Abstract
Toxicity arising from environmental contaminants has attracted global interest in the last few decades, due to the high morbidity and mortality associated with them. Efforts have been made to combat the consequential outcomes of environmental toxicity in humans through traditional remediation techniques and therapeutic measures which have been hampered by one or more limitations. Consequently, this scenario has triggered interest in the medicinal properties of phytochemicals. Thus, this review gives a succinct and in-depth elucidation of the various environmental contaminants and their toxicity effects on humans. It delves into the various classes of phytochemicals and their intervention roles. The study adopted a desk review of existing literatures from scientific reports and peer reviewed articles through triangulation of data sources. “Phytochemicals” are group of secondary metabolites obtained from plants with medicinal properties. These groups of compounds are included but not limited to flavonoids, tannins, saponins, alkaloids, cardenoloids, terpenoids, and phytosteroids. This review corroborates the prophylactic and therapeutics efficacy of these phytochemicals as anti-metastatic, anti-inflammatory, anti-aging, anti-oxidant, anti-microbial and live saving substances with empirical findings from several laboratory, clinical trials and epidemiologic studies. It conclude that given the wide range of medicinal properties of phytochemicals, there is an urgent need for its full optimization in the pharmaceutical industry and future studies should focus on identifying the bioactive molecules in these compounds and its effectiveness against mixer toxicity.
Keywords: Environmental toxicants, phytochemicals, prophylactics, therapeutics, plants, intervention, medicinal properties
Introduction
One of the leading causes of diseases, high morbidity and mortality rates in nations across the globe is environmental contamination. According to the World Health Organization (WHO) approximately 7 million deaths per annum is associated with pollution, mostly due to environmental toxicants which are air borne 1,2; this casualties is greater than the cumulative death figures recorded from communicable diseases such as malaria, tuberculosis and HIV/AIDS altogether.3,4 The greater proportions of these pollution-related deaths take place in developing countries of the world. Exposures to environmental toxicants at early stage in life are primarily precarious.5,6 Susceptibility is most pronounced during the first few weeks of the fetal life when the organs primordial are formed. Vulnerability to environmental toxicants during this delicate period can result in perpetual anatomical, behavioral and metabolic alterations. This anomaly can be expressed as short or long-term disorder at any specific point along an individual lifespan from neonate to senescence. 7 Major acute maladies ascribed to environmental pollution at infancy are diarrheal and pneumonia disease 8 while the chronic non-communicable diseases associated with early exposure to environmental toxicants include dysfunctional neurobehavioral development, childhood and adult asthma, hypertension, diabetes, cardiovascular diseases, obesity, and cancer as reported by Sly et al. 9
In some situations, environmental pollution is a crystal-clear phenomenon, while in others, the perception lies greatly in the eyes of the beholder. Air emission and water discharge release man-made pollutants into the environment. The total transformations of pollutants in the atmospheric, soil and hydrologic environments play a critical role in their toxicity and contamination pathways that result in health and ecological consequences. 10 The principal exposure routes to these contaminants are mainly through the lungs, dermal and oral. Many environmental toxicants cause both local and systemic effects. These effects may take place following apparent recovery from acute exposure or as a consequence of repetitive exposures to low dose of contaminants over a longer period. Nevertheless, the manifested health effects from acute or chronic exposure are a function of the chemical involved and the organ it affected. Even for the same contaminant, the degree of toxicity is not the same but varies from one organ to the other.
Copious reports have linked various health challenges and several age-associated diseases to environmental contaminants and chemical exposure.11–13 The major means by which humans are exposed to inimical chemicals and their combinations are through occupational and environmental sources.14–16 There is no simple “rule” to safeguard or intercede against ailments linked to environmental toxicants exposure. Several contaminants, like heavy metals and persistent organics pollutants (POPs), bioaccumulate in the human system, and remediation techniques in eliminating these pollutants from the surroundings remain a herculean task and are often expensive. Also, treatments aim at obliterating environmental toxicity from the biological compartment are chelation and cleansing procedures, and corroborative techniques, routinely administered in synergy. The treatment may be very complex and personalized, narrowed to an individual’s distinct needs and requiring the adroitness of an expert, often a combination of pundits. 17 Moreover, numerous environmental contaminants induce signaling pathways that lead to oxidative stress which serve as a precursor to the pathogenesis of several long-term maladies. Consequently, techniques that regulate the impact of toxicants on pathophysiologic processes that leads to disease development and escalation will be of immense public health significance. 18 Emerging findings, obtained from fundamental and epidemiologic studies and clinical trials have proven that “phytochemicals” remain the only viable answer to this menace.19–22
Based on the aforementioned discussion, this review presents background information on environmental toxicants and some of their selected toxicity in animals and humans. It further delves into the literature survey of some phytochemicals popularly found in medicinal plants and presents evidence-based studies to accentuate the therapeutic properties of these compounds in combating and ameliorating several health effects due to environmental toxicity. It is our aspiration that this review will trigger public health interest in the beneficial role of phytochemicals and arouses further research that will lead to the discovery of many phytochemicals from the numerous unexplored medicinal plants across the globe.
Methodology
This write-up adopted a systematic review of information primary from available peer-reviewed articles, scientific reports, and grey literatures (reports from international agencies, academia, government documents, and policy statements). The search for literature was conducted utilizing six electronic databases viz Pub med, Web of Science, Google Scholar, Embase, Science Direct, and Cochrane library, for studies published in English from September, 1962 to September, 2022. Studies focused on environmental toxicants and health adversities, pharmacological properties of phytochemicals, and various laboratories, experimental and clinical studies that discussed the intervention roles of phytochemicals. All papers were reviewed using a predesigned data extraction form.
Overview of environmental toxicants
An environmental toxicant can be viewed as any toxic agent or substance produced by humans or introduced into the environment by human activities. Toxicants come in diverse shapes and sizes, and they may emanate from both natural and anthropogenic sources. Thus, toxicants include a wide range of compounds from inorganic substances like metals to numerous organic compounds such as pharmaceutical medicines. Environmental contamination in any possible form has been of primary health interest throughout the globe, because it contributes significantly to the pathophysiology of some human diseases. 23 There are several origins by which chemical toxicants are emitted into the surrounding, although these broadly are grouped into a few classes. This section discusses the diverse kinds of contaminants and the classes they belong.
Types of contaminants
Three primary groups of contaminants exist namely organic, inorganic, and radioactive. 24 Consecutively, there are numerous classes of contaminants under each of these groups. The main categories of contaminants are outlined in Table 1. Numerous chemicals are emitted into the surroundings daily. Therefore, during the conduction of site appraisal surveys, it is imperative to specify the type of contaminants being assessed.
Table 1.
Categories of contaminants.
| S/N | Types | Examples |
|---|---|---|
| 1 | Organic Contaminants | ● Petroleum hydrocarbons (fuels)—benzene, toluene, xylene,
polycyclic aromatics, MTBE. ● Chlorinated solvent-trichloroethene, tetrachloroethene, trichloroethane, carbon tetrachloride ● Pesticides—DDT, toxaphene, atrazine, Polychlorinated biphenyls—PCBs ● Coal tar—polycyclic aromatics ● Pharmaceuticals/food additives/cosmetics—drugs ● Pharmaceuticals/food additives/cosmetics—drugs, surfactants, dyes |
| 2 | Inorganic Contaminants | ● Inorganic “salts”—sodium, calcium, nitrate, sulfate,
chloride ● Heavy/trace metals—lead, zinc, cadmium, mercury, arsenic, selenium |
| 3 | Radioactive Contaminants | ● Solid elements—uranium, strontium, cobalt,
plutonium ● Gaseous elements—radon |
Classes of toxicants
Heavy metals
Heavy metals have no broad or standard yardstick of definition. On the basis of setting or assumption, numerous connotations may be ascribed to the term. 17 In chemistry, its definition is based on chemical behavior, 25 while for the Physicist; the distinct benchmark for heavy metals definition is atomic number, 26 whereas in metallurgy, it is defined by density. 27 The name “Heavy metals” refers to elements with a moderately high density which is lethal even at diminutive concentrations. It is a broad concept that is appropriate to a class of metals and metalloids whose atomic density is more than 4 g/cm3, or five times or heavier than water.25,28 However, heavy metals are basically classified by chemical characteristics other than by specific gravity; and these include copper (Cu), silver (Ag), iron (Fe), lead (Pb), arsenic (As), mercury (Hg), cadmium (Cd), Zinc (Zn), chromium (Cr), and the platinum group elements. 29 They are intrinsically distributed in disproportionate doses in the environment. They either exist in elemental form or are combined in chemical compounds with other elements. Heavy metals are often conveyed over a wide range either as volatile or fine aerosols. This group of metals becomes deleterious to the health of humans when their concentration surpasses the maximum acceptable limits in the surrounding. 30 Their virulence in ecosystem depends exclusively on their chemical properties and behaviors. 17
Heavy metal pollution in the ambiance occurs via diverse pathways. Owning to their persistence, they permeate the environment for several years after their initial deposition; and contamination of the environment often takes place through weathering fraction. 31 They bioaccumulate in soil and crops via natural and man-made processes, and subsequent impacts posed critical problems in the food chain via biomagnification. 32 The principal port of entry in the human community is through ingestion and dermal. 33 A common way by which adults is exposed is through industrial activities whereas in juveniles is by ingestion. 34 Inappropriate therapy, radiological techniques and cracked thermometers are less consequential means of exposure.35,36 Some of the health outcomes of their bioaccumulation in the body are the substitution of essential ions, cell membrane impairment, interference with phosphate ions & sulfhydryl (SH) groups, and competition with vital metabolites for binding sites. 37
Agricultural chemicals (pesticides)
The word “agricultural chemicals” has been widely substituted by the term “pesticides.” Pesticides can be described as a group of chemicals or agglomeration of chemicals that are widely utilized in farming or in quarantine programs to control, annihilate, or fight off infections or nuisance from the plant; and protect humans from disease vectors. Typical classes of pesticides include fumigants, herbicides, fungicides, rodenticides, insecticides, and plant growth regulators.38–40 Pesticides are atypical amidst toxicants because they are applied purposely for the annihilation of certain living things. Ideally, pesticides are expected to be selective, eradicating selected species whilst sparing non-target organism. In reality, most pesticides are unspecific in their action. Taking into consideration the application of pesticides, the gains must be juxtaposed with the threat to environmental safety and mankind salubrity. Important factors to be considered are persistence in the surroundings and propensity for bioaccumulation. Susceptibility to these chemicals can be by inhalation, ingestion, or dermal contact. The possible health consequences are governed by the variety of pesticides, the time span and means of vulnerability, and the underlying health conditions of the individual. Inside the living system, pesticides may be metabolized, excreted, or bio-accumulated in the body’s fat.38,39,41 Numerous inimical health outcomes attributed to chemical pesticides exposure include but not limited to gastrointestinal, carcinogenic, dermatological, endocrine, and neurological effects.42–44 In addition, inadvertently, purposively, or occupational exposure to pesticides can lead to hospitalization and demise.
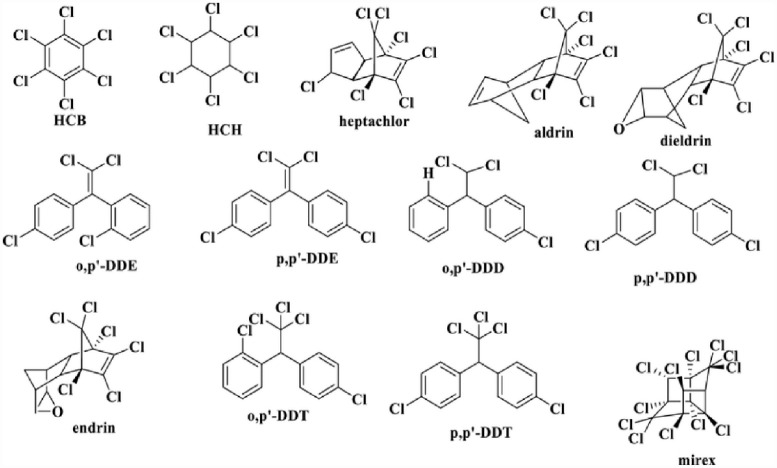
● Organochlorine Pesticides: The chlorinated hydrocarbon insecticides came to limelight in the middle of the 20th century, and include common chemicals like dichlorodiphenyltrichloroethane (DDT), chlordane, heptachlor, dieldrin, aldrin, methoxychlor, toxaphene, mirex, endosulfan, dicofol, lindane, endrin, mirex, and toxaphene. The structures of some familiar organochlorine pesticides are shown in Figure 1. The most popularly known organochlorine pesticide is DDT, whose indiscriminate utilization raised numerous environmental and public health concerns .38,45,46 It is ubiquitous and generally conceived to be present in all living beings where it is primarily bioaccumulated in fatty tissue. These groups of pesticides are toxic to the nervous system where they disrupt nerve impulses transmission.
● Organophosphorus Pesticides (OPs): are among the popularly used pesticides made up of phosphoric acid esters or thiophosphoric acid esters (Figure 2). They include common pesticides, like parathion, dimethoate, and malathion; many of which are famous for their hormone interference potential.43,47,48 Among the OPs, the most widely utilized are glyphosate because of its environmental friendliness over the organochlorines. 49 These groups of pesticides are linked with disruption of cholinesterase enzymes function, 49 the decline in insulin production, disturbance of regular cellular metabolism of carbohydrates, lipids and proteins, 50 genotoxic impacts, 51 and impede the activities of mitochondria, instigating oxidative stress and impairments of the endocrine and nervous system. 50
● Carbamate Pesticides: These are esters of N-methyl (or occasionally N,N-dimethyl) carbamicacid (H2NCOOH). The virulence of this chemical differs based on the alcohol or phenol group. A popularly utilized carbamate insecticide is carbaryl (1-napthylmethylcarbamate), a far-ranging insecticide (Figure 3). It is generally applied as dust in the agriculture, including home gardens. Carbaryl is not regarded as a persistent compound, due to its rapid hydrolysis. Other carbamate pesticides, such as ziram, aldicarb, and carbofuran have been linked with hormone-disrupting property,43,52 probable reproductive defects,52,53 and interference with mitochondrial roles and cellular metabolic actions. 50
● Other Classes of Chemical Pesticides: Botanical insecticides have been in application since ancient times to control insects and are extracts obtained from plants. An example is Nicotine [(S)-3-(1-methyl-2-pyrrolidyl)pyridine], an alkaloid found in several plants, whose insecticidal property was first utilized in 1763. Nicotine is partly toxic dermally and orally. Another botanical insecticide is Pyrethrin (Figure 4), which is an extract obtained from numerous kinds of chrysanthemum, and is among the foremost pesticides utilized by man. It is used at low concentrations and is regarded as non-persistent. Synthetic pyrethroids like permethrin, sumithrin, and fenvalerate, are viewed to be among the innocuous insecticides presently use for agricultural and public health purposes.54,55 Nevertheless, there is proof of their potential to exhibit hormone-interference activity,43,56,57 and to influence reproductive variables in experimental animals.56,58
Figure 1.
Structures of selected organochloride pesticides.
Figure 2.
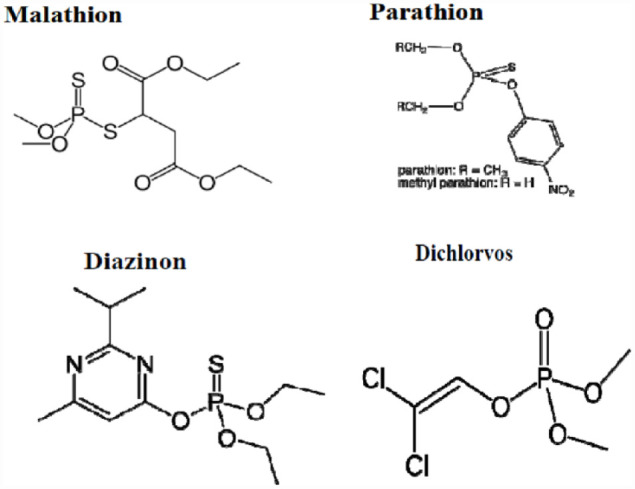
Chemical structures of main organophosphate pesticides.
Figure 3.
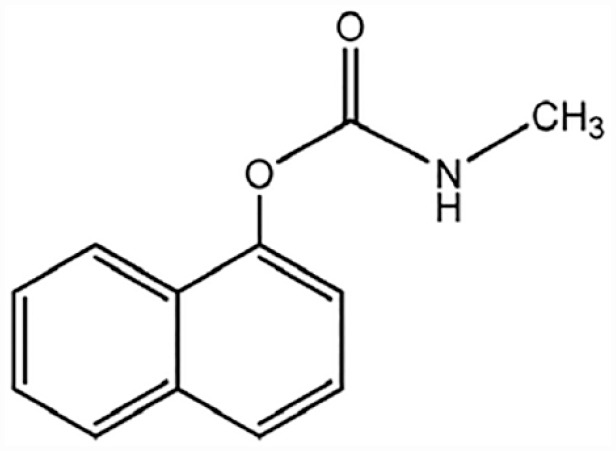
Structure of Carbaryl (insecticide carbamate).
Figure 4.
Structure of Pyrethrin.
Triazines, viz simazine, ametryn, and atrazine, are another group of chemical pesticides. Atrazine is popular among the triazines, and it has been basically linked with oxidative stress that leads to endocrine-disrupting consequences and reproductive toxicity, 59 cell toxicity,52,60 and dopaminergic effects. 61 Neonicotinoid pesticides, like thiacloprid, guadipyr, and imidacloprid, are quite new and widely utilized insecticides, 62 that were popular because of the minimal danger they posed to non-target organism. 63 However, research conducted in 2016 denoted that neonicotinoids are capable of accelerating the activity of aromatase, an enzyme involved in breast cancer, and also plays a vital part in its carcinogenesis timeline. 64
Food additives and contaminants
Chemicals are introduced into food for several reasons: as preservatives with antioxidants, antibacterial, or antifungal features; to modify physical properties, especially for processing; to adjust color; and to modify odor. In totality, food additives have been established to be innocuous and lack long term toxicity. Several of them were introduced at a period when toxicity testing was at its rudimentary; albeit, some of these have been afterward proven to be toxic, such as the type of the inorganic, the most important of which are nitrite and nitrate. Definitely, several of the food additives currently in use globally, lack proper testing. The issue of synergism among these compounds is poorly explored. Moreover, not all toxicants in food are artificial; there are some that are natural. 65
Solvents
These are commonly found in the workplace although they can still be present in residences. In addition to cutaneous consequences, such as local irritation and defatting; several have systemic toxic impacts, such as impacts on the nerves or, as with benzene, on hematopoietic elements. The well-known solvents fall into the below classes 66 :
● Glycols and Glycol Ether: Ethylene and propylene glycols, for instance, in antifreeze give rise to significant exposure in the general population.
● Aliphatic Hydrocarbons: like hexane. These may be straight or branched-chain compounds and often occur in mixtures.
● Aromatic Hydrocarbon: Benzene is probably of greatest interest in this group; however others, like toluene are also of public health interest.
● Halogenated Aliphatic Hydrocarbons: The most popular examples are chloroform, carbon tetrachloride, and methylene dichloride; nevertheless chlorinated ethylenes are also given wider attention.
● Aliphatic Alcohols: Popular examples are methanol and ethanol.
Drugs of abuse
Drugs of abuse (Figure 5) can be defined as drugs that either have no medicinal importance or are used at dose concentrations that exceed the dosage needed for treatment. Albeit many of these substances alter only the higher nervous function like reaction time, coordination, and mood; some cause physical addiction and have consequential physical impacts, with fatal overdoses being a periodic phenomenon. This class of drug includes central nervous system (CNS) depressants, viz secobarbital, ethanol, methaqualone (Quaalude); CNS stimulants, like caffeine, nicotine, cocaine, and methamphetamine (speed); opioids, viz mependine (demerol) and heroin; and hallucinogens like phencyclidine (PCP), lysergic acid diethylamide (LSD), and tetrahydrocannabinol the most active component of marijuana. 67
Figure 5.
Chemical structures of the investigated drugs of abuse.
Cosmetics
The well-known harmful upshots of modern cosmetics are sporadic allergic reactions and contact dermatitis. Although, the organometallics and aromatic amine dyes or azo employed in ancient times are now obsolete. Bromates and Thioglycerol applied in some cold-wave neutralizers and lotion may be markedly injurious if ingested; ditto ethanol applied as a solvent in perfumes and hair dyes. Applied as instructed, cosmetics have seen to pose a limited risk of systemic poisoning, owing partly to the elimination of hazardous constituents in them and partly to the minute amount absorbed. 24
Combustion products
Most air pollutants are from natural and man-made combustion. In terms of human health impacts, the most prominent are the Polycyclic Aromatic Hydrocarbons (PAHs). Albeit they are present in natural products like crude oil and coal, and commonly linked to incomplete oxidation of organic substances. Smoke from coal, wood, tobacco, and oil, for instance, in broiled foods also emits these contaminants. Due to the carcinogenicity of some of these substances, they have been studied diligently from the position of interactions with DNA, metabolic activation, and other facets of chemical carcinogenesis. 24
Toxicity of some selected environmental toxicants
Toxic effects of mercury
Mercury is a popular poisonous metal and its lethal is a frequent cause of acute heavy metal poisoning as reported by the American Association of Poison Control Centers with cases of 3596 in 1997. 68 Methylmercury is a neurotoxic compound that is liable for microtubule ruination, lipid peroxidation, mitochondrial impairment, and accretion of neurotoxic like glutamate, serotonin, and aspartate. 69 It is postulated that 8% to 10% of American females possessed inherent mercury levels that may likely trigger neurological abnormalities in the infants they birthed, as enunciated by both the National Academy of Science and Environmental Protection Agency. 70 Animal studies have affirmed that exposure to toxic mercury caused inimical neurological and behavioral modifications in animals. The primary target organ for mercury is the brain; although it can impair other organs and result in a defect in the muscles, nerves, and kidneys. It leads to derangement of the membrane potential and disrupts intracellular calcium homeostasis. Mercury attaches to free unconjugated thiols as the affinity constants are high. 69 Mercury vapors can result in asthma, bronchitis, and short-term respiratory difficulties. Mercury contributes significantly to the damaging of tertiary and quaternary protein structure and modifies the cellular properties by binding to the selenohydryl and sulfhydryl groups that react with methyl mercury and disrupt the cellular structure. It also interferes with the transcription and translation process leading to the obliteration of ribosomes and destruction of endoplasmic reticulum and natural killer cells properties. In addition, compromise the cell integrity that results in free radical production. 68
Toxic effects of lead
Lead metal brings about injury to the cellular component sequel by ionic mechanism and that of oxidative stress. Numerous studies have demonstrated that oxidative stress in biological systems is instigated by the disequilibrium between the free radicals generation and antioxidants production to neutralize the transitional or mend the outcome impairment.17,68 The potential of lead metal ions to substitute other bivalent cations like Mg2+, Ca2+, Fe2+ and monovalent cations such as Na+ is primarily responsible for its ionic toxicity effects and resultant impairment of the metabolic activities in a biological system. Lead toxicity arising from ionic mechanisms is responsible for adverse alterations in diverse biological activities including protein folding, cell binding, apoptosis, intra- and inter-cellular signaling, maturation, protein folding, enzyme regulation, active transport, and neurotransmitter production. Lead has the capacity to replace calcium even at picomolar concentration altering protein kinase C, which modulates memory storage and neural excitation. 71
Toxic effects of arsenic
The toxic impacts of Arsenic can either be short or long-term. Chronic Arsenic Toxicity (CAT) is termed arsenicosis. Numerous reports of CAT in humans center on dermal externalizations due to its particularity in diagnosis. Keratosis and pigmentation are the unique skin aberrations that point to CAT. 72 Lower exposure levels to arsenic can trigger gastrointestinal disorders, decreased blood cell production, irregular heartbeat, vascular vessel impairment, and prickling sensation at the extremities. Chronic exposure can result in the development of internal cancer, skin lesions, respiratory disease, neurological malignancies, hypertension, vascular disease, diabetes mellitus, and cardiovascular disease. 73 Long-term arsenicosis leads to permanent modifications of the essential organs with an attendant high death rate. Despite the enormity of this imaginably deleterious toxicity, there is the absence of an effective remedy for this malady. 74
Toxicity of persistent organic pollutants (POPs)
The outcomes of acute and chronic exposure to POPs are still ambiguous. However, laboratory probing as well as environmental impact studies in the natural surrounding have stipulated that POPs can lead to immune and reproductive disorders, hormonal disruption, central nervous system (CNS) dysfunction, cancer and developmental anomalies. A few organochlorine chemicals are possibly carcinogenic by aiding tumor development. Polychlorinated Biphenyls (PCBs) [are a group of over 200 anthropogenic and artificial chlorinated and organic chemicals with the same basic chemical structure] are categorized as probably carcinogenic to man, while an additional eight of the 12 other POPs pinpoint in the Stockholm Convention are ranked as possibly carcinogenic to mankind. The leftover three—endrin, aldrin and dieldrin are grouped by the World Health Organisation as very deleterious (class 1b) owing to their short-term toxicity to laboratory animals. Fetuses and newborns are especially susceptible to POPs exposure because of the transmission of POPs from the mother during the crucial period of gestation. Susceptibility in the course of development has been associated with decreased immunity, CNS damage, developmental anomalies, and cancer/tumor induction or promotion in neonates and juveniles. The likelihood of causing human breast cancer has also been reported. 75
Table 2 depicted the list of 12 POPs that are humans’ carcinogenic agents as highlighted by the International Agency for Research on Cancer during the Stockholm Convention.
Table 2.
Classification of POPs according to International Agency for Research on Cancer.
| IARC Classification | POPs |
|---|---|
| Group 1: The agent (mixture) is carcinogenic to humans. | 2,3,7,8-Tetrachlorodibenzo-para-dioxin (TCDD) |
| Group 2A: The agent (mixture) is probably carcinogenic to humans. | Mixtures of polychlorinated biphenyls (PCB) |
| Group 2B: The agent (mixture) is possibly carcinogenic to humans. | Chlordane DDT Heptachlor Hexachlorobenzene Mirex Toxaphene (mixtures of Polychlorinated camphenes) |
| Group 3: The agent (mixture or exposure circumstance) is unclassifiable as to carcinogenicity in humans. | Aldrin Dieldrin Endrin Polychlorinated dibenzo-para-dioxins (other than TCDD) Polychlorinated dibenzofuran |
Toxicity of polycyclic aromatic hydrocarbons (PAHs)
Each of the PAH compounds exhibits unique health outcomes.76,77 Several PAHs are teratogenic, carcinogenic, mutagenic, and immunotoxic to innate beings, such as microbes, animals, and humans.76,78,79 The manner of exposure, period, and dosage are crucial variables for determining the virulence of PAHs’ toxic outcomes. 80 Fatal outcomes of PAHs may differ based on variables such as age and underlying health state. Short-term health impacts include regurgitation, eye soreness, disarray, skin irritation, diarrhea, and inflammation. 78 Anthracene, benzo(a)pyrene, and Naphthalene are explicit skin irritants and skin sensitizers for animals and humans. 76 Long-term impacts are renal and hepatic derangement, eye cataracts, respiratory distress, pulmonary dysfunction, asthma-like symptoms and compromised immunity. 77 Naphthalene can bring about the degradation of erythrocytes if inhaled or ingested in large quantities. 76
Overview of phytochemicals
What are phytochemicals?
The term “phytochemicals” refers to a perplexing number of small molecules from plants, which can be categorized into diverse marked classes based on their biosynthetic origin and structure. On a wider definition, it means plant (Phyto) chemical denoting a large diversity of compounds that are found in plants naturally. A great many bioactive phytochemicals occur in popularly consumed plant foods and products. Broadly, these compounds can be classified into six main categories based on chemical structures and properties. These groups are carbohydrate, lipids, terpenoids, phenolics, alkaloids, and other nitrogen-containing compounds. 81 Each group can be subdivided based on biosynthetic origin or biogenesis into different subgroups. Contemporarily, the word “phytochemical” has been employed to differentiate plant chemicals that do not satisfy the traditional meaning of “essential nutrients.” Many of these phytochemicals trigger reactions in living organisms, including humans, by expressing both therapeutic and prophylactic features that have been associated with the provision of nutrient for normal cell growth and repair, impede carcinogens and acts as antioxidants, and declines the risk of major non-communicable chronic diseases.82,83
Phytochemicals wield their medicinal prowess by acting synergistically or additively and this nullifies the precarious outcomes linked to the high usage of a mono xenobiotic compound, thus giving the herbal drug(s) a broad spectrum of activity, in addition to diminishing the probability of the pathogens developing resistance or adaptive responses.84,85 Some phytochemicals are enunciated in the subsequent sub-section.
Classes of phytochemicals
Flavonoids: are structural derivatives of flavones (see Figure 6). Consisting of conjugated aromatic systems, often bind to sugar(s) as glycosides, and they are phenolic and hydrophilic in nature. 86 Flavonoids are a vital class of natural products; they are in the group of plant secondary metabolites possessing a polyphenolic structure, and are generally found in vegetables, fruits, and some beverages. 87 Flavonoids are linked with a wide range of health-enhancing properties and are invaluable constituents in diverse pharmaceutical, nutraceutical, cosmetic and medicinal applications. This is due to their anti-inflammatory, antioxidative, anti-carcinogenic and anti-mutagenic characteristics coupled with their ability to regulate vital cellular enzyme functions. Likewise, they are regarded as powerful inhibitors for numerous enzymes, viz cyclo-oxygenase (COX), xanthine oxidase (XO), phosphoinositide 3-kinase, and lipoxygenase. 88 Flavonoids have numerous subgroups, such as flavones, isoflavones, flavanones, chalcones, flavanonols, flavonols or catechins, chalcones, and anthocyanins. These subgroups have distinct major sources. 89 Flavonoids lacking hydroxyl (-OH) on their structure are more potent against microbes vis-à-vis those with –OH, and this corroborates the fact that their target in microbes is the membrane. 83
Figure 6.
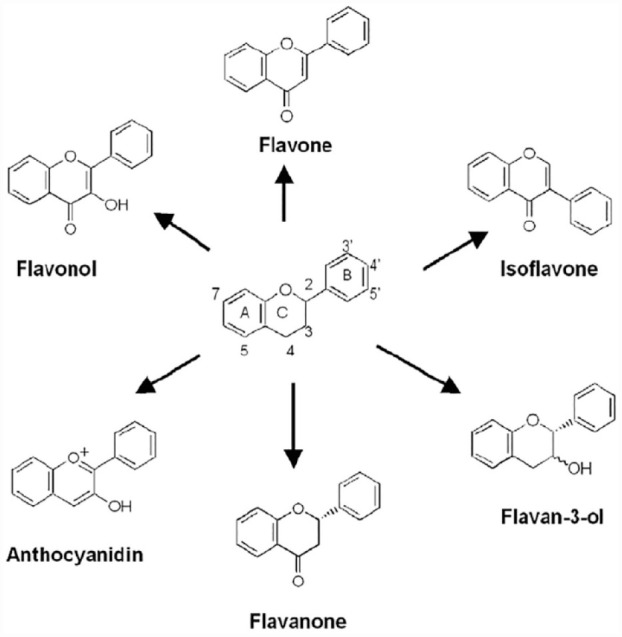
General structure of flavonoids.
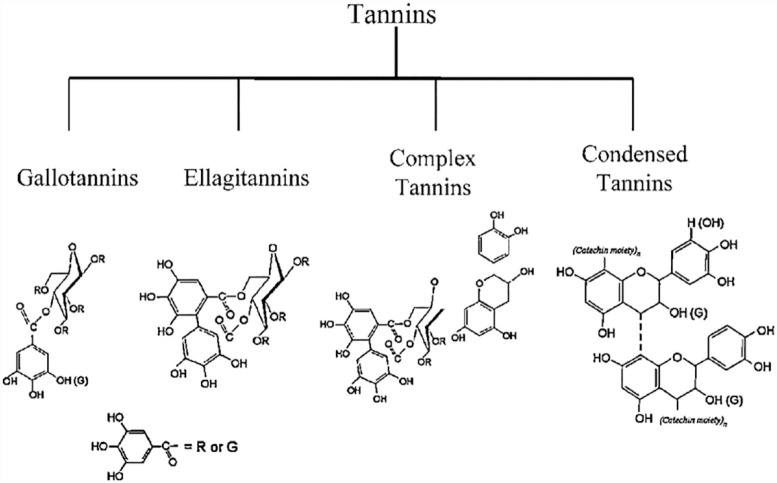
Tannins: these are caustic, pungent phenolic compounds that either coarse or precipitate or contract proteins. They consist of numerous groups of oligomers and polymers. They can adhere to cellulose, minerals, proteins and starch to form complexes. Tannins are hydrophilic compounds with the exclusion of some higher molecular weight structures. Tannins are generally categorized into two groups namely: hydrolyzable tannins such as elligatannin, complex tannins, proanthocyanidins, and gallotannins; and condensed tannins (Figure 7). These compounds are usually found in both gymnosperm and angiosperm. They have been discovered in 180 families of dicotyledons and 44 families of monocotyledons. Tannins are commonly found in the following families of plant species: Actinidiaceae, Burserarceae, Bixaceae, Anacardiceae, Dipterocarpaceae, Combertaceae, Grossulariaceae, Ericaceae, Myriacacea for dicotyledons; whereas Typhaceae and Najadaceae for monocotyledons. They exhibit their physiological function by behaving as antioxidants via free radical scavenging action.83,90 thus regulating oxidative stress and averting deteriorating illness. They also hinder tumor growth by activating cell death 91 and impeding the mutagenicity of carcinogens.83,92
Figure 7.
Main chemical structures of the tannins.
Saponins: refer to a group of bioorganic compounds that are found largely amidst plant biodiversity. More precisely, they are naturally occurring glycosides with soapy properties, and as a result, they generate bubbles when agitated in aqueous solutions. 93 Structurally saponins possess one or more hydrophilic glycoside sugar moieties (Figure 8) aggregated with a lipophilic triterpene molecule. 94 Studies depict that saponins exercise biological function and therapeutic effects like hemolytic factor, anti-inflammatory, antifungal, antibacterial, antiviral, anticancer, cytotoxic, molluscicidal and insecticidal action.95–97 The saponin molecule undergoes hydrolysis to give rise to two fragments, an aglycone and a sugar moiety. Isolated amorphous solid saponins have large molecular weight, and contain 27–30 carbon atoms in the non-saccharide fraction (the hydrocarbon skeleton part lacking a sugar chain). This non-saccharide segment referred to as sapogenin, aglycone, or genin. Based on the kind of sapogenin available, the saponins can be segregated into three distinct groups: triterpenoid glycosides, steroid glycosides, and alkaloid glycosides. 98 The second partition that is, the saccharide moiety—consists of heterogeneity of pentoses or hexoses. 93
Figure 8.
Generic structures of saponins R = sugar moiety.
Alkaloids: These are a large class of naturally existing organic compounds with nitrogen atom or atoms (amino or amido in certain instances) in their structures often located in some cyclic or ring configuration (Figure 9). The presence of nitrogen in these compounds is responsible for their alkalinity. Broadly, based on structure, alkaloids can be classified into groups like quinolones, pyrrolidines, isoquinolines, indoles, tropanes, steroids, pyridines, pyrrolizidines, and trepenoids. Another categorization modality is based on the family of plant species in which these substances are found. A typical example is the opium alkaloids that are found in the opium poppy (Papaver somniferum).99,100 These two dissimilar classification systems create a disparity between their biological distribution and the chemical varieties of alkaloids because there is no distinctive connection. In its pure form, alkaloids are often odorless, crystalline solids, colorless, although often yellowish and have an astringent taste at times. Presently, over 3000 alkaloids are identified in more than 4000 diverse plant species. Alkaloids are a fascinating class of compounds with a divergent range of functionalities in man and animals. Alkaloids have various physiological outcomes: antimitotic, local anesthetic, antibacterial, psychotropic, analgesic, hypnotic, antitumor, anti-inflammatory and several others. Popularly known alkaloids are caffeine, nicotine, ephedrine, strychnine, quinine, atropine, and morphine.99,100
Figure 9.
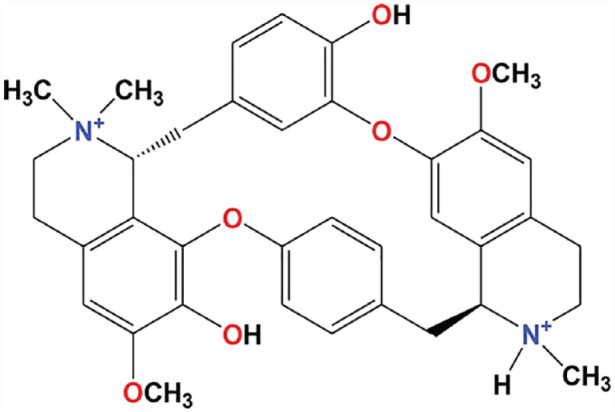
General structure of Alkaloids.
Cardenoloids: These are also known as cardiac glycosides and they consist of the most drug-like molecules subjugated to numerous examinations and were affirmed to be effective in manufacturing efficacious drugs.101,102 These groups are liable for being virulent to livestock and serve as therapy for congestive heart failure. Cardenoloids are steroids capable of exerting a precise forceful effect on the myocardia. A minute quantity can affect a salubrious simulation on an ailing heart. These compounds are beneficial in the treatment of congestive heart failure, flatter, atrial fibrillation, and they behave as emetics and as diuretics. 83 They speed the force of contraction in the heart, which is incapable of meeting the rise in oxygen expenditure. As a result, the myocardium acts a potent pump and is capable of satisfying the need of the vascular system.102,103 Cardiac glycosides consist of two distinct categories of compounds that vary in their aglycone structure (Figure 10). They may be C23 or C24 steroids with a primary nucleus of cyclopentanoperhydro phenanthrene substituted at C17. Cardenolides possessed a five-membered lactone group in the C17 with α, β—unsaturated γ-lactone ring (butenolide), while the other class, the bufadienolides, was first identified in toad as skin lethal. The C17 substituent has a doubly unsaturated six-membered lactone ring (α-pyrone). Plants are capable of producing both cardenolides and bufadienolides. A different group is isocardenolides, which possessed a double bond of butenolide ring at position 21 or 22 rather than at position 20. Most clinical interests were pointed to the cardenoloides due to their medicinal importance. The two commonly utilized digitalis inotropes are Digoxin and digitoxin. Generally, most isocardenolides lack any cardiac activity. 102
Figure 10.
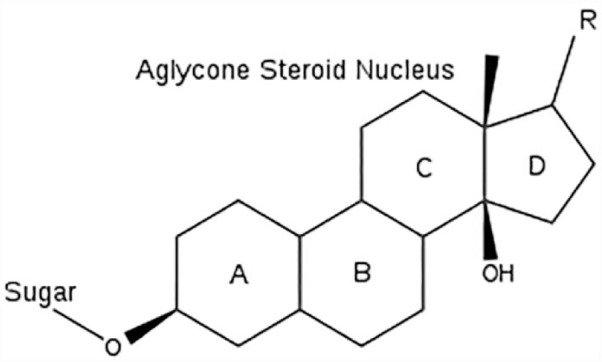
Structure of Cardiac glycosides.
Terpenoids: otherwise called isoprenoids, are a wide and divergent class of naturally existing compounds whose precursors are five carbon isoprene units (Figure 11). Terpenoids distinguished from one another by their basic skeleton and functional groups. Terpenoids are ubiquitous; hence, they occur virtually in all living beings. They add to the scents, color, and flavor of plant’s flowers, leaves and fruits. Terpenoids are also crucial for plant growth and development. The terpenoids generated by plants not only safeguard them from insects and predators but also offer defense from fungal diseases and infections, while in animals, terpenoids serve as precursors of steroids and sterols. Terpenes are simple hydrocarbons, whereas terpenoids are an adjusted class of terpenes with varying functional groups and oxidized methyl groups oscillating at different positions. Terpenoids are categorized into monoterpenes, sesquiterpenes, diterpenes, sesterpenes and triterpenes based on their carbon units. Terpenoids differ in structures and are biologically viable and utilized globally for the therapy of numerous maladies. Several terpenoids impeded diverse human cancer cells and are employed as anticancer medicines such as Taxol and its derivatives. Terpenes and its derivatives are utilized as antimalarial drugs such as artemisinin and associated compounds. They performed various functions viz anti-fungal, anti-protozoan, anti-bacterial, anti-allergens, anti-viral, immune boosters and antineoplastic.83,104,105
Figure 11.
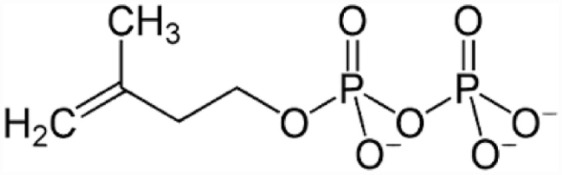
Structure of Terpenoids.
Phytosteroids: these are groups of specialized metabolites obtained from plants that are attached to steroid receptor in animals and can activate or suppress downstream receptor-mediated signaling events. Phytosteroids (Figure 12) have varying structures, occasionally very dissimilar from the animal steroids; however, they behave as antagonists, agonists, or habitually exhibit mixed agonist/antagonist properties for steroid receptors.106,107 Furthermore, some phytosteroids interrelate with several steroid receptors 108 or hamper steroid metabolizing enzymes, 109 hence exhibiting complicated impacts on the reproductive and hormonal systems. They possess a similar basic cyclic configuration to animal steroids, nevertheless they differ due to different chemical groups attached to the central ring in various positions. 83 They are basically used in the treatment of reproductive ailments viz treatment of sexually transmitted diseases, enhance fertility in women and sex drive in males, and ensure safe parturition. They also behave as sex hormones derivatives and thus they are a possible sources of contraceptives.83,98,110 They are also analgesic, anti-microbial, anti-inflammatory, and of use in curing of stomach complications and in reducing serum cholesterol levels.83,98 Phytosteroids are marked as powerful inhibitors of macrophage activation, impeding the generation of pro-inflammatory cytokines and LPS-induced fatality and consequently they can act as immunosuppressive agents particularly the physalins.83,111
Figure 12.
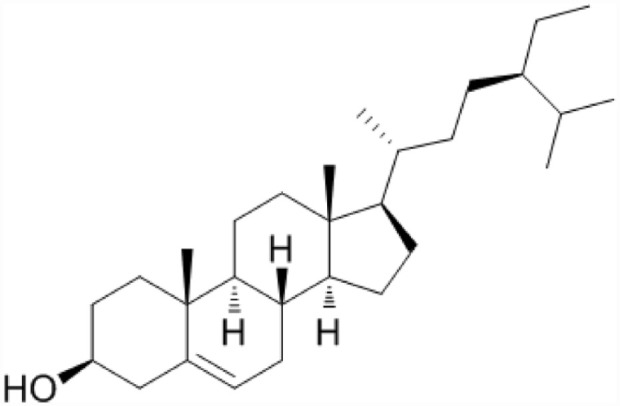
Structure of Phytosterol.
Interventional roles of phytochemicals on various toxicity outcomes
Intervention in oxidative stress, metabolic syndrome and aging
Since time immemorial, it has been known that natural products prolonged the life-span of an organism. 112 Epidemiological and laboratory studies suggested these compounds to be potent antioxidants that ameliorate stress-related ailments. Various studies have postulated the efficacy of these compounds in metabolic symptoms and aging.113,114 Consequently, comprehensive preclinical assessment on the fundamental pharmacology of these natural compounds may serve as strong scientific basis for clinical implementations. Polyphenols and most especially flavonoids have been proven to offer protection from several age-associated morbidities. 115 Numerous studies have shown that augmentation with dietary polyphenols like (-)-epigallocatechin-3-gallate (EGCG) and curcumin can enhance age-related cellular impairment by decreasing production of reactive oxygen species (ROS). 116 Conversely, resveratrol and pterostilbene are regarded as outstanding anti-aging chemicals that can regulate oxidative destruction, cell aging, and inflammation; constituents linked with aging 117 including flavonoids which is proven to aid anti-aging primarily by regulating metabolic syndrome. 118
A few of the proven flavonoids that can handle factors linked to senescence or metabolic syndrome are naringin, hesperidin, and naringenin. 119 Importantly, there is an increase in the data bank of preclinical application of phytochemicals in the therapy of diverse conditions attributed to senescence. Nevertheless, certain limitations exist to their applications such as (1) option of experimental models that is clinical pertinent, (2) nebulous elucidated mechanism of action, and (3) appropriate dosage and duration for data interpretation.
As anti-cancer agent
In spite of the fact that significant progress has been made, some tumor still pose poor prognosis and study is still ongoing toward the utilization of innocuous doses of plant-extracted substances. The innovation to treatment strategy due to natural molecules and drugs was instigated by the recognition and application of natural chemotherapeutic substances vis taxanes, anthracyclines, and vinca alkaloids. 120 Thus, it is reasonable to postulate that compound in foods are presumably to have some protective impacts.
Intriguingly, contemporary research findings suggest an increasing function of polyamines metabolism as an innovative approach against inflammatory ailments. Polyamines are naturally occurring aliphatic compounds, ever-present in all living thing, which interface with nucleotides and proteins and are needed for the growth, viability and specialization of eukaryotic cell.114,115,121,122 Based on the aforementioned roles, polyamines metabolism constitutes an intriguing target for anticancer treatment. 123 Hence, the application of various natural substances, especially polyphenols, emanating from plants constituents may produce auspicious outcomes in antitumor treatment due to their anti-oxidant properties. 124 Interestingly, it has been shown that high flavones intake, particularly quercetin and kaempferol, is capable of inducing a significant decrease in serum IL-6 concentration, a popular inflammation-associated cytokine. 125 Likewise curcumin depicts anti-inflammatory and antioxidant characteristics, and prospective anti-cancer property. 126 Genistein a soybean isoflavone, has exhibited antitumor property in diverse types of cancer including chronic lymphatic leukemia and neuroblastoma and in diverse organs like ovary, prostate, breast, colon, urinary bladder, stomach, and stomach. 127 Genistein is regarded as a phytoestrogen because of its structural similarity with mammalian estradiol. Numerous studies have shown the consequential function of isoflavone in the prevention of cancer development in animal models. Indeed, it is proven that dietary soy supplementation mitigate inflammation associated with prostate carcinogenesis. 128 Another popular anticancer agents possessing both anti-inflammatory and antioxidant feature is lycopene that exhibits treatment actions on numerous tumors. 129 Specifically, it has been established that lycopene ingestion hinders the development of cancer. 130 The recognition of the importance of these natural compounds to the advancement of cancer treatments are well documented nonetheless it is crucial to conduct more meticulous researches and preclinical studies to shed more light on their prospective chemopreventive and antitumor activities.
As therapy for vascular diseases
In this review we shall discuss only the therapeutic impacts of omega 3 polyunsaturated fatty acids (PUFA) and the flavonoid resveratrol due to their prevailing effects on Senescence-Associated Secretory Phenotype (SASP), vascular dysfunction, and cardiovascular disease (CVD).
The effects of omega 3 PUFAs are ascribed to their lower lipid effects which help to decline atherosclerosis development. 131 Omega 3 PUFAs have been demonstrated to mitigate vascular inflammation by down-regulating adhesion molecules and restricting leukocytes binding to the wall of blood vessel. 132 This latter explicitly sway endothelial-derived nitric oxide production owning to stabilization of lipid rafts like the endothelial cells caveolae, as shown in retinal endothelial cells. 133 Nevertheless, the laboratory studies seem to corroborate PUFA’s beneficial roles vis-à-vis the clinical proof. Actually, the omega 3 PUFA outcomes on aggrandized endothelial regenerative ability and preservation of vascular endothelial cells homeostasis by virtue of membrane stabilizing potential were marked to have consequential effects on CVD prevention. 133 A well-detailed appraisal of the literature recently displayed on Cochrane Database Systematic Review, 134 encapsulated the findings of numerous randomized clinical trials investigating the impacts of varying doses of PUFA on CVD manifestations. Findings from this survey depicted that higher PUFA intake only moderately lessen risk of coronary heart disease and CVD acute events (i.e. stroke), but in generality has no important influences on its outcomes. A large number of the positive outcomes were linked to modulation of lipid metabolism. 91 Anyway, even a little but notable decline in 10% of morbidity and death for CVD linked to PUFA supplementation remains a momentous clinical outcome. 135 Conclusively, other phytochemicals with antioxidant properties have been highlighted to contribute a protective action to CVD development and consequently were recommended as significant substances in diet, for example like, β-carotene, curcuma, and others.136,137 Specifically, numerous studies underscore the anti-atherogenic role of lycopene in relation with the interdiction of proinflammatory cytokines release. 138
Role as anti-inflammatory
It has been demonstrated that natural compounds influence numerous pro-inflammatory mediators. Herbal medicines, nutraceuticals, or beneficiary foods with anti-inflammatory properties, can be utilized as an adjunct to anti-inflammatory drugs resulting in the decline of their consumption, leading to the mitigation of their side effects. A comprehensive review on the anti-inflammatory properties of phytochemicals has been done by several researchers. 139 Various anti-inflammatory mechanisms have been linked to numerous flavonoids with diverse chemical configurations. 140 Glycosides of apigenin and luteolin are widely distributed flavones. 141 Apigenin subdue nitric oxide (NO) and prostaglandin production through hampering of inducible nitric oxide synthase (INOS) and COX-2, appropriately. 142 Luteolin was also demonstrated to impede chronic inflammation by in vitro co-culture of adipocytes and macrophages and the phosphorylation of Jun N-terminal kinases (JNK) in macrophage. 143 The most efficacious tumor necrosis factor—α (TNF-α) inhibitors among the flavonoids are luteolin and quercetin. 144 The anti-inflammatory potency of quercetin was clinically assessed in women with rheumatoid arthritis showing an important impact in modulating inflammation and clinical manifestations. 145 In spite of their function in the production of TNF-α, green tea extract has also been shown in contemporary clinical trials to have anti-inflammatory and immunomodulatory properties in autoimmune disease. 146 Consequently, although these compounds cannot replace anti-inflammatory drugs, such as the Disease Modifying Anti-Rheumatic Drugs (DMARDs), but they significantly aid to the decline in their dosage, leading to less costly and innocuous treatment strategy of autoimmune diseases and other inflammation-associated morbidities.
Neurodegenerative diseases prevention
Presently, there is no panacea treatment for neurodegenerative diseases (NDD), and, in a bid to discover new treatment or adjuvant approach for NDDs, numerous natural medicinal plants have attracted interest as prospective neuroprotective agents, and several researches have alluded to the fact that a diet enrich with vegetable products can avert or prolong the initiation of NDD. 147 These attributes may be associated to the presence of polyphenols, a crucial group of phytochemicals that are readily found in vegetables, beverages, fruits, and cereals. In this section we shall discussed the auspicious role of certain phytochemicals in the prevention and treatment of NDD, as illustrated by selected evidence-based studies.
Numerous studies have established that resveratrol possessed important neuroprotective feature both in vitro and in vivo.148,149 In vivo, in a mouse model with cerebral amyloid accumulation, orally administered resveratrol lessen microglial activation related to cortical amyloid plaque development. 150 Moreover, chronic dietary resveratrol decrease cognitive damage and perform a neuroprotective role, reducing the amyloid load and decreasing tau hyperphosphorylation in SAMP8 mice, a model of age-associated Alzheimer’s disease (AD). 151 Rising evidence has also submitted that resveratrol confers increased advantages to cell and animal models with Parkinson’s disease (PD). 152 Curcuminoids comprise three components: curcumin (75%−80%), demethoxycurcumin (15%−20%), and bisdemethoxycurcumin (3%−5%). Curcumin also trigger neuroprotective outcomes via the regulation of pathogenetic oxidative and inflammatory actions both in vitro and in vivo models of AD and PD. In Neuro2a mouse neuroblastoma cells infected with Japanese encephalitis virus, curcumin promotes cell activity by reducing reactive oxygen species (ROS) and hindering proapoptotic signals. 153 Pretreatment of primary hippocampal cultures with quercetin substantially diminished Aβ(1-42)-induced cellular toxicity, lipid peroxidation, protein oxidation, and cell death by regulating oxidative stress. 154 More captivatingly, quercetin diminishes extracellular β-amyloidosis, astrogliosis, microgliosis, and tauopathy in the hippocampus and the amygdala and enhances execution of learning and spatial memory burden in old triple AD model mice. 155 Aggregating, the above findings, point to polyphenols as neuroprotective agents. The addicted consumption of dietary polyphenols is validated to impede diverse secondary sources of ROS and proinflammatory cytokines, hence mitigating of NDDs. 156 An advantageous clinical utilization of polyphenols to lessen oxidative impairment in senescence and age-associated ailments may be a potent and auspicious strategy for NDDs therapy.
As powerful antioxidant agents
The first antioxidant molecule identified is ascorbic acid, also known as vitamin C, which is generated in the course of aerobic metabolism, and react rapidly with O2*, singlet oxygen and ozone (chemically), and H2O2 (enzymatically) via ascorbate peroxidase to detoxify their deleterious effects. Aside this, in plants, such acids likewise takes part in the regeneration of carotenoids and vitamin E (tocopherol). The latter can also behave as antioxidant and vital liposoluble redox system, serving as protection against lipid peroxidation. 157 Carotenoids, which are potent antioxidants, are responsible for several coloring in fruits, flowers and leaves; and they are involved in the scavenging of peroxyl radicals through quenching.158,159
Phenolic compounds are one of the most auspicious molecules for advancing salubrity studies. These phytochemicals comprise of a wide diversity of molecules (circa 8000 numerous structures), playing essential functions in the metabolism of the plant, 160 where they are largely distributed. The far-ranging of organic activities of phenolics, one of which is antioxidant and antitumor characteristics, is widely established in different studies.157,161 The existence of at least a single phenol ring is vital for such role, with methyl, hydroxyl, or acetyl groups substituting the hydrogen. An elevated antioxidant function has been linked to the increased number of free hydroxyls and conjugation of side chains to aromatic rings. 158 Terpenoids constitute additional huge family of plant secondary metabolites. 158 In vitro assays depicted that sesquiterpenes, monoterpenes, and diterpenes derived from aromatic plants have significant antioxidant activity. 159 The hypoglycemic and antioxidant property of alkaloid—vindoline, vindolicine, vindolinine, and vindolidine, gotten from Catharanthus roseus leaves, have also been highlighted. 160
Therapeutic effects on skin pathologies
According to the European Medicines Agency, about 12 herbal substances, preparations, and mixtures are utilized as traditional herbal medicinal products. 161 These 12 herbal substances include—Foeniculum vulgare, Sideritis scardica, Valeriana ofcinalis, Echinacea purpurea, Hamamelis virginiana, Tymus vulgaris, Vitis vinifera, Eleutherococcus senticosus, Melaleuca spp., Calendula ofcinalis, Mentha spp., and Pimpinella anisum. Their inclusion inside such official list is maintained by scientific evidences denoting their treatment efficacy in several diseases conditions vis basal cell carcinoma, carbuncle, allergy, cellulitis, acne, eczema, impetigo, chickenpox, hives, dermatitis, melanoma, rosacea, vitiligo, wart, psoriasis, impetigo, lupus, measles, blister and squamous cell carcinoma.122,157,162–165
A meta-analysis published show Vitis vinifera as an efficacious constituent of medical accessory beneficial in the therapy of local atopic dermatitis. 166 Intriguing outcomes have also been reported in oncological circumstances; Vitis vinifera has demonstrate some effectiveness in decreasing radiotherapy-induced dermatitis 167 and in impeding cell multiplication in melanoma and skin non-melanoma cancer, 168 denoting grape seed proanthocyanidin as an apoptosis and autophagy inducer. Limited allergic reactions are enunciated for Vitis vinifera. 169 In comprehensive elucidation, local utilization of Melaleuca alternifolia derieved oils has been persistently aired to attain a notable amelioration of acne lesions, as reported by numerous autonomous studies.162,170 The clinical efficiency of Melaleuca oil is possibly associated to its familiar antibacterial mechanism163,171 in addition to anti-inflammatory action.164,172 The essential oil from Melaleuca alternifolia also depicts antioxidant properties probably efficacious in dermatitis and skin cancers.165,173 Therapeutic benefits to skin diseases are suggested for Agrimoniae herba (Agrimonia eupatoria; in secondary inflammation and superficial lacerations), Echinacea purpurea (in modest perfunctory wounds and mild acne), Soiae oleum (Glycine max; in benign periodic eczema), Juglandis folium (Juglans regia; in minor skin irritation), Matricariae aetheroleum (in anus and genitals inflammation), Matricariae fos (Matricaria recrutita; in mild skin allergies and sunburns and superfcial wounds), Melaleuca spp (in insects bites, mild acne, prickling, lesser skin irritation), Meliloti herba (Melitotus ofcinalis; in minor skin inflammation), Origani dictamni herba and Origani majoranae herba (Origanum spp.; in petty skin irritation and inflammation). Several others phytochemicals are reported in literature with prospective effects on skin, such as anti-senescence property, 174 photoprotection, 175 wound recuperation, 176 and anti-infection. 177
Conclusions
The review critically highlights the various environmental pollutants and their toxicity on living organisms. It also delves into several classes of phytochemicals and their prophylactic and therapeutic interventions in various maladies challenging humanity. This review enunciates that environmental contaminants are generated from both natural and anthropogenic activities, and they become deleterious to the environment and pose a risk to living things when they bioaccumulate above their threshold limits. They are of significant public health interest across the globe, due to their indispensable role in the pathophysiology of several human diseases. Several remediation techniques and treatment measures employed toward mitigating the outcomes effects of environmental toxicity to humans are characterized by one or many limitations; which make the exploitation of phytochemicals a global interest. Phytochemicals are secondary metabolites found in plants and are popular for their numerous pharmacological and medicinal properties. Hence, they exhibit anti-inflammatory, anti-metastatic, anti-oxidant, immune-modulative, nutritive, prophylactic, anti-microbial and life-saving properties. Current researches in literature focus on expanding the database of the efficacy of phytochemicals in reducing or treating various environmental-induced toxicities. However, mechanistic understanding of their pharmacological action needs to be elucidated. Therefore, future research should focus on identifying the bioactive molecule and testing the effectiveness of phytochemicals against mixer toxicity. In addition, there is also need for extensive studies on the optimization and large-scale production of medicinal formulation from these phytochemicals.
Footnotes
Author contributions: AASA conceived the concept. AASA, MASA, and EDSA were involved data sourcing and collation. AASA, MASA, and EDSA wrote the initial draft and revised the manuscript. ANO, HE, EOT, FAB, MA and OEA critically revised the manuscript. All authors read and approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Significance for public health: This review article is of high significant impact to public health because it creates public awareness on the pharmacological and medicinal importance of phytochemicals from empirical and evidence-based studies against several maladies associated with environmental toxicity. Environmental toxicants are of public health interest as they contribute to high rate of morbidity and mortality globally. Efforts made to ameliorate their impacts on the environment and humans through remediation techniques, chelation and other cleansing procedures are often limited by complicated techniques. Phytochemicals which are secondary metabolites obtained from plants have wide pharmacological and medicinal properties; which can serve as a panacea to this nagging problem. Since Environmental health is a key part of any comprehensive public health system. This review article helps to enlighten people on environmental exposomes and how they can use natural products from plants to safeguard their health, thus promoting healthier environment and communities.
ORCID iD: Muhammad Aledeh  https://orcid.org/0000-0002-6831-9005
https://orcid.org/0000-0002-6831-9005
References
- 1.World Health Organization. 7 million deaths annual linked to air pollution. Geneva: WHO, http://www.who.int/phe/health_topics/outdoorair/databases/en/ (2022, accessed 20 September 2022). [Google Scholar]
- 2.World Health Organization. Burden of disease from household air pollution for 2012. Geneva: WHO, http://www.who.int/phe/health_topics/outdoorair/databases/FINAL_HAP_AAP_BoD_24March2014.pdf?ua¼1 (2014, accessed 20 September 2022). [Google Scholar]
- 3.World Health Organization. Data on the size of the HIV/AIDS epidemic: number of deaths due to HIV/AIDS. Global Health Observatory Data Repository, Geneva, WHO, 2014. [Google Scholar]
- 4.World Health Organization. Deaths: estimated deaths, data by region. Global Health Observatory Data Repository. Geneva: WHO, http://apps.who.int/gho/data/view.main.14117?lang¼en (2014, accessed 9 September 2022). [Google Scholar]
- 5.Olanrewaju JA, Akinte DO, Sokan-Adeaga AA, et al. A survey on faecal management practices and associated health impacts among residents in selected sub-urban communities in Ibadan, Nigeria. J Appl Sci 2022; 22(3): 107–116. [Google Scholar]
- 6.World Health Organization. Environmental Health Criteria 237. Principles for evaluating health risks in children associated with exposure to chemicals. Geneva: WHO, http://www.who.int/ipcs/publications/ehc/ehc237.pdf (2006, Accessed 18 September 2022). [Google Scholar]
- 7.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol 2014; 13: 330e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokan-Adeaga MA, Sokan-Adeaga AA, Sokan-Adeaga ED. Characterization and antibiotics sensitivity pattern of Enterobacteriaceae in Obafemi Awolowo University (OAU) Sewage Oxidation pond. Glob J Med Res 2018; 18(1): 7–17. [Google Scholar]
- 9.Sly PD, Carpenter DO, Van den Berg M, et al. Health consequences of environmental exposures: causal thinking in global environmental epidemiology. Annals Glob Health 2016; 82(1): 3–9. [DOI] [PubMed] [Google Scholar]
- 10.Allen SAA, Ree AG, Ayodeji SAM, et al. Secondary Inorganic Aerosols: Impacts on the global climate system and Human Health. Biodiversity Int J 2019; 3(6): 249–259. [Google Scholar]
- 11.Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect 2005; 113(8): 934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hennig B, Reiterer G, Majkova Z, et al. Modification of environmental toxicity by nutrients: implications in atherosclerosis. Cardiovasc Toxicol 2005; 5(2): 153–160. [DOI] [PubMed] [Google Scholar]
- 13.Needham LL, Barr DB, Caudill SP, et al. Concentrations of environmental chemicals associated with neurodevelopmental effects in U.S. Population. Neurotoxicol 2017; 26(4): 531–545. [DOI] [PubMed] [Google Scholar]
- 14.Olarewaju SO, Sokan-Adeaga AA, Awoaafe FB, et al. Assessment of occupational injuries and safety practices among automobile repair artisans in apo and Gudu area of Abuja, Nigeria. J Epid Soc Nig 2022; 4(2): 71–86. [Google Scholar]
- 15.Center for Disease Control and Prevention (CDC). Third National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Centers for Disease Control and Prevention, http://www.cdc.gov/exposurereport/ (2005, accessed 20 September 2022) [Google Scholar]
- 16.Okareh OT, Akin-Brandom T, Sokan-Adeaga AA. Spatial distribution of heavy metals contamination of groundwater in neighborhood communities of Shagamu Industrial Layout, Nigeria. Glob J Sci Front Res 2018; 18(2:1): 21–38. [Google Scholar]
- 17.Sokan-Adeaga AA, Sokan-Adeaga MA, Sokan-Adeaga, et al. Chemobiokinetics, biotoxicity and therapeutic overview of selected heavy metals poisoning: a review. Biodiversity Int J 2020; 4(5): 211–222. [Google Scholar]
- 18.Hennig B, Ettinger AS, Jandacek RJ, et al. Using nutrition for intervention and prevention against environmental chemical toxicity and associated diseases. Environ Health Perspect 2007; 115: 493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim C, Park K. Association between phytochemical index and inflammation in Korean adults. Antioxidants 2022; 11: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dehghani Firouzabadi F, Jayedi A, Asgari E, et al. The association of dietary phytochemical index with metabolic syndrome in adults. Clin Nutr Res 2021; 10: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghoreishy SM, Aminianfar A, Benisi-Kohansal S, et al. Association between dietary phytochemical index and breast cancer: A case-control study. Breast Cancer 2021; 28: 1283–1291. [DOI] [PubMed] [Google Scholar]
- 22.Delshad Aghdam S, Siassi F, Nasli Esfahani E, et al. Dietary phytochemical index associated with cardiovascular risk factor in patients with type 1 diabetes mellitus. BMC Cardiovasc Disord 2021; 21: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zar HJ, Ferkol TW. The global burden of respiratory disease-impact on child health. Pediatr Pulmonol 2014; 49(5): 430–434. [DOI] [PubMed] [Google Scholar]
- 24.Hodgson E, Mailman RB, Chambers JE. Dictionary of toxicology. 2nd ed.London: Macmillan Reference, 1998. p.162. [Google Scholar]
- 25.Hawkes SJ. What is a “heavy metal”? J Chem Educ 1997; 74(11): 1374. [Google Scholar]
- 26.Gorbachev VM, Zamyatnin YS, Lbov AA. Nuclear reactions in heavy elements: A data handbook. Oxford: Pergamon Press, 1980. p.12. [Google Scholar]
- 27.Morris CG. Academic Press Dictionary of Science and Technology. San Diego: Harcourt Brace Jovanovich, 1992. p.36. [Google Scholar]
- 28.Nriagu JO, Pacyna JM. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 1988; 333(6169): 134–139. [DOI] [PubMed] [Google Scholar]
- 29.Khanna S, P, Assessment of heavy metal contamination in different vegetables grown in and around urban areas. Res J Environ Toxicol 2011; 5(3): 162–179. [Google Scholar]
- 30.Okareh TO, Akin-Brandom T, Sokan-Adeaga AA, et al. Knowledge and perceived health risks associated with heavy metals contamination in groundwater – A case study of Sagamu Local Government Area, Ogun State, Nigeria. Int J Health Safety Environ 2020; 6(03): 506–518. [Google Scholar]
- 31.Nordberg GF, Fowler BA, Nordberg M, et al. Handbook on the Toxicology of Metals. New York, NY: Academic Press, 2005. p.23. [Google Scholar]
- 32.Singh J, Upadhyay SK, Pathak RK, et al. Accumulation of heavy metals in soil and paddy crop (Oryza sativa), irrigated with water of Ramgarh Lake, Gorakhpur, UP, India. Toxicol Environ Chem 2011; 93(3): 462–473. [Google Scholar]
- 33.Türkdoğan MK, Kilicel F, Kara K, et al. Heavy metals in soil, vegetables and fruits in the endemic upper gastrointestinal cancer region of Turkey. Environ Toxicol Pharmacol 2003; 13(3): 175–179. [DOI] [PubMed] [Google Scholar]
- 34.Ngan V. Heavy metal toxicity. Hoboken, NJ: Blackwell Scientific Publications, 2006. p.51. [Google Scholar]
- 35.Nriagu JO. Global metal pollution. Poisoning the biosphere? Environment 1990; 32(7): 7–33. [Google Scholar]
- 36.Smith SR, Jaffe DM, Skinner MA. Case report of metallic mercury injury. Pediatr Emerg Care 1997; 13(2): 114–116. [DOI] [PubMed] [Google Scholar]
- 37.Jaishankar M, Mathew BB, Shah MS, et al. Biosorption of few heavy metal ions using agricultural wastes. J Environ Pollut Hum Health 2014; 2(1): 1–6. [Google Scholar]
- 38.Alewu B, Nosiri C. Pesticides and human health. In: Stoytcheva M. (ed.) Pesticides in the modern world – effects of pesticides exposure. London: InTech, 2011, pp.231–250, http://www.intechopen.com/books/pesticides-in-the-modern-world-effects-of-pesticidesexposure/pesticide-and-human-health. [Google Scholar]
- 39.NSW EPA. What Are Pesticides and How Do They Work?, http://www.epa.nsw.gov.au/pesticides/pestwhatrhow.htm (2013, accessed 10 August 2022).
- 40.Nicolopoulou-Stamati P, Maipas S, Kotampasi C, et al. Chemical Pesticides and human health: the urgent need for a new concept in agriculture. Public Health Front 2016; 4: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pirsaheb M, Limoee M, Namdari F, et al. Organochlorine pesticides residue in breast milk: a systematic review. Med J Islam Repub Iran 2015; 29: 228. [PMC free article] [PubMed] [Google Scholar]
- 42.Sanborn M, Kerr KJ, Sanin LH, et al. Non-cancer health effects of pesticides. Systematic review and implications for family doctors. Can Fam Physician 2007; 53: 1712–1720. [PMC free article] [PubMed] [Google Scholar]
- 43.Mnif W, Hassine AI, Bouaziz A, et al. Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health 2011; 8: 2265–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bassil KL, Vakil C, Sanborn M, et al. Cancer health effects of pesticides: systematic review. Can Fam Physician 2007; 53: 1704–1711. [PMC free article] [PubMed] [Google Scholar]
- 45.Turusov V, Rakitsky V, Tomatis L. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect 2002; 110: 125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van den Berg H. Global status of DDT and its alternatives for use in vector control to prevent disease. Environ Health Perspect 2009; 117: 1656–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKinlay R, Plant JA, Bell JN, et al. Endocrine disrupting pesticides: implications for risk assessment. Environ Int 2008; 34: 168–183. [DOI] [PubMed] [Google Scholar]
- 48.Gasnier C, Dumont C, Benachour N, et al. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 2009; 262: 184–191. [DOI] [PubMed] [Google Scholar]
- 49.Jaga K, Dharmani C. Sources of exposure to and public health implications of organophosphate pesticides. Rev Panam Salud Publica 2003; 14: 171–185. [DOI] [PubMed] [Google Scholar]
- 50.Karami-Mohajeri S, Abdollahi M. Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: a systematic review. Hum Exp Toxicol 2011; 30(9): 1119–1140. [DOI] [PubMed] [Google Scholar]
- 51.Li D, Huang Q, Lu M, et al. The organophosphate insecticide chlorpyrifos confers its genotoxic effects by inducing DNA damage and cell apoptosis. Chemosphere 2015; 135: 387–393. [DOI] [PubMed] [Google Scholar]
- 52.Goad RT, Goad JT, Atieh BH, et al. Carbofuran-induced endocrine disruption in adult male rats. Toxicol Mech Methods 2004; 14: 233–239. [DOI] [PubMed] [Google Scholar]
- 53.Jamal F, Haque QS, Singh S, et al. The influence of organophosphate and carbamate on sperm chromatin and reproductive hormones among pesticide sprayers. Toxicol Ind Health 2016; 32(8): 1527–1536. [DOI] [PubMed] [Google Scholar]
- 54.Kolaczinski JH, Curtis CF. Chronic illness as a result of low-level exposure to synthetic pyrethroid insecticides: a review of the debate. Food Chem Toxicol 2004; 42: 697–706. [DOI] [PubMed] [Google Scholar]
- 55.Ray DE, Fry JR. A reassessment of the neurotoxicity of pyrethroid insecticides. Pharmacol Ther 2006; 111: 174–193. [DOI] [PubMed] [Google Scholar]
- 56.Jaensson A, Scott AP, Moore A, et al. Effects of a pyrethroid pesticide on endocrine responses to female odours and reproductive behaviour in male parr of brown trout (Salmo trutta L. Aquat Toxicol 2007; 81(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 57.Pandey SP, Mohanty B. The neonicotinoid pesticide imidacloprid and the dithiocarbamate fungicide mancozeb disrupt the pituitary-thyroid axis of a wildlife bird. Chemosphere 2015; 122: 227–234. [DOI] [PubMed] [Google Scholar]
- 58.Moore A, Waring CP. The effects of a synthetic pyrethroid pesticide on some aspects of reproduction in Atlantic salmon (Salmo salar L. Aquat Toxicol 2001; 52(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 59.Jin Y, Wang L, Chen G, et al. Exposure of mice to atrazine and its metabolite diaminochlorotriazine elicits oxidative stress and endocrine disruption. Environ Toxicol Pharmacol 2014; 37: 782–790. [DOI] [PubMed] [Google Scholar]
- 60.Huang P, Yang J, Song Q. Atrazine affects phosphoprotein and protein expression in MCF-10A human breast epithelial cells. Int J Mol Sci 2014; 15: 17806–17826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma K, Wu HY, Zhang B, et al. Neurotoxicity effects of atrazine-induced SH-SY5Y human dopaminergic neuroblastoma cells via microglial activation. Mol Biosyst 2015; 11: 2915–2924. [DOI] [PubMed] [Google Scholar]
- 62.Goulson D. REVIEW: an overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol 2013; 50: 977–987. [Google Scholar]
- 63.Jeschke P, Nauen R. Neonicotinoids-from zero to hero in insecticide chemistry. Pest Manag Sci 2008; 64: 1084–1098. [DOI] [PubMed] [Google Scholar]
- 64.Caron-Beaudoin Denison MS, Sanderson JT. Effects of neonicotinoids on promoter-specific expression and activity of aromatase (CYP19) in human adrenocortical carcinoma (H295R) and primary umbilical vein endothelial (HUVEC) cells. Toxicol Sci 2016; 149(1): 134–144. [DOI] [PubMed] [Google Scholar]
- 65.Kotsonis FN, Burdock GA, Flamm WG. Food toxicology, Casarett and Doull’s toxicology: the basic science of poisons. 6th ed.New York, NY: McGraw-Hill, 2001. pp.1049–1088. [Google Scholar]
- 66.Bruckner JV, Warren DA. Toxic effects of solvents and vapors, Casarett and Doull’s Toxicology: the Basic Science of Poisons. 6th ed.New York, NY: McGraw-Hill, 2001. pp.945–964. [Google Scholar]
- 67.Brent J. A review of: medical toxicology. Clin Toxicol 2006; 44(3): 355–355. [Google Scholar]
- 68.Monisha J, Tenzin T, Naresh A, et al. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 2014; 7(2): 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patrick L. Mercury toxicity and antioxidants: Part 1: role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity. Altern Med Rev 2002; 7(6): 456–471. [PubMed] [Google Scholar]
- 70.Haley BE. Mercury toxicity: genetic susceptibility and synergistic effects. Med Veritas 2005; 2(2): 535–542. [Google Scholar]
- 71.Flora SJ, Mittal M, Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res 2008; 128: 501–523. [PubMed] [Google Scholar]
- 72.Martin S, Griswold W. Human health effects of heavy metals. Environ Sci Technol Briefs Citizens 2009; 15): 1–6. [Google Scholar]
- 73.Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ 2000; 78(9): 1093–1103. [PMC free article] [PubMed] [Google Scholar]
- 74.Guha Mazumder DN. Chronic arsenic toxicity & human health. Indian J Med Res 2008; 128(4): 436–447. [PubMed] [Google Scholar]
- 75.Stober J. Health effects of POPs, Proceedings of the Subregional Awareness Raising Workshop on Persistent Organic Pollutants (POPs) Kranjska Gora, Slovenia, 11-14May1998. [Google Scholar]
- 76.Rengarajan T, Rajendran P, Nandakumar N, et al. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac J Trop Biomed 2015; 5: 182–189. [Google Scholar]
- 77.Abdel-Shafy HI, Mansour MSM. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet 2016; 25: 107–123. [Google Scholar]
- 78.Burchiel SW, Gao J. Polycyclic aromatic hydrocarbons and the immune system. In: Vohr H-W. (ed.) Encyclopedia Immunotoxicology. Berlin: Springer, 2014, pp.1192–1194. [Google Scholar]
- 79.Bolden AL, Rochester JR, Schultz K, et al. Polycyclic aromatic hydrocarbons and female reproductive health: a scoping review. Reprod Toxicol 2017; 73: 61–74. [DOI] [PubMed] [Google Scholar]
- 80.Rajpara RK, Dudhagara DR, Bhatt JK, et al. Polycyclic aromatic hydrocarbons (pahs) at the Gulf of Kutch, Gujarat, India: occurrence, source apportionment, and toxicity of pahs as an emerging issue. Mar Pollut Bull 2017; 119: 231–238. [DOI] [PubMed] [Google Scholar]
- 81.Campos-Vega R, Oomah DB. Chemistry and classification of phytochemicals. In: Tiwari BK, Brunton NP, Brennan CS. (eds.) Handbook of plant food phytochemicals: sources, stability and extraction. Hoboken: John Wiley & Sons, Ltd, 2013, pp.5–48. [Google Scholar]
- 82.Ogunwenmo KO, Idowu OA, Innocent C, et al. Cultivars of Codiaeum variegatum (L.) Blume (Euphorbiaceae) show variability in phytochemical and cytological characteristics. J Biotechnol 2007; 6: 2400–2405. [Google Scholar]
- 83.Ngoci SN, Mwendia CM, Mwaniki CG. Phytochemical and cytotoxicity testing of Indigofera lupatana Baker F. J Anim Plant Sci 2011; 11(1): 1364–1373. [Google Scholar]
- 84.Briskin DP. Medicinal plants and phytomedicines. Linking plant Biochemistry and Physiology to human health. Plant Physiol 2000; 124: 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olila D, Olwa-Odyek, Opuda-Asibo J. Antibacterial and antifungal activities of extracts of Zanthoxylum chalybeum and Warburgia ugandensis, Ugandan medicinal plants. J Afri Health Sci 2001; 1: 66–72. [PMC free article] [PubMed] [Google Scholar]
- 86.Harborne JB. Phytochemical methods. London: Chapman and Hall, 1973. pp.52–114. [Google Scholar]
- 87.Ovando C, Hernandez D, Hernandez E, et al. Chemical studies of anthocyanins: a review. Food Chem 2009; 113: 859–871. [Google Scholar]
- 88.Metodiewa D, Kochman A, Karolczak S. Evidence for antiradical and antioxidant properties of four biologically active N,N-diethylaminoethyl ethers of flavaone oximes: a comparison with natural polyphenolic flavonoid rutin action. Biochem Mol Biol Int 1997; 41: 1067–1075. [DOI] [PubMed] [Google Scholar]
- 89.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci 2016; 5: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Navarro MC, Montilla MP, Cabo MM, et al. Antibacterial, antiprotozoal and antioxidant activity of five plants used in izabal for infectious diseases. J Phytotherapy Res 2003; 17: 325–329. [DOI] [PubMed] [Google Scholar]
- 91.Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: Antioxidants and beyond. Am J Clin Nutr 2005; 81: 215S–217S. [DOI] [PubMed] [Google Scholar]
- 92.Okuda T. Systematics and health effects of chemically distinct tannins in medicinal plants. J Phytochemistry 2005; 66: 2012–2031. [DOI] [PubMed] [Google Scholar]
- 93.El Aziz MMA, Ashour AS, Melad ASG. A review on saponins from medicinal plants: chemistry, isolation, and determination. J Nanomed Res 2019; 7(4): 282–288. [Google Scholar]
- 94.Vasudeva RN, Sukhendu BG, Pushpalatha B, et al. Triterpenoid saponins: a review on biosynthesis, applications and mechanism of their action. Int J Pharm Pharm Sci 2015; 7(1): 24–28. [Google Scholar]
- 95.Hassan SM, Haq AU, Byrd JA, et al. Haemolytic and antimicrobial activities of saponin-rich extracts from guar meal. Food Chem 2010; 119: 600–605. [DOI] [PubMed] [Google Scholar]
- 96.Sparg SG, Light ME, van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol 2004; 94(2-3): 219–243. [DOI] [PubMed] [Google Scholar]
- 97.Cheng TC, Lu JF, Wang JS, et al. Antiproliferation effect and apoptosis mechanism of prostate cancer cell PC-3 by flavonoids and saponins prepared from Gynostemma pentaphyllum. J Agric Food Chem 2011; 59(20): 11319–11329. [DOI] [PubMed] [Google Scholar]
- 98.Hostettmann, Marson A. Saponins. Cambridge: Cambridge University Press, 2005. p.21. [Google Scholar]
- 99.Kurek J. Introductory Chapter: Alkaloids – their importance in nature and for human life [internert], 2019Nov13. 10.5772/intechopen.85400 [DOI]
- 100.Chisholm H. Encyclopedia Brittanica. A dictionary of arts, sciences, literature and general information. Vol 22. New York, NY: Sagwan Press, 2015. pp.978134014998. [Google Scholar]
- 101.Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Reviews 2008; 7: 926–935. [DOI] [PubMed] [Google Scholar]
- 102.Morsy N. Cardiac glycosides in medicinal plants. Aromatics and Medicinal Plants – Back to Nature [Internet]. London: Intechopen Publisher, 2017. [Google Scholar]
- 103.Kelly RA. Cardiac glycosides and congestive heart failure. Am J Cardiol 1990; 65: E10–E16. [DOI] [PubMed] [Google Scholar]
- 104.Perveen S. Introductory Chapter: Terpenes and Terpenoids. Terpenes and Terpenoids [internet]. London: Intechopen Publisher, 2018. [Google Scholar]
- 105.Roberts SC. Production and engineering of terpenoids in plant cell culture. Nat Chem Biol 2007; 3: 387–395. [DOI] [PubMed] [Google Scholar]
- 106.Lesovaya E, Yemelyanov A, Swart AC, et al. Discovery of compound A - a selective activator of the glucocorticoid receptor with anti-inflammatory and anti-cancer activity. Oncotarget 2015; 6(31): 30730–30744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toh MF, Mendonca E, Eddie SL, et al. Kaempferol exhibits progestogenic effects in ovariectomized rats. J Steroids Horm Sci 2014; 5: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pihlajamaa P, Zhang FP, Saarinen L, et al. The phytoestrogen genistein is a tissue-specific androgen receptor modulator. Endocrinology 2011; 152: 4395–4405. [DOI] [PubMed] [Google Scholar]
- 109.Blachley JD, Knochel JP. Tobacco chewer’s hypokalemia: Licorice Revisited. N Engl J Med 1980; 302: 784–785. [DOI] [PubMed] [Google Scholar]
- 110.Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 2005; 4: 685–688. [Google Scholar]
- 111.Soares MBP, Brustolim D, Santos LA, et al. Physalins B, F and G, seco-steroids purified from Physalis angulata L., Inhibit lymphocyte function and allogeneic transplant rejection. Int Immunopharmacol 2006; 6: 408–414. [DOI] [PubMed] [Google Scholar]
- 112.Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites 2012; 2(2): 303–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patti AM, Al-Rasadi K, Giglio RV, et al. Natural approaches in metabolic syndrome management. Arch Med Sci 2018; 14(2): 422–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tabatabaei-Malazy O, Larijani B, Abdollahi M. Targeting metabolic disorders by natural products. J Diabetes Metab Disord 2015; 14: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Truong VL, Jun M, Jeong WS. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018; 44(1): 36–49. [DOI] [PubMed] [Google Scholar]
- 116.Queen B, Tollefsbol T. Polyphenols and aging. Curr Aging Sci 2010; 3(1): 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li YR, Li S, Lin CC. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 2018; 44(1): 69–82. [DOI] [PubMed] [Google Scholar]
- 118.Prasain JK, Carlson SH, Wyss JM. Flavonoids and age-related disease: risk, benefits and critical windows. Maturitas 2010; 66(2): 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alam MA, Subhan N, Rahman MM, et al. Effect of Citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutrition 2014; 5(4): 404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nobili S, Lippi D, Witort E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res 2009; 59(6): 365–378. [DOI] [PubMed] [Google Scholar]
- 121.Hussain T, Tan B, Ren W, et al. Polyamines: therapeutic perspectives in oxidative stress and inflammatory diseases. Amino Acids 2017; 49(9): 1457–1468. [DOI] [PubMed] [Google Scholar]
- 122.Forni C, Facchiano F, Bartoli M, et al. Beneficial role of phytochemicals on oxidative stress and Age-Related diseases. Biomed Res Int 2019; 2019: 8748253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lentini A, Tabolacci C, Provenzano B, et al. Phytochemicals and protein–polyamine conjugates by transglutaminase as chemopreventive and chemotherapeutic tools in cancer. Plant Physiol Biochem 2010; 48(7): 627–633. [DOI] [PubMed] [Google Scholar]
- 124.Mao XY, Jin MZ, Chen JF, et al. Live or let die: neuroprotective and anti-cancer effects of nutraceutical antioxidants. Pharmacol Ther 2018; 183: 137–151. [DOI] [PubMed] [Google Scholar]
- 125.Bobe G, Albert PS, Sansbury LB, et al. Interleukin-6 as a potential indicator for prevention of high-risk adenoma recurrence by dietary flavonols in the polyp prevention trial. Cancer Prev Res 2010; 3(6): 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Anand P, Kunnumakkara AB, Newman RA, et al. Bioavailability of curcumin: problems and promises. Mol Pharm 2007; 4(6): 807–818. [DOI] [PubMed] [Google Scholar]
- 127.Mukund V, Mukund D, Sharma V, et al. Genistein: its role in metabolic diseases and cancer. Crit Rev Oncol/Hematol 2017; 119: 13–22. [DOI] [PubMed] [Google Scholar]
- 128.Lesinski GB, Reville PK, Mace TA, et al. Consumption of soy isoflavone enriched bread in men with prostate cancer is associated with reduced proinflammatory cytokines and immunosuppressive cells. Cancer Prev Res 2015; 8(11): 1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carini F, David S, Tomasello G, et al. Colorectal cancer: an update on the efects of lycopene on tumor progression and cell proliferation. J Biol Regul Homeost Agents 2017; 31: 769–774. [PubMed] [Google Scholar]
- 130.Rowles JL, Ranard KM, Smith JW, et al. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2017; 20(4): 361–377. [DOI] [PubMed] [Google Scholar]
- 131.Wahle KW, Caruso D, Ochoa JJ, et al. Olive oil and modulation of cell signaling in disease prevention. Lipids 2004; 39(12): 1223–1231. [DOI] [PubMed] [Google Scholar]
- 132.Baker EJ, Yusof MH, Yaqoob P, et al. Omega-3 fatty acids and leukocyte-endothelium adhesion: novel anti-atherosclerotic actions. Mol Asp Medicine 2018; 64: 169–181. [DOI] [PubMed] [Google Scholar]
- 133.Colussi G, Catena C, Novello M, et al. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: relevance for cardiovascular outcomes. Nutr Metab Cardiovasc Dis 2017; 27(3): 191–200. [DOI] [PubMed] [Google Scholar]
- 134.Payne C, Wiffen PJ, Martin S. Interventions for fatigue and weight loss in adults with advanced progressive illness. Cochrane Database Syst Rev 2012; 1: CD008427. [DOI] [PubMed] [Google Scholar]
- 135.Kones R, Howell S, Rumana U. N-3 polyunsaturated fatty acids and cardiovascular disease: principles, practices, pitfalls, and promises - a contemporary review. Med Princ Pract 2018; 26(6): 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gammone MA, Efhymakis K, Pluchinotta FR, et al. Impact of chocolate on the cardiovascular health. Front Biosci 2018; 23(3): 852–864. [DOI] [PubMed] [Google Scholar]
- 137.Zhang H, Liu H, Chen Y, et al. The curcumin-induced vasorelaxation in rat superior mesenteric arteries. Ann Vasc Surg 2018; 48: 233–240. [DOI] [PubMed] [Google Scholar]
- 138.Gammone MA, Pluchinotta FR, Bergante S, et al. Prevention of cardiovascular diseases with carotenoids. Front Biosci 2017; 9(1): 165–171. [DOI] [PubMed] [Google Scholar]
- 139.Howes MJR. Phytochemicals as anti-infammatory nutraceuticals and phytopharmaceuticals. In: Chatterjee S, Jungraithmayr W, Bagchi D. (eds) Immunity and infammation in health and disease: emerging roles of Nutraceuticals and functional foods in immune support. Elsevier, Cambridge, MA: Academic Press, 2017, pp.363–388. [Google Scholar]
- 140.Gomes A, Fernandes E, Lima J, et al. Molecular mechanisms of anti-inflammatory activity mediated by flavonoids. Curr Med Chem 2008; 15(16): 1586–1605. [DOI] [PubMed] [Google Scholar]
- 141.King HGC. Phenolic compounds of commercial wheat germ. J Food Sci 1962; 27(5): 446–454. [Google Scholar]
- 142.Raso GM, Meli R, Di Carlo G, et al. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci 2001; 68(8): 921–931. [DOI] [PubMed] [Google Scholar]
- 143.Hirai S, Takahashi N, Goto T, et al. Functional food targeting the regulation of obesity-induced inflammatory responses and pathologies. Mediat Inflammation 2010; 2010: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xagorari A, Papapetropoulos A, Mauromatis A, M. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinfammatory cytokine production in macrophages. J Pharmacol Exp Ther 2001; 296(1): 181–187. [PubMed] [Google Scholar]
- 145.Javadi F, Ahmadzadeh A, Eghtesadi S, et al. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: a double-blind, randomized controlled trial. J Am Coll Nutr 2017; 36(1): 9–15. [DOI] [PubMed] [Google Scholar]
- 146.Shamekhi Z, Amani R, Habibagahi Z, et al. A randomized, double-blind, placebo-controlled clinical trial examining the effects of green tea extract on systemic lupus erythematosus disease activity and quality of Life. Phytother Res 2017; 31(7): 1063–1071. [DOI] [PubMed] [Google Scholar]
- 147.Joseph J, Cole G, Head E, et al. Nutrition, brain aging, and neurodegeneration. J Neurosci 2009; 29(41): 12795–12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Granzotto A, Zatta P. Resveratrol acts not through AntiAggregative pathways but mainly via its scavenging properties against aβ and aβ-metal complexes toxicity. PLoS One 2011; 6(6): e21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yan WJ, Liu RB, Wang LK, et al. Sirt3-mediated autophagy contributes to resveratrol-induced protection against ER stress in HT22 cells. Front Neurosci 2018; 12: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Capiralla H, Vingtdeux V, Zhao H, et al. Resveratrol mitigates lipopolysaccharide- and aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J Neurochem 2012; 120(3): 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Porquet D, Casadesús G, Bayod S, et al. Dietary resveratrol prevents alzheimer’s markers and increases life span in samp8. Age 2013; 35(5): 1851–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang F, Shi JS, Zhou H, et al. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol Pharmacol 2010; 78(3): 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dutta K, Ghosh D, Basu A. Curcumin protects neuronal cells from japanese encephalitis virus-mediated cell death and also inhibits infective viral particle formation by dysregulation of ubiquitin-proteasome system. J Neuroimmune Pharmacol 2009; 4(3): 328–337. [DOI] [PubMed] [Google Scholar]
- 154.Ansari MA, Abdul HM, Joshi G, et al. Protective effect of quercetin in primary neurons against aβ(1–42): relevance to Alzheimer’s disease. J Nutr Biochem 2009; 20(4): 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, et al. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacol 2015; 93: 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gardener H, Caunca MR. Mediterranean diet in preventing neurodegenerative diseases. Curr Nutr Rep 2018; 7(1): 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 2005; 17(7): 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jomova K, Valko M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur J Med Chem 2013; 70: 102–110. [DOI] [PubMed] [Google Scholar]
- 159.Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Asp Medicine 2003; 24(6): 345–351. [DOI] [PubMed] [Google Scholar]
- 160.Hassan S, Mathesius U. The role of flavonoids in root-rhizosphere signalling: opportunities and challenges for improving plant-microbe interactions. J Exp Bot 2012; 63(9): 3429–3444. [DOI] [PubMed] [Google Scholar]
- 161.European Medicines Agency. Guideline on declaration of herbal substances and herbal preparations 1 in herbal medicinal products 2 /traditional herbal medicinal products, https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-declaration-herbal-substances-herbal-preparations-herbal-medicinal-products/traditional-herbal-medicinal-products-spc_en.pdf (2010, accessed 16 September 2022).
- 162.Moran JF, Klucas RV, Grayer RJ, et al. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: prooxidant and antioxidant properties. Free Radic Biol Med 1997; 22(5): 861–870. [DOI] [PubMed] [Google Scholar]
- 163.Aharoni A, Jongsma MA, Bouwmeester HJ. Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci 2005; 10(12): 594–602. [DOI] [PubMed] [Google Scholar]
- 164.Baratta MT, Dorman HJD, Deans SG, et al. Chemical composition, antimicrobial and antioxidative activity of Laurel, sage, rosemary, oregano and coriander essential oils. J Essent Oil Res 1998; 10(6): 618–627. [Google Scholar]
- 165.Tiong S, Looi C, Hazni H, et al. Antidiabetic and antioxidant properties of alkaloids from Catharanthus roseus (L.) G. Don. Molecules 2013; 18(8): 9770–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Micali G, Paternò V, Cannarella R, et al. Evidence-based treatment of atopic dermatitis with topical moisturizers. Giornale Italiano di Dermatologia e Venereologia 2018; 153(3): 396–402. [DOI] [PubMed] [Google Scholar]
- 167.Di Franco R, Sammarco E, Calvanese MG, et al. Preventing the acute skin side effects in patients treated with radiotherapy for breast cancer: the use of corneometry in order to evaluate the protective effect of moisturizing creams. Radiat Oncol 2013; 8: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Turk S, Malkan UY, Ghasemi M, et al. Growth inhibitory activity of ankaferd hemostat on primary melanoma cells and cell lines. Sage Open Med 2017; 5: 2050312116689519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brito FF, Gimeno PM, Bartolomé B, et al. Vine pollen allergy in areas with a high density of vineyards. Ann Allergy Asthma Immunol 2008; 100(6): 596–600. [DOI] [PubMed] [Google Scholar]
- 170.Hammer KA. Treatment of acne with tea tree oil (melaleuca) products: a review of efficacy, tolerability and potential modes of action. Int J Antimicrob Agents 2015; 45(2): 106–110. [DOI] [PubMed] [Google Scholar]
- 171.Kwon HH, Yoon JY, Park SY, et al. Comparison of clinical and histological efects between lactobacillus-fermented chamaecyparis obtusa and tea tree oil for the treatment of acne: an eight-week double-blind randomized controlled split-face study. Dermatology 2014; 229(2): 102–109. [DOI] [PubMed] [Google Scholar]
- 172.Ramage G, Milligan S, Lappin DF, et al. Antifungal, cytotoxic, and immunomodulatory properties of tea tree oil and its derivative components: potential role in management of oral candidosis in cancer patients. Front Microbiol 2012; 3: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol 2013; 52(7): 784–790. [DOI] [PubMed] [Google Scholar]
- 174.Kalyana Sundaram I, Sarangi DD, Sundararajan V, et al. Poly herbal formulation with antielastase and anti-oxidant properties for skin anti-aging,. BMC Complement Altern Med 2018; 18(1): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Prasad R, Singh T, Katiyar SK. Honokiol inhibits ultraviolet radiation-induced immunosuppression through inhibition of ultraviolet-induced inflammation and DNA hypermethylation in mouse skin. Sci Rep 2017; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sarandy MM, Novaes RD, Xavier AA, et al. Hydroethanolic extract of strychnos pseudoquina accelerates skin wound healing by modulating the oxidative status and microstructural reorganization of scar tissue in experimental type I diabetes. Biomed Res Int 2017; 2017: 9538351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Houston DMJ, Robins B, Bugert JJ, et al. In vitro permeation and biological activity of punicalagin and zinc (II) across skin and mucous membranes prone to herpes simplex virus infection. Eur J Pharm Sci 2017; 96: 99–106. [DOI] [PubMed] [Google Scholar]