Abstract
BACKGROUND
Chronic stress associates with major adverse cardiovascular events (MACE) via increased stress-related neural network activity (SNA). Light/moderate alcohol consumption (ACl/m) has been linked to lower MACE risk, but the mechanisms are unclear.
OBJECTIVES
The purpose of this study was to evaluate whether the association between ACl/m and MACE is mediated by decreased SNA.
METHODS
Individuals enrolled in the Mass General Brigham Biobank who completed a health behavior survey were studied. A subset underwent 18F-fluorodeoxyglucose positron emission tomography, enabling assessment of SNA. Alcohol consumption was classified as none/minimal, light/moderate, or high (<1, 1-14, or >14 drinks/week, respectively).
RESULTS
Of 53,064 participants (median age 60 years, 60% women), 23,920 had no/minimal alcohol consumption and 27,053 ACl/m. Over a median follow-up of 3.4 years, 1,914 experienced MACE. ACl/m (vs none/minimal) associated with lower MACE risk (HR: 0.786; 95% CI: 0.717-0.862; P < 0.0001) after adjusting for cardiovascular risk factors. In 713 participants with brain imaging, ACl/m (vs none/minimal) associated with decreased SNA (standardized beta −0.192; 95% CI: −0.338 to −0.046; P = 0.01). Lower SNA partially mediated the beneficial effect of ACl/m on MACE (log OR: −0.040; 95% CI: −0.097 to −0.003; P < 0.05). Further, ACl/m associated with larger decreases in MACE risk among individuals with (vs without) prior anxiety (HR: 0.60 [95% CI: 0.50-0.72] vs 0.78 [95% CI: 0.73-0.80]; P interaction = 0.003).
CONCLUSIONS
ACl/m associates with reduced MACE risk, in part, by lowering activity of a stress-related brain network known for its association with cardiovascular disease. Given alcohol’s potential health detriments, new interventions with similar effects on SNA are needed.
Keywords: alcohol consumption, amygdala, brain, cardiovascular disease, chronic stress, stress-associated neural network activity
Several epidemiological studies have identified a U- or J-shaped association between alcohol consumption and the risk of major adverse cardiovascular disease events (MACE).1,2 Specifically, studies have suggested that light/moderate alcohol consumption (≤1 drink/d for women or ≤1-2 drinks/d for men) associates with lower MACE risk compared with abstinence,3,4 whereas more excessive alcohol consumption associates with higher MACE risk and other complications.5 However, it remains unclear whether the potential cardiovascular benefits of light/moderate alcohol consumption result from alcohol itself or whether they may stem from confounders (eg, associated health behaviors, socioeconomic factors).4,6,7 Moreover, the mechanisms by which light/moderate alcohol consumption may attenuate cardiovascular risk remain unclear. A better understanding of the underlying biology is needed to inform novel treatments that could achieve similar benefits without alcohol’s potential adverse effects.
Various physiological effects of alcohol have been hypothesized to explain light/moderate alcohol’s benefits on MACE. Light/moderate alcohol consumption associates with favorable changes in various cardiometabolic markers, including increased high-density lipoprotein cholesterol, decreased fibrinogen, increased adiponectin, and improved insulin sensitivity; yet, such changes do not sufficiently explain alcohol’s impact on MACE.8,9 Rarely mentioned among alcohol’s potentially protective influences are its effects on the central nervous system and its interactions with psychosocial stress, another important risk factor for MACE.10
Alcohol induces a relaxation response in humans and has historically been used for this purpose. Alcohol’s acute anxiolytic effects are mediated through its impact on stress-associated brain regions (eg, the amygdala).11,12 Functional magnetic resonance imaging experiments have shown that alcohol acutely reduces amygdalar reactivity to threatening stimuli.11,12 Nevertheless, alcohol’s chronic effects on the neurobiology of stress are incompletely characterized.
Multisystem imaging with 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) has provided insights into how chronic stress may lead to increased MACE risk. Specifically, chronic stress triggers a serial pathway that involves heightened stress-related neural network activity (SNA) (notably involving heightened amygdalar activity), leading to downstream sympathetic stimulation and leukopoiesis, atherogenesis, and atherosclerotic inflammation, which culminate in MACE.10,13 Given the acute anxiolytic effects of alcohol, we posited that chronic light/moderate alcohol consumption confers cardiovascular benefits, in part, by reducing activation of these pathological, stress-associated mechanisms.
Accordingly, we leveraged a large, well-characterized Biobank cohort to evaluate the hypothesis that light/moderate alcohol reduces MACE by attenuating adverse stress-related neural mechanisms. To explore this, we first evaluated the impact of light/moderate alcohol consumption on MACE after adjusting for a range of genetic, clinical, lifestyle, and socioeconomic confounders that have been inconsistently accounted for previously. Next, we assessed the effect of light/moderate alcohol consumption on resting SNA. Then, we tested whether this effect mediates the beneficial impact of light/moderate alcohol consumption on MACE. Last, we evaluated whether the impact of light/moderate alcohol on MACE is more pronounced among individuals who are expected to have chronically heightened SNA (ie, individuals with a diagnosis of anxiety).14
METHODS
STUDY POPULATION.
Individuals enrolled in the Mass General Brigham (MGB) Biobank were included. The MGB Biobank, established in April 2010, is a biorepository that recruits subjects through hospitals in the MGB network.15 The MGB Human Research Committee approved the study protocol.
ALCOHOL CONSUMPTION.
As of December 23, 2020, 53,064 participants had enrolled in the Biobank and completed an optional comprehensive health behavior survey upon enrollment. The survey included a question on alcohol intake during the year before enrollment (Figure 1, Supplemental Figure 1A). Alcohol consumption was classified for both men and women as none/minimal (<1 drink/wk), light/moderate (1-14 drinks/wk), and high (>14 drinks/wk).16
Figure 1. Study Cohort.
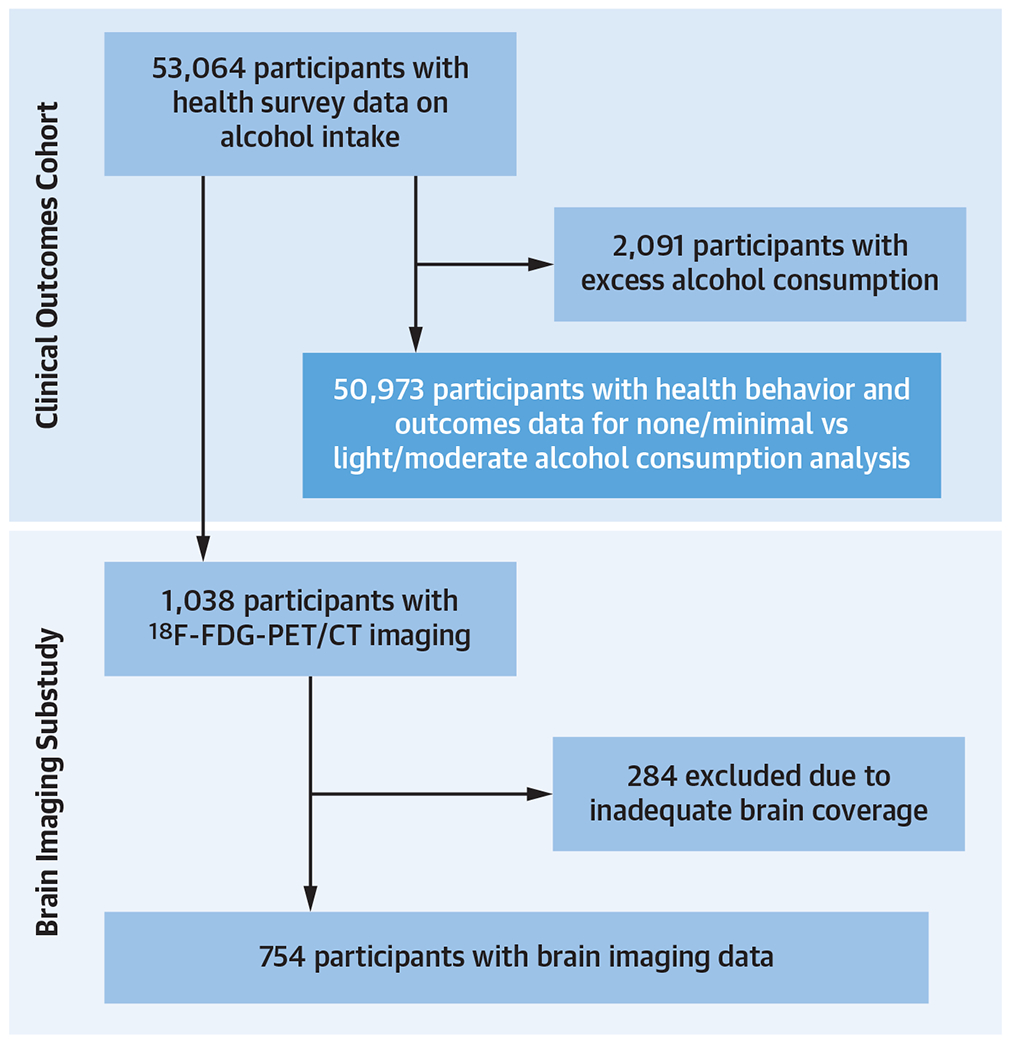
This schematic describes how the patient populations for the clinical outcomes cohort and brain imaging cohort were derived. 18F-FDG-PET/CT = 18F-fluorodeoxyglucose positron emission tomography/computed tomography.
STRESS-ASSOCIATED NEURAL NETWORK ACTIVITY.
Among the subset of 8,734 individuals who provided genetic data, we identified 1,038 participants who underwent clinically indicated 18F-FDG-PET/computed tomography (CT), often for cancer surveillance or diagnosis. Exclusion criteria for this subgroup were inadequate brain imaging or the presence of brain tumors. 18F-FDG-PET/CT imaging was performed in a resting state after an overnight fast per the standard clinical protocol (using Biograph 64, Siemens Healthcare, or equivalent). 18F-FDG was administered ~370 MBq (~10 mCi) intravenously, and PET images were acquired in 3-dimensional mode after ~60 minutes. For attenuation correction, a low-dose, non-contrast-enhanced CT (120 keV, 50 mA) was acquired.
An experienced investigator (S.A.) who was blinded to clinical data measured regional brain activity on 18F-FDG-PET/CT images, as previously described.10 Briefly, using a ~15-mm circular region of interest, 18F-FDG uptake was measured as the maximum standardized uptake values (SUVs) in the left and right amygdalae, which were averaged. These averages were divided by the mean activity of the ventromedial prefrontal cortex (vmPFC) to provide the measure of SNA.17 Whole brain tissue activity was measured by drawing a region of interest around the whole brain on 3 axial planes, 5 mm apart, at the level of the thalamus and averaging the SUVs to provide a background comparison for amygdalar and vmPFC measurements in isolation. Additional details of these measurements are provided in the Supplemental Appendix.
ADVERSE CARDIOVASCULAR DISEASE EVENTS.
MACE was defined according to Framingham Heart Study criteria and included myocardial infarction, coronary revascularization (surgical and percutaneous), unstable angina, cerebrovascular accidents, transient ischemic attacks, peripheral vascular disease, and heart failure.18 MACE data were obtained from the medical records using International Classification of Disease (ICD)-10 codes (Supplemental Table 1). Incident MACE events were assessed for 2 periods: 1) the time from enrollment to the date of data lock (December 23, 2020); and 2) the 10-year period preceding data lock.
COVARIABLES.
Demographic data were determined at the time of Biobank enrollment. Prespecified cardiovascular disease (CVD) risk factors in the primary analysis were defined as hypertension, hyperlipidemia, diabetes mellitus (all derived from ICD codes), and smoking history. Smoking status was derived from survey data upon Biobank entry as current or prior smoking. Using the medical record, the Charlson comorbidity index was computed from ICD codes,15,19 as was a history of sleep disorders, anxiety disorders, or depression (Supplemental Table 2). Physical activity was obtained from questions in the health survey, which assessed the duration that study participants spent on physical and recreational activities, and was quantified as a total metabolic equivalence of task minutes per week for each respondent (Supplemental Figure 1B). Socioeconomic variables (ie, employment status and educational level) were also obtained from the survey (Supplemental Figure 1C). Median income was derived from census data at the zip code level based on each participant’s home address. Additional details are provided in the Supplemental Appendix.
To adjust for possible genetic influences on the relationship between light/moderate alcohol use and brain activity, we leveraged genomic analysis that was performed on the subset of participants who had consented to genetic analysis (8,734 study subjects). A validated polygenic risk score capturing genetic liability for neuroticism that increases the risk for other stress-related syndromes (PRSss) was generated based on single nucleotide polymorphism effect estimates from a large, published genome-wide association study of neuroticism.20 PRSss associates with anxiety disorders and depression.21 Additional details are provided in the Supplemental Appendix.
STATISTICAL METHODS.
Statistical analyses were performed using SPSS version 28 (IBM Corporation). Continuous variables were reported as mean ± SD or, when not normally distributed, as median (IQR). Independent sample Student’s t-tests were used to compare continuous variables between groups for normally distributed data and the Wilcoxon Mann-Whitney test for skewed data. Categorical variables were compared using chi-square or Fisher exact tests as appropriate. All statistical tests were 2-sided with an alpha level of 0.05.
Cox proportional hazards models adjusted for potential confounders were used to calculate HRs and 95% CIs. The covariables in the primary analysis (ie, age, sex, and CVD risk factors) were defined a priori. We performed log-rank tests to generate Kaplan-Meier estimates of MACE-free survival among individuals with none/minimal vs light/moderate alcohol consumption. The interaction of alcohol consumption with baseline anxiety disorders on MACE was tested in Cox models. Additional details of data analysis, including secondary analyses and the implementation of a 10-year event horizon, are described in the Supplemental Appendix.
Linear regression models adjusted for age and sex were used to test for associations of alcohol consumption with the continuous standardized variable of SNA. These models were further adjusted for other variables that may affect SNA, including socioeconomic factors, lifestyle factors, PRSss, and the Charlson index. Logistic regression models adjusting for age, sex, and CVD risk factors were implemented to derive ORs assessing associations between SNA and MACE.
Mediation analysis, which tests a putative causal relationship among variables (ie, a path), was performed to test whether light/moderate alcohol consumption exerts its effect on MACE via SNA. This analysis was carried out with SPSS PROCESS Model 4 macro, which uses ordinary least squares and logistic regression-based path frameworks to estimate direct and indirect effects and produce 95% CIs from 10,000 bias-corrected bootstrap samples.22 Additional details are provided in the Supplemental Appendix.
RESULTS
BASELINE CHARACTERISTICS.
Baseline characteristics are listed in Table 1. Of 53,064 study subjects, 23,920 had <1 drink/wk (none/minimal intake), 27,053 had 1 to 14 drinks/wk (light/moderate intake), and 2,091 subjects had >14 drinks/wk (high intake). The median age was 60 years (IQR: 47-73 years), and 31,762 (59.9%) were women. Subjects with no/minimal alcohol intake were more likely to be female, hypertensive, diabetic, and to have a history of anxiety and depression. Light/moderate drinkers were more likely to be male, smokers, and physically active, and had a higher neighborhood income compared with participants with no/minimal alcohol consumption. Notably, the high alcohol consumption group represented 3.9% of the clinical cohort and 5.4% of the imaging cohort. Because the adverse effects of excess alcohol consumption are well-recognized, and because our hypotheses focused on differences between no/minimal vs light/moderate drinkers, most analyses excluded excess drinkers.
TABLE 1.
Clinical and Demographic Characteristics of Study Subjects
| Total Cohort (N = 50,973; 100%) |
None/Minimal (<1 drink/wk) (n = 23,920; 47%) |
Light/Moderate (1-14 drinks/wk) (n = 27,053; 53%) |
P Value | |
|---|---|---|---|---|
| Age, y | 60 (47, 73) | 59 (46, 72) | 60 (46, 74) | 0.78 |
|
| ||||
| Female | 31,113 (61.0) | 15,738 (65.8) | 15,375 (56.8) | <0.0001 |
|
| ||||
| Hypertension | 23,744 (46.6) | 11,974 (50.1) | 11,770 (43.5) | <0.0001 |
|
| ||||
| Diabetes | 7,662 (15.0) | 4,776 (20.0) | 2,886 (10.7) | <0.0001 |
|
| ||||
| Hyperlipidemia | 24,006 (47.1) | 11,456 (47.9) | 12,550 (46.4) | 0.001 |
|
| ||||
| Current/past smoker | 19,362 (39.9) | 8,807 (38.8) | 10,555 (40.9) | <0.0001 |
|
| ||||
| Exercise in METs-min/wk | 1,155 (269, 2,041) | 817 (1, 1,635) | 1,431 (484, 2,378) | <0.0001 |
|
| ||||
| Sleep disorders | 13,852 (27.2) | 7,190 (30.1) | 6,662 (24.6) | <0.0001 |
|
| ||||
| Depression | 14,115 (27.7) | 7,952 (33.2) | 6,163 (22.8) | <0.0001 |
|
| ||||
| Anxiety disorder | 17,205 (33.8) | 8,995 (37.6) | 8,210 (30.4) | <0.0001 |
|
| ||||
| Educational level | <0.0001 | |||
| Grade school (1-4 y) | 55 (0.1) | 48 (0.2) | 7 (0.03) | |
| Grade school (5-8 y) | 135 (0.3) | 111 (0.5) | 24 (0.1) | |
| Some high school (9-11 y) | 502 (1.0) | 358 (1.5) | 144 (0.5) | |
| High school diploma | 4,045 (8.0) | 2,628 (11.0) | 1,417 (5.2) | |
| Some college | 5,815 (11.4) | 3,341 (14.0) | 2,474 (9.2) | |
| 2-year college or vocational school | 4,631 (9.1) | 2,709 (11.4) | 1,922 (7.1) | |
| 4-year college | 15,820 (31.1) | 6,635 (27.9) | 9,185 (34.0) | |
| Masters, doctoral, or professional degree | 19,824 (39.0) | 7,992 (33.5) | 11,832 (43.8) | |
|
| ||||
| Employed | 38,407 (75.6) | 17,083 (71.8) | 21,324 (79.0) | <0.0001 |
|
| ||||
| Median income | $84,305 ($65,169, $103,441) | $81,216 ($62,492, $99,941) | $86,080 ($68,108, $104,053) | <0.0001 |
|
| ||||
| Charlson index | <0.0001 | |||
| 0 points, 98% 10-y survival | 7,624 (15.0) | 3,222 (13.5) | 4,402 (16.3) | |
| 1 point, 96% 10-y survival | 5,343 (10.5) | 2,420 (10.1) | 2,923 (10.8) | |
| 2 points, 90% 10-y survival | 5,405 (10.6) | 2,385 (10.0) | 3,020 (11.2) | |
| 3 points, 77% 10-y survival | 5,330 (10.5) | 2,428 (10.2) | 2,902 (10.7) | |
| 4 points, 53% 10-y survival | 4,696 (9.2) | 2,099 (8.8) | 2,597 (9.6) | |
| 5 points, 21% 10-y survival | 4,177 (8.2) | 1,913 (8.0) | 2,264 (8.4) | |
| 6 points, 2% 10-y survival | 3,354 (6.6) | 1,531 (6.4) | 1,823 (6.6) | |
| ≥7 points, 0.009% 10-y survival | 14,691 (28.8) | 7,770 (32.5) | 6,921 (25.6) | |
|
| ||||
| PRSss, mean Z-score ± SD | −0.0477 ± 1.0058 | 0.0013 ± 1.0074 | −0.0918 ± 1.0024 | <0.0001 |
|
| ||||
| PRSss, top quintile (%) | 1,747 (20.0) | 900 (21.7) | 847 (18.4) | <0.0001 |
Values are median (Q1, Q3), n (%), or mean ± SD, unless otherwise indicated. P values are reported from chi-square for categorical and Wilcoxon Mann-Whitney test for continuous variables. MET = metabolic equivalence of task; PRSss = polygenic risk score for neuroticism (a genetic index for stress sensitivity).
LIGHT/MODERATE ALCOHOL CONSUMPTION AND SUBSEQUENT MACE.
During a median follow-up of 3.4 years (IQR: 2.0-4.8 years), 1,914 individuals experienced incident MACE after enrollment. Notably, we observed a U-shaped relationship between alcohol consumption and MACE (Supplemental Figure 2), whereby reduced MACE incidence was seen with light/moderate alcohol (vs none/minimal consumption) but not high alcohol consumption. Light/moderate alcohol (vs none/minimal) associated with reduced MACE (HR: 0.786; 95% CI: 0.717-0.862; P < 0.0001) after adjusting for age, sex, and CVD risk factors (Supplemental Table 3, Supplemental Figure 3) and remained associated with reduced MACE risk in models further adjusted for socioeconomic factors, health behaviors, and psychological/medical comorbidities (all P < 0.01) (Supplemental Table 3). Additionally, light/moderate alcohol consumption (vs none/minimal) associated with a reduced incidence of the major subcomponents of MACE (Figure 2). Importantly, however, light/moderate alcohol consumption associated with an increase in cancer (adjusted HR: 1.23; 95% CI: 1.14-1.33; P < 0.0001). Similar findings were seen when 10-year MACE risk was assessed (Supplemental Table 3).
Figure 2. Alcohol Consumption, Cardiovascular, and Cancer Risk.
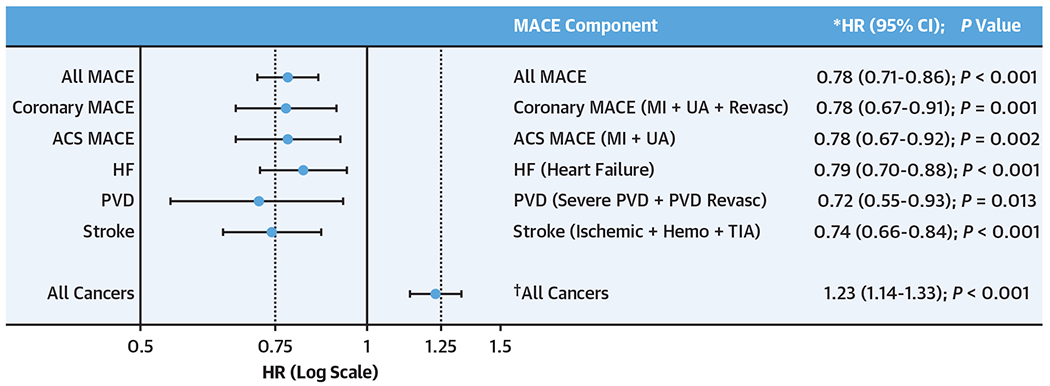
This figure shows HRs (dots) and 95% CIs (bars) for Light/moderate vs no/minimal alcohol consumption and different major adverse cardiovascular events (MACE) components and cancer (in log-scale). For the MACE components: *HRs were obtained from Cox regression models that included age, sex, hypertension, hyperlipidemia, diabetes mellitus, and smoking history. †For the composite cancer outcome: HR was obtained from a Cox regression model that included age, sex, body mass index, smoking, and the Charlson index. ACS = acute coronary syndrome; Hemo = hemorrhagic; HF = heart failure; MI = myocardial infarction; PVD = peripheral vascular disease; revasc = revascularization; TIA = transient ischemic attack; UA = unstable angina.
Next, we sought to account for a potential abstainer bias, in which the nondrinking group may include a disproportionate number of individuals who do not consume alcohol because of existing health concerns. To do so, we conducted sensitivity analyses wherein nondrinkers were excluded. In these analyses, the relationships between light/moderate alcohol consumption (vs minimal) and reduced MACE risk persisted (Supplemental Table 4). Further, there was a similar relationship between light alcohol consumption (vs none/minimal) and reduced MACE risk (Supplemental Table 5).
LIGHT/MODERATE ALCOHOL CONSUMPTION ASSOCIATES WITH DECREASED STRESS-RELATED NEURAL NETWORK ACTIVITY.
To test whether light/moderate alcohol associates with lower SNA, we evaluated the relationship between alcohol consumption and resting SNA in participants with PET imaging data. Among the 754 participants who underwent clinical 18F-FDG-PET/CT imaging, 366 (48.5%), 347 (46.0%), and 41 (5.4%) had none/minimal, light/moderate, and heavy alcohol consumption, respectively. Within this subset, we observed a U-shaped relationship between alcohol consumption and resting SNA (Central Illustration), whereby a reduced SNA was seen with light/moderate alcohol, but not high alcohol consumption. Notably, light/moderate (vs none/minimal) alcohol consumption associated with decreased SNA (ie, the ratio of amygdalar SUV to vmPFC SUV) in a model adjusted for age and sex (standardized beta: −0.192; 95% CI: −0.338 to −0.046; P = 0.01) (Table 2, Central Illustration), remaining robust to further adjustments for socioeconomic factors, lifestyle factors, genetic factors, and the Charlson index.
CENTRAL ILLUSTRATION. Alcohol Consumption, Stress-Related Neural Network Activity, and Cardiovascular Risk.
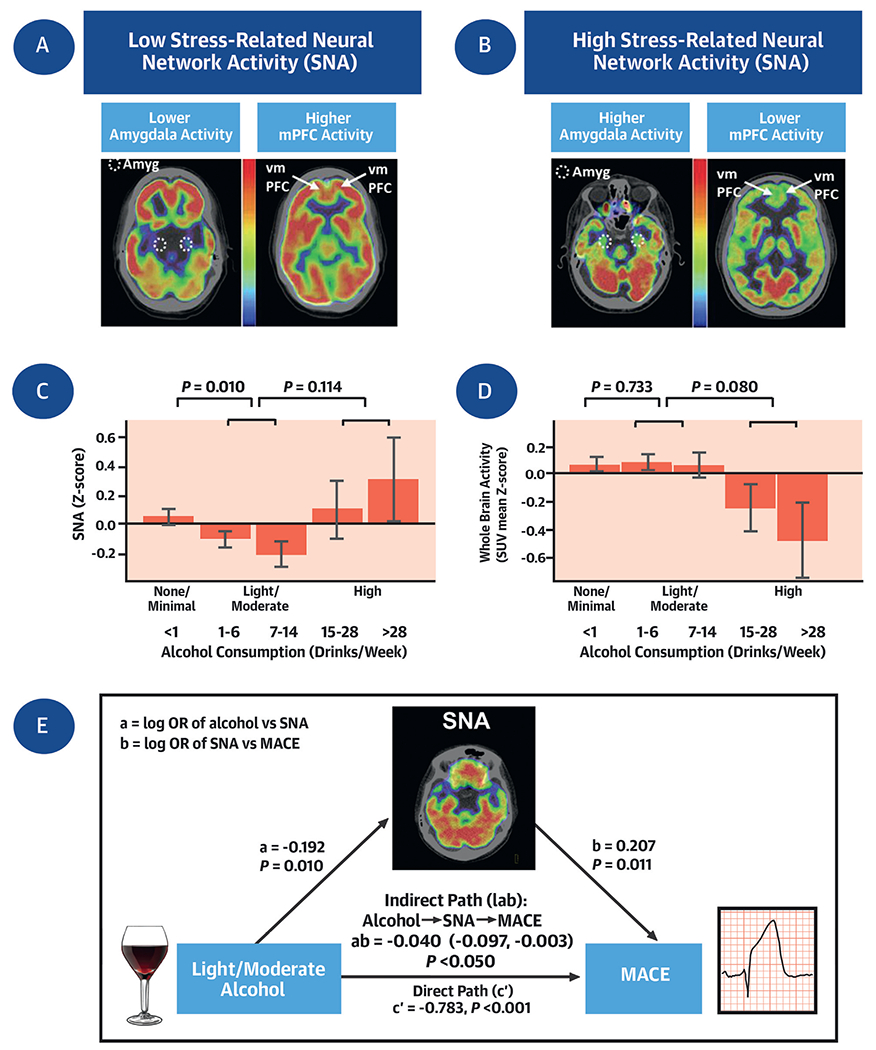
(A and B) Axial brain 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) images with low and high stress-related neural network activity (SNA) (as amygdala activity [circle] divided by ventromedial prefrontal cortical [arrow] activity). (C and D) SNA and whole brain activity by alcohol consumption. Error bars represent SEM. (E) Mediation (path) analysis, which tested whether light/moderate alcohol consumption exerts its effect on major adverse cardiovascular events (MACE) risk via reductions in SNA. The hypothesized indirect path of light/moderate alcohol consumption → ↓SNA → ↓MACE was significant, thus supporting a role for neural pathways in the mechanisms linking light/moderate alcohol consumption to a reduction in MACE events.
Table 2.
Alcohol Consumption vs Stress-Associated Neural Network Activity
| Linear Regression Models | n | Standardized Beta (95% CI) for Light/Moderate vs None/Minimal Alcohol Consumption | P Value |
|---|---|---|---|
| Model 1 | 713 | −0.192 (−0.338 to −0.046) | 0.010 |
| Model 2 | 695 | −0.202 (−0.352 to −0.052) | 0.008 |
| Model 3 | 698 | −0.190 (−0.337 to −0.042) | 0.012 |
| Model 4 | 713 | −0.189 (−0.335 to −0.043) | 0.011 |
| Model 5 | 378 | −0.206 (−0.395 to −0.016) | 0.034 |
Dependent variable: stress-associated neural network activity measured as amygdalar/ventromedial prefrontal cortex standardized uptake values. Independent variable: Alcohol consumption (light/moderate vs none/minimal). Model 1: linear regression adjusted for age and sex. Model 2: model 1 + socioeconomic factors (education + employment + income). Model 3: model 1 + lifestyle factors (exercise + smoking). Model 4: model 1 + Charlson index (medical comorbidities). Model 5: model 1 + PRSss (polygenic risk score for neuroticism).
Because SNA is measured as the ratio of amygdalar activity (Amyg) divided by regulatory activity of the vmPFC, we next assessed the impact of light/moderate alcohol activity on these individual components of SNA (Supplemental Figures 4A and 4B). In an analysis adjusted for age and sex, we observed that light/moderate alcohol (vs none/minimal) associated with lower amygdala activity (standardized amygdala SUV relative to whole brain activity, standardized beta: −0.172; 95% CI: −0.313 to −0.031; P = 0.017) (Supplemental Figure 4A). On the other hand, vmPFC activity (standardized vmPFC SUV relative to whole brain activity) did not differ between the light/moderate alcohol and none/minimal groups (−0.002; 95% CI: −0.147 to 0.144; P = 0.982) (Supplemental Figure 4B). Although whole brain metabolic activity did not change with light/moderate alcohol consumption (vs none/minimal), there was a trend toward decreased brain activity with high alcohol consumption (Central Illustration).
Next, we assessed the impact of SNA on MACE in this cohort, as done previously.10 We again observed that higher SNA predicted greater MACE risk (OR: 1.194; 95% CI: 1.006-1.418; P = 0.042) in a model adjusted for age, sex, and CVD risk factors. Furthermore, SNA positively associated with measures of atherosclerosis (as coronary artery calcium score and arterial 18F-FDG uptake) and with inflammatory and leukopoietic indices. Light/moderate alcohol consumption was generally inversely associated with those measures (Supplemental Table 6).
THE ASSOCIATION BETWEEN LIGHT/MODERATE ALCOHOL CONSUMPTION AND CVD EVENTS IS MEDIATED BY LOWER STRESS-RELATED NEURAL NETWORK ACTIVITY.
Mediation analysis was conducted to evaluate the putative mechanism by which light/moderate alcohol consumption may lead to reduced MACE. Specifically, we evaluated the hypothesized path: light/moderate alcohol consumption (vs none/minimal) → ↓SNA → ↓MACE risk. This analysis demonstrated that the indirect path wherein SNA mediates the link between light/moderate alcohol consumption and MACE was significant in a model adjusted for age and sex (log OR: −0.040; 95% CI: −0.097 to −0.003; P < 0.05) (Central Illustration).
CVD BENEFITS OF LIGHT/MODERATE ALCOHOL CONSUMPTION ARE HIGHER AMONG INDIVIDUALS WITH PRIOR ANXIETY DISORDERS.
Given that the mediation analysis suggested that impact of light/moderate alcohol on CVD events may occur via decreased SNA, we next hypothesized that light/moderate alcohol might have greater CVD benefits among individuals with greater chronic stress (a condition known to associate with higher SNA).14,23,24 Thus, within the overall study population, we compared the effect of light/moderate alcohol consumption (vs none/minimal) on incident MACE among individuals with (vs without) a prior history of anxiety. We observed that light/moderate alcohol consumption was associated with greater relative decreases in incident MACE after enrollment among individuals with (vs without) a history of anxiety in models adjusted for age, sex, and CVD risk factors (HR: 0.71 vs 0.83), although the interaction term was nonsignificant (P interaction = 0.17). Moreover, in analyses that utilized a 10-year MACE horizon, the relative reduction in HR associated with light/moderate alcohol consumption (vs low/none) was substantially greater among those with (vs without) baseline anxiety, with a significant interaction term (HR: 0.60 vs 0.78; P interaction = 0.003) (Figure 3).
Figure 3. Alcohol Consumption and Cardiovascular Risk in Subjects With vs Without Anxiety.
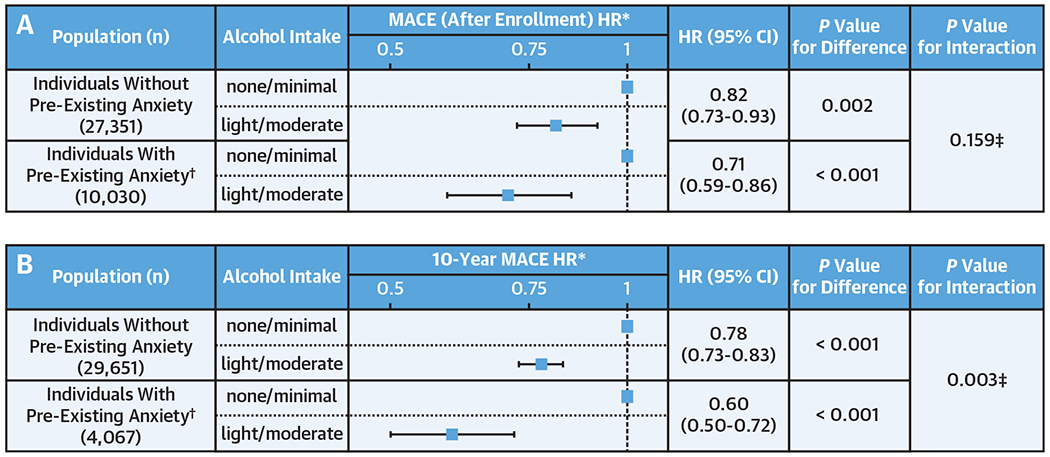
The figure shows the effect of alcohol consumption on MACE among those with vs without anxiety: (A) incident MACE event analysis (date of enrollment to last follow-up) and (B) 10-year MACE event analysis. HRs are shown as boxes with 95% CIs as bars, displayed in log-scale. *Adjusted for age, sex, and cardiovascular risk factors (hypertension, diabetes mellitus, hyperlipidemia, and smoking). †Anxiety after the index date (date of consent and December 2010, respectively for A and B) is excluded. ‡Interaction term: alcohol consumption (none/minimal vs light/moderate) × history of anxiety (yes vs no).
DISCUSSION
Although light/moderate alcohol consumption has repeatedly been found to associate with lower CVD risk (vs none/minimal consumption), the independence of this effect has been challenging to disentangle, and the mechanisms mediating this effect have not been clearly defined. In the current study, we leveraged a large, well-characterized Biobank cohort and observed a U-shaped association between alcohol consumption and MACE. Further, light/moderate alcohol consumption associated with reduced MACE risk (vs none/minimal intake) after accounting for potential confounders, including demographic and socioeconomic factors as well as health behaviors. Moreover, using advanced brain imaging, we observed that light/moderate alcohol consumption (vs none/minimal) associated with decreased resting stress-associated neural network activity (mainly by reducing amygdalar activity) and that this neural effect mediated the beneficial effect of light/moderate alcohol consumption on MACE. As an extension of this finding, we observed that alcohol associates with greater effects on CVD risk reduction among individuals with a history of anxiety in the overall study population. These findings yield insights into mechanisms by which alcohol may improve MACE risk and suggest that interventions targeting stress-associated neural networks may improve CVD outcomes. However, because light/moderate alcohol consumption also associates with adverse noncardiac effects, such as a heightened risk of cancer, alternative approaches to reduce SNA are needed.
Studies over several decades have described a reduction in CVD events with light/moderate alcohol consumption.1–4 Other studies have raised doubts that light/moderate alcohol consumption has protective effects against CVD, citing the possibility of residual confounders to explain the observed association.6,7 Although the effect of confounders has long been debated, a recent study of 330,000 individuals suggested that light/moderate alcohol consumption reduces CVD risk after robust adjustment for socioeconomic variables and health behaviors.5 The current study provides important validation of those findings. Additionally, prior studies raised uncertainty regarding the protective effect of light/moderate alcohol, citing possible abstainer bias.25 However, in the current study, this possibility was also addressed by conducting a sensitivity analysis that excluded abstainers in which similar results were obtained.
The current study provides novel insights into a mechanism by which light/moderate alcohol may reduce CVD. Using 18F-FDG-PET/CT brain imaging, light/moderate alcohol consumption associated with decreased SNA independently of key confounding factors. This effect on SNA appears to be driven by decreased activity of the amygdala rather than by enhanced activity of the regulatory vmPFC. Although several studies previously reported that alcohol ingestion acutely decreases amygdalar activation,11,12 this study is the first to demonstrate a chronic neurobiological effect (as SNA) of light/moderate alcohol on this structure. Further, the observation that light/moderate alcohol consumption did not significantly change resting metabolic activity in the vmPFC (or whole brain) deserves further discussion. It is important to assert that excessive alcohol consumption induces deleterious effects on the hippocampus, corpus callosum, mamillary bodies, and cerebellum, among other brain regions.26 Indeed, in the current study, we observed a trend toward a reduction in whole brain metabolism among heavy drinkers. However, there is equipoise regarding the impact of light/moderate alcohol consumption on brain health. Some studies suggest adverse effects of light/moderate alcohol (eg, increased dementia risks, hippocampal atrophy, and reduced total brain volume),27 whereas others suggest more favorable effects (eg, improved cognition, lower dementia risk, and larger total brain volumes).28 Although the current study was not powered to discern the overall impact of light/moderate alcohol on higher brain function, it was found to associate with a substantial reduction in amygdalar activity relative to vmPFC activity, resulting in overall reductions in SNA. This finding is important, given the established association between heightened SNA and downstream CVD,10,13 and partially explains the protective effect of light/moderate alcohol (vs none/minimal) on CVD risk in this population. In the current study, we again observed that SNA is associated with MACE as well as heightened leukopoietic activity and heightened arterial inflammation. Furthermore, we observed that light/moderate alcohol is associated with reduced leukopoietic activity and high-sensitivity C-reactive protein. Taken together, these results suggest that light/moderate alcohol may reduce MACE through down-regulation of a neural-leukopoietic-arterial axis that otherwise potentiates CVD.10,13
The finding that alcohol reduces CVD risk by attenuating SNA prompted an evaluation of whether alcohol’s CVD benefits are greater among individuals with a history of anxiety (a condition known to associate with higher SNA).14,23,24 We observed that light/moderate alcohol consumption (vs none/minimal) had a larger relative impact on CVD risk among individuals with a pre-existing anxiety disorder. This observation provides important support for the conclusions provided by the imaging substudy, which suggested that lower SNA may mediate light/moderate alcohol’s CVD benefits.
Nevertheless, despite the findings that light/moderate alcohol consumption may improve cardiovascular risk, this benefit must be carefully weighed against its potential adverse impacts on other noncardiac disease processes (eg, malignancy, dependence/abuse). In the current study, we found that even light/moderate alcohol consumption associates with heightened cancer risk. Additionally, with higher intakes of alcohol, we observed decreases in prefrontal and whole brain activity. Such findings may associate with adverse cognitive health.29 Ultimately, an intervention that acts similarly on SNA without alcohol’s potential detrimental effects would be a far more attractive therapeutic option.
STUDY LIMITATIONS.
An important limitation is the current study’s observational design. Although statistical analyses were adjusted for numerous potential confounders, there remains the possibility that the findings may have resulted from factors that were not assessed. Further, ICD codes were used to identify MACE, which might lead to the misclassification of events. We could not fully distinguish individuals who consumed 1 drink/d from those who consumed 2 drink/d because of the limitations of the survey instrument (Supplemental Figure 1A). This distinction is important because the dietary guidelines recommend not more than 1 drink/d for women and 2 drinks/d for men. The alcohol consumption assessment was based on self-reported intake at the time of enrollment; some participants may have underreported their alcohol consumption (which could mean that lowering of MACE risk could occur with higher daily intakes than those reported), and drinking patterns may have changed over the course of the follow-up period. Our survey instrument did not record the status of former drinkers, and this could have introduced an abstainer bias. However, we performed sensitivity analysis with the available data that omitted all abstainers and obtained similar results.
Due to the structure of the health questionnaire, it was also not possible to analyze or distinguish the impact of other aspects of alcohol consumption that contribute substantially to final health outcomes, such as the role of different alcoholic beverages, drinking with or without food, binge drinking vs regular moderate drinking, and so on.
Although SNA tends to be stable over time,30 the imaging findings relied on a single measurement of SNA. Additionally, the subset of participants who underwent 18F-FDG-PET/CT imaging did so for clinical indications (most commonly cancer surveillance). Thus, the related findings may not be generalizable to the broad population. Moreover, although a decrease in vmPFC and whole brain activities with light/moderate alcohol consumption was not observed, the study may have had limited power to observe such an effect. Larger prospective studies could be conducted to overcome several of these limitations and could also extend the imaging evaluations (eg, via PET/cardiac magnetic resonance imaging).
CONCLUSIONS
This study’s results suggest that the benefit of light/moderate (vs none/minimal) alcohol consumption on CVD risk in part stems from its ability to attenuate stress-related neural network activity. As an extension of this finding, we observed that the beneficial impact of light/moderate alcohol intake on MACE in this population was nearly twice as great among individuals with (vs without) anxiety. However, the CVD observations were counterbalanced by adverse findings related to malignancy. New interventions with positive effects on the neurobiology of stress but without the potentially deleterious effects of alcohol (eg, increased risk of malignancy) are needed.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Light/moderate alcohol intake reduces stress-associated neural network activity as assessed by relative amygdala to cortical PET-FDG uptake in association with decreased cardiovascular risk.
TRANSLATIONAL OUTLOOK:
A better understanding of the effect of alcohol on stress-associated neural network activity could pave the way for novel treatments that achieve similar cardiovascular risk reduction without adverse effects.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This study was in part supported by National Institutes of Health grants 1R01AR077187 (to Dr Tawakol), 5P01HL131478 (to Dr Tawakol), K23HL151909 (to Dr Osborne). Dr Osborne has served as a consultant to WCG Intrinsic Imaging, LLC, unrelated to this work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- 18F-FDG-PET
18F-fluorodeoxyglucose positron emission tomography
- CVD
cardiovascular disease
- ICD
International Classification of Diseases
- MACE
major adverse cardiovascular event(s)
- SNA
stress-associated neural network activity
- vmPFC
ventromedial prefrontal cortex
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Alcohol consumption and mortality in patients with cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2010;55:1339–1347. [DOI] [PubMed] [Google Scholar]
- 2.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–2445. [DOI] [PubMed] [Google Scholar]
- 4.Wood AM, Kaptoge S, Butterworth AS, et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018;391:1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xi B, Veeranki SP, Zhao M, Ma C, Yan Y, Mi J. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in U.S. adults. J Am Coll Cardiol. 2017;70:913–922. [DOI] [PubMed] [Google Scholar]
- 6.Rosoff DB, Davey Smith G, Mehta N, Clarke TK, Lohoff FW. Evaluating the relationship between alcohol consumption, tobacco use, and cardiovascular disease: a multivariable Mendelian randomization study. PLoS Med. 2020;17:e1003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biddinger KJ, Emdin CA, Haas ME, et al. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw Open. 2022;5:e223849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du D, Bruno R, Blizzard L, et al. The metabolomic signatures of alcohol consumption in young adults. Eur J Prev Cardiol. 2020;27:840–849. [DOI] [PubMed] [Google Scholar]
- 9.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tawakol A, Ishai A, Takx RA, et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorka SM, Fitzgerald DA, King AC, Phan KL. Alcohol attenuates amygdala-frontal connectivity during processing social signals in heavy social drinkers: a preliminary pharmaco-fMRI study. Psychopharmacology (Berl). 2013;229:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sripada CS, Angstadt M, McNamara P, King AC, Phan KL. Effects of alcohol on brain responses to social signals of threat in humans. Neuroimage. 2011;55:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal A, Dey AK, Chaturvedi A, et al. Chronic stress-related neural activity associates with subclinical cardiovascular disease in psoriasis: a prospective cohort study. J Am Coll Cardiol Img. 2020;13:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox AS, Oler JA, Shelton SE, et al. Central amygdala nucleus (Ce) gene expression linked to increased trait-like Ce metabolism and anxious temperament in young primates. Proc Natl Acad Sci U S A. 2012;109:18108–18113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro VM, Gainer V, Wattanasin N, et al. The Mass General Brigham Biobank Portal: an i2b2-based data repository linking disparate and high-dimensional patient data to support multimodal analytics. J Am Med Inform Assoc. 2022;29:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The U.S. Department of Agriculture and the U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th ed. U.S. Government Printing Office; 2020. [Google Scholar]
- 17.Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry. 2015;77:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 20.Nagel M, Jansen PR, Stringer S, et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat Genet. 2018;50:920–927. [DOI] [PubMed] [Google Scholar]
- 21.de Moor MH, van den Berg SM, Verweij KJ, et al. Meta-analysis of genome-wide association studies for neuroticism, and the polygenic association with major depressive disorder. JAMA Psychiatry. 2015;72:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Third ed. The Guilford Press; 2022. [Google Scholar]
- 23.Liu WZ, Zhang WH, Zheng ZH, et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat Commun. 2020;11:2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He C, Gong L, Yin Y, et al. Amygdala connectivity mediates the association between anxiety and depression in patients with major depressive disorder. Brain Imaging Behav. 2019;13:1146–1159. [DOI] [PubMed] [Google Scholar]
- 25.Fillmore KM, Stockwell T, Chikritzhs T, Bostrom A, Kerr W. Moderate alcohol use and reduced mortality risk: systematic error in prospective studies and new hypotheses. Ann Epidemiol. 2007;17:S16–S23. [DOI] [PubMed] [Google Scholar]
- 26.Zahr NM, Kaufman KL, Harper CG. Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol. 2011;7:284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357:j2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruitenberg A, van Swieten JC, Witteman JC, et al. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281–286. [DOI] [PubMed] [Google Scholar]
- 29.Clergue-Duval V, Questel F, Azuar J, et al. Brain 18FDG-PET pattern in patients with alcohol-related cognitive impairment. Eur J Nucl Med Mol Imaging. 2020;47:281–291. [DOI] [PubMed] [Google Scholar]
- 30.Schaefer SM, Abercrombie HC, Lindgren KA, et al. Six-month test-retest reliability of MRI-defined PET measures of regional cerebral glucose metabolic rate in selected subcortical structures. Hum Brain Map. 2000;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


