Abstract
Background
Laboratory monitoring is not recommended when subcutaneous unfractionated heparin (SQ-UFH) is administered at prophylactic doses. However, aPTT prolongation and associated hemorrhage has been reported in the neurocritically ill. At our institution, Neuroscience Intensive Care Unit (Neuro-ICU) patients with prolonged aPTT are further evaluated with a follow up aPTT and anti-factor Xa.
Purpose
The purpose of this study was to describe concordance between aPTT and anti-factor Xa in neurocritically ill patients receiving prophylactic SQ-UFH with evidence of aPTT prolongation.
Methods
A retrospective chart review of adult patients admitted to the Neuro-ICU from June 2017 to June 2019 was performed. Patients were included if they received SQ-UFH with aPTT levels and at least one anti-factor Xa level drawn within one hour of each other. Concordance between paired aPTT and anti-factor Xa was evaluated using Cohen’s weighted kappa.
Results
Forty two patients with 56 paired aPTT and anti-factor Xa levels were included. The most prescribed SQ-UFH regimen was 5000 units every 8 hours (60.7%) and anti-factor Xa levels were drawn a median (IQR) of 5.7 (3.1-10.7) hours after the SQ-UFH dose. Only 16 (28.6%) pairs were in concordance. The analysis showed a weighted kappa of .09; 95% CI [−.05 to .22] indicating poor agreement.
Conclusions
In neurocritically ill patients receiving prophylactic SQ-UFH with aPTT prolongation, there was poor concordance between aPTT and anti-factor Xa. This suggests that aPTT prolongation may not be solely driven by heparin activity and further evaluation of mechanistic drivers for coagulopathy in this population is necessary.
Keywords: unfractionated heparin, neurocritical care, activated partial thromboplastin time, anti-factor Xa
Introduction
Neurocritically ill patients have multiple risk factors for venous thromboembolism (VTE) including acute brain injury, immobility, and critical illness. 1 Depending on the subset of neurocritically ill patients and varying risk of VTE, studies have found the reduction in VTE events to outweigh the incidence of bleeding with chemical thromboprophylaxis.1-3 VTE prophylaxis guidelines released by the Neurocritical Care Society recommend initiation of chemical VTE prophylaxis within 24 to 72 hours after onset for most neurologic injuries. 1
Subcutaneous unfractionated heparin (SQ-UFH) is a commonly used agent for pharmacologic VTE prophylaxis in critically ill patients. 3 Although monitoring of heparin activity with activated partial thromboplastin time (aPTT) and/or anti-factor Xa levels is recommended when unfractionated heparin (UFH) is administered at therapeutic doses, laboratory monitoring is not recommended when SQ-UFH is administered at prophylactic doses as these doses are not expected to prolong the aPTT. 4 However, prolongation of the aPTT and associated hemorrhage has been reported with prophylactic SQ-UFH administration.4–7 Moreover, the aPTT may be affected by other biological and laboratory factors, independent of UFH’s effects. 8 Therefore the anti-factor Xa assay has been recommended as a preferred laboratory test for monitoring continuous infusions of therapeutic UFH and may help verify presence of therapeutic heparin activity when aPTT is consistently prolonged.7-11 Patients in our Neuroscience Intensive Care Unit (Neuro-ICU) with prolonged aPTT are further evaluated for therapeutic heparin activity with a follow up aPTT and anti-factor Xa.
Historically, studies evaluating concordance of aPTT and anti-factor Xa levels in hospitalized patients receiving therapeutic intravenous UFH infusions have demonstrated modest concordance.9,12-14 With differences in subcutaneous absorption and lower drug exposure, concordance may not be generalizable from studies evaluating intravenous UFH therapy. The primary objective of this study was to describe the concordance between aPTT and anti-factor Xa levels in neurocritically ill patients receiving prophylactic SQ-UFH with prolonged aPTT.
Methods
This was a single-center, retrospective, observational cohort study of patients 18 years and older admitted to the Neuro-ICU at Columbia University Irving Medical Center (CUIMC) of New York-Presbyterian Hospital (NYPH) from June 2017 to June 2019. Patients who received prophylactic SQ-UFH and had an aPTT and at least one anti-factor Xa level drawn within 1 hour of each other were included, and those who received therapeutic anticoagulation at the time of the levels were excluded. Corresponding aPTT and anti-factor Xa levels and SQ-UFH regimens were automatically extracted from the electronic health record. Baseline characteristics and laboratory parameters were extracted by manual chart review. Approval for this study was obtained from the investigational review board at CUIMC.
Patients admitted to the Neuro-ICU and able to receive chemical thromboprophylaxis were initiated on low molecular weight heparin or SQ-UFH. In the absence of contraindications, mechanical thromboembolic prophylaxis in the form of sequential compression devices (SCDs) was also administered. If SQ-UFH was the agent used, it was initiated at 5000 units every 12 hours if the patient weighed less than 50 kg, 5000 units every 8 hours if between 50 to 119 kg, and 7500 units every 8 hours if greater than 119 kg. In patients admitted with intracranial hemorrhages or ischemic stroke post thrombolysis, chemical thromboprophylaxis was initiated after a 24-hour stability computerized tomography (CT) scan confirmed hematoma stability, absence of hematoma expansion or hemorrhagic transformation. In patients without contraindication to anticoagulants, chemical thromboprophylaxis was initiated on admission to the Neuro-ICU.
Coagulation tests were obtained routinely for all patients. Prolonged aPTT values resulted in repeated measurement and persistent aPTT prolongation was followed by measurement of an anti-factor Xa level to evaluate for therapeutic heparin activity (most often to assess peak activity 4 hours after the SQ-UFH dose). In patients with prolonged aPTT, laboratory testing for other causes of the coagulation abnormality (i.e. anti-phospholipid antibodies, lupus anticoagulant) were not routinely obtained. The goal anti-factor Xa level was < .3 IU/mL. If the anti-factor Xa level was subtherapeutic or undetectable (< .3 IU/ml), then it was not checked again. If the anti-factor Xa level was .3 IU/mL or higher, the frequency of SQ-UFH was decreased and an additional anti-factor Xa level was monitored at steady state. Blood samples were drawn into BD Vacutainer Buffered Sodium Citrate .109 M, 3.2% tubes and then centrifuged at 8000 RPMs for 6 minutes to separate blood cells from platelet-poor plasma. The aPTT was then analyzed. Specimens were double spun to ensure platelet depletion and followed by storage at 20°C for a maximum of eight hours until anti-factor Xa assays were performed. Coagulation parameters were measured using a STA-R coagulation instrument using an electromagnetic viscosity detection system.
The primary outcome of this study was concordance between aPTT and anti-factor Xa values in patients receiving prophylactic doses of SQ-UFH. To evaluate concordance, the anti-factor Xa value and the aPTT closest to the time of anti-factor Xa collection were identified and evaluated using the institutional nomogram. Based on the institutional nomogram during that time period, aPTT levels were defined as the following: 23.9 – 34.7 seconds as normal, 34.8 – 75.9 seconds as elevated, 76 – 112 seconds as therapeutic, and greater than 112 seconds as supra-therapeutic. Anti-factor Xa levels were calibrated to report an anti-factor Xa range reflecting therapeutic heparin activity, and therefore were defined as the following: less than .1 IU/mL as undetectable, .1 – .29 IU/ml as elevated, .3-.7 IU/ml as therapeutic, and greater than .7 IU/ml as supra-therapeutic. Paired aPTT and anti-factor Xa levels are grouped as described in Figure 1. Corresponding aPTT and anti-factor Xa levels that were considered concordant were: an aPTT value that fell within normal range paired with an undetectable anti-factor Xa level, an elevated aPTT paired with an elevated anti-factor Xa level, a therapeutic aPTT paired with a therapeutic anti-factor Xa level, and a supra-therapeutic aPTT paired with a supra-therapeutic anti-factor Xa level. A simplified categorization of corresponding aPTT and anti-factor Xa levels was also evaluated and defined concordance as a normal aPTT value paired with an undetectable anti-factor Xa level or an elevated aPTT value paired with an elevated anti-factor Xa level. Elevated values encompassed previous groups defined as elevated, therapeutic, and supra-therapeutic.
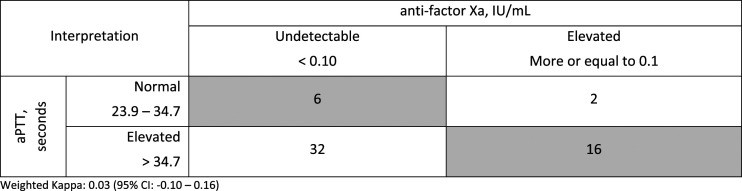
Figure 1.
Concordance of paired aPTT and anti-factor Xa levels (n = 56).
Data Analysis
Data for continuous variables were reported as medians and interquartile ranges, while categorical variables were reported as frequencies and percentage. The Shapiro-Wilk test was performed to evaluate for normality. For bivariate analysis, the Mann-Whitney U test was used for continuous variables; Chi-squared or Fisher exact tests were used for dichotomous variables. A univariable analysis was used to evaluate for differences between levels that were concordant and those that were not. Observed concordance between aPTT and anti-factor Xa tests was calculated. Cohen’s weighted kappa was used to assess concordance between aPTT and anti-factor Xa levels. Statistical significance was set at P-value <.05. Data was analyzed with R software (Version 1.1.423).
Results
A total of 42 patients (56 paired aPTT and anti-factor Xa levels) were included in the analysis. Baseline characteristics are as shown in Table 1. The median (IQR) age was 70 (57-78) years old and 40% of patients were male, with a median (IQR) actual body weight of 66 (62-72) kg. The most common admission diagnoses were intracranial hemorrhage (26%), followed by status epilepticus (24%), and acute ischemic stroke (14%). The most prevalent comorbidities present on admission included hypertension (57%), diabetes (31%), and a history of ischemic stroke (19%). None of the patients included in the analysis had reported positive Factor V Leiden, factor II mutation, elevated serum homocysteine, lupus anticoagulant, elevated anticardiolipin antibody, history of heparin-induced thrombocytopenia, or other congenital or acquired thrombophilia. Most patients (79%) required mechanical ventilation during their Neuro-ICU stay. The most prescribed SQ-UFH regimen administered prior to an anti-factor Xa level was 5000 units every 8 hours (60.7%), followed by 5000 units every 12 hours (37.5 %) as seen in Table 2. Anti-factor Xa levels were drawn at a median (IQR) time of 5.7 (3.1-10.7) hours after the SQ-UFH dose and a median of 6 (3-12) days after SQH-UFH initiation.
Table 1.
Baseline Characteristics (n = 42).
| Age, years | 70 (57-78) |
| Male | 17 (40) |
| BMI, kg/m2 | 23.2 (21.6 - 28.6) |
| Weight, kg | 66 (62 - 72) |
| < 50 kg | 1 (2.4) |
| 50 - 100 kg | 40 (95) |
| > 100 kg | 1 (2.4) |
| Diagnoses | |
| Intracranial hemorrhage | 11 (26) |
| Status epilepticus | 10 (24) |
| Acute ischemic stroke | 6 (14) |
| Other | 15 (36) |
| Glasgow coma scale | 7 (5 - 11) |
| Past medical history | |
| Hypertension | 24 (57) |
| Diabetes | 13 (31) |
| Stroke | 8 (19) |
| Atrial fibrillation | 5 (12) |
| Active systemic cancer | 4 (9.5) |
| End-stage renal disease | 6 (14) |
| Venous thromboembolism | 3 (7.1) |
| Concomitant use of antiplatelets during ICU admission | 17 (40) |
| Mechanical ventilation during ICU admission | 33 (79) |
| Length of hospital stay, days | 32 (18 - 43) |
| Length of ICU stay, days | 16 (8 - 26) |
| ICU Mortality | 6 (14) |
| Platelet count, 103/microliter | 203 (151 - 242) |
| Hemoglobin, g/dL | 11.6 (9.3 - 12.9) |
| aPTT, seconds | 32.0 (26 – 35) |
| INR | 1.2 (1.1 - 1.3) |
| Serum creatinine, mg/dL | 1.0 (.7 - 2.3) |
Data presented as n (%) or median (interquartile range) and is the first value upon ICU admission, unless otherwise indicated.
BMI = body mass index, ICU = intensive care unit, INR = international normalized ratio, aPTT = activated partial thromboplastin time.
Other diagnoses = acute renal failure, brain neoplasm, cardiac arrest, decompensated liver failure, encephalopathy, leptomeningeal disease, meningitis/encephalitis, neuromuscular disease, normal pressure hydrocephalus, respiratory failure, transverse myelitis.
Table 2.
Characteristics of SQ-UFH Regimens and Associated aPTT & Anti-Factor Xa Levels (n = 56).
| SQ-UFH regimen prior to anti-factor Xa levels | 56 (100) |
| 2500 units every 12 hours | 1 (1.8) |
| 5000 units every 12 hours | 21 (37.5) |
| 5000 units every 8 hours | 34 (60.7) |
| Timing of anti-factor Xa level post last SQ-UFH dose, hours | 5.7 (3.1 – 10.7) |
| Timing of anti-factor Xa level from first SQ-UFH dose, days | 6 (3-12) |
| SQ-UFH dosing change after anti-factor Xa level | 16 (28.6) |
| aPTT at time of anti-factor Xa, seconds | 47 (37 – 57) |
| Anti-factor Xa at time of aPTT, IU/ml | .09 (.09 – .12) |
| Time from ICU admission to collection of paired aPTT & anti-factor Xa, days | 6 (3 – 12) |
Only 16 (28.6%) of the 56 paired levels were concordant (Figure 1). The weighted kappa was .09; 95% CI [−.05 to .22] indicating poor overall concordance. The majority (85.7%) of all aPTT levels were prolonged beyond normal range of 23.9-34.7 seconds; 43 (76.7%) were in the elevated range, 4 (7.1%) in the therapeutic range, and 1 (.2%) in the supra-therapeutic range. Although all patients had prolonged aPTT values that led to collection of an anti-factor Xa level, aPTT levels drawn closest to the anti-factor Xa level were designated for analysis and eight aPTT levels included in analysis were normal. The most common reason for non-concordance was an elevated aPTT (34.8-75.9 seconds) with a corresponding undetectable (<.10 IU/ml) anti-factor Xa level (55.4% of all pairs) (Figure 1). Thirty-four paired levels (60.7%) had a disproportionately prolonged aPTT compared to anti-factor Xa levels, while six paired levels (10.7%) had a disproportionately high anti-factor Xa compared to the aPTT level. The SQ-UFH dosing was changed after the result of 16/56 (28.6%) anti-factor Xa levels. When grouped according to a simplified categorization (Figure 2), 22 (39.3%) of the 56 paired levels were concordant and the weighted kappa was .03 (95% CI [−.10 to .16]). An analysis including only the first aPTT and anti-factor Xa pair measured per patient showed similar results, with 15 (35.7%) of the 42 paired levels showing concordance and a weighted kappa of .03 (95% CI [−.16 to .16]) (Figure 3).
Figure 2.
Concordance of paired aPTT and anti-factor Xa levels based on simplified categorization (n = 56).
Figure 3.
Concordance of unique paired aPTT and anti-factor Xa levels based on simplified categorization (n = 42).
A univariable analysis of concordant and non-concordant pairs to evaluate factors that could affect the concordance between aPTT and anti-factor Xa levels is presented in Table 3. In patients with more than one pair, the first pair of aPTT and anti-factor Xa levels was selected. The non-concordant pairs group had a higher baseline serum creatinine (1.26 vs .72, P = .017) and INR (1.2 vs 1.1, P = .023). Due to the small sample size, a multivariable analysis was not performed.
Table 3.
Comparison of Patients with Concordant vs Non-Concordant aPTT & Anti-Xa Pairs (n = 42).
| Concordant pairs (n = 12) | Non-concordant pairs (n = 30) | P-value | |
|---|---|---|---|
| Age, years | 58 (40-74) | 74 (62-80) | .064 |
| Male, sex | 3 (25) | 14 (47) | 0.3 |
| BMI, kg/m2 | 23.0 (21.9-31.7) | 23.5 (21.5-27.8) | 0.8 |
| Weight, kg | 64 (60-76) | 66 (62.0-72.0) | > 0.9 |
| Baseline serum creatinine, mg/dL | .72 (.58-.87) | 1.26 (.77-3.16) | .017 |
| Baseline aPTT, seconds | 31 (28-34) | 33 (26-46) | 0.4 |
| Baseline INR | 1.1 (1.0-1.2) | 1.2 (1.1-1.4) | .023 |
| End-stage renal disease | 1 (8) | 5 (17) | 0.7 |
| Surgery during ICU stay | 5 (42) | 8 (27) | 0.5 |
| ICU mortality | 2 (17) | 4 (13) | > 0.9 |
| Time between SQH dose and anti-factor Xa, hours | 3.7 (2.7-7.0) | 4.6 (3.3-7.3) | 0.5 |
In patients with more than one pair, the first pair of aPTT and anti-factor Xa levels was selected.
Although routine screenings for venous thromboembolism were not performed during the study period, diagnostic tests were performed at the discretion of the team. Tables 4 and 5 describe 12 patients in whom venous thromboembolism and major bleeding was detected. Major bleeding was defined as bleeding requiring blood transfusion or presence of a new or expanding intracranial hematoma.
Table 4.
Bleeding and Thrombotic Events (n = 42).
| VTE, n (%) | |
| PE | 1 (2.4) |
| DVT | 4 (9.5) |
| Upper extremity | 3 (7.1) |
| Lower extremity | 1 (2.4) |
| Major bleeding, n (%) | |
| Transfusion requirement | 7 (17) |
| Hematoma expansion | 5 (12) |
1 patient experienced both major bleeding and a VTE.
Table 5.
Bleeding and thrombotic events and associated aPTT and anti-factor Xa levels (n = 12).
| Patient | Clinical Event | aPTT | Anti-factor Xa |
|---|---|---|---|
| 1 | Required blood transfusion | Elevated | Undetectable |
| 2 | Required blood transfusion | Elevated | Undetectable |
| 3 | New or expanding hematoma, required blood transfusion | Elevated | Undetectable |
| 4 | New or expanding hematoma | Elevated | Elevated |
| 5 | New or expanding hematoma, required blood transfusion | Elevated | Elevated |
| 6 | New or expanding hematoma, required blood transfusion | Elevated | Elevated |
| 7 | New or expanding hematoma, required blood transfusion, diagnosed with pulmonary embolism | Elevated | Elevated |
| 8 | Required blood transfusion | Elevated | Undetectable |
| 9 | Diagnosed with deep vein thrombosis | Elevated | Undetectable |
| 10 | Diagnosed with deep vein thrombosis | Elevated | Undetectable |
| 11 | Diagnosed with deep vein thrombosis | Elevated | Undetectable |
| 12 | Diagnosed with deep vein thrombosis | Elevated | Elevated |
Discussion
Routine monitoring of heparin activity with prophylactic SQ-UFH administration is not generally recommended. However, reports of bleeding associated with aPTT prolongation with SQ-UFH have led to monitoring aPTT levels in high risk patients such as the neurocritically ill. 7 In patients with consistently prolonged aPTT levels, anti-factor Xa may be a more accurate measurement of heparin activity, however data validating a gold standard test is lacking. 7 To our knowledge, our evaluation represents the largest cohort to characterize the concordance between aPTT and anti-factor Xa levels in patients receiving SQ-UFH. Like previous studies in patients receiving intravenous therapeutic UFH, our study showed poor concordance (28.6%) between aPTT and anti-factor Xa levels in patients receiving prophylactic SQ-UFH.9,12-15 Most patients in our study had mild prolongation in their aPTT (classified as elevated, but not therapeutic or supratherapeutic) with undetectable anti-factor Xa levels (Figures 2 and 3). There are multiple possible explanations for this poor concordance.
Previous studies have identified peak heparin activity to be between .05 and .25 IU/mL in patients receiving SQ-UFH.16,17 Like Aggarwal and colleagues, our institutional anti-factor Xa assay during this study was intended to reflect therapeutic heparin activity and had a lower limit of detection of .1 IU/ml. Therefore all anti-Xa activity reported as <.1 IU/mL was considered as zero for analysis. 18 This may have led to imprecise detection of concordance at this end of the spectrum and the large number of non-concordant prolonged aPTT levels paired with undetectable anti-factor Xa levels.
Another possible cause for the poor concordance between aPTT and anti-factor Xa levels is that the aPTT is impacted by multiple laboratory and biologic factors. Laboratory factors include variable concentration of citrate or the underfilling of blood in the sample collection tube, delay in sample analysis, inadequate centrifugation or gross hemolysis of blood sample, and use of different reagents batches.8,19 Biologic factors include coagulopathic deficiencies, consumptive coagulopathy, diurnal variations of coagulation factors, fluctuations in acute phase reactants like factor VIII or fibrinogen, and changes in heparin-binding proteins.8,19 Although the anti-factor Xa level may be more reliable and consistent, it can also be affected by laboratory and biologic factors such as hyperbilirubinemia and hypertriglyceridemia, which may interfere with the colorimetric assay and lead to spurious results. 8 Due to the retrospective nature of this study, the impact of these factors on concordance could not be controlled or measured.
Finally, changes in patient’s subcutaneous absorption, volume of distribution, and clearance of UFH, can affect both aPTT and anti-factor Xa and levels may reflect different amounts of drug exposure if not collected at the same time. An altered absorption of subcutaneous drugs is expected in patients of higher weight and only a single patient was greater than 100 kilograms. Although inclusion of higher weight patients is not expected to improve concordance between aPTT and anti-factor Xa levels, the findings of this study may not be generalizable to patients of higher weight. There is evidence that supports a peak in heparin activity around 3 to 4 hours after SQ-UFH administration. Gallus and colleagues found the mean aPTT to be slightly but significantly prolonged for five hours after administration of 5000 units of SQ-UFH (P < .01), with highest prolongation between 2 to 4 hours. 20 Brozovic and colleagues measured anti-factor Xa levels at 2 hours after administration of 5000 units of SQ-UFH and found levels to be elevated (.05-.15 IU/ml) when compared to levels at 8 hours (< .1 IU/ml). 17 Improved clinical utility may be seen when drawing both aPTT and anti-factor Xa levels during peak heparin activity, around 3 to 4 hours after SQ-UFH administration. The median time between the SQ-UFH dose and anti-factor Xa level in our study was 5.7 hours (3.1-10.7), with the aPTT drawn within 1 hour of the anti-factor Xa level. APTT levels may have been collected either during peak or trough heparin activity and anti-factor Xa levels may have been drawn after the window of highest heparin activity. A univariable analysis of factors that could affect concordance (Table 3) did not show a difference in concordance based on the time between SQ-UFH dose and anti-factor Xa level or weight.
The predominant pattern of non-concordance observed in this study was a disproportionately prolonged aPTT compared to anti-factor Xa levels, as observed in several studies evaluating concordance in patients receiving therapeutic intravenous UFH.9,14 In one such study by Price and colleagues, a disproportionately prolonged aPTT to anti-factor Xa level on at least 2 consecutive occasions was associated with increased 21-day major bleeding rates. 14 A study by Lawlor & colleagues evaluating patients with COVID-19, showed the opposite pattern in patients receiving therapeutic intravenous UFH and suggested the aPTT underestimated heparin activity compared with the anti-Xa assay in most non-concordant observations. However, Lawlor & colleagues concluded that the complex coagulopathy associated with COVID-19 further reduces aPTT reliability, and therefore additional research is needed to explore more accurate assessment of heparin activity. 15 Although multiple studies report non-concordance, the relationship between aPTT and anti-factor Xa remains uncertain as it is also unclear which laboratory test is preferred to best predict the risk of bleeding. Further research to establish the standard reference test in patients who experience major bleeding in the neurocritically ill population may clarify the relationship and guide clinical management of these non-concordant results.
Several limitations accompany this study. This is a single-center retrospective cohort study. The practice of obtaining anti-factor Xa levels to evaluate plasma heparin activity from SQ-UFH after a prolonged aPTT was followed at the multidisciplinary team’s discretion, possibly leading to selection bias. Severity of illness scores such as the SOFA or APACHE were not assessed, which may reduce the generalizability of this study to other critically ill patients. Due to limitations of sample size and selection bias, clinical implications of sequelae such as venous thromboembolism and bleeding were unable to be determined from this data.
Conclusions
Poor concordance between aPTT and anti-factor Xa levels was observed in this neurocritically ill population receiving prophylactic SQ-UFH. A predominant pattern of disproportionately prolonged aPTT compared to anti-factor Xa level was seen. Future prospective studies establishing the standard reference test to evaluate heparin activity and assessing clinical outcomes of non-concordant aPTT and anti-factor Xa patterns in neurocritically ill patients receiving thromboprophylaxis are needed.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
IRB Approval: This retrospective study adheres to ethical guidelines and has been reviewed and approved by Columbia University IRB (Protocol IRB-AAAS0463, Approved 10/04/2018).
ORCID iDs
Karen Berger https://orcid.org/0000-0003-3686-3468
Caroline Der-Nigoghossian https://orcid.org/0000-0001-5445-9421
References
- 1.Nyquist P, Bautista C, Jichici D, et al. Prophylaxis of venous thrombosis in neurocritical care patients: An evidence-based guideline: A statement for healthcare professionals from the neurocritical care society. Neurocrit Care. 2016;24(1):47-60. [DOI] [PubMed] [Google Scholar]
- 2.Attia J, Ray JG, Cook DJ, Douketis J, Ginsberg JS, Geerts WH. Deep vein thrombosis and its prevention in critically ill adults. Arch Intern Med. 2001;161(10):1268-1279. [DOI] [PubMed] [Google Scholar]
- 3.Raslan AM, Fields JD, Bhardwaj A. Prophylaxis for venous thrombo-embolism in neurocritical care: A critical appraisal. Neurocrit Care. 2010;12(2):297-309. [DOI] [PubMed] [Google Scholar]
- 4.Fiebig EW, Jones M, Logan A, Wang CS, Lewis B. Unexpectedly high ptt values after low-dose heparin prophylaxis. Arch Intern Med. 2011;171(7):702-703. [DOI] [PubMed] [Google Scholar]
- 5.Thompson MH, Wilson SH, Toussaint BL, et al. Effect of subcutaneous unfractionated heparin prophylaxis on activated partial thromboplastin time: A retrospective evaluation. J Clin Anesth. 2016;33:346-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahir H, Wani A, Daruwalla V. Life-threatening intracranial hemorrhage with unexpectedly high prothrombin time following venous thromboembolism prophylaxis. J Med Cases. 2015;6(7):313-317. [Google Scholar]
- 7.Shusterman M, Grassl N, Berger K, De Sancho MT. Prolonged activated partial thromboplastin time after prophylactic-dose unfractionated heparin in the post-operative neurosurgical setting: Case series and management recommendations. J Thromb Thrombolysis. 2020;49(1):153-158. [DOI] [PubMed] [Google Scholar]
- 8.Vandiver JW, Vondracek TG. Antifactor xa levels versus activated partial thromboplastin time for monitoring unfractionated heparin. Pharmacotherapy. 2012;32(6):546-558. [DOI] [PubMed] [Google Scholar]
- 9.Samuel S, Allison TA, Sharaf S, et al. Antifactor Xa levels vs. activated partial thromboplastin time for monitoring unfractionated heparin. A pilot study. J Clin Pharm Ther. 2016;41(5):499-502. [DOI] [PubMed] [Google Scholar]
- 10.Olson JD, Arkin CF, Brandt JT, et al. College of american pathologists conference XXXI on laboratory monitoring of anticoagulant therapy: Laboratory monitoring of unfractionated heparin therapy. Arch Pathol Lab Med. 1998;122(9):782-798. [PubMed] [Google Scholar]
- 11.Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(3 suppl l):188S-203S. [DOI] [PubMed] [Google Scholar]
- 12.Van Roessel S, Middeldorp S, Cheung YW, Zwinderman AH, de Pont ACJM. Accuracy of aPTT monitoring in critically ill patients treated with unfractionated heparin. Neth J Med. 2014;72(6):305-310. [PubMed] [Google Scholar]
- 13.Ratano D, Alberio L, Delodder F, Faouzi M, Berger MM. Agreement between activated partial thromboplastin time and anti-Xa activity in critically ill patients receiving therapeutic unfractionated heparin. Thromb Res. 2019;175:53-58. [DOI] [PubMed] [Google Scholar]
- 14.Price EA, Jin J, Nguyen HM, Krishnan G, Bowen R, Zehnder JL. Discordant aPTT and anti-Xa values and outcomes in hospitalized patients treated with intravenous unfractionated heparin. Ann Pharmacother. 2013;47(2):151-158. [DOI] [PubMed] [Google Scholar]
- 15.Lawlor M, Gupta A, Ranard LS, et al. Discordance in activated partial thromboplastin time and anti-factor Xa levels in COVID-19 patients on heparin therapy. Thromb Res. 2021;198:79-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbour LA, Smith JM, Marlar RA. Heparin levels to guide thromboembolism prophylaxis during pregnancy. Am J Obstet Gynecol. 1995;173(6):1869-1873. [DOI] [PubMed] [Google Scholar]
- 17.Brozović M, Stirling Y, Abbosh J. Plasma heparin levels after low dose subcutaneous heparin in patients undergoing hip replacement. Br J Haematol. 1975;31(4):461-466. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal MV, Jarrell AS, Gilmore VT, et al. Anti-Xa activity by weight in critically ill patients receiving unfractionated heparin for venous thromboembolism prophylaxis. J Crit Care. 2019;52:180-185. [DOI] [PubMed] [Google Scholar]
- 19.Takemoto CM, Streiff MB, Shermock KM, et al. Activated partial thromboplastin time and anti-xa measurements in heparin monitoring: Biochemical basis for discordance. Am J Clin Pathol. 2013;139(4):450-456. [DOI] [PubMed] [Google Scholar]
- 20.Gallus AS, Hirsh J, Tutle RJ, et al. Small subcutaneous doses of heparin in prevention of venous thrombosis. N Engl J Med. 1973;288(11):545-551. [DOI] [PubMed] [Google Scholar]