Abstract
Background:
A semiconductor rectal probe was used to compare planned and measured rectal doses during Co-60 high dose rate (HDR) CT-based intracavitary brachytherapy applications (ICBT) of cervical cancer.
Materials and Methods:
A total of 22 HDR brachytherapy applications were included from 11 patients who were first treated with EBRT to the whole pelvis with a total prescribed dose of 50 Gy in 25 fractions. During each application, a PTW 9112 probe rectal probe having a series of five semiconductor diodes (R1 to R5) was inserted into the patient’s rectum and a CT-based HDR ICBT application with a prescribed dose per fraction of 7 or 7.5 Gy to HRCTV was performed. Measurements were carried in water phantom using PTW rectal and universal adaptor plugs. Doses measured in phantom and with patients were compared to those calculated by the treatment planning system.
Results:
The mean percentage dose difference ΔD (%) between calculated and measured values from phantom study were -5.29%, 1.89%, -2.72%, -4.76, and 0.72% for R1, R2, R3, R4, and R3 diodes, respectively and the overall mean ΔD (%) value with standard deviation (SD) was -2.03%±9.6%. From the patient study, a ΔD (%) that ranged from -19.5% to 24.0%, which corresponded to dose disparities between -0.77 Gy and 0.66 Gy. The median ΔD (%) ranged from 0.4% to 1.3%, or -0.03 to 0.05 Gy, respectively. ΔD (%) values exceeded 10% in approximately 26.4% of measurements (29 out of 110 in 22 applications). The location of Rmax in computed and measured values differs in 5 of 22 applications might be due to possible displacement of rectal probe between simulation and treatment.
Conclusion:
Despite the likely geometrical shift of measuring detectors between insertion and treatment, in-vivo dosimetry is feasible and can be used to estimate the dose to the rectum during HDR ICBT.
Key Words: Online rectal does, HDR ICBT, Quality Assurance
Introduction
Cobalt-60 (Co-60) has recently gained popularity as a high dose rate (HDR) brachytherapy source alternative to Iridium-192 (Ir-192). The ability to produce miniaturized Co-60 allows it to be used as an HDR brachytherapy source. Co-60 has a half-life of 5.25 years and can thus be used for approximately 5 years before replacement, making it more cost-effective than Ir-192, which has a significantly shorter half-life of 74 days. Because of this benefit, Co-60 has grown in popularity as an HDR brachytherapy source (Ntekim et al., 2010). The anisotropy, radial dose function, and qualitative isodose distributions produced by the Co-60 source have been reported to be comparable to those produced by the Ir-192 source (Strohmaier et al., 2011). Other studies on the dosimetric parameters and properties of Co-60 as a brachytherapy source have been published (Granero et al., 2007; Ballester et al., 2005).
The risk of radiation toxicity to surrounding normal organs at risk, particularly the rectum, is the main challenge in delivering the prescribed radiation dose to the target during cervical cancer brachytherapy. Despite the use of optimization algorithms that can maximize dose uniformity to the target, dose to the rectum in some clinical situations can be unacceptably high (Chun et al., 2004; Chen et al., 2000; Ogino et al., 1995).
The use of Co-60 as a source for HDR brachytherapy raises the question of whether the rectum will receive a higher radiation dose due to the higher average gamma energy of 1.25 MeV emitted by Co-60 versus 0.38 MeV emitted by Ir-192. Park et al., (2009) compared reference point doses for HDR brachytherapy, Co-60 and Ir-192, and found that rectal doses were 0.8% higher than Ir-192. Palmer et al., (2009) reported that Co-60 plans delivered up to 10% more dose within the rectum along the extension of the applicator axes and lower doses to regions further away from the applicators than Ir-192 plans. As a result, radiation doses to the rectum should be carefully monitored, particularly when using Co-60 for HDR brachytherapy. It is critical to report doses received by the rectum during HDR brachytherapy in order to assess the possibility of toxicity.
The use of real-time in-vivo dosimetry (IVD) is an important method of acquiring doses during brachytherapy. This is the only method for assessing doses to organs at risk (OAR) during actual treatment, and it is especially important in brachytherapy due to treatment planning uncertainties that do not account for inhomogeneity or potential organ or applicator movement between imaging and treatment. IVD during brachytherapy has the potential to reduce treatment errors while also being useful for dose reporting (Tanderkup et al., 2013).
Rectal dose measurement during prostate and vaginal brachytherapy treatment in a number of patients has been assessed for dose discrepancies between planned and delivered doses by Carrara et al., (ICRU., 1985; Allahverdi et al., 2012; Bansal et al., 2013; Uniyal et al., 2013). This follows successful dose verification in both prostate and gynecology phantom under Ir-192 brachytherapy source by Tenconi et al., 2014; Romanuykha et al., 2017) respectively.
Thermoluminescence (TLD), optically stimulated luminescence (OSLD), electron paramagnetic resonance in L-alanine (EPR/Alanine), radiophotoluminescence glass (RPLG), semiconductor diode, and metal-oxide semiconductor field effect transistor (MOSFET) have all been used in IVD in brachytherapy (Anagnostopoulos et al., 2003; Sharma et al., 2013; Schultka et al., 2006; Nose et al., 2008). Among these, diodes and MOSFETs have demonstrated promising results while allowing for more practical applications due to their ability to provide online dose readout (Tanderup et al., 2013). The PTW-Freiburg Model 9112 (PTW 9112) semiconductor diode detector array has been developed for rectal IVD during brachytherapy treatment. This commercially available diode has been extensively used in several studies for real-time rectal dose measurement using Cs-137, Ir-192, and Co-60 sources, demonstrating the detector’s dependability for overall treatment checks (Waldhäusl et al., 2005; Tanderup et al., 2006; Allahverdi et al., 2013; Zaman et al., 2014).
In a study conducted by Jamalludin et al., a PTW 9112 rectal diode detector probe was chosen as the applicator, and a MOSkin detector was attached to the probe (Jamalludin et al., 2000). Both detectors were inserted into the rectum during HDR ICBT cervix treatment, allowing comparison of measured and planned rectal doses from both detectors in cervical cancer patients.
The current study aims to use Co-60 to measure the rectal dose during HDR brachytherapy of cervical cancer for actual patient treatments. Using a commercial semiconductor diode probe, online in-vivo dose measurements were taken on a series of twenty-two HDR brachytherapy applications. The differences between measured rectal doses and doses computed by a treatment planning system (TPS) were evaluated.
Materials and Methods
Brachytherapy Equipment
A Bebig HDR brachytherapy treatment unit model SagiNova® Serial Number 050 (Eckert and Ziegler, Germany), was used in this clinical study. This treatment unit was controlled and monitored using the treatment control computer (TCC) system located in the control console. The radiation source used was Bebig Co-60 model Co0.A86 stepping source. The source strength (reference air kerma strength) was provided on the manufacturer’s source certificate. When commissioning the system, the source strength was checked by using a calibrated well-type ionization chamber (Model - SOURCECHECK4π, Type 33005 from M/s PTW, Germany) and electrometer (Model – PTW VIVODOS from M/s PTW, Germany). This chamber was delivered with a certificate having calibration factors for Co-60 provided by a secondary standard dosimetry laboratory. This verification showed discrepancy of less than 3% compared with source certificate. Annual verification of source strength, to verify the purity of the Co-60 isotope, using this calibrated well type ionization chamber shows discrepancies of less than 3% compared to the decay calculation from the treatment planning system (TPS), which was input by the manufacturer.
The Co-60 source has an active core of 0.5 mm in diameter and a central cylindrical active core length of 3.5 mm. The active core is encapsulated by a cylindrical stainless-steel capsule with an external diameter of 1.0 mm.
PTW 9112 in-vivo dosimetry system
A flexible PTW probe (Type 9,112 from M/s PTW, Germany) was used for rectal dose measurement. This probe is comprised of five separate semiconductor diodes surrounded by a rubber encapsulation. The probe as shown in Figure 1 is a 7 mm diameter comprises of five semiconductor diodes are spaced 15 mm apart. In this study, the first diode is located at the distal end of the probe is labelled as R1 (which is 20 mm from the tip of probe), with the four other consecutive diodes labelled as R2 to R5 as shown in Figure 1. This probe was connected to a built-in PTW Vivo dose electrometer via a single pin channel of the treatment unit, which was controlled by the treatment control computer (TCC) system located at control console. This integrated in-vivo dosimetry (IVD) brachytherapy system is capable of providing simultaneous real-time dose measurement during treatment delivery. The recorded measured doses were stored automatically within the TCC system.
Figure 1.
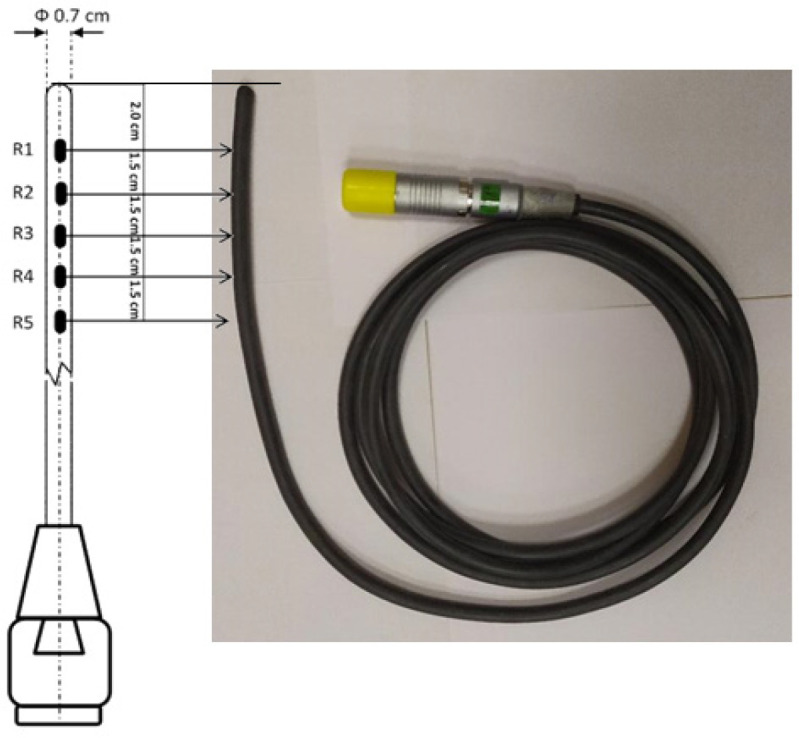
Flexible PTW Rectal Probe (Type 9112 from M/s PTW, Germany) with Semiconductors (5Nos) Position from Distal End
Calibration of PTW 9112 dosimetry system
A polymethyl methacrylate (PMMA) cylindrical afterloading phantom (Type 9193 from PTW, Freiberg, Germany), also known as the Krieger phantom, was used as a medium for the insertion of the rectal probe during measurements prior to the calibration of the PTW 9112 rectal probe under a Co-60 HDR brachytherapy source, as per the recommendation of the rectal probe calibration procedure within the SagiNova® system (Zaman et al., 2014). This phantom, which has a 20 cm diameter and a 12 cm height, was set on a tripod to lessen backscattering. It has four periphery holes that are 8 cm apart from the center.
The setup of rectal diode probe and source applicator on the Krieger phantom during calibration is shown in Figure 2. The aim of diode probe calibration was to obtain calibration factor for each individual diode (R1 to R5), which will be used to calculate the absorbed dose during in-vivo dose measurement. To achieve this, the probe collected the current or charge at a known preset time during Co-60 irradiation. From this measurement, the TCC control software determine the calibration factor for each individual diode. At the TCC control software, real-time measurements taken during patient treatment will be displayed as absorbed dose. An overall uncertainty of 7% has been associated with the use of this probe for in-vivo dosimetry (Waldhäusl et al., 2005). Other physical characteristics for this probe for in-vivo dosimetry have also been reported elsewhere (Ghahramani et al., 2008; Allahverdi et al., 2013).
Figure 2.
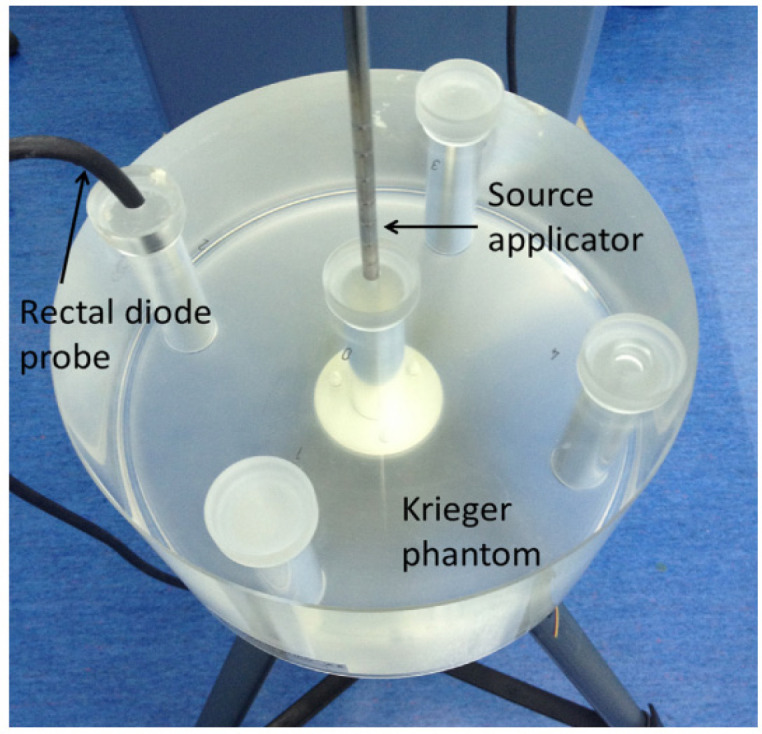
Krieger Phantom (Type 9193 from PTW, Freiberg, Germany) for Calibration of PTW 9112 Rectal Probe Detectors
Phantom in-vivo dose measurement
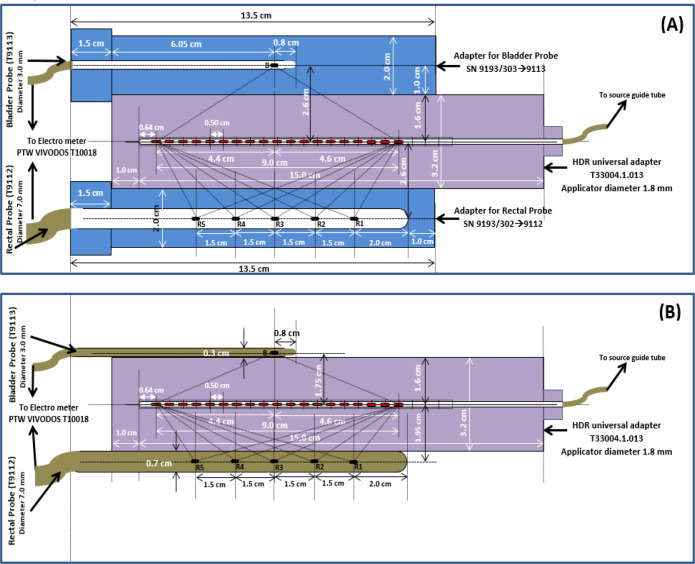
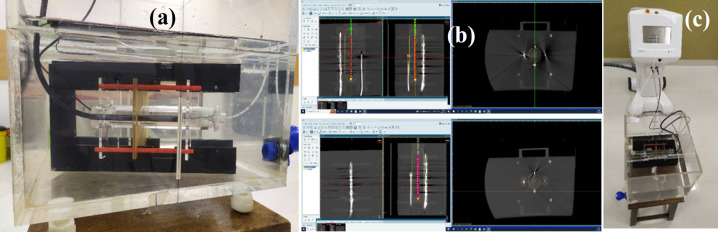
Before proceeding with the patient study, three cylindrical adaptor PMMA plugs, 1) Rectal Probe SN 9193/3029112, 2) Bladder Probe SN 9193/3039113, and 3) HDR universal adapter T33004.1.013 plug, were chosen to assess the response of rectal semiconductor detectors with known doses in water phntom. The rectal and bladder plugs were placed on top and bottom of the universal adapter plug, respectively as shown in Figure 3(a) which depicts the geometric setup, including plugs dimension. The perpendicular distance from the central axis of the universal adapter plug’s axis to the detectors is 2.6 cm with this configuration. In another setup, the rectal and bladder probes were placed on top of the universal adaptor plug (as shown in Figure 3(b)), with the rectum and bladder probes perpendicular to the central axis of the universal adaptor plug at 1.95 cm and 1.75 cm, respectively. The above arrangement was placed in a water phantom (Figure 4(a)), and axial computed tomography (CT) images were obtained with a 16 slice CT Simulator (M/s Wipro GE “High Speed”).
Figure 3.
(a) Rectal and bladder adaptor plugs placement on top of HDR universal adaptor plug. (b) Rectal and bladder probe placement on top of HDR universal adaptor plug
Figure 4.
(a) Assembled rectal, bladder and universal adaptor plugs in water phantom. (b) Representative CT image (coronal and axial) in TPS for dose calculations. (c) The phantom IVD irradiation setup under HDR unit
The obtained CT image data set was then transferred via Digital Imaging and Communications in Medicine (DICOM) network to the SagiPlan® treatment planning system (TPS) (from M/s Eckert and Ziegler, Germany). Rectal dosage points (R1 to R5), which are recognized in axial images, at the middle of each detector, are marked. Three treatment plans were created in TPS with prescribed dosages of 2.0 Gy, 2.5 Gy, and 3.0 Gy to these points while keeping the source dwelling the length of 9.0 cm with step size 0.5 cm in universal adaptor (As shown in Figure 4(b)). The calculated doses by TPS to these points were compared to the actual doses measured while these plans were carried out in the treatment unit (Figure 4(c)). The comparison of bladder dosages is outside the scope of this investigation because the department has access to both plugs (rectal and bladder), which were utilized in the phantom measurement setup described above.
Brachytherapy treatment preparation and simulation
In our center, patients were first treated with 3D external beam radiotherapy (EBRT) to the whole pelvis with prescribed dose of 50 Gy delivered in 25 fractions for 6.5 weeks of treatment duration. After one-week completion of EBRT, three fractionated HDR brachytherapy were administered with a prescribed dose per fraction of 7.0 or 7.5 Gy to high risk clinical target volume (HRCTV).
During each brachytherapy preparation, a Folley catheter balloon containing 7cc of radio-opaque contrast was inserted into the bladder. In each procedure, a computed tomography/magnetic resonance (CT/MR) compatible Fletcher suit applicator set containing different angles of intrauterine tandem and different diameters of ovoid pairs was used to secure the Co-60 source position during irradiation within the uterine cavity and vaginal fornices. The intrauterine tube was inserted into the uterine cavity during each insertion, and the ovoids were placed in the vagina at the level of the fornices.
The rectal diode probe was then inserted into the rectum and secured with an adhesive band to the patient’s body. Following applicator and rectal probe insertion, 3D planning axial images of the pelvis were obtained using a CT simulator with the patient supine and a slice thickness of 3 mm. The image data set was then transferred via DICOM network to the TPS for treatment planning.
Treatment planning, delivery and IVD analysis
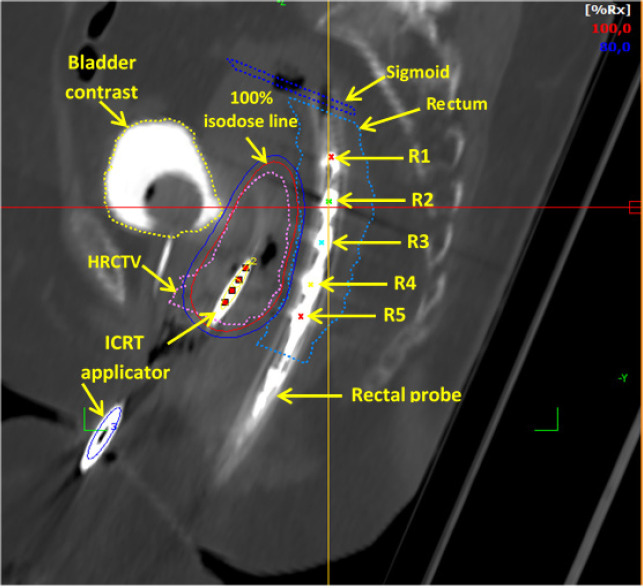
Delineation of target and organs at risk were drawn on 3D CT axial images in TPS. Beginning with applicator reconstruction procedure on digitally reconstruct radiograph (DRR) images, applicators reconstruction was done using applicatory library available in TPS. Center of each semiconductor diode from PTW 9112 probe was identified on the axial CT images, hence being referred as R1, R2, R3, R4 and R5 for diode located at 20, 35, 50, 65 and 80 mm from the probe tip respectively. Figure 5 depicts the identification of detector points (R1 to R5) on TPS images in the reconstructed sagittal plane of CT images. Treatment plans were generated in each application by accounting the EBRT dose into brachytherapy planning to achieve a desired total tumour dose of equivalent dose to 2Gy fractions (EQD2) 80-90 Gy10 (α/β = 10) for high risk CTV (HR-CTV) while keeping the minimum dose to the most exposed 2 cm3 volume (D2cc) of bladder and rectum to total EQD2 90 Gy3 (α/β = 3) and 75 Gy3 (α/β = 3).
Figure 5.
Rectal Probe Diode Positions (R1 to R5) as Seen in the Reconstructed Sagittal Plane of the CT Image
TPS’s auto dwell position and time calculation algorithm was used to determine appropriate source positions with sufficient dwell time for each applicator tube. The dose calculated to the rectal points (R1 to R5) by TPS was recorded. All treatment plans were approved by the treating oncologists, and planned data was sent to the TCC console for treatment execution. Measured doses during treatment delivery were monitored in real-time throughout the session by connecting the PTW 9112 rectal probe to the built-in dosimeter channel to a designated reader. Following the completion of treatment, a report (printout) containing the monitored real-time doses was obtained. The dose deviation, ΔD and percentage dose differences, ΔD% between measured and planned doses are calculated from the equations below:
ΔD= Dmeasured - Dplanned (1)
ΔD (%) = (ΔD/Dplanned)*100 (2)
where
Dmeasured: measured dose during IVD application
Dplanned: TPS planned dose
Results
IVD - Phantom study
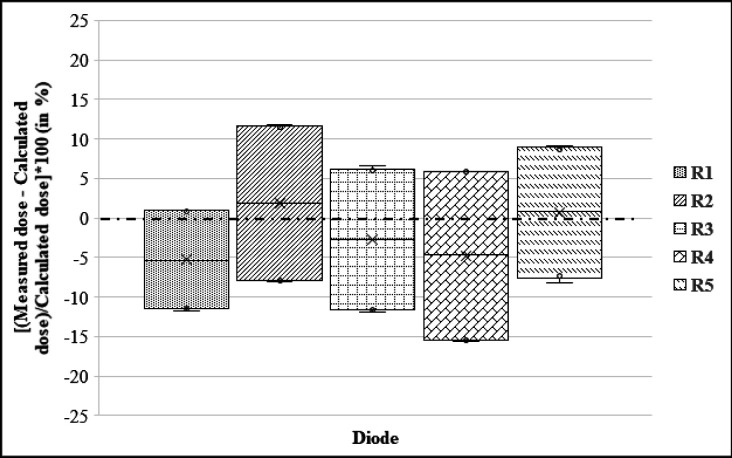
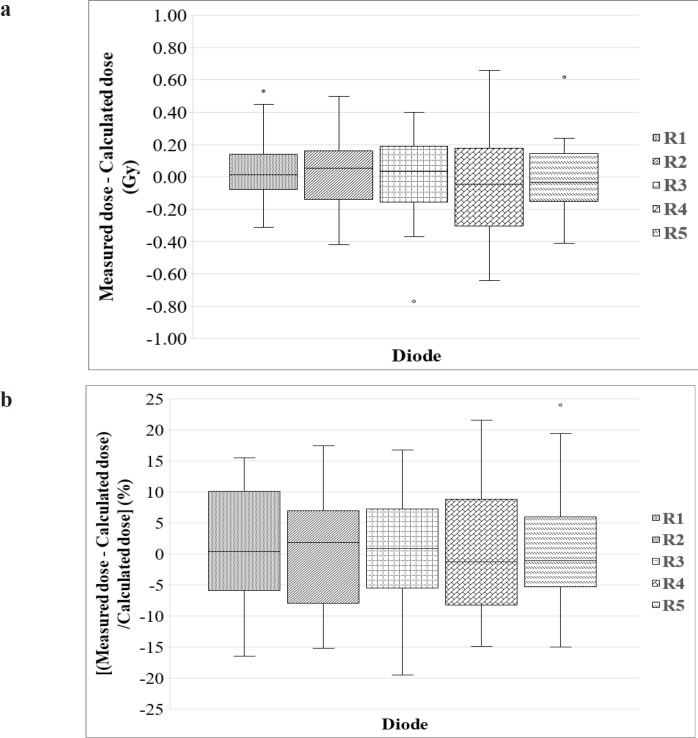
Figure 6 depicts the results of the phantom study, which shows box plots for the percentage difference between measured and calculated dose for each diode (R1 to R5) in the rectal probe from all three prescription dose plans. The median difference is indicated by the dark line inside the box. The box-upper plot’s and lower error bars represent the maximum and minimum values, respectively. As can be seen, the mean ΔD (%) values from these plans were -5.29%, 1.89%, -2.72%, -4.76, and 0.72% for R1, R2, R3, R4, and R3 diodes, respectively. Taking into account all 15 observation points (three prescription dose plans and five rectal points), the overall mean ΔD (%) value with standard deviation (SD) from the phantom study was -2.03%±9.6%.
Figure 6.
Box-Plots of the Percentage Difference between the Measured and Estimated Doses for Each Diode (R1 to R5) in the rectal probe from all three prescription dose plans carried out using the phantom study
IVD - Patients study
Figure 7a and 7b shows the boxplots for the differences between measured and calculated dose for each diode (R1 to R5) in the rectal probe acquired for twenty-two brachytherapy applications respectively. The absolute percentage differences between measured and calculated dose ranged from -19.5% to 24.0% for all diodes. This corresponded to dose differences ranging from -0.77 Gy to 0.66 Gy. Larger differences (as indicated by the range of the dose differences) in the calculated and measured doses were observed for readings recorded by R3 and R4 diodes due to the influence of a number of large maximum dose differences recorded by these diodes. Although the ranges in the dose differences for these diodes were relatively large, the medians were consistent with other diodes. The median percentage differences ranged from 0.4% to 1.3% which corresponded to differences of -0.03 to 0.05 Gy.
Figure 7.
Box-Plots of the (a) absolute difference and (b) percentage difference between measured dose and calculated dose with patients. The dark line inside the box identifies median difference. The upper and lower error bars represent the maximum and minimum values respectively
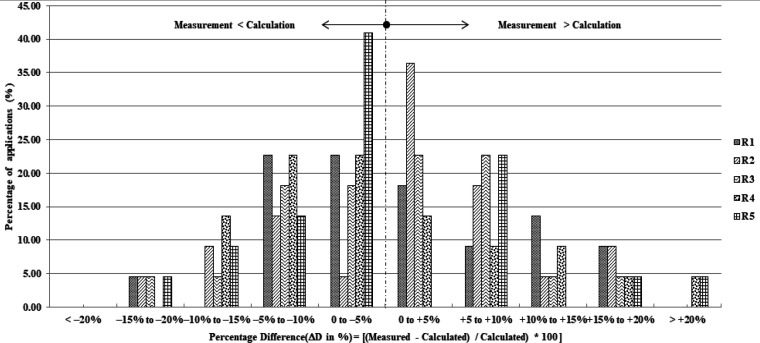
Figure 8 shows a histogram of dose ratios between measured and calculated doses for all diodes. Dose ratio with values larger than zero indicates that the measured dose is larger than the calculated dose. The histogram shows that 11 of 22, 7 of 22, 10 of 22, 13 of 22, and 15 of 22 brachytherapy applications yield higher calculated doses than measured doses at R1, R2, R3, R4, and R5 diodes, respectively. However, 6 of 22, 6 of 22, 4 of 22, 7 of 22, and 5 of 22 brachytherapy applications yield more than 10% at R1, R2, R3, R4, and R5 diodes, respectively.
Figure 8.
Histogram of Dose Ratios between Measured and Calculated Doses for All Diodes
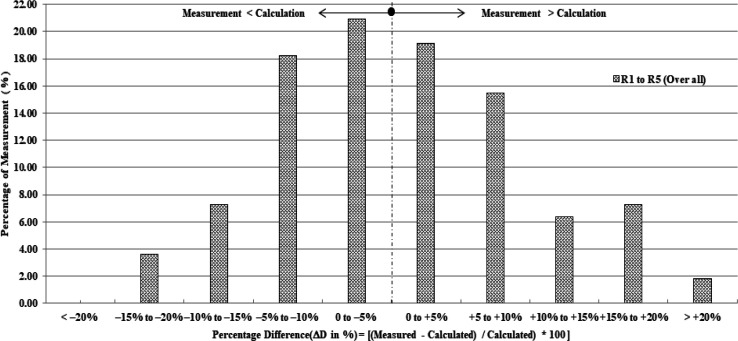
Figure 9 shows the histogram depicts the percentage deviation of measured and calculated doses of all five diodes (categorized into groups of 5% dose difference considering 110 measurements from five diodes of 22 treatment sessions). According to the graph, ΔD (%) values exceeded 10% in approximately 26.4% of measurements (29 out of 110 in 22 applications) when all five diodes were considered.
Figure 9.
Histogram Depicts the Percentage Deviation of Measured and Calculated Doses of All Five Diodes (Categorized into Groups of 5% Dose Difference Considering 110 Measurements from Five Diodes of 22 Treatment Sessions)
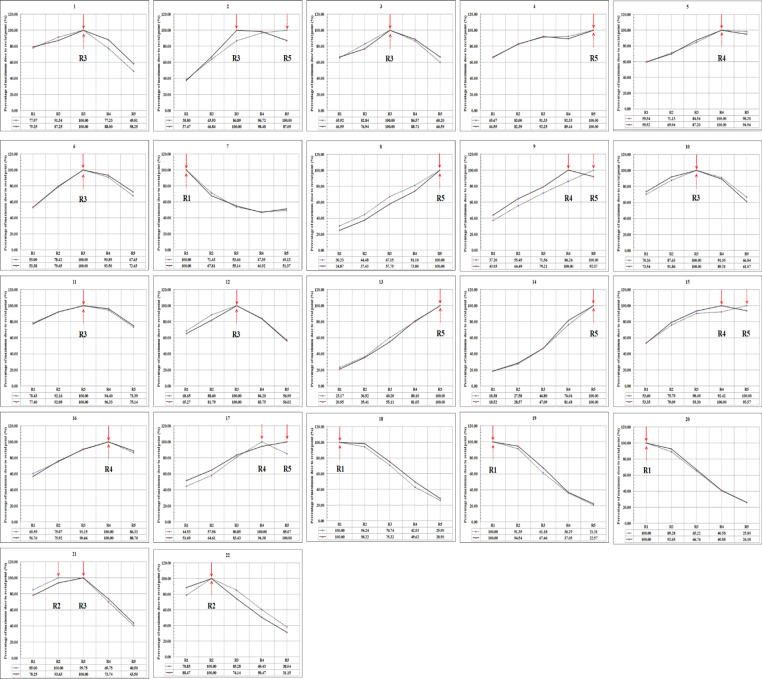
Because the rectal probe was placed within the rectum, there was a possibility of probe displacement between simulation and treatment. Graphs (Figure 10) were created from calculated and measured dose values of each detector by normalizing the dose received by each detector to the maximum dose received (Rmax) in all 22 applications. In each graph, the calculated and measured values were represented by solid and dotted lines, respectively. The location of the Rmax (i.e. normalized value) is indicated by the solid and dotted arrow marks in each graph for computed and measured values, respectively.
Figure 10.
Graphs Depict the Rectal Dose Observed in Calculated (Solid Line) and Measured Data (Dotted Line), normalized to the maximum value (shown with an arrow marks) for each of the 22 ICBT applications with five semiconductor of PTW9112 probe
Figure 10 shows that the Rmax was seen at the same location in computed and measured values at R1, R2, R3, R4, and R5 in 4 of 22, 1 of 22, 6 of 22, 2 of 22, and 4 of 22 applications respectively. However, the location of Rmax in computed and measured values differs in 5 of 22 applications (as seen from the Figure 10, the application nos. 2, 9, 15, 17, and 21). This might be due to the possible displacement of rectal probe between simulation and treatment.
Discussion
The only practical way to check the delivered dose during radiotherapy and brachytherapy is in-vivo dosimetry (Allahverdi et al., 2012). Disparities between doses delivered and doses calculated using TPS can be determined using invivo dosimetry. For in-vivo dosimetry and treatment verification during brachytherapy, various dosimeters have been used (Bansal et al., 2013; Uniyal et al., 2013). The TPS calculation results were used as a reference in this study. If the measured dose does not match the dose calculated during treatment planning, the treatment can be changed to avoid large errors. Unwanted radiation side effects can be avoided, so ICBT should use in-vivo dosimetry (Waldhäusl et al., 2005).
Rectal dosages computed and quantified in this investigation using patient ICBT applications from all diodes had percentage discrepancies ranging from -19.5% to 24.0% (0.7%±9.1%). Although the magnitudes of the percentage variations were somewhat substantial, they were comparable to those of in-vivo rectal dose measurements made while receiving HDR brachytherapy with Ir-192, as reported by other investigators; Waldhäusl et al. reported percentage dose differences of -31% to 90% (mean = 11%) between calculated and measured dose during HDR brachytherapy using Ir- 192 (Waldhäusl et al., 2005). In a similar study, Eich et al. reported differences of -50% to 40% (mean = 4 ± 19%) between calculated and measured doses using diodes (Eich et al., 2000).
A number of factors may contribute to the disparity between calculated and measured rectum doses during brachytherapy. The possibility of diode geometrical shift between simulation and delivery is the most important factor contributing to this. A geometrical shift during treatment can be caused by the patient’s internal organ movement, the diode detector, or the applicators. The shift could be caused by detector movement between the time of CT scan and irradiation. Rectal peristaltic motion or patient movement has also been reported to affect in-vivo dose measurement (Allahverdi et al., 2012; Allahverdi et al., 2013; Waldhäusl et al., 2005; Huh et al., 2007; Alecu et al., 1999). Many studies have reported significant diode displacement, which has been attributed primarily to being a source of error in performing in-vivo dosimetry in brachytherapy.
Rectal dose measurements with a miniature ionization chamber were compared with TPS calculated doses in 86 HDR-ICBT applications of cervical carcinoma in a study by Sha et al., (2011). In 52 patients, the difference between the TPS calculated maximum dose and the measured dose was 5%, 5% to 10% in 26 patients, and 10% to 14% in 8 patients.
Waldhäusl et al. found a more than 10% difference in measured doses versus TPS calculated doses for even minor geometrical shifts of the applicator (Waldhäusl et al., 2005). This was supported by Allahverdi et al., (2012) who confirmed that diode displacement was the cause of the diode’s over response. Furthermore, at close proximity to the source in a high-dose gradient region, any small variation in detector position results in a large difference in dose from what was originally planned. To overcome dose discrepancies caused by geometrical shift, it is recommended that the patient be imaged in real-time using C-arm fluoroscopy to determine the position of diodes in the rectal probe on the TPS just before treatment.
In brachytherapy, using a stainless-steel applicator device is the common procedure. There was a dose attenuation of up to 2% along the transverse plane of the source when the metal applicator was utilised during brachytherapy (Uniyal et al., 2012). This element is not taken into consideration by the TPS algorithm used to calculate dose in brachytherapy planning. As a result, during HDR brachytherapy, differences between calculated and actual doses could happen.
Although the ΔD (%) were significant, the absolute difference was relatively small, with a median difference percentage ranged from 0.4% to 1.3%, or -0.03 to 0.05 Gy, respectively. As seen from the Figure 10, the probable displacement of rectal probe between simulation and treatment can be observed. Table 1 shows the comparison of the results of the current investigation with the available literature for real time in-vivo rectal dose measurements made during HDR/MDR brachytherapy applications using various types of detectors. As seen, our results from this study are in agreement with the published literature.
Table 1.
Comparison of the Results of the Current Investigation with the Available Literature for Real Time in-vivo Rectal Dose Measurements Made During HDR/MDR Brachytherapy Applications Using Various Types of Detectors
| SN | Author (year of publication) |
Source used | Number of applications (n) | Type of Imaging |
Type of detector |
DD (%) Minimum to Maximum (Mean ± SD) |
|---|---|---|---|---|---|---|
| 1 | *Eich et al., (2000) |
Ir-192 | 11 | 2D based | Diodes | -50.0% to 40.0% |
| HDR ICBT | (4.0% ± 19.0%) | |||||
| 2 | *Waldhäusl et al., (2005) |
Ir-192 | 50 | 2D based | Diodes | -31.0% to 90.0% |
| HDR ICBT | (Mean 11.0%) | |||||
| 3 | *Sha et al., (2011) |
Ir-192 | 86 | 2D based | Ionization | <5% (n=52) |
| HDR ICBT | chamber | 5% - 10% (n=26) | ||||
| 10% - 14% (n = 8) | ||||||
| (Mean 3.8%) | ||||||
| 4 | *Allahverdi et al., (2013) |
Cs-137 | 36 | 2D based | Diodes | -85.0% to 36.0% |
| MDR ICBT | -3.00% | |||||
| 5 | *Z.K.Zaman et al., (2014) |
Co-60 | 11 | CT based | Diodes | - 8.5% to 41.2% |
| HDR ICBT | (Mean 2.6%) | |||||
| 6 | **Carrara et al., (2016, 2017) | Ir-192 | 77 | CT based | MOSkin | -16.0% to 19.0% |
| HDR Prostate | ( -- not quoted -- ) | |||||
| 7 | *Jamalludin et al., (2020) |
Co-60 | 18 | CT based | MOSkin | -16.3% to 14.9% |
| HDR ICBT | (-3.2% ± 10.1%) | |||||
| 8 | 18 | Diode | -35.7% to -2.1% | |||
| (RP3) | (-15.5% ± 9.7%) | |||||
| 9 | 48 | Diode | -37.1% to 11.0% | |||
| (RPmax) | (Mean -13.5%) | |||||
| 10 | * Johan et al., | Co-60 | € Phantom | CT | Diodes | -15.6% to 11.8% |
| [Present study] | (-2.0% ± 9.5%) | |||||
| 11 | 22 | CT based | -19.5% to 24.0% | |||
| HDR ICBT | (0.7% ± 9.1%) |
*, Gynecological brachytherapy applications.; **, Prostate brachytherapy applications.; n, number of applications; ICBT, Intracavitary cervix brachytherapy; 2D, Two dimensional (through orthogonal radiographs); HDR, High Dose Rate.; MDR, Medium Dose Rate.; CT, Computed Tomography.; RP3, Dose received to the third diode of rectum probe.; RPmax, Maximum dose received to the rectal point.; DD, Difference of measured and planned doses; SD, Standard Deviation; €, Measurements done in water phantom
In conclusion, in-vivo dosimetry is feasible and can be used to estimate the dose to the rectum during HDR brachytherapy with Co-60, according to our findings. The uncertainties in performing in-vivo dosimetry are similar to those in HDR brachytherapy with Ir-192, with the most significant being the possibility of geometrical shift of measuring detectors between insertion and treatment. Despite these uncertainties, in-vivo dosimetry is useful in providing physicists and other treatment staff with greater confidence in the treatments accuracy. Evaluation of local response of tumor and late rectal toxicities with respect to the cumulative as well maximum rectal doses vs D2cc component will be assessed in the subsequent follow-up of patients. To ensure safe HDR brachytherapy delivery, treating institutions should have their own in-vivo dosimetry quality assurance program.
Author Contribution Statement
JS, CS and DL conducted the experiments. CS was the contributor in literature search, designing and fabrication of the experiment, writing manuscript. CS, JS, AT, SB and AK, has corrected the manuscript subjectively, spelling and grammar checks. CS is the main supervisor of the research and corresponding author. All authors read and approved the final manuscript.
Acknowledgements
Authors would like to express sincere thanks to Ms Pooja, trainee dosimetrist and Mr. Anish undergraduate student of RTT for their active involvement and participation this study.
Ethics approval
This study was approved by the Institutional Ethics Committee (that handles all ethical issues of scientific study proposals), Kasturba Medical College, Mangalore, Karnataka State, India, under the approved protocol No. IEC KMC MLR 03/2022/76.
Consent for publication
Author declares that this study was carried out at Department of Radiation Oncology, Kasturba Medical College (A constituent Institution of Manipal Academy of Higher Education), Mangalore, Karnataka, India. All of the authors declare that they have all participated in the design, execution, and analysis of the paper, and that they have approved the final version. Availability of data and material not applicable.
Availability of data
Not applicable.
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
References
- Alecu R, Alecu M. In-vivo rectal dose measurements with diodes to avoid misadministrations during intracavitary high dose rate brachytherapy for carcinoma of the cervix. Med Phys. 1999;26:768–70. doi: 10.1118/1.598598. [DOI] [PubMed] [Google Scholar]
- Allahverdi M, Jaberi R, Aghili M, Ghahremani F, Geraily G. In vivo dosimetry with semiconductors in medium dose rate (MDR) brachytherapy for cervical cancer. Jpn J Radiol. 2013;31:160–5. doi: 10.1007/s11604-012-0160-x. [DOI] [PubMed] [Google Scholar]
- Allahverdi M, Sarkhosh M, Aghili M, Jaberi R, et al. Evaluation of treatment planning system of brachytherapy according to dose to the rectum delivered. Radiat Prot Dosim. 2012;150:312–5. doi: 10.1093/rpd/ncr415. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos G, Baltas D, Geretschlaeger A, et al. 2003), In vivo thermoluminescence dosimetry dose verification of transperineal 192 Ir high-dose-rate brachytherapy using CT-based planning for the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 57:1183–91. doi: 10.1016/s0360-3016(03)00762-4. [DOI] [PubMed] [Google Scholar]
- Ballester F, Granero D, Perez-Calatayud J, et al. Monte Carlo dosimetric study of the BEBIG Co-60 HDR source. Phys Med Biol. 2005;50:N309. doi: 10.1088/0031-9155/50/21/N03. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Semwal MK, Arora D, et al. A phantom study on bladder and rectum dose measurements in brachytherapy of cervix cancer using FBX aqueous chemical dosimeter. Phys Med. 2013;29:368–73. doi: 10.1016/j.ejmp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Carrara M, Romanyukha A, Tenconi C, et al. Clinical application of MOSkin dosimeters to rectal wall in vivo dosimetry in gynecological HDR brachytherapy. Phys Medica. 2017;41:5–12. doi: 10.1016/j.ejmp.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Carrara M, Tenconi C, Rossi G, et al. In vivo rectal wall measurements during HDR prostate brachytherapy with MOSkin dosimeters integrated on a trans-rectal US probe: comparison with planned and reconstructed doses. Radiother Oncol. 2016;118:148–53. doi: 10.1016/j.radonc.2015.12.022. [DOI] [PubMed] [Google Scholar]
- Chen S-W, Liang J-A, Yang S-N, Liu R-T, Lin F-J. The prediction of late rectal complications following the treatment of uterine cervical cancer by highdose- rate brachytherapy. Int J Radiat Oncol Biol. 2000;47:955–61. doi: 10.1016/s0360-3016(00)00559-9. [DOI] [PubMed] [Google Scholar]
- Chun M, Kang S, Kil H-J, et al. Rectal bleeding and its management after irradiation for uterine cervical cancer. Int J Radiat Oncol Biol. 2004;58:98–105. doi: 10.1016/s0360-3016(03)01395-6. [DOI] [PubMed] [Google Scholar]
- Eich H, Haverkamp U, Micke O, Prott F, Müller R. Dosimetric analysis at ICRU reference points in HDR-brachytherapy of cervical carcinoma. Rontgenpraxis. 2000;53:62–66. [PubMed] [Google Scholar]
- Ghahramani F, Allahverdi M, Jaberi R. Dependency of semiconductor dosimeter responses, used in MDR/LDR brachytherapy, on factors which are important in clinical conditions. Rep Prac Oncol Radiother. 2008;13:29–33. [Google Scholar]
- Granero D, Perez-Calatayud J, Ballester F. Technical note: dosimetric study of a new Co-60 source used in brachytherapy. Med Phys. 2007;34:3485–8. doi: 10.1118/1.2759602. [DOI] [PubMed] [Google Scholar]
- Huh H, Kim W, Loh JJ, et al. Rectum dose analysis employing a multi-purpose brachytherapy phantom. Jpn J Clin Oncol. 2007;37:391–8. doi: 10.1093/jjco/hym032. [DOI] [PubMed] [Google Scholar]
- Jamalludin Z, Jong WL, Malik RA, Rosenfeld AB, Ung NM. Evaluation of rectal dose discrepancies between planned and in vivo dosimetry using MOSkin detector and PTW 9112 semiconductor probe during 60Co HDR CT-based intracavitary cervix brachytherapy. Physica Medica. 2020;69:52–60. doi: 10.1016/j.ejmp.2019.11.025. [DOI] [PubMed] [Google Scholar]
- Nose T, Koizumi M, Yoshida K, et al. In vivo dosimetry of high-dose-rate interstitial brachytherapy in the pelvic region: use of a radiophotoluminescence glass dosimeter for measurement of 1004 points in 66 patients with pelvic malignancy. Int J Radiat Oncol Biol Phys. 2088;70:626–33. doi: 10.1016/j.ijrobp.2007.09.053. [DOI] [PubMed] [Google Scholar]
- Ntekim A, Adenipekun A, Akinlade B, Campbell O. High dose rate brachytherapy in the treatment of cervical cancer: preliminary experience with cobalt 60 radionuclide sourcedA prospective study. Clin Med Insights Oncol. 2010;4:89–94. doi: 10.4137/cmo.s5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino I, Kitamura T, Okamoto N, et al. Late rectal complication following high dose rate intracavitary brachytherapy in cancer of the cervix. Int J Radiat Oncol Biol. 1995;31:725–34. doi: 10.1016/0360-3016(94)00547-8. [DOI] [PubMed] [Google Scholar]
- Palmer A, Hayman O, Muscat S. Treatment planning study of the 3D dosimetric differences between Co-60 and Ir-192 sources in high dose rate (HDR) brachytherapy for cervix cancer. J Contemp Brachyther. 2012;4:52–9. doi: 10.5114/jcb.2012.27952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DW, Kim YS, Park SH, et al. A comparison of dose distributions of HDR intracavitary brachytherapy using different sources and treatment planning systems. Appl Radiat Isot. 2009;67:1426–31. doi: 10.1016/j.apradiso.2009.02.066. [DOI] [PubMed] [Google Scholar]
- Romanyukha AA, Carrara M, Tenconi C, et al. Applications of MOSkin dosimeters for quality assurance in gynecological HDR brachytherapy: an in-phantom feasibility study. Radiat Meas. 2017;106:399–404. [Google Scholar]
- Schultka K, Ciesielski B, Serkies K, et al. EPR/alanine dosimetry in LDR brachytherapy – a feasibility study. Radiat Prot Dosimetry. 2006;120:171–5. doi: 10.1093/rpd/nci528. [DOI] [PubMed] [Google Scholar]
- Sha RL, Reddy PY, Rao R, Muralidhar KR, Kudchadker RJ. Evaluation of rectal dose during high-dose-rate intracavitary brachytherapy for cervical carcinoma. Med Dosim. 2011;36:377–82. doi: 10.1016/j.meddos.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Sharma R, Jursinic PA. In vivo measurements for high dose rate brachytherapy with optically stimulated luminescent dosimeters. Med Phys. 2013:40. doi: 10.1118/1.4811143. [DOI] [PubMed] [Google Scholar]
- Strohmaier S, Zwierzchowski G. Comparison of 60Co and 192Ir sources in HDR brachytherapy. J Contemp Brachyther. 2011;3:199–208. doi: 10.5114/jcb.2011.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanderup K, Beddar S, Andersen CE, Kertzscher G, Cygler JE. In vivo dosimetry in brachytherapy. Med Phys. 2013;40:070902. doi: 10.1118/1.4810943. [DOI] [PubMed] [Google Scholar]
- Tanderup K, Christensen JJ, Granfeldt J, Lindegaard JC. Geometric stability of intracavitary pulsed dose rate brachytherapy monitored by in vivo rectal dosimetry. Radiother Oncol. 2006;79:87–93. doi: 10.1016/j.radonc.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Tenconi C, Carrara M, Borroni M, et al. TRUS probe integrated MOSkin detectors for rectal wall in vivo dosimetry in HDR brachytherapy: In phantom feasibility study. Radiat Meas. 2014;71:379–83. [Google Scholar]
- Uniyal SC, Naithani UC, Sharma SD, Srivastava AK. Radiochromic film dosimetry of rectal inhomogeneity and applicator attenuation in high dose rate brachytherapy of uterine cervix. J Appl Clin Med Phys. 2012;13:3654. doi: 10.1120/jacmp.v13i1.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uniyal SC, Sharma SD, Naithani UC. Dosimetric verification of a high dose rate brachytherapy treatment planning system in homogeneous and heterogeneous media. Phys Med. 2013;29:171–7. doi: 10.1016/j.ejmp.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Waldhäusl C, Wambersie A, Pötter R, Georg D. In-vivo dosimetry for gynaecological brachytherapy: Physical and clinical considerations. Radiother Oncol. 2005;77:310–7. doi: 10.1016/j.radonc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Zaman ZK, Ung NM, Malik RA, et al. Comparison of planned and measured rectal dose in-vivo during high dose rate Cobalt-60 brachytherapy of cervical cancer. Phys Medica. 2014;30:980–4. doi: 10.1016/j.ejmp.2014.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.