Abstract
Background
Skin pigmentation after adrenalectomy occurs due to an increase in adrenocorticotropic hormone (ACTH) following adrenal insufficiency. ACTH-induced pigmentation usually appears as generalized hyperpigmentation and is known to appear after bilateral adrenalectomy. We report a case of unusual transient hyperpigmentation that developed immediately after unilateral adrenalectomy for pheochromocytoma and spontaneously resolved without corticosteroid supplementation.
Case Description
A 29-year-old woman was admitted to the hospital due to sudden-onset chest pain and headache. A 2.8-cm left adrenal mass with heterogeneous enhancement was incidentally found in chest computed tomography during the evaluation. Multiple old infarctions were observed in brain magnetic resonance imaging (MRI), and left ventricular thrombi were found by echocardiography. Biochemical evidence confirmed the diagnosis of pheochromocytoma, while serum ACTH and cortisol levels were within normal ranges. The patient underwent laparoscopic left adrenalectomy via a posterior retroperitoneal approach and recovered without immediate postoperative complications. On day 3 after surgery, a crescent-shaped café-au-lait skin pigmentation occurred on both the subcostal and the lumbar areas of the abdomen. Serial serum cortisol slightly decreased during the immediate postoperative period and recovered on day 3. Serum ACTH was elevated. Under close observation without corticosteroid supplementation, the pigmentation faded on day 8 after surgery. On day 15, the pigmentation clearly disappeared and serum ACTH decreased to within the normal range. A month later, ACTH and all adrenal hormones were within normal range.
Conclusions
We hypothesized that skin pigmentation appeared due to an imbalance of the hypothalamic-pituitary-adrenal (HPA) axis after resection of one adrenal gland. Skin pigmentation may be the first and early manifestation of adrenal insufficiency in patients who undergo unilateral adrenalectomy due to a non-Cushing’s tumor. Therefore, a careful physical examination may allow early detection of adrenal insufficiency and optimal treatment planning.
Keywords: Adrenocorticotropin, pigmentation, adrenalectomy, pheochromocytoma, case report
Highlight box.
Key findings
• We report a case of skin pigmentation after unilateral adrenalectomy in pheochromocytoma.
What is known and what is new?
• ACTH-induced hyperpigmentation is generally known to occur in patients with adrenal insufficiency who do not receive adequate corticosteroid replacement after bilateral adrenalectomy.
• In our case, hyperpigmentation was also observed after unilateral adrenalectomy for a non-Cushing’s tumor.
What is the implication, and what should change now?
• Because atypical skin pigmentation may appear as the first clinical symptom of adrenal insufficiency after adrenalectomy for a non-Cushing tumor, clinicians presented with acute-onset hyperpigmentation following unilateral adrenalectomy should consider adrenal insufficiency.
Introduction
Hyperpigmentation is generally known to occur in patients with adrenal insufficiency who do not receive adequate corticosteroid replacement after bilateral adrenalectomy (1,2). Unilateral adrenalectomy is not generally considered a factor that increases the risk of adrenal insufficiency in patients with normal adrenocortical function, because the reserve function of the remaining adrenal gland maintains homeostasis (1,3). Postoperative adrenal insufficiency after unilateral adrenalectomy has been reported in up to 22–33% of patients without preoperative hypothalamic-pituitary-adrenal (HPA) axis imbalance, which is not uncommon (4,5).
We share a rare case of a patient who showed unusual hyperpigmentation due to adrenal insufficiency following unilateral adrenalectomy for pheochromocytoma. We present this case in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-521/rc).
Case presentation
A 29-year-old woman was admitted to the hospital due to sudden onset chest pain and headache. She complained of a chronic headache that had been present for 1 year. During the examination, a 2.8-cm well-defined round mass on the left adrenal gland was discovered incidentally on chest and abdominal computed tomography (Figure 1). Additionally, a brain magnetic resonance imaging (MRI) and echocardiography found acute infarctions of the left superior frontal gyrus and left ventricular thrombus. Anticoagulant was started. Serum aldosterone, aldosterone/renin ratio, adrenocorticotropic hormone (ACTH), and cortisol levels were within normal range. Twenty-four-hour urinary metanephrine and catecholamine increased, and pheochromocytoma was diagnosed (Table 1). The patient had no family history of pheochromocytoma. Blood pressure was well controlled with 2 mg terazosin before surgery. The patient underwent a laparoscopic left adrenalectomy with a posterior retroperitoneal approach. After the operation, massive hydration and norepinephrine infusion were used to maintain blood pressure on the day of surgery and gradually tapered on the first day after surgery. The patient’s condition gradually improved, and symptoms returned to normal. The histologic examination showed a 3.0-cm pheochromocytoma without tumor necrosis or vascular, lymphatic, or capsular invasion. The pheochromocytoma of the adrenal gland scaled score (PASS) was 0 (Figure 2).
Figure 1.

A horizontal abdominal and pelvic computed tomography image showing a left adrenal mass.
Table 1. 24-h urinary metanephrine and catecholamine measurements.
| Laboratory test | Preoperative (µg/24 h) |
Postoperative (2 weeks) (µg/24 h) |
Reference range (µg/24 h) |
|---|---|---|---|
| 24-h urinary metanephrine | 1,279.8 | 79.2 | <320.1 |
| 24-h urinary normetanephrine | 619.4 | 212.4 | <390.1 |
| 24-h urinary epinephrine | 106.21 | 3.00 | <27.1 |
| 24-h urinary norepinephrine | 106.93 | 37.43 | <97.1 |
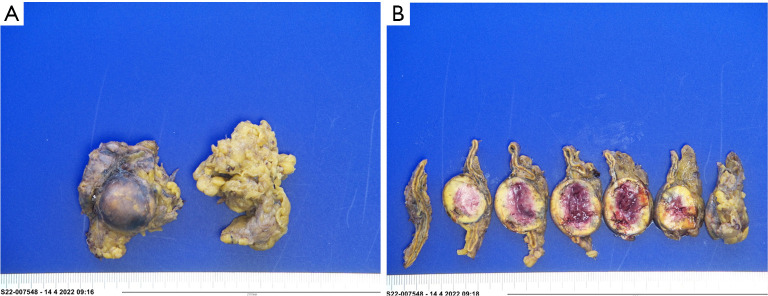
Figure 2.
Gross features of the adrenal specimen.
Patient was discharged on day 3 following surgery with no clinical symptoms and normal blood tests (complete blood count and renal panel). At the time of discharge, on postoperative day 3, a crescent-shaped café-au-lait skin pigmentation occurred on both the hypochondriac area and the lumbar area of the abdomen. The patient complained of mild general weakness. The café-au-lait bilateral spots were symmetric on the hypochondriac and lumbar areas of the abdomen. The pigmentation was flat and not associated with exposure to sunlight (Figure 3). On hormonal evaluation, the postoperative cortisol levels were decreased on the morning of day 1 (8.62 µg/dL; reference range, 9.41–26.06 µg/dL) and returned to normal levels on the day of discharge (13.68 µg/dL). Studies to assess possible imbalance of adrenal hormones revealed that serum ACTH was mildly elevated on the day pigmentation was detected (75.70 pg/mL; reference range, 10.0–60.0 pg/mL).
Figure 3.
Pre- and postoperative photographs show the abdominal skin appearance. (A) No skin pigmentation was observed before adrenalectomy. (B) The café-au-lait bilateral spots appeared to be symmetrical on the hypochondriac and lumbar areas of the abdomen on day 3 after the operation. (C) The skin pigmentation faded slightly on day 8 after the operation and (D) disappeared completely by day 15.
In an outpatient visit on day 8 after surgery, the skin pigmentation had faded slightly. The serum ACTH level was still elevated (64.06 pg/mL), but the cortisol level was within the normal range (9.95 µg/dL). At 10 days after the operation, the ACTH level had increased (86.83 pg/mL), but the serum cortisol level was within the normal range (16.95 µg/dL). On day 15 after surgery, the skin pigmentation had disappeared completely, and the ACTH level had decreased to within the normal range (44.72 pg/mL). At that time, the cortisol level was 5.36 µg/dL, which was lower than the normal range. One month after surgery, ACTH and cortisol levels were normal (59.05 pg/mL and 11.38 µg/dL, respectively) (Table 2), and the synacthen stimulation test (SST) revealed no adrenal insufficiency (Table 3). Twenty-four-hour urinary metanephrine and catecholamine became normal 2 weeks after surgery.
Table 2. Serum ACTH and cortisol levels.
| Cortisol (µg/dL) | ACTH (pg/mL) | |
|---|---|---|
| Reference range | Morning: 9.41–26.06 | 10.0–60.0 |
| Preoperative | 15.63 | 15.73 |
| Postop day 3 | 13.68 | 75.70 |
| Postop day 8 | 9.95 | 64.06 |
| Postop day 10 | 16.95 | 86.83 |
| Postop day 15 | 5.36 | 44.72 |
| Postop day 30 | 11.38 | 59.05 |
ACTH, adrenocorticotropic hormone.
Table 3. Synacthen stimulation test at 1-month postoperative.
| Cortisol (µg/dL) | Aldosterone (pg/mL) | |
|---|---|---|
| Reference range | Morning: 9.41–26.06 | 41.71–208.90 |
| 0 minute | 11.38 | 125.5 |
| 30 minutes | 25.79 | 173.6 |
| 60 minutes | 27.06 | 142.4 |
| 90 minutes | 29.50 | 140.6 |
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Postoperative adrenal insufficiency occurs when adrenalectomy is performed for an autonomously cortisol-secreting tumor, such as Cushing’s syndrome, which secretes cortisol and suppresses the HPA axis (2,3,6). Therefore, corticosteroids should be supplied after adrenalectomy for patients with Cushing’s syndrome to prevent adrenal insufficiency (2,3,6). In contrast, non-Cushing’s syndrome such as pheochromocytoma and primary aldosteronism do not usually require exogenous steroid supplementation after unilateral adrenalectomy, because the HPA axis is not suppressed and the healthy residual adrenal gland maintain homeostasis by producing adequate cortisol (6,7).
However, some researchers have reported that adrenal insufficiency can occur after unilateral adrenal resection for non-Cushing’s syndrome (1,8). The pathophysiology of adrenal insufficiency after unilateral adrenalectomy has not yet been fully identified. One hypothesis is that the contralateral normal adrenal gland may be unable to compensate initially after unilateral adrenalectomy because of decreased adrenocortical reserve (3). Yokoyama et al. reported that patients who underwent nephrectomy with unilateral adrenalectomy for renal cell carcinoma had significantly elevated ACTH levels and lower serum cortisol levels compared with those who underwent nephrectomy alone (9). Honda et al. reported that basal ACTH levels were elevated after unilateral adrenalectomy for non-Cushing’s syndrome, although basal cortisol levels were within normal levels, and the ACTH stimulation test indicated that adrenal reserve function was significantly reduced (10). These findings showed that patients with decreased adrenal reserve function after surgery had increased ACTH secretion from the pituitary gland to compensate for adrenal insufficiency (3,10-12). Although very rare, dual hormone secreting pheochromocytoma with ACTH should also be considered. We will also consider the ACTH immunohistochemistry whether the phenomenon is caused by co-secretion of pheochromocytoma (13,14).
As a compensatory action for hypocortisolism, the secretion of proopiomelanocortin (POMC), a precursor of ACTH, is dramatically increased (3,10). Because POMC is a common precursor of both ACTH and melanocyte-stimulating hormones (MSH), the production of MSH also increased simultaneously in the anterior pituitary (3,10). POMC, ACTH and MSH have potent melanogenesis, and their increase causes hyperpigmentation (2,11). Therefore, hyperpigmentation can be considered a clinical manifestation of adrenal insufficiency (2,11,15,16).
When café-au-lait skin pigmentation occurs in a patient, should be considered including neurofibromatosis type 1 (NF1). The typical café-au-lait skin pigmentation seen in NF1 is round or oval shape, which is different from our patient’s crescent shape. Also, most of the skin pigmentation of NF1 occurred at a young age, but in our case, pigmentation occurred after the event of surgery and was disappeared, NF1 can be clearly excluded as a causative disease (17). There are many patterns of pigmentation related to hormonal disturbance. Pigmentation usually appears as generalized hyperpigmentation, which is more pronounced in areas exposed to sunlight, such as face, neck, and arms (3,16). In our case, skin pigmentation appeared with a focal symmetrical pattern on day 3 post adrenalectomy. It occurred independently of sun exposure and on the trunk rather than the distal parts. It also did not belong to any classification of the lines of Blaschko (18). In laboratory tests, the serum cortisol level decreased slightly immediately after surgery and then increased to within the normal range. ACTH was temporarily elevated for compensation on the days when pigmentation was observed. This atypical skin pigmentation completely disappeared 2 weeks after surgery, coinciding with serum ACTH normalization.
Because of compensatory action, most adrenal insufficiency that occurs after unilateral adrenalectomy is not obvious and is overlooked. Therefore, a diagnostic tool of postoperative adrenal insufficiency is important. The most well-known standard method is the cosyntropin test. However, according to a recent study by Zama and colleagues, cortisol cut offs used in the cosyntropin test for predicting adrenal insufficiency after unilateral adrenalectomy for non-cortisol secreting adrenal tumors can be inaccurate (19). Ortiz et al. showed that routine cortisol testing at 8:00 a.m. after surgery was helpful in diagnosing postoperative adrenal insufficiency and determining steroid replacement (20). The use of routine steroid replacement following adrenalectomy in non-Cushing’s adenoma or subclinical Cushing’s is controversial. Shen et al. showed that steroid replacement is not required when adrenal insufficiency after unilateral adrenalectomy for non-Cushing’s syndrome is not severe (6). In contrast, other researchers recommended considering precautionary steroid substitutive therapy according to the size of the adrenal mass (>4 cm or increasing >1 cm/year) after unilateral adrenalectomy, even if cortisol secretion is normal before surgery (4).
Eller-Vainicher et al. investigated various preoperative parameters to predict postsurgical adrenal insufficiency; however, no definitive parameter can perfectly predict the probability of postsurgical hypocortisolism (4). Furthermore, the SST, known as the most widely used test to diagnosis adrenal insufficiency, tends to overestimate adrenal insufficiency following unilateral adrenalectomy and may lead to unnecessary steroid replacement therapy (19). Because there are no definitive biochemical parameters for diagnosing adrenal insufficiency using blood or urine tests, clinicians must perform a close examination to diagnose and select a group of patients who need steroid treatment.
In this case, we report atypical skin pigmentation as the first clinical manifestation of adrenal insufficiency after adrenalectomy for pheochromocytoma. If hyperpigmentation occurs immediately after unilateral adrenalectomy of a non-Cushing’s adrenal tumor, clinicians should monitor ACTH levels, which may become elevated because of reduced adrenal reserve function and hypocortisolism. Additionally, the adrenal function of the patient should be thoroughly observed to determine whether corticosteroid supplementation is necessary.
Conclusions
Here, we report a case of temporary adrenal insufficiency following unilateral adrenalectomy presenting as unusual acute-onset skin hyperpigmentation caused by increased ACTH secretion. Based on our experience, clinicians presented with acute-onset hyperpigmentation following unilateral adrenalectomy should consider adrenal insufficiency, a possible complication of unilateral adrenalectomy that is not as rare as previously thought.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-521/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-521/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-521/coif). The authors have no conflicts of interest to declare.
References
- 1.Yoshiji S, Shibue K, Fujii T, et al. Chronic primary adrenal insufficiency after unilateral adrenonephrectomy: A case report. Medicine (Baltimore) 2017;96:e9091. 10.1097/MD.0000000000009091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence GD, Bravo E, Bartter FC. Hyperpigmentation on cortisone after adrenalectomy for primary aldosteronism. Arch Intern Med 1974;134:734-7. 10.1001/archinte.1974.00320220136018 [DOI] [PubMed] [Google Scholar]
- 3.Raffaelli M, De Crea C, D'Amato G, et al. Outcome of adrenalectomy for subclinical hypercortisolism and Cushing syndrome. Surgery 2017;161:264-71. 10.1016/j.surg.2016.07.042 [DOI] [PubMed] [Google Scholar]
- 4.Eller-Vainicher C, Morelli V, Salcuni AS, et al. Post-surgical hypocortisolism after removal of an adrenal incidentaloma: is it predictable by an accurate endocrinological work-up before surgery? Eur J Endocrinol 2010;162:91-9. 10.1530/EJE-09-0775 [DOI] [PubMed] [Google Scholar]
- 5.Mitchell J, Barbosa G, Tsinberg M, et al. Unrecognized adrenal insufficiency in patients undergoing laparoscopic adrenalectomy. Surg Endosc 2009;23:248-54. 10.1007/s00464-008-0189-1 [DOI] [PubMed] [Google Scholar]
- 6.Shen WT, Lee J, Kebebew E, et al. Selective use of steroid replacement after adrenalectomy: lessons from 331 consecutive cases. Arch Surg 2006;141:771-4; discussion 774-6. 10.1001/archsurg.141.8.771 [DOI] [PubMed] [Google Scholar]
- 7.Riché F, Laisné MJ, Alves A. Corticosteroid insufficiency in acutely ill patients. N Engl J Med 2003;348:2157-9. 10.1056/NEJM200305223482123 [DOI] [PubMed] [Google Scholar]
- 8.Heinrich DA, Adolf C, Holler F, et al. Adrenal Insufficiency After Unilateral Adrenalectomy in Primary Aldosteronism: Long-Term Outcome and Clinical Impact. J Clin Endocrinol Metab 2019;104:5658-64. 10.1210/jc.2019-00996 [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama H, Tanaka M. Incidence of adrenal involvement and assessing adrenal function in patients with renal cell carcinoma: is ipsilateral adrenalectomy indispensable during radical nephrectomy? BJU Int 2005;95:526-9. 10.1111/j.1464-410X.2005.05332.x [DOI] [PubMed] [Google Scholar]
- 10.Honda K, Sone M, Tamura N, et al. Adrenal reserve function after unilateral adrenalectomy in patients with primary aldosteronism. J Hypertens 2013;31:2010-7. 10.1097/HJH.0b013e3283635789 [DOI] [PubMed] [Google Scholar]
- 11.Marchand L, Lecus A, Lapoirie M, et al. Dramatic change in skin color after bilateral adrenalectomy in Cushing's disease. Ann Endocrinol (Paris) 2016;77:623-4. 10.1016/j.ando.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 12.Giordano R, Balbo M, Picu A, et al. Corticotrope hypersecretion coupled with cortisol hypo-responsiveness to stimuli is present in patients with autoimmune endocrine diseases: evidence for subclinical primary hypoadrenalism? Eur J Endocrinol 2006;155:421-8. 10.1530/eje.1.02222 [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Lian P, Su M, et al. Single-cell transcriptome analysis identifies a unique tumor cell type producing multiple hormones in ectopic ACTH and CRH secreting pheochromocytoma. Elife 2021;10:e68436. 10.7554/eLife.68436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjoeholm A, Li C, Leem C, et al. Adrenal insufficiency in a child following unilateral excision of a dual-hormone secreting phaeochromocytoma. Endocrinol Diabetes Metab Case Rep 2015;2015:150041. 10.1530/EDM-15-0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon HR, Won CH, Chang SE, et al. Generalised hyperpigmentation caused by ectopic adrenocorticotropic hormone syndrome with recurrent thymic neuroendocrine carcinoma. Australas J Dermatol 2015;56:131-3. 10.1111/ajd.12195 [DOI] [PubMed] [Google Scholar]
- 16.Shekhar S, Dharmshaktu P. On the Palms of His Hands: ACTH-Induced Hyperpigmentation. Am J Med 2018;131:144-5. 10.1016/j.amjmed.2017.10.037 [DOI] [PubMed] [Google Scholar]
- 17.Ozarslan B, Russo T, Argenziano G, et al. Cutaneous Findings in Neurofibromatosis Type 1. Cancers (Basel) 2021;13:463. 10.3390/cancers13030463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kromann AB, Ousager LB, Ali IKM, et al. Pigmentary mosaicism: a review of original literature and recommendations for future handling. Orphanet J Rare Dis 2018;13:39. 10.1186/s13023-018-0778-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaman S, Almazrouei R, Sam AH, et al. Synacthen Stimulation Test Following Unilateral Adrenalectomy Needs to Be Interpreted With Caution. Front Endocrinol (Lausanne) 2021;12:654600. 10.3389/fendo.2021.654600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortiz DI, Findling JW, Carroll TB, et al. Cosyntropin stimulation testing on postoperative day 1 allows for selective glucocorticoid replacement therapy after adrenalectomy for hypercortisolism: Results of a novel, multidisciplinary institutional protocol. Surgery 2016;159:259-65. 10.1016/j.surg.2015.05.034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as