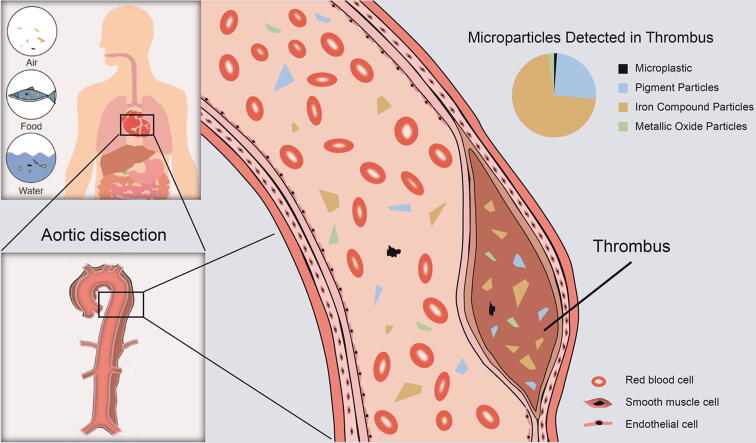
Graphical abstract
Keywords: Microparticle, Pigment, Microplastic, Thrombus, Raman Spectrometer
Highlights
-
•
This study firstly provided detailed photograph and spectrum evidence on particle accumulation in thrombi.
-
•
A quantity of micoparticles including those from synthetic materials such as pigments and plastics were detected in thrombi.
-
•
Particles were all block-shaped and varied from 2.1 to 26.0 μm in size, among which 69% were smaller than 10 μm.
Abstract
Introduction
Environmental microparticle is becoming a global pollutant and the entire population is increasingly exposed to the microparticles from artificial materials. The accumulation of microparticles including microplastics and its subsequent effects need to be investigated timely to keep sustainable development of human society.
Objectives
This study aimed to explore the accumulation of environmental particles in thrombus, the pathological structure in the blood circulation system.
Methods
Patients receiving cardiovascular surgical operations were screened and twenty-six thrombi were collected, digested and filtered. Non-soluble microparticles were enriched on the filter membrane and then were analyzed and identified with Raman Spectrometer. The associations of particle status (presence or absence) or particle number in the thrombus and clinical indicators were examined. One strict quality control-particle detection system was designed to eliminate environmental contaminations.
Results
Among twenty-six thrombi, sixteen contained eighty-seven identified particles ranging from 2.1 to 26.0 μm in size. The number of microparticles in each thrombus ranged from one to fifteen with the median reaching five. All the particles found in thrombi were irregularly block-shaped. Totally, twenty-one phthalocyanine particles, one Hostasol-Green particle, and one low-density polyethylene microplastic, which were from synthetic materials, were identified in thrombi. The rest microparticles included iron compounds and metallic oxides. After the adjustment for potential confounders, a significantly positive association between microparticle number and blood platelet levels was detected (P < 0.01).
Conclusion
This study provides the first photograph and Raman spectrum evidence of microparticles in thrombi. A large number of non-soluble particles including synthetic material microparticles could accumulate in arteries, suggesting that the risk of microparticle exposure was under-estimated and the re-evaluation of its health effects is urgently needed. There will be a series of reports on assessing the health effects of microparticle exposure in humans in the future and this research provided clues for the subsequent research.
Introduction
With the expansion of industrial production and the enhancement of the application of synthetic materials in daily life, the garbage fragments and microparticles resulting from natural degradation are multiplied in the environment. Environmental microparticle is becoming a novel global pollutant and the entire population is increasingly exposed to microparticles from artificial materials. Microparticle pollution and its potential health effects need to be investigated timely in order to keep sustainable development.
Once entering the human body, these microparticles might go into the circulatory system through the digestive organs. Although environmental particles were found in human blood and placenta which is rich in blood vessels [1], [2], the detailed and direct information on microparticles in the blood circulation system has not been elaborated. Due to the physical properties of microparticles, it could be hypothesized that the environmental non-soluble particles have some connections with the thrombus, which is defined as a pathological structure formed within blood vessels.
Thrombosis is the common cause of ischemic heart disease, ischemic stroke, and venous thromboembolism [3] and the etiological cause could be vascular endothelial damage, blood hypercoagulability, and slow blood flow. During the formation and enlargement of the thrombus, it could trap vascular contents and become the reservoir of microparticles [4]. It would be suitable to explore the quantity and types of microparticles in thrombus samples which could reflect the accumulation of particles in the main blood vessels.
With the most updated ultra-precise measuring technique with Raman Spectrometer, particles with the diameter larger than 1 μm could be detected over the last several years. Herein, it is possible to explore the accumulation of non-soluble environmental microparticles in thrombi through rigorous detection and identification. The quantity and types of microparticles in thrombi would be measured and identified to help investigate the accumulation of environmental microparticles in the human body, which provides novel information on promoting human sustainable development in a changing environment.
Material and methods
Ethics statement
This study was carried out in accordance with relevant guidelines and regulations with ethical approval obtained from Nanjing Medical University (Approval No. 2020601).
Quality Control-Particle detection system establishment
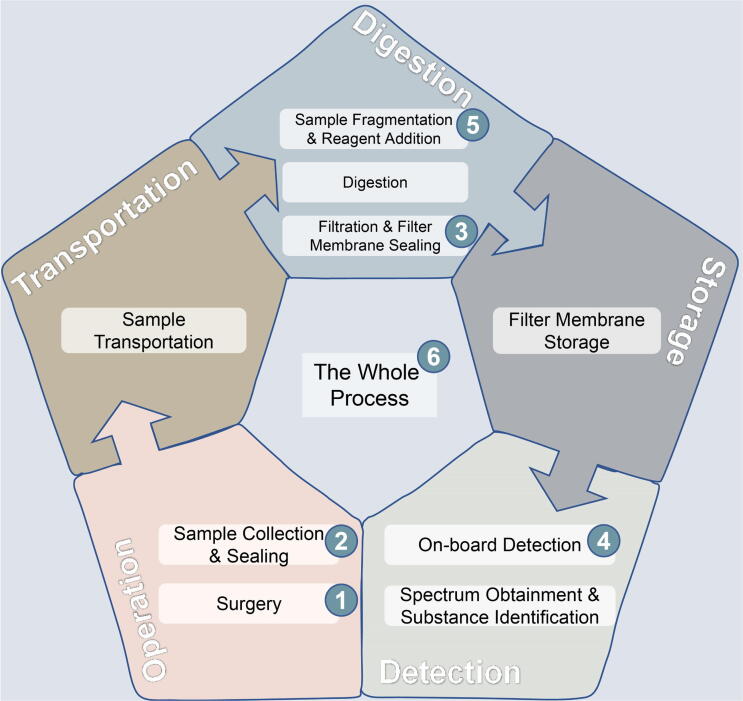
The quality control system was designed before the experiment. Both sample processing and Raman spectrum measurement labs were new on microparticle detection and identification, which excluded the possible contamination from old studies. The spectrum of the filter, which held the samples, was detected. The whole process from sampling to Raman spectrum measurement was considered in detail and a strict Quality Control-Particle Detection (QC-PD) system was designed to inspect five points where there might be risks of contamination: 1. Surgery; 2. Sample Collection & Sealing; 3. Sample Fragmentation & Reagent Addition; 4. Filtration & Filter Membrane Sealing; 5. On-board Detection (Fig. 1). Four kinds of blank controls were set up to control the operation room air, operation of thrombus collection, digestion step air and particle detection room air respectively. In the blank controls, neither samples nor the digestive solution was added. For the operation of thrombus collection control, since microparticles from the environment might enter the sample bottle during the operation of putting one thrombus into that bottle, a control was set at this operation. The bone nail (Double Medical Technology Inc., China) was chosen as the operation object in the control. The bone nails were made of titanium alloy and hence did not contain environmental microparticles including microplastics. They reached the operation room clean-level requirements. After the surgeons finished collecting the thrombus, they put a new bone nail into the control bottle with the same surgical forceps. Two kinds of negative controls were set up (Fig. S1). One was digestive solution control which contained only the digestive solution in the bottles and went to the filtration step directly. The other was the whole process control. In this control, bone nails were used as the operative subjects and were placed in the bottles in the operation room using the same operation tools. The whole process controls went through the whole process including digestion and filtration. Both blank controls and negative controls were operated with thrombus samples in one batch. Five parallels were set for each kind of control. The solutions from the same controls were pooled and filtered. Filter membranes were sealed for particle detection later. More details were provided in supplementary methods. Contrast agent components during thrombus imaging were also analyzed to exclude the possibility of environmental particle contamination from applied agents.
Fig. 1.
Quality Control-Particle Detectionsystem during whole process from sampling to Raman spectrum measurement. Five points of possible contamination were inspected (1–5). The whole process control (6) were set for the whole operation with the bone nails as the operative subjects. The first four controls (1–4) were blank controls, the last two (5–6) were negative controls.
Thrombus samples collection
Participants were patients receiving emergency surgical operations due to arterial dissection or acute arterial embolism of lower extremities in Nanjing First Hospital, Nanjing, China. Inclusion criteria were: 1. The patients whose thrombus could be taken out fully in the surgery (about 1 g in weight) and could be immediately transferred into the sample bottle; 2. The patients who were not implanted with any artificial materials (such as artificial graft, vascular stent and artificial bone) before the surgery; 3. The patients who did not take any diagnostic or therapeutic agents transported by nanomaterials before the surgery. After the screening, a total of twenty-six patients (twenty-four for arterial dissection and two for acute arterial embolism of lower extremities) were included in this study from July 2019 to November 2020. All the participants provided informed consent. Demographic information, medication history, occupation details and lifestyle (including body tattoos, water drinking preferences, smoking and drinking frequencies) were collected by medical personnel. Blood pressures at the upper left limb, left lower limb, upper right limb and right lower limb were measured. An automatic blood analyzer (XN-1000 and CS-5100, SYSMEX, Japan) was used to measure blood lipid (triglycerides, high-density lipoprotein, low-density lipoprotein, apolipoprotein, lipoprotein) levels, platelet level, and D-dimer, plasma prothrombin time, activated partial plasma thromboplastin time, and plasma thrombin time before surgery. Thrombus samples were sealed in glass tubes, transferred to the lab and recoded.
Sample digestion
Thrombus identification numbers were provided by a clinical research associate and thrombus samples were recoded before digestion. Thrombus sample collection, digestion and microparticle detection and identification were double-blinded. The laboratory research associate in charge of sample digestion and Raman spectrum measurement were masked to the information associated with the thrombi. All samples were handled in the biological safety cabinet (BSC-1300IIA2, ISO4, Airtech) in an ultra-clean room to preclude any microparticle exposure from the environment. Clean cotton lab coats and sterile gloves were worn during the whole experiment. Before the experiment, all glass devices and containers were immersed completely in anhydrous ethanol (Analytical reagent, Sinopharm Chemical Reagent Co., Ltd, China), sonicated for 10 min, then thoroughly washed with ultrapure water (Ultrapure water system, Milli-Q, IQ7000, BIOasis) three times and wrapped in aluminum foil for further use. All of the solutions were filtered with 0.7 μm-pore glass filter (0.7 μm glass fiber membrane, Grade GF/F, Whatman, UK) and stored in glass containers. The biosafety cabinet was turned on to run for 20 min before digestion. 30% KOH solution (Analytical reagent, Sinopharm Chemical Reagent Co., Ltd, China) was added in the glass tube containing the thrombus and then alkaline hydrolysis of the thrombus sample was processed at 60 °C for 4 h and room temperature for 48 h. Finally, the hydrolyzed thrombus sample was filtered with the 0.7 μm-pore glass filter. The filter membrane was sealed with specially designed containers and marked. All glass devices and containers were immersed in anhydrous ethanol, sonicated, washed with ultrapure water, and wrapped in aluminum foil again for the next sample digestion.
Raman spectrum measurement and particle identification
The LabRAM HR Evolution Raman spectrometer (HORIBA Scientific, France) equipped with a 785 nm diode laser and 600 lines/mm diffraction grating was first calibrated using an internal silicon wafer with a characteristic band at 520.7 cm-1. Raman spectrum of each particle on the filter was given by Raman spectrometer (spectra range 200–3000 cm−1, acquire time 20 s). Spectra data of 118 particles detected in the thrombi were collected by a LabSpec6 software and were compared with two Raman spectral libraries SLOPP Library of Microplastics [5] and KnowItAll software (BioRad Laboratories, Inc.). The libraries of KnowItAll and SLOPP Library of Microplastics are collections of Raman spectra of compounds. One Raman spectrometer was used to detect the Raman signals of particles and the data were imported into the library software which automatically analyzed the characteristic peaks and peak intensities of the particle spectra with a similarity (classical) algorithm, and compared them with those in the library to obtain a series of spectrum candidates and corresponding HQI values. These matched spectra were filtered based on substance characteristic peaks. The spectrum with the highest HQI value was chosen and the substance was determined.
Microparticle identification was certain when the corresponding Hit Quality Index (HQI) was above 70 [6]. Eighty-seven particles with HQI larger than 70 were analyzed further. Plastic characteristic peaks of the Raman spectrum in most pigment particles were hard to be detected since aging in the environment weakened the peak value and the spectra of pigments and plastics interfered with each other [7]. The identities of phthalocyanine and LDPE microparticles were double-checked manually after being compared to their reference spectra [8], [9]. The associations between microparticle number and demographic information and clinic cardiovascular indicators were analyzed.
Statistical analysis
Baseline characteristics were presented as means (standard deviation) for continuous variables and counts (percentage) for categorical variables. Mann-Whitney U test and chi-squared test were used to compare the distribution of baseline characteristics including demographic information between groups with or without particles in the thrombus. Multiple linear regression was used to examine the associations of particle status (presence or absence) or particle number in the thrombus and clinical indicators. All the multiple linear models were adjusted for age, gender (female/male), smoking status (yes/no) and drinking status (yes/no). The R software (version 4.1.0 for windows, Vienna, Austria) was used for all statistical analyses. Two-sided P < 0.05 was considered to be statistically significant.
Results
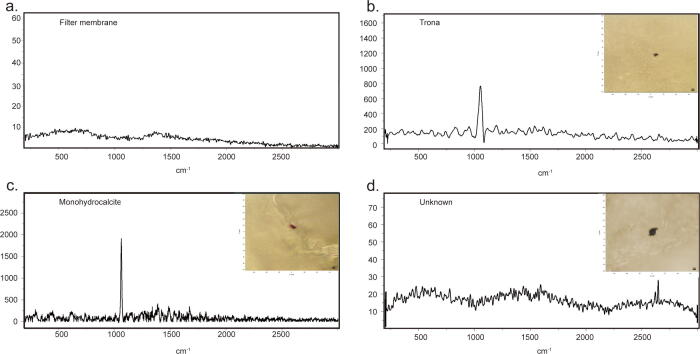
Eleven microparticles were detected in the controls in our Quality Control-Particle Detection System. Eight were identified as trona or monohydrocalcite which might be minerals from the digestion environment [10], and were excluded from the sample particle analysis. The rest three particles were not matched with any reference in the libraries and thus were labeled as “unknown” (Fig. 2). No microparticle was found in thrombus imaging contrast agent components.
Fig. 2.
Raman spectra of filter and particles detected in the controls of Quality Control-Particle Detection system. a. Filter membrane spectrum. b-d. Representative Raman spectra and photos of two types of particles and unknown particles detected in the controls in Quality Control-Particle Detection system.
Patient demographic information, medication history, occupation details, lifestyle (body tattoos, water drinking preferences, smoking and drinking frequencies) were shown in Table 1 and Table S1. The median age of patients enrolled was 56.5 years and nineteen patients (73.08%) were male. The occupation of the patients varied and covered different professions which were not associated with plastic production and processing. Ten patients had working periods of more than ten years. Thirteen patients preferred cold boiled water only and two had body tattoos. According to the diagnostic criteria of hypertension (European Society of Hypertension), five patients were diagnosed to have hypertension stage I, eight were hypertension stage II and seven were hypertension stage III (Table S2-S3). The plasma prothrombin time in patients ranged 1.9–17.6 sec, with a median of 11.6 sec. Activated partial plasma thromboplastin time in patients ranged 21.8–40.4 sec, with a median of 28.1 sec. Plasma thrombin time in patients ranged from 14.6 to 55.4 sec with a median of 18.3 sec. Blood platelet levels in patients ranged 75–312 × 109/L with the median 158 × 109/L. Blood D-dimer levels ranged from 0.30 to more than 50.00 μg/ml with a median of 6.26 μg/ml (Table S4).
Table 1.
Demographic information including occupation, water drinking preference, body tattoos, surgery duration and number of particles detected in thrombus in patients.
| ID | Age | Gender | Occupation | Working years | Daily water drinking preference | Tattoos (Yes / No) | Number of particles detected in thrombus |
|---|---|---|---|---|---|---|---|
| 01 | 49 | Male | Public health physician | greater than 30 | Cold boiled water | N | 0 |
| 02 | 51 | Male | Porter | – | Cold boiled water | N | 0 |
| 03 | 63 | Male | PE teacher | greater than 30 | Green tea | N | 2 |
| 04 | 35 | Male | Office staff | 10 | Cold boiled water | N | 0 |
| 05 | 69 | Male | General practitioner | 40 | Cold boiled water | N | 0 |
| 06 | 72 | Male | Porter | 10 | Cold boiled water | N | 2 |
| 07 | 70 | Female | Farmer | – | Cold boiled water | N | 1 |
| 08 | 38 | Male | Unemployed | – | Tea | Y | 0 |
| 09 | 44 | Male | Garmen | 10 | Cold boiled water | N | 0 |
| 10 | 69 | Male | Porter | 20 | Tea | N | 0 |
| 11 | 45 | Male | Driver | – | Soft drinks; Cold boiled water; Tea | N | 2 |
| 12 | 68 | Female | – | – | – | – | 5 |
| 13 | 55 | Male | Electrician | 2 | Cold boiled water | N | 5 |
| 14 | 70 | Female | Farmer | – | Cold boiled water | N | 9 |
| 15 | 42 | Male | Manager | greater than 10 | Cold boiled water | N | 13 |
| 16 | 66 | Male | Unemployed | – | Tea | N | 11 |
| 17 | 39 | Male | Office staff | – | Tea | N | 5 |
| 18 | 32 | Male | Advertisement designer | 5 | Soft drinks | Y | 15 |
| 19 | 52 | Female | Unemployed | – | Cold boiled water | N | 6 |
| 20 | 65 | Female | Farmer | – | Cold boiled water; Milk | N | 0 |
| 21 | 55 | Female | – | – | – | – | 1 |
| 22 | 57 | Male | Stevedore | greater than 10 | Tea | N | 7 |
| 23 | 74 | Female | – | – | – | – | 0 |
| 24 | 71 | Male | Policeman | 40 | Cold boiled water | N | 0 |
| 25 | 57 | Male | Self-employed | – | Cold boiled water | N | 1 |
| 26 | 58 | Male | Self-employed | – | Tea | N | 2 |
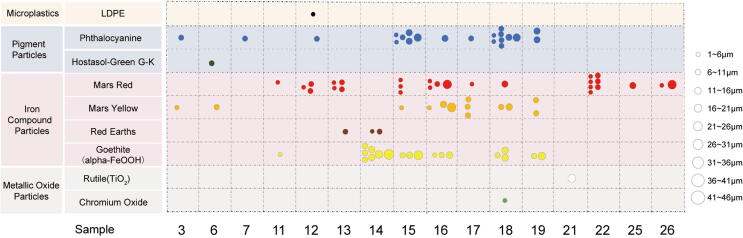
Of the twenty-six thrombi, sixteen contained a total of eighty-seven identified particles after screening (Fig. 3; Table S5; Fig. S2). The maximum of particles in one thrombus was fifteen. The smallest particle was 2.1 μm in diameter. Sixty particles had diameters smaller than 10.0 μm. There were only six particles larger than 20.0 μm and the largest diameter could reach 26.0 μm. The number of microparticles in each thrombus ranged from one to fifteen with the median reaching five. All the particles found in this study were irregularly block-shaped (Fig. S3).
Fig. 3.
Distribution and characteristics of microparticles in thrombus samples.
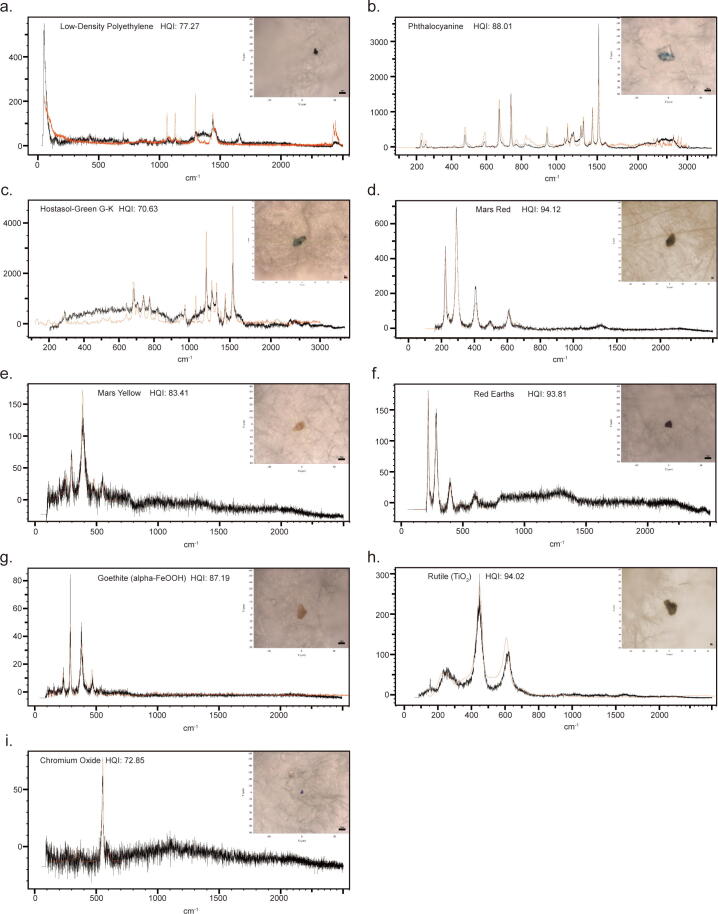
Raman spectrum of each particle was carefully compared with the Raman spectrum reference and twenty-one phthalocyanine particles and one microplastic (Low-Density Polyethylene, LDPE) were identified (Fig. 4). There was one occasional pigment Hostasol-Green G-K. Iron compound group occupied the majority of particles which included Mars Red (twenty-seven particles), Red Earths (three particles), Mars yellow (thirteen particles), and Goethite (nineteen particles). The rest particles were iron-free metallic oxides, including Rutile (TiO2, one particle) and Chromium oxide (one particle). The largest particle was Goethite (alpha-FeOOH) while the smallest one was Mars Red. Thrombus samples could contain only one type of particle (such as seven Mars Red particles found in one thrombus sample) or mixed types of particles. One thrombus sample had as many as five types of particles. Most samples contained Mars Red, Mars Yellow, and phthalocyanine particles. The iron compound pigment group had a large particle size span of 2.1–26.0 µm. The sizes of occasional pigment and iron-free metallic oxide particles were lower than 20 µm.
Fig. 4.
Raman spectra (in black) and photos of representative microparticles of nine kind materials in human thrombi. Reference Raman spectra were shown in red.
The patient having the LDPE particle was not diagnosed with severe gastrointestinal and respiratory diseases and did not have special occupational and lifestyle exposure to plastics. Therefore, the microplastic source could not be inferred. It showed that this microplastic was not accompanied by any specific particles (Fig. 4).
Particle numbers and types in patients were tried to associate with their demographic information such as occupation and lifestyle (daily water drinking preference and body tattoos) (Table S6) but no significant relationships were observed.
Associations of particle status (presence or absence) and clinical indicators were shown in Table S7. Compared to the particles absent group, patients with particle present tended to have higher platelet levels (βadjusted = 41.13, 95 %CI: 9.62–72.64, P < 0.05) after adjusting for potential confounders. Similarly, a positive association between particle number and platelet levels was found (βadjusted = 4.78, 95 %CI: 2.31–7.25, P < 0.01) (Table S8). No other clinical indicators were found to be significantly associated with the particle status (presence or absence) and particle number.
Discussion
In this study, eighty-seven environmental microparticles were detected and identified in thrombi with Raman Spectrometer. Among the microparticles identified, twenty-one phthalocyanine particles and one low-density polyethylene microplastic were definitely from synthetic materials.
Microparticle exposure in the environment was widely investigated. However, the attempt to observe microparticles in humans was just started recently. Some studies applied Inductively Coupled Plasma Mass Spectrometer and Energy Dispersive Spectrometer to detect the elemental composition of tissues, thereby drawing conclusions on the presence of exogenous substances in human, but the direct analysis of the number, size and morphology of exogenous particles were lacking. The detection with Raman spectrometer focuses on the particle’s physical properties, collecting information on particle size, color and surface morphology. Although direct photograph and spectrum evidence can be obtained, this microparticle detection method is very time-consuming due to the large number and variety of particles in the human body, which limits its application in particle observation in human tissue. Another concern was whether the particles detected were from tissue samples or not. In our study, a strict quality control-particle detection system was applied to exclude environmental microparticle contamination. In order to increase the credibility of particle identities, after the comparison between the spectra of detected particles and chemical spectra libraries, only particles with HQI larger than 70 were further analyzed individually.
The majority of the microparticles found in this study were erythrocyte-sized, allowing them to circulate in the blood system before being trapped in the thrombi [11]. Theoretically, large particles could not pass through the epithelial cell layer, thus the detected large particles might probably result from the clotting of tiny particles [12].
Pigment is an organic or inorganic particulate substance added to the polymer matrix to give the plastic a specific color [13], and pigment particles in thrombi might come from daily plastic exposure and usually were classified as microplastics [14]. According to the classification method in the environmental particle detection [15], pigmented microparticles in this study were strictly grouped into microplastics and pigment particles based on their spectra, which increased the preciseness and rigorousness of the microparticle identification.
Fiber-shaped pieces were reported to occupy a high percentage of particles, especially the microplastics found in water systems and fish [16], [17]. However, the particles we found in thrombi were all block-shaped. It could be hypothesized that the slender shape of fibers might lead to very different physical properties which hinder the entrance into the blood system.
Although iron oxide and hydroxide particles could come from the direct oxidation of iron ions [18], the large number and big size of iron compound particles found in this study ruled out the possibility of their natural formation in the body. Since the patients taking any diagnostic or therapeutic agents transported by nanomaterials were not recruited in our study and contrast agent components in thrombus imaging were analyzed to exclude the possible particle contamination, the iron compound particles might be from non-iatrogenic exogenous resources.
Phthalocyanine particles were firstly reported to be detected in thrombi. Phthalocyanine, predominantly copper phthalocyanine, is the synthetic macrocycle with the nature of stability [19], and is commonly used in the chemical pigment, plastic, dye and ink industry. It might enter the body mainly through direct ingestion or inhalation. Phthalocyanine-pigmented microplastics were found in fish meals [20]. The presence of phthalocyanine in thrombi suggests the re-evaluation of its health effect urgently.
Pigment particles Hostasol-Green G-K, iron-free metallic oxide particles of rutile and chromium oxide were occasionally detected while iron compound particles accounted for 71% of the total in thrombi. The high ratio of pigment microparticles to microplastics found in this study drew attention to the daily consumption of these products by the population. However, detailed information on the comparison of pigment and plastic production and usage could not be obtained at present. Karbalaei Lab detected particles larger than 150 µm in commercial marine fish and found that 76.8% of particles were plastic polymers and 5.4% were pigments [21]. Most particles detected in our study were smaller than 20 µm and were pigment microparticles. According to Ivleva and her colleagues’ research on microparticles in limnetic ecosystems, the number of pigment particles was larger than that of microplastics when particle size was below 50 µm, and pigment particles to microplastics ratio continued to increase with the decrease of the particle size [15]. This finding supports the high ratio of pigment particles to microplastics in our detection.
Microplastic pollution has received widespread attention in recent years. However, the detection of microplastics in organisms, especially in humans, has been stalled due to technical limitations. It was the first time to detect the microplastic in the thrombus although only one particle was identified as LDPE which is mainly used in agricultural films, medical devices, pharmaceuticals and food packaging materials and so on [22]. LDPE was also found in water and sediment of the area where this patient lives [23]. However, microplastics were also detected in the soil and water bodies where the other patients live [24], [25]. Compared to the others, the patient with LDPE did not receive any special treatment before and during the surgery. Therefore, it is hard to determine the potential LDPE exposure pathway for this patient. What’s more, the exposure to microparticles such as microplastics causes concern in society and more and more studies focus on environmental microparticles not only in aquatic animals such as fish [26], [27], [28], but also in daily foods and drinking. Scientists found that microparticles could be found in salts, beer, honey and daily drinking water [24], [29], [30]. Recently, one paper published by one corresponding author of this manuscript reported that microplastic could be untaken in the corn plant [31]. As a result, the particle exposure sources of our patients might be varied. Occupational microparticle exposure also is worthy of attention. No occupation-related particle exposure was identified in our study, but expanding sample size and further particle detection in human body fluids might lead to breakthroughs.
This study focused on microparticle internal exposure especially in thrombus and could not provide more information about microparticle resources. However, our results suggested a novel mechanism on thrombosis which included the involvement of exogenous factors.
Two types of thrombi, formed in aortic dissection and acute arterial embolism of lower extremities, were collected in this study when patients were life-threatening and required immediate surgeries. Aortic dissection refers to the intima gradually peeling off and expanding, forming two cavities in the artery [32]. The blood in the false cavity cannot flow quickly and fibrinolysis and clotting reactions happened in the dissection area, forming a thrombus [33]. Combined with the fact that cells tend to grow on artificial materials [34] and environmental particles could adhere to endogenous tissue such as hemoglobin or serum proteins [35], it is reasonable to hypothesize that thrombus could be formed with environmental particles as the core and the initial thrombus could continuously attract the particles in the blood, enlarging the thrombus. What’s more, increasing particles in the blood system might elevate the chance of collision among initial thrombi, platelets and particles, which speeds up thrombosis. Although the positive relationships between blood platelets levels and microparticle number in thrombus found in our study supported this hypothesis (Table S7 and Table S8), the specific correlation between thrombus and particles needs to be verified by further research with a bigger sample size.
In our study, the specific position and shape of the thrombus in the cavity or vein could not be recorded on the spot due to the urgency of the operation, thus the specific location of the particles in the thrombus could not be obtained. Particle identification was determined only based on its spectrum and we did not focus on its chargeability and substances adhered such as heavy metals and organic pollutants. The current lab technique limits the close observation on the interaction of particles and platelets in blood circulation. Although the results of our study could not provide the accurate causes of thrombosis, it proved the existence of environmental microparticles in the thrombus unprecedentedly which allows us to explore the precise nature of thrombus from a perspective of environmental microparticle exposure. The hypotheses and theories speculated from our results could be tested precisely with the increase in sample size and advancement of technology in the near future.
Conclusion
The hazard induced by ultra-fine artificial particles such as microplastics and pigment particles in the environment has not been thoroughly elaborated. In this study, a novel, strict and reliable microparticle detection method with Raman Spectrometer was built up. Clear photographs and Raman spectra of microparticles in this study firstly validated the accumulation of exogenous particles including pigment microparticles and microplastics in thrombi. The results of our findings indicated that the effects of exogenous factors on thrombosis could not be ignored. Whether exogenous non-soluble particles contribute to the formation, enlargement and speeding up of the thrombosis should be investigated to explore the potential involvement of exogenous microparticles in thrombosis.
Since the global environment changes with the fourth industrial revolution, human understanding of environmental pollution is constantly being updated. The development of science and technology has also made the detection of particles more accurate. A general direction of future research is to combine the exposure of microparticle pollutants with cardiovascular disease mechanisms, to re-examine and re-evaluate the effects of microparticle pollution especially pigment microparticles and microplastics on human health. Although up till now the involvement of environmental microparticles on adverse human health effects was only hypothesized, this study provided clues on inspecting environmental microparticles’ health risks brought by global environmental changes.
Compliance with Ethics Requirements.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients included in the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Prof. Xiangliang Pan from Zhejiang University of Technology, China is acknowledged for his kind help on the Raman spectrum measurement. Mr. Chao Dong, Ms. Qiurun Yu, Mr. Qing Wang, Mr. Yuepei Zhang and Mr. Zhaofeng Liu from Nanjing Medical University, China are acknowledged for their great help on data analysis and picture production.
This research was funded by China-U.S. Program for Biomedical Collaborative Research grant NSFC-NIH 81961128022 and Major Project of the National Natural Science Foundation of China 41991330.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.09.004.
Contributor Information
Yankai Xia, Email: yankaixia@njmu.edu.cn.
Yongming Luo, Email: ymluo@issas.ac.cn.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Ragusa A., Svelato A., Santacroce C., et al. Plasticenta: first evidence of microplastics in human placenta. Environ Int. 2021;146 doi: 10.1016/j.envint.2020.106274. [DOI] [PubMed] [Google Scholar]
- 2.Leslie H.A., van Velzen M.J.M., Brandsma S.H., Vethaak A.D., Garcia-Vallejo J.J., Lamoree M.H. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. doi: 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- 3.Violi F., Pastori D., Pignatelli P., Carnevale R. Nutrition, thrombosis, and cardiovascular disease. Circ Res. 2020;126:1415–1442. doi: 10.1161/CIRCRESAHA.120.315892. [DOI] [PubMed] [Google Scholar]
- 4.Santilli F., Marchisio M., Lanuti P., Boccatonda A., Miscia S., Davì G. Microparticles as new markers of cardiovascular risk in diabetes and beyond. Thromb Haemost. 2016;116:220–234. doi: 10.1160/th16-03-0176. [DOI] [PubMed] [Google Scholar]
- 5.Munno K., De Frond H., O'Donnell B., Rochman C.M. Increasing the accessibility for characterizing microplastics: introducing new application-based and spectral libraries of plastic particles (SLoPP and SLoPP-E) Anal Chem. 2020;92:2443–2451. doi: 10.1021/acs.analchem.9b03626. [DOI] [PubMed] [Google Scholar]
- 6.Guedes A., Ribeiro H., Fernandez-Gonzalez M., Aira M.J., Abreu I. Pollen raman spectra database: application to the identification of airborne pollen. Talanta. 2014;119:473–478. doi: 10.1016/j.talanta.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 7.Dong M., Zhang Q., Xing X., Chen W., She Z., Luo Z. Raman spectra and surface changes of microplastics weathered under natural environments. Sci Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139990. [DOI] [PubMed] [Google Scholar]
- 8.Amsterdam S.H., Stanev T.K., Zhou Q., et al. Electronic coupling in metallophthalocyanine-transition metal dichalcogenide mixed-dimensional heterojunctions. ACS Nano. 2019;13:4183–4190. doi: 10.1021/acsnano.8b09166. [DOI] [PubMed] [Google Scholar]
- 9.Liu J., Zheng T., Tian Y. Functionalized h-BN nanosheets as a theranostic platform for SERS real-time monitoring of microRNA and photodynamic therapy. Angew Chem Int Ed Engl. 2019;58:7757–7761. doi: 10.1002/anie.201902776. [DOI] [PubMed] [Google Scholar]
- 10.Baconnier S., Lang S.B., Polomska M., Hilczer B., Berkovic G., Meshulam G. Calcite microcrystals in the pineal gland of the human brain: first physical and chemical studies. Bioelectromagnetics. 2002;23:488–495. doi: 10.1002/bem.10053. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi K., Yamada S., Hayashi H., Sakamoto W., Yogo T. Red blood cell-like particles with the ability to avoid lung and spleen accumulation for the treatment of liver fibrosis. Biomaterials. 2018;156:45–55. doi: 10.1016/j.biomaterials.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 12.Yuge R., Ichihashi T., Miyawaki J., Yoshitake T., Iijima S., Yudasaka M. Hidden caves in an aggregate of single-wall carbon nanohorns found by using Gd2O3 Probes. J Phys Chem C. 2009;113:2741–2744. doi: 10.1021/jp810121a. [DOI] [Google Scholar]
- 13.Glowacki E.D., Voss G., Sariciftci N.S. 25th anniversary article: progress in chemistry and applications of functional indigos for organic electronics. Adv Mater. 2013;25:6783–6800. doi: 10.1002/adma.201302652. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W., Dong Z., Zhu L., Hou Y., Qiu Y. Direct observation of the release of nanoplastics from commercially recycled plastics with correlative raman imaging and scanning electron microscopy. ACS Nano. 2020;14:7920–7926. doi: 10.1021/acsnano.0c02878. [DOI] [PubMed] [Google Scholar]
- 15.Imhof H.K., Laforsch C., Wiesheu A.C., et al. Pigments and plastic in limnetic ecosystems: a qualitative and quantitative study on microparticles of different size classes. Water Res. 2016;98:64–74. doi: 10.1016/j.watres.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Pivokonsky M., Cermakova L., Novotna K., Peer P., Cajthaml T., Janda V. Occurrence of microplastics in raw and treated drinking water. Sci Total Environ. 2018;643:1644–1651. doi: 10.1016/j.scitotenv.2018.08.102. [DOI] [PubMed] [Google Scholar]
- 17.Huang J.S., Koongolla J.B., Li H.X., et al. Microplastic accumulation in fish from Zhanjiang mangrove wetland. South China Sci Total Environ. 2020;708 doi: 10.1016/j.scitotenv.2019.134839. [DOI] [PubMed] [Google Scholar]
- 18.Kashyap S., Woehl T.J., Liu X., Mallapragada S.K., Prozorov T. Nucleation of iron oxide nanoparticles mediated by Mms6 protein in situ. ACS Nano. 2014;8:9097–9106. doi: 10.1021/nn502551y. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J., Li W., Chen Y., et al. A monochloro copper phthalocyanine memristor with high-temperature resilience for electronic synapse applications. Adv Mater. 2021;33 doi: 10.1002/adma.202006201. [DOI] [PubMed] [Google Scholar]
- 20.Germanov E.S., Marshall A.D., Bejder L., Fossi M.C., Loneragan N.R. Microplastics: no small problem for filter-feeding megafauna. Trends Ecol Evol. 2018;33:227–232. doi: 10.1016/j.tree.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Karbalaei S., Golieskardi A., Hamzah H.B., et al. Abundance and characteristics of microplastics in commercial marine fish from Malaysia. Mar Pollut Bull. 2019;148:5–15. doi: 10.1016/j.marpolbul.2019.07.072. [DOI] [PubMed] [Google Scholar]
- 22.Tournier V., Topham C.M., Gilles A., et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020;580:216–219. doi: 10.1038/s41586-020-2149-4. [DOI] [PubMed] [Google Scholar]
- 23.Z, Li, CM, Gao, JL, Yang et al, [Distribution Characteristics of Microplastics in Surface Water and Sediments of Haizhou Bay, Lianyungang]. Huan jing ke xue= Huanjing kexue. 2020; 41: 3212-3221, 10.13227/j.hjkx.201910005. [DOI] [PubMed]
- 24.Yuan F., Zhao H., Sun H., Sun Y., Zhao J., Xia T. Investigation of microplastics in sludge from five wastewater treatment plants in Nanjing. China J environ manage. 2022;301 doi: 10.1016/j.jenvman.2021.113793. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y., Wang J., Zou M., et al. Microplastics in urban soils of Nanjing in eastern China: Occurrence, relationships, and sources. Chemosphere. 2022;303 doi: 10.1016/j.chemosphere.2022.134999. [DOI] [PubMed] [Google Scholar]
- 26.Saad D., Chauke P., Cukrowska E., et al. First biomonitoring of microplastic pollution in the Vaal river using Carp fish (Cyprinus carpio) “as a bio-indicator”. Sci Total Environ. 2022;836 doi: 10.1016/j.scitotenv.2022.155623. [DOI] [PubMed] [Google Scholar]
- 27.Yu X., Huang W., Wang Y., et al. Microplastic pollution in the environment and organisms of Xiangshan Bay, East China Sea: an area of intensive mariculture. Water Res. 2022;212 doi: 10.1016/j.watres.2022.118117. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang W., Zhang Y., Wang L., Barceló D., Wang Y., Lin C. Seasonal relevance of agricultural diffuse pollutant with microplastic in the bay. J Hazard Mater. 2020;396 doi: 10.1016/j.jhazmat.2020.122602. [DOI] [PubMed] [Google Scholar]
- 29.Lee H., Kunz A., Shim W.J., Walther B.A. Microplastic contamination of table salts from Taiwan, including a global review. Sci Rep. 2019;9:10145. doi: 10.1038/s41598-019-46417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liebezeit G., Liebezeit E. Synthetic particles as contaminants in German beers. Food Addit Contam. 2014;31:1574–1578. doi: 10.1080/19440049.2014.945099. [DOI] [PubMed] [Google Scholar]
- 31.Luo Y., Li L., Feng Y., et al. Quantitative tracing of uptake and transport of submicrometre plastics in crop plants using lanthanide chelates as a dual-functional tracer. Nat Nanotechnol. 2022;17:424–431. doi: 10.1038/s41565-021-01063-3. [DOI] [PubMed] [Google Scholar]
- 32.Mussa F.F., Horton J.D., Moridzadeh R., Nicholson J., Trimarchi S., Eagle K.A. Acute aortic dissection and intramural hematoma: a systematic review. JAMA. 2016;316:754–763. doi: 10.1001/jama.2016.10026. [DOI] [PubMed] [Google Scholar]
- 33.Casa L.D.C., Ku D.N. Thrombus formation at high shear rates. Annu Rev Biomed Eng. 2017;19:415–433. doi: 10.1146/annurev-bioeng-071516-044539. [DOI] [PubMed] [Google Scholar]
- 34.Wang L., Zhao Y., Yang F., et al. Biomimetic collagen biomaterial induces in situ lung regeneration by forming functional alveolar. Biomaterials. 2020;236 doi: 10.1016/j.biomaterials.2020.119825. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z., Zhu Q., Song E., Song Y. Characterization of blood protein adsorption on PM2.5 and its implications on cellular uptake and cytotoxicity of PM2.5. J Hazard Mater. 2021;414 doi: 10.1016/j.jhazmat.2021.125499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.