Abstract
Introduction
Breakthrough acute ischemic stroke (AIS) in patients with known, nonvalvular Atrial Fibrillation (AF), on Direct Oral Anticoagulants (DOAC), is an ongoing clinical conundrum. Switching anticoagulants was shown to be ineffective in preventing recurrent AIS. Systematic, patient-level chart review of so-called “DOAC failures” may offer insight into this phenomenon.
Methods
We conducted an IRB-approved, 6-year, retrospective study of AIS admissions, already prescribed DOAC for known AF. We sought plausible, alternative reasons for the AIS using a novel classification schema, CLAMP: C for Compliance concerns, L for Lacunes (small-vessel disease), A for Arteriopathy (atherosclerosis, web, or vasculitis), M for Malignancy, and P for Patent Foramen Ovale (PFO). These categories were labeled as DOAC “Pseudo-failures.” Conversely, absence of CLAMP variables were labeled as DOAC “Crypto-failures” conceivably from AF itself (“atriopathy”) or pharmacokinetic/pharmacogenomic dysfunction (ie, altered DOAC absorption, clearance, metabolism, or genetic polymorphisms). Forward logistic regression analysis was performed on prespecified DOAC subgroups.
Results
Of 4890 AIS admissions, 606 had AF, and 87 were previously prescribed DOAC (14.4% overall DOAC failure rate, 2.4% annualized over 6 years). Pseudo-failures comprised 77%: Compliance concerns (48.9%), Lacunes (5.7%), Arteriopathy (17.0%), Malignancy (26.1%), and PFO (2.3%). Crypto-failures comprised 23%, had lower CHADSVASc scores (AOR = .65, P = .013), and occurred more with rivaroxaban (41%) than apixaban (16%) or dabigatran (5.6%).
Conclusion
In AIS patients with known AF, DOAC Pseudo-failures, with identified alternate etiologies, are 3 times more likely than DOAC Crypto-failures. The CLAMP schema represents a novel approach to diagnostic classification and therapeutic adjustments in patients already prescribed DOAC for AF.
Keywords: stroke < cerebrovascular disorders, ischemic attack, transient < cerebrovascular disorders, electrophysiology/atrial fibrillation < clinical specialty
Introduction
Acute ischemic stroke (AIS) in patients with known, nonvalvular Atrial Fibrillation (AF), already prescribed Direct Oral Anticoagulants (DOAC), is a real-world clinical conundrum. Colloquially known as “DOAC failure,” breakthrough stroke was observed in 4 landmark AF trials1-4 at an annual rate of: 1.1% (dabigatran), 1.7% (rivaroxaban), 1.3% (apixaban), and 1.2% (edoxaban). In 7 subsequent prospective cohort studies, the DOAC failure rate was reported as 4.4% annually. 5 Because minimal data or guidance exists on how best to manage these patients, we aimed to conduct a patient-level, case-by-case, retrospective study of AIS admissions with known AF, previously prescribed DOAC. Our hypothesis was that a novel classification system could parsimoniously subdivide this cohort into distinct causes of “DOAC failure” both quantitatively and qualitatively. Ultimately, alternate diagnostic and therapeutic patterns would emerge as opportunities for adjustments in clinical care and hypotheses for future research.
Previously, a pooled analysis revealed 22.5% of AF patients with AIS or transient ischemic attack (TIA) were already prescribed anticoagulation. 6 Prescribing a different anticoagulant, hoping for a different result (so-called “anticoagulant-switching”) was shown to be ineffective in preventing recurrent AIS.6,7 One study found that adding anti-platelet therapy was linked to worse outcomes. 8 This argues against any widespread biochemical “failure” of the DOAC class, and instead suggests that DOACs may “fail” because of other, more practical explanations, such as non-compliance, or non-AF mechanisms of stroke: carotid atherosclerosis, small-vessel disease, vasculitis, and other causes. DOACs cannot revascularize stenoses, reduce hypertension, or alleviate inflammation. Because these etiologies, like non-compliance, are unrelated to DOAC biochemistry, we labeled them DOAC “Pseudo-failures.”
On the other hand, etiologies of a new AIS, in DOAC-adherent patients with known AF but without alternate etiologies, remains a topic of considerable interest. It is postulated that severe atrial disease “atriopathy” and/or high AF burden could result in AIS despite anticoagulation.6-8 Pharmacogenomic explanations also exist, including: (1). abnormal DOAC metabolism (inability to enzymatically convert DOAC pro-drugs, due to genetic polymorphisms); (2). altered clearance (renally-mediated pharmacokinetic differences in DOAC elimination); and/or (3). DOAC malabsorption (intestinal P-gp efflux pump system interactions with other medicines/herbal remedies).9,10 Because these etiologies rarely are confirmed biochemically, and their true incidence is unknown, we labeled these challenging cases as DOAC “Crypto-failures.”
Theoretically, a novel classification system would mitigate “anchoring bias” (fixating upon AF as the sole etiology) 11 and “premature closure” (omitting tests such as trans-esophageal echocardiogram, conventional cerebral angiogram, or hypercoagulable labs). 11 Reflexive pre-determination that an AF patient’s AIS must be “DOAC failure” can result in “nihilistic decision-making” 11 and repetitive cycles of futile “anticoagulant-switching”. 6
Methods
Study Population
We conducted a 6-year retrospective study of patients with known AF who presented with AIS or TIA to Tampa General Hospital between January 1, 2012 and December 31, 2017. Inclusion criteria were: age ≥18 years, admission diagnosis of TIA or AIS, and prior DOAC prescription (apixaban, dabigatran, rivaroxaban, or edoxaban) for AF. Exclusion criteria were: age <18 years, valvular AF, hemorrhagic stroke, warfarin prescription, or DOAC prescription for non-AF indication, such as venous thromboembolism (VTE).
Data Availability Statement, Case Identification and Data Abstraction
Data collection was performed using our hospital’s Get With The Guidelines-Stroke (GWTG-S) database. Approval was obtained from our Institutional Review Board (IRB). The GWTG-S inventory of all AIS/TIA patients over 6 years was cross-matched with a co-diagnosis of AF, thus providing a manageable number of subjects to abstract and review individually with our electronic medical record (EMR) to identify prior DOAC prescription, dose, and frequency. Demographic information collected included age, sex, NIHSS, CHADSVASc, and comorbidities such as dyslipidemia, diabetes, smoking, hypertension, and prior stroke.
Data Dictionary
To separate Pseudo-failures from Crypto-failures, we composed a novel classification schema called CLAMP: C for Compliance concerns; L for Lacunar small-vessel lipohyalinosis; A for Arterial pathology (intra- or extra-cranial atherosclerosis, vasculitis, or web); M for Malignancy (active cancer or newly detected); and P for Patent Foramen Ovale (PFO). These items were selected because alternate, reasonable treatment options exist other than “anticoagulant-switching.” 4 co-authors (AB, XY, JYC, and DZR) reviewed each case/imaging for non-AF mechanisms with the CLAMP schema to determine the presence of Pseudo-failures. The proposed categorization for each case was discussed and consensus reached by 3 co-authors (AB, XY and DZR) with the senior author making any tie-breaking decision. Subjects without CLAMP variables were deemed Crypto-failure by default. Then finally, the categorization of the entire patient sample was independently reviewed by a fourth co-author (JYC) to re-confirm classification.
Subdivisions of Pseudo-failures
Review of neuroimages and written documentation in the EMR progress notes enabled categorization as a DOAC “Pseudo-failure” if we identified ≥1 CLAMP component as follows:
Compliance Concerns
Compliance included any error in medication management regardless of the person or process that led to the error; examples include: DOAC held/stopped for a procedure/surgery and not restarted; patient non-adherence or refusal to take DOAC for any reason; or incorrect/inappropriate DOAC frequency (ie, once daily instead of twice-daily for dabigatran or apixaban) or dosage based on known criteria from package inserts (ie, prescriber non-compliance with appropriate dose: reduced-dose rather than full-dose as per weight, kidney function and age). 12 Socio-economic factors and/or disparities in care, previously studied specifically in AF patients with AIS, 13 were also included (i.e., inability to pay for medication or reduced access to care).
Lacunar Small-Vessel Disease
This category required confirmation by neuroimaging showing 1 small AIS, defined as ≤1.5 cm on axial Diffusion Weighted Imaging (DWI) sequence of brain MRI, in the brainstem, cerebellum, or subcortex, along with documented elevated blood pressure (systolic ≥180 mmHg and/or diastolic ≥110 mmHg). Lacunar ischemic pathogenicity associates mostly with arterial hypertension), 14 however other small-vessel disease risk factors such as uncontrolled diabetes (HbA1c > 9%) and/or uncontrolled hyperlipidemia (LDL >160) also contribute 15 and hence were also included a priori. Well-controlled (or mostly/partially-controlled) pre-existing hypertension, diabetes, or lipidemia were not deemed Pseudo-failure – nor were tiny, multifocal strokes in several ischemic distributions (especially cortical) because these were less likely to be lacunar and hence more likely to be of cardioembolic origin, and hence were deemed Crypto-failures. Approximately 9-16% of lacunes may actually be AF-related anyway.14,15
Arterial Pathology
AF patients on DOAC with ipsilateral, high-grade, intra-cranial atherosclerosis were considered Pseudo-failure because treatment is antiplatelet(s) and high-dose statin, not “anticoagulant-switching.” Intra- or extra-cranial stenosis, carotid web, or vasculitis, needed to be confirmed by Computed Tomography Angiogram, Magnetic Resonance Angiogram, and/or Digital Subtraction Angiography; specifically 70-99% stenosis for intra-cranial atherosclerosis, based on anticoagulation increasing adverse events without improved efficacy in WASID, 16 and SAMMPRIS 17 utilizing dual antiplatelet therapy (+/- stent), not anticoagulation. Ipsilateral, symptomatic, extra-cranial carotid stenosis 50-99% was also deemed Pseudo-failure: treatment is revascularization (stroke or death reduced by 29% in NASCET 18 ), not “anticoagulant-switching.” Of note, bilateral AIS with only 1 stenotic artery, or those with mild/moderate stenosis, were not deemed Pseudo-failures.
Malignancy
Low-molecular-weight heparin (enoxaparin) is preferred for cancer-associated VTE; research of DOAC efficacy in various cancer types is ongoing. 19 Patients with a history of cancer, smoking, or unexplained weight loss/night sweats, were screened with “pan-CT” (chest/abdomen/pelvis), similar to protocols for cryptogenic stroke or Embolic Stroke of Undetermined Source (ESUS). 20 If a suspicious mass was uncovered in an AF patient with new stroke on DOAC, then they were deemed Pseudo-failure because switching from DOAC to enoxaparin may be more appropriate than from DOAC to DOAC.
PFO
A stroke was deemed “attributable” (likely PFO-related) if RoPE score>6 and echocardiogram bubble study revealed a substantial size/grade shunt based on the consensus cutoff of ≥10 observed bubbles in the left atrium ≤3 cardiac cycles after right atrial opacification during transesophageal study. 21
Statistical Analysis
Descriptive statistics for demographics and clinical information are presented by mean (SD) for continuous variables and frequency (percent) for categorical variables. Student t-tests were used to compare mean values of continuous variables between the Pseudo-failure and Crypto-failure groups; Chi-square analysis or Fisher’s exact test was used to compare categorical variables, as applicable. Comparison of Crypto-failure among 3 observed DOACs (apixaban, dabigatran, or rivaroxaban; none were prescribed edoxaban) and post-hoc comparisons were performed by chi-square analysis with Bonferroni correction applied for post-hoc comparisons. Forward stepwise logistic regression modeling was conducted to identify factors independently associated with Crypto-failure vs Pseudo-failure. Adjusted odds ratios (AOR) and 95% confidence interval were calculated. Except for post-hoc comparisons (P-value <.017), statistical significance was defined as a P-value ≤.05. All analyses were conducted with the SPSS (Version 26.0, IBM Corp., Armonk, NY) and SAS (Version 9.4, SAS Institute Inc., Cary, NC).
Results
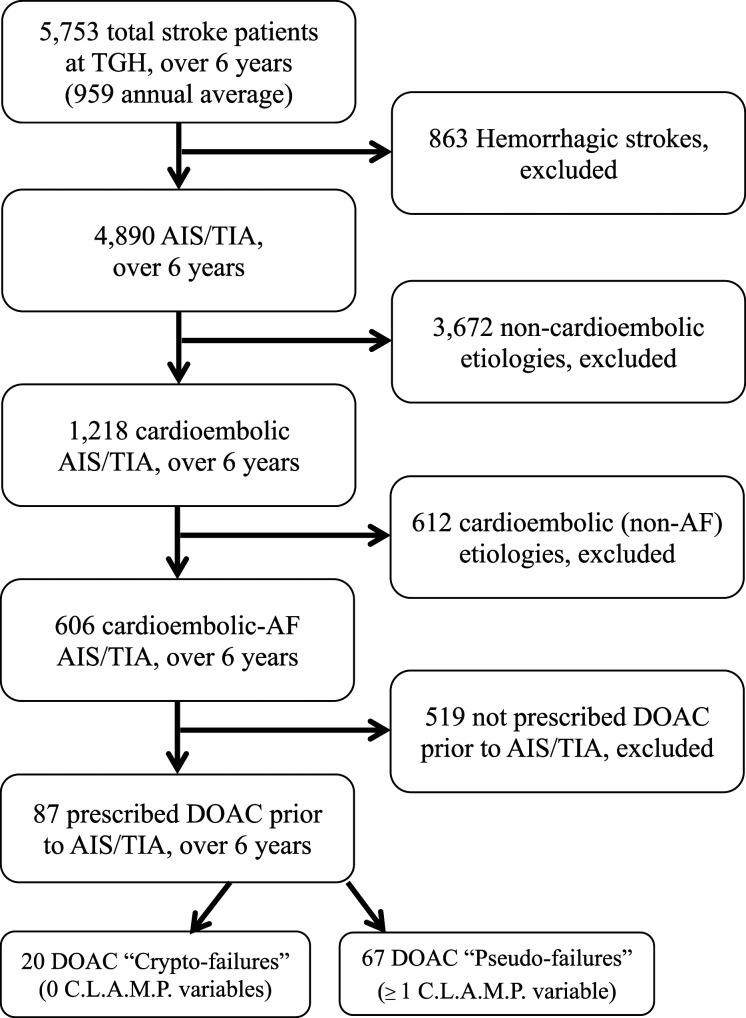
In 6 years, 5753 stroke-diagnosed patients (mean 959 annually) were admitted to our hospital. Excluding 863 hemorrhagic strokes (mean 144 annually), identified 4890 with AIS/TIA (mean 815 annually). Of these, 606 had AF and 87 had already been prescribed DOACs, representing an overall DOAC failure rate of 14.4% (87/606) over 6 years, or 2.4% annualized. The 519 remaining patients were either newly diagnosed AF, or known AF prescribed warfarin, antiplatelet, or neither. The CLAMP classification schema identified ≥1 DOAC “Pseudo-failure” in 77% (67/87). The remaining 23% (20/87) without any CLAMP item were classified as DOAC “Crypto-failures” (Figure 1).
Figure 1.
Flow chart of 6-year retrospective review identifying DOAC failures among patients with AIS/TIA and AF already prescribed DOAC therapy.
Baseline characteristics between the 2 groups were similar (P > .05), including age, sex, NIHSS, stroke location/distribution, and underlying comorbidities of diabetes, prior stroke, and smoking (Table 1). Cancer history was significantly more likely with Pseudo-failure than Crypto-failure (31.8% vs .0%, P = .002).
Table 1.
Patient Demographics among DOAC Pseudo-failures and Crypto-failures.
| Demographic | Pseudo-Failures | Crypto-Failures | P-Value |
|---|---|---|---|
| Age, mean (sd) | 73.9 (12.5) | 73.6 (13.3) | 0.922 |
| Sex, male, n (%) | 35 (52.2%) | 12 (60.0%) | 0.541 |
| Diabetes, n (%) | 20 (29.9%) | 6 (30.0%) | 0.990 |
| Dyslipidemia, n (%) | 39 (58.2%) | 7 (35.0%) | 0.068 |
| Prior stroke, n (%) | 39 (58.2%) | 9 (45.0%) | 0.297 |
| Smoking, n (%) | 12 (18.8%) | 2 (10.0%) | 0.502 |
| Hypertension, n (%) | 60 (89.6%) | 15 (75.0%) | 0.136 |
| Coronary artery disease, n (%) | 24 (35.8%) | 6 (30.0%) | 0.631 |
| Cancer History, n (%) | 21 (31.8%) | 0 (0.0%) | 0.002 |
| DOAC prescribed, n (%) | 0.008 | ||
| Dabigatran | 17 (25.4%) | 1 (5.0%) | |
| Apixaban | 31 (46.3%) | 6 (30.0%) | |
| rivaroxaban | 19 (28.4%) | 13 (65.0%) | |
| CHADSVASc, mean (sd) | 4.9 +/- 1.6 | 3.8 +/- 1.9 | 0.010 |
| Arrived within 4.5 hours, n (%) | 25 (37.3%) | 12 (60.0%) | 0.072 |
| Admission NIHSS, mean (sd) | 8.4 (8.7) | 8.8 (8.8) | 0.872 |
| Last time DOAC taken, n (%) | <0.001 | ||
| <=24 h | 25 (44.6%) | 19 (100%) | |
| >24 h | 31 (55.4%) | 0 (0.0%) | |
| Neuroradiographic territory, n (%) | 0.296 | ||
| No infarct seen (TIA) | 7 (10.6%) | 0 (0.0%) | |
| Left MCA stroke | 12 (18.2%) | 3 (15.0%) | |
| Multifocal strokes | 21 (31.8%) | 10 (50.0%) | |
| Other territory distributions | 26 (39.4%) | 7 (35.0%) | |
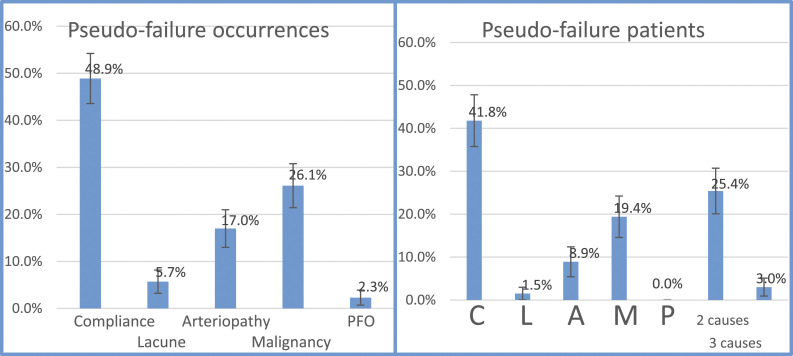
Listed by each CLAMP component (Table 2, Figure 2), Compliance concerns represented 48.9% of all occurrences, 7 (25%) of these were taking an inappropriate dose, 6 (21%) an inappropriate frequency and therefore 13 (46%) had an error with either dosing or frequency of the DOAC; Lacunar small-vessel disease represented 5.7%; Arterial pathology 17.0%; Malignancy 26.1%; and PFO 2.3%. Some Pseudo-failure patients had multiple, simultaneous variables (either 2 or 3), most commonly Compliance with Malignancy. Of malignancies, most were prostate, lung, or gastrointestinal (24% each), breast/endometrial represented 12%, bone 8%, and 8% had rarer cancers.
Table 2.
Pseudo-failure rates listed by occurrences (top, n = 88) with any CLAMP variable; by patients (middle, n = 67) with each CLAMP variable exclusively (only Compliance, only Hypertension, only Arteriopathy, only Malignancy, or only PFO), only 2 variables and only 3 variables; and by multi-factorial CLAMP variables simultaneously (bottom, n =19).
| CLAMP Listed by Pseudo-Failure Occurrence | n (%) |
|---|---|
| Compliance (any) | 43 (48.9%) |
| Lacunar Stroke (any) | 5 (5.7%) |
| Arteriopathy (any) | 15 (17.0%) |
| Malignancy (any) | 23 (26.1%) |
| PFO (any) | 2 (2.3%) |
| Total Pseudo-failure occurrences | 88 (100%) |
Figure 2.
Occurrence of each CLAMP. variable out of all Pseudo-failure occurrences (n = 88, left) and in all Pseudo-failure patients (n = 67, right) with only 1one CLAMP. variable, and multiple variables (2 causes and 3 causes). The bar represents standard error.
DOAC prescriptions overall were: 20.1% dabigatran, 42.5% apixaban, 36.8% rivaroxaban, and none used edoxaban. Pseudo-failures had been mostly prescribed apixaban (46.3%), then rivaroxaban (28.4%) and lastly dabigatran (25.4%). Crypto-failures had been mostly prescribed rivaroxaban (65.0%) much more than apixaban (30.0%) or dabigatran (5.0%). These differences were statistically significant (P = .008, Table 1).
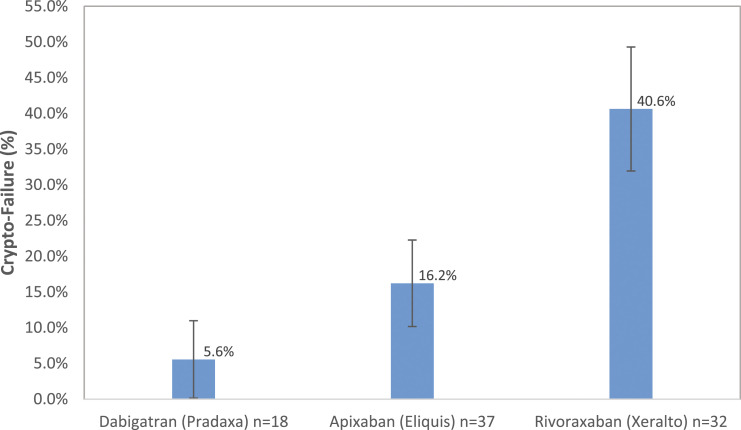
Rivaroxaban was statistically associated more with Crypto-failure than Pseudo-failure (65.0% vs 28.4%), whereas dabigatran statistically more with Pseudo-failure than Crypto-failure (25.4% vs 5.0%). Crypto-failure rates differed significantly (P = .008 < .05) by anticoagulant (Table 3, Figure 3): rivaroxaban was significantly higher than dabigatran (40.6% vs 5.6%, P = .008 < .017) and numerically higher than apixaban (when using a corrected type I error rate for multiple comparisons, this was not statistically significant: 40.6% vs 16.2%, P = .024 > .017). The Crypto-failure rate of dabigatran vs apixaban was relatively similar (5.6% vs 16.2%, P = .406). Using forward stepwise logistic regression modeling (Table 4), dabigatran had statistically significantly lower odds of Crypto-failure than rivaroxaban (AOR = .07, P = .015). Apixaban also had lower odds of Crypto-failure (AOR = .31, P = .054) than rivaroxaban, but this did not reach statistical significance.
Table 3.
DOAC Crypto-failure rate by DOAC comparisons.
| Dabigatran | Apixaban | rivaroxaban | p-Value | |
|---|---|---|---|---|
| DOAC Crypto-failure rate | ||||
| dabigatran vsvs. apixaban vsvs. rivaroxaban | 5.6% | 16.2% | 40.6% | 0.008 |
| dabigatran vsvs. apixaban | 5.6% | 16.2% | 0.406 a | |
| dabigatran vsvs. rivaroxaban | 5.6% | 40.6% | 0.008 a | |
| apixaban vsvs. rivaroxaban | 16.2% | 40.6% | 0.024 a | |
Note: P-values with superscript.
acompare with type I error 0.05/3 = 0.017.
Figure 3.
Comparison of crypto-failure rates among 3 DOACs: dabigatran (5.6%), apixaban (16.2%) and rivaroxaban (40.6%), P-value = .008. The error bar represents standard error.
Table 4.
Adjusted Odds Ratios (AOR) for CHADSVASc score and by each DOAC, with rivaroxaban as the reference, using forward stepwise logistic regression modeling.
| Adjusted Odds Ratio (AOR) | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| CHADSVASc | 0.65 | 0.46, 0.91 | 0.013 |
| DOAC | |||
| dabigatran | 0.07 | 0.01, 0.59 | 0.015 |
| apixaban | 0.31 | 0.09, 1.02 | 0.054 |
| Rivaroxaban | Reference | Reference | reference |
CHADSVASc scores were significantly higher in Pseudo-failures (4.9 +/- 1.6) vs Crypto-failures (3.8 +/- 1.9, P = .010) and multivariate analysis (Table 4) revealed an Adjusted Odds Ratio of .65 (.46-.91, P = .013).
As for timing from stroke symptom onset, Crypto-failures more often presented to the hospital within 4.5 hours (60.0% vs 37.3%), although this trend was nonsignificant (P = .072).
As for timing of anticoagulant use, all (100%) of the evaluable Crypto-failure patients consumed their last anticoagulant pill less than 24 hours (within the day) prior to their AIS/TIA, compared to less than half (44.6%) of Pseudo-failures (P < .001).
Discussion
Rates of “DOAC failure” – a colloquial catch-phrase for breakthrough AIS/TIA in AF patients prescribed DOAC – in 4 landmark randomized trials1-4 varied between 1.1 and 1.7%, while in 7 prospective cohort studies, was 4.4%. 5 Our retrospective review identified a 2.4% annual DOAC failure rate, precisely within range of these trials and studies, supporting its external validity.
Presently, there are no effective solutions for “DOAC failure” – a pooled analysis revealed that “anticoagulant-switching” did not reduce future stroke risk 5 . To evaluate this vexing clinical dilemma, our novel classification schema, CLAMP, systematically identified rates of alternate etiologies for patients’ new event despite having already been prescribed DOAC. Our EMR-based, retrospective review was ideal for a methodical, case-by-case, detailed analysis of individual patient records, with a manageable 87 total “DOAC failures” at our institution over 6 years. Such detail was infeasible in larger trials, meta-analyses, and cohorts. For all 87 charts, we meticulously read notes of discussions with patients/families about use/misuse of DOACs (dose/frequency), insurance coverage lapses, reasons for nonadherence, and reviewed all inpatient stroke workup, neuroimages, tests and vitals to see which (if any) CLAMP category resulted in their AIS/TIA. What we found was congruent with anecdotal clinical practice: most “DOAC failures” arise from either noncompliance (socioeconomic obstacles, prescription errors, or disparities in stroke care) or alternate stroke etiologies 14 – indeed 77% were Pseudo-failures with ≥1 CLAMP variable. Only 23% were Crypto-failures without any CLAMP variable: presumably these were truly AF-related “atriopathy” or genetic polymorphism/DOAC pharmacokinetic failure. Therefore, for three-fourths of “DOAC failures”, the actual cause is identifiable and unrelated to the DOAC itself. Essentially, Pseudo-failures were 3 times more likely than Crypto-failures – for every 4 AIS/TIA patients prescribed DOAC for AF, CLAMP identified 3 who had a plausible, alternate etiology; only 1 out of 4 were unexplainable.
Compliance (C in CLAMP) was the most common (48.9%) Pseudo-failure: this incidence is similar to an insurance database of DOAC users 22 with medication adherence of only 47.5% after 1 year. Elderly patients with dementia may mistime, forget, or accidentally double their DOAC pills; cerebral amyloid angiopathy patients with cerebral microbleeds may forgo anticoagulation fearing brain hemorrhage. 23 DOACs are held for procedures/surgeries, acute/active bleeding, and abnormal laboratory values (thrombocytopenia) but sometimes inadvertently suspended indefinitely – a byproduct of a siloed healthcare system. Some highest-risk AF patients take only antiplatelet (or nothing) as per the PINNACLE Registry. 24 Provider non-prescription/cessation or non-compliance with dosing recommendations (25% in our study, 35% in another study 25 ) is distinct from patient non-compliance, but regardless of who stopped/changed the DOAC, it ultimately resulted in a new stroke. Research on solutions into these “C” subcategories is sorely needed – multiple charts had documented inadequate access to care/prescription coverage; underserved populations are vulnerable to loopholes in the healthcare system, gaping societal inequalities, race-ethnicity and sex disparities, and misunderstanding of DOAC dosing.12,13
Lacunar small-vessel disease, the L in the CLAMP schema, was observed in 5.7% of Pseudo-failures not far off from the 10.4% small-vessel disease detected in a case-control study 25 of unmatched AF patients with stroke on anticoagulation. Lacunar small-vessel disease risk factors did not exclude Crypto-failure: 75% had pre-existing hypertension (Table 1). Likewise, hypertensive emergency, while sufficient, was not necessary for categorization as “lacunar/small-vessel” – uncontrolled diabetes and/or lipidemia also sufficed.25,26 This data reinforces the notion that AF patients with lacunar-type stroke, and uncontrolled risk-factors, need to adjust antihypertensives, lipid-lowering agents, and diabetic medications, reduce psychosocial stress, exercise and enjoy a low-salt/fat diet, 26 not succumb to “anticoagulant-switching.” Objectively, while much progress has been made over the past few decades, primary care risk-factor control still has room for improvement.25,26
Arterial pathology (“A”) represented 17% of all Pseudo-failures; all were moderate-to-severely atherosclerotic, none had web or vasculitis, but these etiologies were included a priori because carotid web stenting/surgery results in less stroke recurrence than medical therapy alone, 27 and cerebral vasculitis treatment is steroids/immunosuppressants, not “anticoagulant-switching.” Likewise, extra-cranial/carotid disease requires surgery/stenting, not changing DOACs. For intra-cranial atherosclerosis, further research on antithrombotic cocktails is warranted (ie, antiplatelet plus low-dose DOAC as per COMPASS criteria 28 ).
Malignancy occurred in one-quarter of Pseudo-failures; most were prostate, lung, or gastrointestinal. Prior cancer diagnosis was significantly more frequent in Pseudo-failures (31.8%) than Crypto-failures (.0%). This data supports the use of pan-CT screening – similar to cryptogenic stroke/ESUS protocols/workup 20 – especially for those with unexplained weight loss, night sweats, cancer history, or smokers; after discovery of any suspicious mass, these unfortunate patients need hematology-oncology referral, radiation, chemotherapy, and/or surgery, 19 not DOAC-switching.
PFO as a Pseudo-failure only comprised 2.3% of cases. As per the 2020 AAN practice advisory update, 29 given the high (∼25%) incidence of PFO in adults, if an alternative mechanism of stroke is identified, PFO is often “an innocent bystander.” However, the PFO may not be incidental in patients with a high RoPE score plus evidence-based criteria (age <60, atrial septal aneurysm, large interatrial shunt).21,29 Indeed, these specific patients may qualify for percutaneous closure of an “attributable” PFO; a meta-analysis of randomized controlled trials comparing PFO closure, anticoagulation, and antiplatelet therapy found that closure may be superior for stroke recurrence. 30
Ultimately, Crypto-failures (without any CLAMP variable) may warrant “anticoagulant-switching” because equipoise exists (this subgroup does not have any identified, competing etiologies) and because DOAC biochemical dysfunction/pharmacogenetic polymorphisms also exist: non-functioning variants of enzymes carboxylesterase 1 or 2 may not metabolize dabigatran into its pro-drug.31,32 Activation is affected by the genetic polymorphism G143E (rs71647871), which confers loss-of-function mutation in carboxylesterase. 32 In this example, “anticoagulant-switching” from dabigatran to another DOAC is reasonable. Our conceptualization of DOAC “Crypto-failures” may encourage genetically-individualized, DOAC-tailored research. Warfarin itself is susceptible to “failure” in patients with variability of genes CYP2C9 and VKORC1, which play a role in efficacy of warfarin to prevent recurrent stroke. 6
Interestingly, all Crypto-failures were fully adherent to their DOAC: 100% swallowed it within hours of their AIS/TIA. Why more Crypto-failures occurred on rivaroxaban than apixaban or dabigatran needs further exploration. Perhaps this is related to rivaroxaban noninferiority to warfarin in ROCKET-AF, 2 and its once-a-day high-peak, low-trough pharmacokinetics whereas dabigatran and apixaban were both superior to warfarin in RE-LY and ARISTOTLE,1,3 respectively, and are administered twice-a-day. Conversely, dabigatran’s association with more Pseudo-failure may be related to self-discontinuation/noncompliance from its side-effect profile (i.e., dyspepsia).
Lastly, Crypto-failures were more likely to present within 4.5 hours of symptom onset. This data may encourage use of DOAC-reversal agents before thrombolysis in AIS – such as idarucizumab neutralizing dabigatran prior to alteplase. 33
Limitations of our study include: (1). its single-center, retrospective nature (DOAC prescribing patterns vary regionally); (2). verification of DOAC timing, dose, frequency, and reason for nonadherence were based on EMR search (potentially incomplete/missed data, or possible incorrect documentation of DOAC compliance); (3). DOACs, antiplatelets, antihypertensives, and statins were reconciled, however unrecorded/unknown drugs/herbal remedy interactions may overestimate Crypto-failures; (4). CLAMP is not exhaustive, and other etiologies exist, such as infective endocarditis, “triple-positive” antiphospholipid antibody syndrome, ventricular thrombus, Paroxysmal Nocturnal Hemoglobinuria and Behcet’s disease9,20,33 – select cases may require screening; (5). definitions of DOAC “Crypto-failures” and “Pseudo-failures” may require further refinement/research in a multi-center, prospective study; (6). Crypto-failures were not tested/confirmed for genetic polymorphisms, such as loss-of-function mutations, because this was beyond the scope of our project; and (7). we did not include left atrial appendage occlusion (LAAO) in the study, and a large trial of AF patients who, after surgery, received LAAO, had statistically fewer strokes than non-LAAO, despite about 75% anticoagulation use in both arms. 34 Hence, our study provides a specific substrate of patients (Crypto-failures) for further LAAO research; a prior study 7 included very few patients (1%) with LAAO in this setting.
In summary, the unwieldy `term “DOAC failures” describes heterogeneous events and assumes DOAC biochemical dysfunction. Lumping all “DOAC failures” together results in “anticoagulant-switching” which is ineffective at preventing recurrent events. 6 Although imperfect, our study is hypothesis-generating and challenges current diagnostic dogma by encouraging a systematic workup of psychosocial, economic, and medical variables. Although nearly half of Pseudo-failures were from noncompliance, future research into socially transmitted health behaviors and the influence of social networks upon medications in general (and upon DOACs specifically) can focus on underserved communities with race-ethnicity and sex disparities 12 and detect modifiable targets for intervention. 35 Vulnerable populations have increased risk of both ischemic and bleeding events, and are currently underrepresented in stroke studies. 36 Our novel classification schema CLAMP may address this unmet need; identifying reasons for noncompliance, plus unearthing non-AF etiologies, may improve long-term outcomes.
Conclusion
AIS in patients with known AF, already prescribed DOAC (so-called “DOAC failures”), is an ongoing clinical conundrum. Our novel classification schema, CLAMP (Compliance concerns, Lacunar small-vessel disease, Arterial pathology, Malignancy, and PFO), found that these “Pseudo-failures” were 3 times more likely than “Crypto-failures” without clear alternate cause, aside from AF/“atriopathy” or pharmacogenomic dysfunction of DOAC absorption, metabolism or clearance. More nuanced evaluation of “DOAC failures” may discourage indiscriminate, ineffective “anticoagulant-switching” and needs further study.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Disclosure: DZR receives honoraria from Atricure, Boston Scientific, Medtronic, Chiesi and CSL-Behring. JYC works with Ascendant Biotech. AB, XY, KK, YL and NCH report no disclosures.
ORCID iDs
David Z. Rose https://orcid.org/0000-0002-9449-6494
Jane Y. Chang https://orcid.org/0000-0002-9178-9881
References
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. [DOI] [PubMed] [Google Scholar]
- 4.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104. [DOI] [PubMed] [Google Scholar]
- 5.Seiffge DJ, Paciaroni M, Wilson D, et al. Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation. Ann Neurol. 2019;85(6):823-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seiffge DJ, De Marchis GM, Koga M, et al. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol. 2020;87:677-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaghi S, Henninger N, Giles JA, et al. Ischaemic stroke on anticoagulation therapy and early recurrence in acute cardioembolic stroke: The IAC study. J Neurol Neurosurg Psychiatry. 2021;92(10):1062-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polymeris AA, Meinel TR, Oehler H, et al. Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. J Neurol Neurosurg Psychiatry. 2022;93(6):588-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stollberger C, Finsterer J. Relevance of P-glycoprotein in stroke prevention with dabigatran, rivaroxaban, and apixaban. Herz. 2015;40(suppl 2):140-145. [DOI] [PubMed] [Google Scholar]
- 10.Kanuri SH, Kreutz RP. Pharmacogenomics of novel direct oral anticoagulants: Newly identified genes and genetic variants. J Pers Med. 2019;9(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose DZ, Falcao D, Martin RC. Seek and Ye shall find fibrillations. Stroke. 2016;47(8):1969-1971. [DOI] [PubMed] [Google Scholar]
- 12.Arbel R, Sergienko R, Hammerman A, et al. Effectiveness and safety of off-label dose-reduced direct oral anticoagulants in atrial fibrillation. Am J Med. 2019;132(7):847-855.e3. [DOI] [PubMed] [Google Scholar]
- 13.Sur NB, Wang K, Di Tullio MR, et al. Disparities and temporal trends in the use of anticoagulation in patients with ischemic stroke and atrial fibrillation. Stroke. 2019;50(6):1452-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norrving B. Long-term prognosis after lacunar infarction. Lancet Neurol. 2003;2(4):238-245. doi: 10.1016/s1474-4422(03)00352-1 [DOI] [PubMed] [Google Scholar]
- 15.Katsi V, Georgiopoulos G, Skafida A, et al. Noncardioembolic stroke in patients with atrial fibrillation. Angiology. 2019;70(4):299-304. [DOI] [PubMed] [Google Scholar]
- 16.Mohr JP, Thompson JL, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345(20):1444-1451. [DOI] [PubMed] [Google Scholar]
- 17.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365(11):993-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American symptomatic carotid endarterectomy trial collaborators. N Engl J Med. 1998;339(20):1415-1425. [DOI] [PubMed] [Google Scholar]
- 19.Fovel LM, Seabury RW, Miller CD, Darko W, Probst LA, Horvath L. Safety and efficacy of direct oral anticoagulant therapy in patients with cancer. J Pharm Pract. 2019;34(5):710-714. [DOI] [PubMed] [Google Scholar]
- 20.Rose DZ, Kasner SE. Forge AHEAD with stricter criteria in future trials of embolic stroke of undetermined source. Neural Regen Res. 2022;17(5):1009-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thaler DE, Di Angelantonio E, Di Tullio MR, et al. The risk of paradoxical embolism (RoPE) study: Initial description of the completed database. Int J Stroke. 2013;8(8):612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao X, Abraham NS, Shah ND, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5(2):e003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soo YOY, Yang SR, Lam WWM, et al. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol. 2008;255(11):1679-1686. [DOI] [PubMed] [Google Scholar]
- 24.Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: Insights from the NCDR pinnacle registry. JAMA Cardiol. 2016;1(1):55-62. [DOI] [PubMed] [Google Scholar]
- 25.Paciaroni M, Agnelli G, Caso V, et al. Causes and risk factors of cerebral ischemic events in patients with atrial fibrillation treated with non-vitamin K antagonist oral anticoagulants for stroke prevention. Stroke. 2019;50(8):2168-2174. [DOI] [PubMed] [Google Scholar]
- 26.Feng Q, Fan S, Wu Y, et al. Adherence to the dietary approaches to stop hypertension diet and risk of stroke: A meta-analysis of prospective studies. Medicine (Baltimore). 2018;97(38):e12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schutt CD, Pesquera JJ, Renati S, Kaplan DJ, Mokin M, Rose DZ. Web browsing: High-speed diagnosis and treatment of carotid artery web. Neurohospitalist. 2022;12(3):498-503. doi: 10.1177/19418744221096650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steffel J, Eikelboom JW, Anand SS, Shestakovska O, Yusuf S, Fox KAA. The Compass trial: Net clinical benefit of low-dose rivaroxaban plus aspirin as compared with aspirin in patients with chronic vascular disease. Circulation. 2020;142(1):40-48. [DOI] [PubMed] [Google Scholar]
- 29.Messe SR, Gronseth GS, Kent DM, et al. Practice advisory update summary: Patent foramen ovale and secondary stroke prevention: Report of the guideline subcommittee of the American academy of neurology. Neurology. 2020;94(20):876-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turc G, Calvet D, Guérin P, et al. Closure, anticoagulation, or antiplatelet therapy for cryptogenic stroke with patent foramen ovale: Systematic review of randomized trials, sequential meta-analysis, and new insights from the CLOSE study. J Am Heart Assoc. 2018;7(12):e008356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose DZ, Burgin WS. Direct oral anticoagulant failure in stroke/transient ischaemic attack: Neurologic and pharmacokinetic considerations. Eur Heart J Case Rep. 2020;4(5):1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi J, Wang X, Nguyen JH, et al. Dabigatran etexilate activation is affected by the CES1 genetic polymorphism G143E (rs71647871) and gender. Biochem Pharmacol. 2016;119:76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amin SKKA, Kasischke KA, Elsayed K, Burgin WS, Rose DZ. No time to lose: Cases of anticoagulant reversal before thrombolysis in acute ischemic stroke patients. Cureus. 2022;14(1):e21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitlock RP, Belley-Cote EP, Paparella D, et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med. 2021;384(22):2081-2091. doi: 10.1056/NEJMoa2101897 [DOI] [PubMed] [Google Scholar]
- 35.Northcott S, Hilari K. Stroke social network scale: Development and psychometric evaluation of a new patient-reported measure. Clin Rehabil. 2013;27(9):823-833. [DOI] [PubMed] [Google Scholar]
- 36.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data collection was performed using our hospital’s Get With The Guidelines-Stroke (GWTG-S) database. Approval was obtained from our Institutional Review Board (IRB). The GWTG-S inventory of all AIS/TIA patients over 6 years was cross-matched with a co-diagnosis of AF, thus providing a manageable number of subjects to abstract and review individually with our electronic medical record (EMR) to identify prior DOAC prescription, dose, and frequency. Demographic information collected included age, sex, NIHSS, CHADSVASc, and comorbidities such as dyslipidemia, diabetes, smoking, hypertension, and prior stroke.