Abstract
Background
Hepatitis B virus infection (HBV) is one of the major healthcare problems in Georgia. To achieve viral hepatitis elimination, gaps in diagnosis and management of chronic HBV infection need to be addressed. The aim of our study was to collect data on clinical and viral characteristics of patients with chronic HBV infection to estimate the proportion of patients who may need antiviral treatment.
Methods
All relevant deidentified data about demographic, clinical, and viral characteristics were extracted from patients’ medical records. Descriptive statistical analyses were done for univariate assessment of demographic, virologic, and clinical characteristics. Chi-square test was used to assess the associations between HBV-DNA level, HBeAg, alanine aminotransferase (ALT), and liver fibrosis.
Results
In total, 96% (124/129) of patients with chronic HBV infection are HBeAg-negative; 84% (145/173) had no or mild fibrosis, and 3% (6/162) had advanced liver fibrosis/cirrhosis. Sixty-five out of 126 (51%) patients were classified as HBeAg-positive or HBeAg-negative chronic HBV infection (without hepatitis); 11 (9%) as chronic hepatitis B; 46 (37%) had not classified in any of the known HBV phases, while 30 of them (24% out of total) had high viral load and normal ALT. Statistically significant association was seen between high HBV-DNA and HBeAg-positivity (P = .043). High ALT level was also associated with liver fibrosis (P = .015). Significant positive correlation between age and the presence of moderate or advanced liver fibrosis was observed (P = .002).
Conclusion
This is the first study about the clinical and viral characteristics of patients with chronic HBV infection in Georgia. The vast majority were HBeAg-negative, only 3% had advanced liver diseases; about half of patients had inactive diseases. However, one out of four patients had a high viral load but normal ALT. By the evaluation of HBV phases, we estimated that 12%–36% of patients with chronic HBV monoinfection require antiviral treatment.
Keywords: viral hepatitis, hepatitis B virus, viral load, hepatitis elimination
Graphical abstract
This is the first study about the clinical and viral characteristics of patients with an initial diagnosis of chronic Hepatitis B virus (HBV) infection in Georgia. We found that the absolute majority (96%) were HBeAg-negative. Only a small number of patients (3%) had advanced liver fibrosis/cirrhosis at the time of diagnosis. Half of them had inactive diseases (HBV infection, without hepatitis), while approximately 1 of 10 patients (9%) had an active disease (chronic hepatitis), which urgently needs antiviral treatment. Surprisingly, one of four (24%) patients had a high viral load but normal alanine aminotransferase (ALT). With these data, we estimated that at least 12%–36% of patients with chronic HBV need antiviral treatment. Further studies are needed to identify the reasons for the high proportion of HBeAg-negative patients with high viral load and normal ALT.
Viral hepatitis is one of the leading causes of liver-related death, with 296 million people worldwide living with Hepatitis B virus (HBV) based on World Health Organization (WHO) estimates in 2019.1 All patients with chronic HBV infection are at increased risk of progression to cirrhosis and hepatocellular carcinoma (HCC), depending on the host and viral factors.2,3
In 2020, WHO released a framework for countries to pursue validation of viral hepatitis elimination. The targets included the elimination of chronic HBV infection as a public health problem by reducing incidence to 95% and mortality to 60%. Except for the improving prevention strategies by increasing HBV vaccine birth dose and three doses of childhood vaccination coverage above 90%, the target is also the identification of 90% of persons with chronic HBV infection and treating/managing at least 80% of them.4,5
Hepatitis B virus infection is one of the major healthcare problems in Georgia. A serosurvey conducted in 2015 provided estimates of the burden of Hepatitis B and C among adults. Georgia appeared to have one of the highest prevalences of Hepatitis C virus (HCV) infection in the world with a 5.4% and 2.9% (95% CI = 2.38–3.51) of chronic HBV.6 In April 2015, the world's first National Hepatitis C Elimination Program was launched, which provides free testing and treatment with direct-acting antivirals for all citizens. In a new “National strategic plan for the elimination of viral hepatitis in Georgia 2021–2025”, Hepatitis B was also incorporated into the elimination efforts to meet WHO goals for viral hepatitis elimination.
The strategy provides additional approaches based on lessons learned during the first 6 years of the HCV eliminations program.
-
•
Promote advocacy, awareness, education, and partnerships for HCV and HBV-associated resource mobilization
-
•
Prevent HCV and HBV transmission
-
•
Identify persons infected with viral hepatitis and link them to care
-
•
Improve HCV and HBV laboratory diagnostics
-
•
Improve HCV and HBV surveillance
Georgia has already achieved some targets for HBV elimination, including a focus on reducing mother-to-child transmission through universal Hepatitis B birth dose administration and high rates of childhood vaccination coverage.
There are big gaps in diagnosing and managing persons with chronic HBV infection. There are no data available on either clinical or viral characteristics of chronic HBV patients and its association with disease progression in Georgia. Also, there are limited data about the prevalence of HBV-related cirrhosis and HCC.
The aim of our study was to collect the data on clinical and viral characteristics of patients with chronic HBV infection. In light of this information, gauge the extent of patients who need antiviral treatment.
Materials and methods
Patient selection
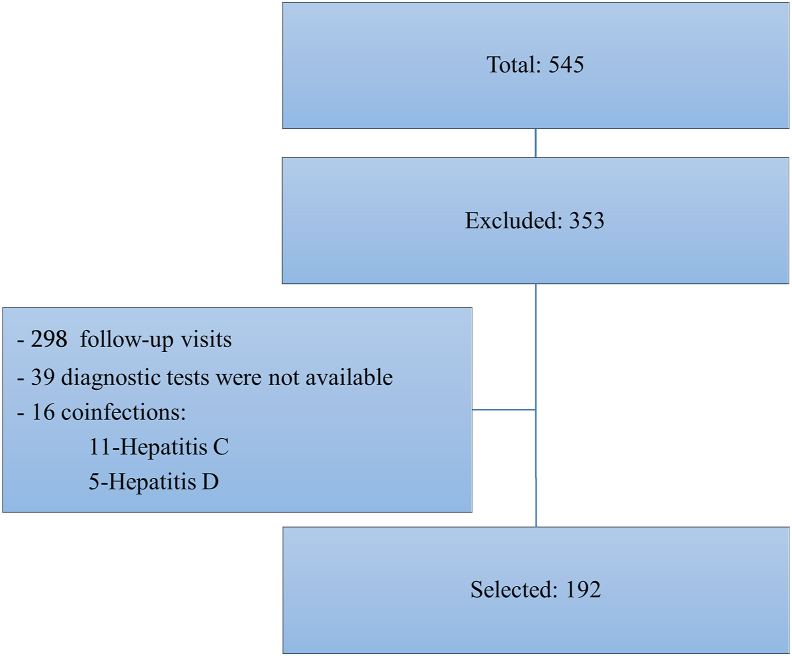
The deidentified data were extracted from the database of Medical Center Mrcheveli. A total of 545 medical records were available with a diagnosis of chronic HBV infection from March 2018 through December 2020. Out of those, 192 HBV monoinfected (ICD-10 code B18.2) patients' medical records were selected, who were recently diagnosed, had no liver-related significant comorbidities, and the necessary diagnostics test results were available for assessment of HBV clinical phases (Figure 1). Additional demographic, epidemiological, and clinical information was also extracted from patients’ medical records.
Figure 1.
Flowchart of Patient Selection.
Parameters assessed
The standard evaluation of chronic HBV patients included polymerase chain reaction (PCR) HBV-DNA, Hepatitis B envelope antigen (HBeAg), liver biochemistry, complete blood count, transient elastography, and abdominal ultrasound. HBsAg and HBeAg tests were performed with an electrochemiluminescence immunoassay. HBV viral load assessment was performed with Roche COBAS 6800 HBV Test, and Aptima HBV Quantitative assays, with a sensitivity of 10 IU/ml. The degree of liver damage was assessed by liver stiffness measurement (LSM) with transient elastography (FibroScan®, Echosens™).
Based on the available literature on noninvasive evaluation of liver fibrosis, for analysis of the data, we stratified patients into the following groups: patients with no/minimal fibrosis (LSM <7 kPa), moderated fibrosis (LSM 7–10 kPa), and advanced fibrosis (LSM >10 kPa). The upper limit of normal (ULN) level of alanine aminotransferase (ALT) was 35 U/L for men and women.
The latest international guideline on the management of HBV infection was used for stratification of patients according to the new classification of clinical phases of chronic HBV infection, which includes (I) HBeAg-positive chronic infection (immune-tolerant), (II) HBeAg-positive chronic hepatitis, (III) HBeAg-negative chronic infection, and (IV) HBeAg-negative chronic hepatitis.7,8
Statistical analyses
Data were entered and analyzed using statistical software IBM SPSS Statistics 23 (IBM; Armonk, New York, USA). Descriptive statistical analysis was done for univariate assessment of demographic, virologic, and clinical characteristics. Chi-square test was used to assess the associations between HBV-DNA level, HBeAg, ALT, and liver fibrosis.
Results
A total number of 192 medical records of chronic HBV infected patients were analyzed: 126 (66%) were males and 66 (34%) were females. The mean age was 35 (17–62). The mean BMI was 26 (16–46). HBeAg test was available in 129 (66%) patients from which 124 (96%) were negative and 5 (4%) were positive. The mean ALT level was 31 (10–179). Of those 162 patients where ALT was available, 125 (77%) of them had within the normal range (<35 IU/ml), 31 (19%) had elevated <2xULN, and 6 (4%) had >2xULN. LSM was done in 173 patients; 145 (84%) had minimal or no fibrosis corresponding to LSM <7 kPa; 22 (13%) had signs of moderate (LSM 7–10 kPa); and 6 (3%) had advanced fibrosis (LSM >10 kPa). Only one patient had decompensated liver cirrhosis.
Of 192 patients, HBV-DNA results were available for 186 patients; 130 (70%) had HBV-DNA less than 2000 IU/ml (low viral load), 46 (25%) had between 2000 and 20,000 IU/ml; and 10 (5%) had more than 20,000 IU/ml (Table 1).
Table 1.
Characteristics of Patients with Chronic HBV Infection.
| Patient characteristics | Total number |
|---|---|
| Gender, n/N (%) | |
| Male | 126/192 (66) |
| Female | 66/192 (34) |
| HBeAg, n/N (%) | 129/192 (67) |
| Positive | 5/129 (4) |
| Negative | 124/129 (96) |
| HBV DNA n/N (%) | 186/192 (97) |
| <2000 | 130/186 (70) |
| 2000–20,000 | 46/186 (25) |
| >20,000 | 10/186 (5) |
| Mean ALT level, n (range) | 31 (10–179) |
| Range n/N (%) | |
| Normal (<35 IU/ml) | 125/162 (77) |
| <2x ULN (35–70 IU/ml) | 31/162 (19) |
| >2x ULN (>70 IU/ml) | 6/162 (4) |
| Liver stiffness measurement n/N (%) | 173/192 (90) |
| Range | |
| <7 KPa | 145/173 (84) |
| 7–10 KPa | 22/173 (13) |
| >10 KPa | 6/173 (3) |
| Ultrasound n/N (%) | 163/192 (85) |
| Fatty liver | 31/163 (19) |
| Splenomegaly (length >120 mm) | 12/163 (7) |
| Ascites | 1/163 (0.6) |
| Mean age, n (range) | 35 (17–62) |
| Mean BMI, n (range) | 26 (16–46) |
ALT, alanine aminotransferase; BMI, body mass index; HBeAg, hepatitis b envelope antigen, HBV, hepatitis B virus; kPa, kilopascal; ULN, upper limit of normal.
Patients with positive HBeAg had significantly higher HBV viral load (P = .043). Four out of five patients had HBV-DNA >20,000 IU/ml, and one patient had between 2000 and 20,000 IU/ml. Elevated ALT level was associated with increased liver fibrosis (P = .015). There was no statistically significant association between HBV viral load and liver fibrosis (P = .916). There was no statistically significant correlation between patients’ age and HBV-DNA level (P = .155). While there was a significant positive correlation between age and the presence of moderate or advanced liver fibrosis (P = .002). BMI was not statistically significantly correlated with HBV-DNA level (P = .466), liver fibrosis (P = .688), or ALT level (P = .106).
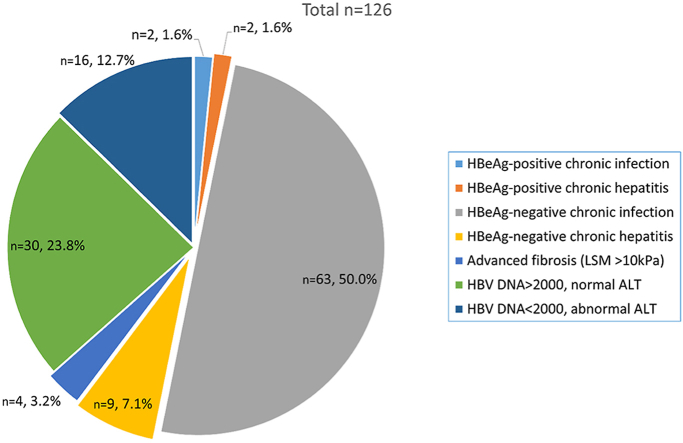
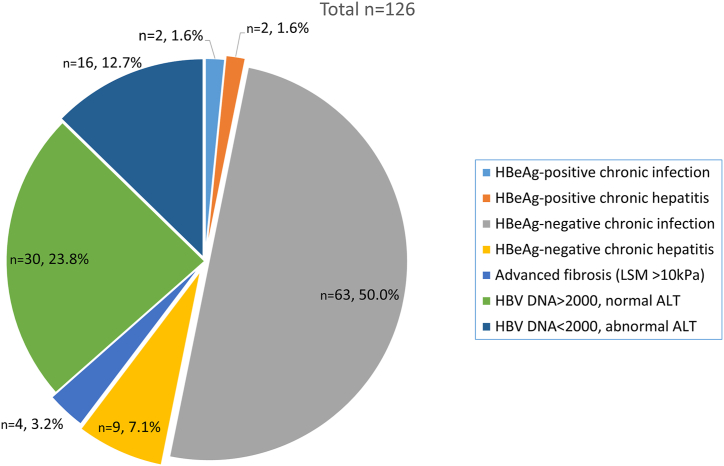
Complete data on viral load, HBeAg, ALT, and LSM were available for 126 patients; 51% (65/126) were classified as either HBeAg-negative or positive HBV infection (without hepatitis) (Figure 2). Two patients with HBeAg-negative chronic hepatitis had evidence of advanced fibrosis (LSM >10 kPa); 9% (11/126) were classified as HBeAg-negative (9/11) and HBeAg-positive (2/11) hepatitis, from which seven had LSM <7 kPa, three −7 to 10 kPa, and one – >10 kPa; 38% (48/126) were not classified in any of the phases.
Figure 2.
Clinical Characteristics of Patients with Chronic HBV Infection. HBV, hepatitis B virus.
The subanalyses of those 46 patients showed that all of them were HBeAg-negative; 16 (13%) had low viral load but abnormal ALT, and 30 had high viral load but normal ALT. In the deeper analyses of 16 patients with low viral load and abnormal ALT, 15/16 (94%) had only mildly elevated ALT (<2xULN), 9/16 (56%) had evidence of fatty liver on abdominal ultrasound; two patients with viral load <2000 and abnormal ALT, had signs of advanced liver fibrosis (LSM >10 kPa). Of 30 patients with normal ALT and high viral load, 25/30 (83%) had HBV-DNA level between 2000 and 20,000 IU/ml, and 2/30 (6%) had signs of moderate or advanced liver fibrosis (LSM >7 kPa).
Discussion
The successful HCV elimination program in Georgia brought a need for HBV elimination. Chronic HBV infection is a dynamic process reflecting the interaction between HBV replication and the host immune response; not all patients with chronic HBV infection need treatment because not all patients have hepatitis.9 The typical indication for HBV antiviral treatment is HBV DNA >2000 IU/ml, elevated ALT, and/or at least moderate fibrosis, while all cirrhotic patients with any detectable HBV DNA should be treated. Except for these typical indications, there are other factors such as age, family history of cirrhosis and HCC, genotypes and mutations, coinfections, and immunosuppression, which should be considered when an antiviral treatment decision is made.
From the 126 patients’ reports, where all pertinent data were available. Eleven classified as HBeAg-positive or HBeAg-negative chronic hepatitis B are the definite antiviral treatment candidates. In addition, four patients with advanced fibrosis (LSM>10 kPa) may also be considered as antiviral treatment candidates (in total 15/126; 12%).
However, in an important proportion of patients (46/126; 37%), a single assessment of HBV viral load and liver disease severity did not allow us to classify within any of the phases (Figure 2). The most challenging patients were 30 patients (24%) who had HBV-DNA level above the cutoff level – 2000 IU/ml, but a normal ALT level. Those are in the “gray area,” which requires additional data or further close surveillance for treatment decision-making. These patients can be considered potential candidates for antiviral treatment.
HBV-DNA cutoff of 2000 and 20,000 IU/ml is an arbitrary value. Mild ALT elevations are often seen in patients with HBeAg-negative chronic HBV, who may sometimes have HBV-DNA between 2000 and 20,000 IU/ml or transiently <2000 IU/ml. It has traditionally been believed that patients who are HBeAg negative with normal ALT have low HBV DNA levels and have no increased risk of disease progression. However, recent studies have shown that this may not always be true. In a study by Papatheodoridis GV et al., 35 out of 399 (8.7%) HBeAg negative patients with chronic hepatitis B have persistently normal ALT and HBV-DNA >2000 IU/ml.10 In a study from India, 35% of HBeAg-negative patients with persistently normal ALT for at least 1 year had HBV-DNA ≥5-log copies/ml (≥18,000 IU/ml), and 13.8% of them had fibrosis stage ≥2 on histology.11
In a systemic review, a total of 246 patients met the criteria of persistently normal ALT and HBV-DNA >2000 IU/ml. On liver biopsy, higher than mild fibrosis was observed in 8% of all patients (moderate fibrosis 7%, severe fibrosis 1%, cirrhosis 0%).12 In our study, despite the unavailability of liver biopsies, we found similar data based on the evaluation of liver fibrosis by transient elastography, 6% (n = 2/30) of patients with HBV-DNA >2000, and normal ALT had evidence of moderate to advanced liver fibrosis (LSM >7 kPa). Multiple other studies suggested the increased long-term risk of clinical events and liver-related death in patients who have inactive HBV infection with high viral load.13, 14, 15 In light of these, we think that these 30 patients with high viral load and normal ALT might be considered current or future candidates for antiviral treatment.
The limitations of this study are that it is a single-center study despite the maximally objectively selected data; the number is too small; for the assessment of necroinflammation and fibrosis, liver biopsy was not used. In addition, there are missing family history of liver cirrhosis or liver cancer, comorbidities (except liver diseases), and medication history. Chronic hepatitis B is a dynamic process, monitoring of serum HBeAg, HBV-DNA, ALT, and liver fibrosis level is required in most cases but even after a complete assessment, some subjects fall into an indeterminate area and management needs to be individualized. Our data include a single assessment, and we do not have follow-up data. For the refinement of the data, we plan to follow-up on these cases and conduct an in-depth virologic examination.
In conclusion, this is the first study about the clinical and viral characteristics of patients with chronic HBV infection in Georgia. We found that the vast majority (96%) of patients with chronic HBV infection were HBeAg-negative. The majority of them had no or mild fibrosis (84%) and only a small number of patients (3%) had advanced liver fibrosis/cirrhosis. Half of the patients (51%) had inactive diseases (HBV infection, without hepatitis), while approximately 1 out of 10 patients (9%) had active disease (chronic hepatitis), which urgently needs antiviral treatment. However, one out of four (24%) had a viral load >2000 IU/ml but normal ALT. With these data, we can estimate the proportion of patients with chronic HBV monoinfection, who needs antiviral treatment (12–36%). Further studies are needed for more precise estimates and to identify the reasons for the high proportion of HBeAg-negative patients with high viral load and normal ALT. This information will help the stakeholders to plan the budget for the HBV elimination program in the country.
Credit authorship contribution statement
MZ, GK, and MB lead the study; Data were contributed by MZ, JZ, and DM and gathered under the supervision of MB and GK. Data analyses were contributed by TA and MB. All authors contributed to the interpretation of the results and writing the report, and approved the final version.
Conflicts of interest
The authors have none to declare.
Funding
The authors and the research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.World Health Organization Global progress report on HIV, viral hepatitis and sexually transmitted infections. 2021. https://www.who.int/publications/i/item/9789240027077external icon Available at:
- 2.Lozano R., Naghavi M., Foreman K., et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schweitzer A., Horn J., Mikolajczyk R.T., et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 4.Interim Guidance for Country Validation of Viral Hepatitis Elimination (who.Int) [DOI] [PubMed]
- 5.Global health sector strategy on viral hepatitis 2016-2021. https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf WHO.
- 6.Kasradze A., Shadaker S., Kuchuloria T., et al. The burden and epidemiology of hepatitis B and hepatitis D in Georgia: findings from the national seroprevalence survey. Publ Health. 2020 Aug;185:341–347. doi: 10.1016/j.puhe.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67 doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeger C., Mason W.S. Molecular biology of hepatitis B virus infection. Virology. 2015;479–480:672–686. doi: 10.1016/j.virol.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papatheodoridis G.V., Manesis E.K., Manolakopoulos S., et al. Is there a meaningful serum HBV DNA cut-off level for therapeutic decisions in HBeAg-negative chronic hepatitis B virus infection? Hepatology. 2008;48:1451–1459. doi: 10.1002/hep.22518. [DOI] [PubMed] [Google Scholar]
- 11.Kumar M., Sarin S.K., Hissar S., et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008 May;134:1376–1384. doi: 10.1053/j.gastro.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 12.Papatheodoridis G.V., Manolakopoulos S., Liaw Y.-F., Lok A. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: a systematic review. J Hepatol. 2012;57:196–202. doi: 10.1016/j.jhep.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Chen J.D., Yang H.I., Iloeje U.H., et al. Risk evaluation of viral load elevation and associated liver disease/cancer in HBV (REVEAL-HBV) study group. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010 May;138:1747–1754. doi: 10.1053/j.gastro.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 14.Choi G.H., Kim G.A., Choi J., et al. High risk of clinical events in untreated HBeAg-negative chronic hepatitis B patients with high viral load and no significant ALT elevation. Aliment Pharmacol Ther. 2019 Jul;50:215–226. doi: 10.1111/apt.15311. [DOI] [PubMed] [Google Scholar]
- 15.Kumada T., Toyoda H., Kiriyama S., et al. Incidence of hepatocellular carcinoma in patients with chronic hepatitis B virus infection who have normal alanine aminotransferase values. J Med Virol. 2010 Apr;82:539–545. doi: 10.1002/jmv.21686. [DOI] [PubMed] [Google Scholar]