Abstract
Introduction
Inertial measurement units (IMUs) may be viable options to collect gait data in clinics. This study compared IMU to motion capture data in individuals who use unilateral lower-limb prostheses.
Methods
Participants walked with lower-body IMUs and reflective markers in a motion analysis space. Sagittal plane hip, knee, and ankle waveforms were extracted for the entire gait cycle. Discrete points of peak flexion, peak extension, and range of motion were extracted from the waveforms. Stance times were also extracted to assess the IMU software’s accuracy at detecting gait events. IMU and motion capture-derived data were compared using absolute differences and root mean square error (RMSE).
Results
Five individuals (n = 3 transtibial; n = 2 transfemoral) participated. IMU prosthetic limb data was similar to motion capture (RMSE: waveform ≤4.65°; discrete point ≤9.04°; stance ≤0.03s). However, one transfemoral participant had larger differences at the microprocessor knee joint (RMSE: waveform ≤15.64°; discrete ≤29.21°) from IMU magnetometer interference. Intact limbs tended to have minimal differences between IMU and motion capture data (RMSE: waveform ≤6.33°; discrete ≤9.87°; stance ≤0.04s).
Conclusion
Findings from this pilot study suggest IMUs have the potential to collect data similar to motion capture systems in sagittal plane kinematics and stance time.
Keywords: Amputee, validation, kinematic, prosthesis, biomechanics
Introduction
Motion capture equipment is considered the gold standard for collecting gait data, but is impractical to use in clinical practice for several reasons: high costs, lack of portability, and the need for specialized personnel. 1 Clinicians, such as prosthetists and physical therapists, evaluate prosthetic alignment and gait deviations using visual observation. Therefore, the patient’s quality of rehabilitation can be subject to factors such as clinician experience, time allotted for the appointment, and patient fatigue. Existing low-cost and portable wearable sensors called inertial measurement units (IMUs) could provide an objective method for practical real-time clinical measurements of gait, such as stance time or sagittal plane asymmetry between intact and prosthetic limbs, that could inform rehabilitation recommendations in individuals who use lower-limb prostheses. 2 For instance, targeting specific muscle groups for strengthening, gait trainings, or prosthetic adjustments. However, IMUs must first be compared to motion capture equipment to determine their validity in prosthetic limbs.
Numerous studies have validated IMUs against motion capture systems in healthy individuals, 3 with a clinical threshold of 5 degrees of error over gait cycle waveforms4,5 and up to 15° in peak flexion-extension sagittal plane data.6,7,8,9,10,11,12,13 Several studies have also validated IMUs against motion capture systems in individuals with various lower-limb pathologies, such as stroke, 14 Parkinson’s Disease, 15 and knee osteoarthritis. 16 However, there is still a need to assess the ability of IMUs to adequately capture motion given additional considerations specific to this population. For instance, the influence of prosthetic technology (e.g. microprocessor knees) has not been determined. It is possible that prosthetic componentry could interfere with IMU componentry, such as magnetometers, which are known to have interference issues when near metal that produce heading errors. Determining the ability of IMUs to collect prosthetic limb data with or without interference could help inform the integration of IMUs into clinical practice.
Further, as wearable sensors become more feasible to use in research and clinic, there is a need to determine how IMU data compares to previous studies that used motion capture data. In individuals with unilateral lower-limb loss, recent studies have used IMUs to measure symmetry and repeatability,17,18 coronal plane kinematics, 19 pose estimation, 20 sensory feedback, 21 spatiotemporal parameters (e.g. stance time), 22 walking speed in daily life, 23 and compare IMU algorithms to detect gait events.24,25,26,27 However, no literature in individuals who use lower-limb prostheses has directly compared IMU-derived data to motion capture, aside from a 2014 case study in a transfemoral user that found IMU-derived knee and ankle flexion data was within 4 degrees of motion capture data. 28
Lower-limb kinematic (e.g. joint angle) and stance time data from motion capture have been associated with adverse clinical events, such as falls, in individuals who use lower-limb prostheses. 29 Improving symmetry between the prosthetic and intact limb in these parameters is typically considered a primary goal in rehabilitation, as more symmetry is viewed as a reduction in gait deviations. However, these gait parameters rely on motion capture equipment to calculate, 30 which require a large unobstructed space, specialized expertise to run, analyze, and maintain, and is cost prohibitive. These barriers make motion capture systems infeasible for use in clinics. Commercially available IMUs have the ability to detect gait events, yet no research has directly compared these data to motion capture. The ability to quantify lower-limb sagittal plane kinematics and stance time (e.g. asymmetry between intact and prosthetic limbs) of prosthesis users in clinical practice could aid prosthetists and physical therapists with decisions regarding prosthetic alignment, targeted rehabilitation exercises, and potentially insurance justification.
Therefore, IMU-derived kinematic and temporal data were compared to motion capture in individuals who use unilateral lower-limb prostheses. Based on a recent meta-analysis in healthy controls, 3 we hypothesized IMU data on the prosthetic limb, compared to motion capture, would show: 1) full gait cycle waveform root mean square errors at the hip, knee, and ankle ≤5.0°, 2) peak parameters at the hip, knee, and ankle ≤15.0°, and 3) stance durations ≤0.02 s (s). Findings could highlight considerations of using IMUs with lower-limb prosthetic devices and comparing IMU to motion capture-derived data in this population.
Methods
Participants
This study was approved under the North Texas Regional Institutional Review Board (#2020-048). Individuals who used unilateral transtibial or transfemoral prostheses were recruited from prosthetic clinics in the Dallas-Fort Worth metroplex to complete a single visit at the University of North Texas Health Science Center. Individuals were included if they: were between the ages of 18 and 95 years, walking with a prosthesis for at least 1 year, and able to walk independently for at least 5 minutes or 100 yards. Individuals were excluded if they had: pain, open wounds or discomfort on their lower-limbs or trunk on the day of testing, limb loss or deficiency on other limbs, major musculoskeletal injury or surgery in the last year besides amputation or revision, or other comorbidities that would make standing, turning, or walking unsafe. All participants provided written informed consent to voluntarily participate in this study, and written permission to use data and images.
Equipment
Seven IMU sensors (iSen, STT Systems, San Sebastian, Spain) were used in this study to collect and store lower-body IMU data within iSen software (v2022.0). The iSen IMU system was selected due to its low cost option in comparison to other more prominent IMU systems, yet still provided similar specifications in components and accuracy. Each IMU (46 g) had a tri-axial accelerometer (±16°g), gyroscope (±2000°/s), and magnetometer (±13G) with company-reported pitch/roll accuracy within 0.5° and heading accuracy within 2.0°. Calibration prior to each walking trial was performed according to iSen instructions, (i.e. standing straight, facing forward, neutral posture). A 14-camera motion capture system (Cortex, Motion Analysis Corp, Santa Rosa, CA) was used to collect and store data from 32 reflective markers using a modified full-body Helen-Hayes marker set. Calibration prior to each participant’s data collection was performed, according to Cortex instructions, with a wand, participant static, and participant dynamic collection. Potential sources of error from using IMU and motion capture equipment generally include movement artifacts between equipment and clothing or skin, as well as environmental factors, such as magnetic interference. All equipment was placed by the same individual (first author, MGF) and all data collections were conducted in the same room. All data was collected at a sampling rate of 100 Hz.
Data collection
Demographic data were collected and managed using Research Electronic Data Capture (REDCap) software hosted at the University of North Texas Health Science Center.31,32,33 Participants also completed the following clinical outcome measures: Socket Comfort Score (SCS), Prosthesis Limb Users Survey of Mobility 12-Item Short Form (PLUS-M), and Amputee Mobility Predictor with Prosthesis (AMPPRO). After demographic and clinical outcome measure data was collected, researchers placed IMUs and reflective markers on participants as shown in Figure 1. Participants wore fitted clothing with their shirt tucked into their shorts or pants.
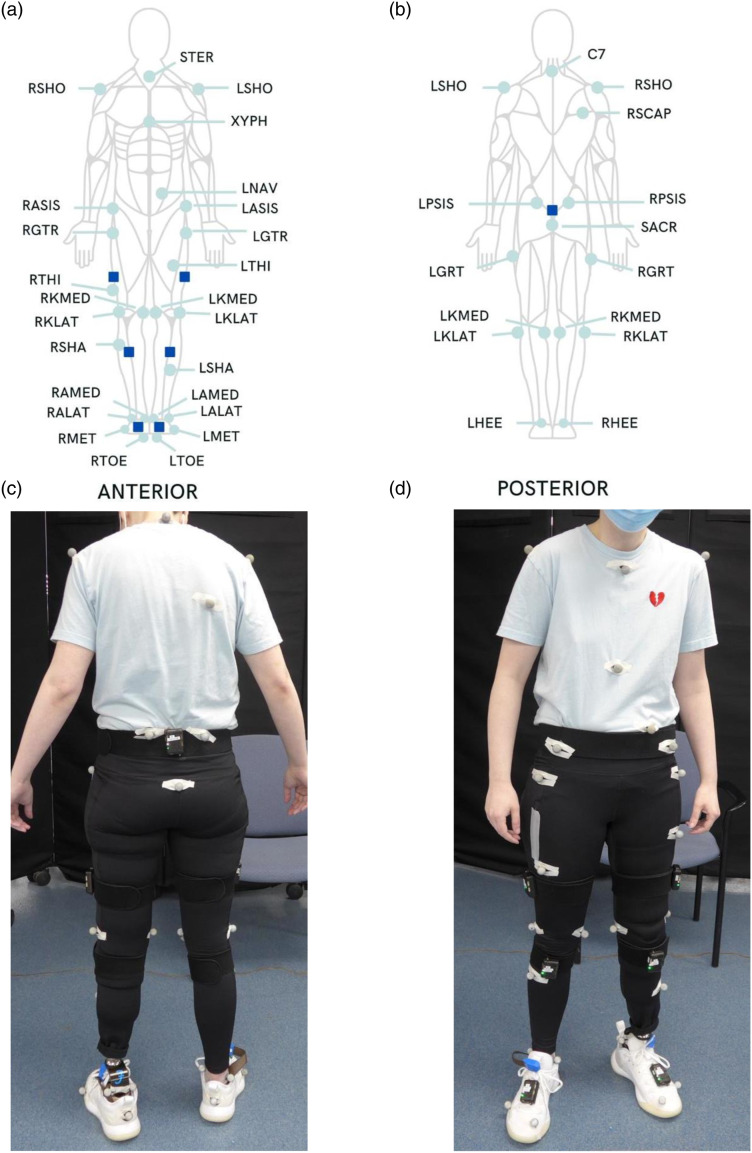
Figure 1.
Equipment placement. Panels A and B depict the location of reflective markers (mint green circles) and IMUs (dark blue squares) on from anterior and posterior views. Panels C and D depict locations of reflective markers and IMUs on a participant. Abbreviations: STER: sternum; RSHO: right shoulder; LSHO: left shoulder; XYPH: xyphoid, LNAV: left offset navel; RASIS: right anterior superior iliac spine; LASIS: left anterior superior iliac spine; RGTR: right greater trochanter; LGTR: left greater trochanter; RTHI: right thigh; LTHI: left thigh; RKMED: right knee medial; LKMED: left knee medial; RKLAT: right knee lateral; LKLAT: left knee lateral; RSHA: right shank; LSHA: left shank; RAMED: right ankle medial; LAMED: left ankle medial; RALAT: right ankle lateral; LALAT: left ankle lateral; RMET: right base of fifth metatarsal; LMET: left base of fifth metatarsal; RTOE: right toe; LTOE: left toe; C7: seventh cervical vertebrae; RSCAP: right scapula; RPSIS: right posterior superior iliac spine; LPSIS: left posterior superior iliac spine; SACR: sacrum; RHEE: right heel; LHEEL: left heel.
IMUs were secured to each participant as recommended by STT Systems for the lower-body model: one on the sacrum, one on each thigh, one on each shank, and one on each foot. IMUs were secured to body segments with elastic straps provided by STT Systems, excluding foot IMUs, which were secured directly to the dorsal aspect of the shoe with velcro. Additionally, due to the conical shape of transfemoral prosthetic sockets, the thigh IMU was secured directly to the transfemoral prosthesis using self-adherent wrap (Coban 1580 Series, 3M™, Saint Paul, MN) to prevent slippage. IMUs and reflective markers on the prosthetic limb were matched to the placement of the intact limb. In a similar manner to the intact limb, the prosthetic knee was bent to visualize a central pivot point for transtibial participants, then compared to the intact limb marker placement for accuracy. The ankle joint marker and knee joint marker for transfemoral participants for the prosthetic limb was placed at the same height as the intact limb and aligned medially. Participants walked across 6 m of level ground at their self-selected habitual walking speed for five trials, with 30 s rest periods between each trial.
Data analysis
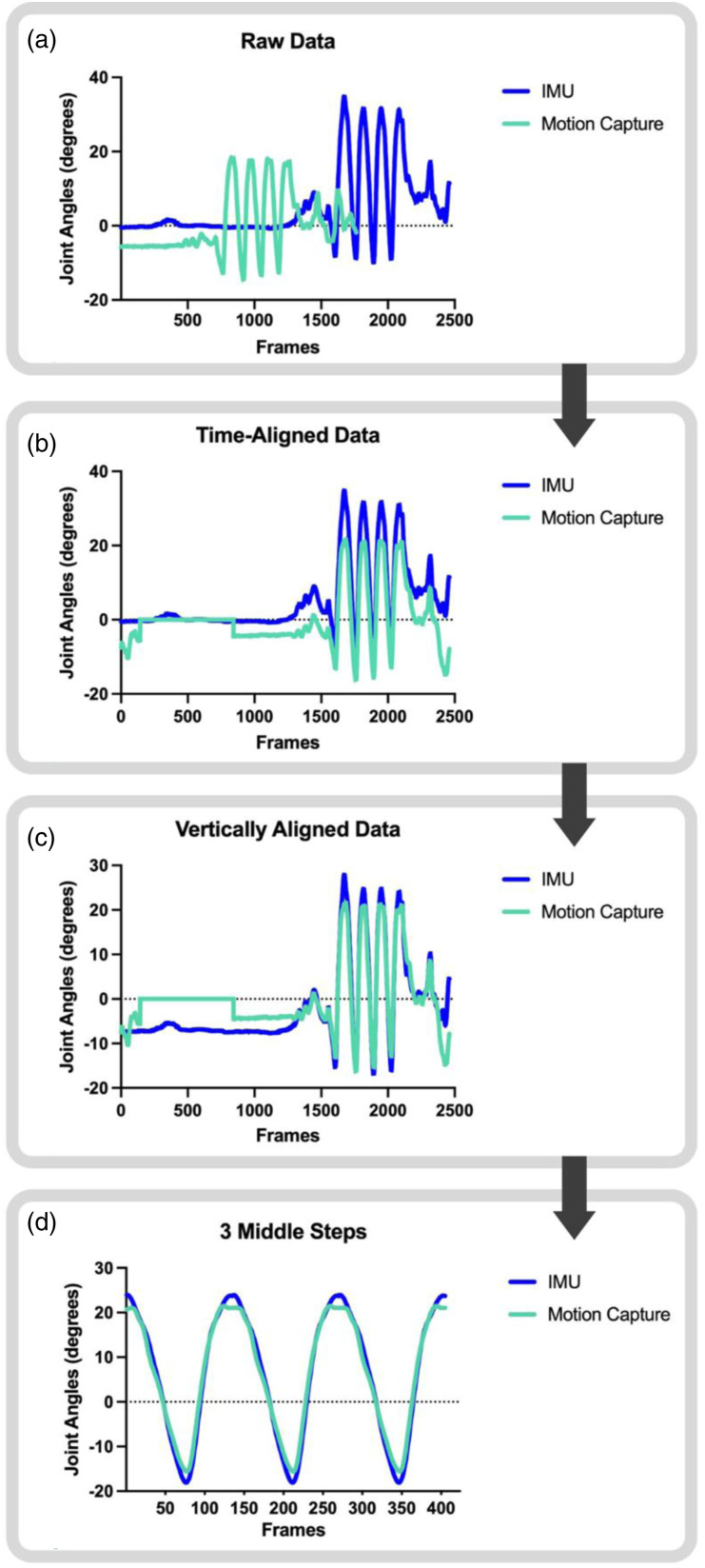
Figure 2 depicts the process used for data analysis. IMU data was processed to calculate joint angles and gait event detection within proprietary iSen software provided by STT Systems and exported to MATLAB for analysis. Motion capture data was processed using a custom MATLAB code based on Cortex definitions to derive joint angle data, with means and standard deviations in Supplemental Table 1. IMU and motion capture data were time-aligned and vertically aligned. Vertical alignment was necessary to make the necessary comparisons across waveforms for this manuscript, as the motion capture system and IMU system would be calibrated to different starting values inherent to differences in how their systems perform calibration. For instance, what an IMU defines as neutral during a walking trial may actually contain some flexion or extension, based on how the participant was standing when the examiner hit the “calibrate” button before the start of the walking trial. Alignments of IMU and motion capture data were manually confirmed by a qualified study personnel (MGF) through visual inspection. If alignments were not deemed appropriate, that trial was not used for analysis, and a different trial was selected to provide a total of three trials. Three of the five trials were chosen to allow for averaging across multiple steps, and is in line with a previous study that found between one and ten trials are needed depending on the motion capture system used and outcome measures collected. 34
Figure 2.
The flow of IMU (dark blue) and motion capture (mint green) data processing. The left hip sagittal plane joint angles from a walking trial of a representative participant was used as an example. Data was exported to MATLAB (Panel A), time-aligned (Panel B), and vertically aligned (Panel C). Then, the 3 middle steps were extracted using initial contact gait events (Panel D). All data was processed using a custom MATLAB code and confirmed by visual inspection.
Three of the participant’s five walking trials were used for analysis. Typically, the first three trials were selected due to the last two trials showing evidence of IMU slippage distally on the thigh segment, determined by visual inspection of the participant walking and noted during the study visit and IMU drift during visual inspection of the data. IMU slippage was only noted on the prosthetic thigh of participants who used a transfemoral prosthesis. Once three walking trials were selected, the three middle steps of each limb from each trial was extracted to minimize effects from the participant accelerating or decelerating, and to minimize IMU drift or distal slippage that tended to occur towards the end of the walking trial.
Each step, for both IMU and motion capture-derived data, was defined as occurring between two initial contact events. For IMU-derived data, steps were extracted based on the automatic detection of gait events identified within iSen IMU software. Additionally, toe-off events were needed to calculate single and double limb support times, which were also automatically detected within iSen IMU software. For motion capture-derived data, steps were extracted based on the maximum distance between the sacrum marker and heel marker for each limb, and toe-off events to calculate single and double limb support times were extracted using the minimum distance between the toe marker for each limb and the sacrum marker. 35 All gait events were confirmed by a qualified study personnel (MFG) and cut steps were deemed appropriate by inspecting the placement of gait events for hip, knee, and ankle waveforms. Any steps that were not appropriate (e.g. large deviations due to IMU slippage) were not used, and a different walking trial was included to ensure three middle steps for each of the three walking trials were analyzed for each limb of each participant.
Lower-limb sagittal plane kinematic and stance time parameters were extracted from each cut step. Lower-limb sagittal plane waveforms at the hip, knee, and ankle, as well as peak flexion and extension values were extracted across the entire gait cycle. Range of motion was calculated from peak flexion and extension values. Stance time parameters included single and double limb support times for both prosthetic and intact limbs. These parameters were extracted from the three middle steps of three walking trials of each limb, then averaged. IMU and motion capture-derived data were then compared by calculating absolute differences in seconds and degrees. Additionally, root mean square error (RMSE) across the entire waveform and at discrete points of peak flexion and extension at the hip, knee, and ankle during the entire gait cycle were also calculated. RMSE waveform values were a single RMSE averaged across the entire waveform, while RMSE discrete point values were taken at single points. Throughout this manuscript, error and RMSE are defined as the difference between the IMU and motion capture data. Peak parameters and stance durations were selected to assess accuracy (e.g. magnitude of difference between peaks for IMUs and motion capture through RMSE) and precision (e.g. consistency of each peak through standard deviation). Peak parameters and stance durations were also selected to provide the ability to compare to previous studies.3,30 Further, stance duration was also chosen to examine the ability of IMUs to accurately detect gait events (e.g. foot strike).
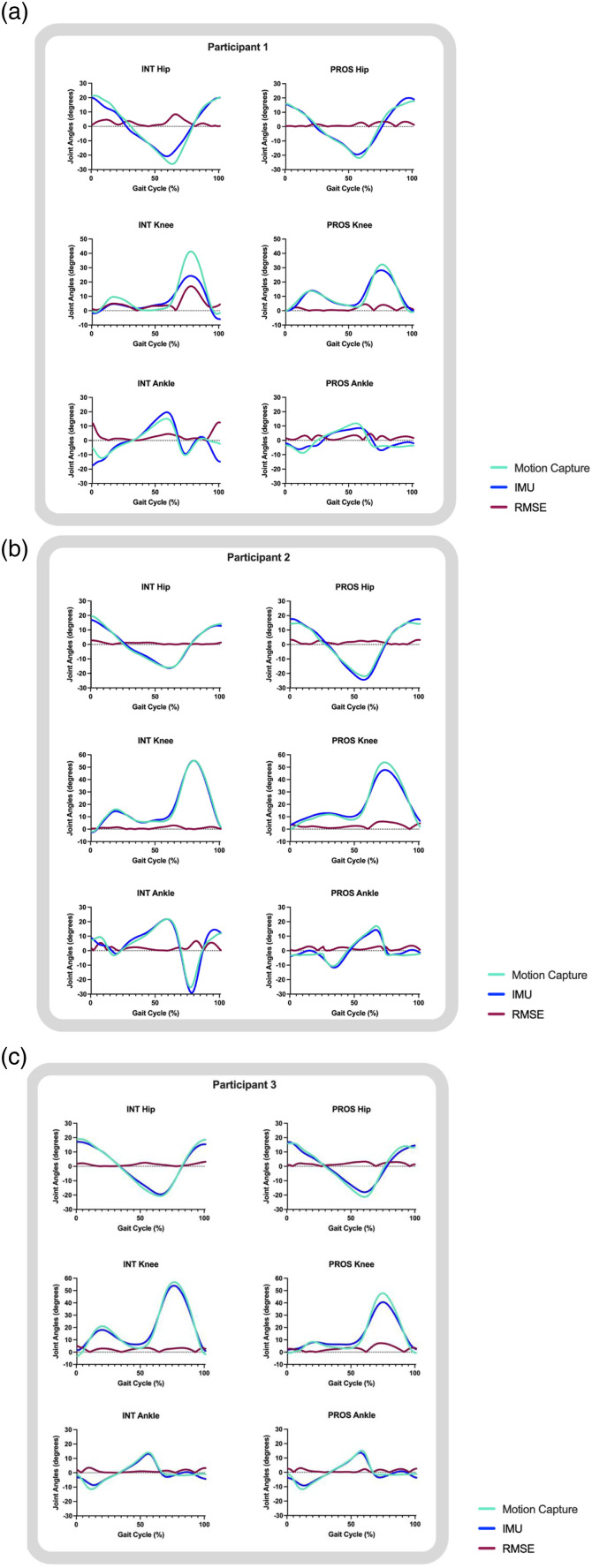
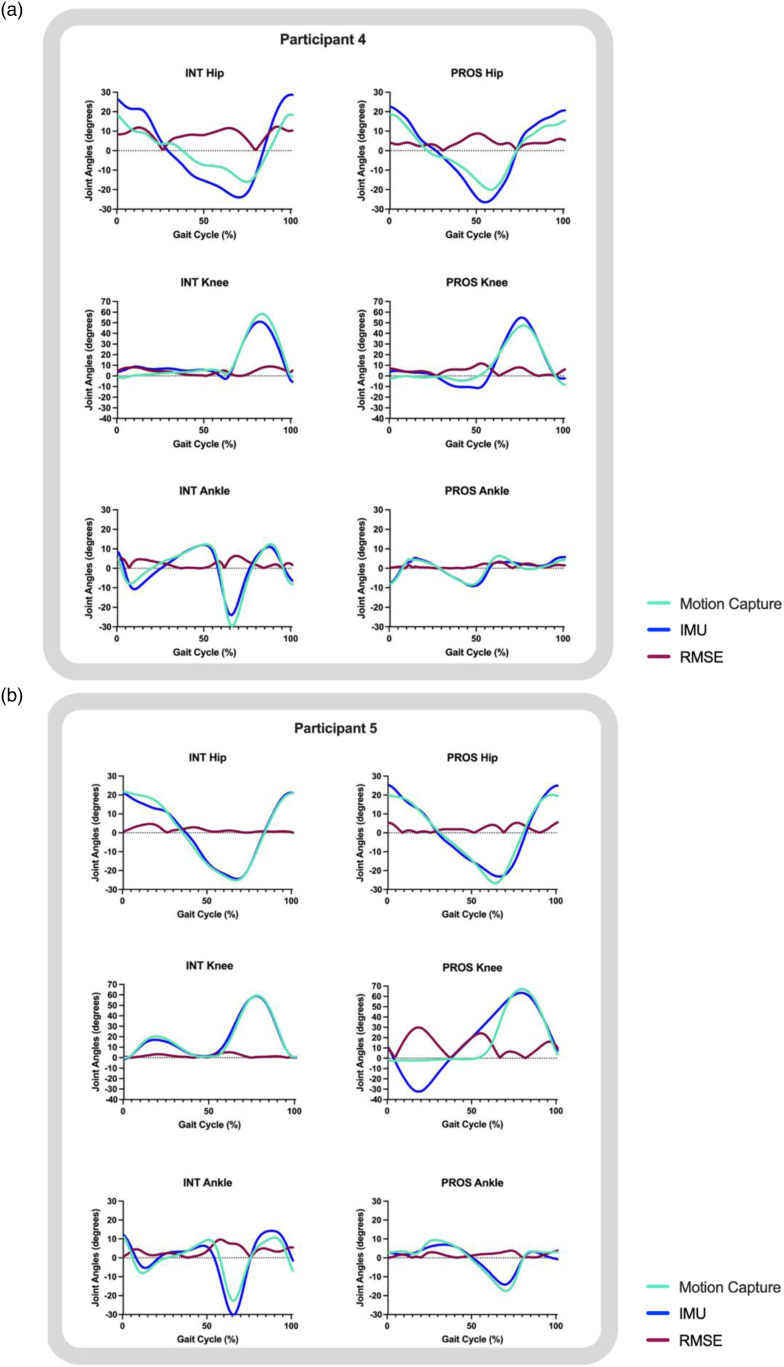
Results
Five individuals who use unilateral lower-limb prostheses (n = 3 transtibial users; n = 2 transfemoral users) participated in this study. Demographic characteristics are reported in Table 1. Absolute differences and group (transtibial and transfemoral) RMSE of discrete points between IMU and motion capture-derived data are reported for each participant in Table 2. Average RMSE values between IMU and motion capture-derived waveform data are reported for each participant in Table 3. Representative joint angle waveforms from the middle step are depicted with RMSE values across the entire gait cycle for transtibial users in Figure 3 and transfemoral users in Figure 4. Following our hypothesis, each results section reports all waveform RMSEs, discrete point RMSEs, and then stance time RMSEs for each participant. RMSEs are further discussed based on their context within our hypothesis based on previous healthy control data (5° for waveform RMSEs, 15° for discrete point RMSEs for peak flexion-extension points, and 0.02s stance time RMSEs). 3
Table 1.
Participant demographics.
| Participants | Age (yrs) | Height (cm) | Weight (kg) | Sex | TSA (yrs) | Etiology | Residual Limb length (cm) | Level of Prosthesis | K-Level | Walking Speed (m/s) | SCS during study visit (score out of 10) | PLUS-M 12-Item Short Form (raw score out of 60) | AMPPRO (score out of 42) | Time using current prosthesis (yrs) | Prosthetic Componentry and Shoes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62.4 | 185.42 | 141.52 | M | 12.0 | Trauma | 18.0 | TT | K3 | 0.64 | 7 | 54 | 40 | 9.0 | Ossur proflex foot; vacuum suspension with a total of 8 ply socks; Merrell tennis shoes |
| 2 | 34.8 | 172.72 | 56.70 | F | 2.5 | Trauma | 21.0 | TT | K3 | 0.63 | 8 | 56 | 42 | 2.0 | Fillauer AllPro Sport foot; suction suspension with 2 ply socks and sleeve; Adidas tennis shoes |
| 3 | 62.1 | 182.88 | 72.12 | M | 6.0 | Vascular | 18.6 | TT | K2 | 0.65 | 9 | 50 | 41 | 5.5 | Kinterra K2 foot; suction suspension with 1 ply sock; bicanno tennis shoes |
| 4 | 38.8 | 167.64 | 89.58 | M | 37.0 | Congenital/vascular (blood clot at 1.5 years of age) | 17.8 | TF | K3 | 0.77 | 9 | 49 | 41 | 1.0 | Ossur powered knee with ossur cheetah xplore foot; iceross 5-ring suction suspension with 0 sock ply; brooks tennis shoes |
| 5 | 37.1 | 167.64 | 54.43 | F | 7.0 | Cancer | 20.3 | TF | K3 | 1.06 | 7 | 51 | 42 | 3.0 | Ottobock C-leg 4 knee; fillauer AllPro foot; magnetic system with vaccuum suspension; brooks tennis shoes |
Table 2.
Discrete point absolute differences and RMSE.
| Transtibial | Transfemoral | |||||||
|---|---|---|---|---|---|---|---|---|
| Participant 1 | Participant 2 | Participant 3 | RMSE | Participant 4 | Participant 5 | RMSE | ||
| Intact limb | Double limb support (s) | 0.03 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.03 |
| Single limb support (s) | 0.03 | 0.01 | 0.05 | 0.04 | 0.02 | 0.01 | 0.02 | |
| Peak hip flex (∘) | 4.83 | 1.01 | 0.88 | 8.00 | 2.14 | 0.63 | 1.70 | |
| Peak hip ext (∘) | 3.75 | 5.13 | 2.97 | 7.36 | 2.36 | 0.71 | 2.62 | |
| Overall hip ROM (∘) | 8.58 | 6.14 | 2.88 | 4.78 | 3.87 | 0.69 | 2.92 | |
| Peak knee flex (∘) | 17.56 | 6.97 | 5.91 | 3.06 | 1.15 | 0.32 | 1.04 | |
| Peak knee ext (∘) | 5.03 | 4.59 | 3.03 | 6.47 | 0.66 | 1.28 | 1.13 | |
| Overall knee ROM (∘) | 22.59 | 11.56 | 8.94 | 9.87 | 0.65 | 0.96 | 1.01 | |
| Peak ankle DF (∘) | 6.62 | 10.39 | 2.24 | 7.64 | 1.22 | 2.56 | 2.10 | |
| Peak ankle PF (∘) | 6.10 | 10.03 | 4.93 | 7.52 | 4.27 | 7.60 | 5.90 | |
| Overall ankle ROM (∘) | 12.72 | 3.67 | 2.68 | 8.14 | 2.04 | 10.16 | 7.36 | |
| Prosthetic limb | Single limb support (s) | 0.04 | 0.01 | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 |
| Peak hip flex (∘) | 6.58 | 3.90 | 0.73 | 4.66 | 4.31 | 4.63 | 4.55 | |
| Peak hip ext (∘) | 1.64 | 8.52 | 3.44 | 5.51 | 4.28 | 2.47 | 4.09 | |
| Overall hip ROM (∘) | 6.40 | 12.42 | 3.20 | 8.76 | 8.59 | 2.81 | 6.86 | |
| Peak knee flex (∘) | 4.94 | 7.20 | 8.26 | 7.19 | 2.69 | 3.68 | 6.16 | |
| Peak knee ext (∘) | 2.12 | 2.81 | 1.86 | 2.51 | 1.36 | 40.87 | 29.21 | |
| Overall knee ROM (∘) | 6.61 | 10.01 | 9.10 | 9.04 | 4.05 | 37.18 | 27.94 | |
| Peak ankle DF (∘) | 0.93 | 1.73 | 2.57 | 1.94 | 1.45 | 0.87 | 1.35 | |
| Peak ankle PF (∘) | 2.46 | 0.26 | 0.78 | 1.57 | 1.71 | 1.95 | 2.04 | |
| Overall ankle ROM (∘) | 2.91 | 1.73 | 3.21 | 2.82 | 2.83 | 1.67 | 2.61 | |
Table 2: Absolute differences in seconds (s) and degrees (∘) for each participant. Root mean square error (RMSE) in seconds (s) and degrees (∘) for each group (transtibial and transfemoral). All values are an average of three steps from 3 walking trials. Abbreviations: DF = dorsiflexion; PF = plantarflexion; ROM = range of motion, Flex = flexion, Ext = extension.
Table 3.
Waveform average RMSE values
| Transtibial | Transfemoral | ||||||
|---|---|---|---|---|---|---|---|
| Participant 1 | Participant 2 | Participant 3 | Participant 4 | Participant 5 | |||
| RMSE | Intact limb | Hip (∘) | 2.74 | 1.83 | 2.05 | 2.27 | 1.82 |
| Knee (∘) | 6.33 | 1.68 | 2.18 | 1.50 | 1.58 | ||
| Ankle (∘) | 3.11 | 2.63 | 3.31 | 2.01 | 3.45 | ||
| Prosthetic limb | Hip (∘) | 2.08 | 3.97 | 2.71 | 2.23 | 2.70 | |
| Knee (∘) | 2.77 | 4.42 | 4.65 | 3.84 | 15.64 | ||
| Ankle (∘) | 2.09 | 1.29 | 1.06 | 1.80 | 3.32 | ||
Table 3: Average root mean square error (RMSE) values in degrees (∘) for each participant over the entire gait cycle. Data depicts the RMSEs across 3 gait cycles over 3 walking trials (9 gait cycles total).
Figure 3.
Transtibial participant waveform data from a representative step. Normalized to 100% of the gait cycle for each participant that used a transtibial prosthesis. Abbreviations: INT: intact limb; PROS: prosthetic limb; RMSE: root mean square error.
Figure 4.
Transfemoral participant waveform data from a representative step. Normalized to 100% of the gait cycle for both participants that used a transfemoral prosthesis. Abbreviations: INT: intact limb; PROS: prosthetic limb; RMSE: root mean square error.
Transtibial participants
Prosthetic limbs
Waveforms of prosthetic limbs in transtibial participants had RMSEs of ≤4.65° (Table 3) at hip, knee, and ankle joints. All three transtibial participants showed the highest prosthetic limb error at the knee joint (RMSEs 2.77°, 4.42°, and 4.65°, respectively), as opposed to hip or ankle joints (Table 3), driven by differences in peak knee flexion values (Table 2). Double limb support times had RMSEs of ≤0.03s. For prosthetic limb support time, Participant 1 had a larger absolute difference (0.04s) than Participants 2 and 3 (0.01s, and 0.03s, respectively) (Table 2).
Intact limbs
With the exception of Participant 1’s knee waveform (RMSEs ≤6.33°), intact limbs had RMSEs ≤2.83° (Table 3, Figure 3). Participant 1 showed the highest intact RMSE at the knee joint, while Participants 2 and 3 showed the highest intact RMSE at the ankle joint (Table 3, Figure 3). These were driven by differences in peak knee flexion values for Participant 1, peak dorsiflexion values for Participant 2, and peak plantarflexion values for Participant 3 (Table 2, Figure 3). Participant 1 had a larger absolute difference in intact limb support time (0.03s) than Participants 2 and 3 (0.01s and 0.02s, respectively) (Table 2).
Transfemoral participants
Prosthetic limbs
For both transfemoral participants, hip, knee, and ankle waveform RMSEs were all ≤3.84° (Table 3), with the exception of Participant 5’s prosthetic knee joint with higher error values of RMSE ≤15.64°, driven by differences in peak knee extension values (Table 2, Figure 4). These higher prosthetic knee joint RMSEs in Participant 5 were caused by magnetometer interference, detailed in the discussion. Double limb support times had RMSEs of ≤0.03s and prosthetic limb support times had RMSEs of ≤0.03s (Table 2).
Intact limbs
Intact limbs had higher error values than prosthetic limbs at the ankle joint (RMSEs ≤3.45°) (Table 3, Figure 4). Higher RMSE in ankle range of motion were driven by peak ankle plantarflexion values (Table 2, Figure 4). Intact limb support times had RMSEs of ≤0.02s (Table 2).
Discussion
To our knowledge, this was the first study to directly compare gait data between IMUs and motion capture systems in unilateral lower-limb prosthesis users. The iSen IMU system provided similar data compared to other IMU systems used in prior studies, such as Xsens,6,8,11 RehaGait, 12 Sparkfun Electronics, 13 and Synertial. 7 IMUs provided similar prosthetic limb data compared to motion capture (RMSE: waveform ≤4.65°; discrete point ≤9.04°; stance ≤0.03s) as previous comparisons in healthy controls (RMSE: waveform ≤5.0°; discrete point ≤15.0°; stance ≤0.02s), 3 with the exception of the microprocessor prosthetic knee joint in one transfemoral user due to magnetometer interference. However, RMSE across participants tended to vary widely, so comparisons across participants should be made with caution. Additionally, prosthetic limb RMSE tended to be less than previous control data from healthy control participants, which could be explained by: 1) differences in how the IMUs were secured to each limb (prosthetic with direct velcro and self-adherent wrap; intact with elastic straps), or 2) intact limb skin and muscle motion that does not occur on the prosthesis. Potentially for similar reasons, lower-limb kinematic IMU data was more similar to motion capture in the stance phase than the swing phase. Overall, findings from this case series indicate IMUs could collect lower-limb sagittal plane kinematic and stance time data to eventually inform rehabilitation (e.g. quantify asymmetry over time to inform considerations for adjustment to the prosthesis over time, prosthetic alignment, muscle group strengthening). It is suggested clinicians not use the IMU system to document absolute values of range of motion (i.e. patient had X degrees of knee hyperextension during gait) but rather for: 1) comparison of range of motion values between intact and prosthetic limbs for symmetry (i.e. patient had X% symmetry) or 2) documentation of absolute differences in stance time in seconds. Future studies should include a larger sample size to determine if findings are generalizable to a larger population of unilateral lower-limb prosthesis users. The portability of IMUs could allow researchers to include participants that have been underrepresented in gait literature (e.g. diabetes, older in age, less active) to better reflect the overall population of lower-limb prosthesis users.30,36
Prosthetic limbs compared to previous control data
Prosthetic limbs had similar or less RMSE than healthy control participants in previous studies. A clinical threshold of 5°(°) of error has been used for motion capture systems and recently applied to IMUs.4,5 Recent meta-analysis 3 found multi-sensor waveform RMSEs in healthy controls at the hip (2.7–6.3°),6,7,8,9 knee (0.7–4.6°),6,7,8,10,11 and ankle (4.0–7.8°).6-8,11 Compared to these values, prosthetic limbs in this study showed similar or lower RMSEs in transtibial participants at the hip (2.05–2.74°), knee (2.77–4.65°), and ankle (1.06–2.09°), and transfemoral participants at the hip (2.23–2.70°), and ankle (1.80° - 3.32°). However, Participant 5’s RMSEs at the microprocessor prosthetic knee tended to be higher than previous control data (15.64°). Discrete points of peak flexion and extension RMSE in control limbs at each joint have ranged 2.7–15°.12,13 Prosthetic limbs in this case series had similar or lower RMSEs of 1.55–13.04°, with the exception of the transfemoral prosthetic knee joint (≤40.87°) of Participant 5 due to magnetometer interference with the microprocessor knee.
In the same meta-analysis, IMU single limb support time RMSEs were 0.02s.6,37,38,39 These values aligned with prosthetic limb support times in this case series of all participants (0.01s - 0.03s) except Participant 1 (0.04s), potentially due to increased IMU slippage and motion in the anatomical limb discussed later. This is the first paper to compare stance time calculations between IMU and motion capture for this population. While healthy populations show stance time differences within 0.02s between IMUs and motion capture, the slightly larger RMSE value of 0.04s in this study may suggest either the proprietary algorithm of stance detection for this IMU system is not as accurate as the software algorithm used in the healthy population, 3 or that the software algorithm was less able to detect heel contact and toe-off due to the prosthetic foot exhibiting different properties than an intact foot.
Prosthetic limbs compared to intact limbs
Control limb data mentioned above was similar to intact limb data in this case series. Therefore, prosthetic limbs also had similar or less RMSE than intact limbs, which may have been influenced by how the IMUs were secured to each limb. IMUs on the intact limb were typically secured using STT systems’ recommended method of elastic straps, which allowed more slippage distally and movement between the IMU and individual’s body segment. However, IMUs on the prosthetic limb were typically secured directly to the prosthesis with velcro and then wrapped tightly in self-adherent wrap to prevent slippage. Additionally, anatomical limbs produce skin and muscle motions that do not occur on the prosthesis. These findings are supported by a 2014 case study of a single transfemoral user that suggested the participant’s intact limb RMSE was nearly four times higher than prosthetic limb RMSE due to skin and muscle motions. 18
Transtibial participant comparisons
Participant 1 had the most conically-shaped limb, so increased IMU slippage down the thigh and anatomical motion could explain why they had higher RMSE values, particularly at the intact knee joint, than the other two transtibial participants. Of the three transtibial participants, Participant 2 was the youngest and most active, potentially explaining why they had the least RMSE at all joints. Participant 3 was the only participant that used a K2 level foot, which is classified by Medicare as typical for the limited community ambulator. 40 This difference in foot componentry may explain why Participant 3 had higher prosthetic limb RMSE at the knee. They may have employed proximal compensation strategies to ensure prosthetic foot clearance, or intact limb compensations due to the reduced range of motion available in the prosthetic foot, which were observed during their visit.
Transfemoral participant comparisons
The only study that has previously compared lower-limb sagittal plane kinematics between IMUs and motion capture was a 2014 case study that reported knee and ankle RMSEs in one transfemoral prosthesis user. 28 Compared to knee error in the 2014 case study (RMSEs: prosthetic ≤1.0°; intact ≤4.0°), participants in this case series had higher prosthetic knee error (RMSE ≤15.64°) and intact knee error (RMSE ≤6.33°). Additionally, compared to ankle error in the 2014 case study (RMSE ≤2.0° at the prosthetic and intact ankle), transfemoral participants in this case series had similar prosthetic ankle error (RMSEs ≤3.32°) but higher intact ankle error (RMSEs ≤3.45°). These differences between the 2014 case study and this case series may have been due to differences in IMU systems. Differences may have also been due to prosthetic foot componentry, as the prosthetic knee was the same as Participant 5, and the 2014 case study did not list the participant’s prosthetic foot.
Participant 4 had larger differences between IMU and motion capture data during hip range of motion compared to all other participants, potentially due to proximal compensatory strategies, such as the circumduction observed during their visit, from undergoing an amputation before reaching walking age. Perhaps these compensatory motions might lead to the IMU not correctly displaying motion that is “out of plane.” Participant 5 had a large RMSE at the prosthetic knee joint due to a technical issue with the IMU magnetometer. The IMU magnetometers were disabled in Participants 4 and 5 to avoid interference with the microprocessor knees. Disabling the magnetometer is available under certain IMU systems such as the one utilized in this study and can be as simple as changing the option in the software. However, this is not true for all systems. We suggest clinicians ensure that the IMU system they purchase allows for magnetometers to be disabled. Upon data analysis, a qualified study personnel (MGF) found the prosthetic knee data still looked as if the magnetometers were enabled, so data files were sent to the IMU company, iSen, for inspection. iSen staff concluded there still appeared to be magnetic interference, but could not explain why, since the display showed the magnetometers were disabled. After troubleshooting, our research group at the University of North Texas Health Science Center found the disabling of the magnetometers only took effect if data collection was initiated with the magnetometers disabled. Participant 4 was able to come in for retesting, while Participant 5 could not be retested due to time constraints. Thus, Participant 4’s data was collected with the magnetometers actually disabled, while Participant 5’s data was collected while the system incorrectly showed the magnetometers were disabled.
Therefore, the magnetometer should be disabled when collecting data from participants who use microprocessor knees. The general purpose of magnetometers is to sense the Earth’s magnetic field for orientation in direction. In Participant 5, the prosthetic knee waveform indicates a steady oscillation from standing (knee extended) to an atypical amount of nearly 40 degrees of hip extension, then knee flexion for an extended period of time. This oscillation is not reflective of how this participant ambulated during visual observation or video. Disabling the magnetometer still provided data similar to motion capture for Participant 4, and was reflective of the gait of the participant from visual observation and video.
Limitations and future work
For clinical use, while the user interface was generally intuitive, experience with biomechanics data was still required in order to process and interpret IMU data. Another limitation is that motion capture data was collected using a marker set and modeling technique that makes inherent assumptions regarding body segments that do not typically hold true for prostheses (e.g. body segment lengths remain constant during movement).41,42 However, differences between IMU and motion capture-derived data were present on both intact and prosthetic limbs, and RMSEs were typically higher in intact limbs compared to prosthetic limbs. This suggests both intact limb and prosthetic limb data was similarly represented, regardless of the motion capture marker set and modeling techniques used in this case series.
While steps are commonly selected in biomechanical analysis to represent steady-state walking, it should be noted this would tend to reflect the least problematic data (e.g., potential IMU slippage during the end of the walking trial), potentially impacting clinical implications. For clinical implementation, it is suggested that IMUs are securely instrumented to minimize slippage. This slippage, similar to marker movement, will change the position data and ultimately the joint angles. IMU slippage, however, will have larger impacts to the output joint angle data than motion capture marker movement. Further, IMUs were only compatible with microprocessor knees when magnetometers were disabled. While the process for disabling the magnetometers was simple using our IMU system, this could limit clinical utility of IMUs for insurance justification and rehabilitation in MPK users. Findings should also be generalized with caution, as this study included a small sample size with data from one IMU company, particularly as iSen software is proprietary.
This study included only five participants from a convenience sample that could complete this study in a limited time frame. Future studies should include more participants to determine if our findings are generalizable to a larger sample of unilateral and bilateral lower-limb prosthesis users. Future work could also determine if IMU RMSEs are influenced by: the method in which they are secured to body segments (e.g. elastic strap compared to direct velcro), motion capture marker modeling techniques, IMU settings, or IMU company. These future directions could help inform a recommended clinical data collection protocol.
Conclusions
Findings from this case series suggest IMUs are capable of providing lower-limb kinematic and stance time data comparable to motion capture systems. Prosthetic limbs tended to have less error than intact limbs or previous control limb data, potentially due to increased movement of the IMUs on anatomical limbs. We suspect for similar reasons, IMU-derived lower-limb kinematic data tended to be more similar to motion capture-derived data in stance than swing. However, error varied across participants, suggesting comparisons within individuals may be more accurate. Therefore, we suggest clinicians not use the IMU system to document discrete peaks of range of motion, but rather for asymmetry or comparison across multiple timepoints within participants. Future studies should include larger sample sizes and other IMU systems to assess generalizability of findings in this case series. Clinicians and researchers could eventually use IMUs to collect gait data that better reflects real-world conditions in prosthesis users to help inform rehabilitation.
Supplemental Material
Supplemental Material for A pilot case series for concurrent validation of inertial measurement units to motion capture in individuals who use unilateral lower-limb prostheses by MG Finco, Rita M Patterson and Sarah C Moudy in Journal of Rehabilitation and Assistive Technologies Engineering
Acknowledgements
The authors would like to thank Tasha Buxton and Shawn Kennedy for assisting with data collection. Thanks also to Cody Logenbaugh at Baker Orthotics and Prosthetics and Mark Ashford at Hanger Clinic for their help with participant recruitment. This project is taken in part from a dissertation MGF submitted to the University of North Texas Health Science Center in partial fulfillment of the requirements for the degree of Doctor of Philosophy.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an American Orthotics and Prosthetics Association research award administered by the Center for Orthotic and Prosthetic Learning and Outcomes/Evidence-Based Practice. MGF was supported by the National Institutes of Health/National Institute on Aging (T32 AG020494) and the Institute for Healthy Aging.
Guarantor: MGF
Contributorship: Conceptualization: SCM, RMP; Data Curation: MGF; Formal Analysis: MGF; Funding Acquisition: MGF, SCM; Investigation: MGF; Methodology: SCM, RMP; Project Administration: MGF: Software: MGF, SCM; Supervision: SCM, RMP; Resources: RMP; Visualization: MGF; Writing- Original Draft Preparation: MGF; Writing- Review and Editing: SCM, RMP.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
References
- 1.Gard SA. Use of quantitative gait analysis for the evaluation of prosthetic walking performance. J Prosthet Orthot 2006; 18: 93–104. [Google Scholar]
- 2.Iosa M, Picerno P, Paolucci S, et al. Wearable inertial sensors for human movement analysis. Expert Rev Med Devices 2016; 13: 641–659. [DOI] [PubMed] [Google Scholar]
- 3.Kobsar D, Charlton JM, Tse CTF, et al. Validity and reliability of wearable inertial sensors in healthy adult walking: a systematic review and meta-analysis. J Neuroeng Rehabilitation 2020; 17: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berner K, Cockcroft J, Morris LD, et al. Concurrent validity and within-session reliability of gait kinematics measured using an inertial motion capture system with repeated calibration. J Bodyw Mov The 2020; 24: 251–260. [DOI] [PubMed] [Google Scholar]
- 5.McGinley JL, Baker R, Wolfe R, et al. The reliability of three-dimensional kinematic gait measurements: a systematic review. Gait and Posture 2009; 29: 360–369. [DOI] [PubMed] [Google Scholar]
- 6.Teufl W, Lorenz M, Miezal M, et al. Towards inertial sensor based mobile gait analysis: event-detection and spatio-temporal parameters. Sensors 2018; 19: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebel K, Boissy P, Nguyen H, et al. Inertial measurement systems for segments and joints kinematics assessment: towards an understanding of the variations in sensors accuracy. Biomed Eng Online 2017; 16: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karatsidis A, Jung M, Schepers HM, et al. Musculoskeletal model-based inverse dynamic analysis under ambulatory conditions using inertial motion capture. Med Eng Phys 2019; 65: 68–77. [DOI] [PubMed] [Google Scholar]
- 9.Ohtaki Y, Sagawa K, Inooka H. A method for gait analysis in a daily living environment by body-mounted instruments. JSME Int J Ser C 2001; 44: 1125–1132. [Google Scholar]
- 10.Cooper G, Sheret I, McMillian L, et al. Inertial sensor-based knee flexion/extension angle estimation. J Biomech 2009; 42: 2678–2685. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J-T, Novak AC, Brouwer B, et al. Concurrent validation of Xsens MVN measurement of lower limb joint angular kinematics. Physiol Meas 2013; 34: N63–N69. [DOI] [PubMed] [Google Scholar]
- 12.Nüesch C, Roos E, Pagenstert G, et al. Measuring joint kinematics of treadmill walking and running: Comparison between an inertial sensor based system and a camera-based system. J Biomech 2017; 57: 32–38. [DOI] [PubMed] [Google Scholar]
- 13.Jaysrichai T, Suputtitada A, Khovidhungij W. Mobile sensor application for kinematic detection of the knees. Ann Rehabil Med 2015; 39: 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuvrier F, Sijobert B, Azevedo C, et al. Inertial measurement unit compared to an optical motion capturing system in post-stroke individuals with foot-drop syndrome. Ann Phys Rehabil Med 2020; 63: 195–201. [DOI] [PubMed] [Google Scholar]
- 15.Romijnders R, Warmerdam E, Hansen C, et al. Validation of IMU-based gait event detection during curved walking and turning in older adults and Parkinson’s disease patients. J Neuroeng Rehabilitation 2021; 18: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hafer JF, Provenzano SG, Kern KL, et al. Measuring markers of aging and knee osteoarthritis gait using inertial measurement units. Journal of Biomechanics 2020; 99: 109567. [DOI] [PubMed] [Google Scholar]
- 17.Valle MS, Casabona A, Sapienza I, et al. Use of a single wearable sensor to evaluate the effects of gait and pelvis asymmetries on the components of the timed up and go test, in persons with unilateral lower limb amputation. Sens 2021; 22: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clemens S, Kim KJ, Gailey R, et al. Inertial sensor-based measures of gait symmetry and repeatability in people with unilateral lower limb amputation. Clin Biomech 2020; 72: 102–107. [DOI] [PubMed] [Google Scholar]
- 19.Leijendekkers RA, Hoogeboom TJ, van Hinte G, et al. Reproducibility and discriminant validity of two clinically feasible measurement methods to obtain coronal plane gait kinematics in participants with a lower extremity amputation. PLoS ONE 2019; 14: e0217046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duraffourg C, Bonnet X, Dauriac, et al. Real time estimation of the pose of a lower limb prosthesis from a single shank mounted IMU. Sens 2019; 19: 2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keri M-I, Shehata AW, Marasco PD, et al. A cost-effective inertial measurement system for tracking movement and triggering kinesthetic feedback in lower-limb prosthesis users. Sens 2021; 21: 1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houdijk H, Appelman FM, Van Velzen JM, et al. Validity of dynaport gaitmonitor for assessment of spatiotemporal parameters in amputee gait. J Rehabil Res Dev 2008; 45: 1335. [PubMed] [Google Scholar]
- 23.Kim J, Colabianchi N, Wensman J, et al. Wearable sensors quantify mobility in people with lower limb amputation during daily life. IEEE Trans Neural Syst Rehabil Eng 2020; 28: 1282–1291. [DOI] [PubMed] [Google Scholar]
- 24.Simonetti E, Villa C, Bascou J, et al. Gait event detection using inertial measurement units in people with transfemoral amputation: a comparative study. Med Biol Eng Comput 2020; 58: 461–470. [DOI] [PubMed] [Google Scholar]
- 25.Maqbool HF, Husman MAB, Awad MI, et al. Heuristic real-time detection of temporal gait events for lower limb amputees. IEEE Sens J 2019; 19: 3138–3148. [Google Scholar]
- 26.Ledoux ED. Inertial sensing for gait event detection and transfemoral prosthesis control strategy. IEEE Trans Biomed Eng 2018; 65: 2704–2712. [DOI] [PubMed] [Google Scholar]
- 27.Bastas G, Fleck JJ, Peters RA, et al. IMU-based gait analysis in lower limb prosthesis users: Comparison of step demarcation algorithms. Gait and Posture 2018; 64: 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seel T, Raisch J, Schauer T. IMU-based joint angle measurement for gait analysis. Sens 2014; 14: 6891–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer ZA, Perry JL, Vanicek N. A personalised exercise programme for individuals with lower limb amputation reduces falls and improves gait biomechanics: a block randomised controlled trial. Gait and Posture. 2018;63:282–289. [DOI] [PubMed] [Google Scholar]
- 30.Finco MG, Moudy S, Patterson R. Normalized kinematic walking symmetry data for individuals who use lower-limb prostheses: considerations for clinical practice and future research, https://journals.lww.com/jpojournal/Fulltext/9900/Normalized_Kinematic_Walking_Symmetry_Data_for.7.aspx (2022). [DOI] [PMC free article] [PubMed]
- 31.Obeid JS, McGraw CA, Minor BL, et al. Procurement of shared data instruments for Research Electronic Data Capture (REDCap). J Biomed Inform 2013; 46: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diss CE. The reliability of kinetic and kinematic variables used to analyse normal running gait. Gait and Posture 2001; 14: 98–103. [DOI] [PubMed] [Google Scholar]
- 35.Zeni JA, Richards JG, Higginson JS. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait and Posture 2008; 27: 710–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of Limb loss in the United States: 2005 to 2050. Arch Phys M 2008; 89: 422–429. [DOI] [PubMed] [Google Scholar]
- 37.Allseits E, Lučarević J, Gailey R, et al. The development and concurrent validity of a real-time algorithm for temporal gait analysis using inertial measurement units. J Biomed Inform 2017; 55: 27–33. [DOI] [PubMed] [Google Scholar]
- 38.Maffiuletti NA, Gorelick M, Kramers-de Quervain I, et al. Concurrent validity and intrasession reliability of the IDEEA accelerometry system for the quantification of spatiotemporal gait parameters. Gait and Posture 2008; 27: 160–163. [DOI] [PubMed] [Google Scholar]
- 39.Sejdic E, Lowry KA, Bellanca J, et al. Extraction of stride events from gait accelerometry during treadmill walking. IEEE J Transl Eng Health Med 2016; 4: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balk E, Gazula A MG. Table 1, lower limb extremity prosthesis medicare functional classification levels (K levels). In: Lower limb prostheses: measurement instruments, comparison of component effects by subgroups, and long-term outcomes. Rockville, MD: Agency for Healthcare Research and Quality (US), https://www.ncbi.nlm.nih.gov/books/NBK531517/table/ch2.tab1/ [PubMed] [Google Scholar]
- 41.Sawers AB, Hahn ME. The potential for error with use of inverse dynamic calculations in gait analysis of individuals with lower limb loss: a review of model selection and assumptions. J Prosthet Orthot 2010; 22: 56–61. [Google Scholar]
- 42.Winter DA. Biomechanics and motor control of human movement. Hoboken: John Wiley and Sons, Inc, 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for A pilot case series for concurrent validation of inertial measurement units to motion capture in individuals who use unilateral lower-limb prostheses by MG Finco, Rita M Patterson and Sarah C Moudy in Journal of Rehabilitation and Assistive Technologies Engineering