Abstract
Burn wound infection is the leading cause of mortality among burn wound patients. One of the most commonly isolated bacterial burn wound pathogens is Pseudomonas aeruginosa , a notorious nosocomial multidrug-resistant pathogen. As a consequence of its recalcitrance to frontline antibiotic therapy, there is an urgent need to develop alternative treatment avenues to tackle this pathogen. One potential alternative infection prevention measure is to seed the wound bed with probiotic bacteria. Several species of Lactobacillus, a common commensal bacterium, have been previously reported to display growth inhibition activity against wound pathogens. Various species of this genus have also been shown to augment the wound healing process, which makes it a promising potential therapeutic agent. Due to the complexity of the burn wound trauma and burn wound infection, an in vivo model is required for the development of novel therapeutics. There are multiple in vivo models that are currently available, the most common among them being the murine model. However, mammalian burn wound infection models are logistically challenging, do not lend themselves to screening approaches and come with significant concerns around ethics and animal welfare. Recently, an invertebrate burn wound and infection model using G. mellonella has been established. This model addresses several of the challenges of more advanced animal models, such as affordability, maintenance and reduced ethical concerns. This study validates the capacity of this model to screen for potential wound probiotics by demonstrating that a variety of Lactobacillus spp. can limit P. aeruginosa burn wound infection and improve survival.
Keywords: burn wound, burn infection, burn wound model, Galleria mellonella, Pseudomonas aeruginosa, probiotic, Lactobacillus
Introduction
Burn wounds can cause significant damage to the integrity of the skin, exposing the affected individual to potential pathogens while also increasing local fluid loss [1]. As a consequence, more than 250 000 deaths worldwide are attributed to fire-induced burns alone, with a vast majority of those taking place in developing countries, where mortality among patients with 40 % of total body surface area (TBSA) reaches 100% [2]. Burn wound infection is the most prevalent complication of burn wound care and is the leading cause of mortality among burn wound patients [3]. In addition, infection can delay healing and lead to autograft failure ultimately resulting in longer treatment courses and hospital stays. This puts a significant burden on healthcare systems, which makes burn research a priority. In 2012–2013 UK’s National Health Service (NHS) was estimated to have managed nearly 90 000 burn injuries resulting in a £90 million cost [4]. One of the most frequently isolated bacteria in burn wounds is Pseudomonas aeruginosa , which is a Gram-negative opportunistic biofilm-forming pathogen [5]. P. aeruginosa is a highly virulent pathogen associated with high mortality rates and frequent outbreaks in burn ICUs [6–8]. The recalcitrance of this pathogen to front-line antibiotic therapy and its potential for causing localized outbreaks in burn treatment centres means novel therapeutic strategies are urgently needed to tackle this pathogen [9].
Several commensal probiotic bacteria, which are present on human skin, have been studied in the context of the burn wound healing and infection treatment. One of the most well-known and established probiotic bacteria is Lactobacillus (some species have been renamed to Lactiplantibacillus spp. and Limosilactobacillus spp.). Lactiplantibacillus plantarum (previously known as Lactobacillus plantarum ) has been shown to decrease the bacterial load in infected burn wounds and improve wound healing in humans [10]. It has also been linked to reducing scar formation in the rabbit burn wound and infection model. L. plantarum probiotic therapy reduced the ability of P. aeruginosa to establish and maintain colonization of the wound and improved the skin restoration at the wound site [11]. Lactobacillus spp. populated alginate gels have also been used to prevent burn wound infection in rats [12]. Improved survival after Lactobacillus acidophilus and Limosilactobacillus reuteri (previously known as Lactobacillus reuteri ) treatment has been observed in Acinetobacter baumannii infections in the mouse burn wound model [13]. L. reuteri has also exhibited protective properties towards epidermal keratinocytes in a Staphylococcus aureus ex vivo burn wound infection [14]. Limosilactobacillus fermentum (previously known as Lactobacillus fermentum ) has also been shown to reduce the bioburden of P. aeruginosa in the murine burn wound infection model [15]. Overall, Lactobacillus spp. is one of the most promising candidates for probiotic-based therapies for burn wound infection. However, the further pre-clinical development of potential probiotic burn wound prophylaxis or treatment is being stymied by the complexities of conducting in vivo burn wound research. This is also limiting further insights into the underlying molecular mechanisms of how Lactobacillus spp. can prevent or limit burn wound infection.
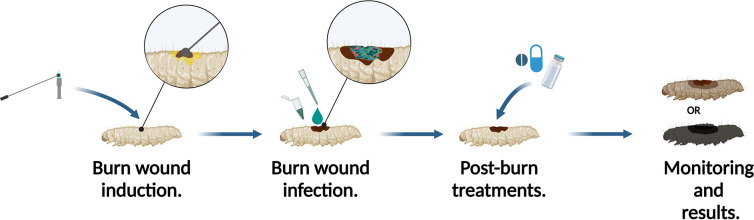
Compared to more conventional wounds such as lacerations, the nature of burn wounds is complex due to the associated multi-system damage; as a result, they are impossible to accurately recreate and study in vitro [16]. Multiple in vivo models have been established to study the burn wound and infection, one of the most widely used models being the murine model. The other in vivo models include porcine, canine and rabbit ear model [17–19]. These models provide invaluable insights into the burn physiology and pathology and are essential for the generation of robust pre-clinical data. These models have several advantages to them, ranging from body system similarities to the versatility of the model organism. However, they have a few disadvantages in common such as (1) they are associated with a high level of ethical and animal welfare concerns; (2) the size of the experimental cohort is very limited; (3) they are costly; (4) they are not suitable for screening-based approaches. In addition to that, the burn wound injury is a severe and morbid condition, which is very distressing to the animal [20]. This creates additional hurdles for researchers and therefore limits in vivo burn wound research. Recently, an invertebrate burn wound, and infection model has been established using Galleria mellonella [21] (Fig. 1). G. mellonella is a robust animal model that has gained significant popularity in the last decade among researchers. It has been firmly established for use in drug toxicity and virulence assays for a wide range of pathogens [22–24]. The G. mellonella burn wound model follows important hallmarks of burn wound trauma and infection, for example, the decrease in survival with the increase of the burn surface area and a significant decrease in survival after topical burn wound infection. Using this invertebrate in burn research addresses several hurdles presented by larger mammalian models such as affordability, cohort sizes, and the ethical and welfare concerns and the need for ethical approval [25]. It also enables high-throughput screening, which is not possible with mammalian burn wound models.
Fig. 1.
Schematic representation of G. mellonella burn wound and infection model. The burn is induced by applying a heated metal element to the back of the G. mellonella. Shortly after the burn wound can be topically infected with a chosen micro-organism. Any treatments can be applied shortly after the procedure. The larvae are then incubated and monitored to observe the survival rates. (Adapted from Maslova et al., 2021 [21].)
This study validates G. mellonella as a model for studying probiotic treatments in a burn wound infection, as well as demonstrating that this model can facilitate inoculation with multiple micro-organisms. This will help fast track the development of novel probiotic-based wound solutions by enabling high-throughput screening of potential candidate probiotics as well as mechanism of action studies. The use of this model in probiotic wound therapeutic development will also help to refine and reduce the number of mammals used in downstream studies.
Methods
Bacterial strains and inoculum preparation
Strains of Lactobacillus spp. L. reuteri CCUG44144, L. casei CCUG2145T, L. jensenii CCUG35572, L. reuteri CCUG33624, L. fermentum CCUG30138, Lactobacillus crispatus CCUG42898 and Lactobacillus gasseri CCUG44046 purchased from Culture Collection University of Gothenburg) and P. aeruginosa PA14 were stored as 20 % glycerol stocks at −80 °C until required [21]. P. aeruginosa was inoculated into a universal 30 ml tube with 5 ml of lysogeny broth and incubated overnight at 37 °C at 180 r.p.m. until it reached OD600=~3.0. Lactobacillus strains were inoculated into 50 ml centrifuge tubes and Petri dishes with De Man, Rogosa and Sharpe (MRS) nutrient media. The cultures were placed into a hermetic chamber with an anaerobe gas generation sachet and incubated anaerobically for 48–72 h at 37 °C until the liquid cultures reached OD600=~1.5.
Preparation of cell-free Lactobacillus spp. supernatant
Lactobacillus spp. 48–72 h liquid cultures were centrifuged for 10 mins at 4500 g at room temperature. The obtained supernatant was filter-sterilized with 0.2 nm filter and the bacterial pellet was discarded. The prepared supernatant was used the same day.
Animal acquisition and preparation
G. mellonella were obtained from a pet-food supplier (LiveFood UK, Somerset, United Kingdom) in plastic containers with wood shavings where they were kept before the experiments. Prior to use, the larvae were stored at +4 °C to minimize the larval movement during procedure. The larvae were sorted into Petri dishes lined with filter paper ensuring all larvae are above 200 mg in weight, which is consistent with them reaching full adulthood and show no signs of melanization (black markings). Only 10 larvae per dish were permitted.
In vivo burn wound induction and P. aeruginosa infection establishment
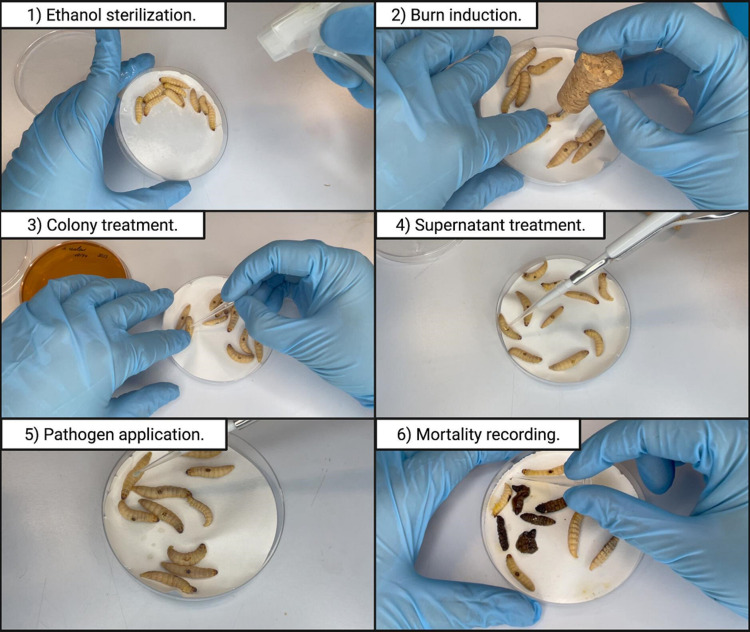
Overall, 70 % ethanol was used to sterilize the larval body surface spraying the entire larval body with the solution (Fig. 2.1, Video S1, available in the online version of this article). The Petri dishes were left open in a sterile environment to allow for the ethanol to evaporate after sterilization. A G. mellonella larva was placed on its ventral side to allow access to the back segment and held down by its head and thorax segments. The burn instrument (a steel nail with a head size of 2 mm2 embedded in cork) was heated in the middle flame of the Bunsen burner until red/white-hot and applied to the middle segment of G. mellonella back for 4 s (Fig. 2.2, Video S2). Any larvae that showed major haemolymph loss or protruding fat body after the procedure was immediately euthanized by placing it at −20 °C for at least 20 min to minimize the suffering. Immediately after the burn is established, 10 µl of overnight P. aeruginosa PA14 culture was pipetted on top of the wound (Fig. 2.5, Video S3). The larvae were allowed to rest for 10 min before introducing any further treatments.
Fig. 2.
A step-by-step outline of the protocol used for the G. mellonella burn wound and infection assay. (1) The larval body is sterilized with 70 % ethanol. (2) The burn is induced by the application of a heated metal element to the back of the larvae. (3), (4) A colony or cell-free supernatant is applied to the established burn wound. (5) The wound is topically inoculated with the pathogen. (6) The mortality is recorded by gently agitating the larvae with a pipette tip to elicit a motility response. Full videos of each step are available as Supplementary Material, available with the onine version of this article.
In vivo L. reuteri CCUG44144 colony and Lactobacillus spp. supernatant treatments of P. aeruginosa PA14 infected wound
After inducing the burn, a sterile 200 µl pipette tip was used to transfer a colony of L. reuteri CCUG44144 from the MRS agar plate to the wound. The tip was gently brushed against the wound to minimize mechanical damage to the wound (Fig. 2.3, Video S4). Following that, 10 µl of an overnight culture of P. aeruginosa PA14 was applied onto the treated wound (Fig. 2.5). In the supernatant treatment experiments, 10 µl of cell-free Lactobacillus spp. supernatant was applied onto the burn wound immediately prior to the establishment of P. aeruginosa PA14 infection (Video S5), as well as to the groups that were not infected with P. aeruginosa (Fig. 2.4). The control groups received no treatment post-burn induction. The larvae were incubated at 37 °C for 72 h. The mortality was recorded every hour. Mortality was recorded upon complete loss of larval movement even with external stimulation (Fig. 2.6, Video S6). Supernatant and colony treatment experiments were performed on different dates with different treatment groups.
In vivo tetracycline treatment of the burn wound infected with P. aeruginosa PA14
A 2.2 mg ml−1 tetracycline solution, a concentration that is in range with therapeutic topical tetracycline ointment concentrations, was prepared [26–28]. After the establishment of burn wound and infection with P. aeruginosa PA14 in G. mellonella, 10 µl of 2.2 mg ml−1 tetracycline solution was applied on top of the wound. The larvae were incubated at 37 °C for 72 h.
Results
L. reuteri colonization and supernatant treatment improve G. mellonella survival
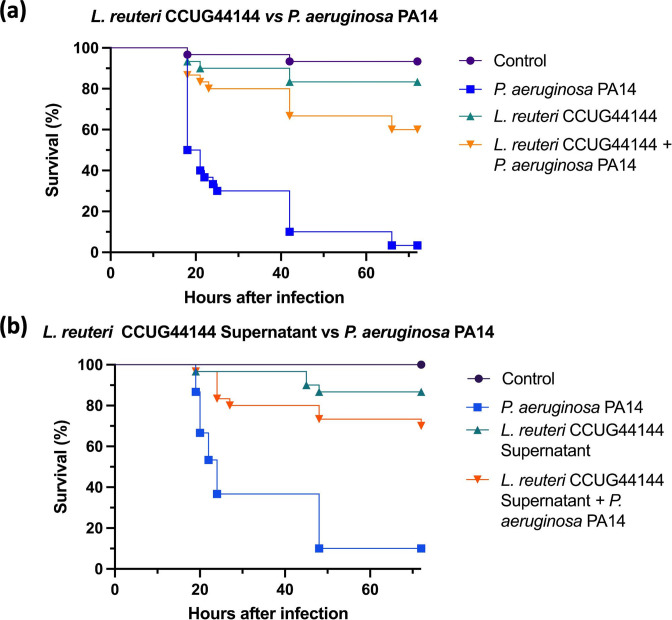
Initially, the G. mellonella larvae burn wound was seeded with L. reuteri , which has been reported to have an antimicrobial activity against P. aeruginosa via the production of reuterin [29, 30]. L. reuteri has also been shown to augment the healing processes in rats [31]. After the inoculation of the wound with L. reuteri colonies, the wound was subsequently infected with P. aeruginosa . Negative controls and L. reuteri CCUG44144 only inoculated groups exhibited 10 % or less mortality rates, which aligns with this strain being a commensal micro-organism. P. aeruginosa infected larvae exhibited above 90 % mortality, which correlates to the expected mortality rates of this clinical isolate [21]. The experimental group with wounds inoculated with L. reuteri CCUG44144 and infected with P. aeruginosa exhibited a significant reduction in mortality of 55 % in comparison to P. aeruginosa only infections (Fig. 3a).
Fig. 3.
Survival curves of in vivo burn wound treated with L. reuteri CCUG44144 colonies (a) and supernatant (b) in the topical infection with P. aeruginosa PA14, n=30. (a) P. aeruginosa PA14 vs L. reuteri CCUG44144 + P. aeruginosa PA14 survival Log rank P value<0.0001. (b) P. aeruginosa PA14 vs L. reuteri CCUG44144 Supernatant + P. aeruginosa PA14 survival Log rank P value<0.0001. Statistical significance was determined with Log rank statistical test with Bonferroni-corrected threshold.
Following the abovementioned findings, L. reuteri supernatant was tested against P. aeruginosa infection to determine if secreted factors were responsible for the observed reduction in virulence. G. mellonella larvae burn wounds were infected with P. aeruginosa and L. reuteri supernatant was topically applied. Negative controls exhibited less than 10 % mortality. Positive controls of P. aeruginosa PA14 infection exhibited more than 90 % mortality. Control groups treated with just L. reuteri supernatant exhibited less than 20 % mortality (Fig. 3). Groups treated with L. reuteri supernatant prior to P . aeruginosa infection showed a significant 60 % reduction of mortality after the treatment (Fig. 3b). This aligned with the findings observed from the colony treatment and the previously reported effects of L. reuteri on P. aeruginosa pathogenicity [29, 30].
Lactobacillus spp. supernatant improves the survival of G. mellonella after P. aeruginosa infection
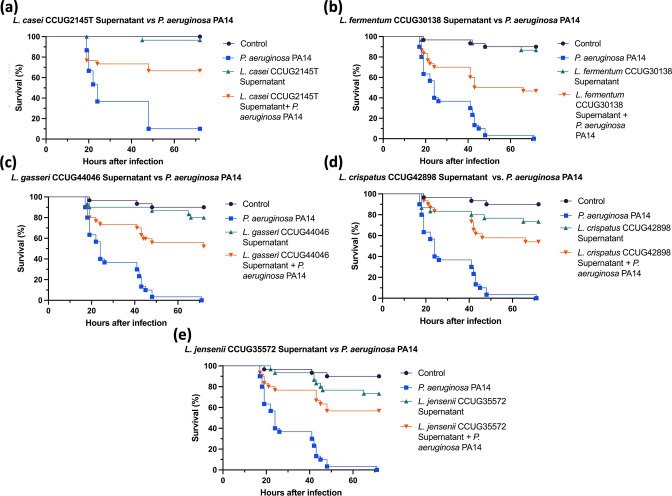
Due to the probiotic effects observed in the larvae treated with L. reuteri colonies and supernatant, more Lactobacillus strains supernatants were tested against P. aeruginosa burn wound infection in G. mellonella. The selected strains have been previously reported to have antimicrobial or probiotic effects in burn wound infections. Groups with P. aeruginosa infected burns showed a significant 60 % reduction of mortality after the treatment with L. casei (Fig. 4a). L. casei has been previously reported to interfere with the adhesion mechanisms of P. aeruginosa in Wistar rats [32]. Infected burn group treated with L. gasseri showed a significant 55 % reduction in mortality, which aligns with reported findings in murine burn infection [33]. P. aeruginosa infected burn wounds treated with L. crispatus and L. jensenii exhibited a significant 50 % reduction in mortality (Fig. 4b, e). However, L. crispatus supernatant only treated group exhibited a 30 % mortality, which is higher than the expected findings (Fig. 4b). L. fermentum treatment resulted in a significant 40 % reduction of mortality (Fig. 4d). Overall, all of the selected strains exhibited a significant reduction of mortality in P. aeruginosa burn wound infection, which is in line with the reported findings in ex vivo and in vivo models [13–15, 32]. An important observation however is that this model was able to distinguish differences in the probiotic potential of the different strains highlighting its versatility.
Fig. 4.
Survival curves of in vivo burn wound P. aeruginosa PA14 infection treated with Lactobacillus spp supernatants, n=30. (a) P. aeruginosa PA14 versus L. casei CCUG2145T, Log rank P value<0.0001. (b) P. aeruginosa PA14 versus L. fermentum CCUG30138, Log rank P value<0.0001. (c) P. aeruginosa PA14 versus L. gasseri CCUG44046, Log rank P value<0.0001. (d) P. aeruginosa PA14 versus L. crispatus CCUG42898, Log rank P value<0.0001. (e) P. aeruginosa PA14 versus L. jensenii CCUG35572, Log rank P value<0.0001. L. casei treatment experiment was performed on the same day as L. reuteri treatment in Fig. 3(b), resulting in the difference in the ‘Control’ and ‘ P. aeruginosa PA14’ control curves between supernatant treatments. Statistical significance was determined with Log rank statistical test with Bonferroni-corrected threshold.
Lactobacillus colonization matches antibiotic activity of tetracycline
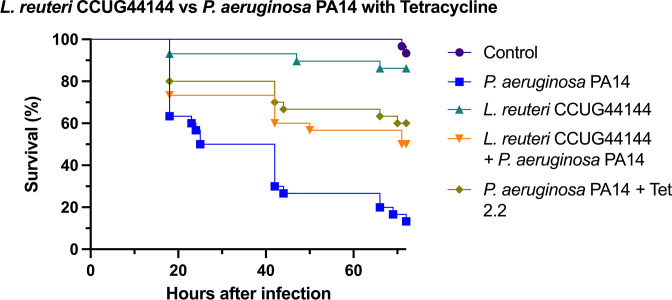
To challenge the model further and compare the probiotics effects to that of a topical antibiotic treatment, L. reuteri CCUG44144 was tested against P. aeruginosa with topical tetracycline treatment (Fig. 5). Topical tetracycline treatment is one of the common clinical strategies against wound infection [34]. The addition of topical tetracycline treatment improved the survival of larvae infected with P. aeruginosa by almost 50 %, which suggests in this model at least, that probiotic prophylaxis is as effective as antibiotic therapy. There was no significant difference observed between tetracycline treated larvae and L. reuteri treated larvae survival, which validates the effectiveness of L. reuteri treatment in G. mellonella burn wound model against P. aeruginosa .
Fig. 5.
Survival curves of in vivo burn wound P. aeruginosa PA14 infection treated with L. reuteri CCUG44144 and topical application of 2.2 mg ml−1 solution of tetracycline, n=30. P. aeruginosa PA14 vs L. reuteri CCUG44144 + P. aeruginosa PA14 survival Log rank P value<0.01. P. aeruginosa PA14 vs P. aeruginosa PA14 + Tet 2.2 mg ml−1 survival Log rank P value<0.0001. P. aeruginosa PA14 + Tet 2.2 mg ml−1 vs L. reuteri CCUG44144 + P. aeruginosa PA14 survival Log rank P value>0.01. Statistical significance was determined with Log rank statistical test with Bonferroni-corrected threshold.
Discussion
This invertebrate burn wound and infection model using G. mellonella addresses several issues that affect in vivo burn wound research, such as limited cohort sizes, strict ethical considerations, affordability and handling and maintenance. The protocol described in this study validates this model as a tool to determine the in vivo efficacy of burn wound probiotic treatments as well as its capacity to be used to study interspecies bacterial interactions. The results obtained from this protocol, demonstrating the probiotic potential of Lactobacillus spp. and their supernatants in the burn wound microenvironment, align with previously reported findings [14, 35]. Despite the previously described advantages this model has several limitations that need to be considered carefully. The anatomy of larval cuticle consists of multiple layers, but indisputably is very different from mammalian skin structure [36]. Even though, its innate immune system has similar elements to its mammalian counterpart, G. mellonella also lacks an adaptive immune system, which plays a major role in the burn trauma and infection pathogenesis [37]. However, due to the challenges associated with currently established in vivo burn wound and infection models, G. mellonella burn wound and infection model could reduce and refine the use of the larger mammalian models as preliminary experiments to optimize the dosing, formulations and timings can be performed in the invertebrate model. G. mellonella burn wound model has been used to assess the effects of antibiotic wound treatments and other antimicrobial therapies against burn wound infections [38–40]. This model will also facilitate high-throughput screening of the commensal skin microbiota to identify potential probiotic strains in vivo, something that is not possible with traditional mammalian models.
Lactobacillus spp. have long been established as one of the most prolific probiotic bacteria. Several mechanisms have been associated with their therapeutic effects, such as their impact on local pH and the production of antimicrobial compounds such as reuterin [29, 41]. Several species of Lactobacillus have been reported to produce bacteriocins, which exhibited an antimicrobial effect on multidrug-resistant P. aeruginosa wound isolates [42]. L. acidophilus and L. casei can produce surfactants that can reduce S. aureus and Staphylococcus epidermidis biofilm development and induce dispersal [43, 44]. The therapeutic effects of Lactobacillus treatments observed in this study could be attributed to several antimicrobial mechanisms deployed by Lactobacillus spp. In the L. reuteri colony treatment (Fig. 3a) some of its therapeutic effect can also be attributed to competitive exclusion within the wound bed as L. reuteri has been previously shown to inhibit S. aureus infection via the same mechanism [14]. Overall, all of the tested Lactobacillus strains have been previously reported for producing antimicrobial compounds or exhibiting antimicrobial or anti-virulence activity against major wound pathogens via multiple mechanisms [15, 32, 33, 45, 46]. This aligns with the therapeutic effects seen when testing the supernatants in Figs 3(b) and 4.
There are several crucial aspects that must be attended to when using this protocol. Larval sizes and health at the beginning of the experiment are important to the survival rates downstream [47]. Larvae that exhibit any sign of melanization or appear to be flaccid upon retrieval from +4 °C incubation should not be used as it is an indication of their declining health condition and will affect their survival [48]. During the burn procedure, attention must be paid to how much haemolymph is lost in the process. Haemolymph should appear as transparent pale-yellow fluid. Due to G. mellonella’s open circulatory system major haemolymph loss will result in an early death from the dehydration [49]. Additionally, due to the placement of the burn wound on the dorsal side of larvae it comes in close proximity with the internal systems of the invertebrate. Therefore, any sign of protruding tissues or leakage of non-transparent fluid from the wound should lead to immediate euthanasation of the animal via incubation at −20 °C. During the step of colony application to the wound, special attention needs to be paid upon touching the wound with the pipette tip. Larval cuticle is thin and fragile, which is only amplified after the burn trauma. In addition to that, the source of G. mellonella larvae needs to be considered. The experiments conducted in this study were performed on shop-grade larvae, meaning that the diet and rearing conditions of the larvae could not be controlled. Using in-house reared or research-grade larvae could limit these variables.
During the application of liquid treatments or inoculating the wound with liquid pathogen cultures, the applications need to be spaced out. G. mellonella forms an eschar on top of the burn wound, which provides a physical protection to the wound. Applying multiple liquid elements to this area in a short period of time could lead to increased permeability of the scab and cause early decline of the larval condition. In addition, during the sterilization step, using any other sterilization agent such as industrial methylated spirits should be avoided and a sufficient time should be allocated for the ethanol to evaporate from the filter paper in the Petri dish to avoid larval alcohol poisoning.
Supplementary Data
Funding information
We thank the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) for funding this work NC/V001582/1 to RRMC and EM. RRMC is supported by a Biotechnology and Biological Sciences Research Council New Investigator Award BB/V007823/1 and the Academy of Medical Sciences/the Wellcome Trust/ the Government Department of Business, Energy and Industrial Strategy/the British Heart Foundation/Diabetes UK Springboard Award [SBF006\1040].
Conflicts of interest
The authors declare no conflict of interest.
Ethical statement
No ethical approval was required for this study.
Footnotes
Abbreviations: ICU, intensive care unit; MRS, De Man, Rogosa and Sharpe; NHS, National Health Service; OD, optical density; TBSA, total body surface area; Tet, tetracycline.
Six supplementary videos are available with the online version of this article.
References
- 1.Rahman MA, Abul Barkat H, Harwansh RK, Deshmukh R. Carbon-based nanomaterials: carbon nanotubes, graphene, and fullerenes for the control of burn infections and wound healing. Curr Pharm Biotechnol. 2022;23:1483–1496. doi: 10.2174/1389201023666220309152340. [DOI] [PubMed] [Google Scholar]
- 2.Forbinake NA, Ohandza CS, Fai KN, Agbor VN, Asonglefac BK, et al. Mortality analysis of burns in a developing country: a CAMEROONIAN experience. BMC Public Health. 2020;20:1269. doi: 10.1186/s12889-020-09372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akers KS, Schlotman T, Mangum LC, Garcia G, Wagner A, et al. 653. Diagnosis of burn sepsis using the FcMBL ELISA: a pilot study in critically ill burn patients. Open Forum Infect Dis. 2019;6:S300. doi: 10.1093/ofid/ofz360.721. [DOI] [Google Scholar]
- 4.Guest JF, Fuller GW, Edwards J. Cohort study evaluating management of burns in the community in clinical practice in the UK: costs and outcomes. BMJ Open. 2020;10:e035345. doi: 10.1136/bmjopen-2019-035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarafdar F, Jafari B, Azimi T. Evaluating the antimicrobial resistance patterns and molecular frequency of blaoxa-48 and blaGES-2 genes in Pseudomonas aeruginosa and Acinetobacter baumannii strains isolated from burn wound infection in Tehran, Iran. New Microbes New Infect. 2020;37:100686. doi: 10.1016/j.nmni.2020.100686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chairat S, Ben Yahia H, Rojo-Bezares B, Sáenz Y, Torres C, et al. High prevalence of imipenem-resistant and metallo-beta-lactamase-producing Pseudomonas aeruginosa in the Burns Hospital in Tunisia: detection of a novel class 1 integron. J Chemother. 2019;31:120–126. doi: 10.1080/1120009X.2019.1582168. [DOI] [PubMed] [Google Scholar]
- 7.Aguilera-Sáez J, Andreu-Solà V, Larrosa Escartín N, Rodríguez Garrido V, Armadans Gil L, et al. Extensively drug-resistant Pseudomonas aeruginosa outbreak in a burn unit: management and solutions. Ann Burns Fire Disasters. 2019;32:47–55. [PMC free article] [PubMed] [Google Scholar]
- 8.Tissot F, Blanc DS, Basset P, Zanetti G, Berger MM, et al. New genotyping method discovers sustained nosocomial Pseudomonas aeruginosa outbreak in an intensive care burn unit. J Hosp Infect. 2016;94:2–7. doi: 10.1016/j.jhin.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Lachiewicz AM, Hauck CG, Weber DJ, Cairns BA, van Duin D. Bacterial infections after burn injuries: impact of multidrug resistance. Clin Infect Dis. 2017;65:2130–2136. doi: 10.1093/cid/cix682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peral MC, Martinez MAH, Valdez JC. Bacteriotherapy with Lactobacillus plantarum in burns. Int Wound J. 2009;6:73–81. doi: 10.1111/j.1742-481X.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satish L, Gallo PH, Johnson S, Yates CC, Kathju S. Local probiotic therapy with Lactobacillus plantarum mitigates scar formation in rabbits after burn injury and infection. Surg Infect. 2017;18:119–127. doi: 10.1089/sur.2016.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brachkova MI, Marques P, Rocha J, Sepodes B, Duarte MA, et al. Alginate films containing Lactobacillus plantarum as wound dressing for prevention of burn infection. J Hosp Infect. 2011;79:375–377. doi: 10.1016/j.jhin.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Stanbro J, Park JM, Bond M, Stockelman MG, Simons MP, et al. Topical delivery of Lactobacillus culture supernatant increases survival and wound resolution in traumatic Acinetobacter baumannii infections. Probiotics Antimicrob Proteins. 2020;12:809–818. doi: 10.1007/s12602-019-09603-z. [DOI] [PubMed] [Google Scholar]
- 14.Prince T, McBain AJ, O’Neill CA. Lactobacillus reuteri protects epidermal keratinocytes from Staphylococcus aureus-induced cell death by competitive exclusion. Appl Environ Microbiol. 2012;78:5119–5126. doi: 10.1128/AEM.00595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandla S, Harjai K, Shukla G. Combinatorial therapeutic strategy of biogenics derived from Lactobacillus fermentum PUM and zingerone against Pseudomonas aeruginosa PAO1-induced surgical site infection: an experimental study. Probiotics Antimicrob Proteins. 2022;14:712–726. doi: 10.1007/s12602-022-09944-2. [DOI] [PubMed] [Google Scholar]
- 16.Fransén J, Huss FRM, Nilsson LE, Rydell U, Sjöberg F, et al. Surveillance of antibiotic susceptibility in a Swedish Burn Center 1994-2012. Burns. 2016;42:1295–1303. doi: 10.1016/j.burns.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Rittié L. Cellular mechanisms of skin repair in humans and other mammals. J Cell Commun Signal. 2016;10:103–120. doi: 10.1007/s12079-016-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gholipour-Kanani A, Mohsenzadegan M, Fayyazi M, Bahrami H, Samadikuchaksaraei A. Poly (ɛ-caprolactone)-chitosan-poly (vinyl alcohol) nanofibrous scaffolds for skin excisional and burn wounds in a canine model. IET Nanobiotechnol. 2018;12:619–625. doi: 10.1049/iet-nbt.2017.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murad HM, Malik ZJ, Umayra AN. Evaluation the skin regeneration by using Kefir production in local dogs. J Phar Sci Res. 2018;10:2653–2658. [Google Scholar]
- 20.Abdullahi A, Amini-Nik S, Jeschke MG. Animal models in burn research. Cell Mol Life Sci. 2014;71:3241–3255. doi: 10.1007/s00018-014-1612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maslova E, Shi Y, Sjöberg F, Azevedo HS, Wareham DW, et al. An invertebrate burn wound model that recapitulates the hallmarks of burn trauma and infection seen in mammalian models. Front Microbiol. 2020;11:998. doi: 10.3389/fmicb.2020.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cutuli MA, Petronio Petronio G, Vergalito F, Magnifico I, Pietrangelo L, et al. Galleria mellonella as a consolidated in vivo model hosts: new developments in antibacterial strategies and novel drug testing. Virulence. 2019;10:527–541. doi: 10.1080/21505594.2019.1621649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernando-Ortiz A, Mateo E, Perez-Rodriguez A, de Groot PWJ, Quindós G, et al. Virulence of Candida auris from different clinical origins in Caenorhabditis elegans and Galleria mellonella host models. Virulence. 2021;12:1063–1075. doi: 10.1080/21505594.2021.1908765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ménard G, Rouillon A, Cattoir V, Donnio P-Y. Galleria mellonella as a suitable model of bacterial infection: past, present and future. Front Cell Infect Microbiol. 2021;11:782733. doi: 10.3389/fcimb.2021.782733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maslova E, Eisaiankhongi L, Sjöberg F, McCarthy RR. Burns and biofilms: priority pathogens and in vivo models. NPJ Biofilms Microbiomes. 2021;7:73. doi: 10.1038/s41522-021-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burton J, The Dermatology Research Group A placebo-controlled study to evaluate the efficacy of topical tetracycline and oral tetracycline in the treatment of mild to moderate acne. Dermatology research group. J Int Med Res. 1990;18:94–103. doi: 10.1177/030006059001800204. [DOI] [PubMed] [Google Scholar]
- 27.Funahara M, Yanamoto S, Ueda M, Suzuki T, Ota Y, et al. Prevention of surgical site infection after oral cancer surgery by topical tetracycline: Results of a multicenter randomized control trial. Medicine. 2017;96:e8891. doi: 10.1097/MD.0000000000008891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon AW, Mohammed Z, Massae PA, Shao JF, Foster A, et al. Impact of mass distribution of azithromycin on the antibiotic susceptibilities of ocular Chlamydia trachomatis . Antimicrob Agents Chemother. 2005;49:4804–4806. doi: 10.1128/AAC.49.11.4804-4806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang H-F, Chen C-N, Chang Y, Sung H-W. Natural antimicrobial agent (reuterin) produced by Lactobacillus reuteri for sanitization of biological tissues inoculated with Pseudomonas aeruginosa . Biotechnol Bioeng. 2003;84:233–239. doi: 10.1002/bit.10764. [DOI] [PubMed] [Google Scholar]
- 30.Knysh OV, Isayenko OY, Voyda YV, Kizimenko OO, Babych YM. Influence of cell-free extracts of Bifidobacterium bifidum and Lactobacillus reuteri on proliferation and biofilm formation by Escherichia coli and Pseudomonas aeruginosa . Regul Mech Biosyst. 2019;10:251–256. doi: 10.15421/021938. [DOI] [Google Scholar]
- 31.Khodaii Z, Afrasiabi S, Hashemi SA, Ardeshirylajimi A, Natanzi MM. Accelerated wound healing process in rat by probiotic Lactobacillus reuteri derived ointment. J Basic Clin Physiol Pharmacol. 2019;30 doi: 10.1515/jbcpp-2018-0150. [DOI] [PubMed] [Google Scholar]
- 32.Abootaleb M, Mohammadi Bandari N, Arbab Soleimani N. Interference of Lactobacillus casei with Pseudomonas aeruginosa in the treatment of infected burns in Wistar rats. Iran J Basic Med Sci. 2021;24:143–149. doi: 10.22038/IJBMS.2020.47447.10920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenzmeier TD, Mudaliar NS, Stanbro JA, Watters C, Ahmad A, et al. Application of Lactobacillus gasseri 63 AM supernatant to Pseudomonas aeruginosa-infected wounds prevents sepsis in murine models of thermal injury and dorsal excision. J Med Microbiol. 2019;68:1560–1572. doi: 10.1099/jmm.0.001066. [DOI] [PubMed] [Google Scholar]
- 34.Ray P, Singh S, Gupta S. Topical antimicrobial therapy: current status and challenges. Indian J Med Microbiol. 2019;37:299–308. doi: 10.4103/ijmm.IJMM_19_443. [DOI] [PubMed] [Google Scholar]
- 35.Hadid MA, Al-Shaibani AB, Al-Mukhtar SA. Treatment of bacteria causing burn wound infections by Lactobacillus Reuteri as a probiotic. World J Pharm Res. 2016;5:02. [Google Scholar]
- 36.Kazek M, Kaczmarek A, Wrońska AK, Boguś MI. Diet influences the bacterial and free fatty acid profiles of the cuticle of Galleria mellonella larvae. PLoS One. 2019;14:e0211697. doi: 10.1371/journal.pone.0211697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira T, de Barros P, Fugisaki L, Rossoni R, Ribeiro F, et al. Recent advances in the use of Galleria mellonella model to study immune responses against human pathogens. J Fungi. 2018;4:128. doi: 10.3390/jof4040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Figueiredo-Godoi LMA, Garcia MT, Pinto JG, Ferreira-Strixino J, Faustino EG, et al. Antimicrobial photodynamic therapy mediated by fotenticine and methylene blue on planktonic growth, biofilms, and burn infections of Acinetobacter baumannii . Antibiotics. 2022;11:619. doi: 10.3390/antibiotics11050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos AL, van Venrooy A, Reed AK, Wyderka AM, García-López V, et al. Hemithioindigo-based visible light-activated molecular machines kill bacteria by oxidative damage. Adv Sci. 2022;9:e2203242. doi: 10.1002/advs.202203242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi Y, Wareham DW, Yuan Y, Deng X, Mata A, et al. Polymyxin B‐triggered assembly of peptide hydrogels for localized and sustained release of combined antimicrobial therapy. Adv Healthcare Mater. 2021;10:2101465. doi: 10.1002/adhm.202101465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rana S, Bhawal S, Kumari A, Kapila S, Kapila R. pH-dependent inhibition of AHL-mediated quorum sensing by cell-free supernatant of lactic acid bacteria in Pseudomonas aeruginosa PAO1. Microb Pathog. 2020;142:104105. doi: 10.1016/j.micpath.2020.104105. [DOI] [PubMed] [Google Scholar]
- 42.Al-Malkey MK, Ismeeal MC, Abo Al-Hur FJ, Mohammed SW, Nayyef HJ. Antimicrobial effect of probiotic Lactobacillus spp. on Pseudomonas aeruginosa . J contemp med sci. 2017;3:218–223. doi: 10.22317/jcms.v3i10.169. [DOI] [Google Scholar]
- 43.Walencka E, Rózalska S, Sadowska B, Rózalska B. The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol. 2008;53:61–66. doi: 10.1007/s12223-008-0009-y. [DOI] [PubMed] [Google Scholar]
- 44.Karska-Wysocki B, Bazo M, Smoragiewicz W. Antibacterial activity of Lactobacillus acidophilus and Lactobacillus casei against methicillin-resistant Staphylococcus aureus (MRSA) Microbiol Res. 2010;165:674–686. doi: 10.1016/j.micres.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Kaur S, Sharma P. Protease-sensitive inhibitory activity of cell-free supernatant of Lactobacillus crispatus 156 synergizes with ciprofloxacin, moxifloxacin and streptomycin against Pseudomonas aeruginosa: an in vitro study. Probiotics Antimicrob Proteins. 2015;7:172–180. doi: 10.1007/s12602-015-9188-4. [DOI] [PubMed] [Google Scholar]
- 46.Sambanthamoorthy K, Feng X, Patel R, Patel S, Paranavitana C. Antimicrobial and antibiofilm potential of biosurfactants isolated from lactobacilli against multi-drug-resistant pathogens. BMC Microbiol. 2014;14:197. doi: 10.1186/1471-2180-14-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jorjão AL, Oliveira LD, Scorzoni L, Figueiredo-Godoi LMA, Cristina A Prata M, et al. From moths to caterpillars: Ideal conditions for Galleria mellonella rearing for in vivo microbiological studies. Virulence. 2018;9:383–389. doi: 10.1080/21505594.2017.1397871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai CJ-Y, Loh JMS, Proft T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence. 2016;7:214–229. doi: 10.1080/21505594.2015.1135289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grizanova EV, Semenova AD, Komarov DA, Chertkova EA, Slepneva IA, et al. Maintenance of redox balance by antioxidants in hemolymph of the greater wax moth Galleria mellonella larvae during encapsulation response. Arch Insect Biochem Physiol. 2018;98:e21460–n. doi: 10.1002/arch.21460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.