Abstract
Osteosarcoma (OS) is a highly prevalent bone malignancy among adolescents, accounting for 40% of all primary malignant bone tumors. Neoadjuvant chemotherapy combined with limb‐preserving surgery has effectively reduced patient disability and mortality, but pulmonary metastases and OS cells' resistance to chemotherapeutic agents are pressing challenges in the clinical management of OS. There has been an urgent need to identify new biomarkers for OS to develop specific targeted therapies. Recently, the continued advancements in genomic analysis have contributed to the identification of clinically significant molecular biomarkers for diagnosing OS, acting as therapeutic targets, and predicting prognosis. Additionally, the contemporary molecular classifications have revealed that the signaling pathways, including Wnt/β‐catenin, PI3K/AKT/mTOR, JAK/STAT3, Hippo, Notch, PD‐1/PD‐L1, MAPK, and NF‐κB, have an integral role in OS onset, progression, metastasis, and treatment response. These molecular classifications and biological markers have created new avenues for more accurate OS diagnosis and relevant treatment. We herein present a review of the recent findings for the modulatory role of signaling pathways as possible biological markers and treatment targets for OS. This review also discusses current OS therapeutic approaches, including signaling pathway‐based therapies developed over the past decade. Additionally, the review covers the signaling targets involved in the curative effects of traditional Chinese medicines in the context of expression regulation of relevant genes and proteins through the signaling pathways to inhibit OS cell growth. These findings are expected to provide directions for integrating genomic, molecular, and clinical profiles to enhance OS diagnosis and treatment.
Keywords: clinic treatment, osteosarcoma, signaling pathway, traditional Chinese medicine
1. INTRODUCTION
Osteosarcoma (OS) is one of the most prevalent primary bone malignancies. 1 In addition to chondrosarcoma and Ewing's tumor, OS, also known as osteogenic sarcoma, originates from the mesenchymal tissue 2 and accounts for 40% of all cases of primary malignant bone tumors, with an annual incidence of one to three cases per million people. 2 OS primarily affects adolescents, mainly in the lower femur, upper tibia, and proximal humeral epiphysis. 3 The pathological characteristics of OS are osteoid tissue produced by spindle‐shaped mesenchymal cells, with extensive tissue heterogeneity, high local invasiveness, rapid infiltration, metastasis, and poor clinical prognosis. 4 Although significant advances in the treatment and prognosis of OS were made in the 1970s and 1980s, there have been no breakthroughs in recent decades. 5 Neoadjuvant chemotherapy combined with limb preservation has become the preferred clinical treatment option for OS, 6 and combined treatment with immunotherapy, molecular targeted therapy, and traditional Chinese medicine (TCM) has also greatly reduced the morbidity and mortality of OS patients. 7 Although the implementation of various treatment options has enhanced the 5‐year survival rate for OS patients to 60−70% without distant metastases, approximately 20−40% of patients still die from distant metastases. 8 Moreover, the adverse effects of radiotherapy and the resistance of OS to chemotherapeutic drugs are urgent problems in the clinical treatment of OS. 9
Signaling pathways are signal networks wherein information is transferred between the interior and exterior regions of cells. This process includes the interaction among multiple molecules and proteins to transmit information through a series of molecules such as signaling molecules, signaling receptors, and signal transduction factors. 10 These signaling pathways regulate cell growth, differentiation, apoptosis, metabolism, and other physiological and pathological processes. 11 Accumulating evidence suggests that abnormal activation of signaling pathways is an important factor contributing to tumorigenesis and progression. 12 , 13 , 14 In OS, the signaling pathways phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR), 15 Wnt/β‐catenin, 16 Janus kinase (JAK)/signal transducers and activators of transcription 3 (STAT3), 17 Hippo, 18 programmed death‐1 (PD‐1)/programmed death ligand‐1 (PD‐L1), 19 Notch, 20 mitogen‐activated protein kinase (MAPK), 21 and nuclear factor kappa B (NF‐κB) 22 perform a fundamental function in OS development. Additionally, multiple factors including drugs, proteins, RNA, and genes either positively or negatively regulate tumor cells proliferation, invasion, and apoptosis in OS by affecting the signaling pathways JAK/STAT3, Hippo, Wnt/β‐catenin, PD‐1/PD‐L1, Notch, MAPK, PI3K/AKT/mTOR, NF‐κB, and others (Figure 1). However, identifying novel biomarkers for OS is urgently needed to develop novel and specific targeted therapies at the molecular level to improve the survival of patients with OS.
FIGURE 1.
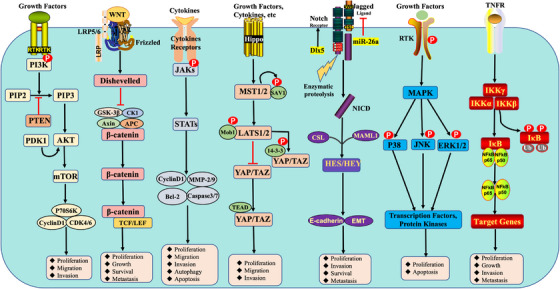
Main signaling pathways and fundamental factors in osteosarcoma. The major signaling of PI3K/AKT/mTOR, Wnt/β‐linked protein, JAK/STAT3, Hippo, PD‐1/PD‐L1, Notch, MAPK, and NF‐κB pathways were elucidated, as well as their regulatory roles in cellular processes.
To offer a thorough overview of the recent research advances on the mechanisms and clinical investigations of OS‐targeted signaling pathways, we examine each of the OS‐related signaling pathways in this review. TCM has become a hot spot for OS treatment because of its broad action, low side effects, and multiple targeting functions. 23 , 24 Therefore, we have focused on the signaling targets involved in the preventive and curative effects of TCM on OS, which act by modulating the expression of relevant proteins and genes through signaling pathways to inhibit OS cells' growth, proliferation, migration, invasion, and metastasis. This review was conducted to provide ideas and discover new treatment options for OS treatment, improve patient prognosis, and increase the overall survival of patients.
2. SIGNALING PATHWAYS IN OS
Recently, research evidence has emerged showing that the dysregulation of various intracellular signaling pathways is closely related to the onset and progression of OS, and to treat OS, it is crucial to research the signaling pathways linked to its development. The PI3K/AKT/mTOR signaling pathway, one of the crucial pathways in cancerous cells, has been demonstrated to have the ability to enhance the cell cycle, inhibit apoptosis, and promote cellular proliferation, invasion, and metastasis. The modulation of OS cellular proliferation, apoptosis, and vascular neogenesis, among other processes, is critically controlled by the JAK/STAT3 axis, a classical intracellular signaling pathway. Numerous mammalian cells and organs are subject to homeostatic modulation by the Hippo signaling pathway, which also controls OS cellular proliferation, apoptosis, invasiveness, and metastasis. The immunosuppression and weakening of immune cell killing effect on tumor cells lead to immune escape of OS cells, consequently culminating in the occurrence and development of OS. Abnormal activation of the MARK signaling pathway leads to loss of apoptosis and differentiation of OS cells, which is intimately correlated with the malignant transformation and abnormal proliferation of OS cells. The OS's ability to proliferate, differentiate, and resist medication is influenced by the Notch signaling pathway. Furthermore, an important factor regulating OS cell proliferation, metastasis, and drug resistance is the NF‐κB pathway. This section examines the possible biomarkers for OS as well as the modulatory functions of the aforementioned signaling pathways.
2.1. PI3K/AKT/mTOR signaling pathway and its role in OS
2.1.1. Overview of PI3K/AKT/mTOR signaling pathway
In 1984, a new signaling pathway intimately correlated with the activity of tyrosine kinase encoded by the viral oncogene was identified. In 1988, Cantley and Downes' team discovered the virus‐associated tyrosine kinase as PI3K, which generates PI3K via the mechanism of phosphorylating the 3‐OH group on the inositol ring. 25 , 26 This signaling pathway involved lipid kinases and was subsequently termed the PI3K signaling pathway. 27 Further research identified AKT, mTOR, and other genes were shown to be present downstream of the PI3K signaling pathway. Thus, this pathway was subsequently named as PI3K/AKT/mTOR signaling pathway. As one of the most crucial intracellular signaling mechanisms, it regulates cellular biological processes like cellular differentiation, proliferation, and migration. 28
The PI3K/AKT/mTOR pathway regulates various cellular functions by integrating multiple extracellular signals. 29 PI3K is an intracellular phosphatidylinositol kinase and a key component of the cell membrane. It has both phosphatidylinositol kinase activity and serine/threonine (Ser/Thr) kinase activity. 30 The PI3K/AKT pathway particularly involves type I PI3K, which is a heterodimer made up of the regulatory subunit (p85) and the catalytic subunit p110 31 and is most closely related to cancer. 32 The structural domains SH2 and SH3 found in the regulatory subunit may interact with target proteins that have matching binding sites. 33 P85 is recruited to the adjoining plasma membrane in response to postsignaling signals from class I PI3K acceptor receptor tyrosine kinase and G‐protein‐coupled receptors. At this point, phosphatidylinositol‐4,5‐bisphosphate (PIP2) is converted to phosphatidylinositol‐3,4,5‐trisphosphate (PIP3) by the binding action of p110 to p85, thus resulting in the further activation of AKT. 34
Phosphatase and tensin homolog (PTEN) is an antagonist of PI3K. PIP3 functions as a second messenger and binds to the intracellular signaling protein AKT through the pleckstrin homology (PH) domain. After binding, the complex undergoes conformational changes, causing AKT to translocate from the cytoplasm to the cell membrane. 35 Consequently, phosphorylation of AKT kinase activity is enhanced by the synergistic action of phosphoinositide‐dependent protein kinase‐1 (PDK‐1) at the Thr308 site. 36 The binding of lipid products to the PH domain of AKT promotes PDK‐1/PDK, a complex that can phosphorylate the Ser473 site of AKT. 37 When the Thr308 and Ser473 sites of AKT are phosphorylated, AKT becomes activated, thereby promoting cell cycle progression by regulating glycogen synthase kinase 3β (GSK3β) and cyclin. 38 , 39
mTOR is an important Ser/Thr protein kinase present downstream of PI3K/AKT. 40 mTOR forms two different protein complexes: mTOR C1 and mTOR C2. Activated AKT derepresses Rheb (ras homolog enriched in the brain) through its direct phosphorylation of mTORC1 or inhibition of the formation of the tuberous sclerosis complex 1/2 (TSC1/2) dimer, thereby enhancing mTORC1 activation. 41 The eukaryotic translation initiation factor 4E‐binding protein‐1 (4EBP‐1) and ribosomal protein S6 kinase (S6K) and are two examples of downstream effectors that are phosphorylated by the activated mTORC1. However, activated S6K phosphorylates ribosomal protein S6 (rpS6) to enhance ribosomal translation efficiency. 42 The activated mTORC1 phosphorylates the translation repressor 4EBP‐1, which attenuates its inhibitory effects on the eukaryotic translation initiation factor 4E (eIF4E) and promotes the initiation of protein translation. 43 Therefore, The PI3K/AKT/mTOR signaling pathway is among the most critical signaling pathways in tumor cells since it stimulates cellular proliferation, invasion, and metastasis while also accelerating cell cycle and inhibiting apoptosis. 44
2.1.2. PI3K/AKT/mTOR signaling pathway and OS development and progression
In OS, the PI3K/AKT/mTOR pathway frequently experiences changes. The high frequency of PI3K mutations and/or their increased catalytic function in tumor cells is a key mechanism of proliferation and metastasis in OS. 45 The class I PI3K p110 catalytic subunit, namely, PI3K p110a, is encoded by the PIK3CA gene. The class I PI3K “PI3K p110a” has both lipid‐like kinase and protein kinase activities, both of which contribute to AKT activation, which can phosphorylate various substrates and participate in the modulation of signaling pathways related to cellular proliferation, differentiation, migration, and apoptosis. 46 Approximately four‐fifths of PIK3CA mutations take place in two hotspot regions: the kinase region (exon 20) and the helix region (exon 9). Mutations in these two regions not only reduce apoptosis but also promote tumor infiltration and increase the activity of the downstream kinase PI3K, contributing to the activation of the PI3K/AKT signaling pathway. 46 , 47 PIK3CA gene mutations increase the risk of OS aggressiveness, and LY294002, a specific inhibitor of PI3K, inhibits PI3K at the adenosine triphosphate (ATP)‐binding site activity, culminating in a decrease in p‐AKT, which is intimately linked to OS cells’ proliferation and apoptosis. 48
The PI3K axis is primarily activated by AKT. 49 Most OS patients have elevated expression levels of AKT and p‐AKT. Aberrant expression of p‐AKT is strongly correlated with the overexpression of PI3K and HER4, and high levels of p‐AKT are a marker of OS progression, metastasis, and poor prognosis. 50 Zhang et al. 51 performed the dual‐luciferase reporter gene assay and confirmed that both long‐stranded noncoding RNA (lncRNA) MSC‐AS1 and CDK6 have binding sites and can therefore bind to miR‐142. Silencing of lncRNA MSC‐AS1 enhanced miR‐142 expression, downregulated CDK6, and decreased phosphorylated protein kinase B (p‐AKT) and phosphorylated PI3K (p‐PI3K), all of which suppressed PI3K/AKT pathway activation, thereby slowing down OS progression and increasing its sensitivity to cisplatin. Silencing miR‐191‐5p upregulated protein expression of stem‐loop‐binding protein, AKT, and p‐AKT and activated the PI3K/AKT signaling pathway, consequently promoting MG63 cells proliferation, migration, and invasion. 52
PTEN chromosomal loss (antagonist of PI3K/AKT) is a common molecule in OS. 35 PTEN overexpression downregulated the expression of p‐AKT and p‐PI3K in OS cells. 53 Overexpression of miR‐524, 54 miR‐191‐5p, 52 miR‐744, 48 and lncRNA HULC 54 enhanced the upregulated PTEN levels and downregulated p‐PI3K and p‐AKT levels in OS cells. Additionally, p‐AKT has an important angiogenic role in OS via the activation of vascular endothelial growth factor (VEGF)‐A. 55
mTOR is a bridge to multiple downstream signaling pathways that could be triggered by a variety of upstream regulatory variables. 56 , 57 As one of the most independent components of the PI3K axis, mTOR is found at the end of the PI3K/AKT/mTOR signaling pathway. 58 Excessive mTOR activation might result from mutations in upstream regulatory factors from several axes, including endothelial growth factor receptor, PI3K, and PTEN. 46 , 59 , 60 Any disturbance in the regulation of mTOR activities can alter the modulation of OS cells’ growth and differentiation. 29 Furthermore, relative to normal bone tissues, OS tissues have substantially greater levels of mTOR expression. 61 , 62 Therefore, we hypothesized that mTOR expression might be employed as a biological marker for tumor invasion and metastasis in addition to OS diagnosis. The significance of the PI3K/AKT/mTOR signaling pathway in the onset and progression of OS suggests that this signaling axis is a viable target for the treatment of cancer.
2.2. Wnt/β‐catenin signaling pathway
2.2.1. Overview of Wnt/β‐catenin signaling pathway
The Wnt gene was discovered during the process of exploring the transcriptional mechanism of mouse mammary tumor virus and was initially named Int‐1. 63 , 64 Further investigation revealed that the wingless gene (from Drosophila) and Int‐1 were homologous and encoded the same protein, both of which were abbreviated as Wnt. 65 Wnt signaling pathways are of two categories: classical Wnt signaling pathways and nonclassical Wnt signaling pathways. 66 The Wnt/PCP (Wnt–c‐Jun N‐terminal kinase [JNK] pathway) and Wnt/Ca2+ pathway are examples of nonclassical Wnt signaling pathway. 67 The classical Wnt signaling pathway, including Wnt/β‐catenin, is one of the most well‐studied signaling pathways. 68
The major components of the Wnt/β‐catenin pathway include ligands (Wnt family proteins), receptors for Wnt family proteins (the primary receptor Frizzled [Fzd] protein and the coreceptor low‐density lipoprotein receptor‐related protein 5/6 [LRP‐5/6]), Dishevelled (Dsh), axin (Axin), glycogen synthase kinase 3β (GSK‐3β), casein kinase 1 (CK1), colorectal adenomatous polyposis coli (APC), and β‐linked protein (β‐catenin). 69 , 70 At rest, GSK‐3β, CK1, APC, and Axin form a “degradation complex,” which binds to free β‐catenin and phosphorylates it. The phosphorylated form is then ubiquitinated and degraded to maintain the cytoplasmic concentration of free β‐catenin at a relatively low level. 71 Upon the activation of the Wnt signaling pathway, Wnt family proteins respond by binding to Fzd proteins and/or LRP‐5/6, thereby activating the pontine protein Dsh, which dissociates the β‐catenin degradation complex comprising Axin, GSK‐3β, and APC. 72 The cytoplasmic β‐catenin is freed from the inhibition of the degradation complex and then accumulates within the cytoplasm and further enters the nucleus. 73 DNA is not directly bound by β‐catenin when it enters the nucleus. Instead, it binds to transcriptional T‐cell factor/lymphocyte enhancer‐binding factor (TCF/LEF), 74 which eventually initiates the transcription of cyclin D1, c‐myc, and other target genes. 75
β‐Catenin is a key signaling protein factor in the classical Wnt signaling pathway. 76 β‐Catenin was originally discovered as an adhesion factor, under normal physiological conditions, most of the β‐catenin molecules in the cytoplasm are bound to E‐cadherin present in the cell membrane to mediate adhesion between cells. 77 Later on, β‐catenin was identified as a multifunctional protein with important effects on normal human physiological functions. 78 Whether or not β‐catenin acts via the accumulation of β‐catenin signals is the main basis for distinguishing between classical and nonclassical pathways. 79 As a critical component of the Wnt/β‐catenin signaling pathway β‐catenin is inhibited by the β‐catenin degradation complex comprising GSK‐3β, CK1, APC, and Axin and is promoted by the β‐catenin antagonistic degradation complex comprising of Dsh, CK1, and GSK‐3β. 80 β‐Catenin expression is an important basis to determine whether there is abnormal activation of the Wnt/β‐catenin pathway. 81
2.2.2. Wnt/β‐catenin signaling pathway and OS development and progression
OS development occurs due to the activation or silencing of several signaling pathways. 82 The signaling pathway is associated with tumorigenesis, and both Wnt3a and Wnt10b can promote metastasis of mouse lesions in OS experiments due to the activation of β‐catenin expression. Experimental results confirm that Wnt signaling can reduce patient survival by regulating the metastasis of OS cells in an autocrine or paracrine manner. 83 In vitro experiments revealed that the activation of Wnt5a and U2OS cell lines showed significant aggressiveness and inhibition of Wnt5a inhibited OS aggressiveness. 84 It is clinically possible to target and intervene in this signaling pathway to treat OS. Human OS samples show a high association between the expression of WNT7B/WNT9A, LOXL2, and c‐FOS, and coexpression with c‐FOS/LOXL2 was linked to OS aggressiveness and shortened patient survival duration. 85 Syndcan2 (SDC2), a modulator of antitumor effects, inhibits SDC2 expression in the Wnt/β‐catenin pathway in OS cells, and reversal of the regulation increases SDC2 expression by secreting SFRP1, a Wnt signaling pathway antagonist, which improves OS disease progression. 86 Secretory Fzd‐related protein 2 (sFRP2) shows high expression in patients with OS and negatively correlates with patient survival. sFRP2 overexpression induces angiogenesis and drives osteoblast precursors into the OS phenotype, 87 , 88 whereas sFRP2 knockdown impairs its metastatic and invasive behavior. 89 sFRP3 blocks the paracrine microsecretory microenvironment provided by Wnt protein expression in OS cells. 90
The positive rate of β‐catenin in OS tissues is 70%, which differs from that in paraneoplastic tissues. β‐Catenin enables the invasion of OS cells and increased β‐catenin content in the nucleus can promote the epithelial‐to‐mesenchymal transition (EMT) of OS cells and increase stem cell formation, both of which could enhance the migration and invasiveness of tumor cells as well as the growth and spread of OS cells. 91 The development of tumor cells might be prevented by regulating the PI3K/AKT/GSK‐3β signaling pathway and inhibiting oxidative stress in the human OS SAOS‐2 cell line. The onset, progression, and metastasis of OS are all tightly linked to β‐Catenin expression in OS, which may be crucial for OS cell invasiveness and metastasis. β‐Catenin can be used as an indicator for OS diagnosis and metastasis prediction. Yang et al. 92 demonstrated that WIF‐1 expression, a suppressor of the Wnt/β‐catenin pathway, significantly reduced in OS cells. Furthermore, in an in vitro study of OS cells, reduced expression of WIF‐1 could lead to an abnormal accumulation of the β‐catenin protein within the cytoplasm, consequently contributing to overactivation of the classical Wnt signaling pathway and promotion of cell growth, cell proliferation, and disease progression. Overexpression of Dickkopf‐3 (DKK‐3), another Wnt signaling antagonist, retards tumor growth and lung metastasis in xenograft models. 93 LRP5 is frequently expressed in OS and is significantly associated with the chondroblast subtype and metastasis in OS. 94 , 95 , 96 Similarly, another in vivo study found that dominantly inactivated LRP5 impedes the tumorigenic potential and metastasis of OS. 97
2.3. JAK/STAT3 signaling pathway
2.3.1. Overview of JAK/STAT3 signaling pathway
The JAK/STAT signaling pathway regulates a variety of cytokines and is crucial for immune and metabolic regulation as well as biological activities including cellular division, differentiation, and apoptosis. 98 , 99 The tyrosine kinase‐related receptors, the JAK family, and the STAT family make up the pathway's three main components. Some of the members of the JAK family include JAK1, JAK2, JAK3, and TYK2. Conversely, the members of the STAT family comprise seven transcription factors (TFs), namely, STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6, 100 a group of over 30 transmembrane proteins that make up a superfamily. Evolutionarily, the JAK2/STAT3 signaling pathway has exhibited high evolutionary conservation and is considered the most important of all JAK/STAT signaling pathways, essentially covering the proinflammatory cytokine spectrum in human antigen‐presenting cells (APCs). 101
Different extracellular signals can activate the JAK/STAT3 signaling pathway in vivo, mainly through the binding of extracellular cytokines (interleukin [IL]‐6, IL‐10, and IL‐11, among others) and growth factors (VEGF, fibroblast growth factor, and EGF, among others) to cell surface receptors as ligands, which dimerize the receptor molecules and induce the polymerization of intracellular glycoprotein 130 (gp130) with the α‐subunit on the receptor. 102 The phosphorylation of tyrosine residues on the receptor is subsequently catalyzed by the activated JAKs. Further, the phosphorylated tyrosine site on the receptor and its surrounding amino acid sequence together form a mooring site in the SH2 region of the STAT3 protein. 103 JAK facilitates the STAT3 protein's binding to the receptor when it binds to this site, resulting in the activation and phosphorylation of STAT3, which, when entering the nucleus as a dimer, binds to DNA through its DNA‐binding domain, regulating the transcription of downstream genes. 104
2.3.2. JAK/STAT3 signaling pathway and OS development and progression
The most significant STAT3 upstream protein is JAK. Additionally, the most predominant class of indirect STAT3 inhibitors that work by preventing STAT3 activation is comprised of JAK inhibitors. Only a few studies have evaluated the role of JAK in OS. In recent years, the JAK/STAT3 signaling pathway has been extensively studied, and researchers have proven that inappropriate activation of JAK/STAT3 in OS is always linked to physiological events such as defective tumor cell migration, invasiveness, metastasis, and vascular neogenesis. 105 , 106
OS cell lines highly express STAT3 and p‐STAT3, and inhibition of STAT3 activation can downregulate Cyclin D1 expression and further inhibit the proliferative ability of OS cells. 107 , 108 , 109 The long‐chain noncoding RNA HOXD‐AS1 increases the levels of matrix metalloproteinases (MMP‐2), Bcl‐2, and Cyclin D1 through the upregulation of STAT3 and its activity, thus achieving a proproliferative effect on OS. 110 These events promote the activation of JAK/STAT3 signaling through integrin α2β1, which triggers sustained tumor growth and cancer recurrence. 106 In a tumor‐bearing mouse model, inhibitors of integrin α2β1 signaling significantly suppressed OS cell proliferation and the tumorigenic ability. Similarly, STAT3 also acts as a downstream effector of miR‐126, 111 miR‐199a‐5p, 112 miR‐19, 113 and miR‐125b, 114 thus regulating the proliferation of OS cells.
Couto et al. 107 explored the apoptosis degree of four canine OS cells lines by using the small‐molecule inhibitor LLL12 to inhibit STAT3 activity and found that the apoptosis level of OS cells increased when STAT3 was inhibited, and the expression of the downstream target genes Bcl‐2, Mcl‐1, Cyclin D1, and survivin was affected by STAT3. In addition, increased apoptosis mediated by Caspase3/7 in OS cells was observed with the use of the STAT3 repressors LLL3 or S31201 or through STAT3 knockdown using small interference RNA (siRNA). 108 , 115
OS‐associated macrophages (tumor‐associated macrophages) promote metastasis and EMT of OS cells by elevating the expression levels of p‐STAT3, 116 MMP‐9, and COX‐2. MSCs can secrete IL‐6 in OS cells and promote their migration and invasiveness through the production of phosphorylated STAT3, suggesting that MSCs in the normal bone tissue surrounding the OS tissue can undergo OS metastasis and inhibit apoptosis of tumor cells through the STAT3 pathway. 117 RNA‐Seq assay has shown that knockdown of KDM5A induces OS cells apoptosis through the IL‐6/JAK/STAT3 pathway. 118 Using microarray and bioinformatics analysis, Lv et al. 119 found that serglycin promotes OS cell proliferation, migration, and invasion through the JAK/STAT signaling pathway.
Metastatic OS involves the formation of a very complex and robust vascular system through angiogenesis. More specifically, metastatic OS cells can form tumor blood vessels directly by mimicking angiogenesis without relying on the involvement of endothelial cells. 120 Exogenous administration of tumor suppressor M, belonging to the IL‐6 cytokine family expressed in canine and human OS cells, promotes phosphorylation of STAT3 and upregulates Src and JAK2 activity, significantly increases VEGF expression in OS cells, and enhances OS neovascularization through STAT3‐specific inhibition of LLL3, which inhibited STAT3 activation and tumor angiogenesis induced by tumor suppressor M. 121
2.4. Hippo signaling pathway
2.4.1. Overview of Hippo signaling pathway
When the Drosophila hippo gene was discovered in 2003, Pan and Hariharan's group discovered that Hippo inactivation regulated tissue development in a negative manner. This brought attention to the Hippo signaling pathway. 122 Subsequent research revealed that the evolutionarily highly conserved Hippo signaling pathway involves a junction protein and a repressive kinase that regulates Yorkie (a growth‐promoting transcriptional regulator). 17 In mammals, the Hippo signaling pathway is responsible for signal transmission from the cytoplasm and plasma membrane to the nucleus, whereby it modulates the expression of multiple different target genes to modulate cell proliferation, differentiation, and apoptosis. 123
Typical Hippo signaling pathways include the mammalian STE20‐like protein kinase 1/2 (MST1/2, a Drosophila Hippo homolog), transcriptional coactivator with PDZ‐binding motif (TAZ), Yes‐associated protein 1 (YAP), Mob kinase activator 1 (MOB1), Salvador homolog 1 (SAV1), and large tumor suppressor 1/2 (Lats1/2). 122 Upon the activation of the Hippo pathway, MST1/2 kinase is phosphorylated to activate Lats1/2 kinase which subsequently stimulates MOB1 with the assistance of Sav1. Thereafter, YAP/TAZ is phosphorylated through the interaction of the Lats1/2 PPxY (PY) motif with the WW domain of YAP/TAZ. Following phosphorylation, YAP/TAZ localizes in the cytoplasm after binding to 14‐3‐3 where it is destroyed by the β‐transducin repeat‐containing E3 ubiquitin‐protein ligase‐dependent proteasome and before being lost via transcriptional coactivation. Once the Hippo pathway is suppressed, YAP/TAZ translocates to the nucleus and it interacts with the TEA domain‐containing sequence (TEADs) and other specific TFs to activate downstream target genes and promote tumor formation. 124 With dysregulation of this pathway, TAZ and YAP oncoproteins, in particular, are often expressed at a high level in OS, suggesting the involvement of the Hippo pathway in OS development. 125
2.4.2. Hippo signaling pathway and OS development and progression
In OS, the dysregulation of the Hippo signaling pathway may depend on Sox‐2 levels. 126 , 127 Basilico showed that Sox2 inhibits two Hippo activators in OS, Nf2 (merlin) and WWC1 (kibra), thus blocking the Hippo pathway. Inhibiting Nf2 and WWC1 induces YAP expression and increases the tumorigenicity of OS. 126 Currently, the same research team validated these results in Sox2 conditional knockout animals. 127 The peroxisome proliferator‐activated receptor‐γ agonist thiazolidinedione (TZD) was also used to illustrate the crucial function of Sox‐2 in promoting OS progression. Only through YAP chelation in the cytoplasm does TZD influence OS cellular proliferation in populations of carcinoma cells that highly express SOX‐2. 128
The TF TEAD1 is implicated in YAP‐driven OS progression transcriptionally. The crucial function of TEAD in the proliferation of YAP‐driven OS cell lines has been shown using knockdown methods. For instance, the YAP1/TEAD1 transcriptional complex was discovered to be a significant dysregulated pathway of Hippo signaling in OS using a knockdown method. 129 USP1 deletion reduced the accumulation of TAZ in the nucleus, inhibited the interaction of TAZ with the TEAD TFs, and decreased the expression of downstream genes involved in the Hippo signaling pathway (CYR61, RUNX2, and c‐Myc). 130 Posttranscriptional or posttransduction changes regulate the expression of YAP in OS. Recent research has shown that several miRNAs perform a carcinogenic function in OS, enhancing the expression and activity of YAP. As an illustration, miR‐375 131 enhances YAP1 activity and miR‐624‐5p induces OS cell proliferation, migration, and invasive capacities by elevating the number of nuclear YAPs. 132
2.5. PD‐1/PD‐L1 signaling pathway
2.5.1. Overview of PD‐1 /PD‐L1 signaling pathway
The expression of PD‐1 is evident in immunocytes like tumor‐infiltrating lymphocytes (TIL), dendritic cells (DCs), monocytes, B cells, and T cells. PD‐1/PD‐L1 mediates immune responses through T cells: APCs and tumor cells both express PD‐L1 and T‐cell dysfunction and even failure may occur when PD‐1 binds to PD‐L1 in T cells. PD‐L1 overexpression in tumors allows self‐escape from cytotoxic T cells (CD8+)‐mediated cell killing. 133 APCs and activated T cells both produce the protein B7‐1 (CD80), which binds to PD‐L1 in cancerous cells to weakly activate effector T cells. 134 Treg CD4+, a different subtype of T cell, maintains PD‐1 expression on its surface to provide a highly immunosuppressive tumor milieu. The PD‐1 protein of Treg cells promotes the conversion of primary CD4+ T cells to Treg cells, thereby suppressing the immune response, particularly by repressing the mTOR‐AKT signaling and increasing the Treg expression and immunosuppressive function of CD4+ T cells. 135 As seen above, PD‐1 expression not only attenuates the immune‐promoting effect of effector T cells but also enhances the suppressive effect of immunosuppressive Treg cells.
The steps of the antitumor immune cycle include the release of tumor antigens (Ags), Ag presentation, trigger and activation, T‐cell trafficking to the tumor, T‐cell infiltration, T‐cell recognition of cancer cells, and the killing of malignant cells. PD‐1/PD‐L1 affects immunity mainly by impairing the activity of cytolytic T cells. 136 Clinical mAb‐based immunotherapy has significant therapeutic effects. APCs are released from cancer cells and present Ag to trigger T cell activation. During this process, the T cells first provide an activation signal through the T‐cell receptor (TCR) containing the Ag peptide complex presented by the major histocompatibility complex (MHC). Then, the TCR activates the signaling pathway resulting in the activation of T‐cells together with costimulation signals of the CD86/CD28 axis. Although it binds to CD86, the cytotoxic T lymphocyte‐associated protein 4 (CTLA‐4) inactivates T cells. When PD‐L1 is present on APC or tumor cells, PD‐1, which is produced on activated T cells, binds to it to dampen immunity. Thus, antitumor immunity is improved by specific blockade of the mAb‐mediated PD‐1/PD‐L1 pathway. 137
2.5.2. PD‐1/PD‐L1 signaling pathway and OS onset and progression
The level of PD‐1/PD‐L1 expression correlates with the prognosis of tumors and is substantially elevated in OS tissues. PD‐1 and PD‐L1 were found to be expressed in 47 and 53% of biopsy specimens, respectively, according to studies examining PD‐1, PD‐L1, and associated immunomarkers in samples of individuals with OS. 138 Additionally, a negative correlation exists between PD‐1/PD‐L1 overexpression and patient survival. 139 , 140 , 141 , 142 Consequently, PD‐1/PD‐L1 overexpression is a poor prognostic indicator for OS.
When OS cells exhibit high immunogenicity, follicular helper T cells overexpress PD‐1 and underexpress the antitumor substance IL‐21, thereby attenuating the ability of CD8+ T cells to release interferon‐γ (IFN‐γ) and degranulation, which, in turn, reduces the toxicity of T cells to OS cells. 143 IFN‐γ is produced by mitogen‐stimulated T lymphocytes, a highly effective antiviral bioactive substance that plays a role in tumor immunity. 144 IFN‐γ was found to induce PD‐L1 expression and consequently immune tolerance in angiosarcoma 145 (Figure 2). Yoshida et al. 146 observed this phenomenon in OS and reported that anti‐PD‐L1 antibody significantly reduced Foxp3+ regulatory cells (Tregs) in CD4+ T cells but increased the number of Tregs and CD8+ T‐cell infiltration, 147 which, in turn, exerted antitumor effects. Markel et al. 148 showed that anti‐PD‐L1 antibodies declined T‐cell depletion, suggesting that T cells perform an instrumental function in the PD‐L1‐mediated development of immune tolerance in OS. PD‐L1 is upregulated in the MG63 cell line, and this cell line is tolerant to the killing effect of natural killer (NK) cells. When PD‐1/PD‐L1 is blocked, the cytotoxicity of NK cells is enhanced, which then exerts the killing effect on OS cells through granzyme B (GZMB). 149 Yoshida et al. 150 studied 19 clinical specimens of OS and reported a significant and positive association of IFN‐γ and GZMB with PD‐L1. The increasing infiltration of CD4+ and CD8+ T cells at the primary site of OS and the correspondingly enhanced PD‐L1 expression, and IFN‐γ stimulation contributed to the upregulation of PD‐L1 in OS cells lines, suggesting that OS can adapt to immune resistance and produce PD‐L1, which, in turn, evades the killing effect of immune cells. 151 In summary, by weakening CD4+ T cells, CD8+ T cells, and NK cells, PD‐1/PD‐L1 could trigger the immune escape of OS cells.
FIGURE 2.
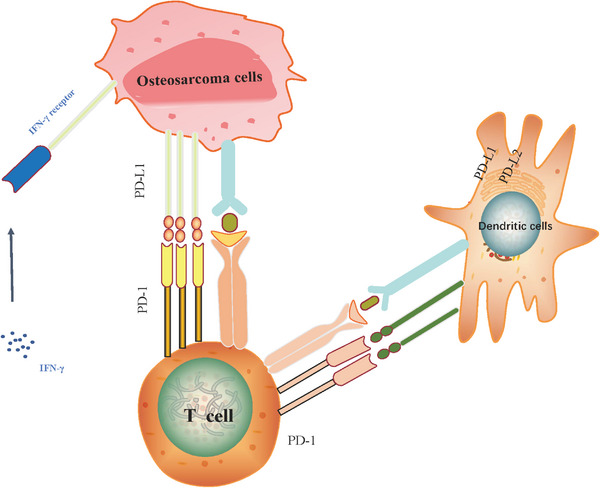
Mechanism of the PD‐1/PD‐L1 signaling pathway in OS. The immune checkpoint proteins PD‐1 on the surface of T cells interact with the ligands PD‐L1/PD‐L2 on OS cells, IFN‐γ produced by T lymphocytes induces PD‐L1 expression in angiosarcoma, resulting in immune tolerance.
PD‐1/PD‐L1 trans‐signaling pathways mediating the immune escape of tumor cells in OS involve relevant signal transduction mechanisms, which have received much attention from researchers. Blocking STAT3 elevated the expression levels of DCs that are triggered by the innate immune system, enhances the production of chemokines and cytokines in tumor tissues, triggers tumor T‐cell responses, 152 , 153 and mediates the body's immune response. STAT3 regulates PD‐L1 expression. 154 Dhupkar et al. 155 observed PD‐L1 expression in both primary and metastatic OS in humans and found that anti‐PD‐L1 antibodies blocked p‐STAT‐3/p‐extracellular signal‐regulated kinase (ERK)1/2 signaling, activated M1 macrophages, and decreased M2 macrophage numbers, resulting in a considerable decrease of OS lung metastatic sites. Histone deacetylase 6 (HDAC6) promotes PD‐L1 upregulation in OS cells, and its mechanism of action in PD‐L1 modulation is primarily driven by STAT3, while selective HDAC6 inhibitors significantly inhibit OS growth. 156 PTEN protein inhibits PD‐L1 expression in cells. 157 FYVE, RhoGEF, and PH structural domain‐containing protein 1 (FGD1) may bind to the N‐terminal region of PTEN, 158 thereby suppressing PTEN function, which subsequently promotes PI3K/AKT activation, PD‐L1 upregulation, and OS progression. 159 Additionally, high expression of myeloid‐derived suppressor cells (MDSCs) in OS can remodel the tumor microenvironment (TME) to promote tumor progression, while PI3Kδ/γ is highly expressed in MDSCs, and application of PI3Kδ/γ inhibitors induces CD8+ T‐cell infiltration and upregulation of PD‐L1 levels in OS cells. 159 , 160
miRNAs are closely associated with PD‐L1 expression. 161 miR‐140, 162 which is weakly expressed in OS, binds to the 3′‐UTR of the downstream targeted gene mRNA and directly downregulates PD‐L1 levels, increases cytotoxic CD8+ T‐cell infiltration, and decreases Tregs expression, which, in turn, inhibits the growth of OS cells, an effect that could be achieved by suppressing the mTOR/S6Ks signaling pathway. miRNA‐200a 163 is involved in OS progression, and its enhanced expression in OS is significantly linked to unfavorable prognosis in OS. miRNA‐200a upregulates PD‐L1 protein levels, reduces the number of CD8+ and CD4+ T cells, and increases the proportion of Foxp3+ Tregs, both in vivo and in vitro. Additionally, PTEN inhibits IFN‐γ secretion by cytotoxic T lymphocytes, thus promoting the growth of OS. miR‐106a interacts with lncRNA (LINC00657) and upregulates PD‐L1 expression, which enhances OS invasion. 164
2.6. Notch signaling pathway
2.6.1. Overview of Notch signaling pathway
There are two types of Notch signaling pathways: classical and nonclassical. The classical Notch signaling pathway mainly includes Jagged/Jagged ligands (Jagged 1 and Jagged 2), Delta‐like ligands (DLL1, DLL3, and DLL4), receptors (Notch 1, Notch 2, Notch 3, and Notch 4), as well as TF C‐promoter‐binding factor (CBF), and its coactivators. 165 The nonclassical Notch signaling pathway either triggers a cascade through a set of nonclassical ligands or activates target genes downstream of the nonclassical Notch signaling pathway. Unlike the Delta‐like ligands and serrate ligands, these nonclassical ligands lack the Delta/serrate/Lag‐2 domains and could be classified into three categories: secreted proteins, membrane proteins anchored to glycosylphosphatidylinositol, and integral membrane proteins. 166 In signal‐receiving cells, receptor protein hydrolysis is triggered by the binding of Notch ligands to receptors. Two further proteolytic cleavages are mediated by the enzymes γ‐secretase and a disintegrin and metalloprotease (ADAM): one in the Notch receptor's transmembrane (ADAM) and the other in the Notch intracellular domain (NICD) from the extracellular domain (γ‐secretase). 167 The NICD activates downstream target genes by stimulating the TF CBF1. 168 Hairy/enhancer‐of‐split 1 (HES1) and YRPW motif‐related hairy/enhancer‐of‐split related with YRPW motif 1 (HEY1) constitute the most significant genes within the Notch pathway. In the Notch pathway, HEY1 is a crucial downstream target gene that can influence tumor cells' fate through the Notch/HES1/HEY1 pathway. 169 In malignancies, the Notch signaling pathway is activated. Direct cell‐to‐cell interface is facilitated by its abnormal activation, which also performs a remarkable function in tumor onset, progression survival, proliferation, invasion, and metastasis. 170
2.6.2. Notch signaling pathway and OS development and progression
Cell migration‐inducible protein (CEMIP) exerts oncogenic effects in several cancers. 171 , 172 , 173 CEMIP promotes OS cell growth and metastasis by activating the Notch pathway, and silencing of CEMIP in vitro and in vivo decreases the protein expression as well as the stimulation of the Notch/Jagged1/Hes1 signaling pathway. 174 Jagged 1, an important ligand of Notch, is an integral part required to activate the Notch pathway through its binding to the Notch1 receptor and performs a fundamental function in tumor cell cycle regulation. 175 , 176 High expression of the lncRNA MEG3 in OS cells substantially lowered the levels of Jagged 1 and Notch 1 and restricted cell proliferation. 177 miR‐26a directly inhibits the Jagged 1 ligand, thus suppressing the activation of the Notch pathway to resist OS proliferation. 178 The TF Dlx5 is expressed during the initial stages of skeletal development and could assume an integral function in controlling the osteogenic process. 179 The findings from dual‐luciferase reporter gene assay and bioinformatics analysis have suggested that Dlx5 may interact directly with Notch 1 to control Notch 1 transcription and the expression of downstream target genes, thereby promoting OS cell proliferation. 180
The Notch signaling pathway affects tumor drug resistance by reducing intracellular drug accumulation and regulating EMT, leading to miRNA dysregulation, apoptosis gene disruption, and regulating tumor stem cells. 181 The expression levels of Notch‐ICN, MRP1, and other proteins were remarkably elevated in hypoxia relative to normoxia, implying that the Notch pathway was activated in MG63 cells. Additionally, the drug resistance of MG63 cells was significantly reduced through siRNA interference with Notch 1 in hypoxia. 182 Reducing intracellular drug accumulation is an important approach to regulate tumor drug resistance, and ATP‐binding cassette (ABC)‐mediated drug efflux is associated with multidrug resistance in OS, and MDR‐related protein‐1 (MRP1, also known as ABCC1) is a major target of ABC. 183 The expression of NICD and PSEN1 (a γ‐secretase catalyst involved in intracellular Notch 1 protein synthesis) significantly increases in OS cells with drug resistance with high ABCC1 expression, and stimulation of the Notch pathway leads to the formation of the NICD/CBF1 complex, which consequently reduces ABCC1 expression, resulting in enhanced drug sensitivity in OS cells. 184
P53 is the most well‐studied oncogene and is intimately linked to diverse biological behaviors like growth arrest, senescence, apoptosis, and DNA repair. 185 Patients with Li–Fraumeni syndrome, caused by congenital p53 gene inactivation, exhibit a considerably elevated risk of developing OS as opposed to those with normal p53 genes. 186 Mutant p53 genes increase the responsiveness of OS‐resistant cells to chemotherapy regimens by upregulating the expression of the proapoptotic genes p21 and Bax. 187 miR‐34c regulates Notch signaling (HEY2, JAG1, and NOTCH1) and p53‐mediated transcription of several genes implicated in the cell cycle and apoptosis (CCNE2, E2F5, E2F2, and HDAC1). 188 EMT is a key process of tumor invasion and metastasis. 189 Su et al. 190 reported that ATG4A could promote EMT in OS by downregulating Notch 1 and Hes 1 expression and upregulating E‐calmodulin expression through ATG4A knockdown in OS with siRNA, thereby activating the Notch signaling pathway.
2.7. MAPK signaling pathway
2.7.1. Overview of MAPK signaling pathway
MAPK is a tightly controlled signaling pathway with Ser/Thr protein kinase activity that performs an instrumental function in diverse biological processes in living organisms, it can be activated by stimuli or cellular signals such as physical stress and inflammatory responses. 191 The MAPK signaling pathway family primarily encompasses ERK1/2, JNK, p38MAPK, and ERK5. 192 ERK1/2 has a fundamental function in cell growth, differentiation, and proliferation. 193 JNK is implicated in cell differentiation, apoptosis, and stress. 194 p38MAPK is involved in cytokine production, transcriptional regulation, and apoptosis. 195 ERK5 performs a modulatory role in cell survival, differentiation, proliferation, and other important pathophysiological processes. 196 Additionally, the MAPK pathway performs a remarkable function in cellular proliferation, angiogenesis, differentiation, apoptosis, and tumor metastasis when compared with the other pathway. 197 However, the MAPK pathway has been less frequently studied as a major target in OS.
2.7.2. MAPK signaling pathway and OS development and progression
VDAC1 independently functions as a prognostic indicator in OS and patients with high VDAC1 expression exhibit a low survival rate. VDAC1 promotes OS cell proliferation and apoptosis through the MAPK signaling pathway. 198 Transcriptome analysis showed that ITGB3 functions in OS through the MAPK and VEGF signaling pathways for cell proliferation and cisplatin resistance. 199 Protein serine kinase H1 (PSKH1) is an autophosphorylated human protein serine kinase. β‐Phenylethyl isothiocyanate induces the proliferation of human OS cells by changing iron metabolism, disrupting redox homeostasis, and activating the MAPK pathway. p38 phosphorylation in OS cells is upregulated by PSKH1, and p38 overexpression promotes the proliferation of OS cells. 200
2.8. NF‐κB signaling pathway
2.8.1. Overview of NF‐κB signaling pathway
NF‐κB belongs to the Rel family of eukaryotic TFs and consists of two subunits: p50 and p65. 201 Under normal conditions, cytoplasmic NF‐κB undergoes inactivation by binding to the repressor protein IκB in a trimeric complex. 202 Upon external stimuli such as tumor necrosis factor‐α signals, inflammatory factors, and ultraviolet light, IκB dissociates from the p50/p65/IκB heterodimer, and the NF‐κB dimer subsequently exposes nuclear localization sequences, thereby rapidly translocating from the cytoplasm to the nucleus to bind to specified intranuclear DNA sequences and to trigger or enhance the transcription of associated genes. 203 Downstream genes of NF‐κB include cyclin D1 and c‐Myc, whose sustained activation may enhance cell growth and contribute to uncontrolled cell proliferation. 204
2.8.2. NF‐κB signaling pathway and OS development and progression
In OS, the NF‐κB pathway performs an indispensable function in modulating tumor growth, metastasis, and chemoresistance. Aberrant expression of NF‐κB is closely associated with OS. Li et al. 205 reported the inhibition of OS cell metastasis by combining the blockade of integrin β1 expression and NF‐κB signaling in MG63 cells. Notably, alpha thalassemia and intellectual disability syndrome X‐linked (ATRX) deficiency promotes OS cell invasion by increasing NF‐κB signaling and integrin β3 binding. 206 The oncogene TRIM10 upregulates nuclear levels of p65, thereby activating classical NF‐κB signaling to promote cisplatin resistance in OS cells. 207
Contemporary research on NF‐κB‐associated mechanisms in OS is centered on the modulation of miRNAs and lncRNAs that are possible upstream modulators of NF‐κB and could exert their oncogenic or their inhibitory properties on tumors by affecting the levels of STAT3 expressed in OS cells. 208 , 209 When the expression of lncRNA NKILA was detected in 60 cases of OS and adjacent tissues, lncRNA NKILA expression was found to be reduced in OS tissues, and the proliferation, invasive rate, and migration of OS cells were enhanced after transfection of NKILA‐siRNA with cells, while the NF‐κB inhibitor (JSH) could reverse the inhibitory impact of NKILA on cell migration and proliferation, implying that lncRNA NKILA is involved in OS development and acts through the NF‐κB/NAIL signaling pathway. 210 Nectin‐4, an oncogene, can activate the PI3K/AKT/NF‐κB signaling pathway by downregulating miR‐520c‐3p expression, thereby promoting OS progression and metastasis. 211
3. MECHANISM OF TARGETING SIGNALING PATHWAYS
The current treatment for patients with OS is mainly neoadjuvant chemotherapy, including high‐dose methotrexate, doxorubicin, and cisplatin, and surgical excision of the lesion with adjuvant chemotherapy, but the treatment for OS is still slightly inadequate compared with the advantages of chemotherapy combined with targeted therapy for lung cancer and breast cancer. This part aims to discuss the molecular biological pathogenesis of OS, from both small molecule inhibitors and TCM, which are strongly correlated with the onset and advancement of OS, and to explore the corresponding therapeutic targets and downstream pathways to provide strong evidence for further research.
3.1. Small molecule inhibitors
A class of organic compounds with molecular weights less than 1000 Da known as small molecule inhibitors can target proteins, reduce protein activity or block biochemical reactions, and are widely used in the study of signaling pathways. 212 Most of the drugs used in clinical practice have small‐molecule inhibitors as their main components. Extensive research has validated the integral function of small molecule inhibitors in OS.
3.1.1. PI3K/AKT/mTOR signaling pathway
LY294002, the first broad‐spectrum PI3K inhibitor, has a broad inhibitory effect on PI3K‐related pathways in OS cells, and it is often used in combination treatment with other oncogenic drugs in clinical studies to improve drug efficacy and to decelerate the developed drug resistance. 48 Preliminary data from a phase 2 trial showed that the pan‐I PI3K inhibitor BKM120 was used as a second‐line therapeutic agent for OS, and BKM120‐treated OS cells showed responses in the form of cell proliferation arrest, increased cellular apoptotic rate, and upregulated caspase‐3 activity. 213 In addition, PI3K inhibitors can reverse anlotinib resistance in OS and restrict OS cell development when used in combination with anrotinib. 214 Further studies on the effects of downstream molecules on PI3K inhibitors in OS are needed. In 143B cells, ZIP10 overexpression increased protein expression levels of p‐AKT and promoted tumor growth and chemoresistance, while this promotion was attenuated by the AKT inhibitor GSK690693. 215 Western blotting results revealed that 2‐methylbenzoyl berbamine inhibited the activation of the AKT pathway in OS. 216 Although a few studies on the use of AKT inhibitors in OS are available, many are only at the cellular experimental stage, necessitating further studies.
With the current development of drugs targeting the mTOR pathway and the limited benefit of monotherapy, multidrug combinations will become the main treatment modality for OS in the future. In human OS cells and mouse OS models in vitro, simultaneous inhibition of PI3K and mTOR targets effectively induced cellular apoptosis, whereas single‐targeted inhibition of PI3K or mTOR was ineffective. 217 When the dual PI3K/mTOR inhibitor CCT128930 is applied to OS cells lines (MG63, U2OS, and Saos‐2), cyclin D1 and/or cyclin B1 expression was downregulated in tumor cells, and the cell cycle was stalled at the G0/G1 phase. 218
3.1.2. Wnt/β⁃catenin signaling pathway
The enzyme COX2 celecoxib can inhibit β‐catenin from translocating to the nucleus by activating the GSK3β signaling pathway, decreasing the abnormal activation of the β‐catenin pathway, and inhibiting the growth, invasive, and migratory capacities of OS cells by increasingly suppressing the Wnt/β‐catenin pathway. 219 TIKI2 functions as an inhibitor protease of the Wnt/β⁃catenin pathway, and its expression is reduced in OS cells. 220 It can inactivate the Wnt/β‐catenin pathway by inhibiting the downstream GSK‐3β pathway and by removing the N‐terminal amino acid. Restoration of TIKI2 expression through adenoviral transfection promoted phosphorylated GSK‐3β expression and reduced β‐catenin levels within the nucleus, which inhibited the proliferation of OS cells. 221
Accumulating evidence suggests that microRNA (miRNA) dysregulation and the Wnt/β‐catenin pathway together trigger cancer development, metastasis, and drug resistance. 222 miRNAs play a targeted role in regulating gene expression, and through gene modification, some miRNA promoters may contain specific binding sites for cellular signaling molecules involved in the Wnt signaling pathway. 223 These molecules can either activate or inhibit various steps of the Wnt signaling pathway. As for tumor pathogenesis and prognosis, miRNAs act as oncogenes and regulate the expression of genes posttranscriptionally, and they are implicated in OS pathogenesis, indicating a more promising application. 224 miR‐429 inhibits OS progression by targeting homologous cassette A9 (HOXA9, HOX gene), thereby suppressing tumor cells proliferation, migration, and invasion and attenuating the Wnt/β‐catenin protein signaling pathway. 225 Yang et al. 226 reported that miR‐183 targets low‐density lipoprotein receptor‐related protein 6 (LRP6), which regulates the Wnt/β‐connexin signaling pathway and is a downstream effector of LRP6. miR‐183 performs the role of a tumor suppressor miRNA in reducing OS cell migration, invasion, and metastasis.
3.1.3. JAK/ STAT3 signaling pathway
Existing research on hindering OS development by inhibiting the JAK/STAT3 pathway is centered on the inhibitory effects of JAK2 inhibitors and STAT3 inhibitors. AG490, a selective inhibitor of JAK2, targets the JAK signaling pathway in Saos‐2 cells and reduces the proliferative and migratory capacities of OS cells by decreasing the phosphorylation level of STAT3 and impeding its activation, which eliminates the stimulatory effect of THAP9‐AS1 on cellular processes. 227 In the Saos‐2 model, AG490 inhibits the development of in situ tumors. 117
Similar to the synthesis‐specific small‐molecule, LLL3, 115 LLL12, 228 and FLLL32 reduce the ability of STAT3 to bind to DNA, downregulate the levels of total STAT3 and p‐STAT3, inhibit cell proliferative and migratory capacities, and contribute to caspase 3‐dependent cell apoptosis. The is also a decrease in the protein and mRNA expression levels of STAT3 downstream target genes like VEGF, MMP‐2, and survivin. 229 , 230 In OS, apatinib suppresses the OS cell growth, blocks cells in the G0/G1 phase, 231 and induces apoptosis and autophagy. 232 NHWD‐870 disrupts the binding affinity of BRD4 to the upstream receptor GP130 promoter region of STAT3, impairs upstream signaling, blocks STAT3 dimerization, and induces apoptosis. 233
3.1.4. Hippo signaling pathway
In xenograft OS, Verteporfin is known to inhibit YAP/TAZ signaling and suppress the proliferation, migration, and invasiveness of spontaneous OS owing to the deficiency of Rb1 and Trp53 in cells that express Ctsk by blocking the Hippo pathway. 234 RhoA knockdown significantly reduces the levels of downstream target genes and unphosphorylated YAP, whereas RhoA knockdown remarkably enhanced the apoptosis in OS cells under both in vivo and in vitro conditions after pyropheophorbide‐α methyl ester‐mediated photodynamic therapy. 235 Fisetin upregulated the levels of leucine zipper‐containing kinase (ZAK) to trigger the Hippo signaling pathway and mediate JNK/ERK stimulation downstream of ZAK, thereby triggering apoptosis in human OS cells in an AP‐1‐dependent manner. Notably, the negatively modulated connective tissue growth factor (CTGF) is targeted by miR‐584‐5p and this regulation results in the inhibition of OS cell metastasis. Moreover, by regulating CTGF in OS, miR‐584‐5p inactivates the Hippo pathway. 236 As a miR‐34c target, PLOD1 promotes cellular growth and metastasis via the inactivation of the Hippo pathway and the inhibition of LATS1 phosphorylation in OS. 237
3.1.5. Notch1 signaling pathway
In vivo experiments have shown that DAPT (GSI‐IX), a common synthetic γ‐secretase inhibitor, specifically blocks γ‐secretase and prevents the activation of the Notch signaling pathway. 238 When DAPT was tested in a mouse para‐tibial tumor model of OS, a K7M2 cell line, and a lung metastasis model, it inhibited ERK pathway phosphorylation by suppressing Notch activation to effectively suppress tumor growth, angiogenesis, and lung metastasis, thereby improving patient survival. 239 Another γ‐secretase inhibitor, RO4929097, reversed the effects of human umbilical vascular endothelial cell‐derived exosomes on Notch 1, Hes 1, and Hey 1 expression by inhibiting the Notch1 signaling pathway. 240 A novel DNA methyltransferase 1 (DNMT‐1) small‐molecule antagonist/inhibitor, named DI‐1 (DNMT‐1 inhibitor), enhances the antitumor effects of the molecularly targeted drug cabozantinib in OS cell lines. 241 Lutetium ethylenediaminetetramethylene phosphonate is a radiopharmaceutical commonly used for its analgesic effect in patients with bone cancer, and relaxin inhibits the Notch‐1 pathway in OS cells through the Notch‐1 pathway. 242 When IL‐24, an oncogene with cytokine properties cloned from human melanoma HO‐1 cells, was administered via injection into the lateral abdomen of nude mice to treat OS, a substantial reduction in the expression levels of Ki‐67, a biomarker of cellular proliferation, was observed. In addition, treatment of the 143B OS mouse model with IL‐24 revealed inhibition of the tumor growth rate and downregulation of Notch 1, Hes 1, and β‐catenin expression in xenograft tumor tissues, suggesting that IL‐24 may be a new target to improve long‐term survival of patients with OS. 243
3.1.6. MAPK signaling pathway
In an in situ OS transplantation model, iron chelators induce apoptosis in OS cells owing to their disruption of the homeostatic balance of intracellular iron and activation of the MAPK pathway. 21 , 244 Theaflavin‐3,3′‐digallate (TF3) possesses strong antitumor characteristics in the treatment of several cancers affecting humans. In both in vivo and in vitro models, TF3 triggers cellular iron death and apoptosis by activating the ROS and MAPK signaling pathways, reducing glutathione depletion, promoting the accumulation of reactive oxygen species (ROS), and enhancing the Fenton response‐induced oxidative response. 245 Additionally, the p38 MAPK inhibitor SB203580 effectively blocks the protumorigenic effect of PSKH1. 200
3.1.7. NF‐κB signaling pathway
The multipotent cytokine macrophage migration inhibitory factor (MIF) inhibitor 4‐iodo‐6‐phenylpyrimidine impedes the binding of MIF with CD74 and p65 and significantly reduces the proliferation and metastasis of OS cells by inhibiting the NF‐κB pathway. 22
3.2. Traditional Chinese medicine
TCM has numerous active monomeric components and their mechanisms of action are complex and diverse. Relying on the synergistic modulation of multiple components, targets, and pathways, TCM plays multiple therapeutic functions in the onset, progression, and metastasis of OS. In recent years, in the study of OS‐targeted signaling pathways, in addition to human‐assembled small molecule inhibitors, more and more studies on natural products have been conducted in recent years (Figures 3 and 4).
FIGURE 3.
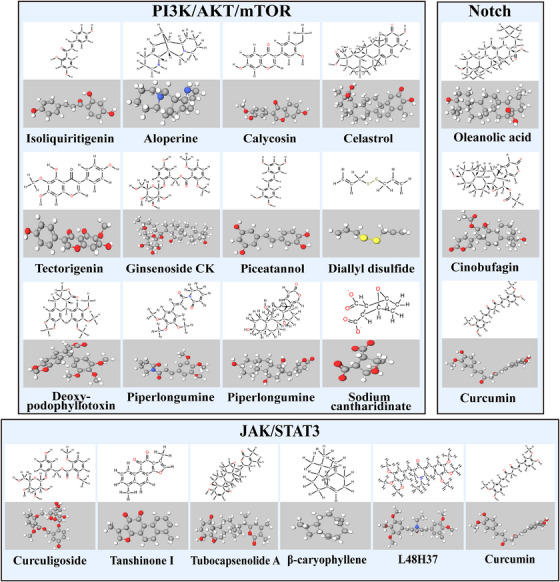
A partial herbal medicine that acts on PI3K/AKT/mTOR, JAK/ STAT3, and NF‐κB signaling pathway in osteosarcoma. Figure are made by Chemdraw. Physakengose G molecular formula is uncertain and not shown in the figure.
FIGURE 4.
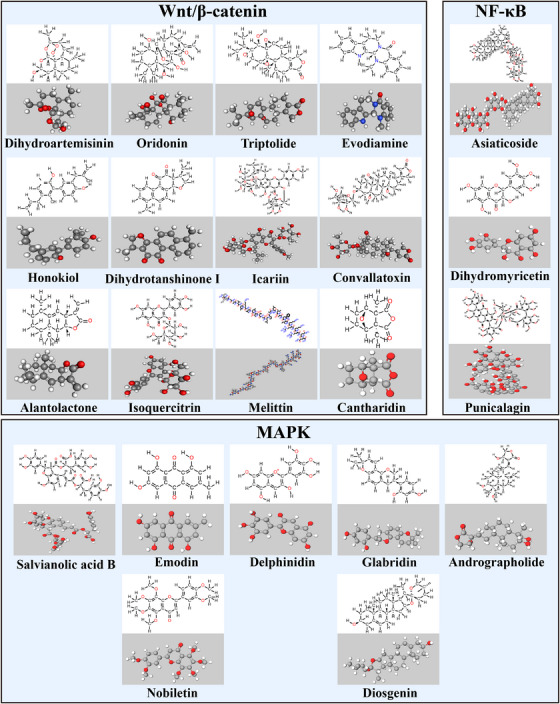
A partial herbal medicine that acts on Wnt/β‐catenin, MAPK signaling pathway in osteosarcoma. Figure are made by Chemdraw. Polyphyllin I and Ganoderma lucidum molecular formula is uncertain and not shown in the figure.
3.2.1. PI3K/AKT/mTOR signaling pathway
Sodium cantharidinate is a semisynthetic derivative of cantharidin hydrolyzed by sodium hydroxide. 246 In MG63 cells, sodium cantharidinate activates the PI3K/AKT pathway, resulting in the activation of p53/p21 and inhibition of the expression levels and activities of cyclin D1 and the protein kinases Cdk‐4 and Cdk‐6, thus leading to the cell block in the G0/G1 phase and suppression of the proliferation of human OS MG63 cells. 247 In human OS cells, Piperlongumine was shown to trigger apoptosis and G2/M phase blockade by modulating the ROS/PI3K/AKT pathway. 248 mTOR performs a critical function in the upstream regulation of autophagy, and the PI3K/AKT pathway is the most characteristic regulatory pathway that activates mTOR. 249 Diallyl disulfide is a natural organic compound extracted from garlic and shallots. Treatment of MG63 cells with diallyl disulfide results in a decrease in PI3K expression and phosphorylation of AKT protein, as well as a decrease in the protein expression of mTOR and its downstream effectors p70S6K and p‐p70S6K. It is suggested that diallyl disulfide blocks the PI3K/AKT/mTOR pathway to induce cellular autophagy. 250 Deoxypodophyllotoxin, a natural compound from herbal medicine, induces cytoprotective autophagy by downregulating p‐AKT and phosphorylating mammalian target of rapamycin protein (p‐mTOR) in U2OS cells and suppressing the activation of the PI3K/AKT/mTOR pathway. 251 In human OS cells, the new compound Physakengose G, which was initially extracted from Physalis alkekengi var, triggers apoptosis via EGFR/mTOR signaling and blocks autophagic flux. 252
Bcl‐2 is a major target molecule for studying the molecular mechanisms of apoptosis. The members of the Bcl‐2 protein family encompass proapoptotic proteins (Bax, Bak, and Bid) and antiapoptotic proteins (Bcl‐xL and Bcl‐2), which control the release of mitochondria‐associated apoptotic factors by controlling the mitochondrial outer membrane permeability. Aloperine, 253 piceatannol, 254 calycosin, 255 and tectorigenin, 256 all increase the levels of caspase‐3 and Bax, downregulate the levels of PI3K, Bcl‐2, and p‐AKT1, and induce apoptosis in OS cells via the PI3K/AKT/mTOR pathway. Upregulation of MMPs can lead to degradation of the basement membrane and extracellular matrix. The invasion‐related proteins MMP‐2 and MMP‐9 are regulated by AKT. 257 Ginsenoside CK downregulates the levels of p70S6K1, MMP2, and MMP9 in MG63 and U2OS cells and plays a synergistic role in cell migration and invasion. It is hypothesized that ginsenoside CK suppresses the viability and proliferation of OS cells, induces apoptosis, and reduces cell migratory and invasive capacities by blocking the PI3K/mTOR/p70S6K1 pathway. 258 Other active ingredients of TCM, such as gamabufotalin, 259 celastrol, 260 and isoliquiritigenin, 261 can reduce the MMP‐2 and MMP‐9 expression levels via the PI3K/AKT pathway, thereby inhibiting OS metastasis.
3.2.2. Wnt/β‐catenin signaling pathway
GSK‐3β can phosphorylate β‐catenin at four N‐terminal sites and is thus a marker of β‐catenin phosphorylation. Following phosphorylation, β‐catenin is degraded by the proteasome through ubiquitination. 262 Dihydroartemisinin inhibits the growth of OS cells and nude mice transplanted tumors by inhibiting the phosphorylation of serine 9 (Ser9) in GSK‐3β, which activates GSK‐3β to degrade β‐catenin. At the same time, artemisinin downregulates the Dvl protein in OS cells, which unregulates the effect of the Dvl protein on β‐catenin growth by GSK‐3β. At the same time, dihydroartemisinin downregulated Dvl protein expression in OS cells, dysregulated GSK‐3β activity, a key component of the degradation complex formed by GSK‐3β, APC, and Axin, and degraded β‐catenin through GSK‐3β phosphorylation. Inactivation of the Wnt/β‐catenin inhibits OS activity. 263 Oridonin inhibits the proliferative effect of 143B cells by increasing GSK3β activity, upregulating Dickkopf‐1 (Dkk‐1) expression, and downregulating β‐catenin expression. Moreover, Dkk‐1 overexpression or β‐catenin knockdown enhanced the inhibitory impact of oridonin on the proliferation of 143B cells. 264 Cantharidin, 265 triptolide, 266 , 267 polyphyllin I, 268 piperidin, 269 and evodiamin 270 can inhibit GSK‐3β phosphorylation and β‐catenin activity, downregulate the Snail, vimentin, N‐cadherin, MMP‐2, MMP‐7, and MMP‐9 expression and upregulate the levels of E‐cadherin, thereby effectively inhibiting OS cell proliferation and metastasis as well as ectopic angiogenesis by regulating the Wnt/β‐catenin pathway.
In vitro trials have illustrated that oleuropein inhibits the proliferation and invasion of OS cells, promotes apoptosis, and exerts good anticancer effects. Additionally, it reduces the expression of the cytoplasmic total β‐catenin in OS cells, reduces the translocation of β‐catenin into the nucleus, and blocks the Wnt/β‐catenin pathway. Notably, the reduction of Wnt‐specific TOP/FOP luciferin reporter gene expression indicated an attenuated transcriptional activity of the TF TCF/LEF in the Wnt/β‐catenin pathway. Furthermore, the expression of the Wnt target genes MMP2 and MMP9 was downregulated to inhibit the migratory invasive ability of cells. 271 Isoquercitrin remarkably suppressed the proliferative rate, triggered EMT‐associated migratory and invasive activity, and promoted apoptosis in OS cells in vitro. Moreover, β‐catenin together with its downstream genes (survivin, cyclin D1, and c‐Myc) were remarkably downregulated. 272 Tie et al. 273 showed that icariin induced apoptosis and inhibited the growth of OS cells by downregulating the Wnt/β‐catenin pathway and attenuating the expression of TOPFlash fluorescence. Conversely, TOPFlash fluorescence expression and adenovirus overexpression of β‐catenin reversed the inhibitory impact of icariin. Ganoderma lucidum, 274 melittin, 275 and dihydrotanshinone I 276 inhibit the Wnt coreceptor LRP5/6 and the Wnt‐associated target genes MMP‐2, MMP‐9, C‐Myc, β‐catenin, and cyclin D1 to block Wnt/β‐catenin signaling and inhibit the EMT process in OS cells, consequently suppressing OS cell proliferation and metastasis. Convallatoxin 277 and alantolactone 278 can inhibit the malignant OS progression by blocking the Wnt/β‐catenin pathway.
3.2.3. JAK/STAT signaling pathway
Besides the human‐assembled small‐molecule inhibitors, natural products have recently gained increasing attention, and their extracts have monomeric components that can inhibit JAK/STAT pathway. In vivo studies suggest that curcumin remarkably and dosage‐dependently lowers the levels of p‐JAK2 and p‐STAT3 expressed in MG63 cells. Also, curcumin suppresses the proliferative, migratory, and invasive capacities of MG63 cells, induces G0/G1 phase arrest, and promotes apoptosis through the inhibition of the p‐JAK2/p‐STAT3 pathway. 279 The curcumin analog L48H37 lowered the phosphorylation level of JAK3, STAT3, JAK2, and JAK1 in U2OS cells, thereby inhibiting the invasion and migration of these cells. 280 Tubocapsenolide A inhibits OS cell proliferation through SHP‐2‐induced JAK/STAT3 pathway inactivation. 105 β‐Caryophyllene regulates the JAK1/STAT3 signaling pathway in MG63 cells through the ROS‐induced apoptotic DNA fragmentation mitochondrial pathway, thereby promoting OS cells apoptosis and inflammation. 281 In vivo and in vitro models revealed that both Tanshinone I, 282 an extract of Danshen, and curculigoside, 17 a natural component of Curculigo orchioides Gaertn, suppressed the growth and metastasis of OS cells by inhibiting the JAK/STAT3 pathway.
3.2.4. Notch signaling pathway
A thorough analysis of the function played by curcumin in OS revealed that Notch 1/2/3 gene signaling was activated in most of the OS cell lines and that the proliferation and metastasis of OS cells were suppressed when Notch 1 signaling and downstream gene expression were downregulated. 283 Oleanolic acid can dosage‐dependently suppress the proliferative capacity of OS cells while suppressing the activation of the Notch signaling pathway. 284 Cisplatin (DDP) is a promising anticancer drug, and cinobufagin combined with low‐dose DDP significantly inhibits OS cells activity and suppresses tumor growth and metastasis, thus increasing survival duration in an OS xenograft model of nude mice, while downregulating Notch 1, Hes 1, Hes 5, and Hey‐L mRNA expression. 285
3.2.5. MAPK signaling pathway
Notably, MAPK pathway proteins play a central function in regulating the expression of MMPs. 286 Emodin, an anthraquinone compound extracted from Polygonum multiflorum and Aloe vera, can suppress the proliferative capacity of OS cells by inhibiting the activity of the ERK signaling pathway and may promote the apoptosis of OS cells by increasing the caspase‐3 and caspase‐9 expression levels. 287 Salvianolic acid B increases the levels of cleaved caspase‐3, phosphorylated p38MAPK (p‐p38MAPK), and phosphorylated p53 (p‐p53) expressed in MG63 cells, suppresses OS cellular proliferation, and triggers apoptosis. In addition, salvianolic acid B increases the level of ROS production, and silencing of p38 expression inhibits the anticancer action of salvianolic acid B on the proliferation level, p‐p53 protein expression, and ROS production level of MG63 cells. 288 Delphinidin reduces the phosphorylation levels of ERK1/2 and p38 MAPK, inhibits the N‐cadherin expression, and promotes the E‐cadherin expression, thereby inhibiting the migration of OS cells, and these effects of delphinidin are enhanced when it is combined with inhibitors of ERK1/2 and p38. 289
The MAPK pathway is regulated by ERK, JNK, and p38 MAPK kinase, which are activated and phosphorylated to regulate downstream MMP gene expression, subsequently promoting the occurrence and progression of OS. 290 Nobiletin 291 suppresses the metastasis of human OS cells upon blocking the expression of ERK and JNK‐mediated MMPs, and Glabridin 292 achieved the same effect by blocking p38 and JNK‐mediated MMPs. Andrographolide causes arrest in the G2/M phase and induces apoptosis by regulating the ROS/JNK signaling pathway in OS cells. 293 Diosgenin induces apoptosis in OS cells by upregulating the ROS‐mediated p38 MAPK signaling pathway. 294
3.2.6. NF‐κB signaling pathway
Punicalagin can interfere with 19 human OS cell lines, including U2OS, MG63, and Saos2. It significantly reduces proliferation and increases apoptosis of OS cells while downregulating IL‐6 and IL‐8 expression levels. 295 As a critical transcriptional modulator of metastatic‐associated genes, aprepitant decreased the expression levels of angiogenic factors, VEGF proteins, and NF‐κB and suppressed the migratory phenotype of OS cells. 296 Dihydromyricetin suppresses the metastasis of human OS cells via the inhibition of SP‐1 and NF‐κB‐regulated urokinase fibrinogen activator. 297 Asiaticoside reverses the malignant characteristics of OS cells induced by the polarization of the M2 phenotype macrophages through the inhibition of TRAF6/NF‐κB. 298
4. ADVANCES IN CLINICAL RESEARCH ON TARGETED THERAPY AND IMMUNOTHERAPY FOR OS
Presently, immunotherapy and targeted therapies are the primary focus of the efforts to develop novel treatment regimens for OS. While mounting evidence on cellular and molecular levels indicated that numerous distinct signaling pathways and genes are implicated in the onset and advancement of OS, the druggable fraction of these markers is extremely limited. The principal drug targets for existing drugs include insulin‐like growth factor (IGF)‐R, EGFR, PDGF/PDGFR, mTOR, VEGFR, and Aurora kinase pathways linked to cellular growth, immune checkpoint pathways linked to immunity, relay immune cell therapy, and tumor vaccines (Figure 5).
FIGURE 5.
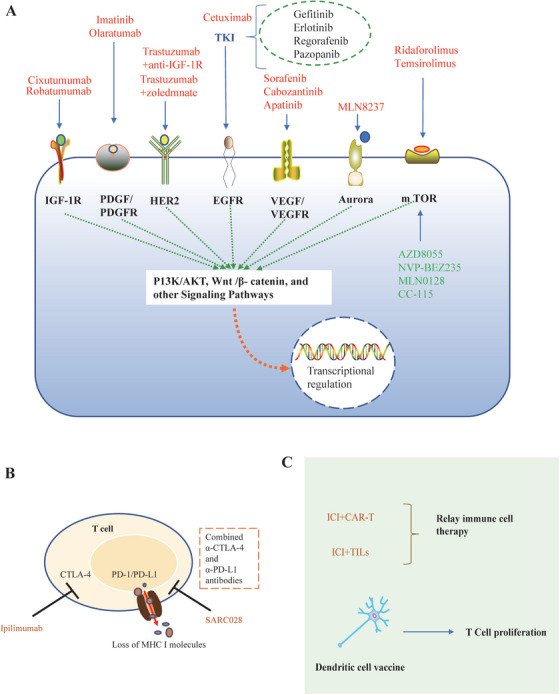
Overview of clinical studies on targeted therapies and immunotherapy for osteosarcoma. The representative therapeutic targets in OS and the corresponding targeted or immunotherapeutic agents that have entered clinical investigations are depicted. (A) Describe targeted therapies in osteosarcoma, (B) describe the immune checkpoint pathways linked to immunity in osteosarcoma, and (C) describe the relay immune cell therapy and tumor vaccines.
4.1. Targeted therapies
4.1.1. IGF‐R targeted therapy
The IGF system includes three main ligands (IGF‐1, IGF‐2, and insulin), three corresponding tyrosine kinase receptors (IGF‐1R, IGF‐2R, and insulin receptor [IR]) and six distinct IGF‐binding proteins (IGF‐BPs1‐6). They promote cell growth and antiapoptosis, mainly through the MAPK pathway and PI3K/protein kinase B pathway, thereby promoting tumor development and progression. Overexpression of IGF1R has been shown in canine OS and is strongly linked to tumor staging and dismal outcome. 299 Therefore, many academic research groups and companies have developed neutralizing antibodies (anti‐IGF1R), small molecule tyrosine kinase inhibitors (TKI), or si‐RNA to target IGF1R as a molecular therapeutic strategy. 300 , 301 , 302 , 303 In a study by WANG et al., 304 IGF‐1R was found to be overexpressed in OS tissues, and this overexpression closely affected the tumor metastasis and OS patients’ prognosis. This study also illustrated that attenuating IGF‐1R overexpression could inhibit the growth of OS cells, promote their apoptosis, and enhance the cells to radiotherapy. Consequently, suppression of the IGF system can block its corresponding pathways of action, thus achieving precision therapy.
Cixutumumab (CIX) is an anti‐IGF‐1R monoclonal antibody that functions by binding to IGF‐1R with strong affinity, thereby blocking the interface between IGF‐1R and its ligand and inducing internalization and degradation of IGF‐1R. 305 Children with refractory solid tumors in phase II trials in 15% of patients with long‐term stable disease showed high tolerance to CIX. When CIX was combined with temsirolimus to treat patients with sarcoma, a remarkable clinical activity was observed; however, no objective responses were identified in phase II trials involving young adults and children with refractory or recurrent sarcomas. 306 Robatumumab (19D12; MK‐7454, often referred to as SCH717454) is an antibody that is only found in humans and whose function involves binding to and inhibiting IGF‐1R. Three of 31 patients with low disease burden had a complete or partial response, according to solid tumor response assessment criteria. 307
Human epidermal growth factor receptor 2 (HER‐2) is a tyrosine kinase receptor and a member of the human epidermal growth factor receptor (HER/EGFR/ERBB) family. HER‐2 is remarkably correlated with tumor growth, and targeted therapies for HER‐2 have been used in some solid tumors. 308 Trastuzumab, an HER‐2‐specific recombinant monoclonal antibody, is currently used in clinical practice to treat patients with HER‐2‐positive breast cancer. Nevertheless, some studies have shown that in patients with HER‐2‐positive OS, trastuzumab combined with chemotherapy did not significantly improve overall survival compared with patients with HER‐2‐negative chemotherapy. 309 However, other studies have shown that when combined with recombinant human IGF‐1R monoclonal antibodies (anti‐IGF‐1R) 310 or zoledronic acid (zoledmnate). 311 They can kill OS cells and significantly increase the growth inhibition rate of HER‐2‐positive OS cells.
Multitarget TKI of EGFR are the fastest advancing class of drugs for targeted therapy of OS. Its mechanism of action is to inhibit the autophosphorylation of intracellular zone receptor tyrosine kinase or competitively suppress the binding of receptor tyrosine kinase ATP to the EGFR site, blocking downstream signaling and is primarily used in the clinical management of advanced non‐small cell lung cancer (NSCLC). Studies on OS have shown that gefitinib significantly reduces the survival of OS cells from starvation stress and can be used as an adjuvant agent to enhance the anti‐OS activity of adriamycin and methotrexate. 312 At the same time, erlotinib can moderately enhance the chemotherapeutic effect of canine OS cells and may be used as a potential EGFR inhibitor to treat some patients with OS. 313 Pazopanib and regorafenib are some of the two drugs that have demonstrated high efficacy in OS therapy in several clinical trials. 314 , 315 Noteworthy, TKI combined with chemotherapy has been considered during the presurgical neoadjuvant phase to ameliorate the toxic effects of chemotherapy and reduced the duration of therapy. Meanwhile, applicable clinical trials are underway. 316 , 317
4.1.2. EGFR targeted therapy
The EGFR family belongs to type I transmembrane tyrosine kinase receptors, including EGFR/ErbBl/Her‐1, ErbB2/Her‐2, ErbB3/Her‐3, and ErbB4/Her‐4. The EGFR also performs a fundamental function in numerous types of cancers. In NSCLC, the driver mutations consist of EGFR mutations, particularly exon 20 insertions and point mutations. 318 , 319 Nevertheless, in other types of cancers such as OS, these EGFR mutations are extremely rare and it is not yet elucidated whether they are significant in clinical settings. Mantovani et al. 313 suggested that activation of the EGFR‐mediated progrowth signaling pathway performs a positive regulatory function in OS onset and advancement. Currently, extensive studies have been conducted on the relevance of EGFR to tumors and have shown positive expression in various malignancies, like lung, breast, cervical, and gastric cancers. 318 Trials have been conducted in patients with OS, but there is still a need for a large amount of research data.
Cetuximab, a monoclonal antibody against EGFR, blocks EGFR pathway signaling mainly by competitively suppressing the binding action of EGFR to related ligands, which can kill EGFR‐positive OS cells by enhancing the cytolytic function of NK cells and inhibit distant tumor invasion and angiogenesis. 319
4.1.3. PDGF/PDGFR targeted therapy
PDGF/PDGFR is a significant signaling pathway with remarkable involvement in the proliferation and differentiation of osteoblasts and osteoclasts. Additionally, PDGF/PDGFR performs a central function in the process of tumor angiogenesis by activating downstream signaling molecules through the formation of dimeric complexes by PDGF and PDGFR binding to trigger receptors. 320 Bozzi et al. 321 concluded that PDGFR overexpression was associated with disease progression and poor prognosis. There is still disagreement regarding the relevance of PDGF‐A expression to the prognosis of OS. Takagi et al. 322 showed that in vivo contact between OS and platelets can result in the aggregation of platelets and stimulation of the PDGF secretion, thereby phosphorylating PDGFR and Akt and promoting the proliferative rate of OS cells. Therefore, preventing PDGF from being bound by ligands can inhibit tumor angiogenesis and thus achieve antitumor effects.
Imatinib is a potent TKI that functions by targeting PDGFR signaling and was originally used to treat chronic myeloid leukemia. 323 Its targets include ABL, PDGFR, and c‐kit. To clarify the inhibitory effect of the PDGF pathway, studies have tested the expression of its downstream signaling proteins and observed that PDGFR phosphorylation and associated downstream signaling molecules were suppressed in >50% of patients after treatment with imatinib. Gobin et al. 324 showed that imatinib mesylate inhibited tumor growth in an animal model of OS in mice. Meanwhile, in two open‐label, single‐arm phase II clinical trials, the effectiveness of imatinib as a monotherapy drug in patients with OS was shown to be unsatisfactory, 325 and its use alone is inappropriate for the treatment of advanced OS, but it is reassuring that a synergistic antiproliferative effect of imatinib in combination with adriamycin for OS has been found in ex vivo experiments. 326 Similar to imatinib, two‐phase I studies of another antibody, olaratumab, evaluated the unsatisfactory efficacy of olaratumab as a single agent in patients with advanced sarcoma. 327 , 328 , 329 However, a phase II study illustrated that when used in tandem with doxorubicin, olaratumab was surprisingly effective as an “add‐on” drug, 330 and the use of olaratumab in conjunction with doxorubicin resulted in a 48% reduction in the mortality risk in contrast with the use of doxorubicin monotherapy.
4.1.4. mTOR targeted therapy
mTOR is a downstream regulator of PI3K, which is implicated in multiple regulatory functions like cell survival, proliferation, metastasis, and vascular regeneration. Studies have confirmed that mTOR signaling is aberrantly activated in OS and its inhibitors can exert antitumor effects by inhibiting cell growth and proliferation. 331 Additionally, the combination of mTOR inhibitors with other forms of drugs, like antiosteoporosis drugs, conventional chemotherapeutic drugs, and super‐terminal domain protein inhibitors enhances their antitumor effects. 332 Another study found that a dual mTOR inhibitor could enhance its antitumor activity in OS via MEK/ERK inhibitors. By blocking the mTOR transduction pathway, it can be used to inhibit the development of OS. 333
Ridaforolimus rapamycin and its analogs are first‐generation inhibitors of mTOR, inhibiting mTORC1 kinase activity by binding to FKBP‐1. 334 The results of preclinical testing illustrated the broad antitumor activity of rapamycin alone in OS xenograft models and in vivo. In addition, administering rapamycin in conjunction with vincristine or cyclophosphamide increased the responsiveness in OS models. 335 Temsirolimus, an analog of rapamycin, exhibited excellent antitumor activity in OS models when combined with cisplatin or bevacizumab. 336 Ridaforolimus, which is a selective mTOR antagonist, was administered as a monotherapy regimen in a phase II trial to treat patients with advanced sarcoma and it was found that these patients experienced partial symptom relief. Moreover, a larger double‐blind phase III trial illustrated that in patients with metastatic sarcoma, administering Ridaforolimus prolonged progression‐free survival (PFS) compared with chemotherapy. 337
Everolimus, a 40‐O‐(2‐hydroxyethyl)‐rapamycin derivative, impairs the proliferative potential of OS cells by stopping the G1 phase of the cell cycle. A phase II clinical trial illustrated that the proportion of patients with high‐grade OS treated with sorafenib in conjunction with everolimus increased from 27 to 45% of patients with a median PFS duration of 6 months compared with sorafenib alone, thus showing that the survival benefit of sorafenib combined with everolimus for high‐grade OS was superior to sorafenib monotherapy. 338 This effect was also confirmed by the experiments of Higuchi et al. 339
In OS xenograft animals, second‐generation dual mTOR inhibitors with weak antitumor activity included AZD8055, which inhibits both mTOR1 and mTOR2. Nonetheless, additional new small molecules inhibitors, like dual PI3K/mTOR antagonists (NVP‐BEZ235, MLN0128) and dual mTOR/DNA‐PK inhibitors (CC‐115), are still being studied in clinical trials to determine their efficacy. 340 , 341
4.1.5. VEGF/VEGFR targeted therapy
Angiogenesis is primarily modulated by VEGF/VEGFR signaling, 342 and there have been attempts to block angiogenesis as part of therapy for OS. Strategies for blocking angiogenic signals include neutralizing VEGF with antibodies, blocking VEGF receptors with antibodies, and inhibiting the intracellular activity of VEGF with small‐molecule TKIs VEGF targeting in OS has, however, not been effective. Bevacizumab, a monoclonal anti‐VEGF antibody, was evaluated in phase II trials as a first‐line treatment for advanced OS. Bevacizumab in conjunction with chemotherapy did not improve OS; however, treatment with bevacizumab was linked to an increase in PFS and an increase in total remission rates. 343
Sorafenib is a specific TKI that targets the VEGFR pathway. Heymann et al. 344 showed that in the OS model, sorafenib is anticipated to limit lung metastasis and attenuate tumor development. A phase II trial showed that sorafenib in patients with OS demonstrated activity as second‐ or third‐line therapy at 4‐month PFS and was the first targeted agent to show activity in patients with OS. 345 The National Comprehensive Cancer Network guidelines now list sorafenib as a second‐line therapy alternative for advanced OS.
Cabozantinib, a VEGFR2 and MET inhibitor, was evaluated in a phase II clinical trial involving 43 patients with OS, 39 of whom had pulmonary metastases, the 6‐month PFS rate was 33% and only five patients had partial responses, a finding similar to that of the sorafenib trial. 346 Apatinib is a new, highly selective oral small‐molecule antiangiogenic drug that binds to and blocks VEGFR‐2, vasculogenesis, and tumor growth. 347 Currently approved for third or more lines of treatment for advanced gastric adenocarcinoma or adenocarcinoma of the gastroesophageal junction, clinical trials of apatinib for OS are now underway. 348
4.1.6. Aurora kinase targeted therapy
The hallmark of tumor cells is the loss of cell cycle regulation, resulting in uncontrolled cell proliferation. Aurora kinase has functions such as regulating cell mitosis, and abnormal expression or activation of aurora kinase results in the transformation of normal cells into tumor cells. 349 The development of many Aurora kinase inhibitors has reached various stages. The Aurora kinase A inhibitor, alisertib (MLN8237), specifically targets AURK‐A by competitive binding to ATP. 350 In human OS U‐2 OS and MG63 cells, it has been shown to exert proapoptotic and proautophagic activities by stimulating the mitochondria‐induced pathway and suppressing the p38 MAPK/PI3K/Akt/mTOR signaling pathway. 351
4.2. Immunotherapy
Over the past 10 years, immunotherapy has revolutionized the way that cancer is treated, and immunotherapy for OS has developed at a breakneck pace. Major cancer immunotherapies include peripatetic immune cell therapy, checkpoint inhibitors, and cancer vaccines, among others. Several solid tumor types can be treated with checkpoint inhibitors. Clinical investigation of other adaptive immune cell therapies and cancer vaccines in solid tumors is ongoing.
4.2.1. Immune checkpoint inhibitors
Researchers have recently developed monoclonal antibodies that are directed against CTLA‐4 and PD‐1/PD‐L1, and they have improved effectiveness in treating malignancies like melanoma 352 and lung cancer. 353 PD‐1/PD‐L1 as an immunotherapeutic target has opened a new era of immunotherapy and accelerated the research on OS treatment. Different immunocytes including CTL, NK cells, B cells, and so on, express PD‐1 on their surfaces. PD‐1 can bind to PD‐L1 indicated by cancer cells to deliver programmed death signals to immune cells. In a mouse model of metastatic OS, significant improvement in OS responsiveness to CTL by using antibodies to block PD‐1/PD‐L1 interaction has been observed to contribute to decreased tumor load and improved patient survival. 354 In addition, blocking the PD‐1/PD‐L1 axis enhances the chemotherapeutic effect of cisplatin in OS. 355 The effectiveness of these studies was only at the animal model stage, and the relevant clinical studies of monoclonal antibodies against PD‐1/PD‐L1 did not show the same therapeutic effect as the animal models. Le Cesne et al. 356 conducted a phase II clinical study involving 17 patients with advanced OS and found a 6‐month PFS rate of only 13.3%.
A phase II clinical trial of camrelizumab in conjunction with apatinib for advanced OS also failed to meet the 6‐month PFS preset goal. 357 A multicenter phase II clinical trial on the effectiveness of pembrolizumab (SARC028) was the first prospective study of the treatment of advanced soft tissue sarcoma and OS by blocking immune checkpoints. Although the study showed some promise for application, the efficacy of the enrolled patients fell far short of expectations. Only 18% of soft tissue sarcoma patients showed a clinical response to pembrolizumab, while only 5% of OS patients showed a clinical response. 358 Research indicates that PD‐L1 expression levels in OS are variable and usually accompanied by the loss of MHC class I molecules leading to immune escape, which may be an important reason for the poor effectiveness of ICIs in OS. 359 Therefore, testing the levels of PD‐L1 and MHC class I molecules expressed in patients with OS before ICI therapy may be important to improve the outcome.
Another important checkpoint is CTLA‐4, a transmembrane glycoprotein receptor expressed in regulatory T cells and memory T cells that inhibits antitumor immune response by interacting with CD80/86 on DCs. 360 Upregulation of T‐cell CTLA‐4 expression levels was found in the blood of 19 patients with OS relative to 16 healthy volunteers, which was the basis for the development of CTLA‐4 inhibitors. 361 Ipilimumab, a CTLA‐4 monoclonal antibody developed in 2011, was approved by the United States Food and Drug Administration as the first next‐generation immune checkpoint inhibitor for the treatment of melanoma. 362 A phase I clinical trial study illustrated that 25% of patients with OS had stable disease after treatment with Iplimma. 363 Combined α‐PD‐L1 and α‐CTLA‐4 antibody blockade immunotherapy performed in a metastatic OS K7M2 mouse model resulted in complete control of most mouse tumors, 364 which offers a novel approach for treating OS.
4.2.2. Relay immune cell therapy
Relay immune cell therapy is another area of immunotherapy that is rapidly evolving, and CAR‐T therapy is at the heart of this. CAR‐T cells are highly specific and targeted for cytotoxicity, using the immune system's capacity to track down, spot, and eliminate cancerous cells. 365 CAR‐T cell therapy has achieved good efficacy in malignant blood diseases, 366 but the poor efficacy in solid cancers is mainly related to the TME, off‐target toxicity, immune escape, and tumor Ag heterogeneity. 367 In preclinical investigations, CAR‐T cells exhibited substantial antitumor efficacy. The combo of ICI may improve the targeted toxicity of CAR‐T cells, and appropriate target Ags including HER2, GD2, and B7‐H3 can enhance their activity and lessen side effects. 368 Nonetheless, because OS is a low‐immunogenic and extremely heterogeneous tumor, it is challenging to choose a precise and secure target.
TIL is another form of passaged cell therapy, and studies have shown satisfactory objective response rates of 40 to 70% in patients with metastatic melanoma to TIL. 369 A preclinical study reported that TILs were detectable in 75% of patients with OS and included CD8+ and CD4+ T‐lymphocytes, CD117 mast cells, and CD20+ B lymphocytes. 370 The advantage of TILs is that they target tumor‐specific Ags and are highly specific for tumors. WANG et al. 371 reported that combining TILs with ICIs was clinically effective in treating metastatic OS, with an efficacy rate nearly five times that of a single ICI, and prolonged PFS and overall survival.
4.2.3. Tumor vaccine
Tumor vaccines are used to achieve antitumor effects by inducing a strong immune response against tumors. A widely used tumor vaccine is the DC vaccine, which is an APC that induces the proliferation of cytotoxic T lymphocytes to achieve antitumor effects. 372 DC vaccines are most commonly used in OS. 373 Although Mackall et al. 374 showed that the 5‐year survival rate was prolonged from 31 to 43% in 30 patients who received the DC vaccine compared to the control group, only two of the 12 OS patients observed by Himoudi et al. 375 received DC vaccine therapy‐induced specific T‐cell immune responses against the tumor. Miwa et al. 376 evaluated the clinical response in 35 of 37 patients with OS; only six patients had stable disease and 28 patients showed tumor progression. These studies suggest that tumor vaccines for OS are safe and can activate the immune system to some extent, but the therapeutic effect on OS or whether it will improve patient prognosis in combination with other immunotherapies remains unclear and needs to be supported by more relevant studies.
5. CONCLUSION AND PROSPECTS
Currently, the signaling pathways related to the development of OS are increasingly being investigated by humans, whether it is PI3K/AKT/mTOR signaling pathway, Wnt signaling pathway, JAK/ STAT3 signaling pathway, Hippo signaling pathway, Notch signaling pathway, PD‐1/PD‐L1 signaling pathway, MAPK signaling pathway, or NF‐κB signaling pathway (Table 1). Related studies have shown their involvement in the development of OS. The mechanism of OS development is a complex signaling network, and in addition to the above eight signaling pathways, some related pathways have not been elucidated in this paper, which needs to be summarized in more depth. Whether one or more pathways are implicated in the regulation of OS signaling pathways has not yet been better demonstrated, and whether single pathways or multiple pathways combine to dominate regulation requires further study. However, there is no doubt that a thorough examination of the mechanisms behind OS evolution will enable us to unlock the secret.
TABLE 1.
The roles and functions of signaling pathways in OS and the identified biomarkers as well as potential therapeutic targets.
| Signaling pathways | Roles and functions: significant roles in OS | Cellular biological processes | Biomarkers and potential therapeutic targets | References |
|---|---|---|---|---|
| PI3K/AKT/mTOR signaling pathway | Diagnosis associated with lung metastasis, related to chemotherapy resistance | Proliferation, survival, migration, invasion, metastasis, cell cycle, apoptosis, angiogenesis | PI3K, p‐PI3K, AKT, p‐AKT, mTOR, PTEN, miR‐142, miR‐191‐5p, miR‐524, miR‐9‐5p, miR‐744, lncRNA HULC, lncRNA MSC‐AS1 | 48 , 51 , 52 , 53 , 54 , 55 , 61 , 62 |
| Wnt/β‐catenin signaling pathway | Diagnosis associated with lung metastasis, related to tumor recurrence and chemotherapy resistance | Proliferation, differentiation, survival, cell cycle, apoptosis, migration, invasion, EMT, angiogenesis | β‐Catenin, ROR2, SDC2, sFRP2, WIF‐1, GSK‐3β, miR‐429, miR‐183 | 84 , 86 , 87 , 88 , 92 , 225 , 226 |
| JAK/ STAT3 signaling pathway | Diagnosis associated with lung metastasis, related to chemotherapy resistance | Proliferation, apoptosis, migration, invasion, metastasis, autophagy, and angiogenesis | JAK, STAT3, P‐STAT3, CyclinD1, Bcl‐2, Mcl‐1, COX‐2, MMP‐2, survivin, lncRNA HOXD‐AS1, miR‐126, miR199a‐3p, miR‐199a‐5p, miR‐125b | 110 , 111 , 112 , 113 , 114 |
| Hippo signaling pathway | Diagnosis and prognosis biomarker, related to chemotherapy resistance | Proliferation, differentiation, apoptosis, migration, and invasion | YAP, TAZ, Sox2, TEAD1, USP1, miR‐375, miR‐624‐5p, miR‐584‐5p, miR‐34c | 126 , 127 , 128 , 129 , 130 , 131 , 132 , 236 , 237 |
| PD‐1/PD‐L1 signaling pathway | Prognosis biomarker, related to immunotolerance | Proliferation, survival, metastasis, apoptosis, immune response | PD‐1/PD‐L1, IFN‐γ, miR‐140, miR‐200a, miR‐106a, Lnc00657 | 143 , 144 , 162 , 163 , 164 |
| Notch signaling pathway | Proliferation, differentiation, survival, migration, invasion, metastasis | Formation, development, survival, proliferation, invasion, and metastasis | Jagged1, Notch1, Notch‐ICN, MRP1, Hes1, E‐cadherin, P53miR‐26a, lncRNA MEG3 | 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 |
| MAPK signaling pathway | Diagnosis associated with lung metastasis, related to chemotherapy resistance | Proliferation, differentiation, apoptosis, ROS, and tumor metastasis | p38, ERK, JNK, VDAC1, ITGB3, PSKH1, MMPs, caspase‐3, caspase‐9 | 198 , 199 , 200 |
| NF‐κB signaling pathway | Related to tumor recurrence and chemotherapy resistance | Growth, proliferation, chemoresistance, invasion, and metastasis | ATRX, p65, TRIM10 | 206 , 207 |
The development of pulmonary metastasis from OS is not something anyone wants to foresee, but the highly active and highly metastatic nature of OS cells leads to early metastasis to distant tissues and can lead to a range of malignant symptoms, which may further impact the prognosis and the rate of survival for OS patients. Although the patients’ prognoses in OS have not changed significantly in recent decades, and pulmonary metastasis of OS remains a major challenge to curing OS and improving patient survival, various therapeutic approaches have made some progress and have yielded important clinical benefits. Targeted therapies for OS are safer and more effective than chemotherapy. For OS‐targeted therapy, the development of more potent medications and the identification of biomarkers with higher sensitivity and specificity remain the major priorities (Table 2). With continuous developments in molecular biology and advances in basic research, many in vivo and in vitro experiments have identified not only the great potential of targeted drugs but also new regulatory mechanisms and targets. Recent research results have identified novel diagnostic/prognostic biological markers and possible therapeutic targets, enabling a clearer knowledge of the molecular mechanisms underlying OS onset, progression, metastasis, and drug resistance. Several molecular targets, including PI3K, mTOR, PD‐1/PD‐L1, and others, have advanced to at least phase II clinical investigations. The molecular processes, however, typically involve crosstalk or feedback loops instead of particular signaling pathways. When using a single targeted therapy, bypass is a key factor in treatment resistance. New combination regimens must thus be created and validated promptly.
TABLE 2.
Clinical trials with potential new molecular targets.
| Trial | Phase | Drug | Treatment regimen | Molecular target | Results and conclusions | References |
|---|---|---|---|---|---|---|
| NCT01016015, NCT01614795 | II | Cixutumumab | Cixutumumab + temsirolimus | IGF‐R | No objective responses were identified in phase II trials involving young adults and children with refractory or recurrent sarcomas. | 306 |
| NCT00617890 | II | Robatumumab | Robatumumab | IGF‐R | Three of 31 patients with low disease burden had a complete or partial response, according to solid tumor response assessment criteria. | 307 |
| Cooperative Children's Oncology Group (COG) | II | Trastuzumab | Trastuzumab + anti‐IGF‐1R or zoledmnate | HER‐2 | Kill OS cells and significantly increase the growth inhibition rate of HER‐2‐positive OS cells. | 310 , 311 |
| Cooperative Children's Oncology Group (COG) | II | Imatinib | Imatinib + adriamycin | PDGF/PDGFR | Synergistic antiproliferative | 325 |
| NCT01185964 | II | Olaratumab | Olaratumab + doxorubicin | PDGF/PDGFR | Reducing the risk of death by 48% | 330 |
| NCT00112372, NCT00093080 | II/III | Ridaforolimus | Ridaforolimus + vincristine or cyclophosphamide | mTOR | OS patient had a PR and prolonged PFS compared with chemotherapy. | 335 , 337 |
| NCT02429973 | II | Everolimus | Everolimus + sirolimus | mTOR | The proportion of median PFS time up to 6 months in patients with single‐agent sorafenib increased from 27 to 45%. | 345 |
| NCT00667342 | II | Bevacizumab | Bevacizumab | VEGF/VEGFR | Associated with increased progression‐free survival and overall remission rate | 343 |
| NCT02429973 | II | Sorafenib | Everolimus + sirolimus | VEGF/VEGFR | Show activity as a second‐ or third‐line treatment in the 4‐month PFS | 345 |
| NCT02243605 | II | Cabozantinib | Cabozantinib | VEGF/VEGFR | The 6‐month progression‐free survival rate was 33%, with only five patients showing a partial response. | 346 |
| NCT03359018 | II | Camrelizumab | Camrelizumab + apatinib | Immune checkpoint inhibitors | The preset target of PFS at 6 months was not reached. | 357 |
| NCT02301039, NCT03013127 | II | Pembrolizumab | Pembrolizumab | Immune checkpoint inhibitors | Only 5% of the patients with osteosarcoma showed a clinical response. | 358 |
| NCT01445379 | I | Ipilimumab | Ipilimumab | Immune checkpoint inhibitors | Twenty‐five percent of patients with osteosarcoma had stable disease after ipllimma. | 363 |
| NCT01241162, NCT01803152 | I/II | DC vaccines | DC vaccines + decitabine or gemcitabine pretreatment | Dendritic cell peptide vaccines | Primary and metastatic tumor growth inhibition and remodeling of tumor microenvironment with reduced Treg and immunosuppressive cytokines and increased CD8+ T lymphocytes, with small outcome benefits in clinical trials. | 374 , 376 |
There are few relatively satisfactory clinical chemotherapy regimens available, and collaborative multiparty studies are needed to identify a chemotherapy regimen that will improve patient prognosis, while basic, translational, and clinical research teams collaborate, leading to the development of new prognostic markers and new therapeutic targets. No single immunotherapy has been definitively shown to be effective in OS, and the clinical efficacy of a single immunotherapy is inadequate, but there are clear studies demonstrating an immune link between OS progression and immunity, so in‐depth studies of immune mechanisms and the development of new immunotherapies are a priority in immunology research. Pain management is also important, not only to improve symptoms but also to benefit patients physically and mentally and their recovery. As immunotherapy and gene therapy are expected to provide more opportunities and possibilities for the treatment of OS, the combination strategy of multiple therapeutic approaches is a hot topic of current research.
This review also focuses on the signaling targets involved in the preventive and curative effects of TCM on OS through the modulation of related protein and gene expression in signaling pathways. Although the research results of TCM for OS are encouraging, most of the current experimental studies focus on the impact of the drugs on OS cells in the context of inhibiting proliferation or promoting apoptosis, and only a few research reports have specifically examined the impact on metabolism and immunity, which is relatively single‐directional research, and it is currently difficult to achieve clear clinical efficacy. Regarding the action mechanism of TCM in OS therapy, most of the TCM compounds are still under the general framework of multitarget and multipathway synergy, and their specific targets have not been fully elucidated. Given the complex and multitarget components of TCM, more extensive research is required. to realize the synergistic effects of multiple TCM extracts to achieve multitarget, multipathway, and multifaceted treatment of OS. Moreover, future studies should focus on comprehensively revealing the mechanism of action of herbal components and compound formulations from a holistic perspective.
AUTHOR CONTRIBUTION
Writing—original draft: Ji Ziyu. Writing—original draft, visualization, funding acquisition: Shen Jianlin. Methodology, writing—review and editing, funding acquisition: Yi Qian. Conceptualization, writing—review and editing, supervision, funding acquisition: Liu Huan. Draw figure: Yujian Lan. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
We thank The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Southwest Medical University, and Affiliated Hospital of Putian University for their support of this study. Thanks to Sanger for providing the text touch‐up service, the molecular structure diagrams are all made with https://molview.org/.
Ji Z, Shen J, Lan Y, Yi Q, Liu H. Targeting signaling pathways in osteosarcoma: Mechanisms and clinical studies. MedComm. 2023;4:e308. 10.1002/mco2.308
Contributor Information
Qian Yi, Email: yiqian2010@yeah.net.
Huan Liu, Email: 20016040@163.com.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Rickel K, Fang F, Tao J. Molecular genetics of osteosarcoma. Bone. 2017;102:69‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang Z, Li X, Yang Y, He Z, Qu X, Zhang Y. Long noncoding RNAs in the progression, metastasis, and prognosis of osteosarcoma. Cell Death Dis. 2016;7(9):e2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pang H, Wu T, Peng Z, et al. Baicalin induces apoptosis and autophagy in human osteosarcoma cells by increasing ROS to inhibit PI3K/Akt/mTOR, ERK1/2 and beta‐catenin signaling pathways. J Bone Oncol. 2022;33:100415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. J A, S C, S D, et al. Circular PVT1: an oncogenic non‐coding RNA with emerging clinical importance. J Clin Pathol. 2019;72(8):513‐519. [DOI] [PubMed] [Google Scholar]
- 5. Yang G, Wu Y, Wan R, Sang H, Liu H, Huang W. The role of non‑coding RNAs in the regulation, diagnosis, prognosis and treatment of osteosarcoma (Review). Int J Oncol. 2021;59(3). [DOI] [PubMed] [Google Scholar]
- 6. Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47(1):283‐292. [DOI] [PubMed] [Google Scholar]
- 7. Grinberg SZ, Posta A, Weber KL, Wilson RJ. Limb salvage and reconstruction options in osteosarcoma. Adv Exp Med Biol. 2020;1257:13‐29. [DOI] [PubMed] [Google Scholar]
- 8. Berner K, Johannesen TB, Berner A, et al. Time‐trends on incidence and survival in a nationwide and unselected cohort of patients with skeletal osteosarcoma. Acta Oncol. 2015;54(1):25‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bajpai J, Chandrasekharan A, Talreja V, et al. Outcomes in non‐metastatic treatment naive extremity osteosarcoma patients treated with a novel non‐high dosemethotrexate‐based, dose‐dense combination chemotherapy regimen ‘OGS‐12’. Eur J Cancer. 2017;85:49‐58. [DOI] [PubMed] [Google Scholar]
- 10. Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev. 2016;96(4):1261‐1296. [DOI] [PubMed] [Google Scholar]
- 11. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qin W, Cao L, Massey IY. Role of PI3K/Akt signaling pathway in cardiac fibrosis. Mol Cell Biochem. 2021;476(11):4045‐4059. [DOI] [PubMed] [Google Scholar]
- 13. Chen R, Zhou L. PD‐1 signaling pathway in sepsis: does it have a future. Clin Immunol. 2021;229:108742. [DOI] [PubMed] [Google Scholar]
- 14. Lee SJ. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J Clin Invest. 2021;131(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu W, Zhao Y, Wang G, et al. TRIM22 inhibits osteosarcoma progression through destabilizing NRF2 and thus activation of ROS/AMPK/mTOR/autophagy signaling. Redox Biol. 2022;53:102344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marini F, Giusti F, Palmini G, Brandi ML. Role of Wnt signaling and sclerostin in bone and as therapeutic targets in skeletal disorders. Osteoporos Int. 2023;34(2):213‐238. [DOI] [PubMed] [Google Scholar]
- 17. Guo H, Zheng L, Guo Y, Han L, Yu J, Lai F. Curculigoside represses the proliferation and metastasis of osteosarcoma via the JAK/STAT and NF‐kappaB signaling pathways. Biol Pharm Bull. 2022;45(10):1466‐1475. [DOI] [PubMed] [Google Scholar]
- 18. Rothzerg E, Ingley E, Mullin B, Xue W, Wood D, Xu J. The Hippo in the room: targeting the Hippo signalling pathway for osteosarcoma therapies. J Cell Physiol. 2021;236(3):1606‐1615. [DOI] [PubMed] [Google Scholar]
- 19. Ge YX, Zhang TW, Zhou L, et al. Enhancement of anti‐PD‐1/PD‐L1 immunotherapy for osteosarcoma using an intelligent autophagy‐controlling metal organic framework. Biomaterials. 2022;282:121407. [DOI] [PubMed] [Google Scholar]
- 20. Zanotti S, Canalis E. Notch signaling and the skeleton. Endocr Rev. 2016;37(3):223‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lv H, Zhen C, Liu J, Shang P. beta‐Phenethyl Isothiocyanate induces cell death in human osteosarcoma through altering iron metabolism, disturbing the redox balance, and activating the MAPK signaling pathway. Oxid Med Cell Longev. 2020;2020:5021983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng L, Feng Z, Tao S, et al. Destabilization of macrophage migration inhibitory factor by 4‐IPP reduces NF‐kappaB/P‐TEFb complex‐mediated c‐Myb transcription to suppress osteosarcoma tumourigenesis. Clin Transl Med. 2022;12(1):e652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yin MC, Wang HS, Yang X, et al. A bibliometric analysis and visualization of current research trends in Chinese medicine for osteosarcoma. Chin J Integr Med. 2022;28(5):445‐452. [DOI] [PubMed] [Google Scholar]
- 24. Chang JL, Wang WY, Li YM, et al. Chinese herbal medicine for osteosarcoma in the mouse: a systematic review and meta‐analysis. Chin J Integr Med. 2019;25(5):370‐377. [DOI] [PubMed] [Google Scholar]
- 25. Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arafeh R, Samuels Y. PIK3CA in cancer: the past 30 years. Semin Cancer Biol. 2019;59:36‐49. [DOI] [PubMed] [Google Scholar]
- 27. Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol‐3‐phosphate. Nature. 1988;332(6165):644‐646. [DOI] [PubMed] [Google Scholar]
- 28. Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol. 2019;59:125‐132. [DOI] [PubMed] [Google Scholar]
- 29. Tapia O, Riquelme I, Leal P, et al. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014;465(1):25‐33. [DOI] [PubMed] [Google Scholar]
- 30. Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hopkins BD, Goncalves MD, Cantley LC. Insulin‐PI3K signalling: an evolutionarily insulated metabolic driver of cancer. Nat Rev Endocrinol. 2020;16(5):276‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Donnell JS, Massi D, Teng MWL, Mandala M. PI3K‐AKT‐mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol. 2018;48:91‐103. [DOI] [PubMed] [Google Scholar]
- 33. Ramakrishnan V, Kumar S. PI3K/AKT/mTOR pathway in multiple myeloma: from basic biology to clinical promise. Leuk Lymphoma. 2018;59(11):2524‐2534. [DOI] [PubMed] [Google Scholar]
- 34. Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170(4):605‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng C, Tang F, Min L, Hornicek F, Duan Z, Tu C. PTEN in osteosarcoma: recent advances and the therapeutic potential. Biochim Biophys Acta Rev Cancer. 2020;1874(2):188405. [DOI] [PubMed] [Google Scholar]
- 36. Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR pathway and its role in cancer therapeutics: are we making headway? Front Oncol. 2022;12:819128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu L, Wei J, Liu P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin Cancer Biol. 2022;85:69‐94. [DOI] [PubMed] [Google Scholar]
- 38. Sirico M, D'Angelo A, Gianni C, Casadei C, Merloni F, De Giorgi U. Current state and future challenges for PI3K inhibitors in cancer therapy. Cancers (Basel). 2023;15(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Majchrzak A, Witkowska M, Smolewski P. Inhibition of the PI3K/Akt/mTOR signaling pathway in diffuse large B‐cell lymphoma: current knowledge and clinical significance. Molecules. 2014;19(9):14304‐14315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karami Fath M, Ebrahimi M, Nourbakhsh E, et al. PI3K/Akt/mTOR signaling pathway in cancer stem cells. Pathol Res Pract. 2022;237:154010. [DOI] [PubMed] [Google Scholar]
- 41. Luciani A, Stagi M. How mTORC1 makes sense of nutrients. Kidney Int. 2021;99(2):295‐298. [DOI] [PubMed] [Google Scholar]
- 42. Kong Y, Si L, Li Y, et al. Analysis of mTOR gene aberrations in melanoma patients and evaluation of their sensitivity to PI3K‐AKT‐mTOR pathway inhibitors. Clin Cancer Res. 2016;22(4):1018‐1027. [DOI] [PubMed] [Google Scholar]
- 43. Hwang SK, Kim HH. The functions of mTOR in ischemic diseases. BMB Rep. 2011;44(8):506‐511. [DOI] [PubMed] [Google Scholar]
- 44. Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. Natural products targeting the PI3K‐Akt‐mTOR signaling pathway in cancer: a novel therapeutic strategy. Semin Cancer Biol. 2022;80:1‐17. [DOI] [PubMed] [Google Scholar]
- 45. Gobin B, Huin MB, Lamoureux F, et al. BYL719, a new alpha‐specific PI3K inhibitor: single administration and in combination with conventional chemotherapy for the treatment of osteosarcoma. Int J Cancer. 2015;136(4):784‐796. [DOI] [PubMed] [Google Scholar]
- 46. Meric‐Bernstam F, Akcakanat A, Chen H, et al. PIK3CA/PTEN mutations and Akt activation as markers of sensitivity to allosteric mTOR inhibitors. Clin Cancer Res. 2012;18(6):1777‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakayama I, Shinozaki E, Matsushima T, et al. Retrospective study of RAS/PIK3CA/BRAF tumor mutations as predictors of response to first‐line chemotherapy with bevacizumab in metastatic colorectal cancer patients. BMC Cancer. 2017;17(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu W, Chen PB, Chen FC, Ding SL, Pan XY. MicroRNA‐744 promotes proliferation of osteosarcoma cells by targeting PTEN. Mol Med Rep. 2020;21(5):2276‐2282. [DOI] [PubMed] [Google Scholar]
- 49. Almhanna K, Strosberg J, Malafa M. Targeting AKT protein kinase in gastric cancer. Anticancer Res. 2011;31(12):4387‐4392. [PubMed] [Google Scholar]
- 50. Sukawa Y, Yamamoto H, Nosho K, et al. Alterations in the human epidermal growth factor receptor 2‐phosphatidylinositol 3‐kinase‐v‐Akt pathway in gastric cancer. World J Gastroenterol. 2012;18(45):6577‐6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang L, Zhao G, Ji S, Yuan Q, Zhou H. Downregulated long non‐coding RNA MSC‐AS1 inhibits osteosarcoma progression and increases sensitivity to cisplatin by binding to microRNA‐142. Med Sci Monit. 2020;26:e921594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen B, Zheng ZY, Yang JZ, Li XG. MicroRNA‐191‐5p promotes the development of osteosarcoma via targeting EGR1 and activating the PI3K/AKT signaling pathway. Eur Rev Med Pharmacol Sci. 2021;25(8):3145. [DOI] [PubMed] [Google Scholar]
- 53. Zhuang M, Qiu X, Cheng D, Zhu C, Chen L. MicroRNA‐524 promotes cell proliferation by down‐regulating PTEN expression in osteosarcoma. Cancer Cell Int. 2018;18:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao R, Zhou X, Zhang W, Li L. Effect of long noncoding RNA HULC on proliferation, migration, and invasion of osteosarcoma cells. J Oncol. 2022;2022:7526731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang L, Lv Z, Xu J, et al. MicroRNA‐134 inhibits osteosarcoma angiogenesis and proliferation by targeting the VEGFA/VEGFR1 pathway. FEBS J. 2018;285(7):1359‐1371. [DOI] [PubMed] [Google Scholar]
- 56. Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17(8):666‐681. [DOI] [PubMed] [Google Scholar]
- 57. Yu G, Wang J, Chen Y, et al. Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of chinese patients with gastric cancer. Clin Cancer Res. 2009;15(5):1821‐1829. [DOI] [PubMed] [Google Scholar]
- 58. Luo Y, Xu W, Li G, Cui W. Weighing In on mTOR complex 2 signaling: the expanding role in cell metabolism. Oxid Med Cell Longev. 2018;2018:7838647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364(23):2187‐2198. [DOI] [PubMed] [Google Scholar]
- 60. Steelman LS, Abrams SL, Whelan J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22(4):686‐707. [DOI] [PubMed] [Google Scholar]
- 61. Lin YC, Chen HY, Hsieh CP, Huang YF, Chang IL. Betulin inhibits mTOR and induces autophagy to promote apoptosis in human osteosarcoma cell lines. Environ Toxicol. 2020;35(8):879‐887. [DOI] [PubMed] [Google Scholar]
- 62. Ray‐Coquard I, Le Cesne A. A role for maintenance therapy in managing sarcoma. Cancer Treat Rev. 2012;38(5):368‐378. [DOI] [PubMed] [Google Scholar]
- 63. Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36(11):1461‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zou G, Park JI. Wnt signaling in liver regeneration, disease, and cancer. Clin Mol Hepatol. 2023;29(1):33‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang Y, Wang X. Targeting the Wnt/beta‐catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bugter JM, Fenderico N, Maurice MM. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer. 2021;21(1):5‐21. [DOI] [PubMed] [Google Scholar]
- 67. Koni M, Pinnaro V, Brizzi MF. The Wnt signalling pathway: a tailored target in cancer. Int J Mol Sci. 2020;21(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhong Z, Virshup DM. Wnt signaling and drug resistance in cancer. Mol Pharmacol. 2020;97(2):72‐89. [DOI] [PubMed] [Google Scholar]
- 69. Huang L, Xiang M, Ye P, Zhou W, Chen M. Beta‐catenin promotes macrophage‐mediated acute inflammatory response after myocardial infarction. Immunol Cell Biol. 2018;96(1):100‐113. [DOI] [PubMed] [Google Scholar]
- 70. MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/beta‐catenin signaling. Cold Spring Harb Perspect Biol. 2012;4(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yu Z, Jiang X, Qin L, et al. A novel UBE2T inhibitor suppresses Wnt/beta‐catenin signaling hyperactivation and gastric cancer progression by blocking RACK1 ubiquitination. Oncogene. 2021;40(5):1027‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liang T, Ye X, Liu Y, et al. FAM46B inhibits cell proliferation and cell cycle progression in prostate cancer through ubiquitination of beta‐catenin. Exp Mol Med. 2018;50(12):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li N, Shi H, Zhang L, et al. miR‐188 inhibits glioma cell proliferation and cell cycle progression through targeting beta‐catenin. Oncol Res. 2018;26(5):785‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hua Y, Yang Y, Li Q, et al. Oligomerization of frizzled and LRP5/6 protein initiates intracellular signaling for the canonical WNT/beta‐catenin pathway. J Biol Chem. 2018;293(51):19710‐19724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ren Y, Guo T, Xu J, Liu Y, Huang J. The novel target of esophageal squamous cell carcinoma: lncRNA GASL1 regulates cell migration, invasion and cell cycle stagnation by inactivating the Wnt3a/beta‐catenin signaling. Pathol Res Pract. 2021;217:153289. [DOI] [PubMed] [Google Scholar]
- 76. Alizadeh A, Jebelli A, Baradaran B, et al. Crosstalk between long non‐coding RNA DLX6‐AS1, microRNAs and signaling pathways: a pivotal molecular mechanism in human cancers. Gene. 2021;769:145224. [DOI] [PubMed] [Google Scholar]
- 77. Katagiri H, Yonezawa H, Shitamura S, et al. A Wnt/beta‐catenin signaling inhibitor, IMU1003, suppresses the emergence of osimertinib‐resistant colonies from gefitinib‐resistant non‐small cell lung cancer cells. Biochem Biophys Res Commun. 2023;645:24‐29. [DOI] [PubMed] [Google Scholar]
- 78. MacDonald BT, Tamai K, He X. Wnt/beta‐catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schunk SJ, Floege J, Fliser D, Speer T. WNT‐beta‐catenin signalling ‐ a versatile player in kidney injury and repair. Nat Rev Nephrol. 2021;17(3):172‐184. [DOI] [PubMed] [Google Scholar]
- 80. Lybrand DB, Naiman M, Laumann JM, et al. Destruction complex dynamics: wnt/beta‐catenin signaling alters Axin‐GSK3beta interactions in vivo. Development. 2019;146(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Perugorria MJ, Olaizola P, Labiano I, et al. Wnt‐beta‐catenin signalling in liver development, health and disease. Nat Rev Gastroenterol Hepatol. 2019;16(2):121‐136. [DOI] [PubMed] [Google Scholar]
- 82. Lin H, Wu T, Peng L, et al. Lnc‐MAP6‐1:3 knockdown inhibits osteosarcoma progression by modulating Bax/Bcl‐2 and Wnt/beta‐catenin pathways. Int J Med Sci. 2020;17(15):2248‐2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lin YC, Haas A, Bufe A, et al. Wnt10b‐GSK3beta‐dependent Wnt/STOP signaling prevents aneuploidy in human somatic cells. Life Sci Alliance. 2021;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Enomoto M, Hayakawa S, Itsukushima S, et al. Autonomous regulation of osteosarcoma cell invasiveness by Wnt5a/Ror2 signaling. Oncogene. 2009;28(36):3197‐3208. [DOI] [PubMed] [Google Scholar]
- 85. Matsuoka K, Bakiri L, Wolff LI, et al. Wnt signaling and Loxl2 promote aggressive osteosarcoma. Cell Res. 2020;30(10):885‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ge Y, Ding S, Feng J, Du J, Gu Z. Diosgenin inhibits Wnt/beta‐catenin pathway to regulate the proliferation and differentiation of MG‐63 cells. Cytotechnology. 2021;73(2):169‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. van Loon K, Huijbers EJM, Griffioen AW. Secreted frizzled‐related protein 2: a key player in noncanonical Wnt signaling and tumor angiogenesis. Cancer Metastasis Rev. 2021;40(1):191‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kim H, Yoo S, Zhou R, et al. Oncogenic role of SFRP2 in p53‐mutant osteosarcoma development via autocrine and paracrine mechanism. Proc Natl Acad Sci U S A. 2018;115(47):E11128‐E11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Techavichit P, Gao Y, Kurenbekova L, Shuck R, Donehower LA, Yustein JT. Secreted Frizzled‐Related Protein 2 (sFRP2) promotes osteosarcoma invasion and metastatic potential. BMC Cancer. 2016;16(1):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bravo D, Salduz A, Shogren KL, et al. Decreased local and systemic levels of sFRP3 protein in osteosarcoma patients. Gene. 2018;674:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Li L, Hobson L, Perry L, et al. Targeting the ERG oncogene with splice‐switching oligonucleotides as a novel therapeutic strategy in prostate cancer. Br J Cancer. 2020;123(6):1024‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yang J, Kitami M, Pan H, et al. Augmented BMP signaling commits cranial neural crest cells to a chondrogenic fate by suppressing autophagic beta‐catenin degradation. Sci Signal. 2021;14(665). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lin CH, Guo Y, Ghaffar S, et al. Dkk‐3, a secreted wnt antagonist, suppresses tumorigenic potential and pulmonary metastasis in osteosarcoma. Sarcoma. 2013;2013:147541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Roslan Z, Muhamad M, Selvaratnam L, Ab‐Rahim S. The roles of low‐density lipoprotein receptor‐related proteins 5, 6, and 8 in cancer: a review. J Oncol. 2019;2019:4536302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hoang BH, Kubo T, Healey JH, et al. Expression of LDL receptor‐related protein 5 (LRP5) as a novel marker for disease progression in high‐grade osteosarcoma. Int J Cancer. 2004;109(1):106‐111. [DOI] [PubMed] [Google Scholar]
- 96. Guo Y, Zi X, Koontz Z, et al. Blocking Wnt/LRP5 signaling by a soluble receptor modulates the epithelial to mesenchymal transition and suppresses met and metalloproteinases in osteosarcoma Saos‐2 cells. J Orthop Res. 2007;25(7):964‐971. [DOI] [PubMed] [Google Scholar]
- 97. Guo Y, Rubin EM, Xie J, Zi X, Hoang BH. Dominant negative LRP5 decreases tumorigenicity and metastasis of osteosarcoma in an animal model. Clin Orthop Relat Res. 2008;466(9):2039‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dodington DW, Desai HR, Woo M. JAK/STAT ‐ emerging players in metabolism. Trends Endocrinol Metab. 2018;29(1):55‐65. [DOI] [PubMed] [Google Scholar]
- 99. Wang G, Zhang B, Wang Y, Han S, Wang C. Crocin promotes apoptosis of human skin cancer cells by inhibiting the JAK/STAT pathway. Exp Ther Med. 2018;16(6):5079‐5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19(49):5662‐5679. [DOI] [PubMed] [Google Scholar]
- 101. Zhong Y, Yin B, Ye Y, et al. The bidirectional role of the JAK2/STAT3 signaling pathway and related mechanisms in cerebral ischemia‐reperfusion injury. Exp Neurol. 2021;341:113690. [DOI] [PubMed] [Google Scholar]
- 102. Xu S, Neamati N. gp130: a promising drug target for cancer therapy. Expert Opin Ther Targets. 2013;17(11):1303‐1328. [DOI] [PubMed] [Google Scholar]
- 103. Rajagopal C, Lankadasari MB, Aranjani JM, Harikumar KB. Targeting oncogenic transcription factors by polyphenols: a novel approach for cancer therapy. Pharmacol Res. 2018;130:273‐291. [DOI] [PubMed] [Google Scholar]
- 104. Xiong A, Yang Z, Shen Y, Zhou J, Shen Q. Transcription factor STAT3 as a novel molecular target for cancer prevention. Cancers (Basel). 2014;6(2):926‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhu D, Chen C, Liu X, et al. Osteosarcoma cell proliferation suppression via SHP‐2‐mediated inactivation of the JAK/STAT3 pathway by tubocapsenolide A. J Adv Res. 2021;34:79‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wei D, Li C, Ye J, Xiang F, Liu J. Extracellular collagen mediates osteosarcoma progression through an integrin alpha2beta1/JAK/STAT3 signaling pathway. Cancer Manag Res. 2020;12:12067‐12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Couto JI, Bear MD, Lin J, et al. Biologic activity of the novel small molecule STAT3 inhibitor LLL12 against canine osteosarcoma cell lines. BMC Vet Res. 2012;8:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang X, Goldstein D, Crowe PJ, Yang JL. Impact of STAT3 inhibition on survival of osteosarcoma cell lines. Anticancer Res. 2014;34(11):6537‐6545. [PubMed] [Google Scholar]
- 109. Yan J, Wang Q, Zou K, et al. Inhibition of the JAK2/STAT3 signaling pathway exerts a therapeutic effect on osteosarcoma. Mol Med Rep. 2015;12(1):498‐502. [DOI] [PubMed] [Google Scholar]
- 110. Qu Y, Zheng S, Kang M, et al. Knockdown of long non‐coding RNA HOXD‐AS1 inhibits the progression of osteosarcoma. Biomed Pharmacother. 2018;98:899‐906. [DOI] [PubMed] [Google Scholar]
- 111. Jiang R, Zhang C, Liu G, Gu R, Wu H. MicroRNA‐126 inhibits proliferation, migration, invasion, and EMT in osteosarcoma by targeting ZEB1. J Cell Biochem. 2017;118(11):3765‐3774. [DOI] [PubMed] [Google Scholar]
- 112. Wang C, Ba X, Guo Y, et al. MicroRNA‐199a‐5p promotes tumour growth by dual‐targeting PIAS3 and p27 in human osteosarcoma. Sci Rep. 2017;7:41456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sun Z, Liu Q, Hong H, Zhang H, Zhang T. miR‐19 promotes osteosarcoma progression by targeting SOCS6. Biochem Biophys Res Commun. 2018;495(1):1363‐1369. [DOI] [PubMed] [Google Scholar]
- 114. Xiao T, Zhou Y, Li H, et al. MiR‐125b suppresses the carcinogenesis of osteosarcoma cells via the MAPK‐STAT3 pathway. J Cell Biochem. 2019;120(2):2616‐2626. [DOI] [PubMed] [Google Scholar]
- 115. Fossey SL, Liao AT, McCleese JK, et al. Characterization of STAT3 activation and expression in canine and human osteosarcoma. BMC Cancer. 2009;9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Han Y, Guo W, Ren T, et al. Tumor‐associated macrophages promote lung metastasis and induce epithelial‐mesenchymal transition in osteosarcoma by activating the COX‐2/STAT3 axis. Cancer Lett. 2019:440‐441. 116‐125. [DOI] [PubMed] [Google Scholar]
- 117. Tu B, Du L, Fan QM, Tang Z, Tang TT. STAT3 activation by IL‐6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett. 2012;325(1):80‐88. [DOI] [PubMed] [Google Scholar]
- 118. Peng D, Lin B, Xie M, et al. Histone demethylase KDM5A promotes tumorigenesis of osteosarcoma tumor. Cell Death Discov. 2021;7(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lv B, Gao G, Guo Y, et al. Serglycin promotes proliferation, migration, and invasion via the JAK/STAT signaling pathway in osteosarcoma. Aging (Albany NY). 2021;13(17):21142‐21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hess AR, Margaryan NV, Seftor EA, Hendrix MJ. Deciphering the signaling events that promote melanoma tumor cell vasculogenic mimicry and their link to embryonic vasculogenesis: role of the Eph receptors. Dev Dyn. 2007;236(12):3283‐3296. [DOI] [PubMed] [Google Scholar]
- 121. Fossey SL, Bear MD, Kisseberth WC, Pennell M, London CA. Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines. BMC Cancer. 2011;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Russell JO, Camargo FD. Hippo signalling in the liver: role in development, regeneration and disease. Nat Rev Gastroenterol Hepatol. 2022;19(5):297‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rothenberg ME, Jan YN. Cell biology: the hippo hypothesis. Nature. 2003;425(6957):469‐470. [DOI] [PubMed] [Google Scholar]
- 124. Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29(6):783‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kovar H, Bierbaumer L. Radic‐Sarikas B. The YAP/TAZ PATHWAY IN OSTEOGENESIS AND BONE SARCOMA PATHOGenesis. Cells. 2020;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Basu‐Roy U, Bayin NS, Rattanakorn K, et al. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat Commun. 2015;6:6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Maurizi G, Verma N, Gadi A, Mansukhani A, Basilico C. Sox2 is required for tumor development and cancer cell proliferation in osteosarcoma. Oncogene. 2018;37(33):4626‐4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Basu‐Roy U, Han E, Rattanakorn K, et al. PPARgamma agonists promote differentiation of cancer stem cells by restraining YAP transcriptional activity. Oncotarget. 2016;7(38):60954‐60970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chai J, Xu S, Guo F. TEAD1 mediates the oncogenic activities of Hippo‐YAP1 signaling in osteosarcoma. Biochem Biophys Res Commun. 2017;488(2):297‐302. [DOI] [PubMed] [Google Scholar]
- 131. Liu G, Huang K, Jie Z, et al. CircFAT1 sponges miR‐375 to promote the expression of Yes‐associated protein 1 in osteosarcoma cells. Mol Cancer. 2018;17(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Luo Y, Liu W, Tang P, et al. miR‐624‐5p promoted tumorigenesis and metastasis by suppressing hippo signaling through targeting PTPRB in osteosarcoma cells. J Exp Clin Cancer Res. 2019;38(1):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zou W, Chen L. Inhibitory B7‐family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467‐477. [DOI] [PubMed] [Google Scholar]
- 134. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death‐1 ligand 1 interacts specifically with the B7‐1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ohaegbulam KC, Assal A, Lazar‐Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD‐1 and PD‐L1 pathway. Trends Mol Med. 2015;21(1):24‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Harb J, Lin PJ, Hao J. Recent development of Wnt signaling pathway inhibitors for cancer therapeutics. Curr Oncol Rep. 2019;21(2):12. [DOI] [PubMed] [Google Scholar]
- 137. Xu Y, Song G, Xie S, et al. The roles of PD‐1/PD‐L1 in the prognosis and immunotherapy of prostate cancer. Mol Ther. 2021;29(6):1958‐1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chen S, Guenther LM, Aronhalt A, Cardillo L, Janeway KA, Church AJ. PD‐1 and PD‐L1 expression in osteosarcoma: which specimen to evaluate? J Pediatr Hematol Oncol. 2020;42(8):482‐487. [DOI] [PubMed] [Google Scholar]
- 139. Huang X, Zhang W, Zhang Z, et al. Prognostic value of programmed cell death 1 ligand‐1 (PD‐L1) or PD‐1 expression in patients with osteosarcoma: a meta‐analysis. J Cancer. 2018;9(14):2525‐2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hashimoto K, Nishimura S, Akagi M. Characterization of PD‐1/PD‐L1 immune checkpoint expression in osteosarcoma. Diagnostics (Basel). 2020;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Groisberg R, Hong DS, Behrang A, et al. Characteristics and outcomes of patients with advanced sarcoma enrolled in early phase immunotherapy trials. J Immunother Cancer. 2017;5(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kushlinskii NE, Alferov AA, Timofeev YS, et al. Key immune checkpoint PD‐1/PD‐L1 signaling pathway components in the blood serum from patients with bone tumors. Bull Exp Biol Med. 2020;170(1):64‐68. [DOI] [PubMed] [Google Scholar]
- 143. Gao W, Zhou J, Ji B. Evidence of interleukin 21 reduction in osteosarcoma patients due to PD‐1/PD‐L1‐mediated suppression of follicular helper T cell functionality. DNA Cell Biol. 2017;36(9):794‐800. [DOI] [PubMed] [Google Scholar]
- 144. Castro F, Cardoso AP, Goncalves RM, Serre K, Oliveira MJ. Interferon‐Gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. 2018;9:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Honda Y, Otsuka A, Ono S, et al. Infiltration of PD‐1‐positive cells in combination with tumor site PD‐L1 expression is a positive prognostic factor in cutaneous angiosarcoma. Oncoimmunology. 2017;6(1):e1253657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yoshida K, Okamoto M, Sasaki J, et al. Anti‐PD‐1 antibody decreases tumour‐infiltrating regulatory T cells. BMC Cancer. 2020;20(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Duan XL, Guo JP, Li F, Xiu C, Wang H. Sunitinib inhibits PD‐L1 expression in osteosarcoma by targeting STAT3 and remodels the immune system in tumor‐bearing mice. Future Oncol. 2020;16(24):1815‐1824. [DOI] [PubMed] [Google Scholar]
- 148. Markel JE, Noore J, Emery EJ, Bobnar HJ, Kleinerman ES, Lindsey BA. Using the spleen as an in vivo systemic immune barometer alongside osteosarcoma disease progression and immunotherapy with alpha‐PD‐L1. Sarcoma. 2018;2018:8694397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhang ML, Chen L, Li YJ, Kong DL. PD‑L1/PD‑1 axis serves an important role in natural killer cell‑induced cytotoxicity in osteosarcoma. Oncol Rep. 2019;42(5):2049‐2056. [DOI] [PubMed] [Google Scholar]
- 150. Yoshida K, Okamoto M, Sasaki J, et al. Clinical outcome of osteosarcoma and its correlation with programmed death‐ligand 1 and T cell activation markers. Onco Targets Ther. 2019;12:2513‐2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Toda Y, Kohashi K, Yamada Y, et al. PD‐L1 and IDO1 expression and tumor‐infiltrating lymphocytes in osteosarcoma patients: comparative study of primary and metastatic lesions. J Cancer Res Clin Oncol. 2020;146(10):2607‐2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7(1):41‐51. [DOI] [PubMed] [Google Scholar]
- 153. Germain D, Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res. 2007;13(19):5665‐5669. [DOI] [PubMed] [Google Scholar]
- 154. Seki N, Kan OK, Matsumoto K, et al. Interleukin‐22 attenuates double‐stranded RNA‐induced upregulation of PD‐L1 in airway epithelial cells via a STAT3‐dependent mechanism. Biochem Biophys Res Commun. 2017;494(1‐2):242‐248. [DOI] [PubMed] [Google Scholar]
- 155. Dhupkar P, Gordon N, Stewart J, Kleinerman ES. Anti‐PD‐1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. 2018;7(6):2654‐2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Keremu A, Aimaiti A, Liang Z, Zou X. Role of the HDAC6/STAT3 pathway in regulating PD‐L1 expression in osteosarcoma cell lines. Cancer Chemother Pharmacol. 2019;83(2):255‐264. [DOI] [PubMed] [Google Scholar]
- 157. Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7‐H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84‐88. [DOI] [PubMed] [Google Scholar]
- 158. Wu W, Jing D, Meng Z, et al. FGD1 promotes tumor progression and regulates tumor immune response in osteosarcoma via inhibiting PTEN activity. Theranostics. 2020;10(6):2859‐2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid‐derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Shi X, Li X, Wang H, Yu Z, Zhu Y, Gao Y. Specific inhibition of PI3Kdelta/gamma enhances the efficacy of anti‐PD1 against osteosarcoma cancer. J Bone Oncol. 2019;16:100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Payandeh Z, Khalili S, Somi MH, et al. PD‐1/PD‐L1‐dependent immune response in colorectal cancer. J Cell Physiol. 2020;235(7‐8):5461‐5475. [DOI] [PubMed] [Google Scholar]
- 162. Ji X, Wang E, Tian F. MicroRNA‐140 suppresses osteosarcoma tumor growth by enhancing anti‐tumor immune response and blocking mTOR signaling. Biochem Biophys Res Commun. 2018;495(1):1342‐1348. [DOI] [PubMed] [Google Scholar]
- 163. Liu Z, Wen J, Wu C, et al. MicroRNA‐200a induces immunosuppression by promoting PTEN‐mediated PD‐L1 upregulation in osteosarcoma. Aging (Albany NY). 2020;12(2):1213‐1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zhang J, Chou X, Zhuang M, et al. LINC00657 activates PD‐L1 to promote osteosarcoma metastasis via miR‐106a. J Cell Biochem. 2020;121(10):4188‐4195. [DOI] [PubMed] [Google Scholar]
- 165. Fryer CJ, White JB, Jones KA. Mastermind recruits CycC:cDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol Cell. 2004;16(4):509‐520. [DOI] [PubMed] [Google Scholar]
- 166. Sprinzak D, Blacklow SC. Biophysics of Notch signaling. Annu Rev Biophys. 2021;50:157‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Shim YS, Lee HS, Hwang JS. Aberrant Notch signaling pathway as a potential mechanism of central precocious puberty. Int J Mol Sci. 2022;23(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Shen Q, Reedijk M. Notch signaling and the breast cancer microenvironment. Adv Exp Med Biol. 2021;1287:183‐200. [DOI] [PubMed] [Google Scholar]
- 169. Nandagopal N, Santat LA, LeBon L, Sprinzak D, Bronner ME, Elowitz MB. Dynamic ligand discrimination in the notch signaling pathway. Cell. 2018;172(4):869‐880 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Reichrath J, Reichrath S. Notch signaling in prevention and therapy: fighting cancer with a two‐sided sword. Adv Exp Med Biol. 2021;1287:1‐7. [DOI] [PubMed] [Google Scholar]
- 171. Ali SR, Jordan M, Nagarajan P, Amit M. Nerve density and neuronal biomarkers in cancer. Cancers (Basel). 2022;14(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Mi C, Zhang D, Li Y, et al. miR‐4677‐3p participates proliferation and metastases of gastric cancer cell via CEMIP‐PI3K/AKT signaling pathway. Cell Cycle. 2021;20(19):1978‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Liu J, Yan W, Han P, Tian D. The emerging role of KIAA1199 in cancer development and therapy. Biomed Pharmacother. 2021;138:111507. [DOI] [PubMed] [Google Scholar]
- 174. Cheng J, Zhang Y, Wan R, et al. CEMIP promotes osteosarcoma progression and metastasis through activating notch signaling pathway. Front Oncol. 2022;12:919108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zhang J, Li N, Lu S, et al. The role of Notch ligand Jagged1 in osteosarcoma proliferation, metastasis, and recurrence. J Orthop Surg Res. 2021;16(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Chrysostomou E, Zhou L, Darcy YL, Graves KA, Doetzlhofer A, Cox BC. The notch ligand jagged1 is required for the formation, maintenance, and survival of hensen's cells in the mouse cochlea. J Neurosci. 2020;40(49):9401‐9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Chen L, Wang J, Li JW, Zhao XW, Tian LF. LncRNA MEG3 inhibits proliferation and promotes apoptosis of osteosarcoma cells through regulating Notch signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(2):581‐590. [DOI] [PubMed] [Google Scholar]
- 178. Lu J, Song G, Tang Q, et al. MiR‐26a inhibits stem cell‐like phenotype and tumor growth of osteosarcoma by targeting Jagged1. Oncogene. 2017;36(2):231‐241. [DOI] [PubMed] [Google Scholar]
- 179. Zhao GQ, Zhao S, Zhou X, Eberspaecher H, Solursh M, de Crombrugghe B. rDlx, a novel distal‐less‐like homeoprotein is expressed in developing cartilages and discrete neuronal tissues. Dev Biol. 1994;164(1):37‐51. [DOI] [PubMed] [Google Scholar]
- 180. Zhang X, Bian H, Wei W, et al. DLX5 promotes osteosarcoma progression via activation of the NOTCH signaling pathway. Am J Cancer Res. 2021;11(6):3354‐3374. [PMC free article] [PubMed] [Google Scholar]
- 181. Dai G, Liu G, Zheng D, Song Q. Inhibition of the Notch signaling pathway attenuates progression of cell motility, metastasis, and epithelial‐to‐mesenchymal transition‐like phenomena induced by low concentrations of cisplatin in osteosarcoma. Eur J Pharmacol. 2021;899:174058. [DOI] [PubMed] [Google Scholar]
- 182. De Francesco EM, Maggiolini M, Musti AM. Crosstalk between Notch, HIF‐1alpha and GPER in breast cancer EMT. Int J Mol Sci. 2018;19(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Tsai HC, Chang AC, Tsai CH, et al. CCN2 promotes drug resistance in osteosarcoma by enhancing ABCG2 expression. J Cell Physiol. 2019;234(6):9297‐9307. [DOI] [PubMed] [Google Scholar]
- 184. Pazhouhandeh M, Sahraian MA, Siadat SD, et al. A systems medicine approach reveals disordered immune system and lipid metabolism in multiple sclerosis patients. Clin Exp Immunol. 2018;192(1):18‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Duffy MJ, Synnott NC, O'Grady S, Crown J. Targeting p53 for the treatment of cancer. Semin Cancer Biol. 2022;79:58‐67. [DOI] [PubMed] [Google Scholar]
- 186. Kovac M, Woolley C, Ribi S, et al. Germline RET variants underlie a subset of paediatric osteosarcoma. J Med Genet. 2021;58(1):20‐24. [DOI] [PubMed] [Google Scholar]
- 187. Ye S, Shen J, Choy E, et al. p53 overexpression increases chemosensitivity in multidrug‐resistant osteosarcoma cell lines. Cancer Chemother Pharmacol. 2016;77(2):349‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Bae Y, Zeng HC, Chen YT, et al. miRNA‐34c Suppresses osteosarcoma progression in vivo by targeting Notch and E2F. JBMR Plus. 2022;6(5):e10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212‐226. [DOI] [PubMed] [Google Scholar]
- 190. Su H, Zhu G, Rong X, Zhou Y, Jiang P, Chen P. Upregulation of ATG4A promotes osteosarcoma cell epithelial‐to‐mesenchymal transition through the Notch signaling pathway. Int J Clin Exp Pathol. 2017;10(7):7975‐7982. [PMC free article] [PubMed] [Google Scholar]
- 191. Wang J, Liu Z, Feng X, Gao S, Xu S, Liu P. Tumor suppressor gene ING3 induces cardiomyocyte hypertrophy via inhibition of AMPK and activation of p38 MAPK signaling. Arch Biochem Biophys. 2014;562:22‐30. [DOI] [PubMed] [Google Scholar]
- 192. Dass CR, Khachigian LM, Choong PF. c‐Jun Is critical for the progression of osteosarcoma: proof in an orthotopic spontaneously metastasizing model. Mol Cancer Res. 2008;6(8):1289‐1292. [DOI] [PubMed] [Google Scholar]
- 193. Lucas RM, Luo L, Stow JL. ERK1/2 in immune signalling. Biochem Soc Trans. 2022;50(5):1341‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Wu Q, Wu W, Fu B, Shi L, Wang X, Kuca K. JNK signaling in cancer cell survival. Med Res Rev. 2019;39(6):2082‐2104. [DOI] [PubMed] [Google Scholar]
- 195. Li Z, Dai A, Yang M, Chen S, Deng Z, Li L. p38MAPK signaling pathway in osteoarthritis: pathological and therapeutic aspects. J Inflamm Res. 2022;15:723‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Paudel R, Fusi L, Schmidt M. The MEK5/ERK5 pathway in health and disease. Int J Mol Sci. 2021;22(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zhu D, Xu X, Zhang M, Wang T. Enhanced expression of KIF4A in osteosarcoma predicts a poor prognosis and facilitates tumor growth by activation of the MAPK pathway. Exp Ther Med. 2021;22(5):1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Cai X, Zhu S. Prognosis‐related VDAC1 regulates the proliferation and apoptosis of osteosarcoma cells via the MAPK signaling pathway. Genomics. 2023:110595. [DOI] [PubMed] [Google Scholar]
- 199. Li Q, Chen G, Jiang H, et al. ITGB3 promotes cisplatin resistance in osteosarcoma tumors. Cancer Med. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zhu X, Jiang C, Wang Z, Zhu X, Yuan F, Yang Y. PSKH1 affects proliferation and invasion of osteosarcoma cells via the p38/MAPK signaling pathway. Oncol Lett. 2023;25(4):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Li CH, Ku MC, Lee KC, et al. Magnolol suppresses ERK/NF‐kappaB signaling and triggers apoptosis through extrinsic/intrinsic pathways in osteosarcoma. Anticancer Res. 2022;42(9):4403‐4410. [DOI] [PubMed] [Google Scholar]
- 202. Neumann M, Naumann M. Beyond IkappaBs: alternative regulation of NF‐kappaB activity. FASEB J. 2007;21(11):2642‐2654. [DOI] [PubMed] [Google Scholar]
- 203. Gilmore TD. Introduction to NF‐kappaB: players, pathways, perspectives. Oncogene. 2006;25(51):6680‐6684. [DOI] [PubMed] [Google Scholar]
- 204. Soukhtanloo M, Mohtashami E, Maghrouni A, et al. Natural products as promising targets in glioblastoma multiforme: a focus on NF‐kappaB signaling pathway. Pharmacol Rep. 2020;72(2):285‐295. [DOI] [PubMed] [Google Scholar]
- 205. Li R, Shi Y, Zhao S, Shi T, Zhang G. NF‐kappaB signaling and integrin‐beta1 inhibition attenuates osteosarcoma metastasis via increased cell apoptosis. Int J Biol Macromol. 2019;123:1035‐1043. [DOI] [PubMed] [Google Scholar]
- 206. Bartholf DeWitt S, Hoskinson Plumlee S, Brighton HE, et al. Loss of ATRX promotes aggressive features of osteosarcoma with increased NF‐kappaB signaling and integrin binding. JCI Insight. 2022;7(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Xi X, Bao Y, Zhou Y, et al. Oncogenic gene TRIM10 confers resistance to cisplatin in osteosarcoma cells and activates the NF‐kappaB signaling pathway. Cell Biol Int. 2021;45(1):74‐82. [DOI] [PubMed] [Google Scholar]
- 208. Lu S, Liao QS, Tang L. MiR‐155 affects osteosarcoma cell proliferation and invasion through regulating NF‐kappaB signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(22):7633‐7639. [DOI] [PubMed] [Google Scholar]
- 209. Zhao J, Ma ST. Downregulation of lncRNA H19 inhibits migration and invasion of human osteosarcoma through the NF‐kappaB pathway. Mol Med Rep. 2018;17(5):7388‐7394. [DOI] [PubMed] [Google Scholar]
- 210. Zhang GD, Li Y, Liao GJ, Qiu HW. LncRNA NKILA inhibits invasion and migration of osteosarcoma cells via NF‐kappaB/Snail signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(10):4118‐4125. [DOI] [PubMed] [Google Scholar]
- 211. Liu Y, Li G, Zhang Y, et al. Nectin‐4 promotes osteosarcoma progression and metastasis through activating PI3K/AKT/NF‐kappaB signaling by down‐regulation of miR‐520c‐3p. Cancer Cell Int. 2022;22(1):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Haag SM, Gulen MF, Reymond L, et al. Targeting STING with covalent small‐molecule inhibitors. Nature. 2018;559(7713):269‐273. [DOI] [PubMed] [Google Scholar]
- 213. Bavelloni A, Focaccia E, Piazzi M, et al. Therapeutic potential of nvp‐bkm120 in human osteosarcomas cells. J Cell Physiol. 2019;234(7):10907‐10917. [DOI] [PubMed] [Google Scholar]
- 214. Chen C, Guo Y, Huang Q, et al. PI3K inhibitor impairs tumor progression and enhances sensitivity to anlotinib in anlotinib‐resistant osteosarcoma. Cancer Lett. 2022;536:215660. [DOI] [PubMed] [Google Scholar]
- 215. Li H, Shen X, Ma M, et al. ZIP10 drives osteosarcoma proliferation and chemoresistance through ITGA10‐mediated activation of the PI3K/AKT pathway. J Exp Clin Cancer Res. 2021;40(1):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Li W, Li Y, Tian W, et al. 2‐methylbenzoyl berbamine, a multi‐targeted inhibitor, suppresses the growth of human osteosarcoma through disabling NF‐kappaB, ERK and AKT signaling networks. Aging (Albany NY). 2020;12(14):15037‐15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Gupte A, Baker EK, Wan SS, et al. Systematic screening identifies dual PI3K and mTOR inhibition as a conserved therapeutic vulnerability in osteosarcoma. Clin Cancer Res. 2015;21(14):3216‐3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Sun JY, Hou YJ, Yin YB, et al. CCT128930 induces G1‐phase arrest and apoptosis and synergistically enhances the anticancer efficiency of VS5584 in human osteosarcoma cells. Biomed Pharmacother. 2020;130:110544. [DOI] [PubMed] [Google Scholar]
- 219. Xia JJ, Pei LB, Zhuang JP, et al. Celecoxib inhibits beta‐catenin‐dependent survival of the human osteosarcoma MG‐63 cell line. J Int Med Res. 2010;38(4):1294‐1304. [DOI] [PubMed] [Google Scholar]
- 220. Li R, Liu J, Wu H, Liu L, Wang L, Zhang S. TIKI2 suppresses growth of osteosarcoma by targeting Wnt/beta‐catenin pathway. Mol Cell Biochem. 2014;392(1‐2):109‐116. [DOI] [PubMed] [Google Scholar]
- 221. Shen W, Zhang X, Tang J, et al. CCL16 maintains stem cell‐like properties in breast cancer by activating CCR2/GSK3beta/beta‐catenin/OCT4 axis. Theranostics. 2021;11(5):2297‐2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103(7):2257‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Vaghari‐Tabari M, Majidinia M, Moein S, et al. MicroRNAs and colorectal cancer chemoresistance: new solution for old problem. Life Sci. 2020;259:118255. [DOI] [PubMed] [Google Scholar]
- 224. Soghli N, Ferns GA, Sadeghsoltani F, Qujeq D, Yousefi T, Vaghari‐Tabari M. MicroRNAs and osteosarcoma: potential targets for inhibiting metastasis and increasing chemosensitivity. Biochem Pharmacol. 2022;201:115094. [DOI] [PubMed] [Google Scholar]
- 225. Sun L, Wang L, Luan S, Jiang Y, Wang Q. miR‐429 inhibits osteosarcoma progression by targeting HOXA9 through suppressing Wnt/beta‐catenin signaling pathway. Oncol Lett. 2020;20(3):2447‐2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Yang X, Wang L, Wang Q, Li L, Fu Y, Sun J. MiR‐183 inhibits osteosarcoma cell growth and invasion by regulating LRP6‐Wnt/beta‐catenin signaling pathway. Biochem Biophys Res Commun. 2018;496(4):1197‐1203. [DOI] [PubMed] [Google Scholar]
- 227. Yang S, Wang B, Liu C, et al. THAP9‐AS1 promotes tumorigenesis and reduces ROS generation through the JAK2/STAT3 signaling pathway by increasing SOCS3 promoter methylation in osteosarcoma. Oxid Med Cell Longev. 2021;2021:5620475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Bid HK, Oswald D, Li C, London CA, Lin J, Houghton PJ. Anti‐angiogenic activity of a small molecule STAT3 inhibitor LLL12. PLoS One. 2012;7(4):e35513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Fossey SL, Bear MD, Lin J, et al. The novel curcumin analog FLLL32 decreases STAT3 DNA binding activity and expression, and induces apoptosis in osteosarcoma cell lines. BMC Cancer. 2011;11:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Onimoe GI, Liu A, Lin L, et al. Small molecules, LLL12 and FLLL32, inhibit STAT3 and exhibit potent growth suppressive activity in osteosarcoma cells and tumor growth in mice. Invest New Drugs. 2012;30(3):916‐926. [DOI] [PubMed] [Google Scholar]
- 231. Liu K, Ren T, Huang Y, et al. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL‐2 signaling in osteosarcoma. Cell Death Dis. 2017;8(8):e3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Zheng B, Ren T, Huang Y, Guo W. Apatinib inhibits migration and invasion as well as PD‐L1 expression in osteosarcoma by targeting STAT3. Biochem Biophys Res Commun. 2018;495(2):1695‐1701. [DOI] [PubMed] [Google Scholar]
- 233. Jiang Y, Wang G, Mu H, et al. Bromodomain inhibition attenuates the progression and sensitizes the chemosensitivity of osteosarcoma by repressing GP130/STAT3 signaling. Front Oncol. 2021;11:642134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Li Y, Yang S, Yang S, Verteporfin inhibits the progression of spontaneous osteosarcoma caused by Trp53 and Rb1 deficiency in Ctsk‐expressing cells via impeding Hippo pathway. Cells. 2022;11(8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Zhan F, He T, Chen Z, et al. RhoA enhances osteosarcoma resistance to MPPa‐PDT via the Hippo/YAP signaling pathway. Cell Biosci. 2021;11(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Lu Q, Wang Y, Jiang X, Huang S. miR‐584‐5p inhibits osteosarcoma progression by targeting connective tissue growth factor. Cancer Biother Radiopharm. 2022. [DOI] [PubMed] [Google Scholar]
- 237. Wu X, Xiang H, Cong W, et al. PLOD1, a target of miR‐34c, contributes to cell growth and metastasis via repressing LATS1 phosphorylation and inactivating Hippo pathway in osteosarcoma. Biochem Biophys Res Commun. 2020;527(1):29‐36. [DOI] [PubMed] [Google Scholar]
- 238. Qin J, Wang R, Zhao C, et al. Notch signaling regulates osteosarcoma proliferation and migration through Erk phosphorylation. Tissue Cell. 2019;59:51‐61. [DOI] [PubMed] [Google Scholar]
- 239. Khan F, Singh VK, Saeed M, Kausar MA, Ansari IA. Carvacrol induced program cell death and cell cycle arrest in androgen‐independent human prostate cancer cells via inhibition of notch signaling. Anticancer Agents Med Chem. 2019;19(13):1588‐1608. [DOI] [PubMed] [Google Scholar]
- 240. Yang J, Hu Y, Wang L, Sun X, Yu L, Guo W. Human umbilical vein endothelial cells derived‐exosomes promote osteosarcoma cell stemness by activating Notch signaling pathway. Bioengineered. 2021;12(2):11007‐11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Wang JH, Zeng Z, Sun J, Chen Y, Gao X. A novel small‐molecule antagonist enhances the sensitivity of osteosarcoma to cabozantinib in vitro and in vivo by targeting DNMT‐1 correlated with disease severity in human patients. Pharmacol Res. 2021;173:105869. [DOI] [PubMed] [Google Scholar]
- 242. Xu J, Wan S, Chen W, Zhang Y, Ji Z. Relaxin inhibits (177)Lu‐EDTMP associated cell death in osteosarcoma cells through notch‐1 pathway. Acta Pharm. 2022;72(4):575‐585. [DOI] [PubMed] [Google Scholar]
- 243. Zhuo B, Wang X, Shen Y, et al. Interleukin‐24 inhibits the phenotype and tumorigenicity of cancer stem cell in osteosarcoma via downregulation Notch and Wnt/beta‐catenin signaling. J Bone Oncol. 2021;31:100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Xue Y, Zhang G, Zhou S, et al. Iron chelator induces apoptosis in osteosarcoma cells by disrupting intracellular iron homeostasis and activating the MAPK PATHWAY. Int J Mol Sci. 2021;22(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. He T, Lin X, Yang C, et al. Theaflavin‐3,3'‐digallate plays a ROS‐mediated dual role in ferroptosis and apoptosis via the MAPK pathway in human osteosarcoma cell lines and xenografts. Oxid Med Cell Longev. 2022;2022:8966368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kang XH, Hu WH, Pei SS, et al. Effects of Sodium Cantharidate and vitamin B6 on apoptosis and survivin expression in human osteosarcoma cells. China Medical Herald. 2017;14:16‐19. [Google Scholar]
- 247. Hua F, Shang S, Hu ZW. Seeking new anti‐cancer agents from autophagy‐regulating natural products. J Asian Nat Prod Res. 2017;19(4):305‐313. [DOI] [PubMed] [Google Scholar]
- 248. Zhou J, Huang Z, Ni X, Lv C. Piperlongumine induces apoptosis and G2/M phase arrest in human osteosarcoma cells by regulating ROS/PI3K/Akt pathway. Toxicol In Vitro. 2020;65:104775. [DOI] [PubMed] [Google Scholar]
- 249. Qin J, Wang W, Sha L, Ge L. Huangqi Fuzheng decoction exerts antitumor activity by inhibiting cell growth and inducing cell death in osteosarcoma. Biomed Pharmacother. 2019;114:108854. [DOI] [PubMed] [Google Scholar]
- 250. Yue Z, Guan X, Chao R, et al. Diallyl disulfide induces apoptosis and autophagy in human osteosarcoma MG‐63 cells through the PI3K/Akt/mTOR pathway. Molecules. 2019;24(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Kim SH, Son KM, Kim KY, et al. Deoxypodophyllotoxin induces cytoprotective autophagy against apoptosis via inhibition of PI3K/AKT/mTOR pathway in osteosarcoma U2OS cells. Pharmacol Rep. 2017;69(5):878‐884. [DOI] [PubMed] [Google Scholar]
- 252. Lin H, Zhang C, Zhang H, et al. Physakengose G induces apoptosis via EGFR/mTOR signaling and inhibits autophagic flux in human osteosarcoma cells. Phytomedicine. 2018;42:190‐198. [DOI] [PubMed] [Google Scholar]
- 253. Chen S, Jin Z, Dai L, et al. Aloperine induces apoptosis and inhibits invasion in MG‐63 and U2OS human osteosarcoma cells. Biomed Pharmacother. 2018;97:45‐52. [DOI] [PubMed] [Google Scholar]
- 254. Wang B, Li J. Piceatannol suppresses the proliferation and induced apoptosis of osteosarcoma cells through PI3K/AKT/mTOR pathway. Cancer Manag Res. 2020;12:2631‐2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Sun H, Yin M, Qian W, Yin H. Calycosin, a phytoestrogen isoflavone, induces apoptosis of estrogen receptor‐positive MG‐63 osteosarcoma cells via the phosphatidylinositol 3‐kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway. Med Sci Monit. 2018;24:6178‐6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Wang JLCL, Yu MY, He JT. Effect of tectorigenin on apoptosis and autophagy of osteosarcoma MG63 cells. Chin J Immunol. 2019;35:3027‐3031. [Google Scholar]
- 257. De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97‐110. [DOI] [PubMed] [Google Scholar]
- 258. Chen K, Jiao J, Xue J, et al. Ginsenoside CK induces apoptosis and suppresses proliferation and invasion of human osteosarcoma cells through the PI3K/mTOR/p70S6K1 pathway. Oncol Rep. 2020;43(3):886‐896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Ma K, Zhang C, Li W. Gamabufotalin suppressed osteosarcoma stem cells through the TGF‐beta/periostin/PI3K/AKT pathway. Chem Biol Interact. 2020;331:109275. [DOI] [PubMed] [Google Scholar]
- 260. Yu X, Wang Q, Zhou X, et al. Celastrol negatively regulates cell invasion and migration ability of human osteosarcoma via downregulation of the PI3K/Akt/NF‐kappaB signaling pathway in vitro. Oncol Lett. 2016;12(5):3423‐3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Li C, Zhou X, Sun C, Liu X, Shi X, Wu S. Isoliquiritigenin inhibits the proliferation, apoptosis and migration of osteosarcoma cells. Oncol Rep. 2019;41(4):2502‐2510. [DOI] [PubMed] [Google Scholar]
- 262. Alomar SY, Mansour L, Abuderman A, et al. beta‐Catenin accumulation and S33F mutation of CTNNB1 gene in colorectal cancer in Saudi Arabia. Pol J Pathol. 2016;67(2):156‐162. [DOI] [PubMed] [Google Scholar]
- 263. Liu Y, Wang W, Xu J, et al. Dihydroartemisinin inhibits tumor growth of human osteosarcoma cells by suppressing Wnt/beta‐catenin signaling. Oncol Rep. 2013;30(4):1723‐1730. [DOI] [PubMed] [Google Scholar]
- 264. Liu Y, Liu YZ, Zhang RX, et al. Oridonin inhibits the proliferation of human osteosarcoma cells by suppressing Wnt/beta‐catenin signaling. Int J Oncol. 2014;45(2):795‐803. [DOI] [PubMed] [Google Scholar]
- 265. Hu S, Chang J, Ruan H, et al. Cantharidin inhibits osteosarcoma proliferation and metastasis by directly targeting miR‐214‐3p/DKK3 axis to inactivate beta‐catenin nuclear translocation and LEF1 translation. Int J Biol Sci. 2021;17(10):2504‐2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Gang W, Hao H, Yong H, et al. Therapeutic potential of triptolide in treating bone‐related disorders. Front Pharmacol. 2022;13:905576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Li X, Lu Q, Xie W, Wang Y, Wang G. Anti‐tumor effects of triptolide on angiogenesis and cell apoptosis in osteosarcoma cells by inducing autophagy via repressing Wnt/beta‐Catenin signaling. Biochem Biophys Res Commun. 2018;496(2):443‐449. [DOI] [PubMed] [Google Scholar]
- 268. Chang J, Li Y, Wang X, et al. Polyphyllin I suppresses human osteosarcoma growth by inactivation of Wnt/beta‐catenin pathway in vitro and in vivo. Sci Rep. 2017;7(1):7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Qi YB, Yang W, Si M, Nie L. Wnt/betacatenin signaling modulates piperinemediated antitumor effects on human osteosarcoma cells. Mol Med Rep. 2020;21(5):2202‐2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Yang S, Chen J, Tan T, et al. Evodiamine exerts anticancer effects against 143B and MG63 cells through the Wnt/beta‐catenin signaling pathway. Cancer Manag Res. 2020;12:2875‐2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Lee IH, Im E, Lee HJ, et al. Apoptotic and antihepatofibrotic effect of honokiol via activation of GSK3beta and suppression of Wnt/beta‐catenin pathway in hepatic stellate cells. Phytother Res. 2021;35(1):452‐462. [DOI] [PubMed] [Google Scholar]
- 272. Wei Z, Zheng D, Pi W, Qiu Y, Xia K, Guo W. Isoquercitrin restrains the proliferation and promotes apoptosis of human osteosarcoma cells by inhibiting the Wnt/beta‐catenin pathway. J Bone Oncol. 2023;38:100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Tie H, Kuang G, Gong X, Jiang R, Wan J, Wang B, Icariin inhibits growth of human osteosarcoma cells by inducing apoptosis via downregulation of beta‐catenin signaling. Article. International Journal of Clinical and Experimental Medicine. 2016;9(8):15437‐15446. [Google Scholar]
- 274. Zhang QH, Hu QX, Xie D, et al. Ganoderma lucidum exerts an anticancer effect on human osteosarcoma cells via suppressing the Wnt/beta‐catenin signaling pathway. Integr Cancer Ther. 2019;18:1534735419890917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Zhu H, Chen D, Xie X, Li Y, Fan T. Melittin inhibits lung metastasis of human osteosarcoma: evidence of wnt/beta‐catenin signaling pathway participation. Toxicon. 2021;198:132‐142. [DOI] [PubMed] [Google Scholar]
- 276. Tan T, Chen J, Hu Y, et al. Dihydrotanshinone I inhibits the growth of osteosarcoma through the Wnt/beta‐catenin signaling pathway. Onco Targets Ther. 2019;12:5111‐5122. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 277. Liu X, Geng Z, Ding X, Lou Y, Zhang X. Convallatoxin suppresses osteosarcoma cell proliferation, migration, invasion, and enhances osteogenic differentiation by downregulating parathyroid hormone receptor 1 (PTHR1) expression and inactivating Wnt/beta‐catenin pathway. Bioengineered. 2022;13(5):13280‐13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Yang C, Zhang L, Huang H, et al. Alantolactone inhibits proliferation, metastasis and promotes apoptosis of human osteosarcoma cells by suppressing Wnt/beta‐catenin and MAPKs signaling pathways. Genes Dis. 2022;9(2):466‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Sun Y, Liu L, Wang Y, et al. Curcumin inhibits the proliferation and invasion of MG‐63 cells through inactivation of the p‐JAK2/p‐STAT3 pathway. Onco Targets Ther. 2019;12:2011‐2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Lu KH, Wu HH, Lin RC, et al. Curcumin analogue L48H37 suppresses human osteosarcoma U2OS and MG‐63 cells' migration and invasion in culture by inhibition of uPA via the JAK/STAT signaling pathway. Molecules. 2020;26(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Annamalai V, Kotakonda M, Periyannan V. JAK1/STAT3 regulatory effect of beta‐caryophyllene on MG‐63 osteosarcoma cells via ROS‐induced apoptotic mitochondrial pathway by DNA fragmentation. J Biochem Mol Toxicol. 2020;34(8):e22514. [DOI] [PubMed] [Google Scholar]
- 282. Wang W, Li J, Ding Z, et al. Tanshinone I inhibits the growth and metastasis of osteosarcoma via suppressing JAK/STAT3 signalling pathway. J Cell Mol Med. 2019;23(9):6454‐6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Farnood PR, Pazhooh RD, Asemi Z, Yousefi B. Targeting signaling pathway by curcumin in osteosarcoma. Curr Mol Pharmacol. 2023;16(1):71‐82. [DOI] [PubMed] [Google Scholar]
- 284. Xu Y, Shu B, Tian Y, et al. Oleanolic acid induces osteosarcoma cell apoptosis by inhibition of Notch signaling. Mol Carcinog. 2018;57(7):896‐902. [DOI] [PubMed] [Google Scholar]
- 285. Dai G, Yu L, Yang J, et al. The synergistic antitumor effect of cinobufagin and cisplatin in human osteosarcoma cell line in vitro and in vivo. Oncotarget. 2017;8(49):85150‐85168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Dai F, Luo F, Zhou R, et al. Calponin 3 is associated with poor prognosis and regulates proliferation and metastasis in osteosarcoma. Aging (Albany NY). 2020;12(14):14037‐14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Wu L, Cai B, Liu X, Cai H. Emodin attenuates calcium overload and endoplasmic reticulum stress in AR42J rat pancreatic acinar cells. Mol Med Rep. 2014;9(1):267‐272. [DOI] [PubMed] [Google Scholar]
- 288. Zeng Z, Zhang H, Wang X, et al. Salvianolic acid B suppresses cell proliferation and induces apoptosis in osteosarcoma through p38‐mediated reactive oxygen species generation. Oncol Lett. 2018;15(2):2679‐2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Kang HM, Park BS, Kang HK, Park HR, Yu SB, Kim IR. Delphinidin induces apoptosis and inhibits epithelial‐to‐mesenchymal transition via the ERK/p38 MAPK‐signaling pathway in human osteosarcoma cell lines. Environ Toxicol. 2018;33(6):640‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Moustardas P, Aberdam D, Lagali N. MAPK pathways in ocular pathophysiology: potential therapeutic drugs and challenges. Cells. 2023;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Cheng HL, Hsieh MJ, Yang JS, et al. Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK‐mediated MMPs expression. Oncotarget. 2016;7(23):35208‐35223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Jie Z, Xie Z, Zhao X, et al. Glabridin inhibits osteosarcoma migration and invasion via blocking the p38‐ and JNK‐mediated CREB‐AP1 complexes formation. J Cell Physiol. 2019;234(4):4167‐4178. [DOI] [PubMed] [Google Scholar]
- 293. Wang S, Li H, Chen S, et al. Andrographolide induces apoptosis in human osteosarcoma cells via the ROS/JNK pathway. Int J Oncol. 2020;56(6):1417‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Zheng GZ, Zhang QH, Chang B, et al. Dioscin induces osteosarcoma cell apoptosis by upregulating ROS‐mediated P38 MAPK signaling. Drug Dev Res. 2023;84(1):25‐35. [DOI] [PubMed] [Google Scholar]
- 295. Huang T, Zhang X, Wang H. Punicalagin inhibited proliferation, invasion and angiogenesis of osteosarcoma through suppression of NF‑kappaB signaling. Mol Med Rep. 2020;22(3):2386‐2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Alsaeed MA, Ebrahimi S, Alalikhan A, Hashemi SF, Hashemy SI. The potential in vitro inhibitory effects of neurokinin‐1 receptor (NK‐1R) antagonist, aprepitant, in osteosarcoma cell migration and metastasis. Biomed Res Int. 2022;2022:8082608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Chou CH, Lu KH, Yang JS, Hsieh YH, Lin CW, Yang SF. Dihydromyricetin suppresses cell metastasis in human osteosarcoma through SP‐1‐ and NF‐kappaB‐modulated urokinase plasminogen activator inhibition. Phytomedicine. 2021;90:153642. [DOI] [PubMed] [Google Scholar]
- 298. Li DK, Wang GH. Asiaticoside reverses M2 phenotype macrophage polarization‐evoked osteosarcoma cell malignant behaviour by TRAF6/NF‐kappaB inhibition. Pharm Biol. 2022;60(1):1635‐1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Sergi C, Shen F, Liu SM. Insulin/IGF‐1R, SIRT1, and FOXOs pathways—an intriguing interaction platform for bone and osteosarcoma. Front Endocrinol (Lausanne). 2019;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Leiphrakpam PD, Agarwal E, Mathiesen M, Haferbier KL, Brattain MG, Chowdhury S. In vivo analysis of insulin‐like growth factor type 1 receptor humanized monoclonal antibody MK‐0646 and small molecule kinase inhibitor OSI‐906 in colorectal cancer. Oncol Rep. 2014;31(1):87‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Song KH, Kang JH, Woo JK, et al. The novel IGF‐IR/Akt‐dependent anticancer activities of glucosamine. BMC Cancer. 2014;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Zovko A, Novak M, Haag P, et al. Compounds from the marine sponge Cribrochalina vasculum offer a way to target IGF‐1R mediated signaling in tumor cells. Oncotarget. 2016;7(31):50258‐50276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Venepalli NK, Emmadi R, Danciu OC, et al. Phase I study of IGF‐methotrexate conjugate in the treatment of advanced tumors expressing IGF‐1R. Am J Clin Oncol. 2019;42(11):862‐869. [DOI] [PubMed] [Google Scholar]
- 304. Wang YH, Han XD, Qiu Y, et al. Increased expression of insulin‐like growth factor‐1 receptor is correlated with tumor metastasis and prognosis in patients with osteosarcoma. J Surg Oncol. 2012;105(3):235‐243. [DOI] [PubMed] [Google Scholar]
- 305. Haddad TC, He J, O'Sullivan CC, et al. Randomized phase II trial of capecitabine and lapatinib with or without IMC‐A12 (Cituxumumab) in patients with HER2‐positive advanced breast cancer previously treated with trastuzumab and chemotherapy: nCCTG N0733 (Alliance). Breast Cancer Res Treat. 2021;188(2):477‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Hattinger CM, Pasello M, Ferrari S, Picci P, Serra M. Emerging drugs for high‐grade osteosarcoma. Expert Opin Emerg Drugs. 2010;15(4):615‐634. [DOI] [PubMed] [Google Scholar]
- 307. Anderson PM, Bielack SS, Gorlick RG, et al. A phase II study of clinical activity of SCH 717454 (robatumumab) in patients with relapsed osteosarcoma and Ewing sarcoma. Pediatr Blood Cancer. 2016;63(10):1761‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Martin V, Cappuzzo F, Mazzucchelli L, Frattini M. HER2 in solid tumors: more than 10 years under the microscope; where are we now? Future Oncol. 2014;10(8):1469‐1486. [DOI] [PubMed] [Google Scholar]
- 309. Ebb D, Meyers P, Grier H, et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the children's oncology group. J Clin Oncol. 2012;30(20):2545‐2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Tomizawa K, Suda K, Onozato R, et al. Prognostic and predictive implications of HER2/ERBB2/neu gene mutations in lung cancers. Lung Cancer. 2011;74(1):139‐144. [DOI] [PubMed] [Google Scholar]
- 311. Liu M, Sun LL, Li YJ, et al. Trastuzumab enhanced the cytotoxicity of Vgamma9Vdelta2 T cells against zoledronate‐sensitized osteosarcoma cells. Int Immunopharmacol. 2015;28(1):160‐167. [DOI] [PubMed] [Google Scholar]
- 312. Sevelda F, Mayr L, Kubista B, et al. EGFR is not a major driver for osteosarcoma cell growth in vitro but contributes to starvation and chemotherapy resistance. J Exp Clin Cancer Res. 2015;34:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Mantovani FB, Morrison JA, Mutsaers AJ. Effects of epidermal growth factor receptor kinase inhibition on radiation response in canine osteosarcoma cells. BMC Vet Res. 2016;12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Oshiro H, Tome Y, Miyake K, et al. An mTOR and VEGFR inhibitor combination arrests a doxorubicin resistant lung metastatic osteosarcoma in a PDOX mouse model. Sci Rep. 2021;11(1):8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Duffaud F, Mir O, Boudou‐Rouquette P, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non‐comparative, randomised, double‐blind, placebo‐controlled, phase 2 study. Lancet Oncol. 2019;20(1):120‐133. [DOI] [PubMed] [Google Scholar]
- 316. Cren PY, Lebellec L, Ryckewaert T, Penel N. Anti‐angiogenic agents in management of sarcoma patients: overview of published trials. Front Oncol. 2020;10:594445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Jiang ZY, Liu JB, Wang XF, Ma YS, Fu D. Current status and prospects of clinical treatment of osteosarcoma. Technol Cancer Res Treat. 2022;21:15330338221124696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Maron SB, Alpert L, Kwak HA, et al. Targeted therapies for targeted populations: anti‐EGFR treatment for EGFR‐amplified gastroesophageal adenocarcinoma. Cancer Discov. 2018;8(6):696‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Gvozdenovic A, Boro A, Born W, Muff R, Fuchs B. A bispecific antibody targeting IGF‐IR and EGFR has tumor and metastasis suppressive activity in an orthotopic xenograft osteosarcoma mouse model. Am J Cancer Res. 2017;7(7):1435‐1449. [PMC free article] [PubMed] [Google Scholar]
- 320. Zhang L, Leeman E, Carnes DC, Graves DT. Human osteoblasts synthesize and respond to platelet‐derived growth factor. Am J Physiol. 1991;261(2):C348‐54. Pt 1. [DOI] [PubMed] [Google Scholar]
- 321. Bozzi F, Tamborini E, Negri T, et al. Evidence for activation of KIT, PDGFRalpha, and PDGFRbeta receptors in the Ewing sarcoma family of tumors. Cancer. 2007;109(8):1638‐1645. [DOI] [PubMed] [Google Scholar]
- 322. Takagi S, Takemoto A, Takami M, Oh‐Hara T, Fujita N. Platelets promote osteosarcoma cell growth through activation of the platelet‐derived growth factor receptor‐Akt signaling axis. Cancer Sci. 2014;105(8):983‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. McGary EC, Weber K, Mills L, et al. Inhibition of platelet‐derived growth factor‐mediated proliferation of osteosarcoma cells by the novel tyrosine kinase inhibitor STI571. Clin Cancer Res. 2002;8(11):3584‐3591. [PubMed] [Google Scholar]
- 324. Gobin B, Moriceau G, Ory B, et al. Imatinib mesylate exerts anti‐proliferative effects on osteosarcoma cells and inhibits the tumour growth in immunocompetent murine models. PLoS One. 2014;9(3):e90795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Chugh R, Wathen JK, Maki RG, et al. Phase II multicenter trial of imatinib in 10 histologic subtypes of sarcoma using a bayesian hierarchical statistical model. J Clin Oncol. 2009;27(19):3148‐3153. [DOI] [PubMed] [Google Scholar]
- 326. Yamaguchi SI, Ueki A, Sugihara E, et al. Synergistic antiproliferative effect of imatinib and adriamycin in platelet‐derived growth factor receptor‐expressing osteosarcoma cells. Cancer Sci. 2015;106(7):875‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Pender A, Jones RL. Olaratumab: a platelet‐derived growth factor receptor‐alpha‐blocking antibody for the treatment of soft tissue sarcoma. Clin Pharmacol. 2017;9:159‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Doi T, Ma Y, Dontabhaktuni A, Nippgen C, Nippgen J, Ohtsu A. Phase I study of olaratumab in Japanese patients with advanced solid tumors. Cancer Sci. 2014;105(7):862‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Chiorean EG, Sweeney C, Youssoufian H, et al. A phase I study of olaratumab, an anti‐platelet‐derived growth factor receptor alpha (PDGFRalpha) monoclonal antibody, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73(3):595‐604. [DOI] [PubMed] [Google Scholar]
- 330. Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft‐tissue sarcoma: an open‐label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Pons‐Tostivint E, Thibault B, Guillermet‐Guibert J. Targeting PI3K signaling in combination cancer therapy. Trends Cancer. 2017;3(6):454‐469. [DOI] [PubMed] [Google Scholar]
- 332. Lee DH, Qi J, Bradner JE, et al. Synergistic effect of JQ1 and rapamycin for treatment of human osteosarcoma. Int J Cancer. 2015;136(9):2055‐2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Chiarini F, Grimaldi C, Ricci F, et al. Activity of the novel dual phosphatidylinositol 3‐kinase/mammalian target of rapamycin inhibitor NVP‐BEZ235 against T‐cell acute lymphoblastic leukemia. Cancer Res. 2010;70(20):8097‐8107. [DOI] [PubMed] [Google Scholar]
- 334. Meng LH, Zheng XF. Toward rapamycin analog (rapalog)‐based precision cancer therapy. Acta Pharmacol Sin. 2015;36(10):1163‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Houghton PJ, Morton CL, Gorlick R, et al. Stage 2 combination testing of rapamycin with cytotoxic agents by the Pediatric Preclinical Testing Program. Mol Cancer Ther. 2010;9(1):101‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Rock A, Ali S, Chow WA. Systemic therapy for chondrosarcoma. Curr Treat Options Oncol. 2022;23(2):199‐209. [DOI] [PubMed] [Google Scholar]
- 337. Demetri GD, Chawla SP, Ray‐Coquard I, et al. Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol. 2013;31(19):2485‐2492. [DOI] [PubMed] [Google Scholar]
- 338. Orosz Z, Rohonyi B, Luksander A, Szanto J. Pleomorphic liposarcoma of a young woman following radiotherapy for epithelioid sarcoma. Pathol Oncol Res. 2000;6(4):287‐291. [DOI] [PubMed] [Google Scholar]
- 339. Higuchi T, Sugisawa N, Miyake K, et al. Combination treatment with sorafenib and everolimus regresses a doxorubicin‐resistant osteosarcoma in a PDOX mouse model. Anticancer Res. 2019;39(9):4781‐4786. [DOI] [PubMed] [Google Scholar]
- 340. Houghton PJ, Gorlick R, Kolb EA, et al. Initial testing (stage 1) of the mTOR kinase inhibitor AZD8055 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2012;58(2):191‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Gobin B, Battaglia S, Lanel R, et al. NVP‐BEZ235, a dual PI3K/mTOR inhibitor, inhibits osteosarcoma cell proliferation and tumor development in vivo with an improved survival rate. Cancer Lett. 2014;344(2):291‐298. [DOI] [PubMed] [Google Scholar]
- 342. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Navid F, Santana VM, Neel M, et al. A phase II trial evaluating the feasibility of adding bevacizumab to standard osteosarcoma therapy. Int J Cancer. 2017;141(7):1469‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Heymann D. Anti‐RANKL therapy for bone tumours: basic, pre‐clinical and clinical evidences. J Bone Oncol. 2012;1(1):2‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Grignani G, Palmerini E, Dileo P, et al. A phase II trial of sorafenib in relapsed and unresectable high‐grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol. 2012;23(2):508‐516. [DOI] [PubMed] [Google Scholar]
- 346. Italiano A, Mir O, Mathoulin‐Pelissier S, et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): a multicentre, single‐arm, phase 2 trial. Lancet Oncol. 2020;21(3):446‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Scott LJ. Apatinib: a review in advanced gastric cancer and other advanced cancers. Drugs. 2018;78(7):747‐758. [DOI] [PubMed] [Google Scholar]
- 348. Li J, Qin S, Xu J, et al. Randomized, double‐blind, placebo‐controlled phase III trial of apatinib in patients with chemotherapy‐refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448‐1454. [DOI] [PubMed] [Google Scholar]
- 349. Borah NA, Reddy MM. Aurora kinase B inhibition: a potential therapeutic strategy for cancer. Molecules. 2021;26(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Maris JM, Morton CL, Gorlick R, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP). Pediatr Blood Cancer. 2010;55(1):26‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Niu NK, Wang ZL, Pan ST, et al. Pro‐apoptotic and pro‐autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U‐2 OS and MG‐63 cells through the activation of mitochondria‐mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway. Drug Des Devel Ther. 2015;9:1555‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Valsecchi ME. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(13):1270. [DOI] [PubMed] [Google Scholar]
- 353. Yang X, Yin R, Xu L. Neoadjuvant PD‐1 blockade in resectable lung cancer. N Engl J Med. 2018;379(9):e14. [DOI] [PubMed] [Google Scholar]
- 354. Lussier DM, O'Neill L, Nieves LM, et al. Enhanced T‐cell immunity to osteosarcoma through antibody blockade of PD‐1/PD‐L1 interactions. J Immunother. 2015;38(3):96‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Liu X, He S, Wu H, Xie H, Zhang T, Deng Z. Blocking the PD‐1/PD‐L1 axis enhanced cisplatin chemotherapy in osteosarcoma in vitro and in vivo. Environ Health Prev Med. 2019;24(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Le Cesne A, Marec‐Berard P, Blay JY, et al. Programmed cell death 1 (PD‐1) targeting in patients with advanced osteosarcomas: results from the PEMBROSARC study. Eur J Cancer. 2019;119:151‐157. [DOI] [PubMed] [Google Scholar]
- 357. Xie L, Xu J, Sun X, et al. Apatinib plus camrelizumab (anti‐PD1 therapy, SHR‐1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: a single‐arm, open‐label, phase 2 trial. J Immunother Cancer. 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft‐tissue sarcoma and bone sarcoma (SARC028): a multicentre, two‐cohort, single‐arm, open‐label, phase 2 trial. Lancet Oncol. 2017;18(11):1493‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Shen JK, Cote GM, Choy E, et al. Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol Res. 2014;2(7):690‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Bang YJ, Cho JY, Kim YH, et al. Efficacy of sequential ipilimumab monotherapy versus best supportive care for unresectable locally advanced/metastatic gastric or gastroesophageal junction cancer. Clin Cancer Res. 2017;23(19):5671‐5678. [DOI] [PubMed] [Google Scholar]
- 361. Hingorani P, Maas ML, Gustafson MP, et al. Increased CTLA‐4(+) T cells and an increased ratio of monocytes with loss of class II (CD14(+) HLA‐DR(lo/neg)) found in aggressive pediatric sarcoma patients. J Immunother Cancer. 2015;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double‐blind, multicentre, phase 2, dose‐ranging study. Lancet Oncol. 2010;11(2):155‐164. [DOI] [PubMed] [Google Scholar]
- 363. Merchant MS, Wright M, Baird K, et al. Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin Cancer Res. 2016;22(6):1364‐1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Lussier DM, Johnson JL, Hingorani P, Blattman JN. Combination immunotherapy with alpha‐CTLA‐4 and alpha‐PD‐L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J Immunother Cancer. 2015;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Fischer JW, Bhattarai N. CAR‐T cell therapy: mechanism, management, and mitigation of inflammatory toxicities. Front Immunol. 2021;12:693016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Sterner RC, Sterner RM. CAR‐T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Ye B, Stary CM, Li X, Gao Q, Kang C, Xiong X. Engineering chimeric antigen receptor‐T cells for cancer treatment. Mol Cancer. 2018;17(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Zhu J, Simayi N, Wan R, Huang W. CAR T targets and microenvironmental barriers of osteosarcoma. Cytotherapy. 2022;24(6):567‐576. [DOI] [PubMed] [Google Scholar]
- 369. Rosenberg SA, Yannelli JR, Yang JC, et al. Treatment of patients with metastatic melanoma with autologous tumor‐infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86(15):1159‐1166. [DOI] [PubMed] [Google Scholar]
- 370. Heymann MF, Lezot F, Heymann D. The contribution of immune infiltrates and the local microenvironment in the pathogenesis of osteosarcoma. Cell Immunol. 2019;343:103711. [DOI] [PubMed] [Google Scholar]
- 371. Wang C, Li M, Wei R, Wu J. Adoptive transfer of TILs plus anti‐PD1 therapy: an alternative combination therapy for treating metastatic osteosarcoma. J Bone Oncol. 2020;25:100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Lu Y, Zhang J, Chen Y, et al. Novel immunotherapies for osteosarcoma. Front Oncol. 2022;12:830546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Dyson KA, Stover BD, Grippin A, et al. Emerging trends in immunotherapy for pediatric sarcomas. J Hematol Oncol. 2019;12(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Mackall CL, Rhee EH, Read EJ, et al. A pilot study of consolidative immunotherapy in patients with high‐risk pediatric sarcomas. Clin Cancer Res. 2008;14(15):4850‐4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Himoudi N, Wallace R, Parsley KL, et al. Lack of T‐cell responses following autologous tumour lysate pulsed dendritic cell vaccination, in patients with relapsed osteosarcoma. Clin Transl Oncol. 2012;14(4):271‐279. [DOI] [PubMed] [Google Scholar]
- 376. Miwa S, Nishida H, Tanzawa Y, et al. Phase 1/2 study of immunotherapy with dendritic cells pulsed with autologous tumor lysate in patients with refractory bone and soft tissue sarcoma. Cancer. 2017;123(9):1576‐1584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


