Abstract
The European Association for the Study of the Liver (EASL) has recently (June 2022) produced new clinical practice guidelines for the investigation and management of haemochromatosis, to replace the previous document published in 2010. Here, we provide an overview of the principal changes recommended for the investigation and management of haemochromatosis arising from these guidelines and highlight particular areas where evidence is lacking and where future focus on specific research would improve patient treatment and outcomes. The guideline provides several important new recommendations that will have a meaningful impact on patient management. Specifically, the use of hepatic elastography as a non-invasive assessment of fibrosis, erythrocytapheresis as an alternative treatment modality to classical phlebotomy, surveillance for hepatocellular carcinoma, dietary recommendations in patients with haemochromatosis and guidance on controversial topics including the management of P.C282Y/p.H63D compound heterozygotes, which have been a source of controversy within the field. It is anticipated that the new guidance will affect the management of haemochromatosis patients commonly seen in gastroenterology, liver and related clinics (e.g. haematology and rheumatology) and with this publication we intend to highlight these changes so as to empower clinicians with the confidence to bring these improvements to their translational practice in the treatment of these patients.
Keywords: haemochromatosis, liver disease, guideline review, EASL
Haemochromatosis is a genetic disorder of iron homeostasis that is inherited in an autosomal recessive pattern. It manifests as deposition of excess iron in body organs, primarily the liver, heart, brain, joints and pancreas. Haemochromatosis is one of the most common inherited genetic conditions in individuals of European descent, but particularly in those of Irish and Celtic ancestry.1 The penetrance is variable and dependent on age, sex and environmental factors (e.g. alcohol, diet) as well as genetic modifiers.
Key advances have been made to our understanding of haemochromatosis in the 12 years since the publication of the previous EASL guidance.2 Improvement in technology behind genetic testing has aided identification of genetic modifiers through genome-wide association studies as well as single gene sequencing. Alongside large scale data from biorepositories such as UK Biobank, this has allowed increased understanding of disease penetrance. Use of this data for the creation of polygenic risk scores may influence future screening, monitoring and treatment of those with at risk genotypes.3
If untreated, haemochromatosis can result in significant mortality and morbidity (e.g. liver cirrhosis, diabetes, arthropathy, hypogonadism and heart failure). The treatment for this condition has remained relatively unchanged since it was first described in 1865 with phlebotomy first trialled in 1947.4 Whilst there are widely accepted treatments, robust, head-to-head, randomised-controlled trials are still required to inform the most appropriate approaches to treatment of the disease.
There is also a significant economic impact from the disease: in the UK, according to a 2022 report released by Haemochromatosis UK, the National Health Service incurs an estimated £487 million in excess costs per year associated with haemochromatosis, much of which is spent on preventable conditions associated with the disease.5
A new clinical practice guideline (CPG)6 has been produced by the European association for the Study of Liver (EASL June 2022) to replace the previous guidance on haemochromatosis published in 2010.2
These guidelines were developed by a panel of experts in the field who were recruited by the EASL governing body. It follows the standard method for creating international CPGs according to the international network guidelines. Evidence and recommendations were scored according to the Oxford Centre for Evidence-Based Medicine (OCEBM) levels of evidence working group system. The Delphi process was used for patient, intervention, comparison and outcomes questions (PICO) and draft recommendation statements. The Delphi process is a well-established technique which is used to answer questions by determining a consensus across a number of experts. This is performed by having ‘rounds’ where each expert is anonymously asked for their opinion regarding the current issue. This system allows opinion on each question to be distilled down to a group consensus by collectively sharing views on the topic. In this case, there were 9 experts on the CPG panel who created the guideline questions and a further 12 expert members on the Delphi panel. The evidence base in specific areas remains limited, reflected by low strength of recommendations and divided expert opinion on the Delphi panel on these points.
We summarise here the key changes since the 2010 guidelines, and the recent understanding and approach to haemochromatosis that the new EASL work promulgates (Table 1). Whilst the guideline is broad in its scope we provide a concise review and its translational relevance but advise clinicians to refer to the original 2022 guideline for a definitive overview.
Table 1.
Summary of Key Changes in New Haemochromatosis EASL Clinical Practice Guidelines.5
| Key changes in updated guidelines |
|---|
| Genetic testing for H63D is no longer recommended |
| Hepcidin concentrations should not be used routinely as a diagnostic tool |
| Liver biopsy is no longer recommended to diagnose hepatic iron overload |
| Lack of evidence to use transferrin saturations to guide monitoring |
| Transient elastography result of <6.4 kPa can rule out presence of advanced fibrosis |
| Increased awareness of non-HFE haemochromatosis |
| HCC screening should include patients with bridging fibrosis, METAVIR F3 (weak evidence) and Ishak stage 4–5 |
| Erthrocytapheresis is an acceptable alternate treatment to phlebotomy where available |
| Target ferritin for induction phase is 50 μg/L, maintenance phase 50–100 μg/L |
| Updated specific guidance for haemochromatosis in pregnancy |
HCC, Hepatocellular carcinoma; HFE, Haemochromatosis; kPa, Kilopascal; METAVIR, meta-analysis of histological data in viral hepatitis.
Diagnosis
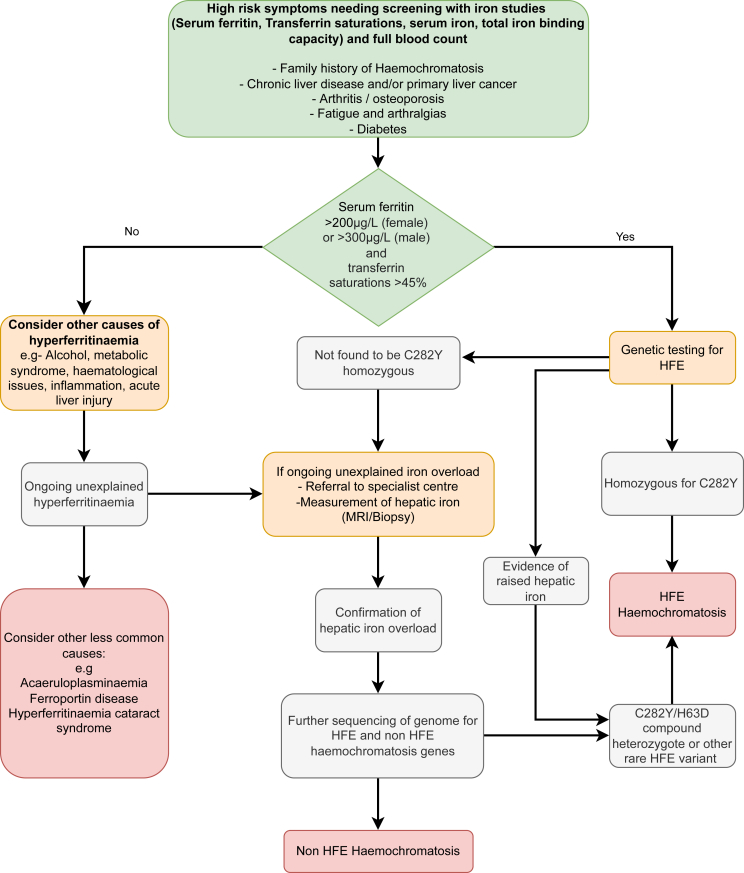
The key recommendations in the EASL guidance for the diagnostic approach to patients with suspected haemochromatosis are summarised in Figure 1.
Figure 1.
Diagnostic pathway of haemochromatosis.4
The diagnosis of haemochromatosis is generally unchanged from the 2010 guidelines.2 Initial testing should include serum ferritin and transferrin saturation. Total iron binding capacity and serum concentrations of transferrin and iron may provide further information for the differential diagnoses. Individuals with elevated serum ferritin concentration (SF > 200 μg/L in females, >300 μg/L in males) and transferrin saturation (TS - females >45%, males >50%), as well as those with unexplained persistently elevated transferrin saturation should be genetically tested for haemochromatosis. All adult first-degree relatives of patients with haemochromatosis, particularly those who are P.C282Y homozygous, should be consented for genetic testing.
It is recommended that hepcidin concentration should not be used routinely in the diagnosis of haemochromatosis. The elevated TS, which results from iron overload leads to inappropriately low hepcidin. Whilst hepcidin can help with differential diagnosis, it has limited diagnostic utility,6 since ranges are assay dependent and there is still no established universal reference range.6
Genetics
Given the multiple confounders in the interpretation of serum iron markers (such as excessive alcohol consumption, inflammation and metabolic syndrome), genetics has an important role in the diagnostic work up for haemochromatosis. HFE C282Y (P.Cys282Tyr) is the main genetic cause of haemochromatosis, however, the influence of other iron overload genes is increasingly recognised. Homozygosity for HFE C282Y is present in >80% of patients with clinically overt haemochromatosis and testing for this genotype remains standard practice in individuals of European descent who have iron indices in an abnormal range (as above).
The diagnostic significance of HFE H63D (P.His63Asp) is controversial and this is underlined by the lack of consensus among the expert panel in this area. The CPG statement concludes that H63D is no longer considered a disease causing variant and that affected patients should be treated according to their phenotypic presentation i.e biochemical, radiological or biopsy evidence of iron overload, rather than genotype alone.6 In patients with iron overload and H63D homozygous or compound heterozygous C282Y/H63D genotypes, environmental aetiology and other genetic risk factors, should be considered. It has therefore been suggested to stop routine testing for H63D with the rationale that it will help prevent confusion.
The management of HFE H63D haemochromatosis is equally controversial and the benefits of phlebotomy for this group remain unclear; compound heterozygous C282Y/H63D individuals represent a significant portion of patients referred for phlebotomy, estimated at 14%–30%.7 Although it has not been assessed in randomised clinical trials, phlebotomy remains the recommended treatment in patients who are compound heterozygous C282Y/H63D or homozygous H63D and have proven iron overload (assessed via MRI R2/T2 or liver biopsy).
In cases without C282Y, and where there is evidence of iron overload, other genetic causes should be considered. Sequencing using gene panels is increasingly accessible and finding mutations can reduce uncertainty and improve family screening. Ethnicity should be reviewed, as loss-of-function HFE variants are a significant genetic cause of haemochromatosis in non-Europeans. Minimum gene panel coverage should include: rare HFE mutation, caeruloplasmin (CP), bone morphogenetic protein (BMP6) and solute carrier family 40 member 1 (SLC40A1) as well as the genes associated with early onset of symptoms, HAMP and haemojuvelin (HJV) and those associated with abnormal transferrin, transferrin receptor 2 (TFR2) and transferrin (TF), and the hyperferritin-cataract syndrome gene (FTL). Deep sequencing studies in haemochromatosis patients, such as whole exome sequencing (WES), continue to identify new candidate genes and are also revealing evidence for partially penetrant mutations; further studies are still required to investigate the relative contribution of these in different populations.8
Management
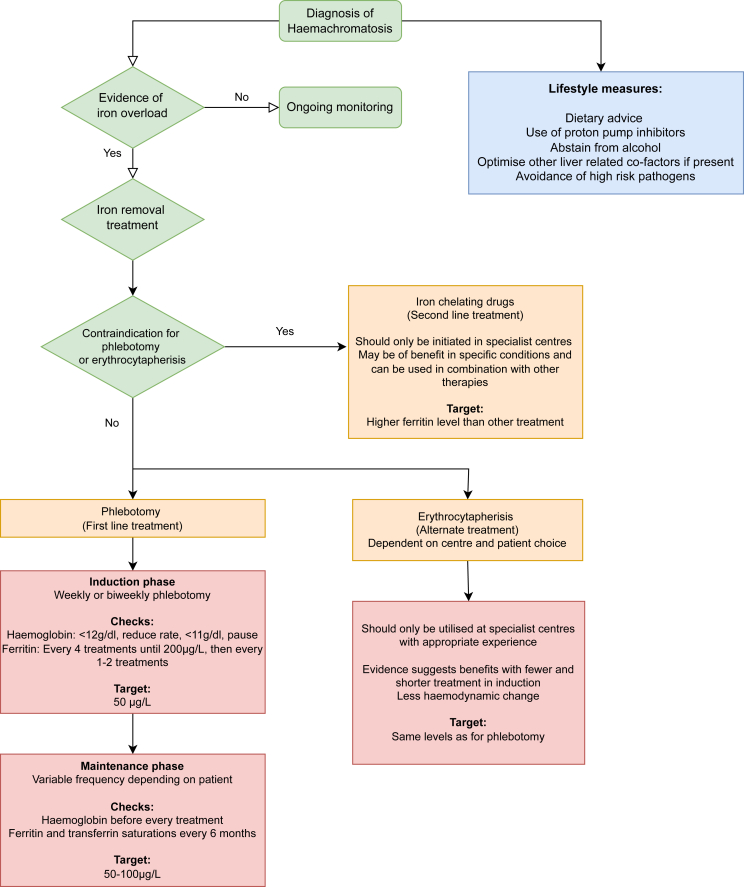
The treatment for haemochromatosis is iron depletion and consists of induction and maintenance phases.6,9 Phlebotomy remains the first line treatment for both phases but erythrocytapheresis has been recommended in the new guidance as a recognised alternative in the induction phase in treatment centres, where it is available along with the required expertise. This technique can also be personalised based on sex, weight, blood volume and haematocrit level. It has been shown that fewer procedures are required to attain the target ferritin, making it cost effective in the maintenance phase. Moreover, it has been associated with reduced fatigue.10,11 Erythrocytapheresis may also be more suitable for some groups of patients such as those with cardiac disease, due to reduced haemodynamic change compared with phlebotomy, and those with thrombocytopenia or hypoproteinaemia due to other blood components being returned to the circulation.12(Figure 2)
Figure 2.
Management pathway of haemochromatosis.4
If neither of these options are feasible or contra indicated (i.e. inaccessible veins, anaemia, life threatening cardiac iron overload), then second line treatment is possible in the form of iron chelators.6 The licensed medication available is deferoxamine, delivered by subcutaneous infusion. However, there is also off-label access to deferasirox and deferiprone, which are both orally administered. The medication with the most treatment evidence in haemochromatosis is deferasirox, which did show a significant reduction in ferritin levels at a tolerated level of 10 mg/kg.13
The use of proton pump inhibitors (PPIs) to reduce non-heme iron absorption can be used as an adjunct therapy and has been shown to reduce the frequency of phlebotomy in the maintenance phase.14
EASL CPG in 2010 advised a serum ferritin (SF) target of <50 μg/L whilst, for example, the most recent UK Haematology national guideline15 advises a target SF of 20–30 μg/L and transferrin saturation <50%. The new CPG takes into account varying global treatment guidelines and advises that to avoid iron deficiency and poor tolerability, a target SF of 50 μg/L, but not lower, is recommended for the induction phase. It also recommends a maintenance phase target SF of 50–100 μg/L, but with some degree of flexibility since a lower SF could be poorly tolerated in elderly populations. The expert panel noted that more relaxed maintenance target ranges of <200 μg/L for women and <300 μg/L for men could be applied; however, these values have not been evaluated in clinical trials. Transferrin saturation is not recommended in monitoring iron overload during treatment, since it has been shown that levels can remain raised even after ferritin has reached the target range.
Hepatic fibrosis is one of the main determinants of morbidity in haemochromatosis and assessment of the presence and degree of fibrosis is important, particularly in guiding the commencement of hepatocellular carcinoma (HCC) surveillance.6 Liver biopsy is only recommended where the presence or absence of cirrhosis is unclear and in patients with SF > 1000 μg/L or abnormal liver enzymes. The current CPG places more emphasis on the role of non-invasive investigations in determining the presence and degree of hepatic fibrosis. It notes a paucity of studies validating diagnostic performance of transient elastography (TE) and serum-based fibrosis scores; it recommends that all patients should be non-invasively investigated for the presence of liver fibrosis at diagnosis, to guide appropriate treatment and follow-up. With a low threshold, TE has a high negative predictive value in diagnosing advanced fibrosis: it is recommended that TE can rule out advanced fibrosis, if the liver stiffness measurement is ≤ 6.4 kPa. Serum-based fibrosis scores and biomarkers have also been evaluated for the diagnosis of advanced fibrosis in haemochromatosis. Of these, FIB-4 (patient age, alanine transferase, aspartate transferase and platelet count) was felt to be the most appropriate; however, it is accepted that the supporting evidence for its use is limited. As with TE, the threshold score for predicting fibrosis in haemochromatosis patients appears to be lower than in those with other forms of liver disease.16
There are important extrahepatic manifestations of haemachromatosis, which should be evaluated in all patients (skeletal, endocrine, reproductive or sexual dysfunction). Skeletal manifestations are particularly common with joint pain or arthritis reported by 86.5% of respondents to a recent survey of haemochromatosis patients in the UK.17 In cases of severe iron overload, cardiac issues are a common finding, and the recommendation is that screening is carried out by electrocardiogram and echocardiography. If cardiac involvement is suspected, this should not delay treatment and should be investigated with cardiac MRI to quantify cardiac iron levels. Cardiac MRI should also be conducted in all patients with juvenile haemochromatosis due to the high prevalence of cardiac involvement in this form of the disease.18,19
HCC is a recognised complication of haemochromatosis.6 Recognised risk factors for HCC in haemochromatosis patients include gender (male), higher age at diagnosis, diabetes mellitus, high iron overload and duration of exposure to iron excess.20,21 As with previous guidelines, six monthly screening with liver ultrasound and serum alpha-fetoprotein for HCC is recommended for all cirrhotic patients who are eligible for cancer treatment. The current CPG goes further to recommend screening those with advanced fibrosis (Bridging fibrosis, METAVIR F3, Ishak stage 4–5). This includes those with pre-treatment fibrosis which has regressed to F2 fibrosis or less, though the risk of developing HCC in this group is reduced.22 This is based on EASL guidance for HCC surveillance which states that all patients with METAVIR F3 fibrosis should be considered for HCC surveillance regardless of aeitiology and on reports of HCC in fibrotic but non-cirrhotic haemochromatotic liver.23,24 However, as the CPG notes, almost all patients with haemochromatosis who are diagnosed with HCC are cirrhotic and the recommendation for screening those with F3 fibrosis is weak.
Regarding management of haemochromatosis in women who are pregnant or planning to conceive, the CPG notes that iron deficiency results in adverse foetal outcomes and should be avoided.25 Pregnancy removes 0.5–1 g of iron26 and in patients with mild to moderate iron overload without signs of advanced liver disease, phlebotomy can be paused for the duration of pregnancy in most cases. Where therapeutic phlebotomy continues, its intensity should be reduced with a target SF ≥ 45 μg/L which is the cut off value for iron deficiency anaemia recommended by the American Gastroenterological Association.27 The CPG also recommends that all pregnant haemochromatosis patients should be assessed for advanced fibrosis and cirrhosis as these are risk factors for poor foetal and maternal outcomes.
Dietary advice is little changed from previous guidance but is more explicit in recommending avoidance of vitamin C and iron supplementation, limitation on eating red meat and restricted alcohol intake with complete abstinence advised in patients with cirrhosis. Patients with haemochromatosis and iron overload are at risk of Vibrio vulnificus infection through handling or consumption of contaminated seafood, and severe infection is more common in this group where fulminant sepsis has a mortality rate of 50%.28 In patients with normal SF and TS, the risk of contracting the infection and of severe disease is minimal.
The EASL CPG offers significant updates for best practice and attempts to clarify areas of controversy but is hampered by a paucity of robust evidence in some areas. Of 43 recommendations, only seven were graded 1 or 2 based on the Oxford 2011 Levels of Evidence and 15 recommendations were made on the basis of expert opinion. The lack of consensus among the expert panel highlights the need for robust randomised controlled trials to clarify points of controversy and unmet need. This includes the impact and significance of iron depletion on HCC risk, validation of non-invasive methods for determining hepatic fibrosis and assessment of more relaxed treatment targets in both induction and maintenance phases of iron depletion against the current recommendations. Additionally, improved screening technologies and availabilities need to be actively considered, as there are newly diagnosed patients who present with end stage disease.
Genetic results and their direct implications need to be actively considered, particularly in those who are not homozygous for C282Y, since the diagnosis of non-classical haemochromatosis is likely much more prevalent than previously appreciated. It is notable that the current CPG has moved away from the term ‘HFE haemochromatosis’ and uses the term ‘haemochromatosis’ as a phenotypic description of iron overload. While this simplifies nomenclature and recognises non-HFE genes, environmental and genetic modifiers, it remains controversial.
Identification of those genetic modifiers and the development of polygenic risk scores are enhancing our understanding of ‘at risk’ genotypes and may lead to improved screening, diagnosis and personalised treatment. Additionally, drugs currently in development, such as hepcidin mimetics, may prompt a move away from more invasive and labour intensive treatments. These developments may feature prominently in future CPGs.
Credit authorship contribution statement
James Liu Yin: Conceptualization, 2nd draft, review and editing, software, references, figures and table. Christopher Cussen: Conceptualization, 1st draft, review and editing, references. Christopher Harrington: Conceptualization, review and editing. Pierre Foskett: Conceptualization, review and editing. Kishor Raja: Conceptualization, review and editing. Aftab Ala: Initial Conceptualization, review and editing, project supervision and administration.
Conflicts of interest
The authors have no conflicting interests in relation to this manuscript.
Acknowledgements
Acknowledgements of grant support: N/A.
Funding
No funding was involved to support this work.
Disclosure of financial agreements
Not applicable.
References
- 1.Porto G., Brissot P., Swinkels D.W., et al. EMQN best practice guidelines for the molecular genetic diagnosis of hereditary hemochromatosis (HH) Eur J Hum Genet. 2016;24:479–495. doi: 10.1038/ejhg.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol [Internet] 2010;53:3–22. doi: 10.1016/j.jhep.2010.03.001. Available from: [DOI] [PubMed] [Google Scholar]
- 3.Atkins J.L., Pilling L.C., Torti S.V., Torti F.M., Kuchel G.A., Melzer D. Hereditary hemochromatosis variant associations with incident nonliver malignancies: 11-year follow-up in UK Biobank. Cancer Epidemiol biomarkers Prev. 2022 Sep;31:1780–1787. doi: 10.1158/1055-9965.EPI-22-0284. a Publ Am Assoc Cancer Res cosponsored by Am Soc Prev Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Čuljak M. Hemochromatosis treatment by venipuncture through history. Southeast Eur Med J. 2018;2:44–48. [Google Scholar]
- 5.York Health Economics Consortium & Haemochromatosis UK . 2022. Report : Evaluating the Cost of Illness of Genetic Haemochromatosis in the UK.https://www.haemochromatosis.org.uk/shop/report-evaluating-the-cost-of-illness-of-genetic-haemochromatosis [Internet] Available from: [Google Scholar]
- 6.Zoller H., Schaefer B., Vanclooster A., et al. EASL clinical practice guidelines on haemochromatosis. J Hepatol. 2022;1–24 doi: 10.1016/j.jhep.2022.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Bentley P., Bell B., Olynyk J. Therapeutic venesection at the Australian red cross blood Service: impact of the high ferritin application on management of hereditary haemochromatosis. Aust Fam Physician. 2015 Aug;44:589–592. [PubMed] [Google Scholar]
- 8.Rametta R., Dongiovanni P., Baselli G.A., et al. Impact of natural neuromedin-B receptor variants on iron metabolism. Am J Hematol [Internet] 2020;95:167–177. doi: 10.1002/ajh.25679. http://europepmc.org/abstract/MED/31724192 Available from: [DOI] [PubMed] [Google Scholar]
- 9.Brissot P. Optimizing the diagnosis and the treatment of iron overload diseases. Expet Rev Gastroenterol Hepatol. 2016;10:359–370. doi: 10.1586/17474124.2016.1119043. [DOI] [PubMed] [Google Scholar]
- 10.Rombout-Sestrienkova E., Winkens B., Essers B.A.B., et al. Erythrocytapheresis versus phlebotomy in the maintenance treatment of HFE hemochromatosis patients: results from a randomized crossover trial. Transfusion. 2016;56:261–270. doi: 10.1111/trf.13328. [DOI] [PubMed] [Google Scholar]
- 11.Rombout-Sestrienkova E., Nieman F.H.M., Essers B.A.B., et al. Erythrocytapheresis versus phlebotomy in the initial treatment of HFE hemochromatosis patients: results from a randomized trial. Transfusion [Internet] 2012 Mar 1;52:470–477. doi: 10.1111/j.1537-2995.2011.03292.x. Available from: [DOI] [PubMed] [Google Scholar]
- 12.Rombout-Sestrienkova E., van Kraaij M.G.J., Koek G.H. How we manage patients with hereditary haemochromatosis. Br J Haematol. 2016 Dec;175:759–770. doi: 10.1111/bjh.14376. [DOI] [PubMed] [Google Scholar]
- 13.Cançado R., Melo M.R., de Moraes Bastos R., et al. Deferasirox in patients with iron overload secondary to hereditary hemochromatosis: results of a 1-yr Phase 2 study. Eur J Haematol. 2015;95:545–550. doi: 10.1111/ejh.12530. [DOI] [PubMed] [Google Scholar]
- 14.Vanclooster A., van Deursen C., Jaspers R., Cassiman D., Koek G. Proton pump inhibitors decrease phlebotomy need in HFE hemochromatosis: double-blind randomized placebo-controlled trial. Gastroenterology. 2017 Sep;153:678–680.e2. doi: 10.1053/j.gastro.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Fitzsimons E.J., Cullis J.O., Thomas D.W., Tsochatzis E., Griffiths W.J.H. Diagnosis and therapy of genetic haemochromatosis (review and 2017 update) Br J Haematol. 2018;181:293–303. doi: 10.1111/bjh.15164. [DOI] [PubMed] [Google Scholar]
- 16.Chin J., Powell L.W., Ramm L.E., Hartel G.F., Olynyk J.K., Ramm G.A. Utility of serum biomarker indices for staging of hepatic fibrosis before and after venesection in patients with hemochromatosis caused by variants in HFE. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2021 Jul;19:1459–1468.e5. doi: 10.1016/j.cgh.2020.07.052. [DOI] [PubMed] [Google Scholar]
- 17.Smith K., Fife-Schaw C., Dibb B.G.W. Haemochromatosis UK; 2018. Living with the Impact of Iron Overload: Report from a Large Survey of People with Haemochromatosis. [Google Scholar]
- 18.De Gobbi M., Roetto A., Piperno A., et al. Natural history of juvenile haemochromatosis. Br J Haematol. 2002 Jun;117:973–979. doi: 10.1046/j.1365-2141.2002.03509.x. [DOI] [PubMed] [Google Scholar]
- 19.Piperno A., Bertola F.B.A. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2022. PMID: 20301349. Adam M.P., Everman D.B., Mirzaa G.M., et al., editors. GeneReviews®; 2005. Juvenile Hemochromatosis. [Google Scholar]
- 20.Deugnier Y.M., Guyader D., Crantock L., et al. Primary liver cancer in genetic hemochromatosis: a clinical, pathological, and pathogenetic study of 54 cases. Gastroenterology. 1993 Jan;104:228–234. doi: 10.1016/0016-5085(93)90856-8. [DOI] [PubMed] [Google Scholar]
- 21.Nowak A., Giger R.S., Krayenbuehl P.-A. Higher age at diagnosis of hemochromatosis is the strongest predictor of the occurrence of hepatocellular carcinoma in the Swiss hemochromatosis cohort: a prospective longitudinal observational study. Medicine (Baltim) 2018 Oct;97 doi: 10.1097/MD.0000000000012886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardou-Jacquet E., Morandeau E., Anderson G.J., et al. Regression of fibrosis stage with treatment reduces long-term risk of liver cancer in patients with hemochromatosis caused by mutation in HFE. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2020 Jul;18:1851–1857. doi: 10.1016/j.cgh.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Goh J., Callagy G., McEntee G., O'Keane J.C., Bomford A., Crowe J. Hepatocellular carcinoma arising in the absence of cirrhosis in genetic haemochromatosis: three case reports and review of literature. Eur J Gastroenterol Hepatol. 1999 Aug;11:915–919. doi: 10.1097/00042737-199908000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Galle P.R., Forner A., Llovet J.M., et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol [Internet] 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. Available from: [DOI] [PubMed] [Google Scholar]
- 25.Kemppinen L., Mattila M., Ekholm E., et al. Gestational iron deficiency anemia is associated with preterm birth, fetal growth restriction, and postpartum infections. J Perinat Med. 2021 May;49:431–438. doi: 10.1515/jpm-2020-0379. [DOI] [PubMed] [Google Scholar]
- 26.Milman N. Iron in pregnancy: how do we secure an appropriate iron status in the mother and child? Ann Nutr Metab. 2011;59:50–54. doi: 10.1159/000332129. [DOI] [PubMed] [Google Scholar]
- 27.Ko C.W., Siddique S.M., Patel A., et al. AGA clinical practice guidelines on the gastrointestinal evaluation of iron deficiency anemia. Gastroenterology [Internet] 2020;159:1085–1094. doi: 10.1053/j.gastro.2020.06.046. Available from: [DOI] [PubMed] [Google Scholar]
- 28.Horseman M.A., Surani S. A comprehensive review of Vibrio vulnificus: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2011 Mar;15:e157–e166. doi: 10.1016/j.ijid.2010.11.003. [DOI] [PubMed] [Google Scholar]