Abstract
Background:
Cholangiocarcinoma (CCA) is the second most frequent hepatobiliary cancer after hepatocellular carcinoma with a poor prognosis and limited treatment options. This study aimed to review existing knowledge on the genetic basis of CCA, molecular targets/signaling pathways involved in the pathogenesis, disease progression and prognosis, including potential targets for targeted therapies of CCA.
Methods:
The systematic review was performed in compliance with PRISMA guidelines. A systematic search in PubMed and Science Direct databases was performed using the following keywords: “cholangiocarcinoma”, AND “molecular target” AND/OR “signaling pathway”, AND/OR “targeted therapy”, AND/OR “cancer chemotherapy.” The eligibility criteria included: i) full-text articles published in English, ii) articles with in vitro and/or in vivo and/or clinical studies of molecular targets/signaling pathwanys related to CCA pathogenesis/disease progression/prognosis and/or targeted therapy. Seventy-three studies that fulfilled the eligibility criteria were finally included in the final data synthesis.
Results:
A total of 833 relevant articles published up to April 2022 were identified and 73 sttudies that fulfilled the eligibility criteria were finally included in the analysis. The molecular biomarkers and drugs targeting signalling pathways were reported. Recent research has been focused on targeting the apoptotic and cell proliferation pathways, and in addition, the angiogenesis and metastasis pathway. More effort focused on testing the efficacy of combination therapies against the cancer cell and specifically CCA. The PI3K (Phosphoinositide 3-kinases)/ERK/Akt (AKT serine/threonine kinase 1)/mTOR (mammalian target of rapamycin) signaling pathway and HER2 (Human epidermal growth factor receptor 2) and EGFR (Epidermal Growth Factor Receptor) pathways are the most potential targets for CCA therapy.
Conclusion:
The information obtained could be exploited for further development of diagnostic tools for early diagnosis of CCA, as well as effective CCA-targeted therapies.
Key Words: PI3K/ERK/Akt/mTOR, HER2, EGFR
Introduction
Cholangiocarcinoma (CCA) is a rare and highly aggressive cancer that accounts for 20% of all hepatobiliary cancers (Ji et al., 2020). It originates from the epithelium lining the biliary tree and is divided into three subtypes, i.e., intrahepatic (IHCCA), extrahepatic (EHCCA), and hilar (HCCA) tumors. Cirrhosis of the liver, biliary stones, age over 65 years, and chronic infection have all been reported as possible risk factors for the development of CCA (Huang et al., 2016; Ji et al., 2020). The prevalence and mortality rates of CCA are increasing worldwide. IHCCA is the second most common form of liver cancer, accounting for 10-15% of all liver cancers (Chen et al., 2015). Several recent epidemiological studies reported that its mortality and prevalence are rising, mainly in the western world (Churi et al., 2014) and northeastern Thailand (Clawson et al., 2010; Dokduang et al., 2013; Dokduang et al., 2016). CCA is characterized by a low early diagnosis rate, marked aggressiveness, and a high rate of metastasis and recurrence (Huang et al., 2015; Chen et al., 2019). The majority of CCA cases have a short median survival time of less than 24 months, which could partially result from the resistance of CCA to currently available treatment (Chan-On et al., 2015; Guest et al., 2016; Hu et al., 2019).
Managing CCA remains challenging due to the limited number of chemotherapy or targeted therapy options (Dokduang et al., 2016; Ji et al., 2020). Identification of tumor-associated signaling molecules/pathways will help developing diagnostic tools or targeted CCA therapies or effective chemotherapeutics. A range of signaling molecules and pathways has been identified as putative mechanistic determinants of CCA pathology, disease progression and prognosis (Hong et al., 2013; Chen et al., 2019; Hu et al., 2019). Clinical trials of targeted therapies, such as erlotinib, lapatinib, cetuximab, sorafenib, and bevacizumab, have been conducted to evaluate their clinical effectiveness for the treatment of CCA (Barad et al., 2012; Hu et al., 2019). Molecular-targeted therapy is superior to traditional chemotherapy due to its ability to suppress cancer cells selectively (Hong et al., 2013). Gene overexpression, amplification and mutation have all been associated with the tumorigenesis and progression of CCA (Gui et al., 2012; Hu et al., 2019). A thorough understanding of the molecular basis of CCA pathogenesis, disease progression and prognosis is required for the development of sensitive and specific diagnostic tools and effective chemotherapeutics for CCA.
In this study, we review existing knowledge on the genetic basis of CCA, molecular targets/signaling pathways involved in the pathogenesis, disease progression and prognosis, including potential targets for CCA targeted therapies.
Materials and Methods
Search strategy and eligibility criteria
The systematic review was performed in compliance with PRISMA guidelines. A systematic search in PubMed and Science Direct databases was performed using the following keywords: “cholangiocarcinoma”, AND “molecular target” AND/OR “signaling pathway”, AND/OR “targeted therapy”, AND/OR “cancer chemotherapy.” The literature search and data extraction were performed by two independent researchers, and by consensus, the final data, including any discrepancies, were resolved.
There was no publication year restriction; the search was last updated until April 2022. All articles published from various journals were retrieved and downloaded in EndNote X9 for further analysis. The inclusion criteria for article selection were i) full-text articles published in English, ii) articles with in vitro and/or in vivo and/or clinical studies of molecular targets/signaling pathways related to CCA pathogenesis/disease progression/prognosis and/or targeted therapy. Duplicates, articles with unclear methodology, articles with unclear data, and articles related to molecular signaling pathways of other types of cancer were excluded from data analysis.
After the removal of duplicated articles, an initial review of titles and abstracts was performed to identify articles of potential interest. They were then retrieved for full-text analysis and independent data extraction by the authors. An additional manual search was performed for further relevant references.
Data extraction and collection
The following study characteristics were extracted from each article that fulfilled the inclusion criteria and had none of the exclusion criteria: molecular target or signaling pathway, targeted therapy or biomarker of CCA, the analytical method applied, type of study, animal or human used, and gene and protein investigated. A final eligibility check of the full-text articles was performed; all articles that were relevant to the review question and keywords were obtained and processed for final analysis.
Results
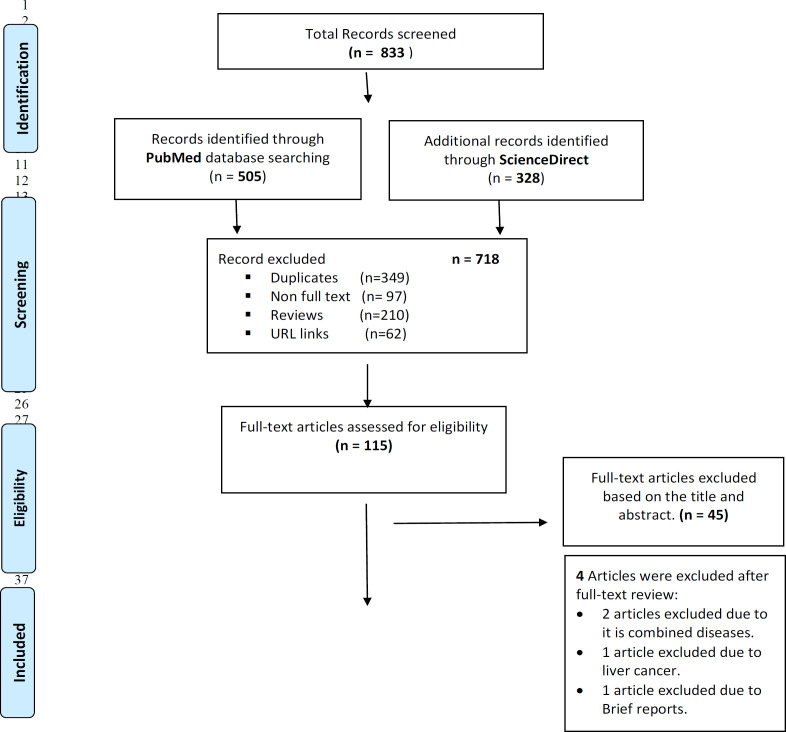
In the initial search, a total of 833 relevant articles published up to April 2021 were identified. Three hundred forty-nine duplicate articles, 210 review articles and 97 non-full-text articles were excluded. Of the remaining 177 articles, 115 articles were available as full-texts for eligibility screening. Irrelevant 42 articles were excluded following abstracts screening. Seventy-three studies that fulfilled the eligibility criteria were finally included in the final data synthesis (Figure 1).
Figure 1.
Flow Diagram Summarizing Steps for Exclusion and Inclusion of the Research Articles Included in the Analysis
The signaling molecules/pathways relevant to CCA pathogenesis, progression, and prognosis, as well as potential targets for CCA-targeted therapies are summarized in Table 1 and Supplement 1. Genetic research shows that polymorphisms in genes encoding enzymes involved in carcinogen metabolism, DNA repair, and inflammation have been found to be either pro-carcinogenic or anticarcinogenic. Recent advances in genome-wide technology have identified several molecular targets, signaling pathways as biomarkers of CCA diagnosis, disease progression, prognosis, as well as targets for CCA-targeted therapies. These include: cell proliferation and apoptosis signaling pathways (PI3K /ERK/Akt/mTOR, HER2 and EGFR signaling, EGFR/ FGFR signaling pathway, RAS/RAF/MEK/ERK, STAT3, S1P, YAP/Hippo, and Wnt/β-catenin signaling pathways); cell autophagy signaling pathway; inflammation signaling pathway; angiogenesis signaling pathway; and metastasis signaling pathway. Promising drugs/candidate molecules have been identified for most pathways, except PI3K /ERK/Akt /Mtor, RAS/RAF/MEK/ERK, STAT3, S1P and Wnt/β-catenin signaling pathways.
Table 1.
Summary of Signaling Molecules/Pathways Identified as Biomarkers for CCA Pathogenesis, Disease Progression and Prognosis, as Well as Targeted Therapies for CCA
| Molecular signaling pathway | Signaling molecule | Chemotherapeutics which targets the pathways/molecules | Type of CCA | Type of gene alteration | References |
|---|---|---|---|---|---|
| 1) Cell proliferation and apoptosis signaling pathway | |||||
| WWOX | - | IHCCA | Suppression | Huang et al., 2015 | |
| EMT | Anlotinib | IHCCA | Suppression | Song et al., 2020 | |
| Akt-NFκB | Xanthohumol (XN) | IHCCA | - | Dokduang et al., 2016; Walden et al., 2017 | |
| PI3K /ERK/AKT /mTOR | D-2-hydroxyglutarate | Ivosidenib | IHCCA | Suppression | Aguado-Frail et al., 2021 |
| AIB1 | - | IHCCA | Over expression Akt / Nrf2 pathways | Chen et al., 2012 | |
| Integrin α6 | - | IHCCA | overexpression | Ding et al., 2013 | |
| Sorafenib, Sunitinib | IHCCA | Suppression | Andersen et al., 2012. Dokduang et al., 2013 | ||
| Rapamycin | IHCCA | Suppression | Hong et al., 2013; Zhao et al., 2016 | ||
| Nimotuzumab | IHCCA | Suppression | Padthaosong et al., 2017 | ||
| Copanlisib, Gemcitabine, Cisplatin | IHCCA EHCCA |
Tan et al., 2020 | |||
| Salubrinal, Rapamycin | IHCCA | Zhao et al., 2016 | |||
| Zileuton | IHCCA | Suppression | Phophai et al., 2018 | ||
| NOTCH3 | - | pCCA IHCCA |
Guest et al., 2016 | ||
| HER2 and EGFR | EGFR | Trastuzumab | IHCCA | Point mutation (KRAS mutations) | Andersen et al., 2012; Yamashita-Kashima et al., 2019 |
| EGFR | Sorafenib | IHCCA | Point mutation | Andersen et al., 2012 | |
| EGFR | Erlotinib | IHCCA | Point mutation | Andersen et al., 2012; Borad et al., 2014 | |
| HER2 and EGFR | Lapatinib | IHCCA | Point mutation | Andersen et al., 2012 | |
| EGFR/ FGFR | EGFR | Ponatinib | SIC | - | Borad et al., 2014 |
| FGFR2 | Erlotinib | Point mutation | |||
| FGFR2 | Pazopanib | Point mutation | |||
| EGF | - | IHCCA | Suppression | Gui et al., 2012 | |
| FGFR | Ponatinib, Dovitinib, BGJ398 | IHCCA | - | Wang et al., 2016 | |
| EGFR | Vandetanib (ZD6474), | IHCCA | - | Yoshikawa et al., 2009 | |
| RAS/RAF/MEK/ERK | EGFR | - | IHCCA EHCCA | Point mutation | Chang et al., 2014 |
| MALT1 | - | IHCCA, EHCCA | Suppression | Yeh et al., 2017 | |
| STAT3 | let-7c, miR-99a, miR-125b | - | EHCCA | Activation | Lin et al., 2016 |
| Fibroblastic FAP | - | IHCCA | - | Lin et al., 2019 | |
| caspase 3/7 | β-eudesmol | EHCCA | - | Srijiwangsa et al., 2018 | |
| (S1P) pathway | SPHK1 | - | IHCCA | Chen et l., 2015 | |
| YAP/Hippo pathway | YAP | - | IHCCA | Regulation of cell proliferation | Marti et al., 2015 |
| FGFR | BGJ398 | IHCCA | Suppression | Rizvi et al., 2016 | |
| YAP, SFK | Dasatinib | IHCCA | Suppression | Sugihara et al., 2018 | |
| Wnt/β-catenin signaling | hUC-MSCs | - | EHCCA | - | Wang et al., 2015 |
| Brg1 | - | IHCCA | - | Zhou et al., 2021 | |
| FoxM1 | FoxM1 | Clioquinol (CQ) | IHCCA | Suppression | Chan-On et al., 2015 |
| nitroxoline NQ) | Suppression | Chan-On et al., 2015 | |||
| 2) Autophagy signaling pathway. | |||||
| Compound C | IHCCA | Zhao et al., 2018 | |||
| 3) Inflammation signaling pathway | |||||
| DcR3 | DcR3, (DcR3siRNA) | EHCCA | - | Xu et al., 2018 | |
| α7-nAChR | - | IHCCA | Overexpression | Chen et al., 2019 | |
| PGE2, Akt | Celecoxib and Rofecoxib | CCA | Suppression | Zhang et al., 2004 | |
| 4) Angiogenesis signaling pathway | |||||
| GATA6/LOXL2 | - | IHCCA | Regulation | Peng et al., 2019 | |
| 5) Metastasis signaling pathway | |||||
| c-MET | MET | LY2801653 | IHCCA | Barat et al., 2016 | |
| MACC1 | IHCCA | Overexpression | Lederer et al., 2015 | ||
Figure 2.
Descriptive Diagram Summarizing Signaling Pathways and Its Molecular Targets
Figure 3.
Descriptive Diagram Summarizing Apoptosis Signaling Pathway and Molecular Targets. Reference : KEGG PATHWAY: Apoptosis - Homo sapiens (human) (genome.jp)
Figure 4.
Descriptive Diagram Summarizing Angiogenesis Signaling Pathway and Molecular Targets
Figure 5.
Descriptive Diagram Summarizing Metastasis Signaling Pathway and Molecular Targets. Reference: www.genome.jp
Discussion
Cell proliferation and apoptosis signaling pathway
Apoptosis is a form of programmed cell death that occurs in multicellular organisms. There are two pathways of apoptosis (i) the intrinsic pathway (Bcl2 inhibitable) activated by cell stress and acts through activation of caspase 9 via cytochrome c and Apaf1, and (ii) the extrinsic pathway (caspase 8/10-dependent) initiated by the death receptors at the cell surface which is promoted by the TNF ligands. Caspases stimulate cell death by activating specific proteins in the cytoplasm and nucleus. Several signalling pathways are active caspases that lead to apoptosis induction, such as the death receptor pathway, the inflammasome pathway, and the kinases pathways, etc.
PI3K /ERK/Akt /mTOR pathway
The PI3K (Phosphoinositide 3-kinases)/ERK/Akt (AKT serine/threonine kinase 1)/mTOR (mammalian target of rapamycin) is essential kinases that control critical cellular activities, including gene transcription and translation, cell proliferation, growth, and survival. PI3K pathway is activated via down regulation of PTEN (Phosphatase and TENsin homolog deleted on chromosome 10), which is an essential multi-functional tumor suppressor, leading to inhibition of apoptosis process. Activation of the PI3K/Akt pathway has been linked to various human cancers, making it an important target for the development of new anticancer drugs.
Amplified in breast cancer 1 (AIB1) is an important transcriptional coactivator involved in the carcinogenesis of several cancers. AIB1 protein is overexpressed in CCA. AIB1 induced the chemoresistance of CCA cells by regulating the expression of the antiapoptotic protein Bcl2. Down-regulation of AIB1 suppressed the Akt pathway, resulting in inhibition of CCA cell proliferation (Chen et al., 2012). Notch is a potential driver of gastric epithelial cell proliferation forced exogenous overexpression of Notch 1 in hepatocytes resulted in biliary tumors. Notch is targeted by toxins that are known to be RBPJ (recombinant signal binding protein for immunoglobulin kappa J region)-dependent. Notch 3 is a key player in EHCCA and IHCCA, as it controls cell survival without the involvement of RBPJ (Guest et al., 2016). After Notch 3 deletion, tumor development was dramatically slowed, and Notch 3 silencing resulted in lower HES (hairy and enhancer-of-split) and HEY (Hes-related repressor Herp, Hesr, Hrt, CHF, gridlock) expression (Guest et al., 2016).
The mTOR signalling pathway is proposed to be an attractive target for cancer therapeutics because it is up-regulated in various cancer types, including CCA (Hong et al., 2013). PI3K cell signaling pathway is activated when growth factors such as hepatocyte growth factor (HGF) and fibroblast growth factors (FGF) bind to their corresponding receptor tyrosine kinases. Following Akt activation, the mTOR pathway is phosphorylated and regulated. The activation of Erk1/2 (extracellular signal-regulated protein kinase) and Akt were higher in CCA tissues than in normal tissues (Dokduang et al., 2013). The use of multi-targeted kinase inhibitors such as sorafenib and sunitinib to suppress kinase activation resulted in considerable cell growth suppression (Chen et al., 2012; Dokduang et al., 2013). Ivosidenib (mIDH1 inhibitors) suppressed AG-120 (oncometabolite D-2-hydroxyglutarate), resulting in disease stability and enhancing progression-free survival (Aguado-Fraile et al., 2021).
Rapamycin, an antitumor and immunosuppressive drug, has been shown to inhibit sarcomatous intrahepatic CCA (SICC) motility by inhibiting mTORC2 assembly and thus, down-regulating p-STAT3 (S727) (Hong et al., 2013; Zhao et al., 2016). Nimotuzumab is a monoclonal antibody that inhibits EGFR (epidermal growth factor receptor) activity. It inhibits CCA cell growth and metastasis through suppression of the EMT (epithelial-mesenchymal transition) process and reducing the expression of MMP9 (matrix metallopeptidase 9), which will also inhibit CCA cell invasion (Pathaisong et al., 2017). Phase II clinical trial performed on six patients with IHCCA and EHCCA indicated that gemcitabine, cisplatin, and copanlisib, including the combination of copanlisib and gemcitabine or cisplatin did not improve progression-free survival (PFS) at six months, and none of the mutations observed was associated with clinical outcomes (Tan et al., 2020). The leukotriene synthesis inhibitor (zileuton) inhibited CCA cell proliferation and migration by inhibiting the Akt signaling pathway (Khopai et al., 2018).
Up to the present, there has been no report of any therapeutic agent that targets the PI3K /ERK/Akt /mTOR pathway.
HER2 and EGFR signaling pathway
HER2 (human epidermal growth factor receptor 2) and EGFR (epidermal growth factor receptor) are cell surface receptor tyrosine kinases. The HER2 gene encodes HER2 protein (HER2/neu protein), which is a receptor on the surface of breast cells that regulates cell growth, division, and repair. EGFR is a member of the ErbB family of receptors EGFR gene encoding a protein involved in cell growth and survival. EGFR mutated gene is associated with some types of cancer.
Aberrant HER2 expression has been found in various malignancies such as ovarian and gastric cancer, but most notably in breast cancer, where it plays a crucial role in malignant transformation and therapy selection (Andersen et al., 2012). HER2 up-regulation was found only in tumors from patients with poor prognosis, who were also characterized by a frequent coactivation of ERBB3 (erythroblastic leukemia viral oncogene homolog) and EGFR (Andersen et al., 2012; Yamashita-Kashima et al., 2019).
Sorafenib and erlotinib are receptor tyrosine kinase (RTK) inhibitors which are the first-line therapy for IHCCA. Treatment of CCA cell lines with trastuzumab and lapatinib, a dual-target TKI (HER2 and EGFR) which activate EGFR and HER2, showed therapeutic potential of these drugs in the subgroup of individuals with HER2 and EGFR signaling activation (Andersen et al., 2012; Yamashita-Kashima et al., 2019). Lapatinib was reported to be more effective in suppressing the growth of CCA cells than trastuzumab (Andersen et al., 2012).
EGFR/ FGFR signaling pathway
EGFR (epidermal growth factor receptor)/FGFR (Fibroblast growth factor receptor 2) signaling pathway is an essential pathway that regulates cellular growth, survival, proliferation, and differentiation. FGFR2 fusions and ERRFI (ERBB receptor feedback inhibitor) mutations may represent novel targets in SIC. Transcriptome sequence analyses and integrated genome-wide were performed on tissue samples from patients with SIC revealed two novel targeted therapies. The anticancer activity of pazopanib was observed in a patient with FGFR2-TACC3 fusion. Erlotinib, an EGFR kinase inhibitor, has rapid and potent efficacy on ERRFI1 inactivated tumor (Borad et al., 2014). EGFR protein expression was markedly downregulated under sustained EGF stimulation (Gui et al., 2012). Recent genomic analysis of CCA patients revealed the presence of FGFR2 fusion proteins in up to 13% of IHCCA.
FGFR inhibitors suppress cell proliferation and induce apoptosis in CCA tumors harboring FGFR2 fusions (expressed only in IHCCA). Antitumor effect of FGFR inhibitors on derived xenograft (PDX) mouse model using an FGFR2-CCDC6 fusion protein from an IHCCA patient was evaluated. Ponatinib, dovitinib, and BGJ398 (most potent) inhibited cell proliferation and induced apoptosis in IHCCA (Wang et al., 2016). Vandetanib (ZD6474), a VEGFR and EGFR inhibitor, is another promising therapeutic agent for CCA; it has the potential to suppress tumor growth at a lower dose. The presence of EGFR amplification and absence of KRAS (Kirsten rat sarcoma gene) mutation may possibly be a predictive molecular indicator of the effectiveness of EGFR-targeted therapy (Yoshikawa et al., 2009).
RAS/RAF/MEK/ERK signaling pathway
The mutation of RAS (small GTPase) B-Raf proto-oncogene, serine/threonine kinase (BRAF) is rare in CCA, while that of EGFR and KRAS are common. EGFR mutation was an independent prognostic marker in CCA in addition to tumor stage and differentiation. No simultaneous EGFR and KRAS mutations in EHCCA and gallbladder carcinoma were found. It was proposed that EGFR and KRAS mutations should be evaluated when tailoring molecular-targeted therapy to patients (Chang et al., 2014). MALT1 (mucosa-associated lymphoid tissue protein 1) is essential for activating NF-κB, the potential target of regorafenib. Regorafenib suppressed CCA growth via down-regulating MALT1 expression through suppression of the Raf/Erk/ Elk1 pathway (Yeh et al., 2017).
Up to the present, there has been no report of any therapeutic agent that targets the RAS/RAF/MEK/ERK signaling pathway.
STAT3 signaling pathway
STAT3 (signal transducer and activator of transcription 3) is one of the seven proteins that transmit signals from active cytokine and growth factor receptors in the plasma membrane to the nucleus, where they control gene transcription. STAT3 plays a critical role in proliferation, survival, apoptosis, angiogenesis, and metastasis.
Let-7c, miR-99a and miR-125b, which are three miRNAs of the same cluster, were down-regulated in CCA. All targeted IL6 (interleukin 6), IL6R and IGF1R (Type 1 insulin-like growth factor) are essential cytokines and receptors of the IL6/STAT3 pathway and have critical roles in inflammation and CCA initiation. Expression of let-7c, miR-99a or miR-125b reduced the activity of STAT3 and suppressed CCA cell migration and tumorigenicity in vivo (Lin et al., 2016).
Possible correlations between miRNAs and inflammation and strategy for miRNA-based therapy for CCA by inhibiting IL6/STAT3 was proposed (Lin et al., 2016). FAP (fibroblast activation protein) expression in fibroblasts was required for STAT3 activation and CCL2 (chemokine ligand 2) production. In IHCCA, the ICCCAFs (intrahepatic cholangiocarcinoma-cancer associated fibroblast) were the primary source of CCL2. The ability of ICCCAFs to promote IHCCA growth, MDSC infiltration, and angiogenesis was significantly reduced when FAP was knocked down in the fibroblasts. IHCCA cell proliferation and apoptotic resistance were unaffected (Lin et al., 2019).
Up to the present, there has been no report of any therapeutic agent that targets the STAT3 signaling pathway.
S1P signaling pathway
S1P (sphingosine-1-phosphate) signaling pathway is an essential intracellular metabolic pathway that plays a role in various biological processes, including cell proliferation, differentiation, apoptosis, and cellular signaling. SPHKs (sphingosine kinases) convert sphingosine to S1P and are essential regulators of cell fate. SPHK1 and SPHK2, the two isoforms of SPHK enzymes with distinct functions, have been discovered. In contrast to ceramide and sphingosine, which induce cell apoptosis and growth arrest, bioactive S1P is now recognized as a critical regulator of cell survival and proliferation. SPHK1 has oncogenic roles in proliferation, angiogenesis, and transformation. It is identified as an independent marker of poor prognosis. SK1I modulated the balance of S1P and induced CCA apoptosis and produced potent antiproliferative effects on CCA cells that were time- and concentration-dependent (Chen et al., 2015).
Up to the present, there has been no report of any therapeutic agent that targets the S1P signaling pathway.
YAP/Hippo signaling pathway
YAP (Yes-associated protein), the Hippo pathway effector, is a transcriptional coactivator implicated in the pathogenesis of CCA. YAP is regulated by a serine/threonine kinase relay module (MST1/2–LATS1/2) and phosphorylated on tyrosine 357 (Y357) in the CCA cell. Microarray expression profiling of CCA cells with down-regulated or overexpressed YAP reveals that YAP regulates genes involved in apoptosis, proliferation, and angiogenesis. The combination of YAP with TEAD (transcription factors) prevented apoptosis induced by cytotoxic drugs. YAP was shown to be a critical regulator of proliferation and anti-apoptotic processes in CCA. Elevated YAP activity in CCA was correlated with increased pro-angiogenic MFAP5 (microfibrillar-associated protein 5) expression and CD311 vasculature. These findings support the role of YAP in promoting angiogenesis by regulating the expression of secreted pro-angiogenic proteins (Marti et al., 2015).
SFK (Sarcoma family kinase) inhibition with Dasatinib resulted in the loss of YAPY357 phosphorylation and promoted its translocation from the nucleus to the cytoplasm (Sukihara et al., 2018). A significant decrease in YAP activation was observed in YAP-positive CCA cell lines treated with BGJ398, which induced cell death due to Mcl1 depletion. Nuclear YAP expression could be used as a biomarker in FGFR-directed therapy (Rizvi et al., 2016).
Wnt/β-catenin signaling pathway
Wnt/β-catenin signaling is a process that causes the β-catenin protein to accumulate in the cytoplasm, which then translocates to the nucleus, where it acts as a transcriptional coactivator. It regulates cellular functions such as cell proliferation, differentiation, migration, genetic stability, apoptosis, and stem cell. A wnt/β-catenin signaling pathway is involved in the initiation and development of CCA.
MSCs (mesenchymal stem cells) are non-hematopoietic stem cells, which are capable of differentiation at least into bone, cartilage, muscle, and adipose tissues. When combined with human CCA cell lines, hUC-MSCs (human umbilical cord-derived mesenchymal stem cells) significantly increased cancer cell proliferation and migration potency through targeting the Wnt/β-catenin signaling. MSCs and their conditioned media (MSC-CM) could improve the drug resistance of tumor when the compound K (CK), a major intestinal bacterial metabolite of panaxoside, was administered. (Wang et al., 2015).
Brg1 (Brahma-related gene 1), an enzyme member of SWI/SNF (SWltch/sucrose non-fermentable) complex that is important in stem cell maintenance and tumor development, is up-regulated in both hepatic progenitor cell (HPC) and IHCCA, although its role is still unclear. The binding of Brg1 with the TCF4 (β-catenin/transcription factor 4) transcription complex is a possible approach for regulating Wnt/β-catenin signaling. Overexpression of Brg1 indicates poor prognosis in IHCCA. Therapies targeting Brg1-expressing, like PFI3, are promising for the treatment of liver cirrhosis and IHCCA (Zhou et al., 2021).
FoxM1 (fork head box M1) signalling pathway is a major transcription factor altered in cancer through altered signal transduction pathways. Activation of FOXM1 via RAS/ERK signaling It is found to be elevated in several types of cancer, including CCA.
The quinoline-based compounds, clioquinol and nitroxoline, were shown to inhibit CCA cell proliferation by decreasing the expression of FoxM1 signaling (Chan-on et al., 2015).
Up to the present, there has been no report of any therapeutic agent that targets the Wnt/β-catenin signaling pathway.
Autophagy signaling pathway
Autophagy is an intracellular apoptotic process triggered by cellular stresses. There are three types of autophagy: (i) macroautophagy, (ii) chaperone-mediated autophagy (CMA), and (iii) late endosomal microautophagy. Autophagy can target proteins, lipids, cell organelles, and pathogens. Autophagy is reported to have both tumor-suppressing and activity-promoting properties.
Compound C, commonly known as dorsomorphin, is a pharmacological AMP-activated protein kinase (AMPK) inhibitor that effectively suppresses AMPK metabolic functions; it inhibits AMPK protective autophagy in CCA. Through the p53 (53-kilodalton (kDa) protein) activation and JNKs (c-Jun N-terminal kinases) pathway, compound C causes protective autophagy in human CCA cells (Zhao et al., 2018).
Inflammation signaling pathway
Inflammation is a process by which the body is protected from harmful outside invaders. Through phagocytosis, macrophages eliminate pathogens and antigens and induce inflammatory responses by generating cytokines and enzymes such as TNF (tumor necrosis factor), IL6 (interleukin 6), iNOS (inducible nitric oxide synthase), and COX2 (cyclooxygenase-2).
TNF and IL6 affect the growth, differentiation, and proliferation of numerous cell types, including CCA. TNF enhances CCA cell motility by increasing the expression of EMT markers. It is suggested that anti-TNF medication might be a viable treatment for CCA
Celecoxib is a COX2 inhibitor that suppresses PGE2 (prostaglandin E2), increases Bax translocation to mitochondria, and increases cytochrome c release into the cytosol. Celecoxib caused CCA cell apoptosis via a mechanism that involved Akt inactivation, Bax translocation, and cytochrome c release (Zhang et al., 2004). DcR3 (Decoy receptor 3) is a protein with the antiapoptotic effect that belongs to the tumor necrosis factor receptor. Down-regulation of DcR3 in CCA decreased CCA cell viability to 61.87% (Xu et al., 2018). Overexpression of 7-nAChR (7-nicotinic acetylcholine receptor) promoted CCA progression by inhibiting apoptosis and promoting EMT (epithelial-mesenchymal transition). 7-nAChR is a promising therapeutic target in CCA because it is an effective molecular biomarker and prognostic factor (Chen e al., 2019).
Angiogenesis signaling pathway
Angiogenesis signaling pathway is important for cell growth, development, wound healing, and carcinogenesis. It is regulated by several growth factors, including VEGF (Vascular endothelial growth factor protein family). VEGFA plays an important role in tumor angiogenesis.
GATA6 (GATA-binding protein 6) is essential for the differentiation, proliferation and development of the digestive system, cardiovascular system, and some tissues during embryonic development. GATA6 and LOXL2 (Lysyl oxidase-like 2) expression influence secreted VEGFA expression, angiogenesis, and tumor development. The interaction of LOXL2 with GATA6 was shown to regulate VEGFA transcription, enhance VEGFA secretion and angiogenesis. Drugs targeting this complex pathway may possess great therapeutic value in the treatment of CCA (Peng et al., 2019).
Targeted therapies under investigation for the angiogenesis signaling pathway include lenvatinib (multi-targeted tyrosine kinase inhibitor that inhibits VEGFR1-3, FGFR1-4, PDGFR, KIT, and RET) and pembrolizumab (humanized antibody that inhibits the programmed cell death 1 (PD-1) receptor). Both significantly decreased the population of immunosuppressive tumor-associated macrophages and increased interferon-γ-producing cluster of differentiation 8+ (CD8+) T cells. The addition of PD-L1 (programmed cell death protein 1/programmed death-ligand) inhibitors helps reverse VEGF-mediated immune suppression, restore T cell function, and promotes T cell tumor infiltration. The combination of lenvatinib and pembrolizumab has demonstrated promising activity with manageable adverse events in various solid tumor types (www.ClinicalTrials.gov).
Metastasis signaling pathway
c-MET (Mesenchymal-epithelial Transition factor) signaling pathway, the proto-oncogene encoding the RTK, plays an important role in cell proliferation, survival, motility, invasion, and metastasis through regulating multiple signaling cascades. c-MET is overexpressed in more than 50% of human CCA and 80% of murine CCA (Barat et al., 2016).
MACC1 (metastasis-associated in colon cancer 1) is a recently identified regulator of the hepatocyte growth factor (HGF)/Met/mitogen-activated protein kinase pathway, which stimulates proliferation, migration, invasion, and metastasis of tumors. MACC1 expression was considerably higher in Klatskin HCA tumor patients with history of tumor recurrence than in those without history of recurrence. MACC1 is considered as a highly predictive biomarker for shorter overall (OS) and disease-free survival (DFS) in Klatskin tumor patients. It is, however, not a prognostic marker for the survival of IHCCA (Lederer et al., 2015).
Although the function of c-MET signaling pathway in CCA remains unclear, it appears to be a promising alternative therapeutic strategy (Barat et al., 2016). LY2801653 (small-molecule kinase inhibitor) suppressed the overactivation of c-MET and p-MET under hypoxic circumstances by partially down-regulating HIF1a (Hypoxia-inducible factor 1-alpha) expression in CCA cell line.
Integrin a6, a cell surface receptor, mediates adhesion to the extracellular matrix (ECM) and enhances cell motility and invasion. The expression of integrin a6 in IHCC tissues was higher than that in nontumor samples. Integrin a6 overexpression was found to be significantly correlated with larger tumors, multiple nodular, microvascular/bile duct invasion, and lymphatic metastasis (Ding et al., 2013).
In summary, identification of signaling molecules/pathways involved in CCA pathogenesis, disease progression and prognosis has been a focus of CCA research in recent years. The information obtained has been exploited for further development of diagnostic tools for early detection of CCA, as well as effective CCA-targeted therapies. Despite the significant advances achieved toward molecularly profiling CCA, there are still substantial gaps in our knowledge. The interactions among signaling pathways and components that induce cancer cell phenotypes are still poorly understood. A better understanding of the epigenetic processes underlying biliary carcinogenesis is likely to lead to the discovery of new therapeutic targets. Evidence suggests that combining different targeted drugs can prevent the development of resistance while also improving efficacy. Recent developments in immunotherapy may create additional treatment options for CCA.
Author Contribution Statement
Rehab Idris, Wanna Chaijaroenkul, and Kesara Na-Bangchang were involved in the design of the experimental study. Rehab Idris performed the experiments. Rehab Idris and Wanna Chaijaroenkul performed data analysis. Rehab Idris drafted the manuscript. Kesara Na-Bangchang revised the manuscript. All authors reviewed and approved the final manuscript for submission. All meet the ICMJE criteria for authorship
Acknowledgements
This study was financially supported by the Research Team Promotion Grant, National Research Council of Thailand (Kesara Na-Bangchang: Grant No. 820/2563), Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma, Thammasat University, the Research Unit in Nutraceuticals and Food Safety, and the Ministry of Higher Education, Science, Research and Innovation of Thailand.
Ethics approval
This article does not involved any human or animal study.
Availability of data and material
The datasets generated during and/or analyzed during this study are available from the corresponding author on reasonable request.
Conflict of Interest
The authors declare no competing interests
References
- Ahn KS, O’Brien D, Kang YN, et al. Prognostic subclass of intrahepatic cholangiocarcinoma by integrative molecular-clinical analysis and potential targeted approach. Hepatol Int. 2019;13:490–500. doi: 10.1007/s12072-019-09954-3. [DOI] [PubMed] [Google Scholar]
- Barat S, Bozko P, Chen X, et al. Targeting c-MET by LY2801653 for treatment of cholangiocarcinoma. Mol Carcinog. 2016;55:2037–50. doi: 10.1002/mc.22449. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xu J, Jhala N, et al. Fas-mediated apoptosis in cholangiocarcinoma cells is enhanced by 3,3’-diindolylmethane through inhibition of AKT signaling and FLICE-like inhibitory protein. Am J Pathol. 2006;169:1833–42. doi: 10.2353/ajpath.2006.060234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Yen CC, Cheng CT, et al. Identification of SPHK1 as a therapeutic target and marker of poor prognosis in cholangiocarcinoma. Oncotarget. 2015;6:23594–608. doi: 10.18632/oncotarget.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Kang X, Liu G, et al. α7-Nicotinic Acetylcholine Receptor Promotes Cholangiocarcinoma Progression and Epithelial-Mesenchymal Transition Process. Dig Dis Sci. 2019;64:2843–53. doi: 10.1007/s10620-019-05609-3. [DOI] [PubMed] [Google Scholar]
- Chan-On W, Huyen NT, Songtawee N, et al. Quinoline-based clioquinol and nitroxoline exhibit anticancer activity inducing FoxM1 inhibition in cholangiocarcinoma cells. Drug Des Devel Ther. 2015;9:2033–47. doi: 10.2147/DDDT.S79313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churi CR, Shroff R, Wang Y, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One. 2014;9:e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokduang H, Yongvanit P, Namwat N, et al. Xanthohumol inhibits STAT3 activation pathway leading to growth suppression and apoptosis induction in human cholangiocarcinoma cells. Oncol Rep. 2016;35:2065–72. doi: 10.3892/or.2016.4584. [DOI] [PubMed] [Google Scholar]
- Dokduang H, Juntana S, Techasen A, et al. Survey of activated kinase proteins reveals potential targets for cholangiocarcinoma treatment. Tumour Biol. 2013;34:3519–28. doi: 10.1007/s13277-013-0930-9. [DOI] [PubMed] [Google Scholar]
- Huang CK, Iwagami Y, Aihara A, et al. Anti-Tumor Effects of Second-Generation β-Hydroxylase Inhibitors on Cholangiocarcinoma Development and Progression. PLoS One. 2016;11:e0150336. doi: 10.1371/journal.pone.0150336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Tian Y, Peng R, et al. Association of downregulation of WWOX with poor prognosis in patients with intrahepatic cholangiocarcinoma after curative resection. J Gastroenterol Hepatol. 2015;30:421–33. doi: 10.1111/jgh.12722. [DOI] [PubMed] [Google Scholar]
- Borad MJ, Champion MD, Egan JB, et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014;10:e1004135. doi: 10.1371/journal.pgen.1004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest RV, Boulter L, Dwyer BJ, et al. Notch3 drives development and progression of cholangiocarcinoma. Proc Natl Acad Sci U S A. 2016;113:12250–5. doi: 10.1073/pnas.1600067113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui A, Kobayashi A, Motoyama H, et al. Impaired degradation followed by enhanced recycling of epidermal growth factor receptor caused by hypo-phosphorylation of tyrosine 1045 in RBE cells. BMC Cancer. 2012;12:179. doi: 10.1186/1471-2407-12-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SM, Park CW, Cha HJ, et al. Rapamycin inhibits both motility through down-regulation of p-STAT3 (S727) by disrupting the mTORC2 assembly and peritoneal dissemination in sarcomatoid cholangiocarcinoma. Clin Exp Metastasis. 2013;30:177–87. doi: 10.1007/s10585-012-9526-9. [DOI] [PubMed] [Google Scholar]
- Hu X, Tan Z, Yang Y, Yang P. Long non-coding RNA MIR22HG inhibits cell proliferation and migration in cholangiocarcinoma by negatively regulating the Wnt/β-catenin signaling pathway. J Gene Med. 2019;21:e3085. doi: 10.1002/jgm.3085. [DOI] [PubMed] [Google Scholar]
- Chen Q, Li W, Wan Y, et al. Amplified in breast cancer 1 enhances human cholangiocarcinoma growth and chemoresistance by simultaneous activation of Akt and Nrf2 pathways. Hepatol. 2012;55:1820–9. doi: 10.1002/hep.25549. [DOI] [PubMed] [Google Scholar]
- Aguado-Fraile E, Tassinari A, Ishii Y, et al. Molecular and morphological changes induced by ivosidenib correlate with efficacy in mutant-IDH1 cholangiocarcinoma. Future Oncol. 2021;2021:2020. doi: 10.2217/fon-2020-1274. [DOI] [PubMed] [Google Scholar]
- Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterol. 2012;142:1021–31. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YT, Chang MC, Huang KW, et al. Clinicopathological and prognostic significances of EGFR, KRAS and BRAF mutations in biliary tract carcinomas in Taiwan. J Gastroenterol Hepatol. 2014;29:1119–25. doi: 10.1111/jgh.12505. [DOI] [PubMed] [Google Scholar]
- Ding YB, Deng B, Huang YS, et al. A high level of integrin α6 expression in human intrahepatic cholangiocarcinoma cells is associated with a migratory and invasive phenotype. Dig Dis Sci. 2013;58:1627–35. doi: 10.1007/s10620-012-2524-6. [DOI] [PubMed] [Google Scholar]
- Gao Q, Yu GY, Shi JY, et al. Neddylation pathway is up-regulated in human intrahepatic cholangiocarcinoma and serves as a potential therapeutic target. Oncotar. 2014;5:7820–32. doi: 10.18632/oncotarget.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki J, Kikuchi K, Mizuguchi Y, et al. MiR-376c down-regulation accelerates EGF-dependent migration by targeting GRB2 in the HuCCT1 human intrahepatic cholangiocarcinoma cell line. PLoS One. 2013;8:e69496. doi: 10.1371/journal.pone.0069496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinawath A, Akiyama Y, Sripa B, Yuasa Y. Dual blockade of the Hedgehog and ERK1/2 pathways coordinately decreases proliferation and survival of cholangiocarcinoma cells. J Cancer Res Clin Oncol. 2007;133:271–8. doi: 10.1007/s00432-006-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima A, Hirsova P, Bronk SF, et al. Fibroblast growth factor receptor inhibition induces loss of matrix MCL1 and necrosis in cholangiocarcinoma. J Hepatol. 2018;68:1228–38. doi: 10.1016/j.jhep.2018.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamigaki M, Sasaki T, Serikawa M, et al. Statins induce apoptosis and inhibit proliferation in cholangiocarcinoma cells. Int J Oncol. 2011;39:561–8. doi: 10.3892/ijo.2011.1087. [DOI] [PubMed] [Google Scholar]
- Kendre G, Marhenke S, Lorz G, et al. The co-mutational spectrum determines the therapeutic response in murine FGFR2 fusion - driven cholangiocarcinoma. Hepatol. 2021;2021:31799. doi: 10.1002/hep.31799. [DOI] [PubMed] [Google Scholar]
- Khoontawad J, Intuyod K, Rucksaken R, et al. Discovering proteins for chemoprevention and chemotherapy by curcumin in liver fluke infection-induced bile duct cancer. PLoS One. 2018;13:e0207405. doi: 10.1371/journal.pone.0207405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khophai S, Thanee M, Techasen A, et al. Zileuton suppresses cholangiocarcinoma cell proliferation and migration through inhibition of the Akt signaling pathway. Onco Targ Ther. 2018;11:7019–29. doi: 10.2147/OTT.S178942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampis A, Carotenuto P, Vlachogiannis G, et al. MIR21 Drives Resistance to Heat Shock Protein 90 Inhibition in Cholangiocarcinoma. Gastroenteron. 2018;154:1066–79. doi: 10.1053/j.gastro.2017.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer A, Herrmann P, Seehofer D, et al. Metastasis-associated in colon cancer 1 is an independent prognostic biomarker for survival in Klatskin tumor patients. JHepatol. 2015;62:841–50. doi: 10.1002/hep.27885. [DOI] [PubMed] [Google Scholar]
- Lin HY, Tey SL, Ho Y, et al. Heteronemin Induces Anti-Proliferation in Cholangiocarcinoma Cells via Inhibiting TGF-β Pathway. Mar Drugs. 2018;16:120–489. doi: 10.3390/md16120489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KY, Ye H, Han BW, et al. Genome-wide screen identified let-7c/miR-99a/miR-125b regulating tumor progression and stem-like properties in cholangiocarcinoma. Oncogene. 2016;35:3376–86. doi: 10.1038/onc.2015.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Li B, Yang X, et al. Fibroblastic FAP promotes intrahepatic cholangiocarcinoma growth via MDSCs recruitment. Neoplasia. 2019;21:1133–42. doi: 10.1016/j.neo.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loilome W, Juntana S, Namwat N, et al. PRKAR1A is overexpressed and represents a possible therapeutic target in human cholangiocarcinoma. Int J Cancer. 2011;129:34–44. doi: 10.1002/ijc.25646. [DOI] [PubMed] [Google Scholar]
- Marti P, Stein C, Blumer T, Abraham Y, Dill MT, Pikiolek M, et al. YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatol. 2015;62:1497–510. doi: 10.1002/hep.27992. [DOI] [PubMed] [Google Scholar]
- Padthaisong S, Thanee M, Techasen A, et al. Nimotuzumab Inhibits Cholangiocarcinoma Cell Metastasis via Suppression of the Epithelial-Mesenchymal Transition Process. Anticancer Res. 2017;37:3591–7. doi: 10.21873/anticanres.11729. [DOI] [PubMed] [Google Scholar]
- Peng F, Jiang J, Yu Y, et al. Direct targeting of SUZ12/ROCK2 by miR-200b/c inhibits cholangiocarcinoma tumourigenesis and metastasis. Br J Cancer. 2013;109:3092–104. doi: 10.1038/bjc.2013.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Deng X, Tian F, et al. The interaction of LOXL2 with GATA6 induces VEGFA expression and angiogenesis in cholangiocarcinoma. Int J Oncol. 2019;55:657–70. doi: 10.3892/ijo.2019.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Yao W, Yang T, et al. aPKC-ι/P-Sp1/Snail signaling induces epithelial-mesenchymal transition and immunosuppression in cholangiocarcinoma. Hepatol. 2017;66:1165–82. doi: 10.1002/hep.29296. [DOI] [PubMed] [Google Scholar]
- Rizvi S, Yamada D, Hirsova P, et al. A Hippo and Fibroblast Growth Factor Receptor Autocrine Pathway in Cholangiocarcinoma. J Biol Chem. 2016;291:8031–47. doi: 10.1074/jbc.M115.698472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubwai W, Wongkham C, Puapairoj A, et al. Aberrant expression of NF-κB in liver fluke associated cholangiocarcinoma: implications for targeted therapy. PLoS One. 2014;9:e106056. doi: 10.1371/journal.pone.0106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DY, Kang JH, Song W, et al. Apoptosis of human cholangiocarcinoma cell lines induced by β-escin through mitochondrial caspase-dependent pathway. Phytother Res. 2011;25:1519–26. doi: 10.1002/ptr.3435. [DOI] [PubMed] [Google Scholar]
- Shirota T, Ojima H, Hiraoka N, et al. Heat Shock Protein 90 Is a Potential Therapeutic Target in Cholangiocarcinoma. Mol Cancer Ther. 2015;14:1985–93. doi: 10.1158/1535-7163.MCT-15-0069. [DOI] [PubMed] [Google Scholar]
- Song F, Hu B, Cheng JW, et al. Anlotinib suppresses tumor progression via blocking the VEGFR2/PI3K/AKT cascade in intrahepatic cholangiocarcinoma. Cell Death Dis. 2020;11:573. doi: 10.1038/s41419-020-02749-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Liu G, Fang T, et al. Expression and prognostic value of glutamate dehydrogenase in extrahepatic cholangiocarcinoma patients. Am J Transl Res. 2017;9:2106–18. [PMC free article] [PubMed] [Google Scholar]
- Sugihara T, Werneburg NW, Hernandez MC, et al. YAP Tyrosine Phosphorylation and Nuclear Localization in Cholangiocarcinoma Cells Are Regulated by LCK and Independent of LATS Activity. Mol Cancer Res. 2018;16:1556–67. doi: 10.1158/1541-7786.MCR-18-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srijiwangsa P, Ponnikorn S, Na-Bangchang K. Effect of β-Eudesmol on NQO1 suppression-enhanced sensitivity of cholangiocarcinoma cells to chemotherapeutic agents. BMC Pharmacol Toxicol. 2018;19 doi: 10.1186/s40360-018-0223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ES, Cao B, Kim J, et al. Phase 2 study of copanlisib in combination with gemcitabine and cisplatin in advanced biliary tract cancers. Cancer. 2020;2020:33364. doi: 10.1002/cncr.33364. [DOI] [PubMed] [Google Scholar]
- Walden D, Kunnimalaiyaan S, Sokolowski K, Clark TG, Kunnimalaiyaan M. Antiproliferative and apoptotic effects of xanthohumol in cholangiocarcinoma. Oncotarget. 2017;8:88069–78. doi: 10.18632/oncotarget.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang H, Peters M, et al. Loss of Fbxw7 synergizes with activated Akt signaling to promote c-Myc dependent cholangiocarcinogenesis. J Hepatol. 2019;71:742–52. doi: 10.1016/j.jhep.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhan M, Li Q, et al. FXR agonists enhance the sensitivity of biliary tract cancer cells to cisplatin via SHP dependent inhibition of Bcl-xL expression. Oncotarget. 2016;7:34617–29. doi: 10.18632/oncotarget.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhong W, Yuan J, et al. Involvement of Wnt/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget. 2015;6:42276–89. doi: 10.18632/oncotarget.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ding X, Wang S, et al. Antitumor effect of FGFR inhibitors on a novel cholangiocarcinoma patient-derived xenograft mouse model endogenously expressing an FGFR2-CCDC6 fusion protein. Cancer Lett. 2016;380:163–73. doi: 10.1016/j.canlet.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YC, Cui J, Zhang LJ, et al. Anti-apoptosis Effect of Decoy Receptor 3 in Cholangiocarcinoma Cell Line TFK-1. Chin Med J (Engl) 2018;131:82–7. doi: 10.4103/0366-6999.221271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi S, Katayose Y, Oda A, et al. ZD1839 (IRESSA) stabilizes p27Kip1 and enhances radiosensitivity in cholangiocarcinoma cell lines. Anticancer Res. 2009;29:1169–80. [PubMed] [Google Scholar]
- Yamashita-Kashima Y, Yoshimura Y, Fujimura T, et al. Molecular targeting of HER2-overexpressing biliary tract cancer cells with trastuzumab emtansine, an antibody-cytotoxic drug conjugate. Cancer Chemother Pharmacol. 2019;83:659–71. doi: 10.1007/s00280-019-03768-8. [DOI] [PubMed] [Google Scholar]
- Yeh CN, Chang YC, Su Y, et al. Identification of MALT1 as both a prognostic factor and a potential therapeutic target of regorafenib in cholangiocarcinoma patients. Oncotarget. 2017;8:113444–59. doi: 10.18632/oncotarget.23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi K, Kobayashi A, Motoyama H, et al. Survival pathway of cholangiocarcinoma via AKT/mTOR signaling to escape RAF/MEK/ERK pathway inhibition by sorafenib. Oncol Rep. 2018;39:843–50. doi: 10.3892/or.2017.6153. [DOI] [PubMed] [Google Scholar]
- Yoshikawa D, Ojima H, Kokubu A, et al. Vandetanib (ZD6474), an inhibitor of VEGFR and EGFR signalling, as a novel molecular-targeted therapy against cholangiocarcinoma. Br J Cancer. 2009;100:1257–66. doi: 10.1038/sj.bjc.6604988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Zhang M, Liu J, et al. Long Non-coding RNA PVT1 Promotes Cell Proliferation and Migration by Silencing ANGPTL4 Expression in Cholangiocarcinoma. Mol Ther - Nucleic Acids. 2018;13:503–13. doi: 10.1016/j.omtn.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Han C, Zhu H, Song K, Wu T. miR-101 inhibits cholangiocarcinoma angiogenesis through targeting vascular endothelial growth factor (VEGF) Am J Pathol. 2013;182:1629–39. doi: 10.1016/j.ajpath.2013.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lai GH, Sirica AE. Celecoxib-induced apoptosis in rat cholangiocarcinoma cells mediated by Akt inactivation and Bax translocation. J Hepatol. 2004;39:1028–37. doi: 10.1002/hep.20143. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Oyesanya RA, Campbell DJ, et al. Preclinical assessment of simultaneous targeting of epidermal growth factor receptor (ErbB1) and ErbB2 as a strategy for cholangiocarcinoma therapy. J Hepatol. 2010;52:975–86. doi: 10.1002/hep.23773. [DOI] [PubMed] [Google Scholar]
- Zhao X, Luo G, Cheng Y, et al. Compound C induces protective autophagy in human cholangiocarcinoma cells via Akt/mTOR-independent pathway. J Cell Biochem. 2018;119:5538–50. doi: 10.1002/jcb.26723. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang C, Zhou H, et al. Synergistic antitumor activity of the combination of salubrinal and rapamycin against human cholangiocarcinoma cells. Oncotarget. 2016;7:85492–501. doi: 10.18632/oncotarget.13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Chen Y, Zhang X, et al. Brg1 Inhibition Prevents Liver Fibrosis and Cholangiocarcinoma by Attenuating Progenitor Expansion. J Hepatol. 2021;2021:31780. doi: 10.1002/hep.31780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during this study are available from the corresponding author on reasonable request.