Abstract
Objective
Early discharge puts neonates at risk of delayed detection of jaundice and resulting neurological injury. In these neonates, we can use cord bilirubin to make predictions. In this meta-analysis, we assessed the diagnostic accuracy of cord bilirubin in predicting the need for phototherapy (AAP-2004 or NICE-2010 charts).
Methods
We searched the databases of PubMed, Embase, Cochrane Library, Google Scholar, and Index Medicus for Southeast Asian Region. We included all observational studies that assessed the diagnostic accuracy of cord bilirubin. A bivariate model was used to pool the data in prespecified range of cord bilirubin levels (<1.5 mg/dl, 1.5–2.0 mg/dl, 2.0–2.5 mg/dl, 2.5–3.0 mg/dl, and >3.0 mg/dl). Data were pooled separately for studies including all neonates (no risk stratification), high-risk neonates (Rh and/or ABO incompatibility only), and low-risk neonates (excluded Rh and ABO incompatibility).
Results
Of the 1990 unique records, we studied 153 full texts and included 54 studies in the meta-analysis. For all the three groups of studies, the highest diagnostic odds ratio was noted for a cord bilirubin cut-off of 2.5–3.0 mg/dl (all neonates: 22.5, 95% CI: 21.1, 22.9; high-risk neonates: 75.5, 95% CI: 63, 85.7; low-risk neonates: 91.9; 95% CI: 64, 134.14). Using the same cut-off, the studies including all neonates without risk stratification had a pooled sensitivity of 0.31 (95% CI: 0.18, 0.47) and a pooled specificity of 0.98 (0.96, 0.99) in predicting the need for phototherapy. In studies on high-risk neonates, the pooled sensitivity was 0.8 (0.39, 0.96) and pooled specificity was 0.95 (0.78, 0.99). In studies on low-risk neonates, the pooled sensitivity was 0.74 (0.39, 0.93) and pooled specificity of 0.97 (0.91, 0.99). We noted significant heterogeneity and a high risk of bias in the index test's conduct.
Conclusion
A cord bilirubin cut-off of 2.5–3 mg/dl has good diagnostic accuracy in predicting the need for phototherapy in neonates.
Registration number
CRD42020196216.
Keywords: cord bilirubin, diagnostic accuracy, high-risk neonates, bivariate model
Graphical abstract
Neonatal jaundice is a common problem in the first week of life, occurring in about 60% of full-term and 80% of preterm neonates.1,2 The global incidence of severe neonatal jaundice varies from as high as 668 per 1,00,000 live births in Africa to as low as 4 per 1,00,000 live births in Europe.3 The risk factors for developing significant jaundice include blood group incompatibility, glucose-6-phosphate dehydrogenase deficiency, genetic polymorphisms in bilirubin metabolism, and postnatal infections. The diagnosis of jaundice is based on clinical examination and serum bilirubin assessment, done at various times, namely, in the hospital, at discharge, and during follow-up visits. The guidelines commonly used to initiate phototherapy are the American Academy of Pediatrics (AAP, 2004)4 and the National Institute for Health and Clinical Excellence (NICE, 2010) charts.5,6 Although several methods are used to predict the need for phototherapy at discharge,7 Bhutani hour-specific nomogram is commonly used worldwide.8
More than a third of the newborns delivered in low- and middle-income countries were discharged within 48 h of birth and half in the first 24 h.9 However, discharge within 24 h of birth is fraught with an increased chance of readmission and non-compliance to follow-up. Hyperbilirubinemia is the commonest reason for readmission following early discharge.10 With this background, predicting newborns at higher risk for developing significant hyperbilirubinemia before discharge is essential, especially in early discharges. Cord bilirubin measurement has the potential to fulfill this need for prediction. Bilirubin levels in cord blood reflect bilirubin synthesis and, indirectly, that of hemolysis. The advantage of cord blood collection is that it is a painless, easy, non-invasive blood collection method that can be clubbed with cord blood screening. Moreover, the results are ready in time to make an informed decision regarding early discharge. Cord bilirubin is used in Rh-negative pregnancies to decide the need for phototherapy and exchange transfusions.11,12
Although several studies have assessed the role of cord bilirubin in predicting the occurrence of significant hyperbilirubinemia, the predictive abilities varied widely, and the thresholds used/derived for prediction were also heterogeneous. So, in this systematic review and meta-analysis, we assessed the diagnostic accuracy of cord blood bilirubin levels in predicting the need for phototherapy in the neonatal period.
Methods
The protocol was registered in the PROSPERO database (registration number CRD42020196216) and is available at https://www.crd.york.ac.uk/PROSPERO/. We followed the Cochrane handbook for systematic reviews of interventions, and the preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagnostic test accuracy (DTA) reviews reporting guidelines. This study was exempted from approval from scientific and ethics committees and patient consent requirements. The differences between the protocol and the study are provided in the supplementary material.
We included all cross-sectional studies that evaluated the diagnostic accuracy of umbilical cord blood bilirubin in predicting significant neonatal jaundice. The guidelines for phototherapy provided by AAP, 2004,4 or NICE, 2010,5,6 were considered the reference standard.
We searched the databases of PubMed, Embase, Cochrane CENTRAL, and Google Scholar in July 2020, and the search was updated in August 2022. We performed a citation search and reviewed the eligible articles' references to identify further studies. For grey literature and unpublished data, we have searched Google and Index Medicus for Southeast Asian Region. The search strategy used is provided below:
-
i.
PubMed: (umbilical OR "cord blood" OR cord OR "placental blood") AND bilirubin
-
ii.
Embase: (umbilical:ti,ab,kw OR 'cord blood':ti,ab,kw OR cord:ti,ab,kw OR 'placental blood':ti,ab,kw) AND bilirubin:ti,ab,kw
-
iii.
Cochrane CENTRAL: (umbilical OR "cord blood" OR cord OR "placental blood") AND bilirubin: Title Abstract and Keywords
-
iv.
Google Scholar: allintitle: (cord bilirubin)
We included the studies when they provided data for constructing a 2 × 2 contingency table showing cross-classification of significant hyperbilirubinemia (defined as the need for phototherapy) and cord bilirubin levels. We included the studies that applied serum bilirubin values corresponding to that given in AAP 2004 or NICE 2010 charts to diagnose the need for phototherapy, even if published before 2004. We excluded conference abstracts and letters to the editor without original data, diagnostic accuracy studies where we could not construct a 2 × 2 contingency table, and where the reference standard was not as described.
One reviewer (RPA) performed the database search and removal of duplicates. Following this, screening of titles and abstracts was done by two reviewers (RP and EAR) in a blinded manner on all the individual studies by using Rayyan software.13 Any discrepancy was sorted out by mutual agreement. We consulted a third reviewer (SD or AK) if a mutual agreement could not be reached. One author (RPA or SD) extracted the data, including study characteristics, participant details, and diagnostic accuracy parameters (true positives, true negatives, false positives, and false negatives). For studies reporting multiple thresholds, the diagnostic accuracy parameters were recorded separately for each of these thresholds. Another reviewer (EAR) supervised the data extraction, and we resolved discrepancies by mutual discussion or involvement of the third reviewer (SD or AK).
Quality assessment was done by two reviewers (RPA and EAR) using the Quality Assessment of Diagnostic Accuracy Studies, version 2 (QUADAS-2) tool,14 customized for the study (as shown in online resource 1). We used pre-stated signaling questions to ascertain the risk of bias and applicability concerns for the four domains of participant selection, index test, reference standard, and flow and timing (online resource 2). Discrepancies were resolved by discussion or involving another reviewer (SD or AK).
Statistical Analysis
Forest plots for sensitivity and specificity with a 95% confidence interval were created using the software RevMan Version 5.4, The Cochrane Collaboration, 2020. We pooled the data to get summary estimates using Stata Statistical Software: Release 14. College Station, TX: StataCorp LP. StataCorp. 2013. We constructed the summary receiver operator characteristic (SROC) curves in RevMan by transferring data from Stata. A bivariate model with the command 'Xtmelogit' was used for pooling diagnostic accuracy of the studies in the prespecified narrow threshold strata (<1.5 mg/dl, 1.5–2.0 mg/dl, 2.0–2.5 mg/dl, 2.5–3.0 mg/dl, and >3.0 mg/dl). When a single study provided more than one dataset in a stratum, the higher cut-offs were included in the analysis. Data were pooled separately for studies including high-risk (Rh or ABO incompatibility only), low-risk (Rh or ABO incompatibility excluded), and all (no risk stratification) neonates. As the thresholds reported varied widely across the studies, we did not use a hierarchical summary ROC (HSROC) model.
We assessed the heterogeneity by examining the forest plots for the variability of sensitivity and specificity and the 95% confidence intervals across the studies. We planned subgroup analysis for gestational age (preterm versus late preterm versus full-term neonates) and study design (prospective versus retrospective studies). We planned a metaregression for the binary covariates of jaundice prevalence (<10% and >10%) and prespecification of thresholds (threshold prespecified and threshold not prespecified). We used Deeks' funnel plot to assess publication bias. To facilitate an easy understanding of the study findings, we applied the diagnostic accuracy parameters (sensitivity and specificity) to a hypothetical cohort of 1000 neonates with a jaundice prevalence of 10% using the GRADEpro Guideline Development Tool [Software]. McMaster University, 2020 (developed by Evidence Prime, Inc.). We registered the meta-analysis prospectively in the PROSPERO database with the registration number CRD42020196216. Differences between the protocol and study are provided in the online resource 2.
Results
The search yielded 1968 studies from electronic databases and 22 studies from grey literature. After excluding duplicates and non-English studies, we performed title and abstract screening for 1344 studies. One hundred and fifty-six full texts were studied, and 54 studies were eligible for inclusion in meta-analysis.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68 The PRISMA flow chart is shown in Figure 1. The details of excluded studies are provided in the supplementary material.
Figure 1.
PRISMA flow diagram.
The characteristics of the included studies (place of study, design, inclusion, and exclusion criteria, the risk groups, and the primary outcome) are shown in Table 1. Forty-three studies included exclusively term neonates,15,16,18, 19, 20,22,24,27, 28, 29, 30, 31, 32,34, 35, 36,38, 39, 40,42, 43, 44, 45, 46, 47,49,51, 52, 53, 54, 55, 56, 57, 58,60, 61, 62, 63, 64, 65, 66, 67, 68 10 studies also included late preterm neonates above 35 weeks,17,21,23,25,26,33,37,41,48,59 and 1 study did not specify the gestational age.50 The details of data extraction are shown in the supplementary material. While 32 studies evaluated a single diagnostic threshold, 22 studies had multiple thresholds, thus providing data for multiple 2 × 2 tables.18,20,21,23,24,28,33,37,38,40,42,44, 45, 46,48,50,51,57,58,60,62,67
Table 1.
Characteristics of the Included Studies.
| Author | Study design | Patient selection | Inclusion criteria | Exclusion criteria | Risk group | Primary outcome studied |
|---|---|---|---|---|---|---|
| Ahire,15 2016, India | Prospective | Consecutive | 37–42 weeks, any delivery mode | Newborns having risk factors for development of severe hyperbilirubinemia like jaundice observed in first 24 h, ABO and/or Rh incompatibility, cephalohematoma or significant bruising, significant co morbidities requiring NICU admission | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >15 mg/dl at 48 h) |
| Aktas,16 2018, Turkey | Prospective | Consecutive | >37 weeks | Known prenatal disease, severe bruising, hematoma, sepsis, Down's syndrome, congenital hypothyroidism, glucose-6-phosphate dehydrogenase deficiency, confirmed liver disease, congenital infections, and major congenital anomalies | No | Need for phototherapy as per the AAP charts |
| Alalfy,17 2018, Egypt | Prospective | Consecutive | >35 weeks, Apgar >7 at 5 min | Significant illness [sepsis, RDS (Respiratory distress syndrome), hypoxia that could aggravate hyperbilirubinemia.] | No | Occurrence of significant hyperbilirubinemia (serum bilirubin >17 mg/dl after 72 h) |
| Arora,18 2015, India | Prospective | Consecutive | >37 weeks, >2.5 kg | Sick newborns with respiratory distress, asphyxia, sepsis, major congenital anomalies, Rh incompatibility, cephalhematoma and G6PD deficiency | ABO setting only | Need for phototherapy as per the AAP charts |
| Bharath,19 2011, India | Prospective | Consecutive | >37 weeks | Jaundice on day 1, sick babies admitted to NICU, On drugs that effect bilirubin metabolism, Apgar <7 at 5 min, major congenital malformations, maternal GDM | No | Occurrence of significant hyperbilirubinemia (serum bilirubin >17 mg/dl after 72 h) |
| Bijari,20 2019, Iran | Prospective | Consecutive | >37 weeks, >2.5 kg | ABO and Rh incompatibility, major congenital malformations, G6PD deficiency, or birth asphyxia, NICU admitted babies due to severe illness or sepsis, cephalohematoma, prolonged rupture of membrane (more than 18 h) and bruising. | Low risk only | Need for phototherapy (started at bilirubin >15 mg/dl at 72 h) |
| Calkins,21 2015, USA | Retrospective | Case-control | ABO/Rh incompatibility | Confirmed liver disease, congenital infections, major congenital anomalies, or a positive maternal hepatitis B screen. | ABO and Rh setting | Need for phototherapy (2 mg/dl below the line in AAP charts is taken as threshold) |
| Carbonell,22 2007, Spain | Prospective | Consecutive | Healthy term neonates | Not mentioned | No | Occurrence of significant hyperbilirubinemia (serum bilirubin >17 mg/dl between days 3 and 4) |
| Castillo,23 2018, USA | Prospective | Consecutive | Admission to newborn nursery | Major congenital anomalies, congenital infections, liver disorders, and maternal history of hepatitis | ABO and Rh setting | Need for phototherapy (3 mg/dl below the line in AAP charts is taken as threshold) |
| Chary,24 2014, India | Prospective | Consecutive | Healthy term newborns | Preterm (<36 weeks), post term (>40 weeks), Rh incompatibility, ABO incompatibility, life threatening congenital anomalies, birth asphyxia and NICU admitted babies | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >5 mg/dl on day 1, >10 mg/dl on day 2, >17 mg/dl thereafter) |
| El Mashad,25 2019, Egypt | Prospective | Consecutive | >35 weeks | Significant illness (sepsis, respiratory distress syndrome, hypoxia, and infant of a diabetic mother) that could aggravate hyperbilirubinemia. | No | Occurrence of significant hyperbilirubinemia (serum bilirubin >17 mg/dl after 72 h) |
| El-Gendy,26 2013, Egypt | Prospective | Consecutive | All newborns of gestational age between 35 and 40 completed weeks | Small for gestational age and large for gestational age, any congenital malformation, respiratory distress, sepsis, newborns with a family history of glucose-6-phosphate dehydrogenase and newborns with suspected hemolytic disease of newborns (ABO and Rh incompatibility). | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >17 mg/dl at 5 days) |
| Elfarargy,27 2021, Egypt | Prospective | Case-control | >37 weeks, APGAR score >7 at 1 and 5 min of life | Neonatal sepsis, perinatal asphyxia, fetal distress or anemia, G6PD deficiency, liver or blood disease in the mother, maternal in- take of EPO, maternal history of diabetes or drug abuse, abruption or infarcts | No | Need for phototherapy (as per AAP recommendations) |
| Farhat,28 2013, Iran | Prospective | Consecutive | >37 weeks, >2.5 kg | Obvious malformations, asphyxia, ecchymosis, cephalohematoma, infants of diabetic mothers and infants with mothers who consumed oxytocin | No | Occurrence of significant hyperbilirubinemia (serum bilirubin >15 mg/dl) |
| Garg,29 2017, India | Prospective | Consecutive | Healthy term newborns | <2.5 kg, significant illness requiring NICU admission, major congenital malformations and Conjugated hyperbilirubinemia | No | Occurrence of significant hyperbilirubinemia (serum bilirubin >12 mg/dl at 24 h, >15 mg/dl at 48 h, and >17 mg/dl at 72 h) |
| Gupta,31 2016, India | Prospective | Consecutive | GA >37, B Wt >2500, Apgar >7 at 1 and 5 min | Preterm babies (GA < 37 weeks), Rh incompatibility, Neonatal sepsis, Instrumental delivery (forceps and vacuum), Birth asphyxia, Respiratory distress, Meconium stained amniotic fluid, and Neonatal jaundice within 24 h of life. | Low risk only | Need for phototherapy (serum bilirubin threshold of >17 mg/dl after 72 h) |
| Gupta N,30 2020, India | Prospective | Consecutive | >37 weeks, >2.5 kg | Development of jaundice in the first 24 h of life, ABO and Rh incompatibility, preterm, direct hyperbilirubinemia, cord blood bilirubin >4 mg/dl and co-morbidities requiring NICU admission (sepsis, asphyxia, respiratory distress etc) | Low risk only | Need for phototherapy (bilirubin >12 mg/dl between 24 and 48 h of age, serum bilirubin >15 mg/dl between 48 and 72 h of age or serum bilirubin >17 mg/dl after 72 h of age) |
| Habeeb,32 2011, India | Prospective | Consecutive | >37 weeks | Significant illness requiring NICU admission, Major congenital anomalies, ABO/Rh incompatibility | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >15 mg/dl at 72 h) |
| Hamdi,33 2012, Egypt | Prospective | Consecutive | >35 weeks | Major CMF, complications that can aggravate neonatal hyperbilirubinemia, conjugated jaundice | No | Need for phototherapy (as per AAP recommendations) |
| Hanasi,34 2022, India | Prospective | Consecutive | >37 weeks, born by LSCS, Mothers blood group of A+, B+ or AB+ | Pathological jaundice due to causes like ABO incompatibility, Rh incompatibility, G6PD deficiency etc, Other Significant illness requiring NICU admission, Major congenital malformations, Chronic maternal illness (like DM), Birth weight less than 2.5 kg, Any maternal complications like Preeclampsia, hypothyroidism etc | Low risk only | Occurrence of significant hyperbilirubinemia (>17 mg/dl at 72 h) |
| Haridas,35 2019, India | Prospective | Consecutive | >37 weeks, >2.5 kg | Newborns with significant illness requiring admission; major congenital malformations; blood group incompatibility. | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >17 mg/dl) |
| Huda,36 2021, India | Prospective | Consecutive | >38 weeks, >2.5 kg, APGAR score ≥7/10 at 1 min, hospital stay of 5 days, LSCS birth | Preterm babies, Rh incompatibility, ABO incompatibility, PROM for >18 h, neonatal sepsis, and respiratory distress | Low risk only | Occurrence of significant hyperbilirubinemia (bilirubin >17 mg/dl) |
| Ingale,37 2018, India | Prospective | Consecutive | >35 weeks, >2 kg | Any complication arising during the Hospital stay that could aggravate the hyperbilirubinemia, direct hyperbilirubinemia, cephalhematoma, prolong rupture of membrane, septicemia, bruising, major congenital malformations | No | Need for phototherapy (as per AAP recommendations) |
| Ipek,38 2011, Turkey | Prospective | Consecutive | >37 weeks, >2.5 kg | Severe bruising, hematoma, sepsis, Down syndrome, hypothyroidism, glucose-6-phosphate dehydrogenase deficiency. | No | Occurrence of significant hyperbilirubinemia (serum bilirubin >17 mg/dl at 72 h) |
| Janaki,39 2018, India | Prospective | Selective | >37 weeks, >2.5 kg | Preterm, asphyxiated, congenital anomalies, born to diabetic mother, delivered by instrumental delivery (vacuum/forceps), history of meconium stained amniotic fluid or at risk of sepsis (premature rupture of membranes>12 h) | ABO setting only | Need for phototherapy (as per AAP recommendations) |
| Jones,40 2017, UK | Retrospective | Consecutive | >37 weeks, data available | Not mentioned | No | Need for phototherapy (as per UK NICE recommendations) |
| Kardum,41 2020, Croatia | Prospective | Consecutive | ≥36 weeks admitted to the well-baby nursery and born to O and/or Rh-negative mothers | None | ABO/Rh setting only | Need for PT (as per NICE guidelines) |
| Kayalvizhi,42 2020, India | Prospective | Consecutive | >37 weeks, 2.5–4 kg, Apgar score >7 | Significant illness or of major congenital malformation; neonatal problems like sepsis, hypothyroidism, respiratory distress syndrome, trauma conditions like cephalhematoma | ABO/Rh setting only | Need for PT (bilirubin >15 mg/dl after 48 h) |
| Khairy,43 2018, Egypt | Prospective | Consecutive | All term neonates | Conditions that could aggravate hyperbilirubinemia (sepsis, respiratory distress syndrome, asphyxia, diabetic mothers, or intrauterine growth retar- dation) or cholestatic jaundice; NICU admission; Major CMF | No | Need for phototherapy (as per AAP recommendations) |
| Kumar,44 2016, India | Prospective | Random | >37 weeks, >2.5 kg, Apgar >7 | Birth asphyxia, sepsis, cephalhematoma, hypothyroidism, congenital malformations, RDS, Congenital infections, maternal GDM or PIH, Rh incompatibility | ABO setting only | Need for phototherapy (as per AAP recommendations) |
| Kumaran,45 2016, India | Prospective | Consecutive | Term, >2.5 kg, Apgar >7 @ 1 min | Sick babies admitted to newborn care unit (Except for phototherapy) including G6PD deficiency, Babies whose mother receiving drugs (AEDs, Antimalarials, sulfonamides), Prematurity, Major congenital anomalies, Birth asphyxia (Apgar <7 at 5 min). | No | Occurrence of significant hyperbilirubinemia (serum bilirubin >15 mg/dl after 72 h) |
| Menon,46 2016, India | Prospective | Selective | Full term, Born to O+ve mother, blood group A or B +ve, Apgar >7 at 1 min, 10 at 5 min | Preterm <37 weeks; Rh incompatibility; Instrumental delivery (forceps and vacuum); significant illness; major congenital malformations | ABO setting only | Need for phototherapy (as per AAP recommendations) |
| Meshram,47 2019, India | Prospective | Consecutive | >37 weeks, >2.5 kg, Apgar >7 at 1 min, 10 at 5 min | ABO/Rh incompatibility; Prematurity (gestational age <37 weeks); Birth trauma, Cephalhematoma, multiple bruises; Meconium stained amniotic fluid; Early onset sepsis; Major congenital malformations | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >10 mg/dl at 24 h or >17 mg/dl at 72 h) |
| Nahar,48 2009, Bangladesh | Prospective | Consecutive | >35 weeks, Apgar >7 at 5 min | Rh incompatibility, any congenital anomaly, babies requiring NICU admission or showing any complication that could aggravate the hyperbilirubinemia, e.g., sepsis | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >17 mg/dl after 72 h) |
| Panneerselvam,49 2018, India | Prospective | Selective | GA >37, B Wt >2500, Apgar >7 at 1 min, Mother BG O+ve, Baby BG A/B/AB+ve | Babies born to mothers with eclampsia, diabetes, and thyroid; and babies with cephalhematoma | ABO setting only | Need for phototherapy (as per AAP recommendations) |
| Peeters,50 2016, Belgium | Retrospective | Consecutive | Not specified | None | No | Occurrence of significant hyperbilirubinemia (serum bilirubin >95th centile on Bhutani chart) |
| Pengoria,51 2018, India | Prospective | Consecutive | Full term healthy neonates | Early onset hyperbilirubinemia (bilirubin ≥10 mg/dl before 24 h of age), maternal ABO incompatibility, G6PD deficiency, cephalohematoma, multiple congenital anomalies, maternal gestational diabetes mellitus, anemia, congenital hypothyroidism, sepsis, cholestasis, RDS and urinary tract infection | Low risk only | Need for phototherapy (as per AAP recommendations) |
| Rajpurohit,52 2015, India | Prospective | Consecutive | Full term healthy neonates | Not mentioned | No | Need for phototherapy (serum bilirubin >17 mg/dl after 72 h, as per AAP recommendations) |
| Rajput,53 2018, India | Prospective | Consecutive | >37 weeks | Significant morbidities that could aggravate hyperbilirubinemia, Rh incompatibility and APGAR score <7 at 1 min | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >15 mg/dl after 72 h) |
| Ramamoorthy,54 2018, India | Prospective | Consecutive | Healthy term newborns, >5 days hospital stay | Clinical jaundice on the first postnatal day; Sick babies or babies admitted to NICU; Babies receiving drugs that are known to affect serum bilirubin levels; More congenital anomalies; Maternal GDM; Pathological jaundice; Rh incompatibility. | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >15 mg/dl after 48 h) |
| Ramteke,55 2018, India | Prospective | Consecutive | >37 weeks, >2.5 kg | Complication arising during the hospital stay that could aggravate hyperbilirubinemia (e.g., neonatal sepsis, ABO, Rh incompatibility, instrumental/traumatic delivery, and birth asphyxia), neonates with antenatal risk factors (meconium stained liquor), gestational hypertension, premature rupture of membrane, preeclampsia, anemia, malaria, maternal fever, chorioamnionitis, any other maternal illness, twins, gross congenital malformations, and/or comorbid illness, and neonates who developed jaundice within 24 h of life. | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >17 mg/dl after 72 h) |
| Reddy JM,56 2021, India | Prospective | Consecutive | >37 weeks, >2000 g, Apgar >7 at 5 min | Newborns with significant illness requiring NICU admission like septicemia, meningitis, ARDS etc, major congenital malformations, and conjugated hyperbilirubinemia | No | Occurrence of significant hyperbilirubinemia (>14 mg/dl at 72 h) |
| Reena,57 2017, India | Prospective | Consecutive | >37 weeks | Major CMF, NICU admission, ABO/Rh incompatibility, maternal drugs interfering bilirubin metabolism, family history, maternal illness, GDM | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >15 mg/dl after 72 h) |
| Rehna,58 2021, India | Prospective | Consecutive | >37 weeks, >2000 g, Apgar >7 at 5 min | Preterm babies, ABO and Rh incompatibility, Apgar score <7 at first minute of life, instrumental delivery, neonates with sepsis, respiratory distress and major congenital anomalies | Low risk only | Need for PT (Bilirubin >13 mg/dl on day 2 or ≥17 mg/dl on day 3) |
| Sehgal,59 2017, India | Prospective | Consecutive | >34 weeks, >2000 g | Neonates with any significant illness (sepsis, respiratory distress syndrome, asphyxia, and infant of diabetic mother) that could aggravate hyperbilirubinemia, babies with Rh and ABO incompatibility were excluded. | Low risk only | Need for phototherapy (as per AAP recommendations) |
| Sharma IK,60 2020, India | Prospective | Consecutive | >37 weeks, >1800 g | Significant illnesses such as neonatal sepsis, birth asphyxia, respiratory distress syndrome, meconium aspiration syndrome, any who were critically ill or haemodynamically unstable | Low risk only | Need for phototherapy (as per AAP recommendations) |
| Shekhar,61 2019, India | Prospective | Consecutive | >37 weeks, healthy | Jaundice at birth or day 1 | No | Need for phototherapy (as per AAP recommendations) |
| Shettigar,62 2017, India | Prospective | Consecutive | Healthy term newborns | ABO/Rh incompatibility; Sick neonates requiring NICU admission; major congenital malformations; cephalohematoma; discharged prior to 72 h of birth. | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >17 mg/dl at 72 h) |
| Singh,63 2019, India | Prospective | Selective | GA >37, B Wt >2500 | Rh incompatibility, Significant illness requiring intensive care management for >12 h, requiring resuscitation (positive pressure ventilation) at birth, excessive weight loss (>10%), cephalohematoma, major congenital malformations, Infant of diabetic mother | ABO setting only | Occurrence of significant hyperbilirubinemia |
| Sundaram,64 2021, India | Prospective | Random | >37 weeks, 2.5–4 kg, Apgar >7 at birth | Prematurity, birth asphyxia, cephalohematoma, sepsis, hypothyroidism and congenital malformation, gestational diabetes. | ABO setting only | Occurrence of significant hyperbilirubinemia (bilirubin level >15 mg/dl on day 4 or any bilirubin level >95th percentile for the age in hours) |
| Taksande,65 2005, India | Prospective | Consecutive | >37 weeks | ABO/Rh incompatibility, G6PD deficiency, NICU admission, major CMF | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >17 mg/dl after 24 h) |
| Thakur,66 2017, India | Prospective | Consecutive | >37 weeks, >2.5 kg, Apgar >7 | Rh incompatibility, Neonatal sepsis, Instrumental delivery (forceps and vacuum), Birth asphyxia, Respiratory distress, Meconium stained amniotic fluid, and Neonatal jaundice within 24 Hours of life. | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >15 mg/dl after 72 h) |
| Vaishnav,67 2014, India | Prospective | Consecutive | exclusively breast fed, appropriate for gestational age newborns | evidences of hemolysis, Rh or blood group incompatibility, septicemia, birth asphyxia, birth injuries, congenital anomalies, metabolic abnormalities (hypothyroidism, hypoglycemia, hypo proteinemia, hypocalcemia or inborn error of metabolism) and those requiring admission and treatment in NICU for causes other than jaundice | Low risk only | Need for phototherapy (as per AAP recommendations) |
| Venkatamurthy,68 2014, India | Prospective | Consecutive | >37 weeks, >2.5 kg | Rh incompatibility, Neonatal sepsis, Instrumental delivery (forceps and vacuum), Birth asphyxia, Respiratory distress, Meconium stained amniotic fluid, and Neonatal jaundice within 24 Hours of life. | Low risk only | Occurrence of significant hyperbilirubinemia (serum bilirubin >17 mg/dl after 72 h) |
AAP, American Academy of Pediatrics; ABO, blood group; RDS, respiratory distress syndrome; NICU, neonatal intensive care unit; EPO, erythropoietin; GA, gestational age; CMF, congenital malformation; LSCS, lower section cesarean section; PT, preterm; GDM, gestational diabetes mellitus; PIH, pregnancy induced hypertension; ARDS, acute respiratory distress syndrome.
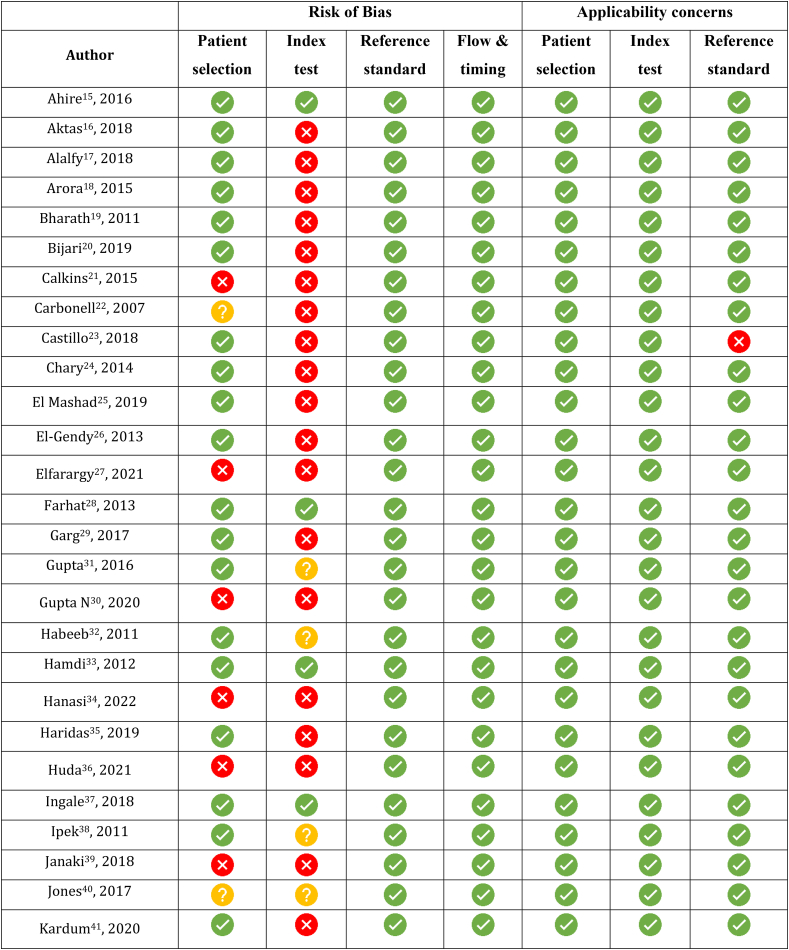
Quality assessment done using QUADAS-2 is shown in Figure 2. None of the studies had a risk of bias in reference standard, flow and timing of the participants, and applicability concerns in patient selection or use of index tests. Seven studies had a high risk of bias in patient selection because of a case–control design,21,27,39 or not including consecutive patients,64 or inappropriate exclusions.30,34,36 Three studies had an unclear risk of bias as exclusion criteria were not stated.22,40,52 Thirty-five studies had a high risk of bias with the index test as the thresholds were not prespecified,16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27,29,30,34, 35, 36,39,41, 42, 43, 44,46,48,49,51,52,54, 55, 56,59, 60, 61 and in nine studies, it was unclear.31,32,38,40,50,53,62,65,68 Two studies had applicability concerns related to the reference standard-one had high risk because of inappropriate application,23 and the other had unknown risk as it was not defined.61
Figure 2.
Quality assessment of the included studies using Quality Assessment for Diagnostic Accuracy Studies version 2 (QUADAS 2).
The summary estimates of diagnostic accuracy of cord bilirubin levels are shown in Table 2. As the cord bilirubin cut-offs increased, the sensitivity decreased, and specificity increased. The cord bilirubin cut-off of 2.5–3 mg/dl has the highest diagnostic odds ratio in all the three subgroups evaluated. At this cut-off, the pooled estimates of specificity were very high (0.95–0.98), while the pooled estimates for sensitivity were variable. On evaluating studies that included all neonates, pooled sensitivity was 0.31 (95% CI: 0.18, 0.47), while it was higher in studies on high-risk (0.8; 95% CI: 0.39, 0.96) and low-risk neonates (0.74; 95% CI: 0.39, 0.93). The post-test probabilities for normal and abnormal cord blood bilirubin levels at an estimated prevalence of significant hyperbilirubinemia of 10% are shown in Table 2. None of the studies assessed the diagnostic accuracy of cord blood fractionated (conjugated or unconjugated) or free bilirubin levels.
Table 2.
Pooled Sensitivity and Specificity of Cord Blood Bilirubin in Predicting the Need for Phototherapy.
| Risk group | Cord bilirubin cut-off | No of studies and no of participants | Sensitivity (95% confidence interval) | Specificity (95% confidence interval) | Diagnostic odds ratio (95% confidence interval) | Post-test probability for an abnormal resulta | Post-test probability for a normal resulta |
|---|---|---|---|---|---|---|---|
| All neonatesb | <1.5 mg/dl | 4; 13,070 | 0.96 (0.7–0.996) | 0.32 (0.13–0.59) | 11.3 (9.63–37.2) | 14% (13%–14%) | 1% (1%–4%) |
| All neonatesb | 1.51–2.0 mg/dl | 13; 15,005 | 0.8 (0.72–0.86) | 0.73 (0.65–0.79) | 10.8 (9.66–11.4) | 25% (22%–28%) | 3% (2%–4%) |
| All neonatesb | 2.01–2.5 mg/dl | 7; 2999 | 0.55 (0.34–0.75) | 0.89 (0.76–0.95) | 9.84 (9.8–9.92) | 36% (30%–42%) | 5% (4%–7%) |
| All neonatesb | 2.51–3.0 mg/dl | 5; 13,420 | 0.31 (0.18–0.47) | 0.98 (0.96–0.99) | 22.5 (21.1–22.9) | 62% (50%–75%) | 7% (6%–8%) |
| All neonatesb | >3.0 mg/dl | 4; 11,860 | 0.11 (0.03–0.34) | 0.999 (0.95–1) | 124 (31–∞) | 92% (59%–99%) | 9% (8%–10%) |
| High riskc | Below 1.5 mg/dl | 3; 1159 | 0.97 (0.83–1) | 0.29 (0.08–0.66) | 13.2 (9.48–∞) | 13% (13%–14%) | 1% (0–3%) |
| High riskc | 1.51–2.0 mg/dl | 8; 3091 | 0.9 (0.81–0.95) | 0.55 (0.33–0.75) | 10.98 (9.46–12.91) | 18% (17%–20%) | 2% (1%–4%) |
| High riskc | 2.01–2.5 mg/dl | 7; 1210 | 0.84 (0.67–0.93) | 0.92 (0.82–0.96) | 60.3 (48.8–60.9) | 55% (48%–59%) | 2% (1%–3%) |
| High riskc | 2.51–3.0 mg/dl | 4; 924 | 0.8 (0.39–0.96) | 0.95 (0.78–0.99) | 75.5 (63–85.7) | 64% (57%–71%) | 2% (2%–3%) |
| Low riskd | 1.51–2.0 mg/dl | 11; 3441 | 0.89 (0.77–0.95) | 0.77 (0.58–0.89) | 26.97 (26.24–27) | 30% (27%–33%) | 2% (1%–3%) |
| Low riskd | 2.01–2.5 mg/dl | 13; 3996 | 0.79 (0.64–0.88) | 0.89 (0.74–0.95) | 30.3 (20.94–33.58) | 44% (39%–50%) | 3% (2%–4%) |
| Low riskd | 2.51–3.0 mg/dl | 10; 2886 | 0.74 (0.39–0.93) | 0.97 (0.91–0.99) | 91.9 (64–134.14) | 74% (65%–80%) | 3% (2%–4%) |
The probabilities are calculated at a prevalence of 10%.
Studies including all neonates without stratifying their risk for jaundice.
Studies including only neonates at risk for blood group incompatibility.
Studies including only low risk neonates (excluded those with blood group incompatibility).
Significant heterogeneity was noted on assessing the forest plots (as shown in supplementary material). The SROC curves are shown in the supplementary material. Publication bias was not assessed as the number of included studies was low.
Discussion
Summary of Main Results
This meta-analysis aimed to evaluate the utility of cord-bilirubin in predicting significant hyperbilirubinemia requiring phototherapy. Of the various cut-offs evaluated, cord bilirubin levels above 2.5–3 mg/dl had better diagnostic accuracy. At this cut-off, the specificity (>95%) was higher than the sensitivity in all the three groups of neonates. As a result, if cord bilirubin levels were to be used, it is prudent to avoid early discharge and reevaluate neonates for jaundice when the bilirubin levels are above 2.5–3 mg/dl. On the other hand, when cord bilirubin is below 1.5 mg/dl, the neonate is unlikely to develop jaundice requiring phototherapy.
The probability of developing significant hyperbilirubinemia requiring phototherapy exceeds 50% at a cord bilirubin cut-off of >2 mg/dl in neonates with a setting for ABO or Rh incompatibility and at a cord bilirubin cut-off of >2.5 mg/dl in other neonates. Similarly, the probability of developing significant hyperbilirubinemia requiring phototherapy exceeds 25% in neonates without a setting for ABO or Rh incompatibility, at a cord bilirubin cut-off of >1.5 mg/dl.
Comparison with Postnatal Bilirubin Assessment
We compared the results of this meta-analysis with the review on the diagnostic utility of bilirubin nomograms.69 The 40th percentile of predischarge nomograms had a sensitivity of 95% (93%–96%), and the 95th centile had a specificity of 96% (95%–97%) for subsequent significant hyperbilirubinemia. These findings are like the diagnostic accuracy estimates for cord bilirubin obtained from this meta-analysis, i.e., a sensitivity of 96% and 97% in studies including all neonates, and high-risk neonates, respectively; and a specificity of 95%–98% in the three groups. However, it is essential to note that factors like placental clearance of bilirubin and maternal hyperbilirubinemia determine cord bilirubin levels, independent of increased bilirubin production in the neonate and are not truly representative of the baby's bilirubin metabolism. On the other hand, postnatal bilirubin assessment predominantly reflects postnatal bilirubin handling of the neonate.
Strengths and Limitations
The strengths of our study include a comprehensive search strategy, including a grey literature search. After deconstructing the data from every study for each of the thresholds specified, we pooled the data using the bivariate model for the narrow cut-off ranges. This approach helped us avoid the problem with the HSROC model, where we cannot specify the appropriate cut-off to be used for good diagnostic accuracy. The limitations included a high risk of bias in the index test and high heterogeneity in the studies.
Implications for Practice
The findings of this study indicate that a cord bilirubin level >2.5–3 mg/dl indicates that the neonate is at a high risk of developing jaundice requiring phototherapy. These findings may be utilized in settings where early discharge (within 24 h) of healthy newborns and lower compliance rates to timely follow-up is predominant (e.g., centers catering to a population from a rural background in LMICs). It is advisable to avoid early discharge when cord blood bilirubin levels exceed 2.5 mg/dl and review neonates with cord blood bilirubin levels between 2 and 2.5 mg/dl at 24 h of age and those with cord blood bilirubin levels between 1.5 and 2 mg/dl at 48 h of age. When cord bilirubin levels are below 1.5 mg/dl, the neonate can be reviewed at 72 h of age.
Implications for Research
Future studies can use a randomized control trial design to understand the downstream consequences of using cord blood bilirubin for predicting future significant hyperbilirubinemia. These include the incidence of bilirubin encephalopathy and mortality, duration of hospital stay, cost of care, breastfeeding rates, and other important neonatal outcomes.
Credit authorship contribution statement
Rajendra Prasad- conceptualised study, database search, title and abstract screening, full text screening, data extraction, analysis, manuscript preparation.
Emine A Rahiman- title and abstract screening, full text screening, data extraction, analysis, manuscript preparation.
Sankalp Dudeja- conceptualised study, full text screening, data extraction, manuscript preparation.
Ashutosh Kumar- full text screening, data extraction, manuscript preparation.
Conflicts of interest
The authors have none to declare.
Acknowledgements
None.
Funding
The study was not funded by any source.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2022.11.011.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Fig. S1.
References
- 1.Bhutani V.K., Stark A.R., Lazzeroni L.C., et al. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J Pediatr. 2013;162:477–482. doi: 10.1016/j.jpeds.2012.08.022. e1. [DOI] [PubMed] [Google Scholar]
- 2.Olusanya B.O., Kaplan M., Hansen T.W.R. Neonatal hyperbilirubinaemia: a global perspective. Lancet Child Adolesc Health. 2018;2:610–620. doi: 10.1016/S2352-4642(18)30139-1. [DOI] [PubMed] [Google Scholar]
- 3.Slusher T.M., Zamora T.G., Appiah D., et al. Burden of severe neonatal jaundice: a systematic review and meta-analysis. BMJ Paediatr Open. 2017;1 doi: 10.1136/bmjpo-2017-000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics Subcommittee on Hyperbilirubinemia Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 5.Amos R.C., Jacob H., Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98) Arch Dis Child Educ Pract Ed. 2017;102:207–209. doi: 10.1136/archdischild-2016-311556. [DOI] [PubMed] [Google Scholar]
- 6.Rennie J., Burman-Roy S., Murphy M.S., Guideline Development Group Neonatal jaundice: summary of NICE guidance. BMJ. 2010;340:c2409. doi: 10.1136/bmj.c2409. [DOI] [PubMed] [Google Scholar]
- 7.Kuzniewicz M.W., Park J., Niki H., Walsh E.M., McCulloch C.E., Newman T.B. Predicting the need for phototherapy after discharge. Pediatrics. 2021:147. doi: 10.1542/peds.2020-019778. [DOI] [PubMed] [Google Scholar]
- 8.Bhutani V.K., Johnson L., Sivieri E.M. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6–14. doi: 10.1542/peds.103.1.6. [DOI] [PubMed] [Google Scholar]
- 9.Kumar P., Dhillon P. Length of stay after childbirth in India: a comparative study of public and private health institutions. BMC Pregnancy Childbirth. 2020;20:181. doi: 10.1186/s12884-020-2839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta P., Malhotra S., Singh D.K., Dua T. Length of postnatal stay in healthy newborns and re-hospitalization following their early discharge. Indian J Pediatr. 2006;73:897–900. doi: 10.1007/BF02859282. [DOI] [PubMed] [Google Scholar]
- 11.Mishra S., Agarwal R., Deorari A.K., Paul V.K. 2007. Jaundice in Newborns- AIIMS NICU Protocols. [Google Scholar]
- 12.Newborn Exchange Transfusion- Neonatal Clinical Practice Guideline. 2015. [Google Scholar]
- 13.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiting P.F., Rutjes A.W.S., Westwood M.E., et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 15.Ahire N., Sonawane R., Gaikwad R., Patil S., Sonawane T. Study of correlation of cord blood bilirubin with neonatal hyperbilirubinemia. MVP J Med Sci. 2016;3:60–66. [Google Scholar]
- 16.Aktas S., Dogan C., Okmen Z.H., Gulec S.G. Is cord blood bilirubin level a reliable predictor for developing significant hyperbilirubinemia? Am J Perinatol. 2019;36:317–321. doi: 10.1055/s-0038-1667368. [DOI] [PubMed] [Google Scholar]
- 17.Alalfy M., Ezzeldin Z., Mansi Y. Role of bilirubin and albumin in cord blood as predictors for neonatal hyperbilirubinemia. J Gynecol Res. 2018;4:208. [Google Scholar]
- 18.Arora S., Amritsar S. Cord serum bilirubin level in predicting the development of significant hyperbilirubinemia in newborns with ABO incompatibility. J Nepal Paediatr Soc. 2015;35:231–236. [Google Scholar]
- 19.Bharath A.P. Rajiv Gandhi university of Health Sciences; Karnataka: 2011. Cord Blood Bilirubin Can Be Used as an Early Predictor of Neonatal Hyperbilirubinemia. PhD Thesis. [Google Scholar]
- 20.Bijari B.B., Jamali Z., Niknafs P., et al. Are cord blood bilirubin values trustable to predict the forthcoming significant hyperbilirubinemia requiring treatment in healthy term newborns? Iran J Pediatr. 2019;29 [Google Scholar]
- 21.Calkins K., Roy D., Molchan L., et al. Predictive value of cord blood bilirubin for hyperbilirubinemia in neonates at risk for maternal-fetal blood group incompatibility and hemolytic disease of the newborn. J Neonatal Perinat Med. 2015;8:243–250. doi: 10.3233/NPM-15814111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carbonell X., Botet F., Figueras J., Riu-Godó A. Prediction of hyperbilirubinaemia in the healthy term newborn. Acta Paediatr Oslo Nor 1992. 2001;90:166–170. doi: 10.1080/080352501300049343. [DOI] [PubMed] [Google Scholar]
- 23.Castillo A., Grogan T.R., Wegrzyn G.H., Ly K.V., Walker V.P., Calkins K.L. Umbilical cord blood bilirubins, gestational age, and maternal race predict neonatal hyperbilirubinemia. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chary E., Bharadwaj N., Kumar P., Vivekand N., Sailaja V., Harika B. Umbilical cord blood bilirubin level measurement in predicting the development of significant hyperbilirubinemia. Indian J Mednodent Allied Sci. 2014;2:144–148. [Google Scholar]
- 25.El Mashad G.M., El Sayed H.M., El Shafie W.A. Cord blood albumin–bilirubin as a predictor for neonatal hyperbilirubinemia. Menoufia Med J. 2019;32:1071–1077. [Google Scholar]
- 26.El-Ghendy F.M., Hassane F.M., Khattab A.A., El-Lahony D.M., Ashour N.M. Predictive ability of first-day serum bilirubin and haptoglobin for subsequent significant hyperbilirubinemia in healthy-term and near-term newborn. Menoufia Med J. 2013;26:127–131. [Google Scholar]
- 27.Elfarargy M.S., Al-Ashmawy G.M., Abu-Risha S., Khattab H. Study of cord blood levels of erythropoietin, bilirubin and reticulocyte count as early predictors of neonatal hyperbilirubinemia. Endocr Metab Immune Disord Drug Targets. 2021;21:1641–1648. doi: 10.2174/1871530321666201229152019. [DOI] [PubMed] [Google Scholar]
- 28.Farhat A., Alizadeh G.A., Mohamadzadeh A., Khodadadi A., Rezaei M. Does umbilical cord bilirubin level have predictive value in pathologic neonatal hyperbilirubinemia? Iran J Neonatol. 2013;4:32–35. [Google Scholar]
- 29.Garg A., Tiwari A.K., Narang S. Umbilical cord bilirubin-an early diagnostic marker of significant neonatal hyperbilirubinemia. J Med Sci Clin Res. 2017;5:20345–20349. [Google Scholar]
- 30.Gupta N., Taran S.J., Gupta S., Kishore Arora K. Role of cord blood albumin and bilirubin for prediction of significant neonatal jaundice. J Nepal Paediatr Soc. 2021;41:239–246. doi: 10.3126/jnps.v41i2.30383. [DOI] [Google Scholar]
- 31.Gupta S. Neonatal hyperbilirubinaemia after induction of labour with oxytocin and cord serum albumin is compared with cord serum bilirubin as a risk indicator. Int J Biomed Res. 2016;7:435–438. [Google Scholar]
- 32.Habeeb A.A. Rajiv Gandhi university of Health Sciences; Karnataka: 2011. Correlation of Cord Blood Bilirubin with Neonatal Hyperbilirubinemia. PhD Thesis. [Google Scholar]
- 33.Hamdi N., Elgayar A., Salah M. Cord blood bilirubin as a predictor of neonatal hyperbilirubinemia. Med J Cairo Univ. 2012;80:31–36. [Google Scholar]
- 34.Hanasi S., Pradeep N., Rudrappa S. Cord blood bilirubin level as a predictor of development of pathological hyperbilirubinemia in newborns. Int J Health Sci. April 8, 2022:2742–2752. doi: 10.53730/ijhs.v6nS2.5669. [DOI] [Google Scholar]
- 35.Haridas K., Shinde R., Belavadi G. Prediction of neonatal hyperbilirubinemia using umbilical cord blood bilirubin. Int J Contemp Pediatr. 2019;6:248–252. doi: 10.18203/2349-3291.ijcp20190676. [DOI] [Google Scholar]
- 36.Huda W.M., Sharma P., Aggarwa J., Agrawal A. A comparative study of cord blood bilirubin and albumin as a predictor for neonatal jaundice in term newborns. J Datta Meghe Inst Med Sci Univ. 2021;16:295–302. [Google Scholar]
- 37.Ingale S., Vaghela P., Patel P. The value of umbilical cord blood bilirubin measurement in predicting the development of significant hyperbilirubinemia in healthy newborn. Int J Med Health Res. 2018;4:69–72. [Google Scholar]
- 38.Ipek I.O., Bozaykut A., Çağrıl S.C., Sezer R.G. Does cord blood bilirubin level help the physician in the decision of early postnatal discharge? J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2012;25:1375–1378. doi: 10.3109/14767058.2011.636089. [DOI] [PubMed] [Google Scholar]
- 39.Janaki A.N. Dr MGR Medical University; 2018. Predictive Value of Umbilical Cord Blood Bilirubin and Albumin for Significant Hyperbilirubinemia in Abo Incompatibility. PhD Thesis. [Google Scholar]
- 40.Jones K.D.J., Grossman S.E., Kumaranayakam D., Rao A., Fegan G., Aladangady N. Umbilical cord bilirubin as a predictor of neonatal jaundice: a retrospective cohort study. BMC Pediatr. 2017;17:186. doi: 10.1186/s12887-017-0938-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kardum D., Serdarušić I., Biljan B., Šantić K., Živković V., Kos M. Cord blood bilirubin and prediction of neonatal hyperbilirubinemia and perinatal infection in newborns at risk of hemolysis. J Pediatr (Rio J). 2021;97:440–444. doi: 10.1016/j.jped.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kayalvizhi S. Dr MGR Medical University; 2020. Cord Blood Bilirubin as a Predictive Marker of Neonatal Hyperbilirubinemia in ABO and Rh Incompatible Babies- A Prospective Study. [Google Scholar]
- 43.Khairy M.A., Abuelhamd W.A., Elhawary I.M., Mahmoud Nabayel A.S. Early predictors of neonatal hyperbilirubinemia in full term newborn. Pediatr Neonatol. 2019;60:285–290. doi: 10.1016/j.pedneo.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Kumar A.T., Sangeeta A., Someshwaran R., Anbu N.A. Diagnostic utility of cord blood bilirubin in early detection of neonatal hyperbilirubinemia among ABO incompatibility cases from a tertiary care medical college hospital. Int J Curr Med Appl Sci. 2016;13:22–27. [Google Scholar]
- 45.Kumaran U., Arya A.K., Rakholia R. Study to predict newborn at risk of developing neonatal hyperbilirubinaemia by measuring cord blood bilirubin. J Evol Med Dent Sci. 2016;5:1676–1683. [Google Scholar]
- 46.Menon M., Sreejyothi G., Raveendranath K. Incidence of early neonatal hyperbilirubinemia in ABO incompatibility and cord bilirubin as a predictor for phototherapy. Pediatr Rev Int J Pediatr Res. 2016;3:221–227. [Google Scholar]
- 47.Meshram R.M., Merchant S., Bhongade S.D., Pathan S.N. Utility of cord blood bilirubin as a predictor of significant neonatal hyperbilirubinemia in the healthy term neonate. Int J Contemp Pediatr. 2019;6:2058–2063. [Google Scholar]
- 48.Nahar Z., Shahidullah M.D., Mannan A., Dey S.K., Mitra U., Selimuzzaman S.M. The value of umbilical cord blood bilirubin measurement in predicting the development of significant hyperbilirubinemia in healthy newborn. Bangladesh J Child Health. 2009;33:50–54. [Google Scholar]
- 49.Panneerselvam K., Mani S., Ramraj B., Pasupathy S., Munusamy M., Sundar S. Predicting pathological jaundice in term babies with ABO setting using cord blood bilirubin. Indian J Child Health. 2018;5:686–688. [Google Scholar]
- 50.Peeters B., Geerts I., Van Mullem M., Micalessi I., Saegeman V., Moerman J. Post-test probability for neonatal hyperbilirubinemia based on umbilical cord blood bilirubin, direct antiglobulin test, and ABO compatibility results. Eur J Pediatr. 2016;175:651–657. doi: 10.1007/s00431-016-2690-1. [DOI] [PubMed] [Google Scholar]
- 51.Pengoria R., Agarwal M. Early markers for the prediction of hyperbilirubinemia in term neonates. J Med Sci Clin Res. 2018;6:623–626. doi: 10.18535/jmscr/v6i11.108. [DOI] [Google Scholar]
- 52.Rajpurohit N., Kumar S., Sharma D., Choudhary M., Purohit S. To assess predictive value of cord blood bilirubin and albumin for significant neonatal hyperbilirubinemia: a prospective study from India. J Pediatr Neonatal Care. 2015;2:60–65. [Google Scholar]
- 53.Rajput G., Dhanawade S. Cord bilirubin as a Predictor of Neonatal Hyper bilirubinemia in healthy term babies. Pediatr Rev Int J Pediatr Res. 2018;5:243–248. [Google Scholar]
- 54.Ramamoorthy K., Abhilash M. Cord blood bilirubin used as an early predictor of hyperbilirubinemia. Int J Contemp Pediatr. 2018;5:1280–1285. doi: 10.18203/2349-3291.ijcp20182075. [DOI] [Google Scholar]
- 55.Ramteke S., Shrivastav J., Agrawal A., Mishra N.R., Saravanan A.T., Tikkas R. Comparison of cord bilirubin and bilirubin albumin ratio to predict significant hyperbilirubinemia in healthy full-term neonates. Indian J Child Health. 2018;5:108–111. [Google Scholar]
- 56.Reddy J.M., Umesh J. To study the predictive value of umbilical cord blood bilirubin levels term neonates as marker of neonatal hyperbilirubinemia. Int J Res Rev. 2021;8:18–23. doi: 10.52403/ijrr.20210603. [DOI] [Google Scholar]
- 57.Reena C.M. Kilpauk Medical College; Chennai: 2017. A Study on Sensitivity of Cord Blood Bilirubin Level in Predicting Neonatal Hyperbilirubinemia. PhD Thesis. [Google Scholar]
- 58.Rehna T., Shiyas K. Comparison of umbilical cord blood bilirubin (UCB) and bilirubin albumin ratio (BAR) in predicting neonatal hyperbilirubinemia: a prospective observational study. Indian J Neonatal Med Res. 2021 doi: 10.7860/IJNMR/2021/52782.2313. [DOI] [Google Scholar]
- 59.Sehgal P., Wasim S., Chandar V., et al. Cord bilirubin levels as a predictive marker for neonatal hyperbilirubinemia: a prospective study. Indian J Child Health. 2017;4:571–574. [Google Scholar]
- 60.Sharma I., Kumar D., Singh A., Mahmood T. Ratio of cord blood bilirubin and albumin as predictors of neonatal hyperbilirubinaemia. Clin Exp Hepatol. 2020;6:384–388. doi: 10.5114/ceh.2020.102170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shekhar M., Kumar V., Bhalke S., Goel A. Umbilical cord albumin and serum bilirubin as predictive factors for hyperbilirubinemia in term neonates. Int J Contemp Pediatr. 2020;7:267–272. doi: 10.18203/2349-3291.ijcp20200012. [DOI] [Google Scholar]
- 62.Shettigar C., Shettigar S., Sanjee S. Umbilical cord bilirubin level as a predictor of subsequent neonatal hyperbilirubinemia in term healthy newborns. J Evid Based Med Healthc. 2017;4:5750–5753. doi: 10.18410/jebmh/2017/1157. [DOI] [Google Scholar]
- 63.Singh R., Jain H. Prediction of significant hyperbilirubinemia by estimating cord blood bilirubin in neonates with ABO incompatibility. Int J Contemp Pediatr. 2019;6:670–675. [Google Scholar]
- 64.Sundaram C.S. Cord bilirubin value as a predictor of significant hyperbilirubinemia in Abo incompatiblity. J Pharm Res Int. December 15, 2021:256–262. doi: 10.9734/jpri/2021/v33i58B34201. [DOI] [Google Scholar]
- 65.Taksande A., Vilhekar K., Jain M., Zade P., Atkari S., Verkey S. Prediction of the development of neonatal hyperbilirubinemia by increased umbilical cord blood bilirubin. Ind Medica. 2005;9:5–9. [Google Scholar]
- 66.Thakur P., Mangashetty R.B., Pawar S.D. Cord blood albumin and cord blood bilirubin in early detection of neonatal hyperbilirubinemia. Int J Sci Res. 2017;6:1327–1329. [Google Scholar]
- 67.Vaishnav D., Ghosh G., Choudhuri T., Bandyopadhyay D. Predictive value of umbilical cord serum bilirubin for postnatal hyperbilirubinemia in term healthy newborns. Child Newborn. 2014;18:5. [Google Scholar]
- 68.Venkatamurthy M., Murali S.M., Mamatha S. A comparison study: cord serum albumin is compared with cord serum bilirubin as a risk indicator in predicting neonatal jaundice. J Evol Med Dent Sci. 2014;3:4017–4023. [Google Scholar]
- 69.Yu Z.B., Han S.P., Chen C. Bilirubin nomograms for identification of neonatal hyperbilirubinemia in healthy term and late-preterm infants: a systematic review and meta-analysis. World J Pediatr. 2014;10:211–218. doi: 10.1007/s12519-014-0495-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.