Abstract
Objective:
Exercise has been reported to suppress colorectal cancer; however, the mechanism of suppression by exercise and its effect on the Wnt pathway, which is particularly involved in the early stage of carcinogenesis, remain unclear. In this study, we subjected ApcMin/+ mice to exercise by shaking stimuli to investigate the mechanisms of suppressing colorectal cancer, and focused on the Ca2+ pathway, which is one of the β-catenin-independent Wnt signaling pathways that suppress the accumulation of β-catenin.
Methods:
Mice in the exercise group were subjected to exercise by shaking stimuli for 30 min/session, 6 sessions/ week, for a total of 11 weeks. The number and diameter of intestinal polyps were calculated. Expression analysis of β-catenin and Pak1 from the intestinal tract and Wnt5a-Pan and Wnt5a-Long from the gastrocnemius muscle was performed by western blotting. The expression of β-catenin and Wnt5a-Pan was observed by immunohistochemical staining.
Result:
The levels of expression of β-catenin and Pak1 in the small intestine were low in the exercise group, indicating that exercise suppressed the accumulation of β-catenin. In the gastrocnemius muscle, the levels of expression of Wnt5a-Pan and Wnt5a-Long were significantly higher in the exercise group (p < 0.05). Histological analysis revealed that the percentage of large polyps was significantly lower in the exercise group than in the control group (p < 0.01), revealing that exercise suppressed the growth of polyps. In addition, the villi/crypt ratio (V/C ratio) was significantly higher in the exercise group, suggesting the suppression of exercise-induced local inflammation in the small intestine.
Conclusion:
We believe that the mechanism of polyp growth suppression is related to the inflammatory and not the Wnt pathway. This study clarified the growth-suppressing effect of a novel exercise method on cancer. We believe that its development and clinical application might open new possibilities for the prevention treatment of colorectal cancer.
Key Words: Colorectal cancer, exercise therapy, myokine, shaking and vibration
Introduction
The leading cause of death in Japan is malignant neoplasms. Among them, colorectal cancer, with an annually increasing mortality rate, is currently ranked first among women and third among men. A large cohort study suggested that exercise affects colorectal cancer prevention (Pham et al., 2012; Samad et al., 2005). In particular, a strong negative association has been shown between weeks of exercise and the prevalence of colorectal cancer (Takahashi et al., 2007). Case-control studies have also shown a link between exercise and the prevalence of colon and rectal cancer (Spence et al., 2009). However, the preventative mechanism of exercise remains unclear along with its effect on the Wnt pathway, which is particularly involved in the early stages of carcinogenesis.
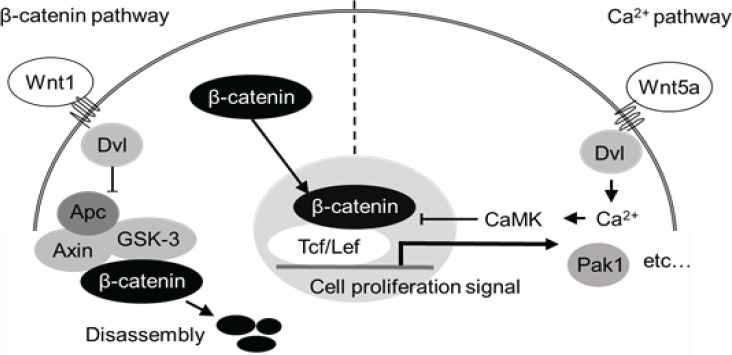
Apc Min/+ mice have a mutation in the APC regulator of Wnt signaling pathway (Apc) gene, which is an important tumor suppressor gene in the development of colorectal cancer. As a result, these mice spontaneously develop polyps throughout their small intestine with aging. This animal model is also widely used for analysis of the mechanism of intestinal polyp formation for human hereditary disease “familial adenomatous polyposis” which is associated with a mutation in the APC gene. A multistage carcinogenesis theory has been proposed for the carcinogenic mechanism of colorectal cancer, with mutations in the Apc gene being among the first steps in the process. This results in the abnormal activation of the β-catenin-dependent Wnt signaling pathway and the formation of polyps. There are 2 Wnt signaling pathways other than the β-catenin pathway, one of which is the Ca2+ pathway (Figure 1). The Ca2+ pathway antagonizes the function of the β-catenin pathway, with Wnt5a acting as its trigger, and shown to have an inhibitory effect on colorectal cancer. However, Wnt5a has also been reported to promote the progression of colorectal cancer (Sun et al., 2021), with its effect differing depending on the respective intracellular signal transduction factors and time of cancer formation. One of these factors might be the difference between the 2 isoforms formed by Wnt5a (Bauer et al., 2013). Due to its structure, the long isoform exerts a tumor-suppressing effect, whereas, the short isoform, which lacks 20 amino acids in the N-terminus (MKKSIGILSPGVALGMAGSA) has a tumor-promoting effect. As such, it has been proposed that different structures produce different actions. One possibility is that the mechanism of polyp growth suppression is related to an inflammatory pathway unlike the Wnt pathway, suggesting the suppression of exercise-induced local inflammation in the small intestine. Therefore, to verify the suppressive effect of exercise on carcinogenesis and progression of colorectal cancer, we conducted a study using a carcinogenic ApcMin/+ mouse model.
Figure 1.
β-catenin and Ca2+ Pathways. The 2 intracellular signaling pathways, β-catenin and Ca2+ pathway. The activated β-catenin pathway in ApcMin/+ mice leads to the expression of genes such as Pak1 and transmits proliferation signals by translocating cytoplasmic β-catenin into the nucleus. Conversely, APC-containing complexes regulate this growth signal by degrading β-catenin. However, if there is a mutation in the Apc gene, this complex cannot be formed and β-catenin cannot be disassembled, resulting in its excessive accumulation in the nucleus, and in turn in an excessive transmission of proliferation signals, leading to the development of polyps. In contrast, when the Ca2+ pathway is activated, the Wnt5a membrane protein suppresses the β-catenin pathway
Materials and Methods
Carcinogenic mouse model
For this study, 8-week-old ApcMin/+ C57BL/6JJcl male and wild-type C57BL/6JJcl female mice (Jackson Laboratory, Bar Harbor, Maine, USA) were bred by natural mating. All male offspring mice were genetically analyzed, and 24 4-week-old ApcMin/+ male mice were obtained. Mice were weighed weekly to evaluate the exercise effect, tumor growth, and overall health; they were also observed for changes in appearance. All experiments were performed according to the guidelines of the Animal Care and Use Committee of the Fujita Health University (approval No. AP20024) and following the US National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Exercise by shaking stimuli
Twelve 4-week-old ApcMin/+ male mice were subjected to exercise with shaking stimuli for 11 weeks at a frequency of 30 min/session and 6 sessions/week. Shaking stimuli uses a variable rotation axis (NR-3; TAITEC Co., Saitama, Japan) that is uniform in all directions to promote movement via horizontal stimuli (movement distance 50 mm, rotation speed 150 times/min). Treadmills and wheel running are mainly used for mouse exercise, both of which provide a strong element of spontaneous motility, which limits the number of mice that can exercise in one session and therefore limits the efficient simultaneous stimulation of a large number of mice. Therefore, considering the future application to humans, we adopted an inexpensive and compact shaking stimulator with passive elements. The stimulator is equipped with a horizontal plate that moves in a circular motion when the axis of the rotation shifts. This allows mice to maintain their balance while providing whole body exercise simulation centered on the lower limbs. In addition, the stimulator can accommodate 24 mice for every session, resulting in good experimental efficiency. The shaking stimulator has been suggested to be effective for muscle hypertrophy (Ito et al., 2010), alleviation of bone density decrease (Yamada et al., 2013), and suppression of hippocampal nerve cell death related to memory (Yao et al., 2020).
Specimen collection
After 11 weeks of shaking stimulation exercise, all 24 15-week-old mice underwent thoracotomy under general anesthesia (isoflurane) and had their blood collected and their small intestine and gastrocnemius muscles removed. The small intestine was cleaned inside and outside with PBS, divided into 3 parts (upper, middle, and lower part), and vertically incised. The inside of the intestine was considered as the surface and attached onto a black sheet, and images were captured to count the number of polyps and measure their diameters. In addition, a total of 12 small intestines from 6 mice in the exercise group and 6 mice in the control group were fixed for tissue analysis using 10 % neutral buffered formalin. The gastrocnemius muscle was weighed and then rapidly frozen and stored at -80 °C. Following centrifugation of the collected blood, the obtained serum was cryopreserved at -30 °C.
Histological analysis of the small intestine
The number and diameter of polyps in the small intestine were measured using image analysis with the ImageJ software (NIH, USA) based on the images captured at the time of excision. The number of polyps was counted separately for those with a diameter of <2 mm and ≥2 mm, and the percentage of polyps by size in the entire small intestine and at each site was calculated and compared.
Protein expression analysis
The gastrocnemius muscle and small intestine were immersed in liquid nitrogen and crushed in a mortar, and then a tissue lysate was prepared using a protein lysing agent (T-PER; Thermo Fisher Scientific, Waltham, Massachusetts, USA). After measuring the protein concentration and adjusting it to a constant level, western blotting (WB) was performed. The lysate from the gastrocnemius muscle was incubated with 1:1000 anti-Wnt5a-Pan (Abcam, US, Cambridge) and anti-Wnt5a-long (Eurofins Genomics K. K, Tokyo, Japan) antibodies, used as primary antibodies. The lysate from the small intestine was incubated with anti-β-catenin (1:5000; Santa Cruz Biotechnology Inc., USA) and anti-Pak1 (1:1000; GeneTex, Irvine, California, USA) antibodies at 4 °C overnight. Chemiluminescence was performed using a ECL Plus substrate (Thermo Fisher Scientific) for color development. After detection using a lumino image analyzer (Image Quant LAS 4000mini; GE Healthcare, Chicago, Illinois, USA), the integrated optical density (IOD) of each band was measured using the ImageJ software (NIH).
Preparation and analysis of tissue specimens
The fixed small intestine was embedded in Swiss roll using paraffin according to the standard method for preparing tissue specimens, and then cut into 4-μm-thick slices. After performing hematoxylin and eosin (HE) staining and measuring the length of villus and the depth of crypt, the V (villus)/C (crypt) ratio was calculated. For immunohistochemical staining, the anti-β-catenin and anti-Wnt5a-Pan antibodies were used as primary antibodies at a dilution ratio of 1:50 and 1:100, respectively, at 25 °C. After that, chemiluminescence was performed in the same manner, and detection was performed using a lumino image analyzer. For β-catenin, the area of the deeply stained area in the polyp was compared in each group, and the positive rate of detection of anti-Wnt5a-Pan was analyzed using the ImageJ software.
Statistical analysis
Analysis was performed using the SPSS software (version 26; IBM, New York, USA). A two-sided Student’s t-test was used for simple comparison between 2 groups. The χ2 test was used to compare the percentage of different-sized polyps. A p < 0.05 was considered statistically significant.
Results
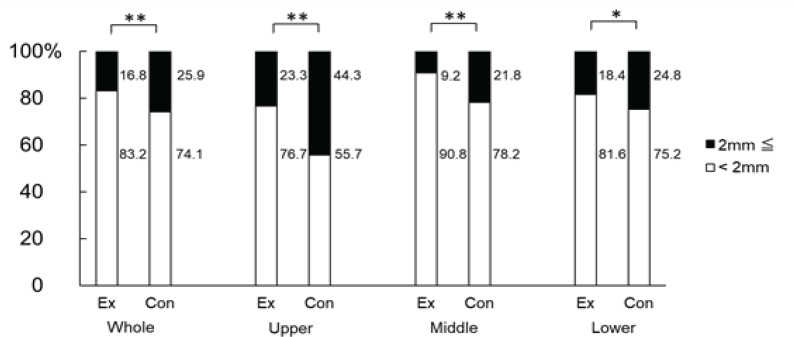
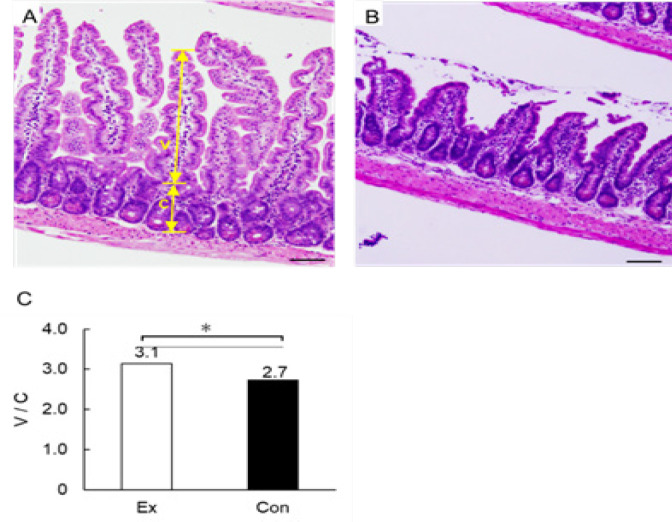
When we counted the number of polyps in the total length of the small intestine, we found that the mean values were 53.5 ± 18.4 mm in the exercise group and 56.8 ± 26.8 mm in the control group; no significant difference was observed between the 2 groups. Similarly, we observed that the mean values of polyp diameters measured were 1.55 ± 0.14 mm in the exercise group and 1.71 ± 0.18 mm in the control group, showing significantly lower values in the exercise group (p < 0.05). In addition, we divided polyps in 2 groups according to their diameter (<2 mm and ≥2 mm), and calculated the percentage of each size to the total number of polyps. We found that in the exercise group, 83.2 % of polyps were <2 mm, whereas 16.8 % were ≥2 mm. In the control group, 74.1 % of polyps were <2 mm, whereas 25.9 % were ≥2 mm. We thus noticed that the percentage of polyps with a diameter of ≥2 mm was significantly lower in the exercise group (p < 0.01). In addition, when we compared all 3 sites, we detected that in the upper part of the small intestine of the exercise group, the percentage of polyps with a diameter of <2 mm was 76.7 %, whereas that of polyps with a diameter of ≥2-mm was 23.3 %. In the control group, the percentage of polyps with a diameter of <2 mm was 55.7 %, whereas that of polyps with a diameter of ≥2 mm was 44.3 %. Hence, the percentage of polyps with a diameter of ≥2 mm was significantly lower in the exercise group (p < 0.01). In the middle part of the small intestine, the percentage of <2 mm polyps was 90.8 and 78.2 %, whereas that of ≥2 mm polyps was 9.2 and 21.8 % in the exercise and control group, respectively. Thus, the percentage of polyps with a diameter of ≥2 mm was significantly lower in the exercise group (p < 0.01). In the lower part of the small intestine, we found that the percentage of <2 mm polyps was 81.6 and 75.2 %, whereas that of ≥2 mm polyps was 18.4 and 24.8 %, in the exercise and control group, respectively. Therefore, the percentage of polyps with a diameter of ≥2 mm was significantly lower in the exercise group (p < 0.05) (Figure 2). We also observed that the V/C ratio in the lower part of the small intestine was significantly higher (3.1 ± 0.1) in the exercise group compared with that in the control group (2.7 ± 0.3) (p < 0.05) (Figure 3).
Figure 2.
Percentage of Polyps by Size at Each Part. The small intestine was cut into 3 parts (upper, middle, and lower) and the ratio of polyps by size was calculated. The white bar graph indicates the percentage of polyps with a diameter <2 mm, whereas the black bar graph indicates that of polyps with a diameter of ≥2 mm. The left end of the graph shows the entire small intestine. The exercise (Ex) and control (Con) groups were compared. *, p < 0.05;**, p < 0.01
Figure 3.
HE-Staining of the Lower Small Intestine. The photograph shows HE-stained sections of villi and submucosa of the small intestine. A shows the exercise group, whereas B shows the control group; Scale bar is 100 µm. V: villus and C: crypt. The V/C ratio was calculated as the length of villus divided by the depth of crypt. C shows and compares the V/C ratio of the lower small intestine. *, p < 0.05
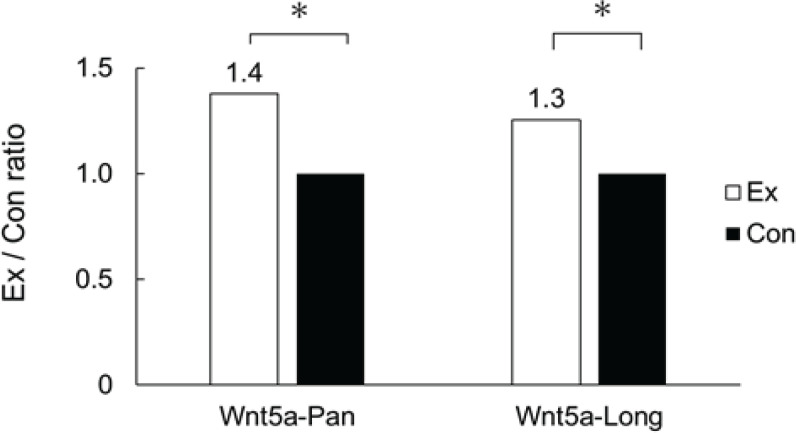
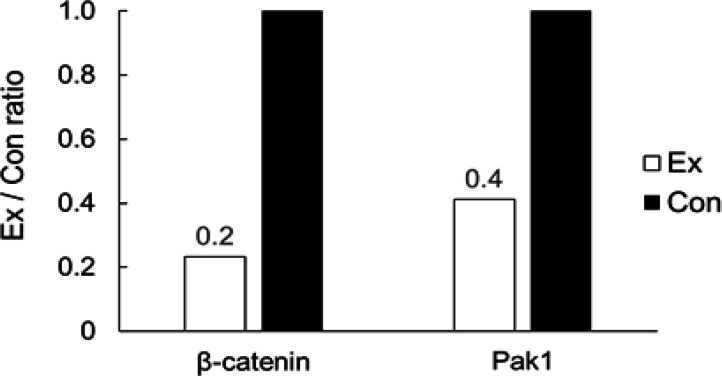
We performed western blotting and compared the levels of expression of Wnt5a-Pan and Wnt5a-Long in the gastrocnemius muscle. We detected that when the level of expression in the control group was set as 1, the levels of Wnt5a-Pan and Wnt5a-Long were 1.37 and 1.25 times higher in the exercise group, showing a significant upregulation (p < 0.05) (Figure 4). We also calculated the area where the anti-β-catenin antibody was concentrated in the polyps of the lower part of the small intestine using immunohistochemical staining. When we compared the mean values, we did not detect any differences between the exercise group (0.95 ± 0.24 mm2) and the control group (0.91 ± 0.50 mm2). We once again performed western blotting and compared the levels of expression of β-catenin and Pak1 in the lower small intestine. We found that when the level of expression in the control group was set as 1, the levels of β-catenin and Pak1 were 0.23 and 0.41 times lower in the exercise group (Figure 5).
Figure 4.
Comparison of the Expression of Wnt5a-Pan and Wnt5a-Long. Comparison of the levels of expression of Wnt5a-Pan and Wnt5a-Long in the gastrocnemius muscle in the exercise group with respect to those in the control group by western blotting. The black bar graph indicates Ex: experimental group, whereas white indicates Con: control group. *, p < 0.05
Figure 5.
Level of Expression of β-catenin and Pak1. Comparison of the levels of expression of β-catenin and Pak1 in the lower small intestine in the exercise group with respect to those in the control group by western blotting. The black bar graph indicates Ex, experimental group, whereas white indicates Con: control group
Discussion
A number of studies have explored the effects of exercise on small intestinal polyps in ApcMin/+ mice (Murphy et al., 2011; Colbert et al., 2000; Colbert et al., 2003). In this study, we investigated the effect of exercise by shaking stimuli, which is a novel exercise stimulator, on the β-catenin pathway of ApcMin/+ mice. When the number of small intestinal polyps and their diameters were compared, both the rate of diameter reduction and number of polyps with a diameter of ≥2 mm were lower in the exercise group, suggesting that exercise by shaking stimuli had an inhibitory effect on the growth of polyps. However, no change was observed in the number of polyps, indicating that exercise did not affect the occurrence of polyps. This result was consistent with the observations by Jamie et al., which used a treadmill-based exercise method (Colbert et al., 2006). It has also been reported that 9 weeks of treadmill exercise reduced the total number of small intestinal polyps in ApcMin/+ mice by 29 % and that of large polyps by 38 % (McClellan et al., 2014). Furthermore, exercise has been reported to lead to a reduction in the total number of small intestinal polyps in ApcMin/+ mice by 50 % and that of large polyps by 67 % (Yu et al., 2019). Regarding the small number of large polyps, the results of this study were consistent with the results of the aforementioned studies. This suggested that exercise by shaking stimuli can be an effective alternative exercise method to that of treadmill running. In addition, as the number of polyps increases rapidly in ApcMin/+ mice from 10 weeks of age, it might be necessary to consider the age of mice for analyzing the effects of exercise in stages rather than transiently. The effects of regular exercise training on ApcMin/+ murine intestinal carcinogenesis models have been shown in various studies. It is clear from this and other studies that exercise plays a significant role in suppressing the progression of polyp growth rather than the onset of polyp development.
We also investigated the effect of exercise on local inflammation of the small intestine. The V/C ratio in the lower part of the small intestine was significantly higher in the exercise group, suggesting that exercise by shaking stimuli suppressed local inflammation of the small intestine. In addition, inflammation caused by oxidized lipids has also been reported to affect the growth of small intestinal polyps (Yu et al., 2019), suggesting that suppression of inflammation is associated with the mechanism by which polyp growth is suppressed.
Furthermore, we considered that exercise by shaking stimuli is related to Wnt5a involved in the β-catenin pathway and the Ca2+ pathway; as such, we focused on these 2 pathways. Immunohistochemical analysis of the expression of β-catenin in polyps of the lower small intestine showed no difference between groups in the area of tissue stained with the anti-β-catenin antibody. Exercise with shaking stimuli did not affect the level of β-catenin accumulated in the polyp (data not shown). Although the expression of β-catenin is useful as a diagnostic marker for early neoplastic changes, it is not an essential step in tumor growth, indicating that there might be other mechanisms underlying the occurring neoplastic changes (Kongkanuntn et al., 1999). We believe that the mechanism of polyp growth suppression by shaking stimuli exercise is not related to lipid metabolism but is rather involved in an inflammatory mechanism different from the β-catenin pathway.
When the levels of expression of β-catenin and Pak1 in the small intestine were analyzed, both β-catenin and Pak1 were low in the exercise group. Although exercise by shaking stimuli did not affect the accumulation of β-catenin in polyps, it might be involved in the regulation of the β-catenin pathway. For instance, exercise has been reported to increase the level of expression of oxidized β-catenin in the small intestine (Baltgalvis et al., 2008), in consistency with the results of this study.
In addition, we performed western blotting in the gastrocnemius muscle using the anti-Wnt5a-Pan and anti-Wnt5a-Long antibodies and immunohistochemical staining in the small intestine using the anti-Wnt5a-Pan antibody. We accordingly found that the expression of Wnt5a-Pan and Wnt5a-Long in the gastrocnemius muscle was significantly higher in the exercise group. This finding showed that the performance or lack of exercise by shaking stimuli in C57BL/6JJcl wild-type mice shows the same tendency as the analysis of the expression of the Wnt5a gene in the gastrocnemius muscle (microarray analysis, data not shown). It is necessary to investigate this because Wnt5a-Pan and Wnt5a-Long in the gastrocnemius muscle might affect the small intestine in an endocrine manner via blood vessels and blood-like humoral factors, such as myokine.
This is the first study to investigate the effect of shaking stimuli on the β-catenin pathway in ApcMin/+ mice as a novel exercise method. Our results revealed that exercise by shaking stimuli reduced the percentage of large polyps (≥2 mm) in the small intestine of ApcMin/+ mice. We believe that exercise by shaking stimuli suppressed the growth of polyps. In contrast, there was no difference between the groups in terms of the expression of β-catenin in the polyps located in the lower part of the small intestine. We believe that the growth mechanism of polyps is related to a pathway different from the β-catenin pathway. Therefore, analysis of the V/C ratio in the lower part of the small intestine revealed the upregulation of local inflammation markers in the exercise group (high V/C ratio). Based on these findings, it was suggested that exercise by shaking stimuli affects several signaling pathways associated with polyp growth. In the protein expression analysis of the signal transduction pathway, the levels of expression of β-catenin and Pak1 in the small intestine were low in the exercise group. These results suggested that exercise by shaking stimuli does not affect the accumulation of β-catenin in polyps, but is involved in the regulation of the β-catenin pathway. Wnt5a-Pan and Wnt5a-Long, which are involved in the Ca2+ pathway, are also involved in its regulation, and analysis of these isoforms also suggested that exercise by shaking stimuli increased the levels of expression of Wnt5a-Pan and Wnt5a-Long in the gastrocnemius muscle. In this study, considering the future application to humans, we examined a new exercise method that might act as an alternative for treadmill and other exercise methods, clarified the tumor growth suppression effect of exercise by shaking stimuli, and suggested the possibility of its use. In addition, it was suggested that Wnt5a-Pan and Wnt5a-Long in the gastrocnemius muscle might reach the small intestine via blood vessels and blood-like humoral factors such as myokine, and its mechanism of action should be analyzed. We expect that further research, development, and eventual clinical application of exercise methods will open new possibilities for the prevention and treatment of colorectal cancer.
Author Contribution Statement
D. Iwata and K. Nishii designed the project. R. Yao and K. Yamada performed exercise stimulation and participated in drafting the work. All the authors took part in the anatomy experiment. H. Sawada, T. Chihara, and T. Kito performed the intestines staining experiment and protein expression analysis. N.Aizu and S. Izawa performed the microscopy experiments and helped with the data analysis. D. Iwata and K. Nishii wrote the manuscript and approved the final version to be published.
Acknowledgements
Funding statement
This work was supported by grant-in-aid for scientific research from the Japan society for the Promotion of Science, and a grant-in-aid from Fujita Health University.
This work was also supported by the Japan Society for the Promotion of Science (No.22500484).
Study
The study is not registered in any registering dataset.
Ethical declaration
All experiments were performed according to the guidelines of the Animal Care and Use Committee of the Fujita Health University (approval No. AP20024) and following the US National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Data availability
All data generated or analyzed during this study are included in this article and can be provided upon request. Further enquiries can be directed to the corresponding author.
Any conflict of interest
The authors have no conflicts of interest to declare.
References
- Baltgalvis KA, Berger FG, Peña MM, Davis JM, Carson JA. Effect of exercise on biological pathways in ApcMin/+ mouse intestinal polyps. J Appl Physiol. 2008;104:1137–43. doi: 10.1152/japplphysiol.00955.2007. [DOI] [PubMed] [Google Scholar]
- Bauer M, Bénard J, Gaasterland T, Willert K, Cappellen D. WNT5A encodes two isoforms with distinct functions in cancers. PLoS One. 2013;8:e80526. doi: 10.1371/journal.pone.0080526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert LH, Davis JM, Essig DA, Ghaffar AB, Mayer EP. Exercise and tumor development in a mouse predisposed to multiple intestinal adenomas. Med Sci Sport Exer. 2000;32:1704–8. doi: 10.1097/00005768-200010000-00007. [DOI] [PubMed] [Google Scholar]
- Colbert LH, Mai V, Perkins SN, et al. Exercise and intestinal polyp development in APC Min mice. Med Sci Sport Exer. 2003;35:1662–9. doi: 10.1249/01.MSS.0000089349.54813.41. [DOI] [PubMed] [Google Scholar]
- Colbert LH, Mai V, Tooze JA, et al. Negative energy balance induced by voluntary wheel running inhibits polyp development in APC Min mice. Carcinogenesis. 2006;27:2103–7. doi: 10.1093/carcin/bgl056. [DOI] [PubMed] [Google Scholar]
- Ito M, Nishii K, Aizu N, et al. Examination of the effect that shaking stimuli gives to mouse psoas major muscle by histological analysis and expression level of the specific proteins. Structure Function. 2010;9:3–11. [Google Scholar]
- Kongkanuntn R, Bubb VJ, Sansom OJ, et al. Dysregulated expression of β-catenin marks early neoplastic change in Apc mutant mice, but not all lesions arising in Msh2 deficient mice. Oncogene. 1999;18:7219–25. doi: 10.1038/sj.onc.1203181. [DOI] [PubMed] [Google Scholar]
- McClellan JL, Steiner JL, Day SD, et al. Exercise effects on polyp burden and immune markers in the ApcMin/+ mouse model of intestinal tumorigenesis. Int J Clin Oncol. 2014;45:861–8. doi: 10.3892/ijo.2014.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EA, Davis JM, McClellan JL, Carmichael MD. Quercetin’s effects on intestinal polyp multiplicity and macrophage number in the Apcmin/+ mouse. Nutr Cancer. 2011;63:421–6. doi: 10.1080/01635581.2011.535954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham NM, Mizoue T, Tanaka K, et al. Physical activity and colorectal cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2012;42:2–13. doi: 10.1093/jjco/hyr160. [DOI] [PubMed] [Google Scholar]
- Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7:204–13. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- Spence RR, Heesch KC, Brown WJ. A systematic review of the association between physical activity and colorectal cancer risk. Scand J Med Sci Spor. 2009;19:764–81. doi: 10.1111/j.1600-0838.2009.00992.x. [DOI] [PubMed] [Google Scholar]
- Sun G, Wu L, Sun G, et al. WNT5a in Colorectal Cancer: Research Progress and Challenges. Cancer Manag Res. 2021;13:2483. doi: 10.2147/CMAR.S289819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Kuriyama S, Tsubono Y, et al. Time spent walking and risk of colorectal cancer in Japan: the Miyagi Cohort study. Eur J Cancer Prev. 2007;16:403–8. doi: 10.1097/01.cej.0000236249.63489.05. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nishii K, Sakai K, Teranishi T. Stimulus in the form of rotation and shaking of a platform and its effect on the formation of trabecular bone in the lumbar vertebrae of mice. Aging Clin Exp Res. 2013;25:625–32. doi: 10.1007/s40520-013-0164-0. [DOI] [PubMed] [Google Scholar]
- Yao R, Nishii K, Aizu N, et al. Maintaining Aging Hippocampal Function with Safe and Feasible Shaking Exercise in SAMP10 Mice. Dement Geriatr Cogn. 2020;49:185–93. doi: 10.1159/000507884. [DOI] [PubMed] [Google Scholar]
- Yu B, Peng XH, Wang LY, et al. Abnormality of intestinal cholesterol absorption in ApcMin/+ mice with colon cancer cachexia. Int J Clin Exp Patho. 2019;12:759–67. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article and can be provided upon request. Further enquiries can be directed to the corresponding author.