Abstract
Jeavons syndrome is a common, often misdiagnosed or overlooked epileptic syndrome presenting with a triad of eyelid myoclonia with or without absence seizures, eye closure-induced EEG paroxysms, and photosensitivity. We present a seven-year-old female who presented with eyelid myoclonia evident since birth with absence seizures and migraines with associated photosensitivity. An EEG with photic stimulation confirmed the diagnosis of Jeavons syndrome. Genetic testing showed a heterozygous mutation in the PLCB1 gene which has been linked to early onset epilepsies and encephalopathic epilepsies. This mutation and her clinical presentation identifies another etiology of Jeavons syndrome and confirms it can begin from birth. Its presence highlights the importance of genetic testing in epileptic patients to better understand the links between genetics and epilepsy syndromes so appropriate treatment can be initiated.
Keywords: epilepsy with eyelid myoclonia, absence seizures, eye tic, ethosuximide, topiramate
Introduction
Jeavons syndrome, recently renamed as epilepsy with eyelid myoclonia (EEM), is characterized by a triad of eyelid myoclonia with or without absence seizures, photosensitivity, and eye closure-induced EEG paroxysms. EEG (Electroencephalography) with photic stimulation is extremely useful for elucidating the diagnosis. 1 The myoclonia is often described as eyelid flickering, fluttering, or trembling and is typically noticeable immediately following a slow eyelid closure. Eyes can remain open or retracted or even twitch along with upward gaze deviation. Head retropulsion may be experienced by some patients. 2 This syndrome was documented by Jeavons in 1977 who noted that “brief absences may occur spontaneously and are accompanied by 3/sec spike and wave discharges… Their presence in a routine EEG is a very reliable warning that abnormality will be evoked by photic stimulation.” 3
EEM was recently recognized as its own diagnosis by the International League Against Epilepsy (ILAE) in 2017 as ‘absences with palpebral myoclonus’ and the ILAE neurophysiology task force classified it as a generalized epileptic syndrome with its own clinical and EEG characteristics. 4 Demographically, this syndrome is twice as common in females and accounts for 7.3-12.9% of generalized epilepsies and 2.5% to 2.7% of epilepsies overall. The diagnosis is delayed an average of 9.6 years and the average time of diagnosis is approximately 7 years.4,5
Case
We report a 7-year-old female referred to the pediatric neurology clinic for “abnormal eye movements” and migraines. At her first visit, the mother stated that the patient has had episodes of eye fluttering with her eyes rolling into the back of her head since she was born. They added that the patient experiences at least 20 episodes of eyelid myoclonia per hour per day and even noticed similar eyelid movements while the patient is asleep. The episodes while awake appear to cause a transient reduction in the patient's awareness and sometimes happen mid-conversation with the patient requiring reorientation. This phenomenon has not interfered with her performance in school thus far although many teachers had been mistaking these episodes as the patient rolling her eyes at them. Of note, she does not have any notable developmental issues and performs above average in school.
The patient's migraines have been apparent since the patient could talk at approximately three years. She described her migraines as an “all-over squeezing” pain with associated photophobia and vomiting. Other medical issues included significant issues falling asleep necessitating melatonin and frequent urinary incontinence.
The patient's physical examination at the initial visit was mostly unremarkable aside from recurrent episodes of eyelid myoclonia during the exam. Hyperventilation for 2–3 min elicited several up to 5-s upward eye deviation absence seizures. Based on the patient's history and clinical presentation we made the diagnosis of Jeavons Syndrome, or eyelid myoclonia with absences, 6 which, as mentioned above, is now known as EEM. We started the patient on topiramate for her seizures and migraine prophylaxis. We also ordered an EEG, brain MRI with epilepsy protocol, and genetic panel with the results to be discussed at the next visit. In the interim, although she became headache free with topiramate titrated up to 10 mg/kg/day, her seizures were not improved therefore ethosuximide was added and titrated up to 40 mg/kg/day. There were no significant adverse medication effects other than mild transient sedation.
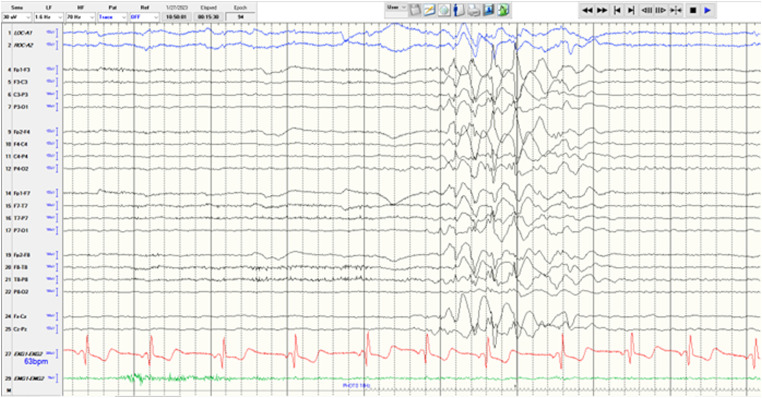
Her 21-channel digital video-EEG results showed a burst of frontally predominant, generalized, 3–4 Hz polyspike-and-slow wave activity during photic stimulation. No seizures were noted. The EEG was consistent with the diagnosis of a genetic generalized epilepsy, specifically EEM (Figure 1). Her MRI scan was unremarkable.
Figure 1.
21-channel digital video-EEG.
Patient followed up in our clinic approximately two months later with a significant >90% improvement of seizure activity per her mother after continuing on the topiramate and ethosuximide combination. Her mother quantified that the patient's seizure frequency now ranged from one to five per hour. Her mother also reported that the patient continues to no longer complain of headaches.
Patient's genetic panel came back with a heterozygous PLCB1 mutation reported as likely pathogenic. She was also heterozygous for SCN1A and SCN9A mutations. Her father's genetic panel was also heterozygous for SCN1A and SCN9A mutations although he has no history of seizures. Her mother's genetic testing came back normal.
Discussion/Conclusion
As mentioned above, based on the patient's presentation we were highly suspicious of EEM, and our EEG findings ended up completing the typical triad. Our patient's unremarkable MRI result is consistent as its typically normal or nonspecific in this syndrome. 4
EEM is suspected to have a genetic origin based on family studies. The patient presented in this case was found to be heterozygous for a PLCB1 mutation, which is a likely genetic cause of her symptoms. Previous studies have shown that mutations in PLCB1 have a strong correlation with early onset epilepsy and have been determined to result in a variety of encephalopathic epilepsies (EE). EE is generally characterized by drug-resistant seizures, psychomotor slowing, and overall poor neurological outcomes. These seizures often present early in life, which can be detrimental to the infant brain and can over time lead to chronic seizures and neurocognitive impairment. 7 In most cases, epileptic spasms and seizures began within 3-5 months of age alongside developmental regression or arrest. 8
The normal function of PLCB1 gene is to encode one of the phosphoinositide-specific phospholipase C (PLC) enzymes; this enzyme is involved in a G-protein coupled receptor signaling pathway, specifically a muscarinic acetylcholine receptor. The PLC protein encoded by PLCB1 is activated by a Gq receptor embedded in the cell membrane, which then hydrolyzes IP2 and generates the second messengers IP3 and DAG. IP3 signals release of Ca2 + from intracellular stores while DAG stimulates the activation of protein kinase C. This signal transduction pathway plays a role in regulating important biological processes such as proliferation, differentiation, and survival. 9
It is also known that the PLCB1 gene is predominantly expressed in the brain, most prominently in the cerebral cortex and the hippocampus. Because of this, it is thought that mutations in this gene lead to problems with growth, development, and intellectual ability. It has been shown that PLCB1 heterozygous mice displayed both hindrance of growth and decreased overall viability and was found that muscarinic signaling impairment in the hippocampus led to onset of epilepsy and death. 9 Similarly, in infants found to have haploinsuffiency of PLCB1, the most common presentation was early-onset seizures as well as profound intellectual disability, hypotonia, hyperreflexia, and microcephaly. 10 It has been shown that the biallelic loss of 20p13, as well as deletions of promoter regions or deletions of different gene regions ranging from exons 1-8 can have detrimental phenotypic variations, all including seizures at a young age. 11 Of note, many of the infants featured in these studies were homozygous for a mutation of PLCB1, but one study included a child noted to have a heterozygous deletion in the promoter region and exons 1-3, resulting in a similar phenotype of early-onset seizures and developmental delay. 9 A search through the literature on this mutation did not elicit any documented associations with EEM specifically. Our patient's heterozygous PLCB1 mutation condition likely resulted in her neurotypical development and relatively milder epilepsy although with extremely frequent and resistant seizures.
Mutations in SCN1A affect modulation of sodium-gated channels and may lead to an increased influx of sodium and a subsequent increase in CNS excitation. These mutations have been linked to generalized epilepsy with febrile seizures which tend to have an autosomal dominant inheritance with incomplete penetrance. 12 SCN1A is a sodium channel expressed in the brain; however, SCN9A is expressed primarily in dorsal root ganglia and sympathetic ganglion neurons in the PNS. SCN9A epilepsy literature review has strongly refuted association between this mutation and epilepsy. Variants in SCN9A are abundant in the normal population and often do not cause disease making it likely that they are benign mutations. 13 Furthermore, as mentioned above, her father is asymptomatic and has the same SCN1A and SCN9A mutations lowering the likelihood that these two mutations are contributing to her specific epilepsy syndrome.
In terms of management, this syndrome can often be drug resistant; 5 however, treatment typically consists of some type of antiepileptic agent with some of the most common being valproate, lamotrigine, ethosuximide, levetiracetam and, in some cases, benzodiazepines. Ethosuximide has shown to be more effective in patients with absence seizures as a predominant manifestation.2,4 This showed to be true in our patient as well with marked symptom improvement using the combination of ethosuximide and topiramate.
Though the predominate clinical manifestation is eyelid myoclonia with or without absence seizures, most patients will eventually experience either spontaneous or light-induced generalized tonic-clonic seizures. Seizures should therefore be properly treated as eyelid myoclonia status epilepticus has been reported without proper treatment. 2 These seizures tend to persist over the patient's lifespan. 14
Continued follow up of this patient over time will give us a better idea of the course of her disease and efficacy of the pharmacologic regimen used. This patient's case was found to be clinically very typical of EEM although her likely pathogenic mutation was atypical for this specific syndrome. This case clearly demonstrates and emphasizes the importance of genetic testing in patients with epilepsy. Collecting this kind of data will help to better understand the genetic components that often accompany seizure disorders. Further studies would need to be conducted to understand how the patient presented in this case's specific gene mutations play a role in her clinical syndrome.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal and written informed consent was obtained from a legally authorized representative for anonymized patient information to be published in this article.
ORCID iD: Alexandria L. Spurgeon https://orcid.org/0009-0008-4020-3407
References
- 1.Madaan P, Jauhari P, Chakrabarty B, Gulati S. Jeavons syndrome: An overlooked epilepsy syndrome. Pediatr Neurol. 2019;93:63. doi: 10.1016/j.pediatrneurol.2018.12.016 [DOI] [PubMed] [Google Scholar]
- 2.Zawar I, Knight EP. Epilepsy with eyelid myoclonia (Jeavons Syndrome). Pediatr Neurol. 2021;121:75-80. doi: 10.1016/j.pediatrneurol.2020.11.018 [DOI] [PubMed] [Google Scholar]
- 3.Jeavons PM. Nosological Problems of Myoclonic Epilepsies in Childhood and Adolescence . Dev Med Child Neurol. 1977;19:3–8. [DOI] [PubMed] [Google Scholar]
- 4.de la Jara J, Vásquez-Hernández C, Ramírez-Rojo E, Moya-Vilches J. Uncommon epileptic syndromes in children: A review. Seizure. 2021;90:17-27. doi: 10.1016/j.seizure.2021.05.005 [DOI] [PubMed] [Google Scholar]
- 5.Smith KM, Youssef PE, Wirrell EC, et al. Jeavons syndrome: Clinical features and response to treatment. Pediatr Neurol. 2018;86:46-51. doi: 10.1016/j.pediatrneurol.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 6.Ng YT. The Hitchhiker’s guide to the role of (Transient) hypoglycemia in refractory seizures and epilepsy. Pediatr Neurol. 2012;47(2):123-124. doi: 10.1016/j.pediatrneurol.2012.04.020 [DOI] [PubMed] [Google Scholar]
- 7.Kurian MA, Meyer E, Vassallo G, et al. Phospholipase C beta 1 deficiency is associated with early-onset epileptic encephalopathy. Brain. 2010;133(10):2964-2970. doi: 10.1093/brain/awq238 [DOI] [PubMed] [Google Scholar]
- 8.Desprairies C, Valence S, Maurey H, et al. Three novel patients with epileptic encephalopathy due to biallelic mutations in the PLCB1 gene. Clin Genet. 2020;97(3):477-482. doi: 10.1111/cge.13696 [DOI] [PubMed] [Google Scholar]
- 9.Schoonjans AS, Meuwissen M, Reyniers E, Kooy F, Ceulemans B. PLCB1 Epileptic encephalopathies; review and expansion of the phenotypic spectrum. Eur J Paediatr Neurol. 2016;20(3):474-479. doi: 10.1016/j.ejpn.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 10.Coppola A, Cellini E, Stamberger H, et al. Diagnostic implications of genetic copy number variation in epilepsy plus. Epilepsia. 2019;60(4):689-706. doi: 10.1111/epi.14683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poduri A, Chopra SS, Neilan EG, et al. Homozygous PLCB1 deletion associated with malignant migrating partial seizures in infancy. Epilepsia. 2012;53:1–8. doi: 10.1111/j.1528-1167.2012.03538.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grill MF, Losey TE, Ng YT. The Hitchhiker’s guide to the child neurologist’s genetic evaluation of epilepsy. Semin Pediatr Neurol. 2008;15(1):32-40. doi: 10.1016/j.spen.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 13.Fasham J, Leslie JS, Harrison JW, et al. No association between SCN9A and monogenic human epilepsy disorders. PLoS Genet. 2020;16(11):1–10. doi: 10.1371/journal.pgen.1009161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Striano S, Capovilla G, Sofia V, et al. Eyelid myoclonia with absences (Jeavons syndrome): A well-defined idiopathic generalized epilepsy syndrome or a spectrum of photosensitive conditions? Epilepsia. 2009;50(SUPPL. 5):15-19. doi: 10.1111/j.1528-1167.2009.02114.x [DOI] [PubMed] [Google Scholar]