Abstract
Background
The multicentre European Bifurcation Club Trial (EBC TWO) showed no significant differences in 12-month clinical outcomes between patients randomised to a provisional stenting strategy or systematic culotte stenting in non-left main true bifurcations.
Aims
This study aimed to investigate the 5-year clinical results of the EBC TWO Trial.
Methods
A total of 200 patients undergoing stent implantation for non-left main bifurcation lesions were recruited into EBC TWO. Inclusion criteria required a side branch diameter ≥2.5 mm and side branch lesion length >5 mm. Five-year follow-up was completed for 197 patients. The primary endpoint was the composite of all-cause mortality, myocardial infarction, or target vessel revascularisation.
Results
The mean side branch stent diameter was 2.7±0.3 mm and mean side branch lesion length was 10.3±7.2 mm. At 5-year follow-up, the primary endpoint occurred in 18.4% of provisional and 23.7% of systematic culotte patients (hazard ratio [HR] 0.75, 95% confidence interval [CI]: 0.41-1.38). No significant differences were identified individually for all-cause mortality (7.8% vs 7.2%, HR 1.11, 95% CI: 0.40-3.05), myocardial infarction (8.7% vs 13.4%, HR 0.64, 95% CI: 0.27-1.50) or target vessel revascularisation (6.8% vs 9.3%, HR 1.12, 95% CI: 0.37-3.34). Stent thrombosis rates were also similar (1.9% vs 3.1%, HR 0.63, 95% CI: 0.11-3.75). There was no significant interaction between the extent of side branch disease and the primary outcome (p=0.34).
Conclusions
In large non-left main true bifurcation lesions, the use of a systematic culotte strategy showed no benefit over provisional stenting for the composite outcome of all-cause mortality, myocardial infarction, or target vessel revascularisation at 5 years. The stepwise provisional approach may be considered preferable for the majority of true coronary bifurcation lesions. ClinicalTrials.gov: NCT01560455.
Introduction
The optimal treatment of bifurcation disease has been widely debated. Early randomised trials comparing the provisional strategy to routine dual stenting showed that two-stent strategies resulted in either no improvement in major adverse cardiovascular events (MACE)1,2 or increased myocardial infarction (MI)3 and mortality4. These studies included first-generation stents implanted within smaller-calibre bifurcations, in which side branch (SB) disease was less likely to be functionally significant.
EBC TWO addressed these concerns with the use of second-generation stents, a mean SB stent diameter of 2.7 mm and mean SB lesion length of 10 mm5. A total of 200 patients with non-left main true bifurcation disease were randomised to either provisional stenting (with T-stent placement in the event of significant SB compromise) or upfront culotte stenting. Twelve-month MACE rates were not significantly different between the provisional and culotte cohorts (7.7% vs 10.3%; p=0.53). Significant reductions in radiation dose and procedural cost were seen in the provisional group.
A concordant result was reported by the Nordic-Baltic Bifurcation Study IV6. The incidence of MACE at 2-years was comparable between the provisional and routine 2-stent strategies (12.9% vs 8.4%, HR 0.63; p=0.12), as was relief of angina. The DKCRUSH-II study compared provisional and double kissing crush (DK-crush) techniques in patients with mean SB lesion lengths of 15 mm7. Five-year follow-up showed a trend towards reduced MACE with DK-crush (15.7% vs 23.8%; p=0.05). This was driven exclusively by reduced target lesion revascularisation (TLR; 8.6% vs 16.2%; p=0.03)8, the majority of which was clustered around mandatory 8-month repeat angiography.
The long-term outcome of provisional versus routine culotte stenting for true bifurcation lesions is unknown. Herein we report the results from the 5-year follow-up of EBC TWO.
Methods
STUDY DESIGN
EBC TWO was an international, multicentre, parallel-group randomised trial, designed and performed by the European Bifurcation Club. Trial oversight was provided by the Cardiovascular European Research Center (CERC, Massy, France). All events were adjudicated by an independent Clinical Events Committee and a Data and Safety Monitoring Board. Procedural details were analysed at an independent core lab. The study protocol was approved by the relevant authorities in all countries involved. Funding was through an unrestricted grant from Terumo Europe, with additional funding for core lab analysis provided by Pie Medical Imaging. The trial was registered on ClinicalTrials.gov: NCT01560455. The CONSORT checklist (Supplementary Appendix 1) was used when writing this manuscript9.
STUDY POPULATION
Patients were recruited if they had non-left main bifurcation disease (Medina 1,1,1, 1,0,1 or 0,1,1) and all limbs of the bifurcation were ≥2.5 mm in diameter. SB ostial disease needed to extend ≥5 mm in length.
REVASCULARISATION PROCEDURE
A detailed outline is provided in the primary outcome publication5. Briefly, provisional stenting began with wire placement into the main vessel (MV) and SB. SB predilation was discouraged to reduce the risk of dissection. The MV stent was sized to the distal main branch (dMB) and deployed with a jailed SB wire in situ. Proximal optimisation technique (POT) was recommended prior to rewiring the SB via a distal cell. Kissing balloon inflation (KBI) was performed with non-compliant balloons sized to the dMB and SB. It was suggested to then postdilate the proximal main branch (pMB) with the kissing balloon pair or a short non-compliant balloon. Further SB treatment was indicated only in the setting of SB Thrombolysis in Myocardial Infarction (TIMI) flow <3, >90% ostial SB stenosis, threatened SB closure or SB dissection >type A. SB stenting was performed with the T-technique, minimising any stent protrusion into the MV. Repeat KBI was mandatory.
Culotte stenting was ideally performed with the pMB-SB stent deployed first, with a jailed wire in the dMB. Following POT, the dMB was rewired through a distal cell and dilated. The pMB-dMB was then stented, and following an optional POT, the SB rewired through a distal cell. Sequential high pressure followed by simultaneous low pressure non-compliant KBI was then required. Final post-dilation of the pMB was optional.
Nobori Biolimus-eluting stents (Terumo Corporation) were implanted, containing biodegradeable polymer coated onto 112 µm stainless steel struts. Aspirin 75-150 mg daily and clopidogrel 75 mg daily were recommended for a minimum of 12 months, as was standard practice at the time.
FOLLOW-UP
Adverse event tracking commenced at randomisation and continued to the end of the 5-year follow-up period. Patients underwent either telephone or hospital follow-up. Routine angiography was not performed.
ENDPOINTS
The primary endpoint was the composite of all-cause mortality, myocardial infarction (MI), or target vessel revascularisation (TVR) at 5 years. Secondary endpoints included the individual components of the primary endpoint and stent thrombosis (based on the Academic Research Consortium [ARC] definition)10. The influence of SB lesion length on the primary outcome was analysed. To isolate the specific impact of bifurcation strategy on clinical outcomes, bifurcation-related adverse cardiac events (the composite of bifurcation-specific procedural acute vessel closure, stent thrombosis, Type 1 myocardial infarction and revascularisation) were also examined.
MI was defined as per European Society of Cardiology/American College of Cardiology (ESC/ACC) guidelines11. Periprocedural MI was defined by the elevation of cardiac troponin (cTn) values (>5×99th percentile upper reference limit [URL]) in patients with normal baseline values (≤99th percentile URL). A rise in cTn values >20% was required if the baseline values were elevated and stable or falling. Additionally, one of the symptoms of myocardial ischaemia, new ischaemic electrocardiogram (ECG) changes, angiographic findings consistent with a procedural complication or imaging demonstration of a new regional wall motion abnormality were also required. TVR involved revascularisation (PCI or coronary artery bypass graft) of the MV±SB or the target vessel, which had demonstrated inadequacy with TIMI flow <3, without repeat revascularisation.
STATISTICAL ANALYSIS
Power calculations have been previously documented5. All analysis was performed on an intention-to-treat basis. Continuous variables are described by mean and standard deviation, and categorical data by raw numbers and percentages. Relationships between categorical data were assessed with Pearson’s Chi-Square or Fisher’s exact test. Continuous variables were assessed with the independent samples t-test. The reported results include recurrent events for individual endpoints and first event only for the composite endpoint. Event-free survival was assessed with Kaplan-Meier analysis and the log-rank test used for comparison. Hazard ratios (HR) of time-to-first-event for the primary composite and secondary endpoints were generated using a Cox regression model. SB lesion length was included as a covariate to check for subgroup interaction. Statistical analysis was performed with SPSS V26.0 (IBM).
Results
Two hundred patients were recruited between 2011 and 2014, and 197 patients completed 5-year follow-up. Baseline demographic and procedural details are summarised in Supplementary Table 1 and Supplementary Table 2. The mean SB lesion length was 10.2 mm. SB stenting was required in 16% of the provisional cohort. The rates of final KBI were approximately 95% in both groups, and procedural success was ≥97%. Of note, significantly more patients in the culotte group underwent additional PCI for bystander disease (40.2% vs 23.3%; p=0.01). As previously reported, radiation dose and procedural cost were significantly lower with provisional stenting.
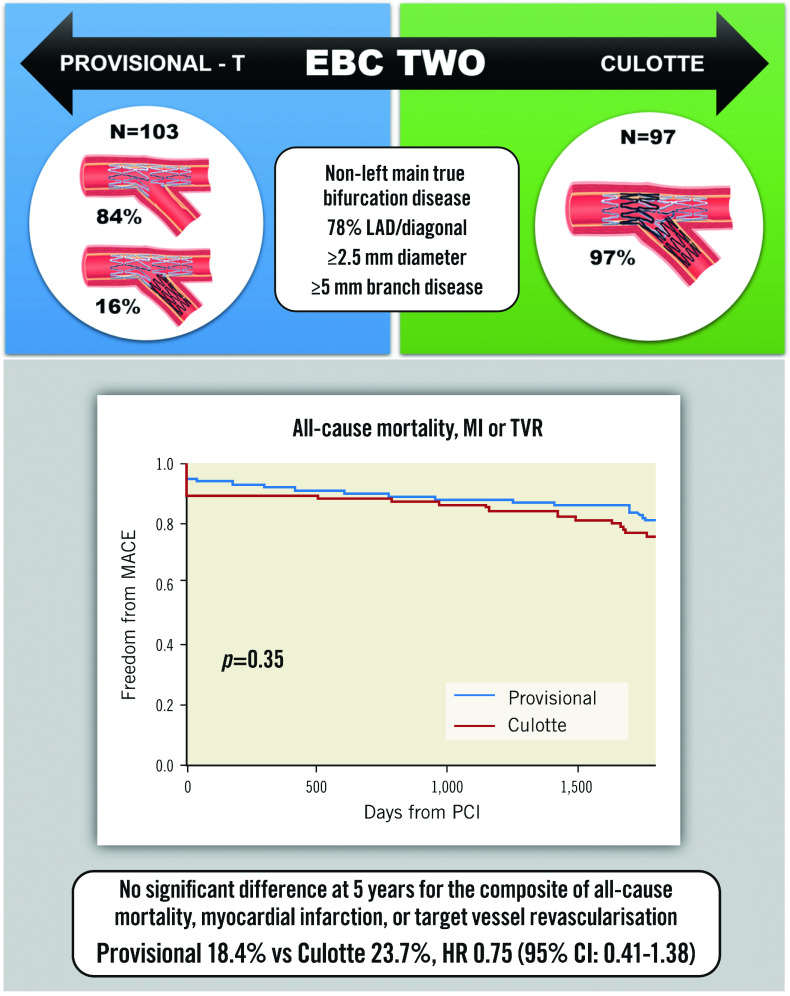
The cumulative 5-year incidence of the composite primary endpoint was not significantly different between the provisional and culotte cohorts (18.4% vs 23.7%, HR 0.75, 95% confidence interval [CI]: 0.41-1.38) (Central illustration). This was consistent within all individual secondary endpoints (Table 1, Figure 1). Progression to SB stent placement in the provisional cohort did not significantly impact the incidence of MACE compared to main vessel stenting only (12.5% vs 19.5%, HR 0.72, 95% CI: 0.17-3.09). Five-year all-cause mortality was 7.5%, with cardiac mortality at 3%. Most MI was periprocedural, which occurred at a numerically higher rate in the culotte group (10.3% vs 4.9%; p=0.16). The overall incidence of Type 1 MI was low at 2.5%. TVR was clinically driven in all cases and occurred in 8% of patients; revascularisation for de novo disease was more frequent at 12%. Definite/probable stent thrombosis occurred in 1.5% of cases.
Central illustration. Provisional-T versus culotte stenting MACE rates at 5-year follow-up.
CI: confidence interval; EBC: European Bifurcation Club; HR: hazard ratio; LAD: left anterior descending artery; MACE: major adverse cardiovascular events; MI: myocardial infarction; PCI: percutaneous coronary intervention; TVR: target vessel revascularisation
Table 1. Trial endpoints.
| Provisional (n=103) | Culotte (n=97) |
Hazard ratio (95% CI) |
p-value | ||
|---|---|---|---|---|---|
| Primary endpoint (MACE) | |||||
| All-cause mortality, MI or TVR | 19 (18.4) | 23 (23.7) | 0.75 (0.41-1.38) | 0.36 | |
| Secondary endpoints | |||||
| Mortality | All-cause mortality | 8 (7.8) | 7 (7.2) | 1.11 (0.40-3.05) | 0.85 |
| Cardiac mortality | 3 (2.9) | 3 (3.1) | 0.93 (0.19-4.59) | 0.92 | |
| Myocardial infarction | All MI | 9 (8.7) | 13 (13.4) | 0.64 (0.27-1.50) | 0.30 |
| Periprocedural MI | 5 (4.9) | 10 (10.3) | 0.47 (0.16-1.36) | 0.16 | |
| Type 1 MI | 3 (2.9) | 2 (2.1) | 1.44 (0.24-8.64) | 0.69 | |
| Revascularisation | All revascularisation events | 16 (15.5) | 24 (24.7) | 0.68 (0.34-1.35) | 0.26 |
| Target vessel revascularisation | 7 (6.8) | 9 (9.3) | 1.12 (0.37-3.34) | 0.84 | |
| Target lesion revascularisation | 7 (6.8) | 7 (7.2) | 1.35 (0.43-4.26) | 0.61 | |
| Stent thrombosis | All stent thrombosis | 2 (1.9) | 3 (3.1) | 0.63 (0.11-3.75) | 0.61 |
| Definite/probable | 1 (1.0) | 2 (2.1) | 0.47 (0.04-5.17) | 0.54 | |
| Possible | 1 (1.0) | 1 (1.0) | 0.97 (0.06-15.4) | 0.98 | |
| Values are n (%). Recurrent events are included for the secondary endpoints. Hazard ratios are calculated from time-to-first-event only. CI: confidence interval; MACE: major adverse cardiovascular events; MI: myocardial infarction; TVR: target vessel revascularisation | |||||
Figure 1. Secondary endpoints at 5 years.
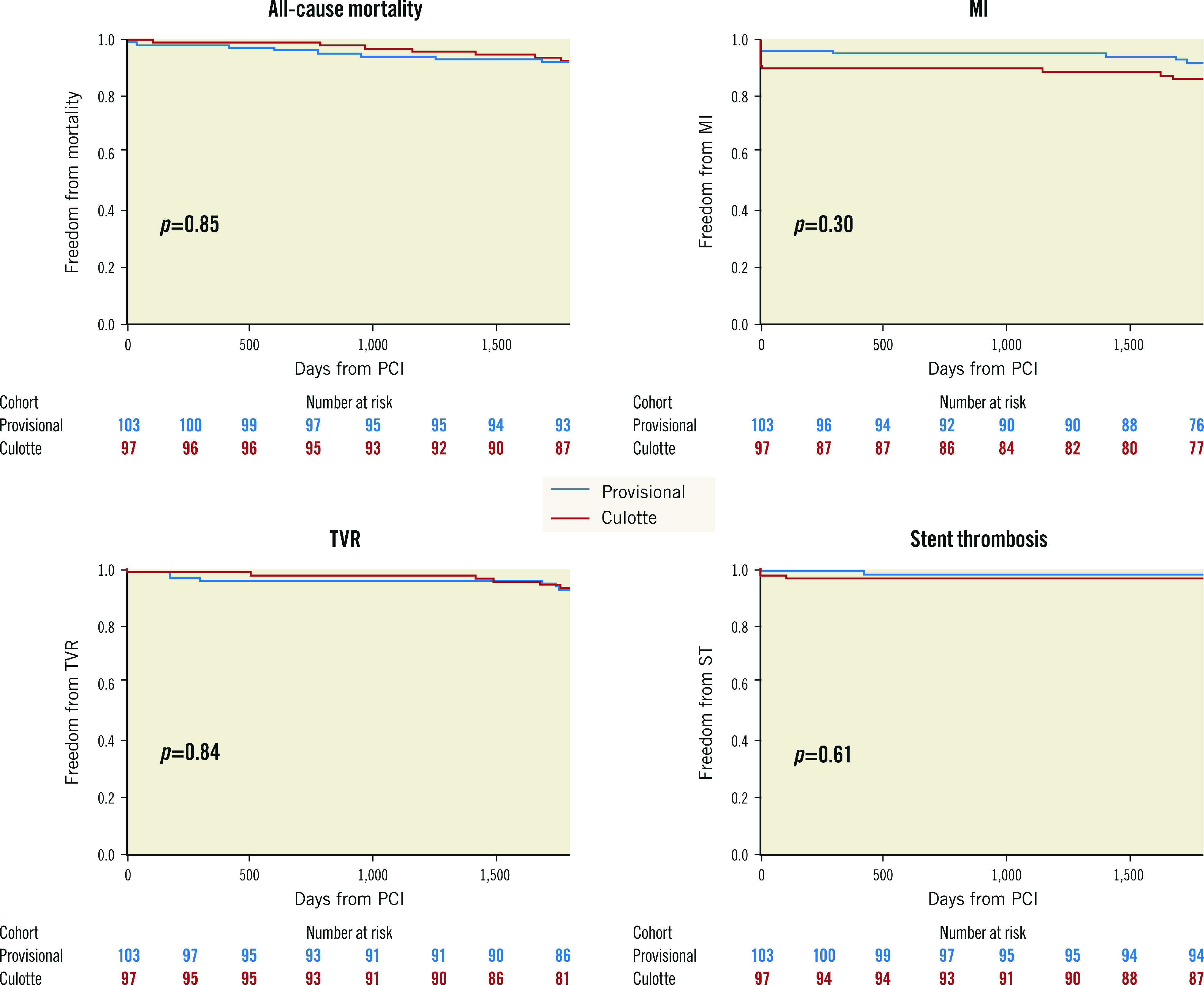
MI: myocardial infarction; PCI: percutaneous coronary intervention; ST: stent thrombosis; TVR: target vessel revascularisation
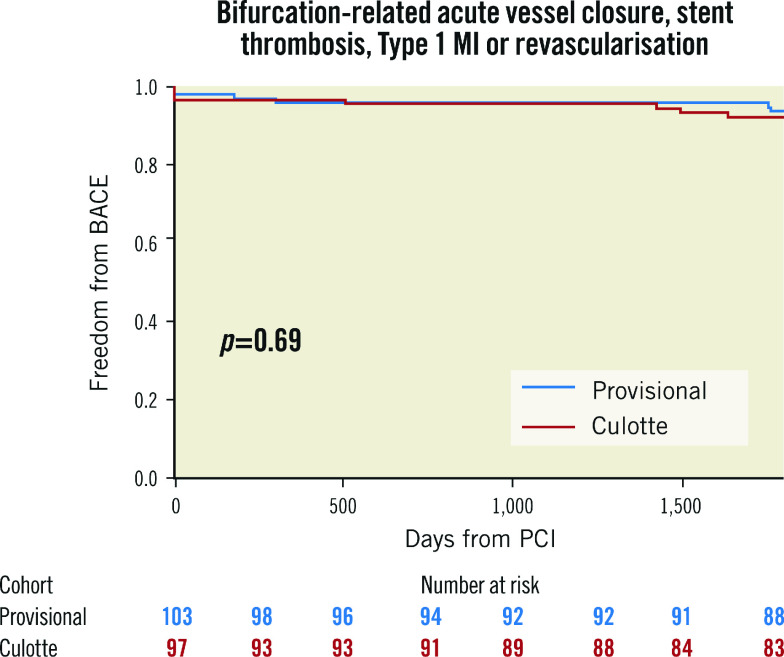
The presence of SB disease with a lesion length ≥10 mm did not show significant interaction with the primary outcome (Table 2, Figure 2). Bifurcation-related adverse cardiac events occurred in 5.8% of provisional and 7.2% of culotte patients (Table 3, Figure 3). There were no significant differences between groups for the individual endpoints, and the overall clinical restenosis rate was 3.5% at 5 years. The site of bifurcation failure was evenly distributed.
Table 2. Influence of SB lesion length on the primary endpoint.
| Provisional | Culotte | Hazard ratio (95% CI) | p-value (interaction) | |
|---|---|---|---|---|
| SB lesion <10 mm | 7/59 (11.9) | 11/54 (20.4) | 0.53 (0.21-1.36) | 0.34 |
| SB lesion ≥10 mm | 12/44 (27.3) | 12/42 (28.6) | 0.99 (0.45-2.21) | |
| Values are n/N (%). CI: confidence interval; SB: side branch | ||||
Figure 2. Influence of SB disease length on the primary endpoint.
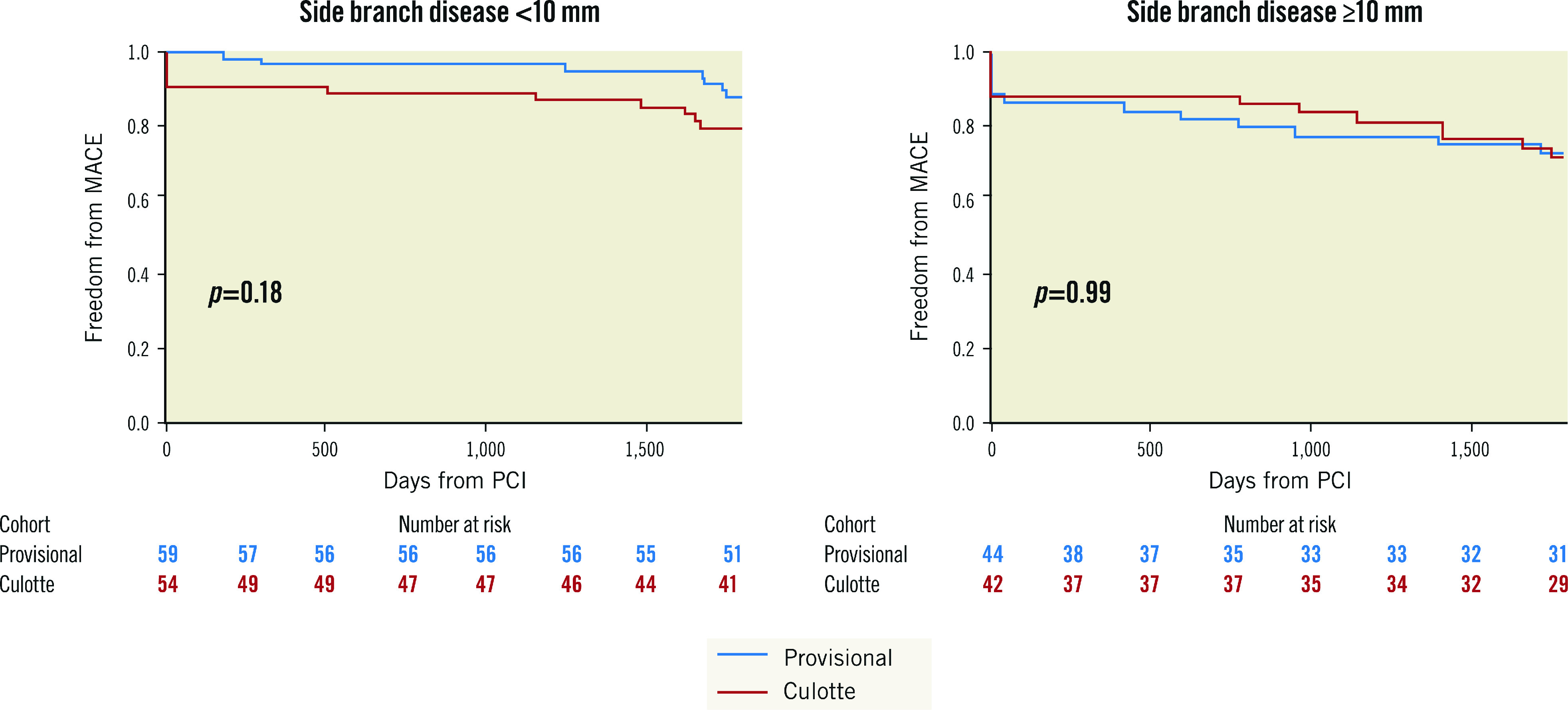
MACE: major adverse cardiovascular events; PCI: percutaneous coronary intervention; SB: side branch
Table 3. Bifurcation-related endpoints.
| Provisional (n=103) | Culotte (n=97) | Hazard ratio (95% CI) | p-value | ||
|---|---|---|---|---|---|
| Bifurcation-related adverse cardiac events (BACE) | |||||
| Bifurcation-related acute vessel closure, stent thrombosis (probable or definite), Type 1 MI or revascularisation. | 6 (5.8) | 7 (7.2) | 0.80 (0.27-2.39) | 0.69 | |
| Individual endpoints | |||||
| Periprocedural | Acute vessel closure | 1 (1.0) | 1 (1.0) | 0.95 (0.06-15.13) | 0.97 |
| Stent thrombosis | Definite/probable | 1 (1.0) | 2 (2.1) | 0.47 (0.04-5.16) | 0.54 |
| Myocardial infarction | Type 1 MI | 0 (0) | 1 (1.0) | 0.02 (0->100) | 0.61 |
| Clinically significant restenosis | Target lesion revascularisation | 4 (3.9) | 3 (3.1) | 1.28 (0.29-5.73) | 0.75 |
| Site of bifurcation failure | |||||
| Main vessel | 1 (1.0) | 2 (2.1) | p=ns | ||
| Side branch | 3 (2.9) | 2 (2.1) | |||
| Both | 1 (1.0) | 1 (1.0) | |||
| Values are n (%). CI: confidence interval; MI: myocardial infarction; ns: non-significant | |||||
Figure 3. Bifurcation-related adverse cardiac events (BACE) at 5 years.
MI: myocardial infarction; PCI: percutaneous coronary intervention
Discussion
This study provides the longest follow-up of patients undergoing provisional versus culotte bifurcation PCI with contemporary stents. The results confirm that systematic culotte treatment does not offer any long-term clinical benefit beyond the provisional strategy in non-left main lesions (Central illustration). This is in concordance with 12-month outcomes and remains true regardless of the extent of SB disease.
Multiple variables influence the results of bifurcation PCI, including patient risk factors, equipment, operator expertise and stenting technique. Important lesion characteristics include location, MV and SB size, Medina class and SB lesion severity and length. EBC TWO included 32% acute coronary syndrome (ACS) patients, a high proportion of left anterior descending artery disease (78%) and large-calibre SBs (mean SB stent diameter >2.5 mm). Patients were also required to have significant (>50%) SB stenosis and had a mean SB lesion length of 10 mm. Therefore, this cohort was specifically selected for disease that was highly likely to be functionally significant in both the MV and SB. As such, it is notable that excellent clinical outcomes were achieved with MV stenting and KBI alone. The fact that the provisional strategy achieved similar outcomes to upfront culotte, with only a 16% rate of SB stenting, suggests that the overwhelming majority of upfront SB stenting is unnecessary. This remains true even when a SB lesion is over 10 mm in length. Avoidance of a second stent reduces both procedural cost as well as the potential for complications. Of interest, a trend towards favourable outcomes was apparent with the provisional strategy for SB lesion lengths under 10 mm. These results are largely consistent with a network meta-analysis of 21 randomised trials which reported no difference in MACE between provisional and culotte techniques12. Whilst 2-stent techniques showed benefit for SB lesion lengths above 10 mm, these results were driven entirely by studies using DK-crush.
DK-crush is an alternative technique to culotte and has been reported to reduce rates of TLR compared to provisional stenting for non-left main bifurcations7. However, there are important factors to consider when comparing data. In DKCRUSH-II, patients had a mean SB lesion length of 15 mm, compared to 10 mm in EBC TWO, and 29% of provisional patients progressed to second stent insertion, compared to 16% in EBC TWO. This suggests greater SB lesion complexity in DKCRUSH-II. Main vessel stent post-dilation was more common in the DK-crush group than the provisional group (100% vs 87.6%; p=0.008). KBI was performed in only 79.5% of the provisional group in DKCRUSH-II, compared to 94% in EBC TWO. Therefore, there were also technical differences in the performance of the procedures. Finally, follow-up included a scheduled 8-month angiogram for DKCRUSH-II patients compared to standard clinical practice in EBC TWO. The clustering of TLR events at the time of scheduled angiography, with parallel survival curves before and after, results in difficulty interpreting this outcome in real-world practice.
The trend towards increased periprocedural MI with upfront culotte stenting in EBC TWO should be interpreted with caution. While it may be related to increased procedural complexity, the culotte cohort also underwent significantly more bystander disease PCI during the index procedure. The clinical significance of periprocedural MI based on current definitions is also debatable, and no difference in the critical endpoints of cardiac death or Type 1 MI was seen.
It is notable that bifurcation-related adverse cardiac events (6.5%) represented a minority of overall MACE (21%) at 5 years. In the first instance, this demonstrates that modern PCI techniques offer robust long-term results for large-calibre true bifurcation lesions. Rates of probable and definite bifurcation stent thrombosis were very low in both strategies (0.3% annually), with one event recorded in a patient who received an off-protocol bifurcation-specific stent. It also emphasises the importance of cardiovascular risk factor optimisation for secondary prevention in these high-risk patients. With modern advances in medical therapy, including potent P2Y12 inhibition and multipronged lipid management (high intensity statins, ezetimibe and proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibition), it is likely that adverse event rates have declined further.
Limitations
The trial was run with an open design, and patient and operator awareness of therapy could theoretically have led to bias in the interpretation of outcomes. Patients were treated with clopidogrel, and event rates may have been reduced by the use of newer P2Y12 agents. The duration of dual antiplatelet therapy use over the 5-year period is unknown. Use of intracoronary imaging was not recorded. Culotte patients underwent significantly greater bystander PCI at the index procedure, which may have influenced the overall MACE outcomes.
While the rate of follow-up was high, the overall sample size was limited. Only a few trials have reported 5-year outcomes, and it may require even longer follow-up and greater patient recruitment for small differences between bifurcation strategies to become evident13. However, when there are minimal differences in outcomes, it is likely that operator proficiency with a given technique outweighs any intrinsic benefit of a specific strategy.
Since the completion of EBC TWO, modifications to the culotte technique have been recommended. These include minimising stent overlap length and performing double kissing balloon inflation after rewiring the first stent (DK mini-culotte)14. Benchtop testing has identified reduced malapposition and side branch ostial stenosis with these adaptations15, although clinical data are lacking. Finally, rates of initial and repeat POT were not recorded, and may have influenced target lesion failure16.
Conclusions
In patients with large-calibre (≥2.5 mm) non-left main bifurcation lesions with significant side branch disease (lesion length ≥5 mm), upfront culotte stenting did not offer any benefit over provisional stenting for the composite endpoint of all-cause mortality, myocardial infarction, or target vessel revascularisation at 5 years. Side branch lesion lengths ≥10 mm did not influence this finding.
Impact on daily practice
Long-term follow-up of EBC TWO has shown that patients with large-calibre non-left main true bifurcation lesions do not gain any benefit from systematic culotte stenting over a provisional approach. MACE rates were similar at 5 years, and bifurcation-specific adverse cardiac events remained infrequent in both cohorts. This study demonstrates that percutaneous coronary intervention offers robust long-term results for the treatment of bifurcation lesions and that the stepwise provisional approach should remain the standard technique.
Supplementary data
CONSORT checklist.
Patient demographics.
Procedural details.
Acknowledgments
Funding
EBC TWO was funded by an unrestricted grant from Terumo Europe. Additional funding for core lab analysis was provided by Pie Medical Imaging.
Conflict of interest statement
D. Hildick-Smith is a proctor to and is on the advisory board of Boston Scientific, Abbott, Medtronic, Terumo, Edwards Lifesciences, Occlutech, Gore, and CERC. A. Chieffo is a consultant for Abiomed, Biosensor, and Magenta; and has received speakers’ fees from Abbott Vascular, Abiomed, Boston Scientific, and Cardinal Health. T. Lefèvre is a proctor for Abbott, Edwards Lifesciences, and Terumo; and has received lecture fees from Boston Scientific and Terumo. M. Pan has received lecture fees from Abbott and Boston Scientific. J.F. Lassen has received lecture fees and honoraria from Medtronic, Boston Scientific, Biotronik, and Biosensors. F. Burzotta has received lecture fees from Abiomed, Abbott, Medtronic, and Terumo. G. Stankovic has received speaker fees from Boston Scientific, Medtronic, Abbott Vascular, and Terumo. M.S. Spence has received honoraria from Medtronic, Edwards Lifesciences, Abbott, Boston Scientific, and Gore. A. Baumbach has received institutional research support from Abbott Vascular; and honoraria from AstraZeneca, Sinomed, MicroPort, Medtronic, Faraday, and Pi-Cardia. The other authors have no conflicts of interest to declare.
Abbreviations
- ACS
acute coronary syndrome
- dMB
distal main branch
- KBI
kissing balloon inflation
- MACE
major adverse cardiovascular events
- MI
myocardial infarction
- MV
main vessel
- pMB
proximal main branch
- POT
proximal optimisation technique
- SB
side branch
- TLR
target lesion revascularisation
- TVR
target vessel revascularisation
Contributor Information
Sandeep Arunothayaraj, Sussex Cardiac Centre, University Hospitals Sussex NHS Trust, Brighton, UK.
Miles W. Behan, Edinburgh Heart Centre, Edinburgh, UK.
Thierry Lefèvre, Institut Cardiovasculaire Paris Sud, Hôpital privé Jacques Cartier, Ramsay Santé, Massy, France.
Jens F. Lassen, Department of Cardiology B, Odense University Hospital, Odense, Denmark and University of Southern Denmark, Odense, Denmark.
Alaide Chieffo, Interventional Cardiology Unit, San Raffaele Scientific Institute, Milan, Italy.
Goran Stankovic, Department of Cardiology, University Clinical Centre of Serbia, Belgrade, Serbia and Faculty of Medicine, University of Belgrade, Belgrade, Serbia.
Francesco Burzotta, Fondazione Policlinico A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy.
Manuel Pan, Department of Cardiology, Reina Sofia Hospital, University of Cordoba, (IMIBIC), Cordoba, Spain.
Miroslaw Ferenc, University Heart Center Freiburg – Bad Krozingen, Bad Krozingen, Germany.
Thomas Hovasse, Institut Cardiovasculaire Paris Sud, Hôpital privé Jacques Cartier, Ramsay Santé, Massy, France.
Mark S. Spence, Department of Cardiology, Royal Victoria Hospital, Belfast, UK.
Philippe Brunel, Hôpital privé Dijon Bourgogne, Clinique Valmy, Dijon, France.
James M. Cotton, Royal Wolverhampton University Hospital NHS Trust, Wolverhampton, UK.
James Cockburn, Sussex Cardiac Centre, University Hospitals Sussex NHS Trust, Brighton, UK.
Didier Carrié, Department of Cardiology, Toulouse University, Rangueil Hospital, Toulouse, France.
Andreas Baumbach, Centre for Cardiovascular Medicine and Devices, William Harvey Research Institute, Queen Mary University of London and Barts Heart Centre, London, UK.
Michael Maeng, Department of Cardiology, Aarhus University Hospital & Aarhus University, Aarhus, Denmark.
Yves Louvard, Institut Cardiovasculaire Paris Sud, Hôpital privé Jacques Cartier, Ramsay Santé, Massy, France.
David Hildick-Smith, Sussex Cardiac Centre, University Hospitals Sussex NHS Trust, Brighton, UK.
References
- Maeng M, Holm NR, Erglis A, Kumsars I, Niemelä M, Kervinen K, Jensen JS, Galløe A, Steigen TK, Wiseth R, Narbute I, Gunnes P, Mannsverk J, Meyerdierks O, Rotevatn S, Nikus K, Vikman S, Ravkilde J, James S, Aarøe J, Ylitalo A, Helqvist S, Sjögren I, Thayssen P, Virtanen K, Puhakka M, Airaksinen J, Christiansen EH, Lassen JF, Thuesen L Nordic-Baltic Percutaneous Coronary Intervention Study Group. Long-term results after simple versus complex stenting of coronary artery bifurcation lesions: Nordic Bifurcation Study 5-year follow-up results. J Am Coll Cardiol. 2013;62:30–4. doi: 10.1016/j.jacc.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Ferenc M, Ayoub M, Büttner HJ, Gick M, Comberg T, Rothe J, Valina CM, Hochholzer W, Neumann FJ. Long-term outcomes of routine versus provisional T-stenting for de novo coronary bifurcation lesions: Five-year results of the Bifurcations Bad Krozingen I study. EuroIntervention. 2015;11:856–9. doi: 10.4244/EIJV11I8A175. [DOI] [PubMed] [Google Scholar]
- Hildick-Smith D, de Belder, Cooter N, Curzen NP, Clayton TC, Oldroyd KG, Bennett L, Holmberg S, Cotton JM, Glennon PE, Thomas MR, Maccarthy PA, Baumbach A, Mulvihill NT, Henderson RA, Redwood SR, Starkey IR, Stables RH. Randomized trial of simple versus complex drug-eluting stenting for bifurcation lesions: the British Bifurcation Coronary Study: old, new, and evolving strategies. Circulation. 2010;121:1235–43. doi: 10.1161/CIRCULATIONAHA.109.888297. [DOI] [PubMed] [Google Scholar]
- Behan MW, Holm NR, de Belder, Cockburn J, Erglis A, Curzen NP, Niemelä M, Oldroyd KG, Kervinen K, Kumsars I, Gunnes P, Stables RH, Maeng M, Ravkilde J, Jensen JS, Christiansen EH, Cooter N, Steigen TK, Vikman S, Thuesen L, Lassen JF, Hildick-Smith D. Coronary bifurcation lesions treated with simple or complex stenting: 5-year survival from patient-level pooled analysis of the Nordic Bifurcation Study and the British Bifurcation Coronary Study. Eur Heart J. 2016;37:1923–8. doi: 10.1093/eurheartj/ehw170. [DOI] [PubMed] [Google Scholar]
- Hildick-Smith D, Behan MW, Lassen JF, Chieffo A, Lefèvre T, Stankovic G, Burzotta F, Pan M, Ferenc M, Bennett L, Hovasse T, Spence MJ, Oldroyd K, Brunel P, Carrie D, Baumbach A, Maeng M, Skipper N, Louvard Y. The EBC TWO Study (European Bifurcation Coronary TWO): A Randomized Comparison of Provisional T-Stenting Versus a Systematic 2 Stent Culotte Strategy in Large Caliber True Bifurcations. Circ Cardiovasc Interv. 2016;9:e003643. doi: 10.1161/CIRCINTERVENTIONS.115.003643. [DOI] [PubMed] [Google Scholar]
- Kumsars I, Holm NR, Niemelä M, Erglis A, Kervinen K, Christiansen EH, Maeng M, Dombrovskis A, Abraitis V, Kibarskis A, Trovik T, Latkovskis G, Sondore D, Narbute I, Terkelsen CJ, Eskola M, Romppanen H, Laine M, Jensen LO, Pietila M, Gunnes P, Hebsgaard L, Frobert O, Calais F, Hartikainen J, Aarøe J, Ravkilde J, Engstrøm T, Steigen TK, Thuesen L, Lassen JF Nordic Baltic bifurcation study group. Randomised comparison of provisional side branch stenting versus a two-stent strategy for treatment of true coronary bifurcation lesions involving a large side branch: The Nordic-Baltic Bifurcation Study IV. Open Heart. 2020;7:e000947. doi: 10.1136/openhrt-2018-000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Santoso T, Zhang JJ, Ye F, Xu YW, Fu Q, Kan J, Paiboon C, Zhou Y, Ding SQ, Kwan TW. A randomized clinical study comparing double kissing crush with provisional stenting for treatment of coronary bifurcation lesions: results from the DKCRUSH-II (Double Kissing Crush versus Provisional Stenting Technique for Treatment of Coronary Bifurcation Lesions) trial. J Am Coll Cardiol. 2011;57:914–20. doi: 10.1016/j.jacc.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Chen SL, Santoso T, Zhang JJ, Ye F, Xu YW, Fu Q, Kan J, Zhang FF, Zhou Y, Xie DJ, Kwan TW. Clinical Outcome of Double Kissing Crush Versus Provisional Stenting of Coronary Artery Bifurcation Lesions: The 5-Year Follow-Up Results From a Randomized and Multicenter DKCRUSH-II Study (Randomized Study on Double Kissing Crush Technique Versus Provisional Stenting Technique for Coronary Artery Bifurcation Lesions). Circ Cardiovasc Interv. 2017;10:e004497. doi: 10.1161/CIRCINTERVENTIONS.116.004497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es, Zuckerman B, Fearon WF, Taggart D, Kappetein AP, Krucoff MW, Vranckx P, Windecker S, Cutlip D, Serruys PW Academic Research Consortium. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:2635–50. doi: 10.1161/CIRCULATIONAHA.117.029289. [DOI] [PubMed] [Google Scholar]
- Antman E, Bassand JP, Klein W, Ohman M, Lopez Sendon, Rydén L, Simoons M, Tendera M, Chaitman BR, Clemmensen P, Falk E, Fishbein MC, Galvani M, Garson A. J, Grines C, Hamm C, Hoppe U, Jaffe A, Katus H, Kjekshus J, Klein W, Klootwijk P, Lenfant C, Levy D, Levy RI, Luepker R, Marcus F, Naslund U, Ohman M, Pahlm O, Poole-Wilson P, Popp R, Pyorala K, Ravkilde J, Rehnquist N, Roberts W, Roberts R, Roelandt J, Ryden L, Sans S, Simoons ML, Thygesen K, Tunstall-Pedoe H, Underwood R, Uretsky BF, Van de Werf F, Voipio-Pulkki LM, Wagner G, Wallentin L, Wijns W, Wood D. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- Di Gioia, Sonck J, Ferenc M, Chen SL, Colaiori I, Gallinoro E, Mizukami T, Kodeboina M, Nagumo S, Franco D, Bartunek J, Vanderheyden M, Wyffels E, De Bruyne, Lassen JF, Bennett J, Vassilev D, Serruys PW, Stankovic G, Louvard Y, Barbato E, Collet C. Clinical Outcomes Following Coronary Bifurcation PCI Techniques: A Systematic Review and Network Meta-Analysis Comprising 5,711 Patients. JACC Cardiovasc Interv. 2020;13:1432–44. doi: 10.1016/j.jcin.2020.03.054. [DOI] [PubMed] [Google Scholar]
- Ninomiya K, Serruys PW, Garg S, Gao C, Masuda S, Lunardi M, Lassen JF, Banning AP, Colombo A, Burzotta F, Morice MC, Mack MJ, Holmes DR, Davierwala PM, Thuijs DJFM, van Klaveren D, Onuma Y SYNTAX Extended Survival Investigators. Predicted and Observed Mortality at 10 Years in Patients With Bifurcation Lesions in the SYNTAX Trial. Cardiovasc Interv. 2022;15:1231–42. doi: 10.1016/j.jcin.2022.04.025. [DOI] [PubMed] [Google Scholar]
- Lassen JF, Albiero R, Johnson TW, Burzotta F, Lefèvre T, Iles TL, Pan M, Banning AP, Chatzizisis YS, Ferenc M, Dzavik V, Milasinovic D, Darremont O, Hildick-Smith D, Louvard Y, Stankovic G. Treatment of coronary bifurcation lesions, part II: implanting two stents. The 16th expert consensus document of the European Bifurcation Club. EuroIntervention. 2022;18:457–70. doi: 10.4244/EIJ-D-22-00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Tu S, Cai W, Jiang Z, Zheng H, Xiao L, Qiu C, Xiong C, Yao Y, Chen L. Double kissing mini-culotte versus mini-culotte stenting: Insights from micro-computed tomographic imaging of bench testing. EuroIntervention. 2019;15:465–72. doi: 10.4244/EIJ-D-18-00688. [DOI] [PubMed] [Google Scholar]
- Chevalier B, Mamas MA, Hovasse T, Rashid M, Gomez JA, Pan M, Witkowski A, Crowley J, Aminian A, McDonald J, Beygui F, Portales JF, Roguin A, Stankovic G. Clinical outcomes of the proximal optimisation technique (POT) in bifurcation stenting. EuroIntervention. 2021;17:e910–8. doi: 10.4244/EIJ-D-20-01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT checklist.
Patient demographics.
Procedural details.