Key Points
-
•
CMV reactivation is associated with a reduced risk of relapse in both AML and ALL.
-
•
The mild antileukemia effect of CMV reactivation is independent of acute GVHD but lacks effect modification by baseline characteristics.
Visual Abstract
Abstract
Cytomegalovirus reactivation (CMVR) after allogeneic hematopoietic cell transplantation (HCT) is a frequent complication related to survival outcomes; however, its impact on relapse remains unclear, especially in acute lymphoblastic leukemia (ALL). In this nationwide retrospective study, we included patients with acute myeloid leukemia (AML) and ALL in the first or second complete remission who underwent their first HCT using a pre-emptive strategy for CMVR. Because 90% of cases with CMVR had occurred by day 64 and 90% of cases with grades 2 to 4 acute graft-versus-host disease (GVHD) had occurred by day 58, a landmark point was set at day 65. In landmark analyses, 3793 patients with AML and 2213 patients with ALL who survived without relapse for at least 65 days were analyzed. Multivariate analyses showed that CMVR was associated with a lower incidence of relapse in both AML (hazard ratio [HR], 0.81; 95% confidence interval [CI], 0.69-0.95; P = .009) and ALL (HR, 0.81; 95% CI, 0.66-0.99; P = .045). These findings were confirmed when CMVR was used as the time-dependent covariate. Moreover, our study suggests that the protective effect of CMVR on relapse was independent of acute GVHD. A post-hoc subgroup analysis of combined AML and ALL showed that CMVR had a mild antileukemia effect without effect modification, in contrast to the impact of CMVR on NRM. Our findings may provide important implications for strategies used for CMV prophylaxis after HCT.
Introduction
Cytomegalovirus (CMV) is an important cause of morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT).1, 2, 3, 4, 5 Over the past few decades, the incidence of CMV disease has dramatically decreased with the introduction of a pre-emptive strategy for CMV reactivation (CMVR).6, 7, 8, 9, 10, 11 However, CMVR requiring pre-emptive treatment is still considered to be harmful due to delayed immune reconstitution,12 subsequent increased risk of graft-versus-host disease (GVHD),13,14 and the side effects of antiviral agents such as bone marrow suppression.15 Many studies have shown that CMVR was associated with a higher risk of nonrelapse mortality (NRM).14,16, 17, 18, 19, 20
Despite the disadvantages of CMVR in NRM, many studies have reported a protective effect of CMVR on relapse in acute myeloid leukemia (AML),16,18,21,22 but not in acute lymphoblastic leukemia (ALL). This has been explained by speculating that the graft-versus-leukemia (GVL) effect targets virus-derived antigens on AML cells that are infected by CMV.23 However, because CMV infects lymphocytes24 and malignant cells of ALL as well,25 CMVR might have a protective effect on relapse even in ALL. The Seattle group and Center for International Blood and Marrow Transplantation Research have failed to demonstrate that CMVR has a significant impact on relapse in ALL (Seattle, n = 289, P = .18; Center for International Blood and Marrow Transplantation Research, n = 1864, P = .08),16,17 but the relatively wide confidence interval (CI) in ALL compared with that in AML does not exclude the null hypothesis of a protective effect of CMVR on relapse.
The recent epoch-making clinical trial of letermovir demonstrated its safety profiles and its ability to significantly reduce CMVR.26 If CMVR itself has a protective effect on relapse, an increased risk of relapse through the use of letermovir prophylaxis is a significant concern. The results of our recent study, limited to patients with acute GVHD, suggested that the use of letermovir was related to an increased risk of relapse, but it is difficult to draw a definitive conclusion because of the lack of stratification by disease and relapse risk.27 Direct evidence comparing the risk of relapse between patients with and without letermovir limited to a homogeneous population requires the accumulation of massive amounts of real-world data. Therefore, before such evidence can be available, we conducted a retrospective study to reevaluate the impact of CMVR on relapse in patients with AML and ALL using a nationwide Japanese data set with a sufficient number of HCT recipients who underwent a pre-emptive strategy.
Methods
Data source and patient selection
Clinical data were obtained from the Transplant Registry Unified Management Program, which is the registry database of the Japan Society for Transplantation and Cellular Therapy.28 Patients were included in this analysis if they were aged 16 years or older, had AML or ALL in first or second complete remission, and had undergone their first allogeneic HCT from HLA-matched related donors, HLA 1-antigen-mismatched related donors, HLA-matched unrelated donors, HLA-mismatched unrelated donors, umbilical cord blood, or haploidentical donors between 2006 and 2019 with complete information for all covariates. Only patients with a CMV-seropositive donor or recipient (D/R) and who achieved neutrophil engraftment were included. We considered the donor CMV serological status with umbilical cord blood to be negative. Letermovir became available in Japan in May 2018 and patients who received letermovir were excluded (n = 441). We also excluded patients who received prophylactic anti-CMV agents such as ganciclovir, valganciclovir, or foscarnet (n = 55).
This study was approved by the data management committee of the Japan Society for Transplantation and Cellular Therapy and the Institutional Review Board of Jichi Medical University Saitama Medical Center. This study was conducted following the Declaration of Helsinki.
Definitions
The patients underwent weekly CMV monitoring using the pp65 antigenemia assay at the time of engraftment. CMVR was defined as the initiation of CMV pre-emptive therapy.14,18,19 In most centers, the threshold for the start of pre-emptive therapy was 3 antigenemia-positive cells per 2 slides, which was comparable to real-time PCR with a threshold of 300 CMV DNA copies per ml.29 Disease risk index (DRI), hematopoietic cell transplantation-specific comorbidity index (HCT-CI) scores, and conditioning intensity were categorized as previously reported.30, 31, 32 HLA compatibilities in related and unrelated donors were evaluated with a 6/6 antigen match of HLA-A, -B, and –DR and with an 8/8 allele match of HLA-A, -B, -C, and –DRB1, respectively. The use of antithymocyte globulin or alemtuzumab was considered for in vivo T-cell depletion.
Statistical analysis
The primary end point was the effect of the CMVR on relapse. We defined relapse as recurrent blasts in the peripheral blood or increased blasts in the bone marrow by >5%. We used a landmark method to assess the impact of CMVR on the long-term outcomes. The landmark day was set at the time when 90% of the patients developed CMVR or grades 2 to 4 acute GVHD (G24GVHD). The univariate Gray’s method and multivariate Fine and Gray method were used to evaluate the impact of CMVR on relapse or NRM. The competing event for relapse was death without relapse and the competing event for NRM was relapse. The Kaplan-Meier method was used to estimate the probability of overall survival (OS). Cox proportional hazards regression models were used to evaluate the effect of CMVR on OS in the multivariate analysis or to treat CMVR and acute GVHD as time-dependent covariates. The proportional hazard assumption of a main effect for each variable was tested based on Schoenfeld residuals.33 None of the variables in this study violated the proportional hazard assumption.
The following variables were adjusted in the multivariate analyses: recipient’s age at HCT (< 50 vs ≥ 50 years), sex mismatch (female to male vs others), recipient/donor CMV serological status (R−/D+ vs R+/D− vs R+/D+), DRI (low vs intermediate vs high risk), HCT-CI (< 2 vs ≥ 2), donor source (HLA-matched related vs HLA 1-antigen-mismatched related vs HLA-matched unrelated vs HLA-mismatched unrelated vs umbilical cord blood vs haploidentical), conditioning intensity (myeloablative vs reduced-intensity), GVHD prophylaxis (cyclosporine-based vs tacrolimus-based), in vivo T-cell depletion (no vs yes), year of HCT, and G24GVHD. In the analyses of ALL, the positivity of Philadelphia chromosome was also included.
All statistical tests were 2-sided and a P value < .05 was considered statistically significant. All statistical analyses were performed with EZR version 1.53 (Jichi Medical University Saitama Medical Center), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 3.2.2).34
Results
Patient characteristics
In this study, we identified 3947 patients with AML and 2292 patients with ALL. Because 90% of cases with CMVR occurred by day 64 (median, day 40), and 90% of cases with grades 2 to 4 acute GVHD occurred by day 58 (median, day 29), a landmark point was set at day 65. A total of 3793 patients with AML and 2213 patients with ALL who survived without relapse for at least 65 days were analyzed in this study (Table 1). The median ages of patients with AML and ALL were 49 years (range, 16-74) and 42 years (range, 16-76), respectively. By day 65, 1864 (49.1%) and 1046 (47.3%) developed CMVR, respectively (supplemental Table 1). The median observation periods for survivors in AML and ALL were 5.4 years and 5.2 years, respectively.
Table 1.
Patient characteristics
| AML |
ALL |
|
|---|---|---|
| N = 3793 | N = 2213 | |
| Median age at HCT, y (range) | 49 (16-74) | 42 (16-76) |
| Age, category | ||
| <50 | 1927 (50.8) | 1494 (67.5) |
| ≥50 | 1866 (49.2) | 719 (32.5) |
| Sex match between recipient and donor | ||
| Female to male | 781 (20.6) | 456 (20.6) |
| Others | 3012 (79.4) | 1757 (79.4) |
| Recipient/donor CMV serostatus | ||
| Negative/Positive | 351 (9.3) | 256 (11.6) |
| Positive/Negative | 1367 (36.0) | 781 (35.3) |
| Positive/Positive | 2075 (54.7) | 1176 (53.1) |
| DRI | ||
| Low | 549 (14.5) | 0 (0.0) |
| Intermediate | 3046 (80.3) | 1927 (87.1) |
| High | 198 (5.2) | 286 (12.9) |
| Disease status | ||
| First complete remission | 2797 (73.7) | 1927 (87.1) |
| Second complete remission | 996 (26.3) | 286 (12.9) |
| HCT-CI | ||
| <2 | 2910 (76.7) | 1762 (79.6) |
| ≥2 | 883 (23.3) | 451 (20.4) |
| Donor source | ||
| HLA matched related | 1031 (27.2) | 663 (30.0) |
| HLA 1-antigen-mismatched related | 106 (2.8) | 52 (2.3) |
| HLA matched unrelated | 1070 (28.2) | 616 (27.8) |
| HLA mismatched unrelated | 978 (25.8) | 565 (25.5) |
| Umbilical cord blood | 402 (10.6) | 212 (9.6) |
| Haploidentical | 206 (5.4) | 105 (4.7) |
| Conditioning intensity | ||
| Myeloablative | 2863 (75.5) | 1669 (75.4) |
| Reduced intensity | 930 (24.5) | 544 (24.6) |
| GVHD prophylaxis | ||
| CSA-based | 1241 (32.7) | 779 (35.2) |
| TAC-based | 2552 (67.3) | 1434 (64.8) |
| In vivo T-cell depletion | ||
| No | 3489 (92.0) | 2055 (92.9) |
| Yes | 304 (8.0) | 158 (7.1) |
| Median y of HCT, (range) | 2013 (2006-2019) | 2013 (2006-2019) |
| Year of HCT | ||
| 2006-2012 | 1629 (42.9) | 976 (44.1) |
| 2013-2019 | 2164 (57.1) | 1237 (55.9) |
| Ph-chromosome | ||
| Ph-chromosome negative | - | 1283 (58.0) |
| Ph-chromosome positive | - | 930 (42.0) |
| Grades 2-4 Acute GVHD by day 65 | ||
| No | 2522 (66.5) | 1371 (62.0) |
| Yes | 1271 (33.5) | 842 (38.0) |
| Grades 3-4 acute GVHD by day 65 | ||
| No | 3463 (91.3) | 2015 (91.1) |
| Yes | 330 (8.7) | 198 (8.9) |
| CMV reactivation by day 65 | ||
| No | 1929 (50.9) | 1167 (52.7) |
| Yes | 1864 (49.1) | 1046 (47.3) |
DRI, disease risk index; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; CSA, cyclosporine; TAC, tacrolimus.
Impact of CMV reactivation on long-term outcomes
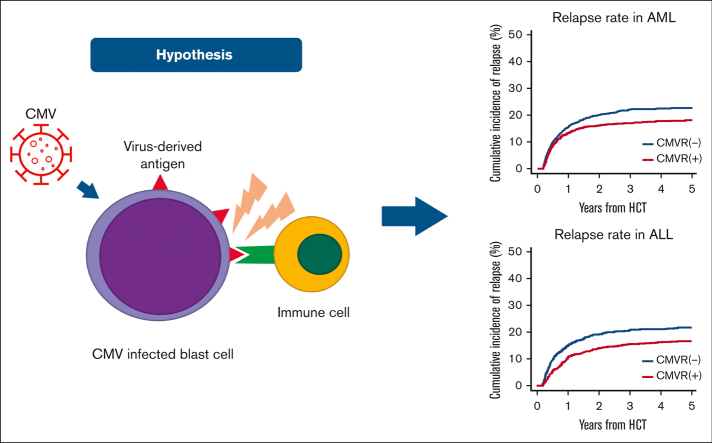
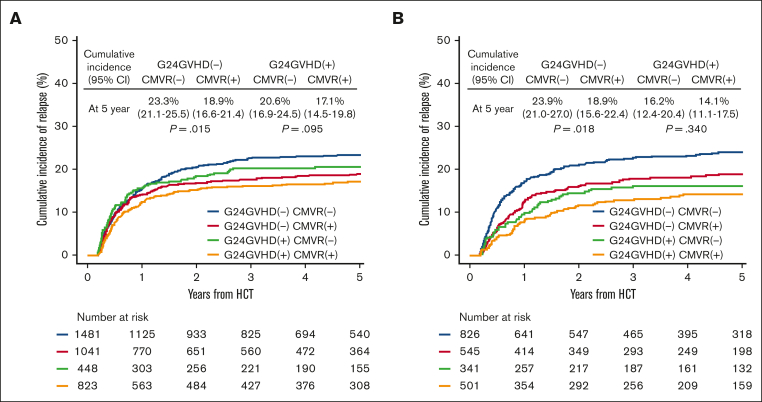
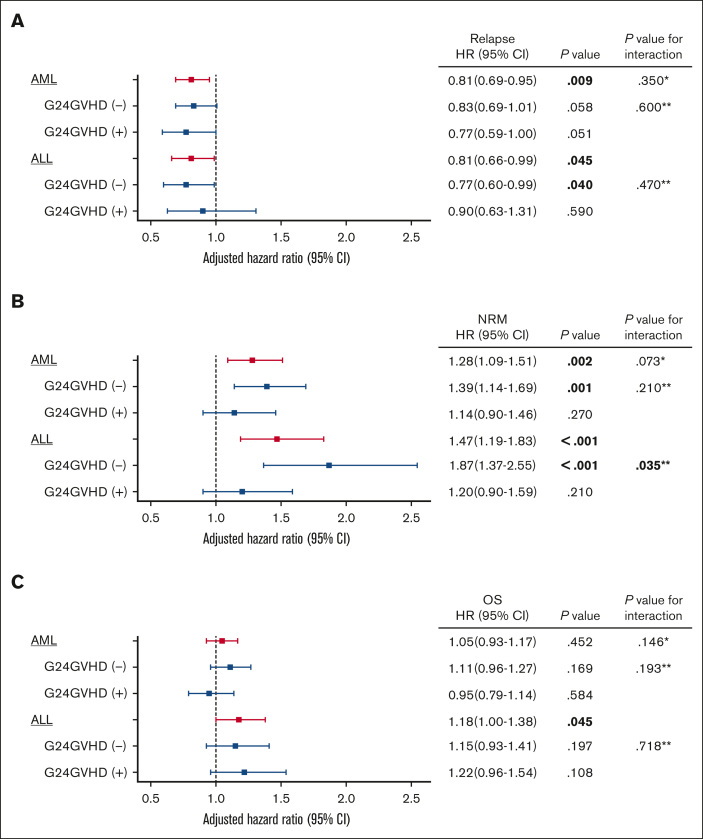
The cumulative incidences of relapse at 5 years in patients with and without CMVR were 22.6% (95% CI, 20.8%-24.6%) and 18.1% (95% CI, 16.4%-19.9%) in AML (P < .001), and 21.7% (95% CI, 19.3%-24.1%) and 16.6% (95% CI, 14.3%-19.0%) in ALL (P = .001), respectively (Figure 1). Sensitivity analyses using the 1 minus Kaplan-Meier method confirmed these findings (supplemental Figure 1). Because it is well known that the development of acute GVHD decreases the risk of relapse in acute leukemia,35, 36, 37, 38 we performed prespecified analyses stratified by the presence of G24GVHD. In univariate analyses, CMVR was associated with a decreased risk of relapse in patients without G24GVHD (AML, P = .015; ALL, P = .018), but this association was not statistically significant in patients with G24GVHD (AML, P = .095; ALL, P = .340) (Figure 2). In the multivariate analyses, CMVR was associated with a reduced risk of relapse in AML (hazard ratio [HR], 0.81; 95% CI, 0.69-0.95; P < .001) and ALL (HR, 0.81; 95% CI, 0.66-0.99; P < .001) (Table 2 and Figure 3). The interactions between G24GVHD and CMVR on relapse in AML and ALL were not statistically significant (AML, P = .600; ALL, P = .470) (Figure 3). These results were also confirmed when CMVR and G24GVHD were treated as time-dependent covariates in 3947 and 2292 patients with AML and ALL, respectively (supplemental Table 2). In contrast, when the landmark point was set at day 100 or day 180, CMVR was associated with the risk of relapse neither in AML (day 100: HR, 0.88; 95% CI, 0.74-1.05, P = .150; day 180: HR, 0.81; 95% CI, 0.65-1.01, P = .063) nor in ALL (day 100: HR, 0.88; 95% CI, 0.71-1.11, P = .290; day 180: HR, 0.96; 95% CI, 0.73-1.26, P = .760). This is because the landmark point on day 100 or day 180 excluded patients who developed relapse early after HCT, which resulted in an underestimation of the impact of CMVR on relapse.
Figure 1.
Cumulative incidence of relapse stratified according to CMV reactivation. The unadjusted cumulative incidence of relapse in patients with AML (A) and ALL (B). The landmark point was set at day 65.
Figure 2.
Cumulative incidence of relapse stratified according to CMV reactivation and grades 2 to 4 acute GVHD. The unadjusted cumulative incidence of relapse in patients with AML (A) and ALL (B). The landmark point was set at day 65.
Table 2.
Impact of CMV reactivation on outcomes in the multivariate analyses
| HR (95% CI) | P value | P value for interaction∗ | |
|---|---|---|---|
| Relapse | |||
| AML | .350 | ||
| CMV reactivation(−) | 1 | Ref | |
| CMV reactivation(+) | 0.81 (0.69-0.95) | .009 | |
| ALL | |||
| CMV reactivation(−) | 1 | Ref | |
| CMV reactivation(+) | 0.81 (0.66-0.99) | .045 | |
| NRM | |||
| AML | .073 | ||
| CMV reactivation(−) | 1 | Ref | |
| CMV reactivation(+) | 1.28 (1.09-1.51) | .002 | |
| ALL | |||
| CMV reactivation(−) | 1 | Ref | |
| CMV reactivation(+) | 1.47 (1.19-1.83) | <.001 | |
| OS | |||
| AML | .146 | ||
| CMV reactivation(−) | 1 | Ref | |
| CMV reactivation(+) | 1.05 (0.93-1.17) | .452 | |
| ALL | |||
| CMV reactivation(−) | 1 | Ref | |
| CMV reactivation(+) | 1.18 (1.00-1.38) | .045 |
All models were adjusted for recipient’s age, sex mismatch, CMV serological status, DRI, HCT-CI, donor source, GVHD prophylaxis, conditioning intensity, in vivo T-cell depletion, year of HCT, and grades 2-4 acute GVHD by day 65. In ALL, a positivity of Ph-chrosomosome was also included in the model.
Bold indicates statistical significance.
P value for the interaction between primary disease (AML vs ALL) and CMV reactivation.
Figure 3.
Impact of CMV reactivation on outcomes according to development of acute GVHD in multivariate analyses. Forest plots show the adjusted HR of CMV reactivation on relapse (A), nonrelapse mortality (B), and OS (C). All models were adjusted for recipient age, sex mismatch, CMV serological status, DRI, HCT-CI, donor source, GVHD prophylaxis, conditioning intensity, in vivo T-cell depletion, year of HCT, and grades 2-4 acute GVHD (G24GVHD) on day 65. In ALL, positivity of Ph-chromosome was also included in the model. Bold indicates statistical significance. ∗P value for the interaction between primary diseases (AML vs ALL) and CMV reactivation. ∗∗P value for the interaction between G24GVHD and CMV reactivation.
In the multivariate analyses, CMVR was associated with an increased risk of NRM in patients with AML (HR, 1.28; 95% CI, 1.09-1.51; P = .002) and ALL (HR, 1.47; 95% CI, 1.19-1.83; P < .001) (Table 2, Figure 3, and supplemental Figure 2). When stratified according to the development of G24GVHD, CMVR was associated with an increased risk of NRM in patients with AML and ALL without G24GVHD, but not in patients with G24GVHD (Figure 3 and supplemental Figure 3). Especially in ALL, we detected a significant interaction between G24GVHD and CMVR (P = .035).
On the other hand, in the multivariate analyses, CMVR was related to inferior OS in ALL (HR, 1.18; 95% CI, 1.00-1.38; P = .045), but not in AML (HR, 1.05; 95% CI, 0.93-1.17; P = .452) (Table 2, Figure 3, and supplemental Figure 4). When stratified according to the development of G24GVHD, there was no significant difference in the effect of CMVR on OS or interactions between G24GVHD and CMVR (Figure 3 and supplemental Figure 5).
Subgroup analysis
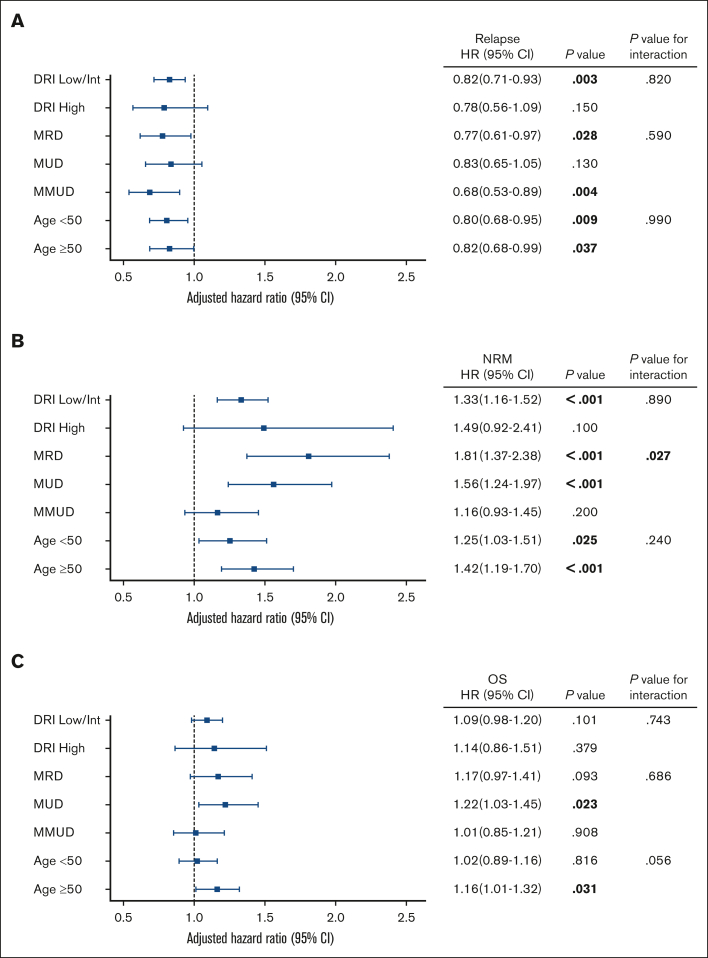
To determine the effect of CMVR modification on outcomes based on patient characteristics, we performed post-hoc analyses. Because of the similar effect of CMVR on relapse between AML and ALL (interaction P value between primary diasease and CMVR was 0.350), we combined patients with AML and ALL for subgroup analyses. To minimize multiple comparisons, we selected 5 subgroups with more than 1000 cases, in which we previously showed the potential effects of CMVR on NRM,14 in addition to DRI related to the risk of relapse. We did not adjust the threshold of statistical significance for multiple comparisons because of the exploratory nature of subgroup analyses.
In the multivariate analyses of the 7 subgroups, HRs on relapse varied within a limited range (0.68 to 0.83), and we did not observe any significant interactions (Figure 4). In contrast, HRs on NRM varied within a relatively wide range (from 1.16 to 1.81), and there was a significant interaction between CMVR and donor source on NRM (P = .027). The interaction between CMVR and recipient age on OS was borderline significant (P = .056).
Figure 4.
Impact of CMV reactivation on outcomes according to subgroups in multivariate analyses. Forest plots show the adjusted HR of CMV reactivation on relapse (A), nonrelapse mortality (B), and OS (C). Patients with AML and ALL were combined in all analyses. All models were adjusted for recipient age, sex mismatch, CMV serological status, DRI, HCT-CI, donor source, GVHD prophylaxis, conditioning intensity, in vivo T-cell depletion, year of HCT, and grades 2-4 acute GVHD by day 65. In ALL, positivity of Ph-chromosome was also included in the model. Bold indicates statistical significance. DRI, disease risk index; MRD, HLA matched related donors; MUD, HLA matched unrelated donors; MMUD, HLA mismatched unrelated donors.
Discussion
The suppression of relapse induced by CMVR, especially in patients with ALL is unclear due to the limited sample size of patients with ALL compared with patients with AML in previous studies.16,17 In this large retrospective study, we identified a statistically significant reduction of the risk of relapse with CMVR in both AML and ALL, but the effect size was not large (HR was 0.81 in each group). In addition, there were no significant effect modifications of CMVR on relapse depending on the development of acute GVHD or baseline characteristics, in contrast to NRM.
Because management after CMV has changed dramatically worldwide since the FDA approved letermovir,26,27,39,40 it is important to understand the impact of CMVR on relapse when using letermovir as CMV prophylaxis. Over the last decade, significant progress has been made in research regarding the immune control system during CMV exposure after HCT.41, 42, 43 Yeh et al. recently suggested that expanded CD57+/CD27- CD4+ cells during CMV exposure serve to eradicate CMV-infected antigen-presenting cells.44 Because CMV also infects AML and ALL malignant cells,25 these adopted immune cells induced by CMV exposure might eliminate CMV-infected leukemic cells in both AML and ALL. This hypothesis may also support the idea that delayed CMV-specific T-cell reconstitution after the use of letermovir45 impairs the protective effect of CMVR on relapse. Another possible mechanism of the antileukemic effect involves natural killer cells after CMV exposure.46, 47, 48, 49 Further investigations regarding the mechanism of the antileukemia effect of CMVR are warranted.
Although acute GVHD is a major cause of morbidity and mortality after HCT,50 acute GVHD itself contributes to a reduced risk of relapse.35, 36, 37, 38 The development of G24GVHD increases the subsequent risk of CMVR, and the presence of CMVR also increases the risk of G24GVHD.13,14 Therefore, we performed analyses stratified according to the development of G24GVHD. There was no significant effect modification of CMVR on relapse according to the development of G24GVHD, suggesting that the different targets of the GVL effect (virus-derived antigen and alloantigen) by CMVR and acute GVHD might induce independent antileukemia effects. In contrast, a heterogeneous effect of CMVR on NRM was observed according to the development of acute GVHD or baseline characteristics, and our study suggests that these harmful effects surpass the benefit of the modest antileukemia effect in some cases. Our findings emphasize the importance of considering the balance between the risk of relapse and NRM due to CMVR, based on the development of acute GVHD or baseline characteristics, when making a clinical decision regarding the prophylactic use of letermovir. For example, CMVR was associated with inferior OS in older patients but not in younger patients, suggesting that the use of letermovir might be more beneficial in older patients.
In Japan, pp65 antigenemia monitoring is still used, as opposed to the practice in most other countries. There was no significant difference in the preventive effect of CMV disease between ganciclovir prophylaxis and a pre-emptive strategy using antigenemia7 or between antigenemia and quantitative polymerase chain reaction (qPCR) monitoring.29 However, because the qPCR assay might show positive results earlier than antigenemia due to the higher sensitivity to CMVR,8,51 the timing of pre-emptive therapy might be different according to the detection technique used. Even in the qPCR assay, because there is no established threshold for starting pre-emptive therapy,8,40 the timing of pre-emptive therapy depends on physician or institutional preferences. Chen et al previously suggested that a low level of CMVR promotes CMV-specific T-cell reconstitution, which is inhibited by early pre-emptive therapy.52 Therefore, the discrepancy in the timing of pre-emptive therapy based on the detection assay and/or different cutoff values might affect the outcomes. Our findings need to be confirmed in other large-scale studies using qPCR assays with a unified threshold for treatment initiation.
This study has several limitations. First, minimal residual disease (MRD) and/or molecular profiles of tumor cells were not available for the majority of patients. Because the presence of MRD and high-risk molecular features are highly related to the subsequent risk of relapse,53, 54, 55, 56, 57, 58 future studies that incorporate these predictive factors for relapse instead of DRI are warranted. Second, we performed post hoc analyses in only 7 subgroups because of the limited sample size in each and to avoid multiple comparisons. P values were not corrected for multiple comparisons in exploratory analyses. Third, although the clearance speed of CMVR after pre-emptive therapy might affect the risk of relapse as well as NRM,59 the kinetics of CMVR are not available in our database.
In summary, CMVR was associated with a decreased risk of relapse in patients with ALL and AML. The effect of CMVR on relapse was modest and did not differ depending on the development of acute GVHD or baseline characteristics, in contrast to the effect of CMVR on NRM. Our findings will serve as the basis for future strategies to prevent CMVR.
Conflict-of-interest disclosure: H.N. has received honoraria from Pfizer and Takeda Pharmaceutical, outside the submitted work. K.T. and Y.K. received honoraria from Merck, Sharp & Dohme, outside the submitted work. S.K. received honoraria from Pfizer and Takeda Pharmaceutical, outside the submitted work. The remaining authors declare no competing financial interests.
Acknowledgments
The authors greatly appreciate the contributions of many physicians and data managers throughout the Japan Society for Transplantation and Cellular Therapy, the Japan Marrow Donor Program (JMDP), and the Japan Cord Blood Bank Network (JCBBN), who made this analysis possible. The authors thank the members of the Transplant Registry Unified Management Committees at the Japan Society for Transplantation and Cellular Therapy, JMDP, and JCBBN, for their dedicated management of data. Y. Akahoshi is a recipient of a Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad. H.N. received grants from the Japan Program for Infectious Diseases Research and Infrastructure from the Japan Agency of Medical Research and Development (AMED) (JP21wm0325046).
Authorship
Contribution: Y. Akahoshi designed the study, analyzed the data, and wrote the manuscript; H.N., K.T., S.Y., M.N., S.K., M.Y., and Y. Arai. reviewed and revised the manuscript; N.D., M.T., Y.O., N.U., T.A., H.N., S.O., M.O., and Y.S. provided important clinical data; J.T., T.F., Y.K., and Y. Atsuta collected the patient data; and all authors contributed to the writing of the manuscript and approved its final version.
Footnotes
Requests for data sharing may be submitted to the corresponding author, Yu Akahoshi (akahoshiu@gmail.com).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711–5719. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin N Am. 2011;25(1):151–169. doi: 10.1016/j.hoc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ljungman P, Brand R, Hoek J, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis. 2014;59(4):473–481. doi: 10.1093/cid/ciu364. [DOI] [PubMed] [Google Scholar]
- 4.Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9(9):543–558. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt-Hieber M, Labopin M, Beelen D, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013;122(19):3359–3364. doi: 10.1182/blood-2013-05-499830. [DOI] [PubMed] [Google Scholar]
- 6.Kanda Y, Mineishi S, Saito T, et al. Pre-emptive therapy against cytomegalovirus (CMV) disease guided by CMV antigenemia assay after allogeneic hematopoietic stem cell transplantation: a single-center experience in Japan. Bone Marrow Transplant. 2001;27(4):437–444. doi: 10.1038/sj.bmt.1702805. [DOI] [PubMed] [Google Scholar]
- 7.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88(10):4063–4071. [PubMed] [Google Scholar]
- 8.Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7) Lancet Infect Dis. 2019;19(8):e260–e272. doi: 10.1016/S1473-3099(19)30107-0. [DOI] [PubMed] [Google Scholar]
- 9.Einsele H, Ljungman P, Boeckh M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood. 2020;135(19):1619–1629. doi: 10.1182/blood.2019000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green ML, Leisenring W, Stachel D, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1687–1699. doi: 10.1016/j.bbmt.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ljungman P, Perez-Bercoff L, Jonsson J, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 2006;91(1):78–83. [PubMed] [Google Scholar]
- 12.Suessmuth Y, Mukherjee R, Watkins B, et al. CMV reactivation drives posttransplant T-cell reconstitution and results in defects in the underlying TCRbeta repertoire. Blood. 2015;125(25):3835–3850. doi: 10.1182/blood-2015-03-631853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantoni N, Hirsch HH, Khanna N, et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(9):1309–1314. doi: 10.1016/j.bbmt.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Akahoshi Y, Kimura SI, Inamoto Y, et al. Effect of cytomegalovirus reactivation with or without acute graft-versus-host disease on the risk of nonrelapse mortality. Clin Infect Dis. 2021;73(3):e620–e628. doi: 10.1093/cid/ciaa1871. [DOI] [PubMed] [Google Scholar]
- 15.Goodrich JM, Mori M, Gleaves CA, et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Engl J Med. 1991;325(23):1601–1607. doi: 10.1056/NEJM199112053252303. [DOI] [PubMed] [Google Scholar]
- 16.Green ML, Leisenring WM, Xie H, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122(7):1316–1324. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427–2438. doi: 10.1182/blood-2015-11-679639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takenaka K, Nishida T, Asano-Mori Y, et al. Cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation is associated with a reduced risk of relapse in patients with acute myeloid leukemia who survived to day 100 after transplantation: the Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol Blood Marrow Transplant. 2015;21(11):2008–2016. doi: 10.1016/j.bbmt.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Kaito S, Nakajima Y, Hara K, et al. Heterogeneous impact of cytomegalovirus reactivation on nonrelapse mortality in hematopoietic stem cell transplantation. Blood Adv. 2020;4(6):1051–1061. doi: 10.1182/bloodadvances.2019000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3(3):e119–e127. doi: 10.1016/S2352-3026(15)00289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118(5):1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 22.Turki AT, Tsachakis-Muck N, Leserer S, et al. Impact of CMV reactivation on relapse of acute myeloid leukemia after HCT is dependent on disease stage and ATG. Blood Adv. 2022;6(1):28–36. doi: 10.1182/bloodadvances.2021005509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koyama M, Hill GR. Alloantigen presentation and graft-versus-host disease: fuel for the fire. Blood. 2016;127(24):2963–2970. doi: 10.1182/blood-2016-02-697250. [DOI] [PubMed] [Google Scholar]
- 24.Rice GP, Schrier RD, Oldstone MB. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermouet S, Sutton CA, Rose TM, et al. Qualitative and quantitative analysis of human herpesviruses in chronic and acute B cell lymphocytic leukemia and in multiple myeloma. Leukemia. 2003;17(1):185–195. doi: 10.1038/sj.leu.2402748. [DOI] [PubMed] [Google Scholar]
- 26.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377(25):2433–2444. doi: 10.1056/NEJMoa1706640. [DOI] [PubMed] [Google Scholar]
- 27.Akahoshi Y, Kimura SI, Tada Y, et al. Cytomegalovirus gastroenteritis in patients with acute graft-versus-host disease. Blood Adv. 2022;6(2):574–584. doi: 10.1182/bloodadvances.2021005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atsuta Y. Introduction of transplant registry unified management program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data) Int J Hematol. 2016;103(1):3–10. doi: 10.1007/s12185-015-1894-x. [DOI] [PubMed] [Google Scholar]
- 29.Kanda Y, Yamashita T, Mori T, et al. A randomized controlled trial of plasma real-time PCR and antigenemia assay for monitoring CMV infection after unrelated BMT. Bone Marrow Transplant. 2010;45(8):1325–1332. doi: 10.1038/bmt.2009.337. [DOI] [PubMed] [Google Scholar]
- 30.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 34.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Storb R, Gyurkocza B, Storer BE, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31(12):1530–1538. doi: 10.1200/JCO.2012.45.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ringden O, Labopin M, Ciceri F, et al. Is there a stronger graft-versus-leukemia effect using HLA-haploidentical donors compared with HLA-identical siblings? Leukemia. 2016;30(2):447–455. doi: 10.1038/leu.2015.232. [DOI] [PubMed] [Google Scholar]
- 37.Yeshurun M, Weisdorf D, Rowe JM, et al. The impact of the graft-versus-leukemia effect on survival in acute lymphoblastic leukemia. Blood Adv. 2019;3(4):670–680. doi: 10.1182/bloodadvances.2018027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akahoshi Y, Igarashi A, Fukuda T, et al. Impact of graft-versus-host disease and graft-versus-leukemia effect based on minimal residual disease in Philadelphia chromosome-positive acute lymphoblastic leukemia. Br J Haematol. 2020;190(1):84–92. doi: 10.1111/bjh.16540. [DOI] [PubMed] [Google Scholar]
- 39.Ljungman P, Schmitt M, Marty FM, et al. A mortality analysis of letermovir prophylaxis for cytomegalovirus (CMV) in CMV-seropositive recipients of allogeneic hematopoietic cell transplantation. Clin Infect Dis. 2020;70(8):1525–1533. doi: 10.1093/cid/ciz490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hakki M, Aitken SL, Danziger-Isakov L, et al. American society for transplantation and cellular therapy series: #3-prevention of cytomegalovirus infection and disease after hematopoietic cell transplantation. Transplant Cell Ther. 2021;27(9):707–719. doi: 10.1016/j.jtct.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Degli-Esposti MA, Hill GR. Immune control of cytomegalovirus reactivation in stem cell transplantation. Blood. 2022;139(9):1277–1288. doi: 10.1182/blood.2020010028. [DOI] [PubMed] [Google Scholar]
- 42.Martins JP, Andoniou CE, Fleming P, et al. Strain-specific antibody therapy prevents cytomegalovirus reactivation after transplantation. Science. 2019;363(6424):288–293. doi: 10.1126/science.aat0066. [DOI] [PubMed] [Google Scholar]
- 43.Nakasone H, Kusuda M, Terasako-Saito K, et al. Features of repertoire diversity and gene expression in human cytotoxic T cells following allogeneic hematopoietic cell transplantation. Commun Biol. 2021;4(1):1177. doi: 10.1038/s42003-021-02709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeh AC, Varelias A, Reddy A, et al. CMV exposure drives long-term CD57+ CD4 memory T-cell inflation following allogeneic stem cell transplant. Blood. 2021;138(26):2874–2885. doi: 10.1182/blood.2020009492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zamora D, Duke ER, Xie H, et al. Cytomegalovirus-specific T-cell reconstitution following letermovir prophylaxis after hematopoietic cell transplantation. Blood. 2021;138(1):34–43. doi: 10.1182/blood.2020009396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fletcher JM, Prentice HG, Grundy JE. Natural killer cell lysis of cytomegalovirus (CMV)-infected cells correlates with virally induced changes in cell surface lymphocyte function-associated antigen-3 (LFA-3) expression and not with the CMV-induced down-regulation of cell surface class I HLA. J Immunol. 1998;161(5):2365–2374. [PubMed] [Google Scholar]
- 47.Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119(11):2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Della Chiesa M, Falco M, Muccio L, Bertaina A, Locatelli F, Moretta A. Impact of HCMV infection on NK cell development and function after HSCT. Front Immunol. 2013;4:458. doi: 10.3389/fimmu.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Della Chiesa M, Falco M, Podesta M, et al. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus? Blood. 2012;119(2):399–410. doi: 10.1182/blood-2011-08-372003. [DOI] [PubMed] [Google Scholar]
- 50.Ferrara JLM, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boeckh M, Gallez-Hawkins GM, Myerson D, Zaia JA, Bowden RA. Plasma polymerase chain reaction for cytomegalovirus DNA after allogeneic marrow transplantation: comparison with polymerase chain reaction using peripheral blood leukocytes, pp65 antigenemia, and viral culture. Transplantation. 1997;64(1):108–113. doi: 10.1097/00007890-199707150-00020. [DOI] [PubMed] [Google Scholar]
- 52.Chen GL, Wallace PK, Zhang Y, et al. Low-level cytomegalovirus antigenemia promotes protective cytomegalovirus antigen-specific T cells after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2020;26(11):2147–2154. doi: 10.1016/j.bbmt.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131(12):1275–1291. doi: 10.1182/blood-2017-09-801498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Dongen JJM, van der Velden VHJ, Bruggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood. 2015;125(26):3996–4009. doi: 10.1182/blood-2015-03-580027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akahoshi Y, Arai Y, Nishiwaki S, et al. Minimal residual disease (MRD) positivity at allogeneic hematopoietic cell transplantation, not the quantity of MRD, is a risk factor for relapse of Philadelphia chromosome-positive acute lymphoblastic leukemia. Int J Hematol. 2021;113(6):832–839. doi: 10.1007/s12185-021-03094-x. [DOI] [PubMed] [Google Scholar]
- 56.Dohner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–1377. doi: 10.1182/blood.2022016867. [DOI] [PubMed] [Google Scholar]
- 57.Pfeifer H, Raum K, Markovic S, et al. Genomic CDKN2A/2B deletions in adult Ph(+) ALL are adverse despite allogeneic stem cell transplantation. Blood. 2018;131(13):1464–1475. doi: 10.1182/blood-2017-07-796862. [DOI] [PubMed] [Google Scholar]
- 58.Paietta E, Roberts KG, Wang V, et al. Molecular classification improves risk assessment in adult BCR-ABL1-negative B-ALL. Blood. 2021;138(11):948–958. doi: 10.1182/blood.2020010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takeshita J, Kimura SI, Nakasone H, et al. Association between the kinetics of cytomegalovirus reactivation in terms of the area under the curve of cytomegalovirus antigenemia and non-relapse mortality after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2021;23(5) doi: 10.1111/tid.13715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.