Abstract
Background:
Fatigue is a typical consequence of cancer that can affect one’s quality of life (QOL). The goal of this review is to provide comprehensive data on the impact of fatigue on QOL of cancer patients.
Methods:
An electronic data search in Web of Science, SCOPUS, and PubMed for relevant papers; those written in English; those reporting quantitative data; and those including more than one hundred patients who received just chemotherapy were included. Studies involving participants that received other kinds of anti-neoplastic therapies were excluded.
Results:
A total of 35 papers published between January 2000 and December 2021 were retrieved from the search databases of which (11612 patients) met the inclusion criteria. Findings showed that fatigue negatively affected QOL with a pooled prevalence of 49% (95% CI; 25.00-74.00) and the significant heterogeneity between articles was (I²=98%, P <0.001). Further, breast cancer contributed to the majority of selected articles with about 55 % (95%CI; 9:00- 94:00), followed by cancer (unspecified) 44% (95%CI; 5:00 – 92:00). Most studies (71%) (95%CI; 4:00 – 99:00) used the brief fatigue inventory (BFI) tool to assess severity of fatigue and 39% (95%CI; 17:00 -68:00) employed the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire to evaluate QOL among cancer survivors.
Conclusion:
The prevalence of fatigue in cancer patients is high and fatigue has a negative impact on QOL of cancer patients receiving chemotherapy. Directionality, fatigue needs to be addressed and treated properly for better improvement of health status and QOL in cancer patients.
Key Words: Fatigue, cancer patients, quality of life, prevalence
Introduction
Fatigue in cancer is a subjective feeling of tiredness experienced by cancer patients and can be described as an unpleasant symptom, which ranges from body weakness to exhaustion and influences normal daily life (Ream and Richardson, 1996). In the general population, fatigue is common, but it is more prevalent in cancer patients (Ericsson et al., 2013; Neefjes et al., 2013). It is also frequently reported in cancer survivors (Curt et al., 2000; Flechtner and Bottomley, 2003; Langeveld et al., 2003) without any distinction based on age, gender or method of treatment (Winningham, 2001).
Cancer-related fatigue (CRF) is a medical issue that influences cancer patients before and after treatment (Curt and Johnston, 2003; Curt et al., 2000). It differs from normal fatigue in that cancer patients rarely get relief from their fatigue by resting and/or sleeping (Morrow et al., 2005).
The underlying cause of increased fatigue in cancer may be related to tumor type, location, stage of treatment, and various types of anti-neoplastic therapies (Monga et al., 1999). For instance, lung cancer patients who received radiotherapy experienced more fatigue compared to individuals suffering from other cancer types (Smets et al., 1998) and gynecologic patients who prescribed with chemotherapy experienced side effects e.g nausea vomiting and fatigue which affect their QOL (Viriyasiri et al., 2020). Studies found that CRF could be due to tumor-related cytokine production, factors related to neuroendocrine, pain and management (Rosen and Brand, 2011). Moreover, fatigue is also one of the well-known symptoms of radiotherapy or antineoplastic in cancer patients as about 65% to 100% and 82% to 96% of patients receiving radiotherapy and chemotherapy, respectively developed severe fatigue (Rosen and Brand, 2011). Cancer patients undergoing chemotherapy experienced a significantly high level of severe fatigue which may last several months and even years (Iop et al., 2004). Until recently, CRF has gained the attention of both patients and healthcare providers with more focus on how it affects patients’ quality of life (QOL) (Amarsheda and Bhise, 2021). In medical field, quality of life is described as an evaluation of how a disease can affect various parts of a life of the individual (Testa and Simonson, 1996). Regular assessment of QOL may lead to the maintenance of appropriate drugs selections, minimise side effects and delay or prevent diseases progression.
Several tools are available for the evaluation of quality of life among individuals diagnosed with cancer, such as EORTC- QOL, functional assessment of cancer therapy–general (FACT-G) and 36-Item Short-Form Survey (SF-36). Some of these tools, such as the SF-36 and FACT-G, are used to measure QOL in general, whereas others, such as the EORTC QLQ-BR23 (for breast cancer patients), the Functional Assessment of Cancer Therapy of Anaemia (FACT-An) (used to assess QOL among anemic cancer patients ), and the Functional Assessment of Chronic Illness of Fatigue (FACIT-F) (used to assess QOL in fatigue cancer patients), are used for specific condition or disease. Similarly, numerous tools are also available to assess severity of fatigue among patients with cancer , which includes the Cancer fatigue scale (CFS), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Fatigue Assessment Questionnaire (FAQ), Multifunctional Fatigue Scale (MFS), Piper Fatigue Scale-Revised (PFS-R) (Piper et al., 1998) and Brief Fatigue Inventory (BFI) (Iwase et al., 2015).
CRF has an immense negative effect on QOL of cancer patients and daily life activities (Amarsheda and Bhise, 2021). Aside from causing physical problems, severe and intense fatigue has a negative effect on QOL, social status, ability to work, and, may consequently affect mental health (Stasi et al, 2003). The main goal of this comprehensive analysis is to ascertain the prevalence of CRF and its effect on QOL in cancer patients undergoing chemotherapy.
Materials and Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) was used as the framework for this systematic review (Moher et al., 2015).
Inclusion and exclusion criteria
The inclusion criteria included in this analysis were: (1) patients diagnosed with cancer , regardless of age or type of cancer, (2) interventions: cancer patients receiving only chemotherapy, (3) outcomes of interest: the prevalence of CRF, the effect of fatigue on cancer patients’ QOL who were treated with chemotherapy, (4), study design: only full-text English papers with at least 100 participants, cross-sectional, randomised trials (RCTs), case-control, or descriptive studies. The exclusion criteria were articles that do not fit the above characteristics, such as in-vitro or in-vivo studies, as well as those involving other therapeutic modalities rather than chemotherapy, such as surgery, radiotherapy, hormonal therapy (targeted therapy), traditional and complementary treatment for fatigue. Likewise, non-English articles, abstracts, reviews, meta-analyses, conference papers, book chapters, and thesis were not considered in the present study.
Literature search strategy
This study aims to search and locate relevant studies regarding the association between CRF and QOL. Web of Science (WoS), PubMed, and SCOPUS electronic databases were used to find the relevant articles from January 2000 and December 2021 that fits the inclusion criteria. The titles and abstracts were searched using various keywords and Medical Subject Headings (MeSH) terms such as “cancer” OR “neoplasm” AND “fatigue” OR “clinical trial”, “fatigue” AND “quality of life” AND “cancer”, and “prevalence” AND “fatigue” AND “cancer” AND “chemotherapy”. The search results were filtered to identify the studies written in English. All appropriate references were uploaded into the EndNote software X9 (Thomson Reuter CA, USA), and duplicates were removed.
Selection of studies and quality assessment
The primary articles were reviewed by two reviewers. All studies were screened independently by assessing their titles and abstracts. The selected articles were categorized into three sections: relevant, irrelevant and unsure. Thereafter, the irrelevant articles were removed from the systematic review. Subsequently, the two reviewers also looked at full-text articles using the eligibility criteria. If the two reviewers disagree, they will examine their choices before reaching a final conclusion. If the two reviewers could not agree on a decision due to misunderstanding or confusion, a third viewpoint was requested from the other reviewer in order to reach a conclusion and a final decision.
Data extraction and analysis
Data was extracted from articles that matched the inclusion criteria. Trials, title, abstract, and full text were screened and the extracted data were recorded consistently using a standardised data extraction form. The following information was gleaned from the selected studies: the number of patients and articles, year of publications, study design, cancer type, trial length, tools to assess QOL, tools to measure fatigue severity, outcomes, and countries.
Statistical analysis
We analysed the data using R software, and the heterogeneity of the relevant publications was determined using the I2 (%) test. The Egger’s test was performed to detect publication bias, with a significance level of 0.05, and the associated Forest plots were created.
Results
Literature selection
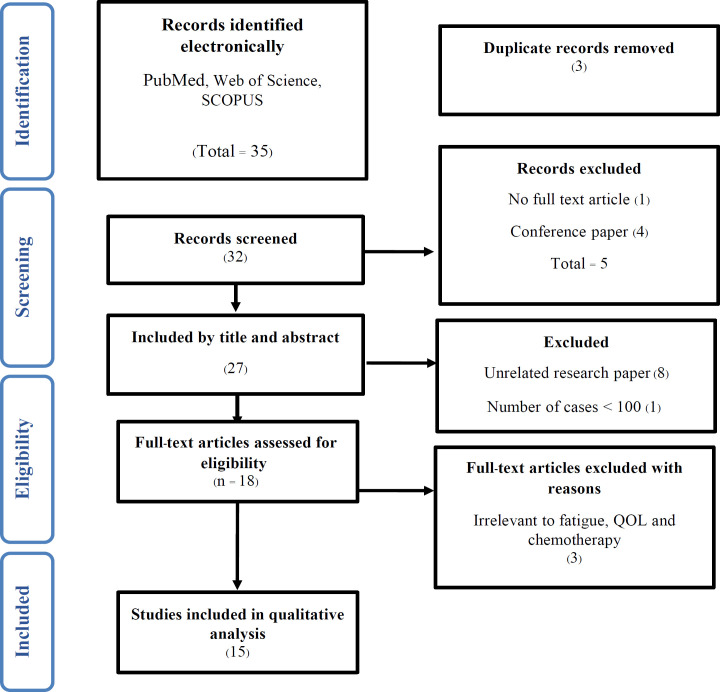
During the analysis procedure, 35 studies were extracted from selected databases. Following the removal of duplicate publications and unrelated subjects and abstracts, 32 studies were chosen for final evaluation. During the screening process, five studies were removed because they were either irrelevant or lacked full-text articles. A total of 27 relevant published articles were further evaluated for eligibility. Twelve papers (8 articles were unrelated, 1 paper contained number of patients ≤ 100 and 3 articles were not relevant) were also excluded because they were not relevant to fatigue, QOL and chemotherapy. Finally, 15 articles were selected in this analysis as they met the inclusion criteria (Figure 1).
Figure 1.
Flow Chart of Study Selection Strategies According to Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) Guidelines
Characteristics of selected articles
Tables 1 and 2 summarize the features and characteristics of the included articles in this meta-analysis. A total of 15 studies were selected from 10 countries across seven continents. The countries that have studied the effect of fatigue on cancer patients QOL undergoing antineoplastic chemotherapy were Brazil (n= 3, 20%), Germany (n= 2, 13.3%), Turkey (n= 2, 13.3%), India (n= 2, 13.3%), Spain (n= 2, 13.3%), Finland (n= 1, 6.6%), France (n= 1,6.6%), Malaysia (n= 1, 6.6%) and the United States (n=2, 6.6%) (Table 2). Most of the articles were published in 2015 and 60% of the studies used a combination of self-administered or surveys, telephone and in-person interviews methods for data collection and assessment of cancer patients’ QOL (Table 2). Most of the studies were cross-sectional designs (n= 12, 80%), whereas 3 (20%) studies used observational, cohort and prospective designs. Furthermore, a detailed description of the articles included is shown in (Table 2). The majority of the patients were diagnosed with breast cancer (n= 8, 53.3%), followed by prostate and lung cancers (n= 4, 26.6%), and other studies include patients diagnosed with unspecified cancer and treated with chemotherapy.
Table 1.
A Detail Description of Selected Studies in the Systematic Review
| Number of patients/study design /Setting /Age mean | Cancer type | Trial length and data collection method | Quality of life variable | Tools to measure fatigue | Tools to measure QOL | Prevalence of fatigue % (n) | Outcomes | References/ country |
|---|---|---|---|---|---|---|---|---|
| N =172 | Breast cancer | 8 months/self-administered | PWB,SWB,EWB,FWB | BFI | FACIT-F | 100% (172/172) | Fatigue strongly decrease QOL | Muthanna et al., 2021 |
| Observational prospective | ||||||||
| Setting = Malaysia | ||||||||
| Age mean = 52.6 | ||||||||
| N = 180 | Cancer | Written | PWB,SWB,EWB,FWB | BFI | FACT-G | 67.07% (121/180) | Fatigue affected QOL negatively | Poort et al., 2020 |
| RCTs | ||||||||
| Age mean = 53.31 | ||||||||
| N = 318 | Epithelial & ovarian cancer | 1.5 years/self-administered | Physical, Social, Emotional, Functional domains | FACIT-F | FACT-G | 26% (82/318) | Prevalence of fatigue was double in cancer survivor’s comparison to control. Also, fatigue disturbed the QOL | Joly et al., 2019 |
| Cross-sectional | ||||||||
| Setting = France | ||||||||
| Age mean = 53.6 | ||||||||
| N = 235 | Prostate cancer | self-administered | PWB, EWB,SWB,FWB | BFI | FACT-G/FACT-P | 74% (174/235) | Fatigue reduced QOL negatively | Rodríguez Antolín et al.,2019 |
| Cross-sectional/multicenter | ||||||||
| Setting = Spain | ||||||||
| Age mean = 77.3 | ||||||||
| N = 440 | Breast cancer | 3 years/self-administered | Physical, cognitive, social function | BSI | EORTC QLQ-C 30 | 4.20% (19/440) | Fatigue had an adverse effect on QOL and functionality | Calderon et al., 2019 |
| Prospective/multicenter/ | ||||||||
| cross-sectional | ||||||||
| Setting = Spain | ||||||||
| Age mean = 53.2 | ||||||||
| N = 236 | Breast, lung, & gastrointestinal | 14 months/self-administered | PWB, EWB,SWB,FWB | FACIT-F | FACT-G | 23.25% (55/236) | Self-care and self-efficiency improved the QOL and lowered fatigue in cancer patients | Akin & Kas Guner, 2019 |
| Descriptive cross-sectional | ||||||||
| Setting = Turkey | ||||||||
| Age mean = 57.37 | ||||||||
| N = 148 | Prostate cancer | 3.5 years/self-administered, face-to-face | Physical, affective, cognitive | CFS | EORTC QLQ-C 30, EORTC QLQ-PR25 |
66.90% (99/148) |
Fatigue negatively affect the QOL of prostate cancer patients | Charalambous & Kouta, 2016 |
| Cross-sectional | ||||||||
| Setting = Finland | ||||||||
| Age mean = 37 | ||||||||
| N = 402 | Cancer | 6 months/ telephone or face to face | Physical, cognitive, social function | ESAS | EORTC-QOL PAL15 | 80.80% (325/402) | A significant correlation was reported between fatigue and QOL. | Ghoshal et al., 2016 |
| Prospective cohort | ||||||||
| Setting = India | ||||||||
| Age mean = 52 | ||||||||
| Number of patients/study design /Setting /Age mean |
Abbreviations: BFI (Brief Fatigue Inventory), CFS (Cancer fatigue scale), ( EORTC QLQ-C30 (the European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire), EORTC QLQ-C15-PAL (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 15-Palliative), FACIT-F (Functional Assessment of Chronic Illness Therapy-Fatigue), FAQ (Fatigue Assessment Questionnaire), MFS (Multifunctional Fatigue Scale), PFS-R (Piper Fatigue Scale-Revised), QOL (quality of life), WHOQOL-BREF (World Health Organisation Quality of Life Instrument).
Table 2.
Characteristics of Fifteen Studies Included in the Systematic Review
| Characteristics | Frequency (percentage) |
|---|---|
| Total number of studies | 15 (100%) |
| Total number of patients | 11612 [100(0.86%) -8478 (73%)] |
| Country | |
| Brazil | 3 (20%) |
| Turkey | 2 (13.3%) |
| Spain | 2 (13.3%) |
| USA | 2 (13.3%) |
| India | 1 (7%) |
| Finland | 1 (7%) |
| France | 1 (7%) |
| Japan | 1 (7%) |
| Germany | 1 (7%) |
| Malaysia | 1 (7%) |
| Study design | |
| Self-administered | 6 (40%) |
| Combination (self-administered /telephone/face-to-face interview) | 9 (60%) |
Prevalence of CRF
The prevalence of fatigue severity was recorded in all cancer patients undergoing chemotherapy, which ranged from 4.2% (Calderon et al., 2019) to 100% (Muthanna et al., 2021). The prevalence of fatigue varied depending on the patient’s age and types of cancer. In this review, cancer patients belonging to the age category of 35 to 60 years old (Charalambous and Kouta, 2016; Kluthcovsky and Urbanetz, 2015) , Karthikeyan et al., 2012) had the highest prevalence of fatigue , whereas the lowest prevalence was recorded in the age group of 61 to 70 years old (Calderon et al., 2019) (Table 1).
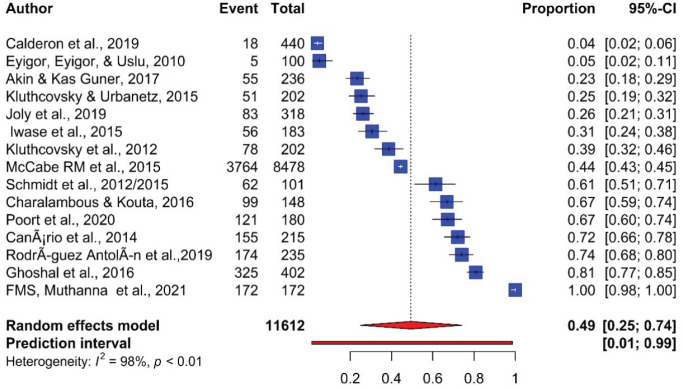
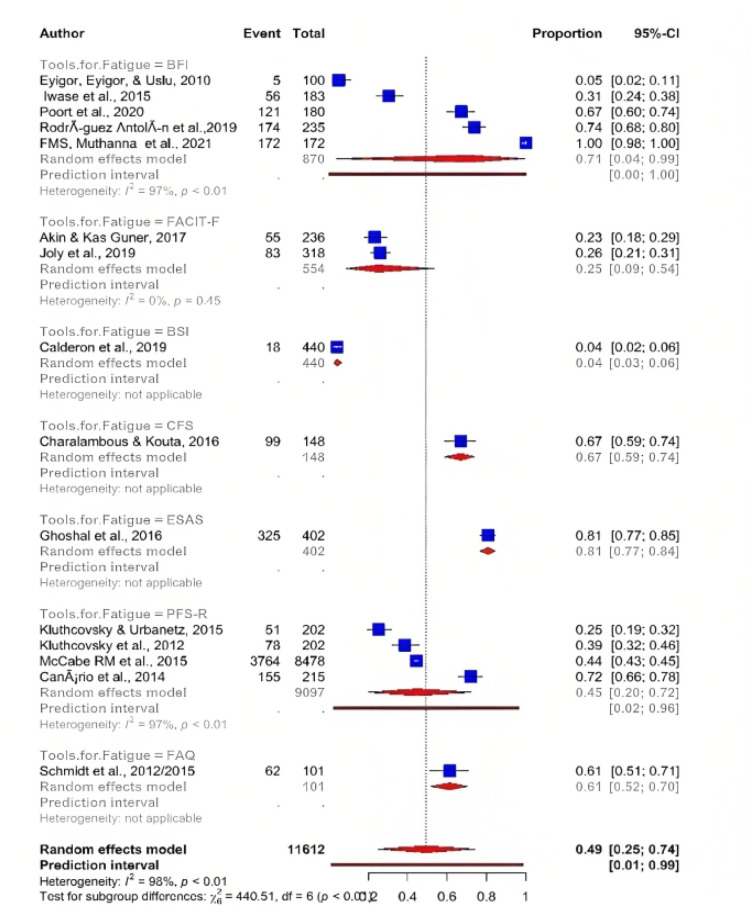
Furthermore, using the random-effect model, meta-analysis revealed the pooled prevalence and showed significant heterogeneity between the studies. The pooled prevalence of fatigue reported by the 15 articles was 49% (95% CI; 25.00-74.00) with significant heterogeneity between articles (I²=98%, P <0.001) (Figure 2).
Figure 2.
Prevalence of Fatigue in Cancer Patients Receiving Chemotherapy
Subgroup analyses
Cancer Type
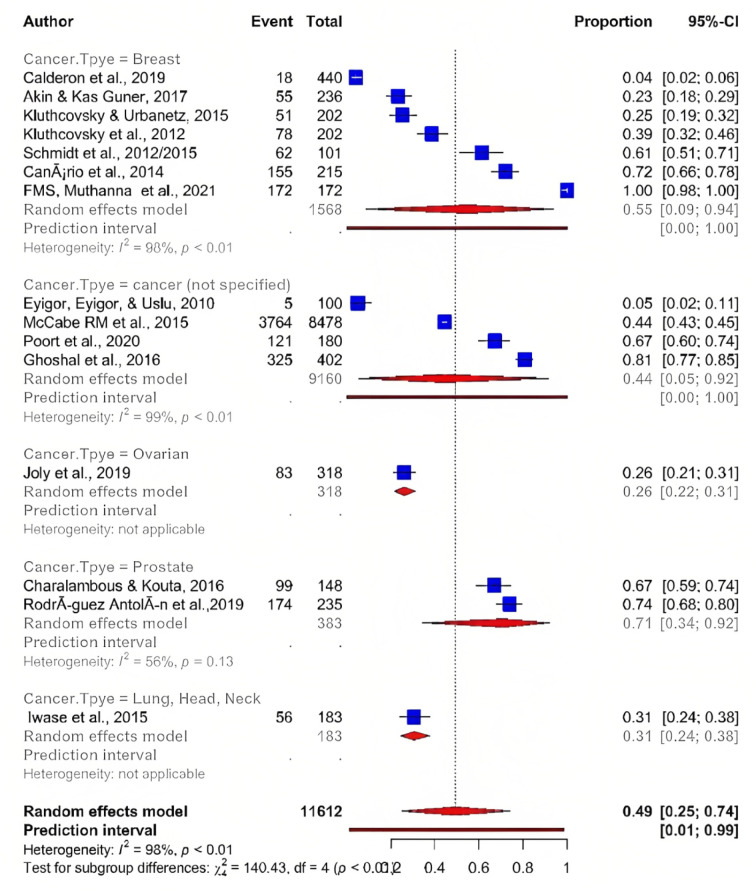
To evaluate potential heterogeneity among study results, subgroups analysis by type of tumour was performed. Of the 15 articles, the highest estimated overall prevalence of fatigue was found among patients diagnosed with breast cancer 55 % (95% CI; 9 00: 94 00), followed by cancer (unspecified) 44% (95% CI; 5.00 : 92.00), while the overall lowest prevalence of fatigue was estimated among patients suffering from ovarian cancer 26% (95% CI; 21.00 : 31.00) and neck, head, and lung cancer 31% (95% CI; 24.00 : 38.00) (Figure 3). These differences might be due to variations in the study objectives, methodologies or instrument used, as well as the criteria of inclusion and exclusion. Similarly, the intensity of fatigue severity was different among various types of cancer patients. The majority of selected articles revealed that a higher proportion of cancer patients showed overall fatigue, two studies reported that the fatigue score ranged from mild to severe (Ghoshal et al., 2016; Muthanna et al., 2021) whereas one study indicated moderate to severe fatigue symptoms (Iwase et al., 2015).
Figure 3.
Prevalence of Fatigue among Different Cancer Type
Assessment Tools of QOL in cancer patients
Several tools were used to assess and measure QOL in cancer patients. Some instruments are generic while some are specific. FACT-G and EORTC QLQ-C 30 are examples of generic tools which are used in assessing general QOL of cancer patients, whereas disease-specific QOL measuring instruments include FACIT-F (for fatigue patients), FACT-An (for the anaemic patient), FACIT-Br (for breast cancer), EORTC QLQ-BR23 (for breast cancer) and EORTC QLQ-PR25 (for prostate cancer) (Akin and Kas Guner, 2019; Cella, 1997; Nunes et al., 2017).
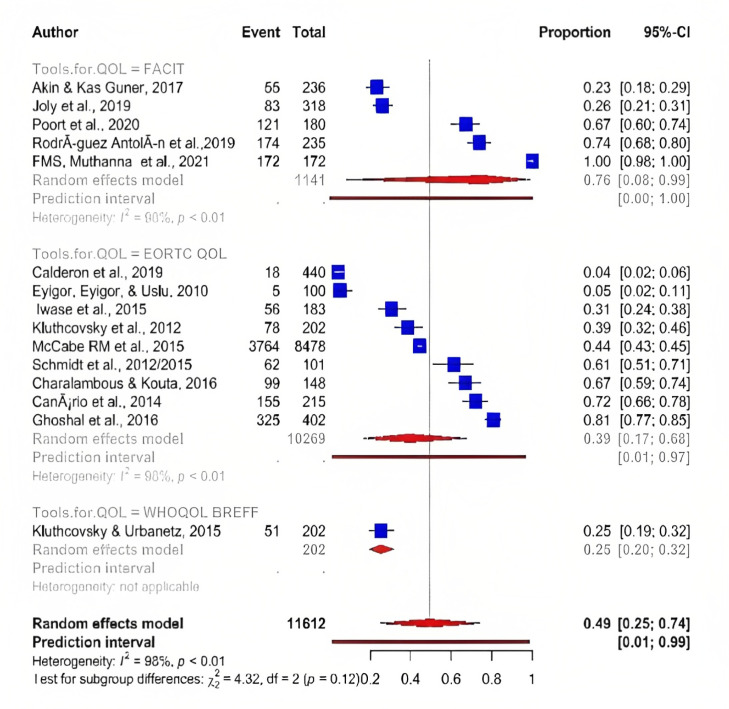
Meta-analysis showed that EORTC QLQ-C 30 tool was the most frequent assessment tool used to determine QOL in cancer patients suffering from fatigue with an overall prevalence 39% (95% CI; 17.00 : 68.00) which was lower than prevalence of fatigue assessed by FACT-G which indicated overall prevalence 76% (95% CI; 8.00 : 99.00). Furthermore, WHOQOL BREFF was the least frequently used assessment tool, with only two studies with a prevalence of 25% (95% CI; 20.00 : 32.00) (Figure 4 ) .
Figure 4.
Tools Used to Assess QOL among Fatigue Cancer Patients
EORTC QLQ-C30
The EORTC-QOL-C30 is a more valid and dependable instrument for assessing QOL among cancer patients (Alawadhi and Ohaeri, 2010; Snyder et al., 2013). It contains 30-items and used to assess cancer patients’ quality of life. The EORTC-QOL-C30 comprises symptom scale (nausea or vomiting, pain and fatigue), global health scale (physical, emotional, role, cognitive and social) and functional scale (diarrhoea, appetite loss, dyspnoea, constipation and sleep disturbances). The highest score for the global health and functional scale signifies a good QOL, whereas the highest score in the symptom scale indicates a poor QOL (Aaronson et al., 1993). Similar reliability and validity can be expected from the electronic version of patient-related outcome (e-PRO) of EORTC-QLQ-C30 (Wallwiener et al., 2017). Furthermore, 7 items in the questionnaire from the EORTC QLQ-BR23 breast cancer-specific module are used to evaluate the issue with the affected breast (Peter Fayers et al., 1995).
The EORTC QLQ-C30 is available in a variety of languages in most parts of the world and shows acceptable psychometric properties (Aaronson et al., 1993). Two studies in this review utilised EORTC QLQ-C30, which was translated to Portuguese among Brazilian cancer patients (Santos et al., 2006).
EORTC QLQ-PR25
The EORTC QLQ-PR25 is a tool specially developed to measure the QOL among localised and metastatic prostate cancer patients. It contains 25 items which include bowel symptoms (four items), urinary symptoms (nine items), sexual functioning (six items) and treatment-related symptoms (six items) (Charalambous and Kouta, 2016). It is validated and translated into multiple languages, including Greek (Kontodimopouloset al., 2012).
EORTC QLQ-C15-PAL
The EORTC QLQ-C15-PAL is a questionnaire established to measure QOL in palliative care (Miyazaki et al., 2012). It contains 15 items which include a 5-item functional scale (role, emotional, social, cognitive, and physical functioning), 9-item symptom subscale (measuring pain, insomnia, fatigue vomiting, nausea, constipation, diarrhoea, appetite loss, and functional difficulties). The subscale item scores range from 0 to 10. A higher score in the functional subscale (greater than 60) represents a better function, whereas a lower score in the symptom subscale (less than 40) represents better physical conditioning (Miyazaki et al., 2012).
FACT-G, FACT-An and FACIT-F
FACT-G is a generic tool used to assess health-related QOL among cancer patients (Schmidt et al., 2015). Its latest version (Version 4) contains 27 Likert-type items, which are further categorised into separate subscale’s formulation: emotional (six items), physical (seven items), functional (seven items), and family/social well-being (seven items). The score ranges from 0 to 4 (where 0= not at all, 1 a little bit, 2= somewhat, 3= quite a bit, and 4= very much) and the highest score represents better QOL (Mast, 1998). FACT-An is a specific instrument used to measure QOL among anaemic cancer patients. FACT-An consists of 47 items (27 FACT-G) plus 20 items (AnS) specific for anaemic patients.
FACIT-F is a tool specified for evaluation of QOL among cancer patients suffering from fatigue. It comprises FACT-G (27 items) and 13 items that are specific for fatigue symptoms. The 40 items of FACIT-F comprise four subscales, including social wellbeing, physical wellbeing, functional wellbeing, and emotional wellbeing. Additionally, it contains a fatigue subscale with 13 items to measure fatigue, especially in cancer patients. Respondents are asked about their feeling of tiredness in the previous seven days. The subscale has a 5-point Likert scale ranging from 0 (not at all) to 4 (very much). The sum of the score from four subscales is represented as the FACT-G total score; hence, the minimum score is while the maximum score is 108. However, the total score of the FACIT-F subscale (13 items) ranges from 0 (the worst) to 52 (the best), whereas that of FACIT-F score ranges from 0 to 160 with the highest score representing good QOL. FACIT-F is available and validated in many languages in countries such as Turkey, China, and Japan (Cella, 1997).
WHOQOL-BREF
It is a tool developed by the World Health Organization (WHO) to assess general QOL. This tool contains four domains namely psychological (six items), physical (seven items), environmental (eight items), and social relationships (three items). The highest score represents a good QOL (Skevington et al., 2004), and it has been translated and validated in many languages and the psychometric properties have been deemed satisfactory (Fleck et al., 2000).
Assessment Tools of fatigue severity
In the present systematic review, different questionnaires were used to assess the severity of fatigue in patients diagnosed with cancer receiving chemotherapy. The majority of the studies used BFI (n= 5, 33.3%), PFS-R (n= 3, 20.0%), FACIT-F (n= 2, 13.3%), CFS (n= 2, 13.3%) and ESAS (n= 1, 6.6%) (Table 1).
Meta-analysis indicated that BFI was the most frequent assessment too used to detect fatigue severity in cancer patients with an overall prevalence 71% (95% CI; 4 - 99), followed by FACIT-F with a total prevalence of 25% (95% CI; 9 -54). Furthermore, BSI tool was the least frequent assessment tool used, as only two studies with a prevalence of 4 % (95% CI; 3.00 : 6.00 ) (Figure 5 ).
Figure 5.
Tools Used to assess Fatigue Severity of Cancer Patients
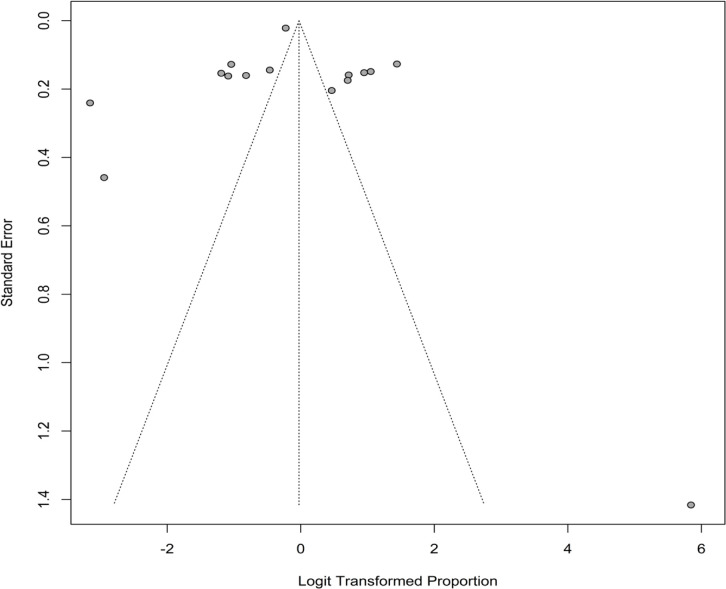
Publication bias
A visual examination of the funnel plot (Figure 6) reveals publication bias as a result of some asymmetry. The publishing bias is also revealed by the Egger’s test (P <0.001).
Figure 6.
Funnel Plot for Visual Detection of Publication Bias
Discussion
The goals of this study were to determine the prevalence of fatigue and the association between fatigue and QOL in cancer patients receiving chemotherapy.
Fatigue is a medical undocumented side effect that is experienced by cancer patients. In general, the prevalence of fatigue is high among cancer patients, ranging from 4.2% (Calderon et al., 2019) to 90 % (Karthikeyan et al., 2018) with a higher risk among cancer patients with advanced stages or those undergoing chemotherapy. The development of instruments to evaluate fatigue in patients diagnosed with cancer assists in determining better and more appropriate ways to treat the medical issue. The intensity of fatigue in patients diagnosed with cancer increases among the breast cancer patients (46.6%) compared to other cancer types. However, it should be noted that most studies in this review focused on determining the prevalence of fatigue among breast cancer patient population, which might explain the higher reported prevalence rate. This denotes data paucity on fatigue in other cancer patient populations. Besides, breast cancer patients undergoing chemotherapy experienced fatigue due to the underlying illness and the damaging effects of the chemotherapy on healthy cells. These events could intensity fatigue in response to the damage caused by anti-neoplastic therapy (Muthanna et al., 2022).
The severity of CRF was also prevalent in patients of advanced age. According to Giacalone and colleagues, the association between age and intensity of fatigue would be easily detectable among 65-year-old elderly patients, who have been reported to be extremely susceptible to cancer (Giacalone et al., 2013). Similarly, a significant relationship has been reported between CRF and cancer patients aged 35 to 60 years old (Su et al., 2011).
Fatigue has been described as the most popular unpleasant health illness experienced by cancer patients prior to or following anti-neoplastic treatment. It is also a long-term symptom, as some cancer patients suffer from cancer related fatigue for a long period of time after finishing chemotherapy (Weis, 2015). Despite being regarded as the most serious medical symptom affecting QOL in patients with cancer, evaluation, information about its prevalence, and impact on QOL remains scarce, particularly in advanced stages of cancer (Charalambous and Kouta, 2016; Salca et al., 2015). Strong and clear evidence depicts a negative association between CRF and QOL among cancer patients who have received chemotherapy as indicated by this review. Fatigue was reported to affect multiple factors concerning QOL, including social activity, cognitive task, employment, emotional, physical, physiological, role, environmental, school functioning, and behavioural functioning (Canário et al., 2016; Karthikeyan et al., 2012). CRF was a common health issue in cancer patients and it caused difficulties for patients to perform cognitive tasks and participate in social activities (Akin and Kas Guner, 2019; Kluthcovsky et al., 2012). Fatigue also had an adverse effect on the employment status of both patients and caregivers (Curt, 2000). Additionally, fatigue adversely affected QOL parameters relating to emotional, physiological and social aspects in lung and breast cancer patients (Dagnelie et al., 2007).
In this systematic review, in terms of pain, sleep, and QOL, elderly cancer patients did not differ significantly from those of a younger age group (Eyigor et al., 2010). Considerable fatigue and depression decreased the QOL among breast cancer survivors’ populations than the general population. Furthermore, younger women suffered more fatigue than the older ones while fatigue women had poor health-related QOL (Kluthcovsky and Urbanetz, 2015; Kluthcovsky et al., 2012). The severity of fatigue in breast cancer patients has been reported to be higher in those receiving chemotherapy alone, followed by those receiving a combination of concurrent chemotherapy and radiotherapy (Kluthcovsky et al., 2012). Breast cancer patients who experienced persistent fatigue experienced significant long-term loss in QOL in terms of financial, physical, cognitive, and social aspects (Schmidt et al., 2015; Zaker et al., 2019).
CRF and its effect on QOL varied depending on the type of cancer. Breast cancer patients experienced a higher level of fatigue severity compared to prostate cancer patients (Calderon et al., 2019; Charalambous and Kouta, 2016; Kluthcovsky and Urbanetz, 2015; Kluthcovsky et al., 2012). Moreover, breast cancer reduced QOL more significantly than other types of cancer patients (Muthanna et al., 2022). This might be explained by the inclusion criteria and research objectives as most participants in the reviewed studies were diagnosed with breast cancer. Furthermore, most studies sought to identify the occurrence of fatigue and its impact on QOL. Some studies have found that an increase in inflammatory cytokines such as interleukin 6 (IL-6), tumor necrosis factor (TNF), and IL-1 receptor antagonist (IL-1RA), particularly IL-8, is associated with an increase in the prevalence and severity of fatigue (Reyes-Gibby et al., 2013).These factors were found to be significant determinants of pain and fatigue in cancer patients. Fatigue is more common in breast cancer patients than in prostate cancer patients, and it is associated with other symptoms such as depression , pain, and sleep disturbance.
CRF occurred more frequently in breast, lung, neck, head and pancreases cancer patients. It was successfully evaluated with BFI for the first time in Japan, and also found to influence patients’ QOL in terms of emotional functioning (Iwase et al., 2015). On the other hand, physical activity reduced fatigue and improved the QOL, especially for functional capacity in cancer patients (Canário et al., 2016). Furthermore, prostate cancer patients with CRF had a lower level of QOL concerning physical, affective, and cognitive as compared to non-fatigue patients (Charalambous and Kouta, 2016). Three types of fatigue (general, sleep, cognitive) in children and adolescents with cancer had severe negative impacts on the subjects’ QOL in terms of physical, emotional and social aspects (Nunes et al., 2017). Recently, a study suggested that self-care and self-efficiency led to improved QOL and lower fatigue in cancer patients during chemotherapy (Akin and Kas Guner, 2019).
In summary, the large data summarised above revealed that fatigue significantly affects the QOL among cancer patients receiving chemotherapy. Most of the articles in the present systematic review indicated that fatigue is prevalent most frequently among solid cancer patients and showed a significant negative association with QOL. Charalambous and Kouta, 2016 indicated that fatigue reduced QOL in all domains among a total of 148 prostate cancer patients enrolled in the study that lasted for 3.5 years. The researchers used the CFS to measure fatigue severity and EORTC QOQ-30 to assess general QOL while EORCT QOQ-PR-25 was applied to assess QOL among prostate cancer patients. Similar findings were reported by Calderon et al., 2019, where 440 breast cancer patients were enrolled and EORTC QLQ-C 30 was used to evaluate the subjects’ QOL.
All domains of QOL are negatively affected by fatigue with a slight effect on psychological well-being as indicated by the majority of studies (Canário et al., 2016; Kluthcovsky and Urbanetz, 2015). This may be attributed to psychological factors such as personality traits and depression, which are difficult to measure and are rarely detected during observational studies, coupled with the fact that most studies were conducted among breast cancer patients.
Strength and limitation
We used the PRISMA guidelines to perform our analysis, and we were able to discover all research that had the possibility to be considered. Subgroup analyses were conducted to compare the cancer type and assessment tools results from each study. Despite the fact that we were limited in our ability to analyze numerous significant variations due to a lack of existing evidence on the factors that cause fatigue in cancer patients and among assessment tools, our pooled assessments demonstrated a significantly increased risk of fatigue among cancer patients regardless of cancer type or instruments used to assess fatigue severity or QOL. One of the review’s limitations was that only studies with populations of more than 100 people were included. Increasing the sample size yield more precise mean values, detect deviations that could significantly skew data in a sample size, and have a lower error margin. Other limitations include the fact that this study was limited to determining the prevalence of CRF and its impact on QOL. There are no factors linked to an increased prevalence of fatigue in cancer patients (e.g gender, history of illness, marital status , cancer type.etc).
Even though our findings showed that breast cancer was the most common type of tumor to have a negative association with CRF, we cannot ignore the fact that other types of cancer also reduce QOL. Similarly, while we found a statistically significant decline in QOL among cancer patients who used the FACT-G, FACT-F, and BFI tools, QOL among cancer patients who used other assessment tools may also be reduced. Finally, we looked at the relationship between health-related QOL and fatigue in studies from three databases (PubMed, Web of Science, and Scopus), which limited the number of studies included. Other databases (for example, Google Scholar) may contain more results
Overall, the prevalence of CRF is very high among cancer patients and commonly results in an overall deterioration of QOL, especially among patients undergoing chemotherapy. According to the findings of this review, there is a significant negative association between QOL and CRF in cancer patients undergoing chemotherapy. It is critical to find an appropriate treatment that can help cancer patients with fatigue. CRF should be managed correctly in order to improve QOL in cancer patients. We propose that oncologists and other healthcare providers involved in the care of cancer patients require a comprehensive management guideline (pharmacological and non-pharmacological) in order to effectively monitor, assess, treat CRF and improve QOL.
Author Contribution Statement
Conceptualization: Fares M.S Muthanna; Data curation: Egbal Abdulrahman; Formal analysis: Hamza Khalifa Ibrahim; Methodology: Bassam Abdul Rasool Hassan; Writing and editing: Ali Haider Mohammed; Writing – original draft: Fares M.S Muthanna, Mahmathi Karuppannan; Supervision: Mahmathi Karuppannan
Acknowledgements
The authors would like to thank Dr Sameh Samir Elawady for his assistance and generosity in analyzing data.
Availability of data
All data are available upon request from the first or corresponding author.
Conflicts of interest
The authors have declared no conflicts of interest for this review.
References
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- Akin S, Kas Guner C. Investigation of the relationship among fatigue, self-efficacy and quality of life during chemotherapy in patients with breast, lung or gastrointestinal cancer. Eur J Cancer Care. 2019;28:e12898. doi: 10.1111/ecc.12898. [DOI] [PubMed] [Google Scholar]
- Alawadhi SA, Ohaeri JU. Validity and reliability of the European Organization for Research and Treatment in Cancer Quality of Life Questionnaire (EORTC QLQ): experience from Kuwait using a sample of women with breast cancer. Ann Saudi Med. 2010;30:390–6. doi: 10.4103/0256-4947.67083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarsheda SB, Bhise AR. Association of fatigue, quality of life and functional capacity in breast cancer patients receiving adjuvant chemotherapy. Asian Pac J Cancer Care. 2021;6:59–64. [Google Scholar]
- Antolín AR, Martínez-Piñeiro L, Romero MJ, et al. Prevalence of fatigue and impact on quality of life in castration-resistant prostate cancer patients: the VITAL Study. BMC Urol. 2019;19:1–8. doi: 10.1186/s12894-019-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon C, Carmona-Bayonas A, Hernández R, et al. Effects of pessimism, depression, fatigue, and pain on functional health-related quality of life in patients with resected non-advanced breast cancer. Breast J. 2019;44:108–12. doi: 10.1016/j.breast.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Canário ACG, Cabral PUL, Paiva LCd, et al. Physical activity, fatigue and quality of life in breast cancer patients. Rev Assoc Med Bras. 2016;62:38–44. doi: 10.1590/1806-9282.62.01.38. [DOI] [PubMed] [Google Scholar]
- Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34:13–9. [PubMed] [Google Scholar]
- Charalambous A, Kouta C. Cancer related fatigue and quality of life in patients with advanced prostate cancer undergoing chemotherapy. Biomed Res Int. 2016:2016. doi: 10.1155/2016/3989286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curt GA, Breitbart W, Cella D, et al. Impact of cancer related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353–60. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- Curt G, Johnston PG. Cancer fatigue: the way forward. Oncologist. 2003;8:27–30. doi: 10.1634/theoncologist.8-suppl_1-27. [DOI] [PubMed] [Google Scholar]
- Dagnelie P, Pijls-Johannesma M, Lambin P, et al. Impact of fatigue on overall quality of life in lung and breast cancer patients selected for high-dose radiotherapy. Ann Oncol. 2007;18:940–4. doi: 10.1093/annonc/mdm057. [DOI] [PubMed] [Google Scholar]
- Donovan KA, Jacobsen PB, Andrykowski MA, et al. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manag. 2004;28:373–80. doi: 10.1016/j.jpainsymman.2004.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Bremell T, Mannerkorpi K. Usefulness of multiple dimensions of fatigue in fibromyalgia. J Rehabi Med. 2013;45:685–93. doi: 10.2340/16501977-1161. [DOI] [PubMed] [Google Scholar]
- Eyigor S, Eyigor C, Uslu R. Assessment of pain, fatigue, sleep and quality of life (QoL) in elderly hospitalized cancer patients. Arch Gerontol Geriatr. 2010;51:57–61. doi: 10.1016/j.archger.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Flechtner H, Bottomley A. Fatigue and quality of life: lessons from the real world. Oncologist. 2003;8:5–9. doi: 10.1634/theoncologist.8-suppl_1-5. [DOI] [PubMed] [Google Scholar]
- Fleck M, Louzada S, Xavier M, et al. Aplicação da versão em português do instrumento abreviado de avaliação da qualidade de vida” WHOQOL-bref”. Rev Saúde Publica. 2000;34:178–83. doi: 10.1590/s0034-89102000000200012. [DOI] [PubMed] [Google Scholar]
- Ghoshal A, Salins N, Deodhar J, Damani A, Muckaden MA. Fatigue and quality of life outcomes of palliative care consultation: A prospective, observational study in a tertiary cancer center. Indian J Palliat Care. 2016;22:416. doi: 10.4103/0973-1075.191766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacalone A, Quitadamo D, Zanet E, et al. Cancer-related fatigue in the elderly. Support Care Cancer. 2013;21:2899–911. doi: 10.1007/s00520-013-1897-1. [DOI] [PubMed] [Google Scholar]
- Iop A, Manfredi AM, Bonura S. Fatigue in cancer patients receiving chemotherapy: an analysis of published studies. Ann Oncol. 2004;15:712–20. doi: 10.1093/annonc/mdh102. [DOI] [PubMed] [Google Scholar]
- Iwase S, Kawaguchi T, Tokoro A, et al. Assessment of cancer-related fatigue, pain, and quality of life in cancer patients at palliative care team referral: a multicenter observational study (JORTC PAL-09) PLoS One. 2015;10:e0134022. doi: 10.1371/journal.pone.0134022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly F, Ahmed-Lecheheb D, Kalbacher E, et al. Long-term fatigue and quality of life among epithelial ovarian cancer survivors: a GINECO case/control VIVROVAIRE I study. Ann Oncol. 2019;30:845–52. doi: 10.1093/annonc/mdz074. [DOI] [PubMed] [Google Scholar]
- Karthikeyan G, Jumnani D, Prabhu R, Manoor UK, Supe SS. Prevalence of fatigue among cancer patients receiving various anticancer therapies and its impact on quality of life: a cross-sectional study. Indian J Palliat Care. 2012;18:165. doi: 10.4103/0973-1075.105686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluthcovsky ACGC, Urbanetz AA. Fatigue and quality of life in breast cancer survivors: a comparative study. Rev Bras Ginecol Obstet. 2015;37:119–26. doi: 10.1590/SO100-720320150005247. [DOI] [PubMed] [Google Scholar]
- Kontodimopoulos N, Samartzis A, Papadopoulos AA, Niakas D. Reliability and validity of the Greek QLQ-C30 and QLQ-MY20 for measuring quality of life in patients with multiple myeloma. Sci World J. 2012:2012. doi: 10.1100/2012/842867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeveld N, Grootenhuis M, Voute P, De Haan R, Van Den Bos C. No excess fatigue in young adult survivors of childhood cancer. Eur J Cancer. 2003;39:204–14. doi: 10.1016/s0959-8049(02)00629-9. [DOI] [PubMed] [Google Scholar]
- Mast ME. Correlates of fatigue in survivors of breast cancer. Cancer Nurs. 1998;21:136–42. doi: 10.1097/00002820-199804000-00007. [DOI] [PubMed] [Google Scholar]
- McCabe RM, Grutsch JF, Braun DP, Nutakki SB. Fatigue as a driver of overall quality of life in cancer patients. PLoS One. 2015;10:e0130023. doi: 10.1371/journal.pone.0130023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Suzukamo Y, Shimozuma K, Nakayama T. Verification of the psychometric properties of the Japanese version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 15 palliative (EORTCQLQ-C15-PAL) Qual Life Res. 2012;21:335–40. doi: 10.1007/s11136-011-9939-y. [DOI] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1–9. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga U, Kerrigan AJ, Thornby J, Monga TN. Prospective study of fatigue in localized prostate cancer patients undergoing radiotherapy. Radiat Oncol Investig. 1999;7:178–85. doi: 10.1002/(SICI)1520-6823(1999)7:3<178::AID-ROI7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Morrow GR, Shelke AR, Roscoe JA, Hickok JT, Mustian K. Management of cancer-related fatigue. Cancer Invest. 2005;23:229–39. doi: 10.1081/cnv-200055960. [DOI] [PubMed] [Google Scholar]
- Muthanna FM, Iqbal MS, Karuppannan M, et al. Prevalence and associated factors of fatigue among breast cancer patients in Malaysia—A prospective study. J Appl Pharm Sci. 2022;12:131–9. doi: 10.1155/2022/7611733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthanna F, Karuppannan M, Abdulrahman E, et al. Prevalence and Associated Factors of Anemia among Breast Cancer Patients Undergoing Chemotherapy: A Prospective Study. Adv Pharmacol Pharm Sci. 2022;2022:7611733. doi: 10.1155/2022/7611733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthanna FMS, Karuppannan M, Hassan BAR, Mohammed AH. Impact of fatigue on quality of life among breast cancer patients receiving chemotherapy. Osong Public Health Res Perspect. 2021;12:115. doi: 10.24171/j.phrp.2021.12.2.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes EC, van der Vorst MJ, Blauwhoff-Buskermolen S, Verheul HM. Aiming for a better understanding and management of cancer-related fatigue. Oncologist. 2013;18:1135. doi: 10.1634/theoncologist.2013-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes MDR, Jacob E, Bomfim EO, et al. Fatigue and health related quality of life in children and adolescents with cancer. Eur J Oncol Nurs. 2017;29:39–46. doi: 10.1016/j.ejon.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BF, Dibble SL, Dodd MJ, et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25:677–84. [PubMed] [Google Scholar]
- Poort H, Jacobs JM, Pirl WF, Temel JS, Greer JA. Fatigue in patients on oral targeted or chemotherapy for cancer and associations with anxiety, depression, and quality of life. Palliat Support Care. 2020;18:141–7. doi: 10.1017/S147895151900066X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ream E, Richardson A. Fatigue: a concept analysis. Int J Nurs Stud. 1996;33:519–29. doi: 10.1016/0020-7489(96)00004-1. [DOI] [PubMed] [Google Scholar]
- Reyes-Gibby CC, Wang J, Spitz M, et al. Genetic variations in interleukin-8 and interleukin-10 are associated with pain, depressed mood, and fatigue in lung cancer patients. J Pain Symptom Manage. 2013;46:161–72. doi: 10.1016/j.jpainsymman.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen G, Brand S. Sleep in children with cancer: case review of 70 children evaluated in a comprehensive pediatric sleep center. Support Care Cancer. 2011;19:985–94. doi: 10.1007/s00520-010-0921-y. [DOI] [PubMed] [Google Scholar]
- Salca A, Checiches A, Irsay L. The Approach of Cancer Related Fatigue in Rehabilitation Medicine: Part I – mechanisms, symptoms, clinical evaluation and screening. Balneo Rese J. 2015;6:79–85. [Google Scholar]
- Santos FR, Kozasa EH, Maria de Lourdes L, Colleoni GW, Leite JR. Psychosocial adaptation and quality of life among Brazilian patients with different hematological malignancies. J Psychosom Res. 2006;60:505–11. doi: 10.1016/j.jpsychores.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Schmidt ME, Wiskemann J, Armbrust P, et al. 2015 ) Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int J Cancer. 137:471–80. doi: 10.1002/ijc.29383. [DOI] [PubMed] [Google Scholar]
- Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial A report from the WHOQOL group. Qual Life Res. 2004;13:299–310. doi: 10.1023/B:QURE.0000018486.91360.00. [DOI] [PubMed] [Google Scholar]
- Smets E, Visser M, Willems-Groot A, et al. Fatigue and radiotherapy:(A) experience in patients undergoing treatment. Br J Cancer. 1998;78:899–906. doi: 10.1038/bjc.1998.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder CF, Blackford AL, Okuyama T, et al. Using the EORTC-QLQ-C30 in clinical practice for patient management: identifying scores requiring a clinician’s attention. Qual Life Res. 2013;22:2685–91. doi: 10.1007/s11136-013-0387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer related fatigue: evolving concepts in evaluation and treatment. Cancer. 2003;98:1786–801. doi: 10.1002/cncr.11742. [DOI] [PubMed] [Google Scholar]
- Testa MA, Simonson DC. Assessment of quality-of-life outcomes. N Engl J Med. 1996;334:835–40. doi: 10.1056/NEJM199603283341306. [DOI] [PubMed] [Google Scholar]
- Viriyasiri P, Phutthikiat P, Phonmak P, et al. Symptom and anxiety assessment in gynecologic cancer patients receiving chemotherapy. Asian Pac J Cancer Care. 2020;5:95–100. [Google Scholar]
- Wallwiener M, Matthies L, Simoes E, et al. Reliability of an e-PRO tool of EORTC QLQ-C30 for measurement of health-related quality of life in patients with breast cancer: prospective randomized trial. J Med Internet Res. 2017;19:e322. doi: 10.2196/jmir.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis J. Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Rev Pharmacoecon Outcomes Res. 2011;11:441–6. doi: 10.1586/erp.11.44. [DOI] [PubMed] [Google Scholar]
- Winningham ML. Strategies for managing cancer related fatigue syndrome: A rehabilitation approach. Cancer. 2001;92:988–97. doi: 10.1002/1097-0142(20010815)92:4+<988::aid-cncr1411>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Zaker MR, Hazrati-Marangaloo A, Hosseini SR. Quality of life in Iranian breast cancer survivors and affecting factors: A Review Article. Asian Pac Environ Cancer. 2019;2:5–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request from the first or corresponding author.