar]
- 242.Wu X., Meng G., Wang S., Wu F., Huang W., Gu Z. Zn and Sr incorporated 64S bioglasses: material characterization, in-vitro bioactivity and mesenchymal stem cell responses. Mater Sci Eng C Mater Biol Appl. 2015;52:242–250. doi: 10.1016/j.msec.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 243.Wu C.T., Zhou Y.H., Fan W., Han P.P., Chang J., Yuen J., Zhang M.L., Xiao Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials. 2012;33(7):2076–2085. doi: 10.1016/j.biomaterials.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 244.Bhattacharjee A., Gupta A., Verma M., Anand M.P., Sengupta P., Saravanan M., Manna I., Balani K. Antibacterial and magnetic response of site-specific cobalt incorporated hydroxyapatite. Ceram. Int. 2020;46(1):513–522. doi: 10.1016/j.ceramint.2019.08.291. [DOI] [Google Scholar]
- 245.Kargozar S., Lotfibakhshaiesh N., Ai J., Mozafari M., Brouki Milan P., Hamzehlou S., Barati M., Baino F., Hill R.G., Joghataei M.T. Strontium- and cobalt-substituted bioactive glasses seeded with human umbilical cord perivascular cells to promote bone regeneration via enhanced osteogenic and angiogenic activities. Acta Biomater. 2017;58:502–514. doi: 10.1016/j.actbio.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 246.Kermani F., Mollazadeh Beidokhti S., Baino F., Gholamzadeh-Virany Z., Mozafari M., Kargozar S. Strontium- and cobalt-doped multicomponent mesoporous bioactive glasses (MBGs) for potential use in bone tissue engineering applications. Materials. 2020;13(6) doi: 10.3390/ma13061348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Anand A., Sengupta S., Kankova H., Svancarkova A., Beltran A.M., Galusek D., Boccaccini A.R., Galuskova D. Influence of copper-strontium Co-doping on bioactivity, cytotoxicity and antibacterial activity of mesoporous bioactive glass. Gels. 2022;8(11) doi: 10.3390/gels8110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Shepherd J.H., Shepherd D.V., Best S.M. Substituted hydroxyapatites for bone repair. J. Mater. Sci. Mater. Med. 2012;23(10):2335–2347. doi: 10.1007/s10856-012-4598-2. [DOI] [PubMed] [Google Scholar]
- 249.Pajor K., Pajchel L., Kolmas J. Hydroxyapatite and fluorapatite in conservative dentistry and oral implantology-A review. Materials. 2019;12(17) doi: 10.3390/ma12172683. Article 2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ganjali M., Mousavi S., Nikzamir S., Milan P.B., Mozafari M. Effect of laser cladded co-doped strontium fluorapatite nanopowder coating on the antibacterial and cell attachment of Ti-6Al-4V implants for bone applications. Mater. Technol. 2021;37(8):829–841. doi: 10.1080/10667857.2021.1898716. [DOI] [Google Scholar]
- 251.Shahrouzifar M.R., Salahinejad E., Sharifi E. Co-incorporation of strontium and fluorine into diopside scaffolds: bioactivity, biodegradation and cytocompatibility evaluations. Mater Sci Eng C Mater Biol Appl. 2019;103 doi: 10.1016/j.msec.2019.109752. [DOI] [PubMed] [Google Scholar]
- 252.Huang Y., Qiao H., Nian X., Zhang X., Zhang X., Song G., Xu Z., Zhang H., Han S. Improving the bioactivity and corrosion resistance properties of electrodeposited hydroxyapatite coating by dual doping of bivalent strontium and manganese ion. Surf. Coating. Technol. 2016;291:205–215. doi: 10.1016/j.surfcoat.2016.02.042. [DOI] [Google Scholar]
- 253.Miola M., Brovarone C.V., Maina G., Rossi F., Bergandi L., Ghigo D., Saracino S., Maggiora M., Canuto R.A., Muzio G., Verne E. In vitro study of manganese-doped bioactive glasses for bone regeneration. Materials Science & Engineering C-Materials for Biological Applications. 2014;38:107–118. doi: 10.1016/j.msec.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 254.Zhang X.Y. Interactions between cancer cells and bone microenvironment promote bone metastasis in prostate cancer. Cancer Commun. 2019;39(1):76. doi: 10.1186/s40880-019-0425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kretschmann K.L., Welm A.L. Mouse models of breast cancer metastasis to bone. Cancer Metastasis Rev. 2012;31(3–4):579–583. doi: 10.1007/s10555-012-9378-4. [DOI] [PubMed] [Google Scholar]
- 256.Lang J., Zhao Q., He Y., Yu X. Bone turnover markers and novel biomarkers in lung cancer bone metastases. Biomarkers. 2018;23(6):518–526. doi: 10.1080/1354750X.2018.1463566. [DOI] [PubMed] [Google Scholar]
- 257.Zhang W., Liu D., Feng C., Zhou C., Zhan C., Que R., Chen L. Management of differentiated thyroid carcinoma with bone metastasis: a case report and review of the Chinese literature. J. Zhejiang Univ. - Sci. B. 2014;15(12):1081–1087. doi: 10.1631/jzus.B1400124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kuroda I. Effective use of strontium-89 in osseous metastases. Ann. Nucl. Med. 2012;26(3):197–206. doi: 10.1007/s12149-011-0560-5. [DOI] [PubMed] [Google Scholar]
- 259.Manafi-Farid R., Masoumi F., Divband G., Saidi B., Ataeinia B., Hertel F., Schweighofer-Zwink G., Morgenroth A., Beheshti M. Targeted palliative radionuclide therapy for metastatic bone pain. J. Clin. Med. 2020;9(8):2622. doi: 10.3390/jcm9082622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Eastell R., O'Neill T.W., Hofbauer L.C., Langdahl B., Reid I.R., Gold D.T., Cummings S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Prim. 2016;2 doi: 10.1038/nrdp.2016.69. Article 16069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.