Abstract
Background
Our elderly population is growing and the number of spine fractures in the elderly is also growing. The elderly population in general may be considered as poor surgical candidates experience a high rate of fractures at C1 and C2 compared with the general population. Nonoperative management of upper cervical fractures is not benign as there is a high nonunion rate for both C1 and C2 fractures in the elderly, and orthosis compliance is often suboptimal, or complicated by skin breakdown. The optimal technique for upper cervical stabilization in the elderly may be different than in younger populations as the bone quality is inferior in the elderly. The objective of this basic science study is to determine whether the bone mineral density (BMD) of C1 and C2 vary by region, and if this is a gender difference in this elderly age group.
Methods
Twenty cadaveric spines from 45 to 83 years of age were used to obtain BMD using quantitated computed tomography (QCT). BMD was measured using a QCT. For C1, 8 regions were determined: anterior tubercle, bilateral anterior and medial lateral masses, bilateral posterior arches, and posterior tubercle. For C2, 7 regional BMDs were determined: top of odontoid, base of odontoid-body interface, mid body, bilateral lateral masses, anterior inferior body near the discs space, and the C2 spinous process.
Results
The BMD was greatest at the C1 anterior tubercle (564.4±175.8 mg/cm3) and C1 posterior ring (420.8±110.2 mg/cm3), and least at the anterior and medial lateral masses (262.8±59.5 mg/cm3, 316.9±72.6 mg/cm3). At C2 QCT BMD was greatest at the top of the dens (400.6±107.9 mg/cm3) decreasing down through the odontoid-C2 body junction (267.8±103.5 mg/cm3) and least in the mid C2 body 249.1±68.8 mg/cm3). The posterior arch of C1 and the spinous process of C2 had higher BMD's 420.8±110.2 mg/cm3 and 284.1±93.0 mg/cm3, respectively. A high correlation was observed between the BMD at the interface of the dens-vertebral body with the vertebral body with a Pearson correlation coefficient of 0.86. The BMD of the top of dens was significantly higher (p<.05) than all the regions in C2.
Conclusions
Regional and segmental BMD variations at C1 and C2 have clinical implications for surgical constructs in the elderly population. Given the higher BMDs of the C1 and C2 spinous process and posterior arches, consideration should be given to incorporate these areas using various C1–C2 wiring techniques. In the elderly, lateral masses particularly at C1 with lower BMD may result in potential screw loosening and nonunion in this age group. Old-school wiring techniques have a track record of efficacy and safety with less blood loss, reduced operative time, reduced X-ray exposure, and should be considered in the elderly as a primary stabilization technique or a belt-over suspenders approach based on regional variations in BMD in the elderly.
Keywords: Cervical spine, Bone mineral density, Regional variation, Osteoporosis, Segmental variation, Geriatric
Introduction
Epidemiology
Cervical spine injuries are one of the leading causes of mortality and morbidity in the elderly and a burden on the health-care systems. It is projected that there will be an increase in the number of C1 and C2 fractures in the elderly as our population ages [1,2]. The upper cervical region C1–C2 is responsible for 25% to 40% of all the cervical spine injuries. Falls and trauma are the leading causes for upper cervical spine fractures. Motor vehicle accidents and low-velocity falls are frequent causes for these fractures [3,4]. Recent epidemiological studies have shown that the rate of atlas fractures is higher in the elderly population (63.8% of atlas fracture occurred in patients over the age of 70 years) [5]. Based on several literature studies, the rate of atlas fractures is in the range of 11% to 13% [5,6].
Odontoid fractures are the most common type of fractures of C2 (accounting for 60% of C2 fractures). The rate of C2 fracture in the Medicare population increased by 135% from the years 2000 to 2011 [3]. Given the trend in increasing geriatric population and better imaging modalities, these numbers are projected to increase [2].
Osteoporosis is rarely discussed in cervical spine except in the context of revision surgery [7], [8], [9]. It is characterized by a decrease in bone density and an increase in fracture risk. In the elderly population, there are higher incidences of osteoporosis and revision surgery compared with younger populations. Hence, it is important to understand the regional variation in bone mineral density (BMD) in the upper cervical spine for the elderly.
Literature supports operative treatment but the ideal fixation in the elderly may be different than in younger populations due to segmental and regional differences in BMD [10], [11], [12]. Screw fixations are the most common technique used to stabilize the unstable segments. In this technique, screws such as pedicle screws or lateral mass or pars are inserted in their corresponding regions and connected with rods to maintain alignment for subsequent biomechanical stabilization or fusion. Biomechanical studies have shown that the pullout strength of these screws is dependent on BMD, insertion angle, and screw length [13], [14], [15].
The vertebral column is designed to respond to mechanical loads by allowing for adaptation to external physiologic stresses such as carrying and alterations in posture associated with movement, in addition to adaptations to body morphology. Bone tends to adapt to these internal and external stress by modifying the BMD. Dual X-ray absorptiometry (DXA) scans have been the traditional gold standard for measurement of BMD but there are inherent problems using this technique. DXA scans do not allow for the regional variations encountered within the various regions of the spine [16,17]. Use of quantitated computed tomography (QCT) aids in both visualization of the vertebral structures and assess the regional BMD. CT scans are usually performed in trauma protocols to assess the fracture and surgical decision making/planning. Hence, QCTs are ideal imaging protocol for determining regional BMD measurements in the cervical spine.
Based on Wolff's law, given the higher loads the thoracic and lumbar spines experience compared with the cervical spine, one would anticipate higher BMD in the thoracic and lumbar spine to support the torso and abdomen, but this is not the case for the cervical spine. Paradoxically the cervical spine has the highest BMD within the spine followed by the thoracic or lumbar spine, respectively [18], [19], [20]. A 2006 study demonstrated that the trabecular bone in the cervical spine was significantly different than the lumbar spine [20]. QCTs were used to determine BMD on C2–C7, T1 and L2–4 on 57 healthy male volunteers with an age range of 18 to 41. A decreasing trend in BMD was observed from the cervical spine to the lumbar spine. This trend showed significance as the mean cervical spine BMD was 1.5 times higher than the lower lumbar vertebrae. A 2017 study [19] used QCT to determine the regional and spinal level variations within the cervical spine. This study used 31 young healthy volunteers (16 males and 15 females) and showed BMD variations with both spinal level and regional variations within each level. The study was also demonstrated that females had a higher BMD in the cervical spine than males and attributed it to increased BMD in the posterior regions [18].
Bone mineral density should influence surgical decision making in any fracture case and particularly in the elderly. Upper cervical fractures and C1–C2 instability cases can be very challenging in the elderly as the weighing of comorbidities and ethical issues often complicate the surgical decision process. Treatment options vary depending on the type of fracture(s), degree of displacement, patient age, and comorbidity factors. While the elderly population may often be considered suboptimal surgical candidates, they experience a high rate of fractures at C1 and C2 from low-velocity falls and motor vehicle accidents. The fracture incidence rates for the elderly at C1, C2, and the C1–C2 complex range in the 25% to 40% [21]. Cervical orthosis treatment is often pursued due to fragility in the elderly. But cervical orthosis treatment itself is not benign nor has a particularly good success rate for many upper cervical fractures. For the group that fails to heal with nonoperative management, this typically results in delayed healing, a weak fibrous union, or a delay in surgical intervention. To stabilize these fractures, C1–C2 screws (pars, pedicle, transpedicular) are commonly used.
From a biomechanical perspective, external loads and potentially compromised vascular supply and suboptimal bone quality attributed to this region of C2 are associated with the poor healing rates for type II odontoid fractures. For this reason, it is important that BMD be incorporated into the surgical planning of C1 and C2 fractures to optimize the surgical construct to prevent screw loosening and nonunion due to poor bone quality in this age group.
The objective of the current study is to determine if there are regional variations in C1 and C2 vertebra with an emphasis on a geriatric population from a larger sample size. The results of the study can aid in finding the optimal construct for C1–C2 stabilization.
Methods
Regional BMDs of C1 and C2 vertebrae using QCT were obtained from 20 unembalmed human cadaver spines. A QCT software (Midways Inc., San Francisco, CA) was used to determine bone density. For C2: BMDs were determined at 7 regions: the top of the odontoid, the base of an odontoid-body interface, the anterior-inferior body, the mid-body, the bilateral body, and the posterior spinous process. For C1: 8 regions were studied: anterior tubercle, bilateral anterior and medial lateral masses, bilateral posterior arches, and posterior arch (Fig. 1). The spine surgeon author outlined the regions on the CT scans for determining the BMDs.
Fig. 1.
Left shows C1 vertebra regional QCT bone mineral density measurement sites with regions marked (1–8) where 1=anterior tubercle; 2 and 8=anterior lateral mass (right and left); 3 and 7=midline lateral mass (right and left); 4 and 6=posterior arch (right and left), and 5=posterior tubercle. Right shows C2 vertebra regional QCT bone mineral density measurement sites with regions marked (1–8) where 1=distal dens; 2=interface of dens and vertebral body; 3=vertebral body; 4=inferior anterior mid body; 5 and 6=lateral mass; 7=spinous process.
The selection of the anatomic regions was based on commonly used screw trajectories or clinical fixation points based on common surgical constructs (Fig. 1 left and right). Repeated measure linear mixed model was used for statistical analysis with p<.05 for significance. Tukey's post hoc test was performed to analyze the regional variations in the BMD. Analysis was done for the entire group and for a subgroup with 11 specimens age ≥65 years, to discuss the applicability to the elderly populations.
Results
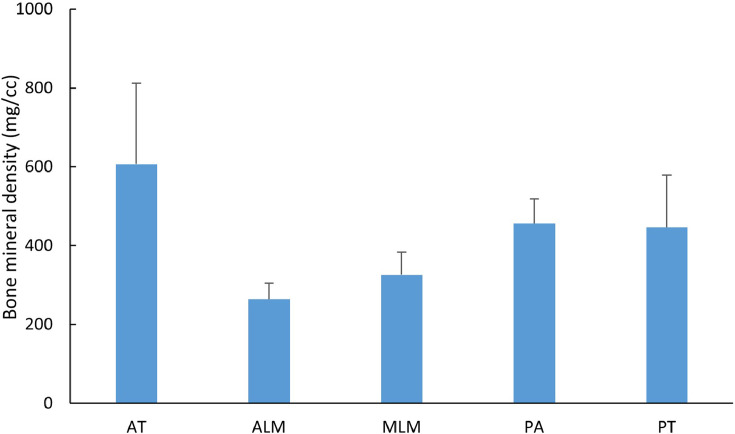
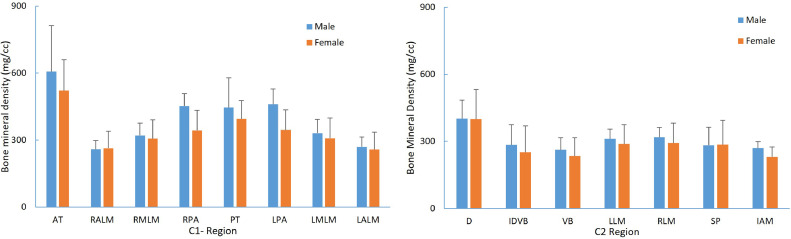
The demographics of the cadaveric (PMHS) used for the BMD measurements are shown in the Table 1. For C1, BMD (Fig. 2) was greatest at the anterior tubercle (564.4±175.8 mg/cm3) and posterior tubercle (420.8±110.2 mg/cm3), and least at the anterior and medial lateral masses (262.8±59.5 and 316.9±72.6 mg/cm3). For C2 (Fig. 3), there were no statistical differences (p>.5) between left and right lateral mass BMDs, and hence lateral masses were averaged for analysis. BMD was significantly greater (p<.05) at the top of the dens (400.6±107.9 mg/cm3) compared with the other 6 regions (average 249.1–303.0 mg/cm3). It was the least in the midbody (249.1±68.8 mg/cm3). The BMD was greater (p<.05) at the C2 lateral mass than odontoid-vertebral body interface or midbody. The C1 posterior arch (400.9±93.6 mg/cm3) and C2 spinous process (284.1±93.0 mg/cm3) had higher BMDs than C1 lateral (262.8±59.5 mg/cm3) and C2 lateral masses (303.30±67.0 mg/cm3). We were able to demonstrate spinal level and regional variations within spinal levels that attain statistical significance, but no statistically significant gender differences were noted in this elderly population (Fig. 4).
Table 1.
Demographics of the cadavers
| All data (n=20) |
Male (n=10) |
Female (n=10) |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 66.1 | 10.3 | 64.2 | 10.8 | 67.9 | 9.9 |
| Height (cm) | 170.3 | 10.8 | 177.3 | 8.2 | 163.3 | 8.5 |
| Weight (kg) | 76.7 | 15.1 | 83.8 | 11.6 | 69.6 | 15.2 |
Fig. 2.
Regional variation of BMD of C1 vertebra.
Fig. 3.
Regional variation of BMD of C2 vertebra.
Fig. 4.
Gender-based comparison of BMD in C1 and C2 vertebrae.
Discussion
The objective of the current study was to determine if regional variation in BMD exists in the upper cervical spine and if there were gender differences in this older age group. We used QCT for determination of BMD in unembalmed human cadaver spines as DXA has been shown to be inaccurate in the cervical spine [22]. Using QCT or Hounsfield units is more practical as clinically CTs are commonly obtained in trauma protocols and in deformity cases in the diagnostic and planning stages for fracture/deformity treatment [23].
Data were analyzed for the whole group and for the elderly subgroup. For the entire ensemble, our findings for C1 show that the anterior and medial lateral mass have the lowest BMD of both C1 and C2 (Fig. 2). For C2, there were no statistical differences (p>.5) between left and right lateral mass, C2 BMD was significantly greater (p<.05) at the top of the dens compared with the other 6 regions, C2 BMD was greater (p<.05) at the C2 lateral mass than odontoid-vertebral body interface. C2 BMD was greater (p<.05) at the C2 lateral mass than at the midbody. The hypothesis that the BMD shows a regional response and decreases caudally from the top of the odontoid down through the vertebral body was proven in the present study with human cadaver cervical vertebrae. Statistical results remained the same for the subgroup, except the C2 lateral mass did not attain the same level of significance with respect to the other regions. This finding may have clinical/surgical implications.
From a clinical perspective, given that type II and type III odontoid fractures would occur in the region of lowest BMD within C2, an indirect stabilization method should be considered in the elderly. Avoiding the softer bone encountered in an anterior odontoid screw fixation avoids the lower BMD encountered in this geriatric age group and avoids the higher incidence of dysphagia experienced in elderly populations with anterior approaches [24,25]. For younger patients with well aligned and “fresh” Type 2 odontoid fractures, the anterior approach is favored to maintain and optimize postoperative range of motion [26]. The screw trajectory for an anterior odontoid screw stabilization extends from the anterior inferior C2 body near the disc space through the lower BMD bone found in the C2 vertebral body and incorporates the higher BMD within the odontoid as the screw projects toward the distal odontoid tip. This anterior odontoid screw trajectory for type II odontoid fractures goes through better bone quality and bone with higher BMD at the distal aspect giving a more secure construct and less chance of screw loosening [9].
In younger populations care should be taken to avoid using a short screw with the anterior odontoid approach as the inferior odontoid may be in contact with softer bone near the actual fracture site (an area of lower BMD), which may potentiate a delayed screw loosening. If there is C1–C2 instability and the posterior arches are fractured, for pedicle, pars, lateral mass, or transarticular screws regional variations in BMD should be considered to optimize screw length to obtain a secure construct. Multiple biomechanical studies both cadaveric and finite element modeling have been performed to compare construct stability in atlantoaxial fixation with heterogenous results based on mode of testing [27], [28], [29], [30], [31], [32]. However, these studies are performed on younger specimens with higher BMD along the lateral masses and pedicles.
In elderly patients with more complex fractures involving both the odontoid and C1, the lateral mass of C1, both anteriorly and medially, may have lower BMD than in younger patients. Commonly used C1–C2 screw fixation techniques in the elderly population may encounter lower BMD which causes concern for potential screw loosening with the lower BMD's confronted along the trajectory of the C1 screw in particular. With ectatic vertebral arteries commonly found in the elderly, and potential for higher blood loss as the need for a more lateral exposure for initiation of the screw placement, additional consideration for screws use in the upper cervical spine should be considered for surgical planning. Bone mineral density has been previously studied using QCT, and regional variations were determined by the spinal level and by anatomic regions within the vertebra in a healthy younger population (ages 20–35) [19].
The BMD data from the present study added to the prior literature are more applicable to the elderly because of the geriatric age of the current specimens. The screw fixation techniques both anteriorly for type II odontoid fractures and for C1–C2 constructs for instability and complex fractures of either C1, C2, or both posterior arches at C1 and C2 should incorporate the BMD to optimize surgical constructs. In our study focused on the geriatric population, we did not identify any significant gender differences in BMD which is different than cited study that showed an increased BMD in the posterior elements of C1 and C2 in women as compared with men in a younger age group. Additional human cadaver specimens may be needed to detect the presence of gender bias in the geriatric populations, and this will be a future study topic.
There are segmental and regional variations in BMD in the cervical spine. Biomechanically the C1–C2 screw constructs in the younger population have been shown to be biomechanically superior to wiring constructs [29,33,34]. Most biomechanical studies are performed on younger healthy cadaveric specimens. The BMD observed in this population tends to be superior to that encountered in the geriatric population. Placing screws at C1 and C2 in elderly patients cannot be assumed to have the same biomechanical strength as in younger spines given the decrease in BMD along the lateral mass and pedicle. Surgical constructs for C1 and C2 fractures particularly in the elderly need to consider the regional and segmental variations to strengthen surgical constructs in the geriatric population.
The BMD's encountered in the cervical spine of geriatric patients is suboptimal and cannot be directly compared with the screw-rod constructs studied in younger populations. By considering posterior C1–C2 wiring/cable constructs which use the C1 posterior arch and C2 spinous process/posterior arch to allow for incorporation of bone with higher BMD and better bone quality to be integrated into the surgical construct this offers a stronger construct in the elderly population. Depending on the surgeon's comfort with placing sublaminar wires or cables and the patient's anatomy, the use of various C1–C2 wiring/cable techniques [35], [36], [37] should still be considered particularly in the elderly. If the posterior elements of C1 and C2 are fractured or the patient is younger, surgeons need to recognize the regional variations in BMD to optimize the surgical approach and screw length to obtain a secure construct.
Conclusions
Regional and segmental BMD variations at C1 and C2 have clinical implications for surgical constructs in the elderly population. Given the higher BMDs of the C1 and C2 spinous process and posterior arches, consideration should be given to incorporate these areas using various C1–C2 wiring techniques. In the elderly, lateral masses particularly at C1 with lower BMD coupled with suboptimal nonunion and failure rates in C1 and C2 fractures may result in potential screw loosening and nonunion in this age group. Old-school wiring techniques have a track record of efficacy and safety with less blood loss, reduced operative time, reduced X-ray exposure, and should be considered in the elderly as a primary stabilization technique or a belt-over suspenders approach based on regional variations in BMD in the elderly.
Declarations of Competing Interest
The authors have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: JLB: Grant: DOD (Paid directly to institution/employer), Nonfinancial. VV: Nothing to disclose, Nonfinancial. AB: Grants: NIH (Paid directly to institution/employer), Nonfinancial. NY: Grant: DOD (Paid directly to institution/employer), Nonfinancial.
References
- 1.Delcourt T, Begue T, Saintyves G, Mebtouche N, Cottin P. Management of upper cervical spine fractures in elderly patients: current trends and outcomes. Injury. 2015;46(suppl 1):S24–S27. doi: 10.1016/S0020-1383(15)70007-0. [DOI] [PubMed] [Google Scholar]
- 2.Fehlings MG, Tetreault L, Nater A, et al. The aging of the global population: the changing epidemiology of disease and spinal disorders. Neurosurgery. 2015;77(suppl 4):S1–S5. doi: 10.1227/NEU.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 3.Pearson AM, Martin BI, Lindsey M, Mirza SK. C2 vertebral fractures in the medicare population: incidence, outcomes, and costs. J Bone Joint Surg Am. 2016;98(6):449–456. doi: 10.2106/JBJS.O.00468. [DOI] [PubMed] [Google Scholar]
- 4.Yoganandan N, Pintar F, Baisden J, Gennarelli T, Maiman D. Injury biomechanics of C2 dens fractures. Annu Proc Assoc Adv Automot Med. 2004;48:323–337. [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons JG, Mian HM. Epidemiology of atlas fractures in the United States: a 20-year analysis. J Craniovertebr Junct Spine. 2022;13(1):85–93. doi: 10.4103/jcvjs.jcvjs_164_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Passias PG, Poorman GW, Segreto FA, et al. Traumatic fractures of the cervical spine: analysis of changes in incidence, cause, concurrent injuries, and complications among 488,262 patients from 2005 to 2013. World Neurosurg. 2018;110:e427–e437. doi: 10.1016/j.wneu.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Guzman JZ, Feldman ZM, McAnany S, Hecht AC, Qureshi SA, Cho SK. Osteoporosis in cervical spine surgery. Spine (Phila Pa 1976) 2016;41(8):662–668. doi: 10.1097/BRS.0000000000001347. [DOI] [PubMed] [Google Scholar]
- 8.Emohare O, Dittmer A, Morgan RA, Switzer JA, Polly, Jr DW. Osteoporosis in acute fractures of the cervical spine: the role of opportunistic CT screening. J Neurosurg Spine. 2015;23(1):1–7. doi: 10.3171/2014.10.SPINE14233. [DOI] [PubMed] [Google Scholar]
- 9.Oitment C, Thornley P, Koziarz F, Jentzsch T, Bhanot K. A review of strategies to improve biomechanical fixation in the cervical spine. Global Spine J. 2022;12(7):1596–1610. doi: 10.1177/21925682211063855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catalino MP, Pate V, Sturmer T, Bhowmick DA. Comparative propensity-weighted mortality after isolated acute traumatic axis fractures in older adults. Geriatr Orthop Surg Rehabil. 2020;11 doi: 10.1177/2151459320911867. 2151459320911867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YR, Boakye M, Arrigo RT, et al. Morbidity and mortality of C2 fractures in the elderly: surgery and conservative treatment. Neurosurgery. 2012;70(5):1055–1059. doi: 10.1227/NEU.0b013e3182446742. discussion 9. [DOI] [PubMed] [Google Scholar]
- 12.Fan L, Ou D, Huang X, et al. Surgery vs conservative treatment for type II and III odontoid fractures in a geriatric population: a meta-analysis. Medicine (Baltimore) 2019;98(44):e10281. doi: 10.1097/MD.0000000000010281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varghese V, Saravana Kumar G, Krishnan V. Effect of various factors on pull out strength of pedicle screw in normal and osteoporotic cancellous bone models. Med Eng Phys. 2017;40:28–38. doi: 10.1016/j.medengphy.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Barnes AH, Eguizabal JA, Acosta, Jr FL, Lotz JC, Buckley JM, Ames CP. Biomechanical pullout strength and stability of the cervical artificial pedicle screw. Spine (Phila Pa 1976) 2009;34(1):E16–E20. doi: 10.1097/BRS.0b013e3181891772. [DOI] [PubMed] [Google Scholar]
- 15.Reitman CA, Nguyen L, Fogel GR. Biomechanical evaluation of relationship of screw pullout strength, insertional torque, and bone mineral density in the cervical spine. J Spinal Disord Tech. 2004;17(4):306–311. doi: 10.1097/01.bsd.0000090575.08296.9d. [DOI] [PubMed] [Google Scholar]
- 16.Choi MK, Kim SM, Lim JK. Diagnostic efficacy of Hounsfield units in spine CT for the assessment of real bone mineral density of degenerative spine: correlation study between T-scores determined by DEXA scan and Hounsfield units from CT. Acta Neurochir (Wien) 2016;158(7):1421–1427. doi: 10.1007/s00701-016-2821-5. [DOI] [PubMed] [Google Scholar]
- 17.Pennington Z, Ehresman J, Lubelski D, et al. Assessing underlying bone quality in spine surgery patients: a narrative review of dual-energy X-ray absorptiometry (DXA) and alternatives. Spine J. 2021;21(2):321–331. doi: 10.1016/j.spinee.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Anderst WJ, Thorhauer ED, Lee JY, Donaldson WF, Kang JD. Cervical spine bone mineral density as a function of vertebral level and anatomic location. Spine J. 2011;11(7):659–667. doi: 10.1016/j.spinee.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderst WJ, West T, Donaldson WF, 3rd, Lee JY. Cervical spine bone density in young healthy adults as a function of sex, vertebral level and anatomic location. Eur Spine J. 2017;26(9):2281–2289. doi: 10.1007/s00586-017-5119-2. [DOI] [PubMed] [Google Scholar]
- 20.Yoganandan N, Pintar FA, Stemper BD, et al. Trabecular bone density of male human cervical and lumbar vertebrae. Bone. 2006;39(2):336–344. doi: 10.1016/j.bone.2006.01.160. [DOI] [PubMed] [Google Scholar]
- 21.Barrey CY, di Bartolomeo A, Barresi L, et al. C1-C2 injury: factors influencing mortality, outcome, and fracture healing. Eur Spine J. 2021;30(6):1574–1584. doi: 10.1007/s00586-021-06763-x. [DOI] [PubMed] [Google Scholar]
- 22.Link TM, Lang TF. Axial QCT: clinical applications and new developments. J Clin Densitom. 2014;17(4):438–448. doi: 10.1016/j.jocd.2014.04.119. [DOI] [PubMed] [Google Scholar]
- 23.Alawi M, Begum A, Harraz M, et al. Dual-energy X-ray absorptiometry (DEXA) scan versus computed tomography for bone density assessment. Cureus. 2021;13(2):e13261. doi: 10.7759/cureus.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson S, Rodrigues M, Olerud C. Odontoid fractures: high complication rate associated with anterior screw fixation in the elderly. Eur Spine J. 2000;9(1):56–59. doi: 10.1007/s005860050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Josten C, Jarvers JS, Glasmacher S, Spiegl UJ. Odontoid fractures in combination with C1 fractures in the elderly treated by combined anterior odontoid and transarticular C1/2 screw fixation. Arch Orthop Trauma Surg. 2018;138(11):1525–1531. doi: 10.1007/s00402-018-3013-y. [DOI] [PubMed] [Google Scholar]
- 26.Yuan S, Wei B, Tian Y, et al. The comparison of clinical outcome of fresh type II odontoid fracture treatment between anterior cannulated screws fixation and posterior instrumentation of C1-2 without fusion: a retrospective cohort study. J Orthop Surg Res. 2018;13(1):3. doi: 10.1186/s13018-017-0702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrey C, Mertens P, Rumelhart C, Cotton F, Jund J, Perrin G. Biomechanical evaluation of cervical lateral mass fixation: a comparison of the Roy-Camille and Magerl screw techniques. J Neurosurg. 2004;100(3 suppl spine):268–276. doi: 10.3171/spi.2004.100.3.0268. [DOI] [PubMed] [Google Scholar]
- 28.Chun DH, Yoon DH, Kim KN, Yi S, Shin DA, Ha Y. Biomechanical comparison of four different atlantoaxial posterior fixation constructs in adults: a finite element study. Spine (Phila Pa 1976) 2018;43(15):E891–E897. doi: 10.1097/BRS.0000000000002584. [DOI] [PubMed] [Google Scholar]
- 29.Du JY, Aichmair A, Kueper J, Wright T, Lebl DR. Biomechanical analysis of screw constructs for atlantoaxial fixation in cadavers: a systematic review and meta-analysis. J Neurosurg Spine. 2015;22(2):151–161. doi: 10.3171/2014.10.SPINE13805. [DOI] [PubMed] [Google Scholar]
- 30.Gluf WM, Schmidt MH, Apfelbaum RI. Atlantoaxial transarticular screw fixation: a review of surgical indications, fusion rate, complications, and lessons learned in 191 adult patients. J Neurosurg Spine. 2005;2(2):155–163. doi: 10.3171/spi.2005.2.2.0155. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y, Lee YP, Bhatia NN, Lee TQ. Biomechanical comparison of C1 lateral mass-C2 short pedicle screw-C3 lateral mass screw-rod construct versus Goel-Harms fixation for atlantoaxial instability. Spine (Phila Pa 1976) 2019;44(7):E393–E3E9. doi: 10.1097/BRS.0000000000002868. [DOI] [PubMed] [Google Scholar]
- 32.Su BW, Shimer AL, Chinthakunta S, et al. Comparison of fatigue strength of C2 pedicle screws, C2 pars screws, and a hybrid construct in C1-C2 fixation. Spine (Phila Pa 1976) 2014;39(1):E12–E19. doi: 10.1097/BRS.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 33.Cadena G, Duong HT, Liu JJ, Kim KD. Atlantoaxial fixation using C1 posterior arch screws: feasibility study, morphometric data, and biomechanical analysis. J Neurosurg Spine. 2018;30(3):314–322. doi: 10.3171/2018.8.SPINE18160. [DOI] [PubMed] [Google Scholar]
- 34.Elliott RE, Tanweer O, Boah A, et al. Atlantoaxial fusion with screw-rod constructs: meta-analysis and review of literature. World Neurosurg. 2014;81(2):411–421. doi: 10.1016/j.wneu.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Larsen AMG, Grannan BL, Koffie RM, Coumans JV. Atlantoaxial fusion using C1 sublaminar cables and C2 translaminar screws. Oper Neurosurg (Hagerstown) 2018;14(6):647–653. doi: 10.1093/ons/opx164. [DOI] [PubMed] [Google Scholar]
- 36.Tran M, Wadhwa R, Ziewacz J, Mummaneni P, Chou D. Comparison between C1-2 fixation with and without supplemental posterior wiring. Evid Based Spine Care J. 2014;5(1):12–15. doi: 10.1055/s-0034-1371972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Xue R, Wu L, Ding W, Ma L. Comparison of clinical and radiological outcomes between modified Gallie graft fusion-wiring technique and posterior cervical screw constructs for Type II odontoid fractures. Medicine (Baltimore) 2018;97(29):e11452. doi: 10.1097/MD.0000000000011452. [DOI] [PMC free article] [PubMed] [Google Scholar]